Abstract
Context
Transgender youth may initiate GnRH agonists (GnRHa) to suppress puberty, a critical period for bone-mass accrual. Low bone mineral density (BMD) has been reported in late-pubertal transgender girls before gender-affirming therapy, but little is known about BMD in early-pubertal transgender youth.
Objective
To describe BMD in early-pubertal transgender youth.
Design
Cross-sectional analysis of the prospective, observational, longitudinal Trans Youth Care Study cohort.
Setting
Four multidisciplinary academic pediatric gender centers in the United States.
Participants
Early-pubertal transgender youth initiating GnRHa.
Main Outcome Measures
Areal and volumetric BMD Z-scores.
Results
Designated males at birth (DMAB) had below-average BMD Z-scores when compared with male reference standards, and designated females at birth (DFAB) had below-average BMD Z-scores when compared with female reference standards except at hip sites. At least 1 BMD Z-score was < -2 in 30% of DMAB and 13% of DFAB. Youth with low BMD scored lower on the Physical Activity Questionnaire for Older Children than youth with normal BMD, 2.32 ± 0.71 vs. 2.76 ± 0.61 (P = 0.01). There were no significant deficiencies in vitamin D, but dietary calcium intake was suboptimal in all youth.
Conclusions
In early-pubertal transgender youth, BMD was lower than reference standards for sex designated at birth. This lower BMD may be explained, in part, by suboptimal calcium intake and decreased physical activity–potential targets for intervention. Our results suggest a potential need for assessment of BMD in prepubertal gender-diverse youth and continued monitoring of BMD throughout the pubertal period of gender-affirming therapy.
Keywords: transgender youth, gender-affirming medical treatment, gonadotropin-releasing hormone agonists, bone mineral density, DXA, bone QCT
An estimated 0.7% to 2.7% of American teenagers identify as transgender or gender nonconforming [1-3], and gender-affirming hormone therapy (GAH) for transgender and gender-diverse (TGD) youth in the United States has been provided for more than a decade [4]. Since then, access to pediatric GAH has rapidly expanded, with many prominent academic institutions establishing multidisciplinary clinics, often in partnership with community centers [5]. For youth who meet diagnostic criteria for gender dysphoria [6], current guidelines recommend GnRH agonists (GnRHa) to pause puberty as early as Tanner stage 2 to prevent physical changes inconsistent with the affirmed gender and to allow additional time for gender exploration [7, 8]. Little is known, however, about bone mineral density (BMD) or long-term consequences of early pubertal suppression on skeletal health in these youth.
Data from the Netherlands have shown that pretreatment BMD Z-scores determined by dual-energy X-ray absorptiometry (DXA) were significantly low in late-pubertal transgender girls before GnRHa and failed to normalize upon treatment with estradiol [9, 10]. Adult studies have similarly shown low BMD Z-scores in transgender women before and after GAH [11-13]. A UK study showed late-pubertal transgender boys had lower pretreatment BMD Z-scores by DXA at the spine and hip [14]. In contrast, another Dutch study that focused on transgender boys in late or postpuberty (median age, 16.5 years) showed normal mean pretreatment BMD Z-scores by DXA at the spine and hip [15]. Little is known, however, about BMD in early-pubertal transgender youth or about factors that impact skeletal health in this population, such as dietary calcium intake, vitamin D status, and weight-bearing exercise. Based on the low BMD Z-scores observed in the previously noted studies in late-pubertal adolescents and adults, further investigation of transgender youth in earlier stages of puberty is needed to determine when this disparity in BMD emerges.
We present pretreatment BMD data from our multisite cohort of 63 American TGD youth initiating puberty suppression in early puberty. Selected determinants of bone health were also examined to identify potential targets for intervention.
1. Materials and Methods
TGD youth, defined as those whose gender identity is atypical of the sex designated at birth, nonbinary, or fluid [16], were prospectively enrolled from 4 study sites (Children’s Hospital Los Angeles, Lurie Children’s Hospital, Boston Children’s Hospital, and University of California San Francisco Benioff Children’s Hospital) in the observational Trans Youth Care Study as previously described [17]. Eligible participants in this cohort were at Tanner stages 2-3 (based on breast or testicular examination) and initiating pubertal blockade with GnRHa. Primary outcomes were areal BMD (aBMD) and volumetric BMD (vBMD) Z-scores assessed by DXA and quantitative computed tomography (QCT), respectively. BMD was assessed before or no more than 2 months after the puberty blocker was initiated. Of the 95 participants in the puberty-blocker cohort, 13 were excluded because no DXA or QCT was performed, 13 were excluded because they were at Tanner stage 4 of puberty, and 6 were excluded because DXA was assessed more than 2 months after puberty blocker initiation. After these 32 participants were excluded, a total of 63 participants were included in the data analyses. More than 90% (57/63) of included participants had BMD assessed before initiation of GnRHa.
Because of the observational nature of this study, methods and machines used for assessment of BMD varied among the study sites. At Children’s Hospital Los Angeles, cortical and trabecular vBMD were assessed by QCT at midshaft femur and L1-L3 vertebral bodies, respectively [18]. Lurie Children’s Hospital used a GE/Lunar iDXA machine with scans of total body less head (TBLH) and lumbar spine. Boston Children’s Hospital and University of California San Francisco Benioff Children’s Hospital used Hologic Discovery A DXA machines with scans of TBLH, lumbar spine, and/or total hip (TH) and femoral neck. Because of insurance coverage considerations, 17% (11/63) of participants obtained their DXA scans from outside institutions. Pretreatment BMD Z-scores were analyzed according to the sex designated at birth, and adjustments according to height Z-scores were calculated for Hologic DXA scans to allow data comparison across sites [19]. Because of the significant differences in imaging modalities and body sites evaluated, aBMD and vBMD Z-scores were analyzed separately. After separate analyses of Lunar BMD Z-scores and height-adjusted Hologic BMD Z-scores yielded similar results [20], we pooled aBMD Z-score results. Serum 25-hydroxyvitamin D was measured by standard clinical assays, dietary calcium intake was assessed with a 1-week food inventory questionnaire, and physical activity was assessed with the Physical Activity Questionnaire for Older Children (PAQ-C) [21, 22], which rates physical activity for a variety of activities on a Likert scale (1 = lowest activity, 5 = highest activity).
All data analyses were performed using Stata, v16 (College Station, TX) [23] and were stratified by sex designated at birth or by whether low BMD was present, as defined by at least 1 BMD Z-score < -2. Comparisons between groups were performed using Student t tests. After verifying the assumption of normally distributed residuals and assessing departure from linearity, a linear regression model was used to determine whether chosen predictors were statistically significant predictors of BMD Z-scores. We set a significance level of α = 0.05 for all statistical analyses. Participant characteristics were compared among the 4 sites using ANOVA and did not exhibit statistically significant differences by site [20]; participants from all sites were therefore grouped for analyses.
2. Results
A.Demographics
Demographics of the cohort show essentially balanced sex designated at birth, majority white race (56%), majority total household income greater than $100 000 (75%), and majority of guardians/parents having completed undergraduate or graduate/professional degrees (68%) (Table 1). The majority of the participants reported a binary gender identity (92%), and 64% of the participants were in Tanner stage 2 of puberty. Differences in age at time of puberty blocker initiation between designated females at birth (DFAB) and designated males at birth (DMAB) reflect the expected pubertal timing of sex designated at birth, 11.0 ± 1.4 years vs. 12.1 ± 1.3 years (P = 0.002).
Table 1.
Demographics (n = 63 Participants)
| Percentage | |
|---|---|
| Sex designated at birth Male | 52.4% (n = 33) |
| Gender identity | |
| Binary | 92.1% (n = 58) |
| Nonbinary/fluid/queer | 7.9% (n = 5) |
| Race/ethnicity | |
| Non-Hispanic White | 55.6% (n = 35) |
| Hispanic/Latinx | 19.1% (n = 12) |
| Black/African-American | 3.2% (n = 2) |
| Multirace | 11.1% (n = 7) |
| Native American, Asian, Native Hawaiian | 4.8% (n = 3) |
| Unknown/no answer | 6.4% (n = 4) |
| Tanner stage | |
| 2 | 63.5% (n = 40) |
| 3 | 36.5% (n = 23) |
| Total household income (past 12 months) | |
| <$10 000 | 0% (n = 0) |
| $10 000-$14 999 | 1.6% (n = 1) |
| $15 000-$24 999 | 0% (n = 0) |
| $25 000-$34 999 | 6.4% (n = 4) |
| $35 000-$49 999 | 4.8% (n = 3) |
| $50 000-$74 999 | 6.4% (n = 4) |
| $75 000-$99 999 | 4.8% (n = 3) |
| $100 000-$149 999 | 30.2% (n = 19) |
| ≥$150 000 | 44.4% (n = 28) |
| Unknown/No Answer | 1.6% (n = 1) |
| Highest level of education of parents/guardians (n = 126) | |
| Some high school, no degree | 1.6% (n = 2) |
| High school graduate (or equivalent) | 0.8% (n = 1) |
| Some college, no degree | 7.9% (n = 10) |
| Vocational or technical program certificate | 6.3% (n = 8) |
| Associate’s degree | 4.0% (n = 5) |
| Bachelor’s degree | 30.2% (n = 38) |
| Master’s degree | 30.2% (n = 38) |
| Professional school degree | 7.9% (n = 10) |
| Doctorate degree | 7.1% (n = 9) |
| Unknown/no answer | 4.0% (n = 5) |
B. Primary BMD outcomes
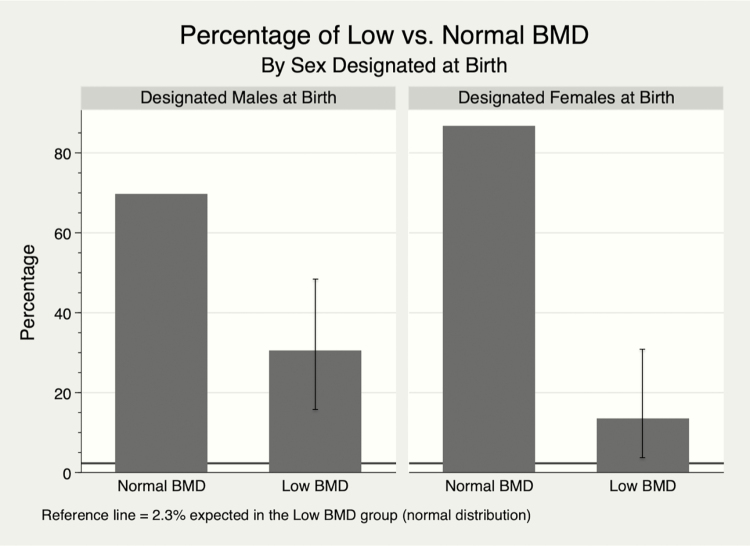
A low aBMD or vBMD Z-score, defined as < -2, was observed in 30% (10/33, 95% confidence interval [CI], 15.6-48.7) of DMAB and 13% (4/30, 95% CI, 3.8-30.7]) of DFAB, significantly higher rates than the 2.3% expected in a normal distribution (Fig. 1). When reviewing the subset of participants with low BMD, 25-hydroxyvitamin D levels were 28.7 ± 10.8 ng/mL, daily calcium intake was 520 ± 383 mg/d, PAQ-C scores were 2.32 ± 0.71, and BMI Z-scores were 0.08 ± 1.57. The average age at initiation of puberty blockers in this low BMD subset was 12.0 ± 1.7 years of age, at an average Tanner stage of 2.43 ± 0.51 (Table 2). Further stratification by sex designated at birth was performed; these data are separately reported [20]. Only 1 participant in this low BMD subset had a serum 25-hydroxyvitamin D < 20 ng/mL, and only 1 participant had a daily calcium intake ≥ 1300 mg/d. In comparison to the normal BMD group, the low BMD group had statistically significantly lower PAQ-C scores, 2.32 ± 0.71 vs. 2.76 ± 0.61 (P = 0.01).
Figure 1.
Percentage of low vs. normal BMD. Bar graph demonstrating markedly higher percentages of low BMD, as defined as at least one BMD Z-score < -2, in our cohort of transgender/gender diverse youth. Low BMD error bars denote 95% confidence intervals. Data are stratified by sex designated at birth and show that DMAB had a higher frequency of pretreatment low BMD than DFAB youth (0.30 ± 0.47 vs. 0.13 ± 0.35, P = 0.0545). Horizontal reference lines indicate the expected 2.3% to have BMD Z-score < -2 in a normal distribution. BMD, bone mineral density; DFAB, designated females at birth; DMAB, designated males at birth.
Table 2.
Selected Determinants of Bone Health, by Lowa vs. Normal BMD
| Low BMD Group (n = 14) |
Normal BMD Group (n = 49) |
P Value | |
|---|---|---|---|
| Age at blocker placement, years, mean ± SD (95% CI) |
12.0 ± 1.7 (11.1-13.0) |
11.5 ± 1.4 (11.1-11.8) |
0.9 |
| Tanner stage, mean ± SD (95% CI) | 2.43 ± 0.50 (2.25-2.62) |
2.30 ± 0.47 (2.14-2.47) |
0.3 |
| PAQ-C score, (1 = low, 5 = high), mean ± SD (95% CI) | 2.32 ± 0.71 (1.91-2.73) |
2.76 ± 0.61 (2.58-2.93) |
0.01 |
| Serum 25-hydroxyvitamin D, ng/mL, mean ± SD (95% CI) | 28.7 ± 10.8 (21.0-36.4) |
28.8 ± 9.3 (26.0-31.7) |
0.5 |
| Daily calcium intake, mg/day, mean ± SD (95% CI) |
520 ± 106 (289-752) |
637 ± 334 (541-733) |
0.1 |
| BMI Z-score, mean ± SD (95% CI) | 0.08 ± 1.57 (-0.83 to 0.99) |
0.40 ± 0.99 (0.12-0.68) |
0.2 |
| Height Z-score, mean ± SD (95% CI) | -0.27 ± 1.02 (-0.86 to 0.32) |
0.22 ± 1.12 (-0.10 to 0.54) |
0.07 |
aAt least 1 BMD Z-score < -2.
Abbreviations: BMD, bone mineral density; BMI, body mass index; CI, confidence interval; PAQ-C, Physical Activity Questionnaire for Older Children.
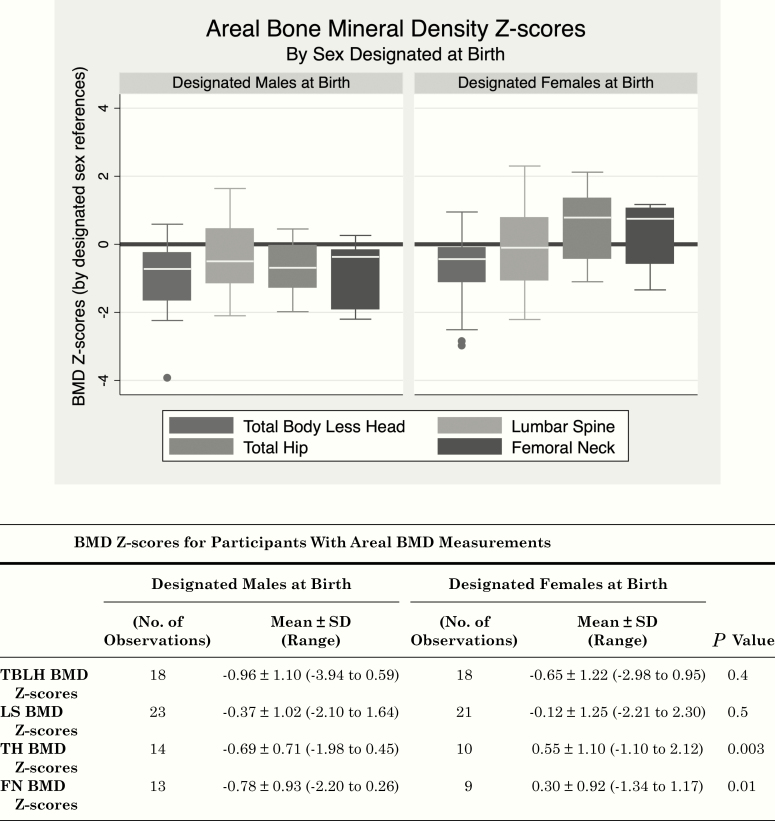
Both aBMD and vBMD Z-scores (Figs. 2 and 3) revealed mean BMD Z-scores consistently lower in DMAB than in DFAB, with a statistically significant difference at the hip sites, which primarily reflect measurements of cortical bone.
Figure 2.
Areal bone mineral density Z-Scores. Boxplots of areal BMD Z-scores (determined by reference standards for sex designated at birth) at 4 sites (FN, femoral neck; LS, lumbar spine; TBLH, total body less head; TH, total hip) are shown for designated males at birth (left) and designated females at birth (right). Boxes represent the interquartile ranges (IQR, 25th-75th percentile), white bars mark the median values, the whiskers show minimum (quartile 1-1.5 * IQR) and maximum values (quartile 3 + 1.5 * IQR), and points show outliers. BMD Z-scores from Hologic DXA machines (31/47) are height Z-score adjusted and combined with BMD Z-scores from Lunar DXA machines (16/47). BMD, bone mineral density. BMD, bone mineral density; FN, femoral neck; LS, lumbar spine; TBLH, total body less head; TH, total hip.
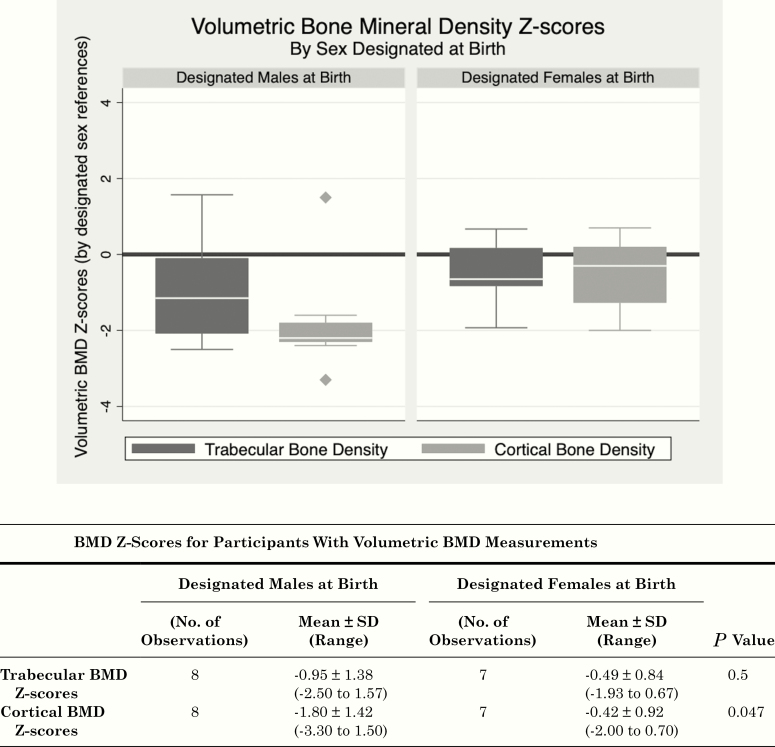
Figure 3.
Volumetric bone mineral density Z-Scores. Boxplots of volumetric BMD Z-scores (determined by reference standards for sex designated at birth) at two sites (trabecular bone density: L1-L3 vertebral bodies; cortical bone density: midshaft femur) are shown for designated males at birth (left) and designated females at birth (right). Boxes represent the interquartile ranges (IQR, 25th-75th percentile), white bars mark the median values, the whiskers show minimum (quartile 1-1.5 * IQR) and maximum values (quartile 3 + 1.5 * IQR), and points show outliers. BMD, bone mineral density.
C. Selected determinants of bone health
Review of the selected determinants of bone health by sex designated at birth showed that 15% (5 DMAB and 3 DFAB, 8/53 of TGD youth who had serum 25-hydroxyvitamin D measured) had vitamin D insufficiency (<20 ng/mL). Notably, the daily calcium intake of all TGD youth was suboptimal with mean 613 ± 345 mg daily, far below the recommended 1300 mg per day [24, 25]. Although these recommended dietary allowance values for calcium intake may be considered ambitious, prior literature based on National Health and Nutrition Examination Study data from 2003 to 2006 reported that 9- to 13-year-old children consumed approximately 1000 mg of calcium per day [26]. There were no statistically significant differences based on sex designated at birth in serum 25-hydroxyvitamin D, daily calcium intake, or BMI Z-scores (Table 3).
Table 3.
Selected Determinants of Bone Health, by Sex Designated at Birth
| Designated Females at Birth | Designated Males at Birth | P Value | |
|---|---|---|---|
| Age at blocker placement, years, mean ± SD (95% CI) |
11.0 ± 1.4 (10.5-11.5) |
12.1 ± 1.3 (11.7-12.6) |
0.002 |
| Tanner stage, mean ± SD (95% CI) | 2.43 ± 0.50 (2.25-2.62) |
2.30 ± 0.47 (2.14-2.47) |
0.3 |
| PAQ-C score, (1 = low, 5 = high), mean ± SD (95% CI) | 2.83 ± 0.57 (2.63-3.04) |
2.50 ± 0.69 (2.26-2.74) |
0.04 |
| Serum 25-hydroxyvitamin D, ng/mL, mean ± SD (95% CI) | 30.8 ± 7.3 (28.0-33.7) |
26.9 ± 11.0 (22.6-31.1) |
0.1 |
| Daily calcium intake, mg/day, mean ± SD (95% CI) |
540 ± 269 (441-640) |
676 ± 393 (540-813) |
0.1 |
| BMI Z-score, mean ± SD (95% CI) | 0.28 ± 1.05 (-0.11 to 0.67) |
0.38 ± 1.22 (-0.06 to 0.81) |
0.7 |
| Height Z-score, mean ± SD (95% CI) | -0.03 ± 1.17 (-0.46 to 0.39) |
0.25 ± 1.05 (-0.12 to 0.61) |
0.3 |
Abbreviations: BMD, bone mineral density; BMI, body mass index; CI, confidence interval; PAQ-C, Physical Activity Questionnaire for Older Children.
There were statistically significant differences in PAQ-C physical-activity scores between DFAB and DMAB, with DFAB reporting higher activity scores than DMAB, 2.83 ± 0.57 vs. 2.50 ± 0.69 (P = 0.04) (Table 3). For reference, the original validation studies of the PAQ-C in 1997 examined scores in 125 boys and 90 girls ages 9 to 15 years, in whom gender identity was not assessed, and showed mean PAQ-C physical-activity scores of 3.44 ± 0.68 for boys and 2.96 ± 0.69 for girls [21]. A more recent Canadian school-based assessment of 643 fifth graders, also without specific assessment of gender identity, showed mean PAQ-C physical-activity scores of 3.36 ± 0.72 for boys and 3.21 ± 0.72 for girls [27]. Given the population studies regarding prevalence of TGD individuals, we assume that the aforementioned studies [21, 27] primarily describe cis-gender youth.
D. Significant predictors of BMD Z-scores
Using a conceptual framework of determinants of BMD and bone health as previously described [28], a multivariate linear regression analysis was performed to evaluate for significant variables contributing to BMD Z-scores. The following predictors were included in the linear regression model: sex designated at birth, PAQ-C score, BMI Z-score, Tanner stage, age at puberty blocker placement, dietary calcium intake, and serum 25-hydroxyvitamin D (Table 4). BMI Z-scores were positive contributors to BMD Z-scores at the TBLH site (P < 0.0001). Female sex designated at birth (P = 0.04) and serum 25-hydroxyvitamin D (P = 0.048) were positive predictors and age at puberty blocker placement (P = 0.049) was a negative predictor of TH BMD Z-scores. Age at puberty blocker placement (P = 0.02) was a negative predictor of femoral neck BMD Z-scores. No predictors reached statistical significance for the trabecular and cortical vBMD Z-scores.
Table 4.
Predictors of BMD Z-scores: Multivariate Linear Regression Models
| Predictors of aBMD Z-scores | TBLH | LS | TH | FN | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β |
95% CI |
P | β | 95% CI | P | β |
95% CI |
P | β |
95% CI |
P | |
| (Constant) | -2.1 | -6.6 to 2.5 | 0.4 | -0.5 | -6.1 to 5.1 | 0.9 | 3.6 | -3.1 to 10.3 | 0.3 | 4.4 | -2.6 to 11.5 | 0.2 |
| Female sex designated at birth | 0.4 | -0.3 to 1.0 | 0.3 | 0.07 | -0.7 to 0.9 | 0.9 | 0.9 | 0.03 to 1.7 | 0.04 | 0.6 | -0.3 to 1.5 | 0.2 |
| PAQ-C score (1 = low, 5 = high) |
0.3 | -0.3 to 0.9 | 0.3 | 0.002 | -0.7 to 0.7 | 1.0 | -0.05 | -0.7 to 0.6 | 0.9 | 0.2 | -0.5 to 0.8 | 0.6 |
| BMI Z-score | 0.7 | 0.4 to 1.1 | <0.0001 | 0.3 | -0.05 to 0.7 | 0.09 | -0.2 | -0.2 to 0.6 | 0.4 | 0.2 | -0.2 to 0.6 | 0.4 |
| Tanner stage | -0.2 | -1.0 to 0.6 | 0.6 | 0.4 | -0.5 to 1.3 | 0.4 | 0.3 | -0.7 to 1.2 | 0.6 | 0.3 | -0.7 to 1.4 | 0.5 |
| Age at blocker placement, years | 0.06 | -0.3 to 0.4 | 0.7 | -0.1 | -0.5 to 0.3 | 0.6 | -0.5 | -0.9 to 0.002 | 0.049 | -0.6 | -1.1 to -0.1 | 0.02 |
| Daily calcium intake, mg/d | 0.0003 | -0.0008 to 0.001 | 0.6 | -0.00003 | -0.001 to 0.001 | 1.0 | -0.001 | -0.003 to 0.0006 | 0.2 | -0.0009 | -0.003 to 0.0008 | 0.3 |
| Serum 25-OH D, ng/mL | -0.0007 | -0.04 to 0.03 | 1.0 | 0.001 | -0.02 to 0.05 | 0.4 | 0.04 | 0.0006 to 0.08 | 0.048 | -0.0009 | -0.008 to 0.08 | 0.1 |
| Predictors of vBMD Z-scores | TBD | CBD | ||||||||||
| β | 95% CI | P | β |
95% CI |
P | |||||||
| (Constant) | -1.2 | -21.2 to 18.8 | 0.9 | -0.09 | -20.1 to 19.9 | 1.0 | ||||||
| Female sex designated at birth | -0.2 | -6.6 to 6.1 | 0.9 | 2.0 | -4.4 to 8.3 | 0.4 | ||||||
| PAQ-C score (1 = low, 5 = high) | 0.1 | -4.1 to 4.4 | 0.9 | 0.2 | -4.1 to 4.5 | 0.9 | ||||||
| BMI Z-score | -0.5 | -3.1 to 2.1 | 0.6 | -0.06 | -2.7 to 2.6 | 0.9 | ||||||
| Tanner stage | 1.2 | -4.0 to 6.5 | 0.5 | 0.8 | -4.5 to 6.0 | 0.7 | ||||||
| Age at blocker placement, y | -0.06 | -1.4 to 1.2 | 0.9 | -0.2 | -1.5 to 1.1 | 0.7 | ||||||
| Daily calcium intake, mg/d | -0.001 | -0.005 to 0.003 | 0.5 | -0.0009 | -0.004 to 0.003 | 0.5 | ||||||
| Serum 25-OH D, ng/mL | -0.04 | -0.4 to 0.3 | 0.7 | -0.06 | -0.4 to 0.3 | 0.6 |
Abbreviations: aBMD, areal bone mineral density; BMD, bone mineral density; BMI, body mass index; CI, confidence interval; PAQ-C, Physical Activity Questionnaire for Older Children; vBMD, volumetric bone mineral density.
In summary, female sex designated at birth and higher serum 25-hydroxyvitamin D were associated with higher TH BMD Z-scores, and later age at puberty blocker placement was associated with lower BMD Z-scores at the DXA hip sites. At the TBLH site, higher BMI Z-scores were associated with higher BMD Z-scores.
3. Discussion
We identified a high prevalence of low BMD (Z-score < -2) in early-pubertal TGD youth before starting GnRHa therapy, with higher rates in DMAB than in DFAB. Our findings extend prior studies in late-pubertal transgender youth [9], by demonstrating that low BMD is already present by early puberty and thus this disparity could arise before puberty. Earlier identification of low BMD in prepubertal TGD youth could therefore expand the time for potential interventions to mitigate this pretreatment discordance in BMD and, in turn, the expected further decrease in BMD Z-scores with GnRHa [9, 10, 14, 15]. Our linear regression results support this concept because age at puberty blocker placement was negatively associated with BMD Z-scores at the hip sites, suggesting that underlying factors contributing to low BMD may potentially have more time to exert negative effects. This negative association can also be explained, in part, by the differential timing of puberty in DMAB versus DFAB individuals, as DMAB youth had both lower BMD Z-scores and later ages at pubertal onset. Additionally, because eligibility was based on early-pubertal status, older individuals in the study cohort started puberty at the later end of the usual age range, so it is expected that they would have lower BMD compared with reference ranges based on youth who largely had more typical timing of puberty and thus had significant exposure to sex steroids by that age. Contribution of Tanner stage at time of blocker initiation to BMD Z-scores was not statistically significant at any anatomical sites, but the mostly positive β-coefficients in our regression models suggest that later Tanner stage at time of puberty blocker placement had a positive effect on BMD Z-scores, reflecting the positive effect of pubertal hormones on bone mineralization.
We additionally noted that there were statistically significant differences in BMD Z-scores at the hip sites between DMAB and DFAB groups. Although the International Society for Clinical Densitometry notes that the hip is not a preferred site for pediatric DXA measurements [29], the hip is primarily cortical bone, whereas the lumbar spine is primarily trabecular bone. The hip mineralizes earlier than the spine [30], such that we may be able to observe differences at the hip before they are apparent in other regions. The hip is also a weight-bearing site, and the lower BMD Z-scores in the DMAB youth make sense given the findings of lower PAQ-C scores in the DMAB youth. Finally, the International Society for Clinical Densitometry does suggest potential utility in proximal femur DXA measurements for assessing children with reduced weight-bearing of the lower extremities who would benefit from serial DXA measurements into adulthood [29].
With respect to determinants of skeletal health, PAQ-C scores were low overall and significantly lower in DMAB than DFAB youth, providing a potential explanation for the lower pretreatment BMD Z-scores in the DMAB group. However, regression models showed that PAQ-C scores could not completely account for these differences, suggesting that other factors may also contribute to this difference. Further reinforcing the postulation that lower physical activity contributes to the low BMD Z-scores are the statistically significantly lower PAQ-C scores in the group with low BMD when compared with the group with normal BMD. Prior studies according to recorded sex (gender identity was not ascertained) have reported that boys have higher PAQ-C scores than girls [21, 22]; thus, TGD youth in our study tended to have physical activity levels that correspond to gender identity.
In contrast to physical activity, no significant differences were found in serum 25-hydroxyvitamin D, dietary calcium intake, and BMI Z-scores between DFAB and DMAB groups, or between low-BMD and normal-BMD groups. However, daily calcium intake was globally suboptimal in our early-pubertal TGD youth cohort. The majority of the literature supports adequate calcium intake in improving BMD [28], with greater gains seen in those who begin supplementation at earlier stages of puberty [31] and who have lower baseline daily calcium intake [32]. However, there are still gains seen in those who are later in puberty and have higher baseline daily calcium intakes [33]. These results suggest that potential interventions for improving BMD could include standard recommendations for optimizing dietary calcium and vitamin D intake as well as increasing weight-bearing exercise, which could be initiated in the prepubertal to early-pubertal time period [34-36]. Additionally, BMI Z-scores were a significant positive predictor of BMD Z-scores at the TBLH site, reinforcing that careful assessment of physical activity and dietary history to screen for eating disorders should be done [37], particularly if low BMD is found.
Strengths of our study include assessments of dietary calcium intake, physical activity, and vitamin D status, which have not been reported previously in transgender youth. A limitation of this study is related to the observational and multisite nature of the Trans Youth Care study, such that BMD measurements were not standardized across all sites. Despite this limitation, we obtained comparable results across the different imaging modalities, lending robustness to our findings. As of yet, fracture data have not been reported in transgender adolescents and, thus, BMD Z-scores are the only current proxy for estimating future fracture risk.
It has been shown that significant bone mineralization occurs after linear growth is complete [30]. Because timing of puberty influences peak bone mineral content, such that later pubertal onset leads to lower adult bone mineral content [38-40], longitudinal follow-up of this cohort with continued skeletal imaging will be critical for understanding the trajectory of bone mineral accrual as these youth are treated with GnRHa and progress to treatment with gender-affirming sex steroids. Findings from this pretreatment analysis will be followed up by longitudinal assessments over time and will further inform our current treatment and monitoring protocols.
Acknowledgments
We thank all the study participants and their families for participating in the Trans Youth Care Study, as well as the clinical research coordinators. We thank Bo Fan, MD, and Ellen Fung, PhD, RD, CCD, for their invaluable advice regarding analysis of the bone density measurements.
Financial Support: Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (award numbers R01HD082554 and F32HD098763). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
Abbreviations
- aBMD
areal bone mineral density
- BMD
bone mineral density
- DFAB
designated female at birth
- DMAB
designated male at birth
- DXA
dual-energy X-ray absorptiometry
- GAH
gender-affirming hormone therapy
- GnRHa
GnRH agonist
- PAQ-C
Physical Activity Questionnaire for Older Children
- QCT
quantitative computed tomography
- TBLH
total body less head
- TGD
transgender and gender-diverse
- TH
total hip
- vBMD
volumetric bone mineral density
Additional Information
Disclosures Summary: J.Y.L., C.F., J.O.-K., R.G., and D.V.G. have no relevant financial interests to disclose. Y.-M.C. and S.M.R. have served on an advisory board for Endo Pharmaceuticals.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References and Notes
- 1. Herman JL, Flores AR, Brown TNT, Wilson BDM, Conron KJ. Age of Individuals Who Identify as Transgender in the United States. Los Angeles, CA: The Williams Institute: UCLA School of Law; 2017:1-13. [Google Scholar]
- 2. Rider GN, McMorris BJ, Gower AL, Coleman E, Eisenberg ME. Health and care utilization of transgender and gender nonconforming youth: a population-based study. Pediatrics. 2018;141(3):1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Johns MM, Lowry R, Andrzejewski J, et al. Transgender identity and experiences of violence victimization, substance use, suicide risk, and sexual risk behaviors among high school students - 19 states and large urban school districts, 2017. MMWR Morb Mortal Wkly Rep. 2019;68(3):67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Edwards-Leeper L, Spack NP. Psychological evaluation and medical treatment of transgender youth in an interdisciplinary “Gender Management Service” (GeMS) in a major pediatric center. J Homosex. 2012;59(3):321-336. [DOI] [PubMed] [Google Scholar]
- 5. Hsieh S, Leininger J. Resource list: clinical care programs for gender-nonconforming children and adolescents. Pediatr Ann. 2014;43(6):238-244. [DOI] [PubMed] [Google Scholar]
- 6. American Psychiatric Association. American Psychiatric Association. DSM-5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed Washington, D.C.: American Psychiatric Association. [Google Scholar]
- 7. Hembree WC, Cohen-Kettenis PT, Gooren L, et al. Endocrine treatment of gender-dysphoric/gender-incongruent persons: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab.. 2017;102(11):1-35. [DOI] [PubMed] [Google Scholar]
- 8. Coleman E, Bockting W, Botzer M, et al. Standards of care for the health of transsexual, transgender, and gender-nonconforming people, version 7. Int J Transgender. 2012;13(4):165-232. [Google Scholar]
- 9. Klink D, Caris M, Heijboer A, van Trotsenburg M, Rotteveel J. Bone mass in young adulthood following gonadotropin-releasing hormone analog treatment and cross-sex hormone treatment in adolescents with gender dysphoria. J Clin Endocrinol Metab. 2015;100(2):E270-E275. [DOI] [PubMed] [Google Scholar]
- 10. Vlot MC, Klink DT, den Heijer M, Blankenstein MA, Rotteveel J, Heijboer AC. Effect of pubertal suppression and cross-sex hormone therapy on bone turnover markers and bone mineral apparent density (BMAD) in transgender adolescents. Bone. 2017;95:11-19. [DOI] [PubMed] [Google Scholar]
- 11. Haraldsen IR, Haug E, Falch J, Egeland T, Opjordsmoen S. Cross-sex pattern of bone mineral density in early onset gender identity disorder. Horm Behav. 2007;52(3):334-343. [DOI] [PubMed] [Google Scholar]
- 12. Van Caenegem E, Taes Y, Wierckx K, et al. Low bone mass is prevalent in male-to-female transsexual persons before the start of cross-sex hormonal therapy and gonadectomy. Bone. 2013;54(1):92-97. [DOI] [PubMed] [Google Scholar]
- 13. Wierckx K, Mueller S, Weyers S, et al. Long-term evaluation of cross-sex hormone treatment in transsexual persons. J Sex Med. 2012;9(10):2641-2651. [DOI] [PubMed] [Google Scholar]
- 14. Joseph T, Ting J, Butler G. The effect of GnRH analogue treatment on bone mineral density in young adolescents with gender dysphoria: findings from a large national cohort. J Pediatr Endocrinol Metab. 2019;32(10):1077-1081. [DOI] [PubMed] [Google Scholar]
- 15. Stoffers IE, de Vries MC, Hannema SE. Physical changes, laboratory parameters, and bone mineral density during testosterone treatment in adolescents with gender dysphoria. J Sex Med. 2019;16(9):1459-1468. [DOI] [PubMed] [Google Scholar]
- 16. Turban JL, Ehrensaft D. Research review: gender identity in youth: treatment paradigms and controversies. J Child Psychol Psychiatry. 2018;59(12):1228-1243. [DOI] [PubMed] [Google Scholar]
- 17. Olson-Kennedy J, Chan YM, Garofalo R, et al. Impact of early medical treatment for transgender youth: protocol for the longitudinal, observational trans youth care study. JMIR Res Protoc. 2019;8(7):e14434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gilsanz V, Perez FJ, Campbell PP, Dorey FJ, Lee DC, Wren TA. Quantitative CT reference values for vertebral trabecular bone density in children and young adults. Radiology. 2009;250(1):222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96(10):3160-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee JY, Finlayson C, Olson-Kennedy J, et al. Supplemental data: Low bone mineral density in early pubertal transgender/gender diverse youth: findings from the Trans Youth Care Study. Dryad Digital Repository 2020. Deposited 7 May 2020. 10.7272/Q6N29V5N [DOI] [Google Scholar]
- 21. Crocker PR, Bailey DA, Faulkner RA, Kowalski KC, McGrath R. Measuring general levels of physical activity: preliminary evidence for the physical activity questionnaire for older children. Med Sci Sports Exerc. 1997;29(10):1344-1349. [DOI] [PubMed] [Google Scholar]
- 22. Kowalski KC, Crocker PRE, Faulkner RA. Validation of the physical activity questionnaire for older children. Pediatr Exerc Sci. 1997;9(2):174-186. [Google Scholar]
- 23. StataCorp. Stata Statistical Software. Vol Release 16 College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 24. Munns CF, Shaw N, Kiely M, et al. Global consensus recommendations on prevention and management of nutritional rickets. J Clin Endocrinol Metab. 2016;101(2):394-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140(4):817-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smith C, Clark AF, Wilk P, Tucker P, Gilliland JA. Assessing the effectiveness of a naturally occurring population-level physical activity intervention for children. Public Health. 2020;178:62-71. [DOI] [PubMed] [Google Scholar]
- 28. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int. 2016;27(4):1281-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. 2019 ISCD Official Positions - Pediatric. The International Society for Clinical Densitometry. 2019. [Google Scholar]
- 30. McCormack SE, Cousminer DL, Chesi A, et al. Association between linear growth and bone accrual in a diverse cohort of children and adolescents. JAMA Pediatr. 2017;171(9):e171769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cameron MA, Paton LM, Nowson CA, Margerison C, Frame M, Wark JD. The effect of calcium supplementation on bone density in premenarcheal females: a co-twin approach. J Clin Endocrinol Metab. 2004;89(10):4916-4922. [DOI] [PubMed] [Google Scholar]
- 32. Dibba B, Prentice A, Ceesay M, Stirling DM, Cole TJ, Poskitt EM. Effect of calcium supplementation on bone mineral accretion in Gambian children accustomed to a low-calcium diet. Am J Clin Nutr. 2000;71(2):544-549. [DOI] [PubMed] [Google Scholar]
- 33. Prentice A, Ginty F, Stear SJ, Jones SC, Laskey MA, Cole TJ. Calcium supplementation increases stature and bone mineral mass of 16- to 18-year-old boys. J Clin Endocrinol Metab. 2005;90(6):3153-3161. [DOI] [PubMed] [Google Scholar]
- 34. Kannus P, Haapasalo H, Sankelo M, et al. Effect of starting age of physical activity on bone mass in the dominant arm of tennis and squash players. Ann Intern Med. 1995;123(1):27-31. [DOI] [PubMed] [Google Scholar]
- 35. Gunter K, Baxter-Jones AD, Mirwald RL, et al. Impact exercise increases BMC during growth: an 8-year longitudinal study. J Bone Miner Res. 2008;23(7):986-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lappe JM, Watson P, Gilsanz V, et al. The longitudinal effects of physical activity and dietary calcium on bone mass accrual across stages of pubertal development. J Bone Miner Res. 2015;30(1):156-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Coelho JS, Suen J, Clark BA, Marshall SK, Geller J, Lam PY. Eating disorder diagnoses and symptom presentation in transgender youth: a scoping review. Curr Psychiatry Rep. 2019;21(11):107. [DOI] [PubMed] [Google Scholar]
- 38. Gilsanz V, Chalfant J, Kalkwarf H, et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr. 2011;158(1):100-105, 105 e101-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elhakeem A, Frysz M, Tilling K, Tobias JH, Lawlor DA. Association between age at puberty and bone accrual from 10 to 25 years of age. JAMA Netw Open. 2019;2(8):e198918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cousminer DL, Mitchell JA, Chesi A, et al. Genetically determined later puberty impacts lowered bone mineral density in childhood and adulthood. J Bone Miner Res. 2018;33(3):430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]