Abstract
Background
PD-1 and PD-L1 are frequently expressed in T-cell lymphomas. This provides a rationale for exploration of immune checkpoint inhibitors in the management of T-cell lymphomas.
Patients and Methods
In this phase II single-arm multicenter trial, patients with relapsed or refractory systemic T-cell lymphoma were treated with 200mg pembrolizumab intravenously every 21 days. Primary end point was progression-free survival (PFS). Secondary endpoints were response rate, overall survival (OS), response duration, and safety. We assessed PD-L1, p-AKT expression and peripheral blood immune cells as potential predictive biomarkers.
Results
Of 18 enrolled patients, 13 were evaluable for the primary endpoint. The trial was halted early after a preplanned interim futility analysis. The overall response rate (95%CI) was 33% (9–55%); 4 patients achieved a complete response (27%; 5–49%). Median PFS (95%CI) was 3.2 months (1.2–3.7), and median OS 10.6 months (3.2–100). Median duration of response was 2.9 months (0–10.1). Two of the 4 complete responders remain in remission >15 months. Rash was the most common adverse event (17%; n=3). The most common ≥grade 3 treatment emergent adverse events were rash and pneumonitis (11%; n=2 each). Neither PD-L1 nor p-AKT expression were associated with outcomes. However, a higher relative frequency of CD4+ T lymphocytes pre-treatment was associated with improved PFS (HR 0.15; 0.03–0.74).
Conclusion
Pembrolizumab demonstrated modest single agent activity in relapsed or refractory T-cell lymphoma.
Keywords: peripheral T cell lymphoma, angioimmunoblastic lymphoma, PD-1 inhibitor
MICROABSTRACT
The PD1/PD-L1 pathway may be a target for the treatment of T-cell lymphomas. Here, we treated 18 patients with relapsed or refractory T-cell lymphoma with the PD1-inhibitor pembrolizumab. Although we did not meet our primary objective of improving progression-free survival from 3 to 6 months, 5 of 13 evaluable patients showed a response providing a benchmark for future studies.
INTRODUCTION
Mature NK and T-cell lymphomas are a heterogeneous disease group comprised of over 25 subtypes based on the 4th revised edition of the World Health Organization (WHO) classification of lymphoid neoplasms 1. While clinically, phenotypically, genetically and molecularly diverse, outcomes, with very few exceptions, are worse than their B-cell counterparts and typically characterized by primary chemorefractoriness and frequent relapses 2, 3. Survival following relapse is generally poor and has not significantly improved over the last 2 decades 4, 5. With the exception of the CD30-directed immunoconjugate brentuximab vedotin, for the uniformly CD30-positive anaplastic large cell lymphoma (ALCL)6, response rates of approved single agents in the relapsed or refractory setting are suboptimal and range between 25–29% with progression-free survival usually ≤4 months 7–9, indicating an unmet need for new therapeutic approaches..
The rationale for exploring immune checkpoint inhibitors for T-cell lymphoma is supported by increased expression of PD-1 in a variety of NK/T-cell lymphomas by malignant T cells and within the tumor microenvironment 10, 11. Furthermore, immune “graft-versus-lymphoma” effect following allogeneic hematopoietic cell transplantation can result in durable remissions for patients with T-cell lymphoma 12, 13. Lesokhin and colleagues provided proof of the principle that immune checkpoint blockade by PD-1 inhibition can induce responses in T-cell lymphomas 14. Since then PD-1 inhibitors have shown significant activity in extranodal NK/T-cell lymphoma (ENKTL) and mycosis fungoides/Sézary syndrome 15, 16. Herein we report the results of the first prospective clinical trial assessing the safety and efficacy of the PD-1 inhibitor pembrolizumab in the management of relapsed or refractory systemic mature T-cell lymphomas.
PATIENTS AND METHODS
Study Design and Participants
In this investigator-initiated, multicenter, single-arm phase II trial we enrolled patients 18 years or older with relapsed or refractory mature T-cell lymphoma, who had received ≥1prior line of systemic therapy; had measurable disease; had an Eastern Cooperative Oncology Group performance status of ≤1; and had adequate hematologic, renal, pulmonary and liver function. Patients were excluded if they carried a diagnosis of immunodeficiency; were receiving any form of immunosuppressive therapy; had an active, recent or history of severe autoimmune disease; had known Human Immunodeficiency Virus (HIV) infection or active Hepatitis B or C; or were within ≤100 days of having undergone an allogeneic stem cell transplant. More detailed eligibility criteria are available online.
The study was performed at 5 US centers. Approval was obtained from the institutional review board at each institution. All patients provided written informed consent before study enrollment. The trial was registered at www.clinicaltrial.gov as #NCT 02535247.
Procedures
Patients received pembrolizumab at a flat dose of 200mg given intravenously every 3 weeks for up to 2 years, until disease progression, or unacceptable toxicity, whichever occurred first. Disease assessment was performed using computed tomography (CT), or CT combined with positron emission tomography (PET) 12 weeks after initiating treatment and every 12 weeks thereafter during the treatment phase. Responses were evaluated at each institution using the Revised Response Criteria for Malignant Lymphoma 17. To account for tumor flares, patients who at the first imaging time point demonstrated ≤2 new lesions, with the sum of all lesions < 70% greater than baseline, and were clinically stable without evidence of rapid clinical deterioration, were allowed to continue on treatment for 1 month; if reimaging at that time showed further evidence of progression by the response criteria, they were removed from the study and the date of progression was the date of the initial scan showing progression. Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 and classified as being unrelated, unlikely, possibly, probably, or definitely related to study treatment.
Pathology was reviewed centrally by a hematopathologist (E.A.) according to the WHO 2017 classification of lymphoid malignancies.
Correlative Biomarkers
PD-L1 and p-AKT expression in tumor samples
PD-L1 expression was determined on formalin-fixed, paraffin-embedded archival tumor samples at a central laboratory (QualTek Molecular Laboratories©) using the anti-human PD-L1 antibody (clone 22C3) generated at Merck Research Laboratories (Kenilworth, New Jersey). PD-L1 staining was scored as either 0, 1+, 2+, 3+, and an overall percentage (%) of reactive cells expressing PD-L1 was estimated. An H-Score was calculated, where H-Score was the product of score x % tissue stained positive for PD-L1 as previously described 18. AKT phosphorylation (p-AKT) level, a putative marker for PD-1 activated T-cell receptor signaling 19, was measured by immunohistochemical (IHC) staining performed at the Fox Chase Cancer Center Histopathology Facility using p-AKT antibody (Cell signaling. Cat# 3787). The intensity and percentage of positive malignant cells were evaluated by a hematopathologist and a p-AKT H-score similar to PD-L1 was calculated for each case.
Immune cell biomarkers
PD-1 and PD-L1 expression, leukocyte activation markers as well as NK cell function were determined on circulating lymphocytes pre-treatment, during treatment (prior to doses 2 and 3) and at time of relapse as previously described (details in supplement) 20. An NK cell degranulation assay was modified from Bryceson et al (details in supplement) 21. Flow Cytometry was performed using a Becton Dickson FACSAria II flow cytometer (details in supplement).
Outcomes
The primary outcome was progression-free survival (PFS) defined as time from date of registration to disease progression or death, whichever occurred first. Secondary objectives included overall survival (OS; defined as time from date of registration to death), overall response rate (ORR; defined as complete response or partial response), duration of response (time from first response to time of relapse or death), safety and tolerability. Exploratory objectives included the association of PD-L1 expression and treatment response. For biomarker analysis, we defined non-progressors as everyone with stable disease or better on their first response assessment at 12 weeks.
Statistical Analysis
The study used a two-stage design with early stopping for futility rules based on median PFS testing the null hypothesis that median PFS was ≤3 months versus the alternative that it was ≥6 months 22. An initial cohort of 13 patients evaluable for progression at 3 months needed to be enrolled; if more than 6 patients were progression-free at 3 months, an additional 11 patients would be enrolled. If 10 out of 24 evaluable patients were progression-free at 6 months, the null hypothesis could be rejected. Patients who were not evaluable for PFS at 3 months were replaced. The study had an 80% power and 4.7% type I error. The chance of early stopping, after 13 patients, was 50% under the null and 5.5% in error. All patients who received ≥1 dose of the study drug were included in the safety analysis. The early stopping rules for safety mandated study interruption and/or termination if 3 of the first 13 treated patients or 4 of the first 24 treated patients had to discontinue study treatment due to treatment related adverse events. The chance of early termination with a true toxicity of 25% or 5% was 67% and 2.5%, respectively. The overall chance of study termination under the null of 5% true toxicity was 4.2% and 89% if true toxicity were 25%.
The Kaplan-Meier method was used to characterize the OS and PFS distributions. For survival analysis, patients were censored on the date of progression, death or last follow-up, respectively. Analyses of correlative biomarkers were exploratory. Pearson’s coefficients were computed to estimate correlations between continuous variables. Wilcoxon rank sum tests were used to compare pre-treatment biomarker values between groups (e.g., responders vs. non-responders) and Wilcoxon sign-rank tests were computed to assess changes from baseline in sequential samples drawn from the same patient. Cox proportional hazard models were used to estimate the effects of correlative biomarkers on PFS, where biomarker values were scaled by their standard deviations to facilitate the comparison of relative hazards between covariates with different ranges. Storey’s method 23 was used to account for multiple testing, and cell surface markers with p-values < 0.01 and a false discovery rate (FDR) <20% were considered significant. Statistical analyses were performed using SAS V9.4, Matlab Statistics and Machine Learning Toolbox (The Mathworks).
RESULTS
Patient Characteristics and Disposition
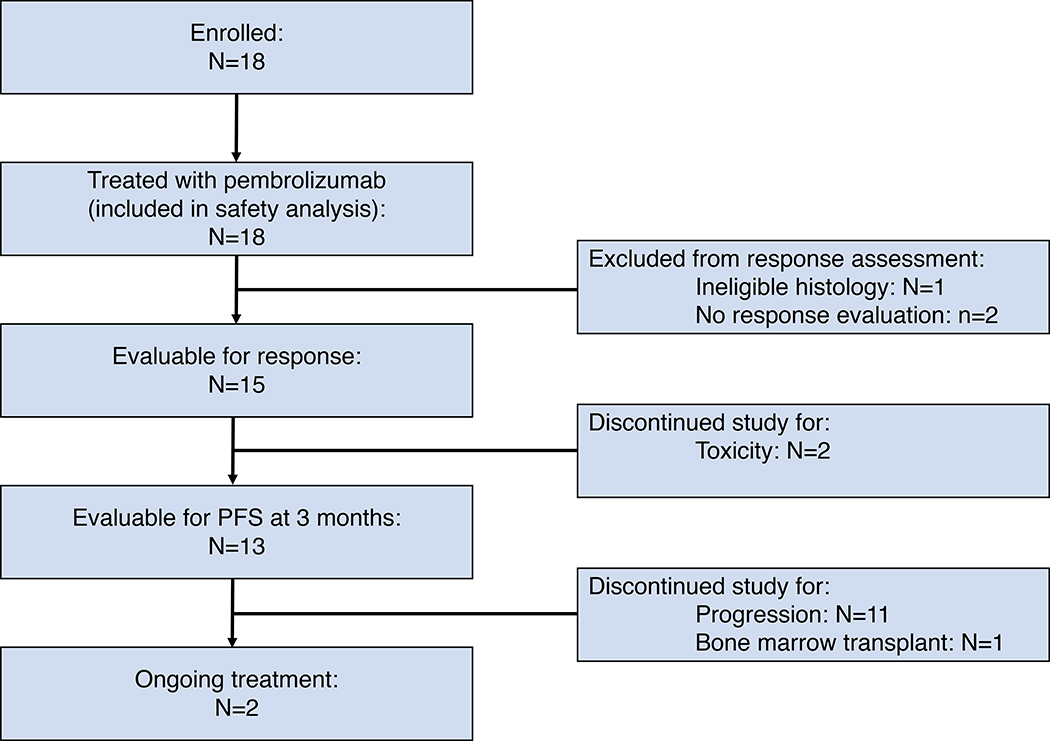
Eighteen patients were enrolled between February 2016 and August 2017. All enrolled patients were included in the safety analysis; 1 patient was excluded from efficacy analysis for ineligible histology after central pathology review. Only 15 patients of the 17 eligible patients had response data available (2 patients were taken off study for toxicity after only 1 dose of pembrolizumab – see below) and 13 patients were evaluable at 3 months for the primary endpoint PFS (Figure 1). The trial was halted early for futility as less than 6 of 11 patients evaluable for PFS at 3 months were free of progression. The median patient age was 71 years and 47% were male. The most common histologies were Peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS; n=7), follicular T-cell lymphoma (FTL; n=4) and transformed mycosis fungoides (MF; n=3). The median number of prior therapies was 2 and 65% of patients were refractory to their last treatment (see Table I).
Fig. 1.
CONSORT diagram indicating patient flow.
Table I.
Patient Characteristics (n=17)*
| Age, years (median; range) | 71 (18–88) |
| Sex (male; %) | 8 (47) |
| Histology (n; %) | |
| PTCL-NOS | 7 (41) |
| FTL | 4 (18) |
| tMF | 3 (18) |
| Other† | 3 (23) |
| Number of prior therapies (median; range) | 2 (1–9) |
| >2 prior therapies (n; %) | 6 (35) |
| Refractory to last therapy (%)‡ | 11 (65) |
Abbreviations: PTCL-NOS, peripheral T-cell lymphoma, not otherwise specified; FTL, follicular T-cell lymphoma (includes angioimmunoblastic T-cell lymphoma); tMF, transformed mycosis fungoides.
Patient with ineligible histology not included
“Other” includes monomorphic epitheliotropic T-cell lymphoma, hepatosplenic T-cell lymphoma, Alk-negative anaplastic large cell lymphoma (n=1 each)
defined as either not responding to or relapse within 3 months of prior therapy
Response Rate
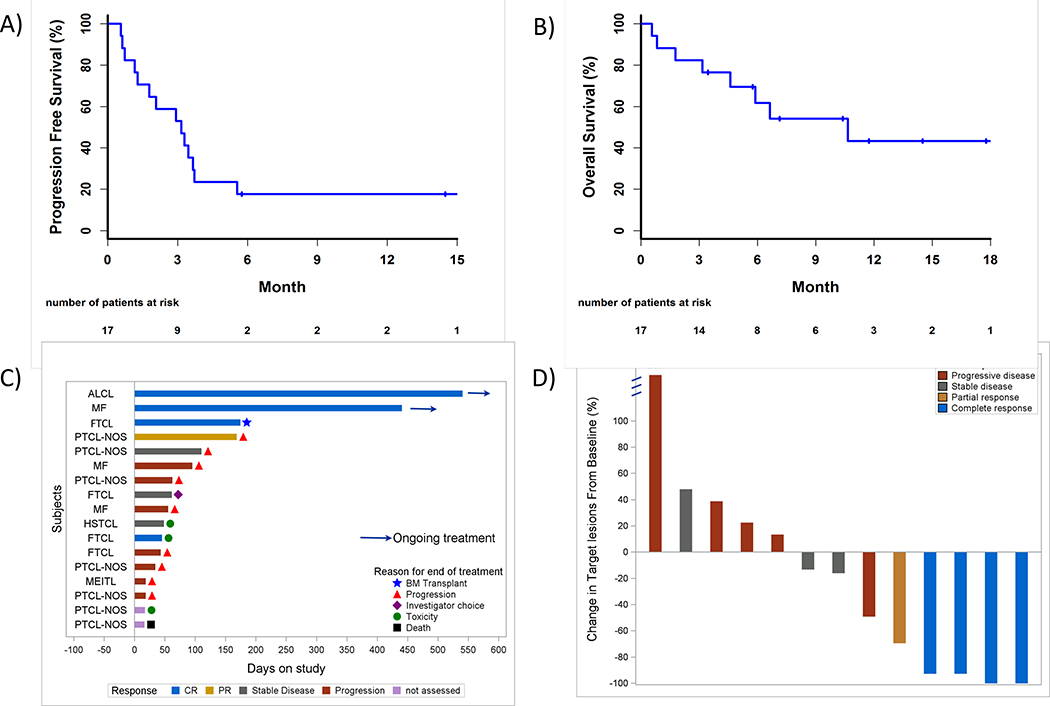
Two of the 17 eligible patients came off study without response assessment (1 patient developed a toxicity (pneumonitis) after 1 cycle and started a new therapy; 1 patient died of an unrelated toxicity after only 1 dose). The ORR was 33% (5/15 patients; 95%CI 9–57%) with 4 patients achieving a complete response (CR: 27%; 95%CI 4–49%). The median time to best response was 114 days (range 82–146). Figure 2D shows the changes of tumor volume at time of best response.
Fig. 2. Treatment outcomes.
Kaplan-Meier curves for A) Progression-free survival and B) Overall survival. Swimmer plot in C) demonstrates response duration, where each bar represents 1 subject and the length of the bar represents length of time on study. Best percent change in tumor volume is shown in D).
Survival
After a median follow up of 5.9 months (95%CI 0–18), median PFS and OS were 3.2 (95%CI 1.2–3.7) and 10.6 months (95%CI 3.2–100), respectively (Figure 2A–B). Out of 17 evaluable patients, 88% discontinued the study: 11 patients due to progression; 1 due to death; 2 patients due to toxicities; 1 each by patient choice, investigator choice and for hematopoietic cell transplantation (Figure 2C). None of the responders experienced progression on treatment: one responder discontinued treatment due to a toxicity after only 1 dose (vasculitis); another responder came off in CR for a hematopoietic cell transplant; two responders remain in remission after >15 months and are still receiving treatment at the time of manuscript preparation (median duration of response 2.9 months, 95% C 0–10.1).
Safety
A summary of toxicities attributed to pembrolizumab (possibly, probably, or definitively related) that occurred in >1 patient is provided in Table II. The most common adverse events (AE) were rash (n=3, 17%), followed by hypothyroidism, watering eyes, chills, edema, fatigue, fever, injection reactions, dyspnea, and pneumonitis (n=2; 11% each). While 12 of 18 (67%) treated patients experienced grade ≥3 AE, the investigators attributed only 22% (n=4) of the grade ≥3 AE as treatment related (Table III) with pneumonitis and rash being the most common (n=2; 11% each). There were no treatment attributed deaths. Two patients discontinued treatment for toxicities (n=1 for pneumonitis and vasculitis each). Immune-related AEs occurred in 12 patients (Supplemental Table II) and included rash (17%), hypothyroidism, pneumonitis (11% each), adrenal insufficiency, diarrhea, liver test abnormalities, and vasculitis (6% each). One patient developed hemophagocytic lymphohistiocytosis at the same time as disease progression. One patient died while on treatment. This patient experienced septic shock shortly after receiving the first dose of pembrolizumab secondary to a presumed soft tissue infection following an excisional lymph node biopsy.
Table II.
Adverse events at Least Possibly Related to Pembrolizumab that Occurred in at Least 2 Patients in the Safety Population (N=18)
| Toxicity | Total N (%) | Grade N (%) | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Hypothyroidism | 2 (11) | 1 (6) | 1 (6) | |||
| Watering eyes | 2 (11) | 1 (6) | 1 (6) | |||
| Chills | 2 (11) | 2 (11) | ||||
| Edema | 2 (11) | 2 (11) | ||||
| Fatigue | 2 (11) | 2 (11) | ||||
| Fever | 2 (11) | 2(11) | ||||
| Injection reaction | 2 (11) | 2 (11) | ||||
| Dyspnea | 2 (11) | 1 (6) | 1 (6) | |||
| Pneumonitis | 2 (11) | 1 (6) | 1 (6) | |||
| Rash | 3 (17) | 1 (6) | 2 (11) | |||
Table III.
Grade ≥3 Adverse Events at Least Possibly Related to Pembrolizumab in the Safety Population (N=18)
| Toxicity | Total N (%) | Grade N (%) | ||
|---|---|---|---|---|
| 3 | 4 | 5 | ||
| Febrile Neutropenia | 1 (6) | 1 (6) | ||
| Hemophagocytic Lymphohistiocytosis (HLH) | 1 (6) | 1 (6) | ||
| Lung Infection | 1 (6) | 1 (6) | ||
| Hyperglycemia | 1 (6) | 1 (6) | ||
| Hyponatremia | 1 (6) | 1 (6) | ||
| Muscle Weakness | 1 (6) | 1 (6) | ||
| Peripheral Sensory Neuropathy | 1 (6) | 1 (6) | ||
| Pleural Effusion | 1 (6) | 1 (6) | ||
| Pneumonitis | 2 (11) | 1 (6) | 1 (6) | |
| Rash | 2 (11) | 1 (6) | 1 (6) | |
| Hypotension | 1 (6) | 1 (6) | ||
| Vasculitis | 1 (6) | 1 (6) | ||
Biomarker Evaluation
PDL-1 and p-AKT Expression and Response
As assessed by IHC on FFPE tumor biopsy samples, median PD-L1 H-scores were 115 in patients who achieved a complete response vs 30 in those who did not (p=0.38). Similarly, median p-AKT-expression was not significantly higher in responders (H-score 30 (responders) vs. 0 (non-responders), p=0.73). Of note, PD-L1 and p-AKT expression were highly correlated (Pearson correlation coefficient 0.99).
Immune Cell Biomarkers and Outcomes
Baseline Predictive Markers
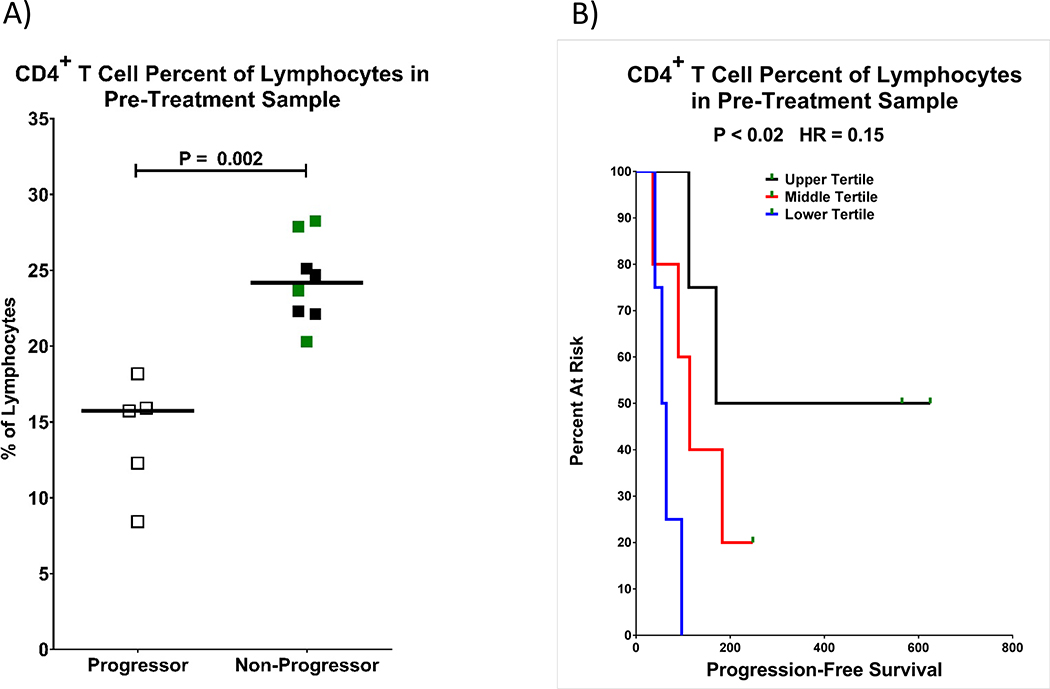
When comparing the baseline peripheral blood lymphocyte composition between progressors and non-progressors by flow cytometry, we found that the median percentage of CD4+ T cells within the total lymphocyte population at baseline was significantly higher in non-progressors (24% vs. 16%, p≤0.002 FDR 3%). In general, patients with higher pre-treatment CD4+ T cell % had longer PFS (Hazard Ratio (HR) 0.15, 95%CI 0.03–0.74, p<0.02; FDR 49%; Fig 3). While the FDR for this test did not meet our criteria for significance, we view the association between CD4+ T cell percentages with PFS as additional support for this biomarker’s association with subsequent clinical outcomes. Unfortunately, we were unable definitively establish the influence of contaminating tumor cells in the lymphocyte gate of peripheral blood in seven of the 13 analyzed patients, due to the lack of clearly defined biomarker features based on pathology reports. Therefore, our calculated values of % CD4+ T cells within lymphocytes may be slightly over- or under-estimated for some patients.
Fig. 3. Relative percentage of CD4+ T lymphocytes and outcomes.
Patients who started out with a higher relative percentage of peripheral blood CD4+ T cells had a lower risk of progression. CD4+ T cells are gated as viable, CD45+ SSClow,CD3+ CD4+. A) CD4+ T cell percent of total lymphocytes (CD45+ SSClow) are shown with progressors as open squares and non-progressors as filled squares. Patients with a complete response are shown in green, while those with stable disease or partial response are shown in black. Statistical significance was measured with a Wilcoxon rank-sum test. B) Kaplan-Meyer curves of progression-free survival are shown as a function of CD4+ T cell percent of lymphocytes with the upper tertile in black, middle tertile in red, and lower tertile in blue. Green rectangles indicate censored data points. The P-value and hazard ratio were determined by a Cox proportional hazard regression.
Improved survival was not associated with % CD8+ T cells of lymphocytes, nor CD4/CD8 ratio (data not shown).
Dynamic changes with treatment
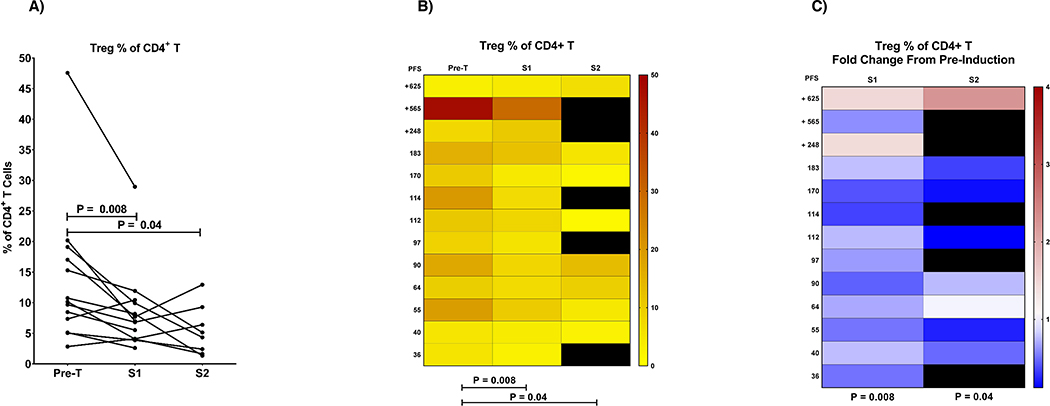
We also observed a significant reduction in the percentage of regulatory T cells (Treg) within the total CD4+ T cell population during treatment (% reduction after first dose (p=0.008; FDR 19%); Figure 4). This reduction however was not significantly associated with response or survival.
Fig. 4. Dynamic changes of regulatory CD4+ T cells (Treg) during treatment.
Percentage of regulatory CD4+ T cells (Treg) was reduced by pembrolizumab treatment in most patients. Regulatory T cells were gated as viable, CD45+, CD3+, CD4+, CD25high, CD127low. The percentages of CD4+ T cells that are CD25high and CD127low are shown here, a population that is essentially absent in CD8+ T cells. All three plots are different representations of the same data. A) Filled circles indicate the Treg percentage of CD4+ T cells with data points from pretreatment (pre-T) and post-treatmentsamples1and2 (S1 and S2) of the same patient connected by lines. B) shows a heat map representation of the Treg percentage of CD4+ T cells according to the scale on the right. C) shows the fold change in Treg percentage of CD4+T cells as a fold change from the pre-treatment sample according to the scale on the right. Black areas in both B) and C) represent missing data.
DISCUSSION
Our study represents the first prospective clinical trial evaluating a PD-1 inhibitor exclusively in mature systemic T-cell lymphomas. Single agent pembrolizumab resulted in an overall response rate of 33% in patients with relapsed or refractory mature T-cell lymphoma, which is comparable to other single agents in this setting. The observed adverse events were in line with the previously described toxicity profile of PD-1 inhibitors. Some responders exhibited long response durations. Specifically, patients with higher relative frequency of CD4+ T-cells pre-treatment appeared to derive the greatest benefit.
Tumor control in patients treated with PD1-inhibitors is related to lifting suppressed host immunity. Tumor related biomarkers associated with response to PD1-inhibitors include increased PD-L1 expression on tumor cells 24, an immune-inflamed phenotype of the tumor microenvironment 25, tumor mutational landscape and load 26, and DNA mismatch repair deficiency 27. There is reason to believe that PD1-blockade may have a higher impact on tumor control in NK- or T-cell lymphomas that are associated with viral infections, which have been linked to evasion of immune detection. Epstein-Barr virus (EBV) can be detected not only in the majority of ENKTL and AITL, but also in up to 40% of other T-cell lymphomas 28, 29. EBV infection can induce PD-L1 expression by stimulating JAK/STAT pathways 30. As such, a high response rate to PD-1 inhibition has been shown in ENKTL 15. In our study with limited number of patients, we did not observe an association between response and disease subtype, EBV expression (though it was not an information consistently available), PD-L1 expression, or p-AKT expression, which we studied because PD-1 activation putatively inhibits T-cell receptor-mediated signaling through AKT 19.
On the other hand, certain pre-treatment and dynamic immune biomarkers were associated with response and progression in our study. We found that patients with a higher percentage of CD4+ T cells at baseline appeared to have longer PFS. This observation has to our knowledge not been described previously and may have significant implications on selecting patients who may have the best chance of benefitting from PD1-blocking immunotherapy. While tumor-derived biomarkers are being explored extensively, the impact of host-derived predictive biomarkers is less known, especially in the peripheral blood. Patients with primary or acquired immunodeficiencies have been commonly excluded from trials with immune checkpoint inhibitors. Nevertheless, responses in patients infected with HIV have been described 31, 32. A trial specifically exploring the impact of immune checkpoint blockade in people living with HIV and cancer is currently ongoing (ClinicalTrials.gov ID NCT02408861). Cytotoxic chemotherapy is associated with various degrees of CD4+ T cell- and lymphodepletion, which has been associated with worse cancer-specific outcomes 33, 34. This may be of particular relevance in immunotherapy. Higher relative lymphocyte counts are associated with favorable OS in patients with melanoma treated with checkpoint inhibitors 35, 36. Therefore, if the amount of lymphocytes and specifically CD4+ T cells pre-treatment predicts response to immunotherapy, usage in earlier lines of therapy, where lymphopenia is less prevalent, may be important 37. In our study, we observed higher odds of achieving a CR in patients who had received 2 or less prior therapies compared to patients receiving >2 prior therapies (OR 9.0, 95% CI 0.4–203.3; p=0.08). It must be noted that the % T cells within lymphocytes in our PTCL patients is substantially lower (<30%) compared to that of healthy individuals (about 40%, which is also consistent with our historic controls; data not shown). We conclude that the T cell compartment is compromised in these patients, and we cannot exclude the possibility that those patients with CD4+ T cell frequencies closer to the normal range inherently have better overall prognosis. In fact, increased frequency of CD4+ T cells in peripheral blood has been shown to predict improved overall survival in B cell lymphomas38, 39.
We also identified a relative reduction of Tregs in the peripheral blood after treatment, although the degree of change was not associated with outcomes. The reduction of Tregs in the tumor environment has been described as an important mechanism leading to cancer control by immune checkpoint inhibitors 40. A higher relative frequency of Tregs in peripheral blood has been shown to be associated with favorable outcomes with checkpoint inhibition 36. Also, an increase in Tregs after therapy with pembrolizumab has been associated with progression in melanoma 41. These findings all support an important role of host immunity in cancer immunotherapy, but need to be confirmed in a larger cohort.
While we observed a response rate of pembrolizumab akin to other single agents in this setting 7–9, there is concern that PD-1 inhibition may accelerate T-cell lymphoma progression. Wartewig and colleagues described a murine T-cell lymphoma xenograft model where oncogenic T cell signaling upregulated PD-1 expression and PD-1 functions via PTEN/PI3K/AKT as an inhibitory loop to suppress oncogenic effector pathways. PD-1 ligation as well as treatment with PD-1 and PD-L1 blocking antibodies promoted tumorigenesis in vivo, while PI3K inhibition reversed the effect of “PD-1 deficiency” and led to prolonged survival 42. Supporting the notion that PD-1 blockade in T-cell lymphoma may be harmful, a recent report described 3 patients on a phase 2 clinical trial for adult T-cell Lymphoma/Leukemia (ATLL), who experienced rapid disease progression after a single dose of the PD1 inhibitor nivolumab 43. Although we did not observe unexpectedly rapid disease progression in our trial, a potential detrimental effect in a subset of patients cannot be excluded. Whether this is specific for ATLL, which is caused by the human T-cell leukemia virus type 1(HTLV-1) and is frequently associated with PD1 overexpression, remains unclear 44. An explanation for the harmful effect of PD1 Inhibition in ATLL may be the interference of the HTLV-1 basic zipper protein with PD1-signaling 45.
CONCLUSIONS
Despite the limitations of our study related to the small sample size and heterogeneity of T-cell lymphoma subtypes, we were able to demonstrate encouraging responses to pembrolizumab in the treatment of T-cell lymphomas. Progress in moving the bar in the treatment of T-cell lymphoma has been hampered by the heterogeneity and rarity of the disease. Our findings, in the context of recently published reports that show the potential for benefit and harm with PD1 inhibition, emphasize that better understanding of the effects of PD1 inhibition on malignant T cells and the lymphoma tumor microenvironment will be required to optimize immunotherapy in T-cell lymphoma. A combinatorial approach to dampen the potential for accelerating tumorigenesis may be warranted. Follow up study combining pembrolizumab other agents are planned. Correlative biomarker studies assessing the host immune system in addition to tumor-related factors will be essential to identify patients who will benefit most from immunotherapy.
Supplementary Material
CLINICAL PRACTICE POINTS.
PD-1 inhibitors have shown activity in mycosis fungoides and extranodal NK/T-cell lymphoma, but less is known about their activity in other T-cell lymphoma subtypes. There is concern however, that administration of PD-1 inhibitors may accelerate tumor progression in some T-cell lymphomas, such as adult T-cell leukemia/lymphoma. In this phase 2 single arm study of the PD1-inhibitor pembrolizumab, we found that 33% of evaluable patients responded to pembrolizumab therapy with some patients achieving durable responses. The observed adverse events were in line with the known side effect profile of PD-1 inhibitors. Interpretation of the study is limited by the small patient number and the multiple different T-cell lymphoma subtypes in participants. Nevertheless, the results provide a benchmark for single agent activity of pembrolizumab in this population and furthermore support future exploration of immune checkpoint inhibitors as treatment for T-cell lymphoma either alone or in combination with other agents in well-defined and -characterized T-cell lymphoma subgroups.
ACKNOWLEDGMENTS
This clinical trial was supported by a research grant from Merck and by grant #15-175-22 from the American Cancer Society.
CONFLICTS-OF-INTEREST
S.K.B. is an investigator funded by Merck to perform this study. R. S. is currently an employee of GlaxoSmithKline. Y.O. is currently an employee of Jazz Pharmaceuticals. The other authors declare no competing financial interests.
REFERENCES
- 1.Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vose J, Armitage J, Weisenburger D. International peripheral T-cell and natural killer/T-cell lymphoma study: pathology findings and clinical outcomes. J Clin Oncol. 2008;26:4124–4130. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Feldman T, Kroll-Desrosiers AR, et al. Peripheral T-cell lymphomas in a large US multicenter cohort: prognostication in the modern era including impact of frontline therapy. Ann Oncol 2014;25:2211–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mak V, Hamm J, Chhanabhai M, et al. Survival of patients with peripheral T-cell lymphoma after first relapse or progression: spectrum of disease and rare long-term survivors. J Clin Oncol. 2013;31:1970–1976. [DOI] [PubMed] [Google Scholar]
- 5.Chihara D, Fanale MA, Miranda RN, et al. The survival outcome of patients with relapsed/refractory peripheral T-cell lymphoma-not otherwise specified and angioimmunoblastic T-cell lymphoma. Br J Haematol. 2017;176:750–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pro B, Advani R, Brice P, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30:2190–2196. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor OA, Pro B, Pinter-Brown L, et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. Journal of Clinical Oncology. 2011;29:1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coiffier B, Pro B, Prince HM, et al. Results From a Pivotal, Open-Label, Phase II Study of Romidepsin in Relapsed or Refractory Peripheral T-Cell Lymphoma After Prior Systemic Therapy. Journal of Clinical Oncology. 2012;30:631–636. [DOI] [PubMed] [Google Scholar]
- 9.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results of the Pivotal Phase II BELIEF (CLN-19) Study. Journal of Clinical Oncology. 2015;33:2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox RA, Wada DA, Ziesmer SC, et al. Monocytes promote tumor cell survival in T-cell lymphoproliferative disorders and are impaired in their ability to differentiate into mature dendritic cells. Blood. 2009;114:2936–2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andorsky DJ, Yamada RE, Said J, Pinkus GS, Betting DJ, Timmerman JM. Programmed death ligand 1 is expressed by non-hodgkin lymphomas and inhibits the activity of tumor-associated T cells. Clin Cancer Res. 2011;17:4232–4244. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg JD, Chou JF, Horwitz S, et al. Long-term survival in patients with peripheral T-cell non-Hodgkin lymphomas after allogeneic hematopoietic stem cell transplant. Leuk Lymphoma. 2012;53:1124–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith SM, Burns LJ, van Besien K, et al. Hematopoietic Cell Transplantation for Systemic Mature T-Cell Non-Hodgkin Lymphoma. Journal of Clinical Oncology. 2013;31:3100–3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. Journal of Clinical Oncology. 2016;34:2698–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwong YL, Chan TSY, Tan D, et al. PD1 blockade with pembrolizumab is highly effective in relapsed or refractory NK/T-cell lymphoma failing l-asparaginase. Blood. 2017;129:2437–2442. [DOI] [PubMed] [Google Scholar]
- 16.Khodadoust M, Rook AH, Porcu P, et al. Pembrolizumab for Treatment of Relapsed/Refractory Mycosis Fungoides and Sezary Syndrome: Clinical Efficacy in a Citn Multicenter Phase 2 Study. Blood. 2016;128:181–181. [Google Scholar]
- 17.Cheson BD, Pfistner B, Juweid ME, et al. Revised Response Criteria for Malignant Lymphoma. Journal of Clinical Oncology. 2007;25:579–586. [DOI] [PubMed] [Google Scholar]
- 18.Dolled-Filhart M, Locke D, Murphy T, et al. Development of a Prototype Immunohistochemistry Assay to Measure Programmed Death Ligand-1 Expression in Tumor Tissue. Archives of pathology & laboratory medicine. 2016;140:1259–1266. [DOI] [PubMed] [Google Scholar]
- 19.Parry RV, Chemnitz JM, Frauwirth KA, et al. CTLA-4 and PD-1 Receptors Inhibit T-Cell Activation by Distinct Mechanisms. Molecular and cellular biology. 2005;25:9543–9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacFarlane AWt, Jillab M, Plimack ER, et al. PD-1 expression on peripheral blood cells increases with stage in renal cell carcinoma patients and is rapidly reduced after surgical tumor resection. Cancer immunology research. 2014;2:320–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bryceson YT, Fauriat C, Nunes JM, et al. Functional analysis of human NK cells by flow cytometry. Methods Mol Biol. 2010;612:335–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litwin S, Wong YN, Hudes G. Early stopping designs based on progression-free survival at an early time point in the initial cohort. Statistics in medicine. 2007;26:4400–4415. [DOI] [PubMed] [Google Scholar]
- 23.Storey JD. A Direct Approach to False Discovery Rates. Journal of the Royal Statistical Society. Series B (Statistical Methodology). 2002;64:479–498. [Google Scholar]
- 24.Patel SP, Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Molecular cancer therapeutics. 2015;14:847–856. [DOI] [PubMed] [Google Scholar]
- 25.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. [DOI] [PubMed] [Google Scholar]
- 26.Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dupuis J, Emile JF, Mounier N, et al. Prognostic significance of Epstein-Barr virus in nodal peripheral T-cell lymphoma, unspecified: A Groupe d’Etude des Lymphomes de l’Adulte (GELA) study. Blood. 2006;108:4163–4169. [DOI] [PubMed] [Google Scholar]
- 29.Mourad N, Mounier N, Brière J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111:4463–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green MR, Rodig S, Juszczynski P, et al. Constitutive AP-1 activity and EBV infection induce PD-L1 in Hodgkin lymphomas and posttransplant lymphoproliferative disorders: implications for targeted therapy. Clin Cancer Res. 2012;18:1611–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ostios-Garcia L, Faig J, Leonardi GC, et al. Safety and Efficacy of PD-1 Inhibitors Among HIV-Positive Patients With Non–Small Cell Lung Cancer. Journal of Thoracic Oncology. 2018;13:1037–1042. [DOI] [PubMed] [Google Scholar]
- 32.Heppt MV, Schlaak M, Eigentler TK, et al. Checkpoint blockade for metastatic melanoma and Merkel cell carcinoma in HIV-positive patients. Annals of Oncology. 2017;28:3104–3106. [DOI] [PubMed] [Google Scholar]
- 33.Ding Z-C, Zhou G. Cytotoxic Chemotherapy and CD4+ Effector T Cells: An Emerging Alliance for Durable Antitumor Effects. Clinical and Developmental Immunology. 2012;2012:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grossman SA, Ellsworth S, Campian J, et al. Survival in Patients With Severe Lymphopenia Following Treatment With Radiation and Chemotherapy for Newly Diagnosed Solid Tumors. Journal of the National Comprehensive Cancer Network : JNCCN. 2015;13:1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weide B, Martens A, Hassel JC, et al. Baseline biomarkers for outcome of melanoma patients treated with pembrolizumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:5487–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martens A, Wistuba-Hamprecht K, Foppen MG, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clinical cancer research : an official journal of the American Association for Cancer Research. 2016;22:2908–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saida Y, Watanabe S, Tanaka T, et al. Critical Roles of Chemoresistant Effector and Regulatory T Cells in Antitumor Immunity after Lymphodepleting Chemotherapy. J Immunol. 2015;195:726–735. [DOI] [PubMed] [Google Scholar]
- 38.Shafer D, Smith MR, Borghaei H, et al. Low NK cell counts in peripheral blood are associated with inferior overall survival in patients with follicular lymphoma. Leuk Res. 2013;37:1213–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judd J, Dulaimi E, Li T, et al. Low Level of Blood CD4(+) T Cells Is an Independent Predictor of Inferior Progression-free Survival in Diffuse Large B-cell Lymphoma. Clinical lymphoma, myeloma & leukemia 2017;17:83–88. [DOI] [PubMed] [Google Scholar]
- 40.Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A. 2010;107:4275–4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weber JS, Kudchadkar RR, Yu B, et al. Safety, Efficacy, and Biomarkers of Nivolumab With Vaccine in Ipilimumab-Refractory or -Naive Melanoma. Journal of Clinical Oncology. 2013;31:4311–4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wartewig T, Kurgyis Z, Keppler S, et al. PD-1 is a haploinsufficient suppressor of T cell lymphomagenesis. Nature. 2017;552:121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med. 2018;378:1947–1948. [DOI] [PubMed] [Google Scholar]
- 44.Kataoka K, Iwanaga M, Yasunaga J-i, et al. Prognostic relevance of integrated genetic profiling in adult T-cell leukemia/lymphoma. Blood. 2018;131:215–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kinosada H, Yasunaga JI, Shimura K, et al. HTLV-1 bZIP Factor Enhances T-Cell Proliferation by Impeding the Suppressive Signaling of Co-inhibitory Receptors. PLoS pathogens 2017;13:e1006120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.