Abstract
Purpose
Anti‐CD19 chimeric antigen receptor T (CAR‐T) cell therapy has demonstrated remarkable efficacy for refractory and relapsed diffuse large B cell lymphoma (R/R DLBCL). However, this therapy failed in nearly 25% patients mainly due to antigen loss. The authors performed a phase Ⅱ trial by coadministration of anti‐CD19 and anti‐CD20 CAR‐T cells treatment for R/R DLBCL and evaluated its efficacy and toxicity.
Methods
Totally 21 patients with DLBCL were enrolled in this study. The patients were conditioned with fludarabine and cyclophosphamide before the infusion of anti‐CD19 and anti‐CD20 CAR‐T cells. Treatment response, toxicity, and persistence were monitored continuously.
Results
Of the 21 patients received the treatment, the objective response rate (ORR) is 81.0% (95% confidence interval [CI], 58.1%‐94.6%) with four cases of bulk (4/5) and one case of testis involvement; 52.4% (95% CI, 29.8%‐74.3%) had a complete response (CR). Peak levels of anti‐CD19 and anti‐CD20 CAR cells were associated with response (P = .007 and .002). Grade 3‐4 cytokine release syndrome (CRS) and neurologic events occurred in 28.5% and 9.5% patients, respectively. Median overall survival (OS) and progression‐free survival (PFS) were 8.1 and 5.0 months, respectively. The maximum standard uptake value (SUVmax) of CD4/CD8 ratio before and after infusion were associated with responses, and the total lesion glycolysis (TLG) before infusion correlates with cytokines level.
Conclusions
Coadministration of anti‐CD19 and CD20 CAR‐T cells therapy for DLBCL is feasible with manageable toxicity. Cytokine markers are related to toxicity and SUVmax could predict efficacy. This trial was registered at www.clinicaltrials.gov as NCT03207178.
Keywords: CAR‐T, CD19, CD20, clinical trial, DLBCL
In this study, we confirmed that combination of anti‐CD19 and anti‐CD20 CAR‐T cells therapy strategy was efficient (81% ORR and 52.4% CR respectively) for R/R DLBCL, and that the toxicities was transient and manageable. To our knowledge, this is the first report that combination of anti‐CD19 and anti‐CD20 double‐target CAR‐T cell therapy for R/R DLBCL, and our data demonstrated the feasibility of combination of anti‐CD19 and anti‐CD20 CAR‐T cells therapy for R/R DLBCL.
1. INTRODUCTION
Diffuse large B cell lymphoma (DLBCL) accounts for approximately 40% of non‐Hodgkin lymphoma (NHL). Due to high heterogeneity, nearly 40% of DLBCL patients did not benefit from rituximab‐based immunochemotherapy. 1 , 2 R‐CHOP plus X regimen or rituximab‐based high‐dose regimen also failed to improve overall survival (OS) for some patients, especially those with MYC and BCL‐2/BCL‐6 double hit/expression, activated B cell (ABC), and CD5‐positive DLBCL. 3 , 4 In addition, elder patients tend to be more ABC subtype, complexed molecular genetic background, and poor tolerance to chemotherapy, resulting in worse prognosis. 5 , 6 For cases of R/R DLBCL, the 3‐year progression‐free survival (PFS) after high‐dose chemotherapy and hematopoietic stem cell transplantation (HSCT) is only 30%‐40% and the median OS is 4.4 months for those frail and unfit for HSCT patients. 7 , 8 Although novel drugs have improved the survival of individuals with R/R DLBCL, the objective response rate (ORR) is still less than 40%. 9 , 10 , 11
Chimeric antigen receptor (CAR) T cell therapy targeting CD19 has achieved great success in R/R DLBCL treatment with about 50% CR and a greater than 80% ORR. 12 , 13 , 14 However, since nearly 25% of anti‐CD19 CAR‐T cell therapy for R/R DLBCL failed due to antigen loss, 15 and CAR‐T cell therapy targeting other targets such as CD20 and CD22 has achieved encouraging results, 16 , 17 we speculated that a combined‐target CAR‐T cell treatment to cover more than one target might potentially increase the efficacy by remedying antigen loss‐associated failure. We have previously demonstrated both efficacy and feasibility of combined anti‐CD19 and anti‐BCMA CAR‐T cells for R/R multiple myeloma (ChiCTROIC‐17011272). 18 These findings prompted us to explore the efficacy and potential adverse effects including CRS and CAR‐T cell therapy‐related encephalopathy syndrome (CRES) in R/R DLBCL when multiple CAR‐T cells were coadministered. Therefore, we treated 21 R/R DLBCL patients with coadministration of anti‐CD19 and anti‐CD20 CAR‐T cells after lymphodepleting and evaluated the safety and efficacy of the dual‐targeted CAR‐T cell therapy.
2. MATERIALS AND METHODS
2.1. Study design and patients
This trial was designed by Blood Disease Institute of Xuzhou Medical University with a registration in ClinicalTrials.gov (NCT03207178), and the CAR was assembled in a lentivirus encoding a murine CD19 or CD20‐specific single chain variable fragment combined with the 4‐1BB costimulatory domain and the CD3ζ domain (Figure 1A). The enrolled patients met the following criteria: (a) DLBCL was confirmed by pathology and immunohistochemistry; (b) Patients have previously underwent immunochemotherapy based on anti‐CD20 antibody and anthracycline; (c) At least one measurable lesion was present (refer to the International Working Group [IWG] standard); (d) Nether radiotherapy nor systemic therapy has been administered two weeks before lymphocyte collection; (e) Physical score >50 (Karnofsky Performance Status); and (f) Normal liver, kidney, and cardiac function. Patients were excluded if (a) with a serious autoimmune disease or other tumor disease; (b) participated in other clinical trials of new drugs within the past 3 months; (c) with systemic or local uncontrollable infection; (d) with active hepatitis B and C infection; (e) with HIV infection; and (f) with psychiatric disorders. The CAR‐T cell therapy were conducted between March 2017 and July 2018. The end of follow‐up was October 31, 2018, and the median follow‐up time was 6.6 months (ranged 0.3‐16.4). Clinical trial was approved by the Institutional Review Board of the Affiliated Hospital of Xuzhou Medical University in accordance with Declaration of Helsinki principles. All enrolled patients signed the informed consent prior to the study. Generation of CAR‐T cells, quantitative polymerase chain reaction, image analysis, and cytokine assays are described in the Supplementary Material.
FIGURE 1.
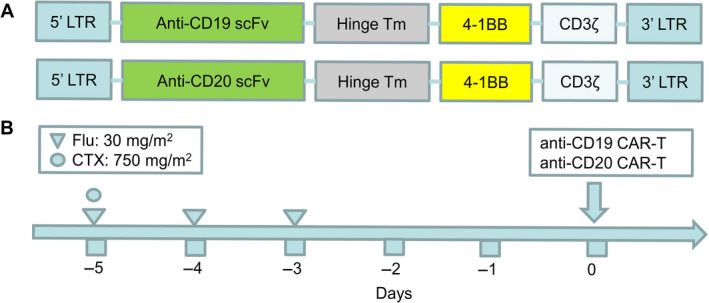
Design of chimeric antigen receptor (CAR) targeting CD19 and CD20. (A) Schematic of anti‐CD19 CAR and anti‐CD20 CAR. Single‐chain (sc) Fv region recognizes CD19 or CD20, CAR contained 4‐1BB costimulatory domain and CD3‐ζ T cell activation domain. (B) Fludarabine combined with cyclophosphamide was used as the pretreatment regimen. Cells were ready for infusion on day 0
2.2. Lymphodepletion chemotherapy and CAR‐T cells infusion
Of the 21 patients, 19 received fludarabine (30 mg/m2/d) at days of −5 to −3 and cyclophosphamide (750 mg/m2/d) at day −5; and the remaining two patients were treated with ifosfamide (2 g/d) at days of −5 to −3. None of the patients underwent additional chemotherapy as a bridging therapy. The median number of anti‐CD19 CAR‐T cells anti‐CD20 CAR‐T cells was 1.0 × 106/kg (0.2‐4.0 × 106/kg) and 1.0 × 106/kg (0.1‐4.0 × 106/kg), respectively.
2.3. Assessment of clinical response and toxicity
The primary end point of the study was ORR. The secondary end point of the study was the CR status at month 6, median OS and PFS, safety, and expansion of CAR‐T cells in vivo. Response of treatment was defined according to the standard international criteria. 19 For CRS diagnosis and severity assessment, the grading standard were based on that revised criteria by Lee et al. 20 Other adverse events were evaluated according to the Common Nervous Adverse Events Standards (CTCAE) Version 4.03. 21
2.4. Statistical methods
The study was determined to provide 80% power to test the null hypothesis that the ORR would be 20% or lower vs the alternative hypothesis that it would be 40% or higher. A prespecified rate of response of 20% on the basis of historical values for refractory diffuse large B cell lymphoma. 22 , 23 , 24 , 25 This design minimized the maximum sample size among all participants in the Simon 2‐stage optimal design with 1‐sided 10% type I error rate. Under the assumption of 5% ineligibility, 21 patients were planned. The 95% CIs for the ORR were calculated using the binomial exact method.
Patients' demographics and clinical characteristics have been summarized using descriptive statistics. PFS was defined as the time from CAR‐T cell infusion until disease progression or death, whichever occurred first. OS was determined as the time from CAR‐T cell infusion until death from any cause. Patients were censored at the date on which they were last known to be alive and/or progression‐free. The median 2‐sided PFS and OS with the 95% CIs were estimated using the Kaplan‐Meier method. For continuous variables that conform to normal distribution, t test was used. For those that do not conform to normal distribution, Wilcoxon signed‐rank test was used for paired samples, and Mann‐Whitney U test was used for independent samples. P values less than .05 were considered significant.
3. RESULTS
3.1. Patients characteristics
A total of 25 patients with R/R DLBCL were originally enrolled. However, one of them failed to collect sufficient T lymphocytes; CAR‐T cell expansion in vitro failed in two of them, and another patient died due to rapid progressive disease (PD) before CAR‐T cell infusion. Therefore, 21 patients received CAR‐T cell infusion according to the treatment schema (Table 1; Table S1, and Figure 1B). Among them, four patients were MCY/BCL2 double expression, five patients were with bulky mass (≥7.5 cm), and one patient was with MCY/BCL2 rearrangement, one CD5 positive patient had testicular involvement, and one patient received autologous HSCT before CAR‐T cell therapy. Fourteen patients were immunochemotherapy refractory as defined as the best response was stable disease (SD) or PD after two cycles of a standard or conventional first‐line treatment regimen, or failed to achieve CR after two cycles. Seven patients met the criteria of relapse that CR was achieved after treatment, but relapse occurred within 1 year after treatment. Patients received a median of 3‐line (range, 1‐6) regimens before protocol enrollment (Table S2).
TABLE 1.
Characteristics of the patients at baseline
| Characteristic | Patients, n (%) |
|---|---|
| Total patients | 21 (100%) |
| Median age, years (range) | 55 (23‐72) |
| Sex | |
| Male | 13 (61.9%) |
| Female | 8 (38.1%) |
| Disease stage | |
| I‐II | 3 (14.3%) |
| III‐IV | 18 (85.7%) |
| NCCN‐IPI group | |
| Low‐intermediate: 2‐3 | 6 (28.6%) |
| High‐intermediate: 4‐5 | 13 (61.9%) |
| High: ≥6 | 2 (9.5%) |
| Extranodal disease | 6 (28.6%) |
| BM involved | 5 (23.8%) |
| CNS involved | 3 (14.3%) |
| Bulky | 5 (23.8%) |
| Cell of origin | |
| GCB | 16 (76.2%) |
| NON‐GCB | 5 (23.8%) |
| MCY/BCL2 DE | 4 (19.0%) |
| Refractory DLBCL | 15 (71.4%) |
| Median previous therapies (range) | 3 (1‐6) |
| Conditioning therapy | |
| FC | 19 (90.5%) |
| Others | 2 (9.5%) |
Abbreviations: BM, bone marrow; CNS, central nervous system; DE, double expression; FC, fludarabine and cyclophosphamide; GCB, germinal center B cell; NCCN‐IPI, National Comprehensive Cancer Network‐International Prognostic Index.
3.2. Response assessment
The median time between recruitment and infusion was 32 days (range, 16‐45). The ORR and CR rates at 3 months were 81.0% (17 patients, 95% CI, 58.1%‐94.6%) and 52.4% (11 patients), and the 6‐month sustained ORR and CR rates were 46.2% (6 patients, 95% CI, 19.2%‐74.9%) and 40.0% (4 patients, 95% CI, 12.2%‐73.84%), respectively. The median PFS, OS, and duration of response were 5.0 months (95% CI, 2.3‐7.7), 8.1 months (95% CI, 6.5‐9.6), and 6.8 months, respectively (Figure 2A‐C). Of the five patients with bulky mass, four patients responded, and two of them achieved CR (Table 2). Of the four patients with MYC/BCL‐2 expression, three achieved CR, and one achieved PR. The patient of MYC/BCL2 rearrangement developed PD. CR was achieved in a CD5 positive case with testicular involvement. There were three patients with central nervous system (CNS) involvement, including two of primary and one secondary cases. Of the two patients with primary CNS lymphoma, one achieved PR (P12) and the other showed SD (P18). The patient with secondary CNS involvement (P13) achieved PR at 3 months. At the end of follow‐up, 10 patients died due to PD. Two patients died without relapse, one was due to pulmonary infection at day 42 and the other suffered from severe intestinal infection at day 121 after CAR‐T cell infusion (Figure 2D,E).
FIGURE 2.
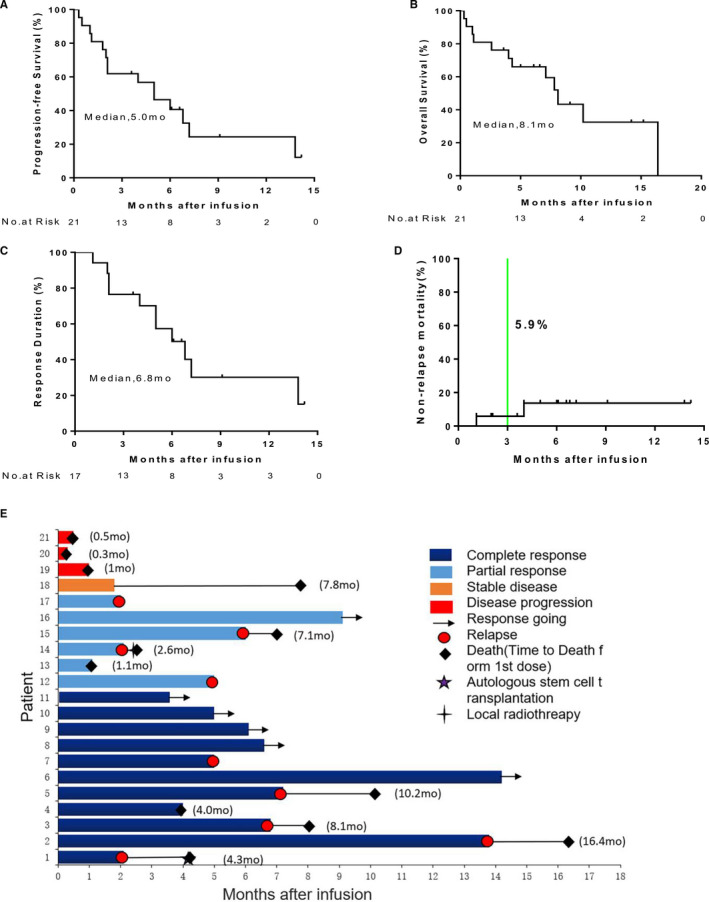
Function of anti‐CD19 and anti‐CD20 CAR‐T cells in R/R DLBCL. (A, B) The median PFS and OS of 21 patients are shown. (C) The response duration and non‐relapse mortality (NRM) (D) of 17 patients with response are shown. The survival fractions were calculated by the Kaplan‐Meier method, and the (green) lines indicate censored patients. (E) The clinical responds and survival condition of anti‐CD19 combined with anti‐CD20 CAR‐T cells therapy
TABLE 2.
Baseline characteristics of patients with bulky
| No. | Age (y) | Sex | Stage/status | Previous therapies | Site involved | Response | The longest diameter (cm) | |
|---|---|---|---|---|---|---|---|---|
| Before | After | |||||||
| 1 | 58 | F | ⅣB/PD | 3 | Abdomen | CR | 11.0 | 1.3 |
| 2 | 70 | M | ⅣA/PD | 3 | Abdomen | CR | 8.0 | 1.2 |
| 3 | 48 | M | ⅣA/PD | 5 | Neck (L) | PR | 11.9 | 5.1 |
| 4 | 46 | F | ⅣA/PD | 3 | Abdomen | PR | 7.8 | 3.2 |
| 5 | 57 | M | ⅢA/PD | 1 | Iliac fossa (L) | PD | 9.1 | 9.3 |
Abbreviations: CR, complete remission; F, female; L, left; M, male; PD, progressive disease; PR, partial remission.
3.3. Toxicities of CAR‐T cell
The adverse events were detailed in Table 3. CRS occurred in all 21 patients with 6 (28.5%) of them as grade 3‐4 CRS. The median time from CAR‐T cell infusion to CRS occurrence was 2 days (ranged 0‐5) and the median CRS duration was 5 days (ranged 2‐14). Four patients with CRS returned to normal after dexamethasone treatment and none of them needed tocilizumab. Five patients were with CRES, and the median duration was 5 days (ranged 3‐8). For the two patients with grade 3‐4 CRES, the duration were 7 and 8 days, respectively. The main symptoms of CRES were dysphoria (n = 3) and delirium (n = 2) with a median duration of 3 days (ranged 2‐7). Of the five patients over 60 years old, one had grade four CRS and another had CRES.
TABLE 3.
Treatment‐emergent adverse events
| Adverse events | No. of patients, (%, n = 21) | |
|---|---|---|
| All grades | Grade ≧3 | |
| Fatigue | 16 (76.2%) | 5 (23.8%) |
| Dyspnea | 7 (33.3%) | 3 (14.3%) |
| Nausea | 7 (33.3%) | 0 |
| Anorexia | 11 (52.4%) | 3 (14.3%) |
| Diarrhea | 2 (9.5%) | 0 |
| Vomit | 5 (23.8%) | 0 |
| Constipation | 4 (19.0%) | 0 |
| Acute kidney injury | 2 (9.5%) | 0 |
| Pulmonary infection | 4 (19.0%) | 1 (4.8%) |
| a CRS, specific symptoms | ||
| Fever | 20 (95.2%) | 2 (9.5%) |
| Rigors | 6 (28.6%) | 0 |
| Hypotension | 10 (47.6%) | 4 (19.0%) |
| Hypoxia | 10 (47.6%) | 3 (14.3%) |
| Tachycardia | 13 (61.9%) | 4 (19.0%) |
| Neurotoxicity, specific symptoms | ||
| Encephalopathy | 5 (23.8%) | 2 (9.5%) |
| Delirium | 2 (9.5%) | 2 (9.5%) |
| Somnolence | 1 (4.8%) | 0 |
| Restlessness | 3 (14.3%) | 2 (9.5%) |
| Dysmnesia | 1 (4.8%) | 0 |
| Tremor | 1 (4.8%) | 0 |
| Hematologic events | ||
| Leukopenia | 16 (76.2%) | 10 (47.6%) |
| Neutropenia | 16 (76.2%) | 11 (52.4%) |
| Anemia | 17 (81.0%) | 6 (28.6%) |
| Thrombocytopenia | 6 (28.6%) | 6 (28.6%) |
| Laboratory abnormalities | ||
| ALT elevation | 4 (19.0%) | 0 |
| AST elevation | 4 (19.0%) | 0 |
| Hyperuricemia | 3 (14.3%) | 0 |
| Hypoalbuminemia | 7 (33.3%) | 3 (14.3%) |
| Hypokalemia | 8 (38.1%) | 2 (9.5%) |
| Hyponatremia | 6 (28.6%) | 0 |
| Hypocalcemia | 4 (19.0%) | 0 |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase.
CRS was graded per a modified grading system proposed by Lee et al. 20
3.4. Cytokine and laboratory assessment
The levels of IL‐6, ferritin, C‐reactive protein (CRP), and IFN‐γ reached their peak between days of 7 and 14 after CAR‐T cell infusion. The peak levels of these factors in patients with grade 1‐2 CRS (n = 15) were significantly lower than those with grade 3‐4 CRS (n = 6) (Figure 3A‐D). Hematologic toxicities were found in 17 patients, including 16 cases of neutropenia (76.2%), 17 cases of anemia (81.0%), 6 cases of thrombocytopenia (28.6%), and 16 cases with a decreased white blood cell count (76.2%). Grade 3 hematologic toxicities of neutropenia, anemia, and thrombocytopenia were 52.4%, 28.6%, and 28.6%, respectively. Eleven patients received an injection of recombinant human granulocyte macrophage‐stimulating factor to increase leukocyte levels, four and three patients required platelet and red blood cell transfusion, respectively.
FIGURE 3.
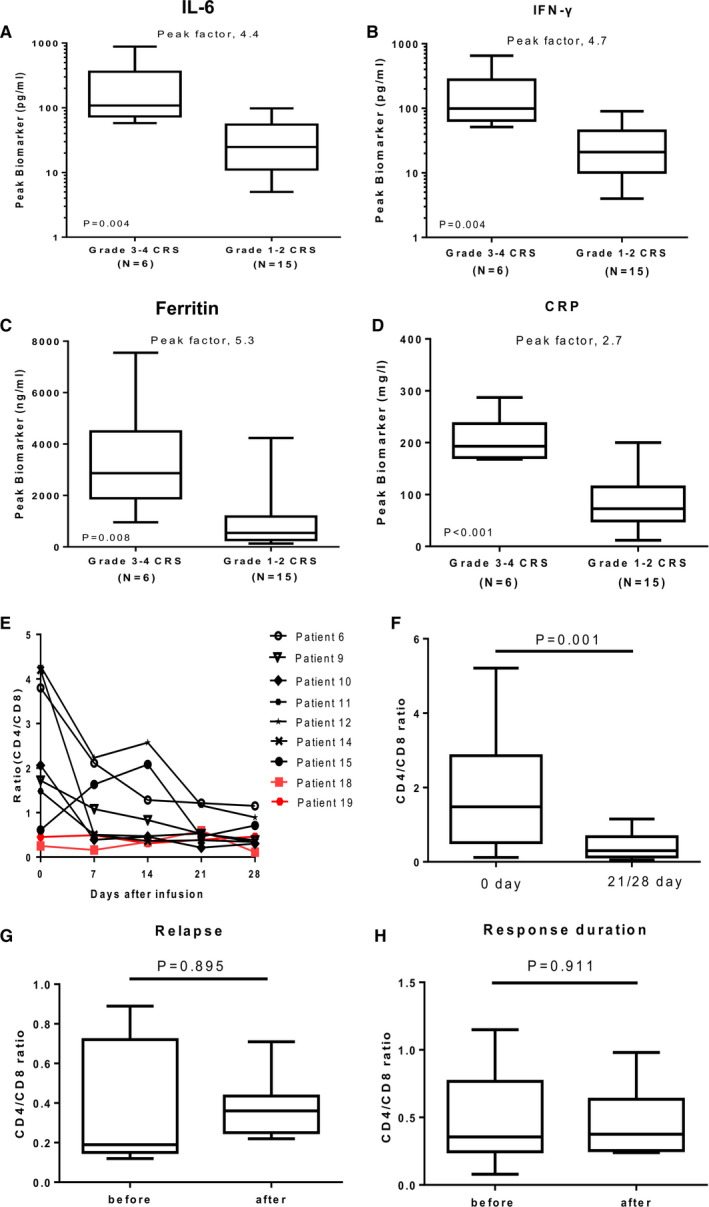
Changes of serum biomarkers and CD4/CD8 ratio after CAR‐T cell infusion. (A‐D) IL‐6, IFN‐γ, ferritin, and CRP were associated only with CRS. The peak value is defined as the maximum level of the cytokine after baseline within a month of cell infusion. The peak factor is the value in patients with CRS of grade 3‐4 vs those with events of grade 1‐2. The horizontal line within each box represents the median, the lower and upper borders of each box represent the 25th and the 75th percentiles, respectively, and the I bars represent the minimum and maximum range. The Mann‐Whitney U test or t test was used for statistical analysis. (E) Dynamic changes of CD4/CD8 ratio in patients after CAR‐T cell infusion. P6, 9, 10, and 11 were CR patients, P12, 14, and 15 were PR patients, P18 was SD patient, and P19 was PD patient. (F) Dynamic changes of CD4/CD8 ratio in 17 patients with response. (G) Dynamic changes of CD4/CD8 ratio in 9/17 patients with relapse after CAR‐T cell infusion. (H) Dynamic changes of CD4/CD8 ratio in patients with response duration. The Wilcoxon rank‐sum test or t test were used for statistical analysis
3.5. Assessment of B cells and immunoglobulin
B cells and immunoglobulin were measured to assess the immune status of B cells after CAR‐T cell therapy (data not shown). In five (23.8%) patients, B cells were not detected in peripheral blood before CAR‐T cell infusion, including four patients with response and one patient without response eventually. Two weeks after CAR‐T cell infusion, B cells were undetectable in 11 responsive patients and one nonresponsive patient; and detectable in two non‐responsive patients. B cells recurred in five of nine relapsed patients, and the median time is 6.1 months (ranged 4‐13.7) after CAR‐T cell infusion. Of the 17 patients with response, 14 (82.4%) showed a progressive reduction of serum immunoglobulin levels one week after infusion. Eight of the 21 (38.1%) patients received intravenous immunoglobulin during CAR‐T cell therapy.
3.6. Assessment of T cells and CD4/CD8 ratio
T cells in the peripheral blood of patients were measured to assess cellular immune status after CAR‐T cell therapy (data not shown). A dynamic reduction of CD4/CD8 ratio occurred in 15 of 17 responsive patients. Among the four nonresponsive patients, the ratio did not change in three and declined in one (Figure 3E). The CD4/CD8 ratio in the 17 responsive patients at 4 weeks after CAR‐T cell infusion was significantly lower than that before infusion (Figure 3F, P = .001). Of the 17 responsive patients, nine relapsed later but their CD4/CD8 ratio displayed no change before and after the relapse (Figure 3G, P = .895). Of note, the CD4/CD8 ratio in the six patients with continuous response did not change even by the end of the follow‐up (Figure 3H, P = .911).
3.7. In vivo expansion and persistence of CAR‐T cells
Significant CAR‐T cell expansion occurred in 17 responsive patients and both anti‐CD19 and anti‐CD20 CAR peaked between 7‐14 days after CAR‐T cell infusion. Of the four non‐responders, three had anti‐CD19 CAR‐T expansion, two had anti‐CD20 CAR‐T expansion, and one amplified neither of them. The expansion of anti‐CD19/CD20 CAR‐T cells in patients with response (n = 17) was significantly higher than those without response (n = 4) (Figure 4A,B, P = .007 and .002). For the 11 patients achieving CR, there was no difference between the peaks of anti‐CD19 and anti‐CD20 CAR‐T cells (Figure 4C). In the six patients with grade 3‐4 CRS, the median copies of anti‐CD19 and anti‐CD20 CAR were 65 680 and 23 715 copies/100 ng total DNA, respectively, which were not significantly different from those with grade 1‐2 CRS (n = 15) (Figure 4D‐I). CAR‐T cells could not be detected in the patients after relapse and the two patients with continuous remission, respectively, at 6 and 9 months after CAR‐T cell infusion.
FIGURE 4.
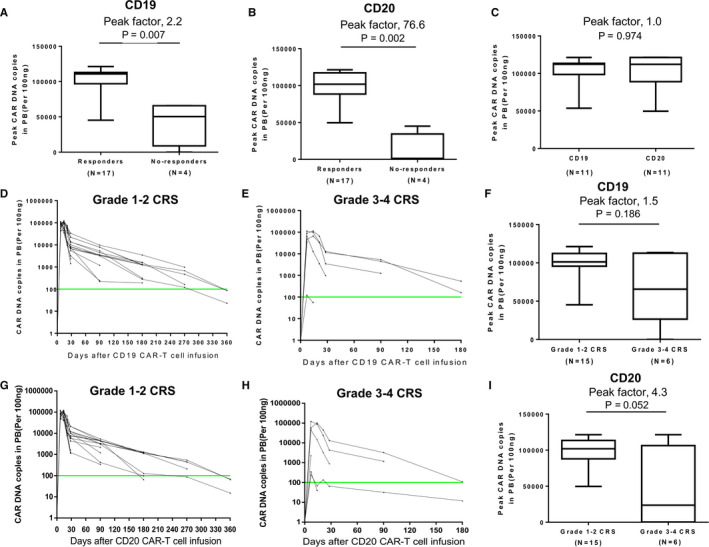
Expansion and persistence of anti‐CD19 and anti‐CD20 CAR‐T cells in vivo. (A, B) Peak CAR DNA copies of anti‐CD19 and anti‐CD20 CAR‐T cells in the groups with response and without response. (C) Peak CAR DNA copies of anti‐CD19 CAR‐T cell and anti‐CD20 CAR‐T cell in patients of CR. (D, E) CAR DNA copies of anti‐CD19 CAR‐T cell at serial time points after infusion in patients who developed grade 1‐2 CRS and those who developed grade 3‐4 CRS. (F) Peak CAR DNA copies of anti‐CD19 CAR‐T cell in the groups with grade 1‐2 and grade 3‐4 CRS. (G, H) CAR DNA copies of anti‐CD20 CAR‐T cell at serial time points after infusion in patients who developed grade 1‐2 CRS and those who developed grade 3‐4 CRS. (I) Peak CAR DNA copies of anti‐CD20 CAR‐T cell in the groups with grade 1‐2 and grade 3‐4 CRS. The horizontal line at 100 copies per microgram of DNA represents the lower limit of quantification of this assay. The Mann‐Whitney U test was used for statistical analysis
3.8. Impact of SUVmax and TLG on response, CRS and CAR‐T cell expansion
Based on PET‐CT, we evaluated the SUVmax and TLG before CAR‐T cell therapy. The SUVmax (g/ml) of 15 evaluable patients with treatment response (median of 12.23, range: 6.49‐35.71) was significantly lower than that of three patients without response (median of 24.8, range: 18.6‐42.29) (Figure 5A, P = .038), but the SUVmax is comparable in the six patients with durable remission and the other nine cases (P = .541). There was no significant difference in TLG values between responders and non‐responders (Figure 5B). The SUVmax and TLG of evaluable patients with grade 1‐2 CRS (n = 13) were not significantly different from those with grade 3‐4 CRS (n = 5) (Figure 5C,D). SUVmax and cytokine levels were not correlated (Figure 5E‐H). However, high levels of TLG correlate with the levels of IL‐6, ferritin, and IFN‐γ but not the CRP level (Figure 5I‐L). In addition, we evaluated the correlations of SUVmax and TLG with the expansion of CAR‐T cells in vivo. There was no significant difference in the anti‐CD19 CAR‐T and anti‐CD20 CAR‐T expansion between patients whose SUVmax or TLG was over the median and those below (Figure 5M‐P).
FIGURE 5.
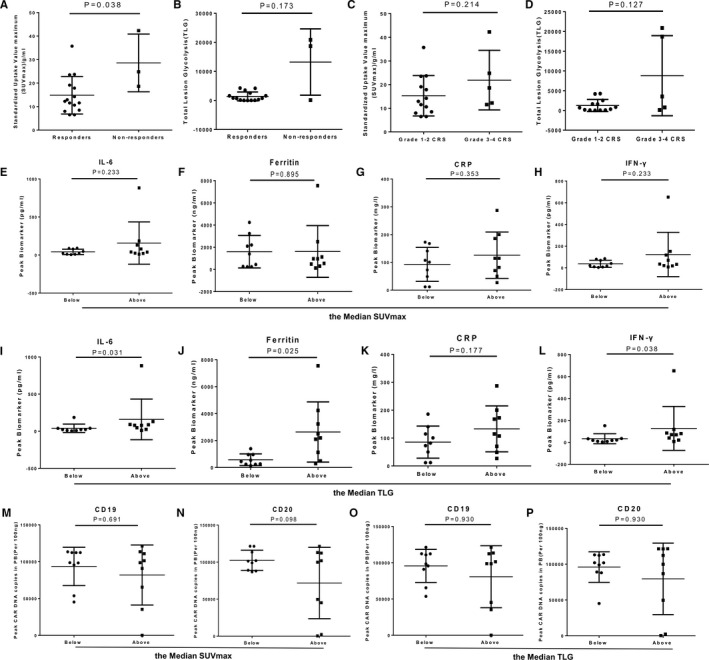
The values of SUVmax and TLG in response, CRS and CAR‐T cells expansion. (A, B) The vaule of SUVmax and TLG in the groups with and without response. (C, D) The vaule of SUVmax and TLG in grade 1‐2 and grade 3‐4 CRS. (E‐H) Peak CAR DNA copies of anti‐CD19 and anti‐CD20 in below and above the median SUVmax/TLG groups. (I‐P) The peak value of serum biomarkers (IL‐6, IFN‐γ, ferritin, and CRP) in below and above the median SUVmax/TLG groups. The peak value is defined as the maximum level of the cytokine after baseline within a month. The data represent the means ± SD. The Mann‐Whitney U test or t test were used for statistical analysis
4. DISCUSSION
To our knowledge, this is the first reported clinical trial of coadministration of anti‐CD19 and anti‐CD20 CAR‐T cell therapy for R/R DLBCL. Although anti‐CD19 CAR‐T cell therapy is effective for R/R DLBCL, 12 , 13 , 14 about 50%‐60% patients relapse after CAR‐T treatment with as high as 27% are antigen loss‐associated. 15 Based on the expectation that multi‐target CAR‐T cell therapeutic strategy should improve efficacy and potentially solve the problem with antigen loss‐associated failure, we treated R/R DLBCL with the combination of anti‐CD19 and anti‐CD20 CAR‐T cells and achieved a promising response with CR rate of 52.4% and ORR rate of 81% albeit the PFS and DOR were not as encouraging. This could be the results of immune escaping, lacking patient‐originated memory CAR‐T cells, or failure of continued CAR‐T cell expansion. Generally, MYC and BCL‐2/BCL‐6 double‐expression/rearrangement and CD5‐positive DLBCL are resistant to rituximab‐based immunochemotherapy. We noticed in this trail that three out of four patients with MYC/BCL‐2 double expression achieved CR. Due to the limited number of cases, we cannot make solid conclusion that co‐administration of anti‐CD19 and anti‐CD20 CAR‐T cells can overcome the adverse prognosis caused by double expression. In addition to that multiple cases with bulk achieved 80% ORR, we have also noticed that in one patient of CD5‐positive DLBCL with testicular involvement also achieved CR, suggesting that anti‐CD19 and anti‐CD20 CAR‐T cells are capable of passing the blood‐testis barrier.
The universal application of CAR‐T cell therapy is limited mainly due to the complications of CAR‐T cell therapy, especially for ≥grade 3 CRS and CRES. 26 , 27 In our trial, 23.8% and 9.5% patients had CRS (≥grade 3) and CRES (≥grade 3), respectively, four patients received emergent glucocorticoid therapy due to hemorheological instability, but none of the patients received tocilizumab. Interestingly, of the 21 patients receiving CAR‐T cells, six elderly achieved 83% ORR with only one of them had grade 3 CRS. More importantly, the toxicity was transient and manageable while the median dose of 1.0 × 106/kg CAR‐T cells were administered and the levels of cytokine peaked 7‐14 days after CAR‐T cell infusion. We have also noticed that the peak levels of the inflammatory cytokines in patients with grade 1‐2 CRS were significantly lower than those with grade 3 CRS and these notifications were consistent with the reports in the literature. 12 , 13 , 14 The hematologic toxicities primarily manifested as manageable hemocytopenia and none of the patients died due to CAR‐T cell treatment‐associated hematologic complications although off‐target effects were noticeable. All responders and a few nonresponders lost B cells two weeks after CAR‐T cell infusion. However, B cells recurred in five of nine relapsed patients with the median time of 6.1 months post CAR‐T cell infusion. Consistently, immunoglobulin levels reduced progressively in the responders and some of the nonresponders and 38.1% patients received intravenous immunoglobulin infusion during CAR‐T cell therapy but none of them had serious infection.
Great expansion of CD8 positive CAR‐T cells was closely related to efficacy, and IL‐2 produced by CD4 positive T cells was important in sustaining its response. 28 As reported by Turtle and his colleagues, CAR‐T cell products in a 1:1 CD4/CD8 ratio was supposed to be worthy of recommendation. 29 However, the correlation of CD4/CD8 ratio after CAR‐T cell infusion and efficacy is not clear. As shown in this study, the CD4/CD8 ratio in 15 of 17 responders decreased gradually, whereas the ratios did not change in three of the four nonresponders. So, we propose that the CD4/CD8 ratio could serve as a predictor for CAR‐T cell therapy. We also analyzed the expansion and persistence of CAR‐T cells in patients. We found that all responders exhibited expansion with the peak values of anti‐CD19 and CD20 CAR‐T cells in responders significantly higher than that of the nonresponders. However, there is no significant difference between the peak values of anti‐CD19 and anti‐CD20 CAR‐T cells in the CR patients.
PET‐CT is of great value in assessment of tumor burden and treatment efficacy. 30 To evaluate the role of metabolic activity of lymphoma on CAR‐T therapy, we analyzed the correlation of SUVmax, and we also evaluated the correlation of TLG, which was determined by SUVmean and TMV. We found that lower SUVmax correlates with better response suggesting high levels SUVmax predict high risk of CAR‐T cells treatment. However, the levels of TLG have nothing to do with efficacy but highly associated with IFN‐γ, IL‐6, and the ferritin levels. Nevertheless, neither SUVmax nor TLG correlate with the occurrence of CRS or CAR‐T cell expansion.
In summary, this trial demonstrated that coadministration of anti‐CD19 and anti‐CD20 CAR‐T cell therapy is safe and feasible with manageable toxicity even for those with bulk, MYC/BCL‐2 double expression, and CD5 positive DLBCL. We also found that CAR‐T cells are capable of passing the blood‐brain and blood‐testis barriers. Finally, reduced CD4/CD8 ratio and lower SUVmax predict a better treatment efficacy.
CONFLICT OF INTEREST
No potential conflict of interest was disclosed.
AUTHORS CONTRIBUTIONS
Conception and design: Kailin Xu, Wei Sang, Zhenyu Li, Junnian Zheng. Development of methodology: Kailin Xu, Wei Sang, Jiang Cao, Ming Shi. Acquisition of data (provided animals, acquired and managed patients, provide facilities, etc): Kailin Xu, Wei Sang, Ming Shi, Jingjing Yang, Jiang Cao, Linyan Xu, Dongmei Yan, Meixue Yao, Hui Liu, Hai Cheng, Zhiling Yan, Depeng Li, Haiying Sun, Feng Zhu, Ying Wang. Analysis and interpretation of data (eg, statistical analysis, biostatistics, computational analysis): Wei Sang, Jingjing Yang, Bing Zhang, Kemeng Sun, Xuguang Song, Cai Sun, Jun Jiao, Tingting Sang, Xiang Gao, Lingyu Zeng. Writing, review, and/or revision of the manuscript: All authors. Administrative, technical, or material support (ie, reporting or organizing data, constructing databases): Kailin Xu, Wei Sang, Jiang Cao, Zhenyu Li, Junnian Zheng.
Supporting information
Table S1‐S2
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by Natural Science Foundation of Jiangsu Province (BK20171181), Young Medical Talents of Jiangsu Science and Education Health Project (QNRC2016791) and Jiangsu Key Research and Development Project of Social Development (BE2019638). We thank Xin Wang in Shanghai Longyao Biotechnology Co., Ltd for assistance with providing partial technical support. We also thank the attending fellows, nurses, and other staff of the Department of Hematology, the Affiliated Hospital of Xuzhou Medical University.
Sang W, Shi M, Yang J, et al. Phase II trial of co‐administration of CD19‐ and CD20‐targeted chimeric antigen receptor T cells for relapsed and refractory diffuse large B cell lymphoma. Cancer Med. 2020;9:5827–5838. 10.1002/cam4.3259
Wei Sang, Ming Shi and Jingjing Yang contributed equally to this work.
Contributor Information
Junnian Zheng, Email: jnzhengxzmu@126.com.
Kailin Xu, Email: lihmd@163.com.
DATA AVAILABILITY STATEMENT
The data will be provided upon the request.
REFERENCES
- 1. Sehn LH, Donaldson J, Chhanabhai M, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B‐cell lymphoma in British Columbia. J Clin Oncol. 2005;23:5027‐5033. [DOI] [PubMed] [Google Scholar]
- 2. Coiffier B, Thieblemont C, Van Den Neste E, et al. Long‐term outcome of patients in the LNH‐98.5 trial, the first randomized study comparing rituximab‐CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040‐2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thakral B, Medeiros LJ, Desai P, et al. Prognostic impact of CD5 expression in diffuse large B‐cell lymphoma in patients treated with rituximab‐EPOCH. Eur J Haematol. 2017;98:415‐421. [DOI] [PubMed] [Google Scholar]
- 4. Staiger AM, Ziepert M, Horn H, et al. Clinical impact of the cell‐of‐origin classification and the MYC/BCL2 dual expresser status in diffuse large B‐cell lymphoma treated within prospective clinical trials of the German High‐Grade Non‐Hodgkin's Lymphoma Study Group. J Clin Oncol. 2017;35:2515‐2526. [DOI] [PubMed] [Google Scholar]
- 5. Mareschal S, Lanic H, Ruminy P, Bastard C, Tilly H, Jardin F. The proportion of activated B‐cell like subtype among de novo diffuse large B‐cell lymphoma increases with age. Haematologica. 2011;96:1888‐1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klapper W, Kreuz M, Kohler CW, et al. Patient age at diagnosis is associated with the molecular characteristics of diffuse large B‐cell lymphoma. Blood. 2012;119:1882‐1887. [DOI] [PubMed] [Google Scholar]
- 7. Crump M, Kuruvilla J, Couban S, et al. Randomized comparison of gemcitabine, dexamethasone, and cisplatin versus dexamethasone, cytarabine, and cisplatin chemotherapy before autologous stem‐cell transplantation for relapsed and refractory aggressive lymphomas: NCIC‐CTG LY.12. J Clin Oncol. 2014;32:3490‐3496. [DOI] [PubMed] [Google Scholar]
- 8. Van Den Neste E, Schmitz N, Mounier N, et al. Outcome of patients with relapsed diffuse large B‐cell lymphoma who fail second‐line salvage regimens in the International CORAL study. Bone Marrow Transplant. 2016;51:51‐57. [DOI] [PubMed] [Google Scholar]
- 9. Oki Y, Kelly KR, Flinn I, et al. CUDC‐907 in relapsed/refractory diffuse large B‐cell lymphoma, including patients with MYC‐alterations: results from an expanded phase I trial. Haematologica. 2017;102:1923‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lesokhin AM, Ansell SM, Armand P, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. J Clin Oncol. 2016;34:2698‐2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilson WH, Young RM, Schmitz R, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922‐926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abramson JS, Gordon LI, Palomba ML, et al. Updated safety and long‐term clinical outcomes in TRANSCEND NHL 001, pivotal trial of lisocabtagene maraleucel (JCAR017) in R/R aggressive NHL. J Clin Oncol. 2018;36:7505. [Google Scholar]
- 13. Locke FL, Ghobadi A, Jacobson CA, et al. Long‐term safety and activity of axicabtagene ciloleucel in refractory large B‐cell lymphoma (ZUMA‐1): a single‐arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20:31‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B‐cell lymphoma. N Engl J Med. 2019;380:45‐56. [DOI] [PubMed] [Google Scholar]
- 15. Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T‐cell therapy in refractory large B‐cell lymphoma. N Engl J Med. 2017;377:2531‐2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou H, Luo Y, Zhu S, et al. The efficacy and safety of anti‐CD19/CD20 chimeric antigen receptor‐ T cells immunotherapy in relapsed or refractory B‐cell malignancies:a meta‐analysis. BMC Cancer. 2018;18:929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fry TJ, Shah NN, Orentas RJ, et al. CD22‐targeted CAR T cells induce remission in B‐ALL that is naive or resistant to CD19‐targeted CAR immunotherapy. Nat Med. 2018;24:20‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yan Z, Cao J, Cheng H, et al. A combination of humanised anti‐CD19 and anti‐BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single‐arm, phase 2 trial. Lancet Haematol. 2019;6:e521‐e529. [DOI] [PubMed] [Google Scholar]
- 19. Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059‐3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188‐195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Department of Health and Human Services . Common Terminology Criteria for Adverse Events Version 4.03. Bethesda, MD: National Institutes of Health; 2010. [Google Scholar]
- 22. Hitz F, Connors JM, Gascoyne RD, et al. Outcome of patients with primary refractory diffuse large B cell lymphoma after R‐CHOP treatment. Ann Hematol. 2015;94:1839‐1843. [DOI] [PubMed] [Google Scholar]
- 23. Matasar MJ, Czuczman MS, Rodriguez MA, et al. Ofatumumab in combination with ICE or DHAP chemotherapy in relapsed or refractory intermediate grade B‐cell lymphoma. Blood. 2013;122:499‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nagle SJ, Woo K, Schuster SJ, et al. Outcomes of patients with relapsed/refractory diffuse large B‐cell lymphoma with progression of lymphoma after autologous stem cell transplantation in the rituximab era. Am J Hematol. 2013;88:890‐894. [DOI] [PubMed] [Google Scholar]
- 25. Telio D, Fernandes K, Ma C, et al. Salvage chemotherapy and autologous stem cell transplant in primary refractory diffuse large B‐cell lymphoma: outcomes and prognostic factors. Leuk Lymphoma. 2012;53:836‐841. [DOI] [PubMed] [Google Scholar]
- 26. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127:3321‐3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hay KA, Hanafi L‐A, Li D, et al. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor‐modified T‐cell therapy. Blood. 2017;130:2295‐2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sommermeyer D, Hudecek M, Kosasih PL, et al. Chimeric antigen receptor‐modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia. 2016;30:492‐500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turtle CJ, Hanafi L‐A, Berger C, et al. CD19 CAR‐T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123‐2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsuchiya J, Yamamoto M, Bae H, et al. Tumor identification of less aggressive or indolent lymphoma with whole‐body 11C‐acetate PET/CT. Clin Nucl Med. 2019;44:276‐281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1‐S2
Supplementary Material
Data Availability Statement
The data will be provided upon the request.


