Abstract
Agricultural expansion and intensification are major threats to tropical biodiversity. In addition to the direct removal of native vegetation, agricultural expansion often elicits other human-induced disturbances, many of which are poorly addressed by existing environmental legislation and conservation programmes. This is particularly true for tropical freshwater systems, where there is considerable uncertainty about whether a legislative focus on protecting riparian vegetation is sufficient to conserve stream fauna.
To assess the extent to which stream fish are being effectively conserved in agricultural landscapes, we examined the spatial distribution of assemblages in river basins to identify the relative importance of human impacts at instream, riparian and catchment scales, in shaping observed patterns. We used an extensive dataset on the ecological condition of 83 low-order streams distributed in three river basins in the eastern Brazilian Amazon.
We collected and identified 24,420 individual fish from 134 species. Multiplicative diversity partitioning revealed high levels of compositional dissimilarity (DS) among stream sites (DS = 0.74 to 0.83) and river basins (DS = 0.82), due mainly to turnover (77.8% to 81.8%) rather than nestedness. The highly heterogeneous fish faunas in small Amazonian streams underscore the vital importance of enacting measures to protect forests on private lands outside of public protected areas.
Instream habitat features explained more variability in fish assemblages (15%–19%) than riparian (2%–12%), catchment (4%–13%) or natural covariates (4%–11%). Although grouping species into functional guilds allowed us to explain up to 31% of their abundance (i.e. for nektonic herbivores), individual riparian - and catchment - scale predictor variables that are commonly a focus of environmental legislation explained very little of the observed variation (partial R2 values mostly <5%).
Policy implications. Current rates of agricultural intensification and mechanization in tropical landscapes are unprecedented, yet the existing legislative frameworks focusing on protecting riparian vegetation seem insufficient to conserve stream environments and their fish assemblages. To safeguard the species-rich freshwater biota of small Amazonian streams, conservation actions must shift towards managing whole basins and drainage networks, as well as agricultural practices in already-cleared land.
Keywords: Amazon, Brazilian Forest Code, functional guilds, human-modified landscapes, multiplicative diversity partitioning, physical habitat, species turnover, tropical landscapes, watershed management
1. |. INTRODUCTION
Agricultural expansion and its associated forest disturbances are major threats to the biodiversity of the humid tropics (Barlow et al., 2016; Laurance, Sayer, & Cassman, 2014). Environmental legislation and conservation programmes help countries to minimize these losses and to meet their commitments to the Convention on Biological Diversity (CBD, 2010). However, the focus of legislative efforts has been largely based on maintaining terrestrial forest extent and has paid little heed to the critical features of hydrological systems such as the size and distribution of river catchments (Castello & Macedo, 2016). As such, it remains unclear the extent to which existing environmental regulations safeguard the ecological integrity of stream systems, which accumulate human impacts from many different terrestrial activities, and whose biodiversity may be more imperilled than their terrestrial equivalents (Strayer & Dudgeon, 2010).
There are few places on Earth where the conservation of aquatic diversity is more important than in the Amazon Basin, which has the world’s most diverse freshwater fish fauna (Castello & Macedo, 2016; Reis, Kullander, & Ferraris, 2003). One of the most poorly studied elements of this fauna is the fish diversity of small, wadable streams (Mojica, Castellanos, & Lobón-Cerviá, 2009). Those streams are the most extensive and widespread freshwater ecosystems in the basin (Beighley & Gummadi, 2011), consisting of up to 90% of the total channel length in some sub-basins (McClain & Elsenbeer, 2001).
Brazil contains 60% of the Amazon Basin, and its environmental regulations seek to conserve freshwater ecosystems in three ways: (1) establishing protected areas; (2) controlling forest cover on private properties; and (3) regulating water resources that are considered to be of high economic importance. Yet all of these approaches have important limitations. Although protected areas comprise 54% of the Brazilian Amazon, their distribution takes little account of connectivity in and among watercourses, many of which extend across biomes and jurisdictional boundaries (Castello et al., 2013). Effective protection of transboundary river basins is particularly challenging because countries have different levels of international cooperation, conservation priorities and conservation budgets (Dolezsai, Sály, Takács, Hermoso, & Erős, 2015). Environmental regulation on Brazilian private lands, which make up about half of the country’s native vegetation (Ferreira et al., 2012; Soares-Filho et al., 2014), is through the Forest Code (FC; Law 12.651; Brasil, 2012). Although the FC stipulates minimum-width riparian forests along streams and limits deforestation outside riparian zones, it does not provide guidance for forest protection at catchment or basin scales or for agricultural practices, both of which affect the freshwater biota (Leitão et al., 2017; Roth, Allan, & Erickson, 1996). Lastly, the two Brazilian legal instruments directly concerned with streams, the Fisheries Code (Law 11.959; Brasil, 2009) and the Water Resources Regulation (Law 9.433; Brasil, 1997), focus on aquaculture and fishing activities and water for human consumption, respectively. As such, they do not directly address the biodiversity values of freshwater ecosystems (Castello et al., 2013). Moreover, all three of these areas of legislation to conserve freshwater systems in the Brazilian Amazon suffer from being poorly coordinated and weakly enforced (Castello & Macedo, 2016).
Given the potential shortcomings in existing legislation to conserve stream biota, there is an urgent need to assess the effectiveness of existing regulatory mechanisms for conserving the fish assemblages in the Amazon Basin. Our current understanding of their effectiveness is limited by three key knowledge gaps. First, there is a lack of data on the responses of freshwater biota to human pressures across the biome. The vast majority of research on the effects of habitat degradation in the Amazon is on terrestrial biota. For example, a review of 62 studies assessing faunal responses to land-use change in Amazonia (Peres et al., 2010) included just one on fish (Dias, Magnusson, & Zuanon, 2010). Second, where fish responses to human impacts have been studied in Amazonia, they have focused on large rivers, hydropower plants, and commercially important species (Barthem, de Brito Ribeiro, & Petrere, 1991; Hurd et al., 2016; Tregidgo, Barlow, Pompeu, de Almeida Rocha, & Parry, 2017). Very few studies have examined the consequences of human impacts on the heterogeneous Amazonian fish assemblages in small streams. As such, little is known about the responses of stream fauna to deforestation, agricultural intensification and other sources of forest degradation (Dias et al., 2010; Issues, 2002; Leitão et al., 2017; Prudente, Pompeu, Juen, & Montag, 2017).
Third, we lack large-scale empirical studies evaluating the relative importance of pressures affecting biotic change in streams at different spatial scales, and how amenable such pressures are to changes in the management regime (Hughes, Wang, & Seelbach, 2006). There is uncertainty regarding whether catchment disturbances (Allan, Erickson, & Fay, 1997; Marzin, Verdonschot, & Pont, 2013; Roth et al., 1996) or local riparian disturbances (Macedo et al., 2014; Sály, Takács, Kiss, Bíró, & Eros, 2011; Wang et al., 2003) are the most critical drivers of changes in the biotic condition of streams. Similarly, it is unknown to what extent management practices at local, small scales are constrained by ecological processes at catchment scales (Castello & Macedo, 2016; Mantyka-Pringle et al., 2016; Palmer, Menninger, & Bernhardt, 2010). Answers lie largely in the types and relative degrees of disturbance and natural variability at these two scales and the biotic indicators of condition (Terra, Hughes, & Araújo, 2016; Wang, Seelbach, & Lyons, 2006).
We address these knowledge gaps using a large-scale assessment of the fish fauna among 83 stream sites in the human-modified landscapes of the eastern Brazilian Amazon. First, we examine the importance of forest reserves on private lands for conserving fish diversity by assessing patterns of species turnover among stream sites within three river basins and among those basins. Second, we examine the effectiveness of the FC for protecting Amazonian stream biota by investigating how fish assemblages are affected by human disturbances assessed at three spatial scales: (1) the riparian scale, reflecting the explicit focus of the FC in conserving aquatic systems; (2) the catchment scale, accounting for the requirement of private landholders to conserve 50%–80% of their forest cover outside the riparian zone, although the FC does not explicitly regulate at the catchment scale; (3) the instream habitat scale, characterizing conditions that are strongly affected by riparian and catchment disturbances, and that have a direct impact on fish assemblages, but for which there is virtually no legislative protection (Figure 1). We use our findings to discuss the challenges involved in understanding the links between human disturbances and fish assemblages in tropical streams, the effectiveness of the FC in protecting stream biota and the implications for large-scale conservation planning in human-modified tropical forest landscapes more generally.
FIGURE 1.
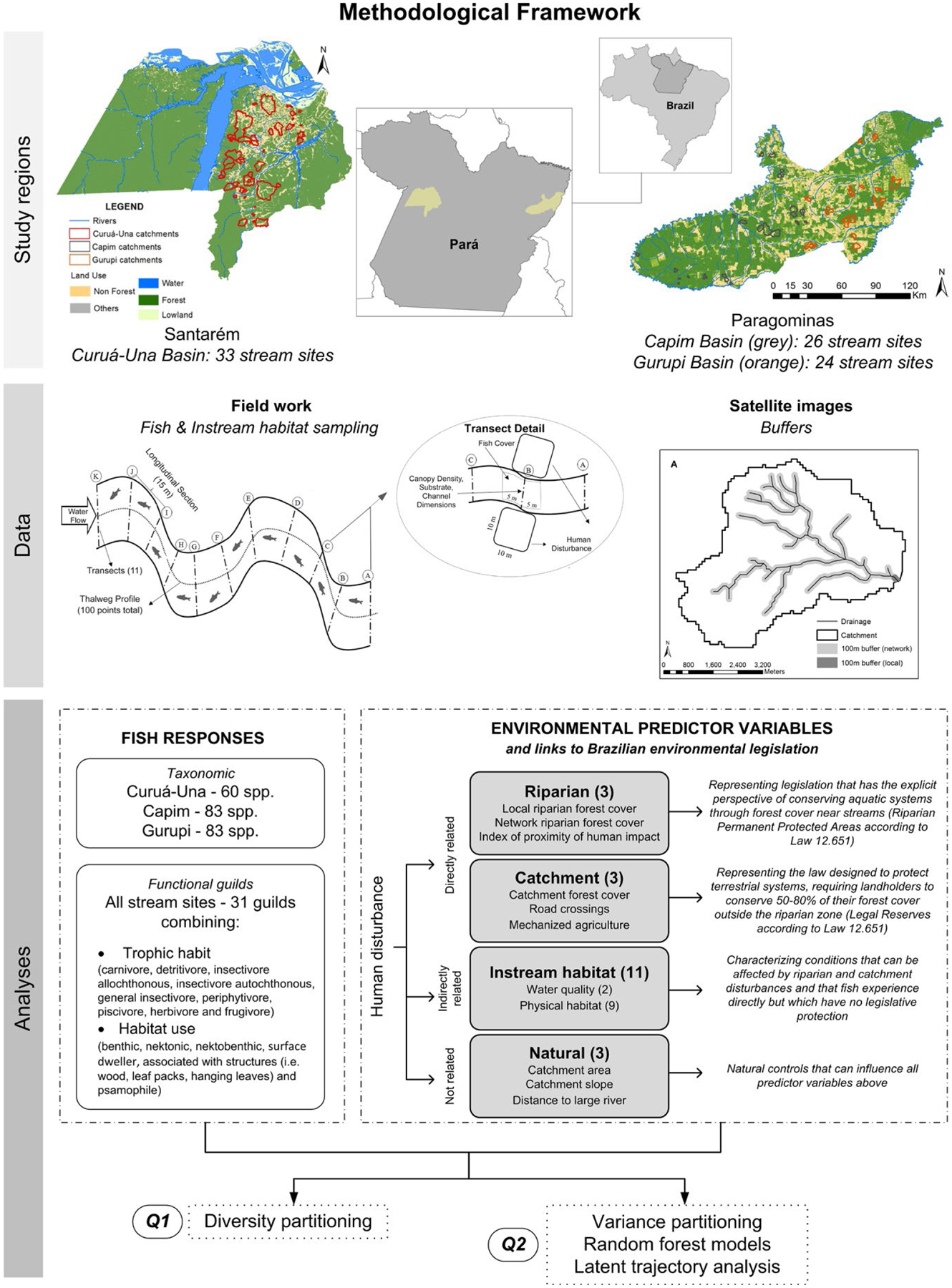
Methodological framework to investigate fish species responses to human disturbances in Amazonian landscapes
2 |. MATERIALS AND METHODS
2.1 |. Sampling design
We studied two regions in the eastern Brazilian Amazon state of Pará. Santarém (STM) covers 1 million ha at the confluence of the Amazonas and Tapajós Rivers; Paragominas (PGM) covers 1.9 million ha in the far eastern Amazon Basin. Both regions are characterized by a patchwork of pasture (3.9% in STM and 21.1% in PGM, data from 2010), annual crops (2.0% and 3.5%), including mechanized agriculture, and secondary forest (10.4% and 17.6%) and retain around two-thirds of their native primary forest, albeit in varying stages of degradation from fragmentation, logging, and fires (Almeida et al., 2016; Gardner et al., 2013). Wadable stream sites (1st to 3rd Strahler order on a digital 1:100,000 scale map) were chosen to encompass a gradient in the extent of riparian and catchment forest cover, resulting in 33, 26 and 24 sites in the Curuá-Una (STM), Capim (PGM) and Gurupi (PGM) River Basins, respectively (Figure 1).
We sampled fish during the Amazonian dry season June–August 2010 (STM) and 2011 (PGM). Each 150-m-long site was subdivided into 10 continuous sections by 11 cross-sectional transects (isolated by block nets) (Figure 1). Three people sampled fish for 120 min (12 min per section) with seines (6.0 × 1.5 m, 5 mm stretched mesh size) and semi-circular hand nets (0.8 m in diameter, 2 mm stretched mesh size) (Appendix S4). Specimens were euthanized in Eugenol and then fixed in 10% formalin. In the laboratory, all sampled fishes were transferred to 70% ethanol and identified to species. Voucher specimens from all species are deposited at the fish collections of the Instituto Nacional de Pesquisas da Amazônia (INPA) and the Museu Paraense Emílio Goeldi (MPEG), Brazil.
Physical habitat data were collected along the thalweg and from 11 transects every 15 m (Figure 1; Appendix S1; Hughes & Peck, 2008). Between the transects, we quantified large wood volume in the channel and measured thalweg depth and substrate size at 10 equidistant points. At each of the 11 transects, we measured bankfull width and depth, and at five equidistant points along each transect, we measured water depth and assigned a surficial bed particle diameter class. Cover for fish was assessed at each transect along 10-m-long plots inside the stream channel using semi-quantitative estimates of the areal cover of leaf packs, roots, overhanging vegetation, wood, undercut banks, boulders, filamentous algae and aquatic macrophytes. Forest canopy cover above the channel was measured with a convex densiometer at the centre of each transect (facing upstream, downstream, left and right margins), and the mean values were used as a proxy for channel shading. We measured conductivity and temperature with a portable digital meter placed below the water surface in the centre of the site. From these measurements, we calculated 11 metrics (Table 1; Kaufmann, Levine, Robison, Seeliger, & Peck, 1999) representing complementary attributes of instream conditions likely affected by land-use changes (Leal et al., 2016) and influencing stream-fish assemblages (Leitão et al., 2017).
TABLE 1.
Environmental variables used to predict fish assemblage composition in Amazonian stream sites
| Environmental predictor variables | ||
|---|---|---|
| Group | Code | Definition |
| Riparian | NET_FOR | % riparian network forest |
| LOC_FOR | % local riparian forest | |
| W1_HALL | Proximity weighted tally of riparian/stream side disturbances (Kaufmann et al., 1999) | |
| Catchment | CAT_FOR | % catchment forest |
| CAT_MAG | % mechanized agriculture | |
| DEN_RCS | Number of road crossings within a 5 km circular buffer upstream and downstream the stream site divided by catchment area | |
| Instream habitat | Water quality | |
| TEMP | Water temperature (°C) | |
| COND | Electrical conductivity (μ/cm) | |
| Substrate | ||
| FINE | Streambed surficial fines <0.6 mm diameter—% areal cover | |
| Cover and wood | ||
| AMCV | In-channel algae and macrophytes—% areal cover | |
| NTCV | In-channel natural cover (wood, live trees and roots, leaf packs, overhanging vegetation, undercut banks, boulders)—% areal cover | |
| WOOD | Wood volume—m3/m2 wetted channel area | |
| Channel morphology | ||
| DPTH | Standard deviation of thalweg depth (cm) | |
| BKWD | Ratio: Bankfull width to bankfull thalweg depth—dimensionless | |
| RP100 | Mean residual depth at thalweg ([m2/m]/cm) | |
| Other | ||
| LRBS | Log10 of relative bed stability estimated at bankfull flow conditions (Kaufmann, Faustini, Larsen, & Shirazi, 2008; Kaufmann, Larsen & Faustini 2009) | |
| SHAD | Canopy density (shading) measured at mid-channel (%) | |
| Natural | CAT_ARE | Catchment area (ha) |
| CAT_SLO | Catchment slope | |
| DST_RIV | Distance to large river (≥4th Strahler order) (m) | |
2.2 |. Riparian-and catchment-scale measures
We mapped the drainage network using the hydrological model ArcSWAT (Di Luzio, Srinivasan, & Arnold, 2004), allowing us to calculate hydrological distance between each site and the main river downstream (4th order reaches). We determined catchment boundaries, mean elevation and slope through use of digital elevation models (SRTM images, 90 m resolution).
We assessed site pressures at three spatial scales (Figure 1): (1) whole catchment upstream from a site (catchment); (2) 100 m buffer along the entire drainage network upstream from the site (riparian network); and (3) 100 m riparian buffer along the site (local riparian). Riparian buffer widths and the basis for their definition vary greatly among ecological studies and environmental regulations world-wide (e.g. Lee, Smyth, & Boutin, 2004). The FC establishes a minimum buffer width of riparian vegetation to be protected (or restored in case of illegal deforestation) alongside watercourses inside private properties. However, this width is based on several criteria (e.g. size of the property, stream width, when deforestation occurred, etc.) and there is no set width that could be applied across the landscape in the absence of data on land tenure and deforestation history. Therefore, we selected 100 m buffers to provide estimates of land use within the riparian zone considering the resolution of the land-use maps and the digital elevation models (30 to 90 m), and what is considered in other studies (e.g. Van Sickle et al., 2004), without linking these to the requirements specified by Brazilian laws.
We calculated forest-cover proportion for 2010 using classified Landsat images with 30 m of resolution (Gardner et al., 2013). Forest cover included primary forest (whether undisturbed or disturbed from fire or logging), and secondary forest older than 10 years, which was considered sufficiently developed to provide important hydrological services (e.g. soil stabilization, sediment and nutrient filtration). The history of mechanized agriculture was calculated from annual MODIS data from 2001 to 2010 (Gardner et al., 2013).
We recorded the human activities in the local riparian zone (e.g. pipes, buildings and trash; Hughes & Peck, 2008) and calculated an index of proximity of human impact (W1_HALL; Kaufmann et al., 1999). We used RapidEye images (2010 for STM and 2011 for PGM, 5 m resolution) to estimate riverscape fragmentation from upstream and downstream road crossings within a 5 km circular buffer from the stream site. All landscape analyses were conducted in ArcGIS 9.3© (Environmental Systems Research Institute, Redlands, CA, USA).
2.3 |. Linking environmental predictors with Brazilian legislation
Our direct (riparian and catchment) and indirect (instream habitat) measures of human disturbance reflect different aspects of Brazilian legislation regulating the protection of watercourses (Figure 1, Table S1). The forest-cover variables and the index of proximity of human impact represent the FC regulation on the protection of riparian vegetation and Legal Reserves elsewhere in the properties. Roads alter both the streams they cross (Leal et al., 2016; Leitão et al., 2017; Macedo et al., 2013) and the riparian forests adjacent to the crossing; however, the FC regulates only the forests. The extent and type of agricultural mechanization are not governed by the FC or any other regulation in the country. Measures of instream habitat are very difficult to regulate because they reflect both natural characteristics of the landscape and the outcomes of human disturbances. However, dissolved oxygen is used for water body classification by Law No 9.433 (Brasil, 1997).
2.4 |. Data analyses
2.4.1 |. Diversity partitioning
We used multiplicative diversity partitioning to analyse the spatial distribution of fish diversity considering the following decompositions: γregion = αriver basin × βriver basin (for PGM) and γriver basin = αstream site × βstream site (for the Curuá-Una, Capim, and Gurupi Basins). We compared the magnitude of variation in βriver basin and βstream site using the relative compositional dissimilarity (DS) following Arroyo-Rodríguez et al. (2013). DS varies from 0 (identical assemblages) to 1 (completely different assemblages). Next, we decomposed the components of βstream site to investigate whether variation in species composition across sites in each river basin was a result of turnover (species replacement) or nestedness (species loss or gain) using Sørensen (βSOR) and Simpson (βSIM) indices (Baselga, 2010).
2.4.2 |. Assemblage–environment modelling
We conducted variance-partitioning analysis (Borcard, Legendre, & Drapeau, 1992) for each river basin separately, which allowed us to estimate the amount of variation in taxonomic composition in assemblages explained by the four sets of environmental predictors. We performed variance partitioning for functional guilds by combining fish trophic and habitat-use characteristics for all river basins together (Appendix S2). Species biological traits can help to uncover responses to human disturbances (Mouillot, Graham, Villéger, Mason, & Bellwood, 2013), especially in systems dominated by rare species. Several species were singletons (e.g. 12 species in Capim) or occurred at very few sites (e.g. 50% of the Curuá-Una species occurred in three or fewer sites) (Appendix S3), which hindered development of robust species-specific models.
We used values from adjusted redundancy analysis, which account for the number of predictor variables in each group and the number of observations in the response variables to produce unbiased estimates (Peres-Neto, Legendre, Dray, & Borcard, 2006). Explained variance was split into 16 fractions using partial ordination methods: four individual components explained independently by each group of predictor variables, 11 fractions for the explained variance shared by two or more groups and a residual fraction of the unexplained variance (Borcard et al., 1992).
2.4.3 |. Relative effects of policy-relevant environmental predictors
To examine the influence of variables that are frequently targeted by environmental legislation, we used random forest models (RF; from Breiman, 2001) to evaluate changes in functional guild abundance for the combined river basins. We considered riparian and catchment predictors and natural covariates in the models to investigate the effect of those governed by the FC (CAT_FOR, LOC_FOR, NET_FOR, W1_HALL) and possibly governable (DNS_RDS, CAT_MAG) (see Table 1 for variable codes). RF incorporates interactions among predictors and nonlinear response–predictor relationships. We calculated a pseudo-r2 value as 1-MSE/Var(y), where MSE is the mean squared error of the out-of-bag predictions (Ellis, Smith, & Roland Pitcher, 2012). This value estimates the reliable proportion of variation predicted by the ensemble model. All models were fitted with 10,000 trees, with one-third of variables randomly sampled as candidates at each split (one variable selected if total variables <3).
Next, we used RF to model the partial responses of functional guilds to the six predictor variables listed above. Those partial responses show the relative odds of detecting each guild along a predictor gradient while holding all other predictors constant (Barlow et al., 2016). Last, we used latent trajectory analysis (LTA) to group guild partial responses into homogeneous classes, which summarize the main types of response to the predictors and the extent of species turnover. We considered LTA models with up to five classes and selected the model with the lowest Bayesian Information Criterion score. We show the LOWESS smoothed response of each guild class along the associated predictor variable with bandwidth set to the default value of 0.75.
All analyses were performed in R (R Core Team, 2013) and are outlined in Appendix S2. Diversity partitioning (beta.multi function) and variance partitioning (varpart function) were performed using the vegan library (Oksanen et al., 2013). Random forest models and the relative importance (RI) of individual predictor variables were calculated using the conditional permutation method in the randomForest function of the extendedForest library (Smith, Ellis, & Pitcher, 2011). Latent trajectory analysis used the lcmm library (Proust-Lima, Philipps, Amadou, & Liquet, 2016).
3 |. RESULTS
3.1 |. Diversity partitioning to assess landscape patterns of stream-fish diversity
We collected 24,420 individual fish from 134 species, with 60 species (5,846 specimens) in Curuá-Una, 83 in Capim (7,421 specimens) and 83 in Gurupi (11,153 specimens) (Table S2, Appendix S4). The relative compositional dissimilarity for the PGM basins was DS = 0.46. Among stream sites, DS = 0.82 for PGM, 0.74 for Gurupi, 0.78 for Capim and 0.83 for Curuá-Una, indicating that river basins and stream sites within river basins are distinct from each other (Figure 2a), showing the high level of environmental heterogeneity in Amazonian streams. The contribution of turnover to the βstream site component was much higher than nestedness in all river basins: 81.8% (Curuá-Una), 78.6% (Capim) and 77.8% (Gurupi) (Figure 2b). All values were significantly different from those expected by chance obtained from 1,000 permutations (p < .001).
FIGURE 2.
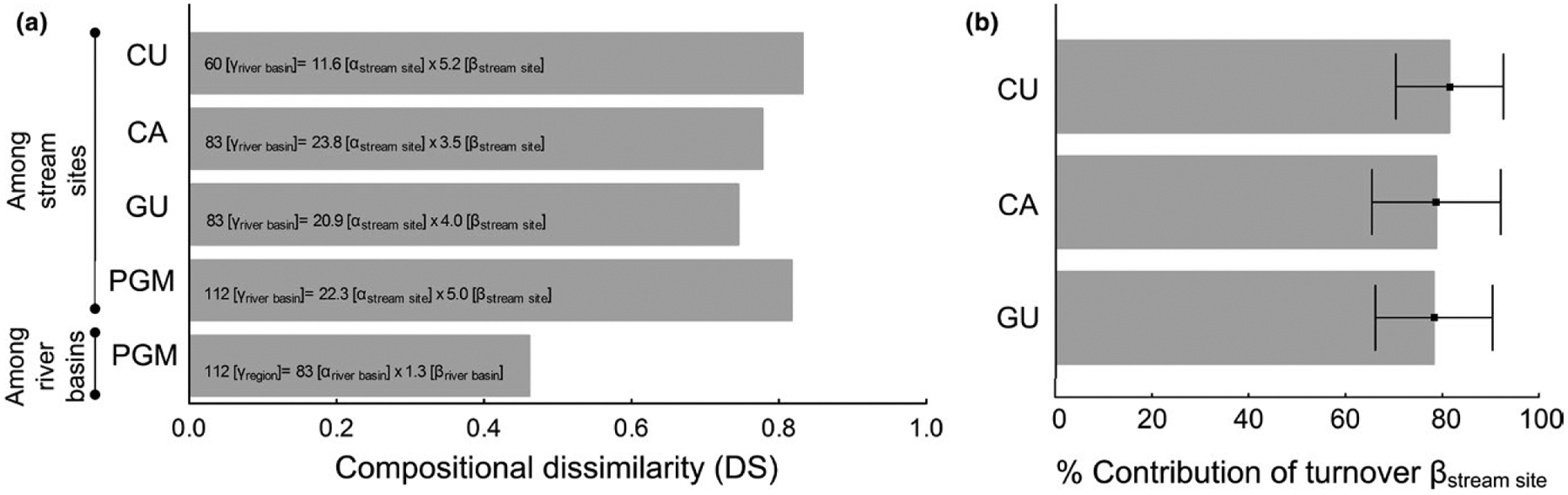
Multiplicative diversity partitioning for Amazonian stream sites and river basins: Curuá-Una (CU), Capim (CA) and Gurupi (GU). (a) Relative compositional dissimilarity among stream sites and river basins; DS varies from 0 (identical assemblages) to 1 (completely different assemblages). (b) Percentage contribution of turnover to βstream site with standard deviation bars
3.2 |. Assemblage–environment relationships to assess the effectiveness of current legislation to protect stream-fish diversity
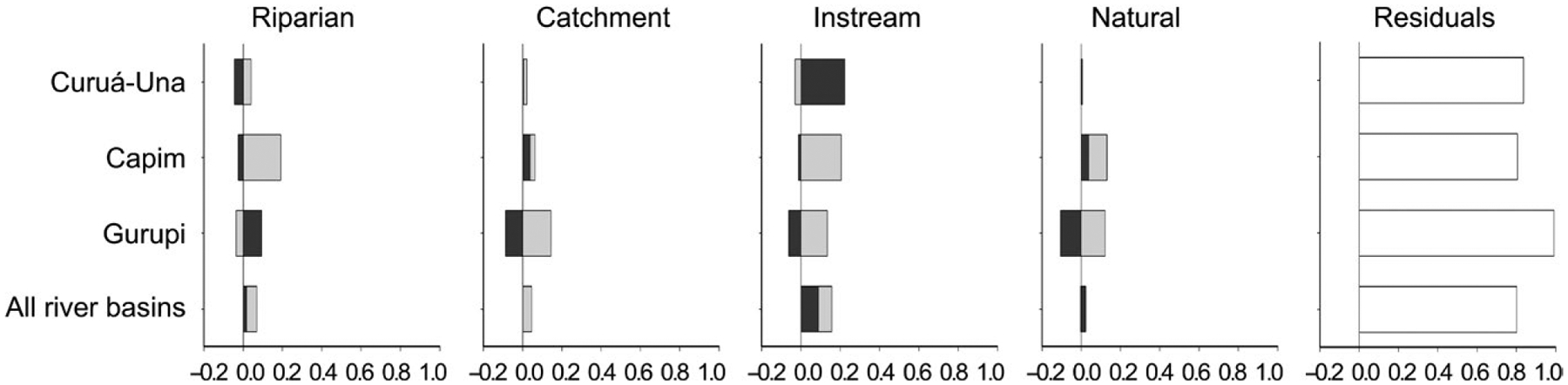
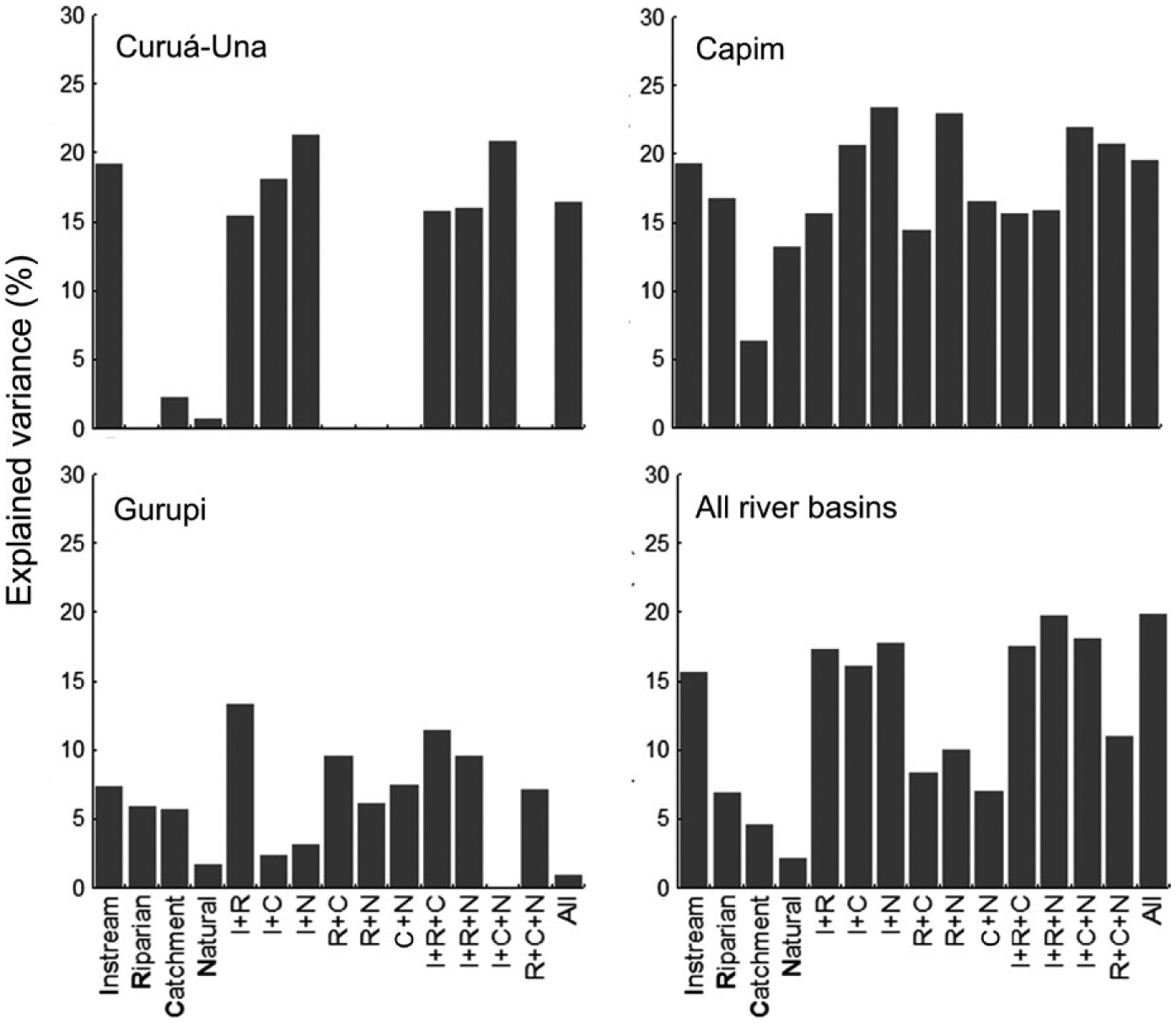
Despite the diverse set of environmental predictor variables included in our analysis, together they explained only 0.9%–19.5% of the variation in taxonomic and 19.8% in functional guild assemblage composition (Figure 3). Instream habitat was the most important predictor in the Curuá-Una Basin (22.3%) and for all stream sites (8.8%) (Figure 3). In the Capim and Gurupi basins, the effect of instream habitat was through its interactions with other predictor variables. Riparian and catchment predictors explained smaller proportions of assemblage variation for both species and guild abundance, and mostly through interactions with other predictor variables. Natural characteristics of stream sites were mainly important in the Capim River Basin (3.8%).
FIGURE 3.
Partitioning of the variation in occupancy of stream fish assemblages in Curuá-Una, Capim and Gurupi River Basins (species abundance), and all river basins together (functional guilds abundance) showing the variance explained by each group of predictor variables (dark grey) when partitioning out the effects of the other groups through redundancy analysis (partitions [a], [b], [c] and [d] according to variance partition analysis) and the fractions shared between the groups (light grey). Unexplained variance is represented in white. Negative values of indicate that the predictor variables explain less variation than random normal variables, and should be interpreted as zeros (Legendre, 2008)
Assessing the effects of each group of predictor variables independently showed a similar pattern of responses (Figure 4). Instream habitat had the greatest contribution in explaining the observed variability in fish assemblages from the Curuá-Una (19.2%), Capim (19.2%) and Gurupi (7.3%) basins, and in the functional guild composition for all stream sites combined (15.7%). The contribution of riparian pressures differed greatly accounting for 16.5% in the Capim Basin, 5.8% in the Gurupi Basin and 6.8% in all river basins together, but effectively none of the variability in the Curuá-Una Basin. Overall, catchment disturbance was associated with smaller proportions of the variability in assemblage composition than riparian pressures, except for the Curuá-Una Basin. Natural characteristics were only important in the Capim Basin (13.2%); however, they accounted for variability in the other assemblages through interactions with other predictor variables.
FIGURE 4.
Individual and joint effects of instream habitat (I), riparian (R), catchment (C), and natural (N) predictor variable groups on taxonomic (Curuá-Una, Capim, and Gurupi River Basins) and functional guild (all river basins together) composition
3.3 |. Functional guild responses to policy-relevant measures of human impact
Random forest models explained up to 31% (for the nektonic herbivore guild) of the observed variation in guild abundance (Table S3). Four of the 31 guilds had no variation explained, and another ten could not be modelled because they occurred at too few sites or were represented by too few individuals. Single riparian- and catchment-scale predictor variables explained very little of the observed variation (partial R2 values mostly <5%) in most functional guilds (Figure 5). This result reflects the low level of assemblage turnover relative to most of our measures of human disturbance, which was shown by the LTA on guild partial responses (Figure 6, Table S4). Guild responses mainly were to forest-cover variables. Most guilds responded negatively to network forest cover (Figure 6b), and some showed a positive increase at ca 70%. Few guilds responded to local forest cover, and those mainly decreased in more forested streams (Figure 6a). Catchment forest accounted for sharp increases of guilds at ca 60%. However, most responses also related to guilds decreasing in abundance along the gradient of human impact (Figure 6d). We did not find consistent changes in guild abundance in response to the index of proximity of human impact, road density or the proportion of mechanized agriculture in catchments (Figure 6c,e,f).
FIGURE 5.
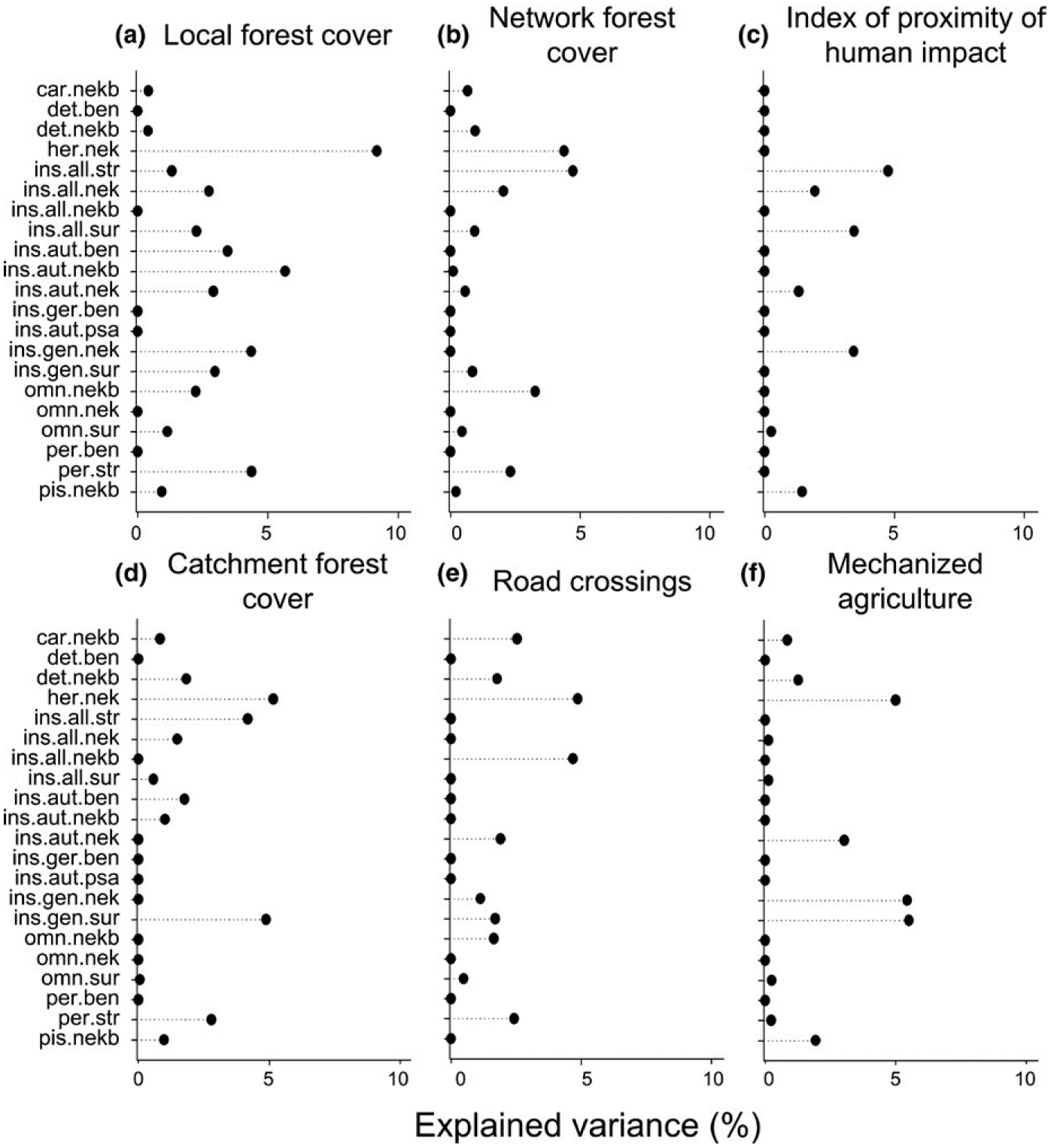
Partial effects from random forest models showing the percentage of functional guild variation explained by the environmental predictors
FIGURE 6.
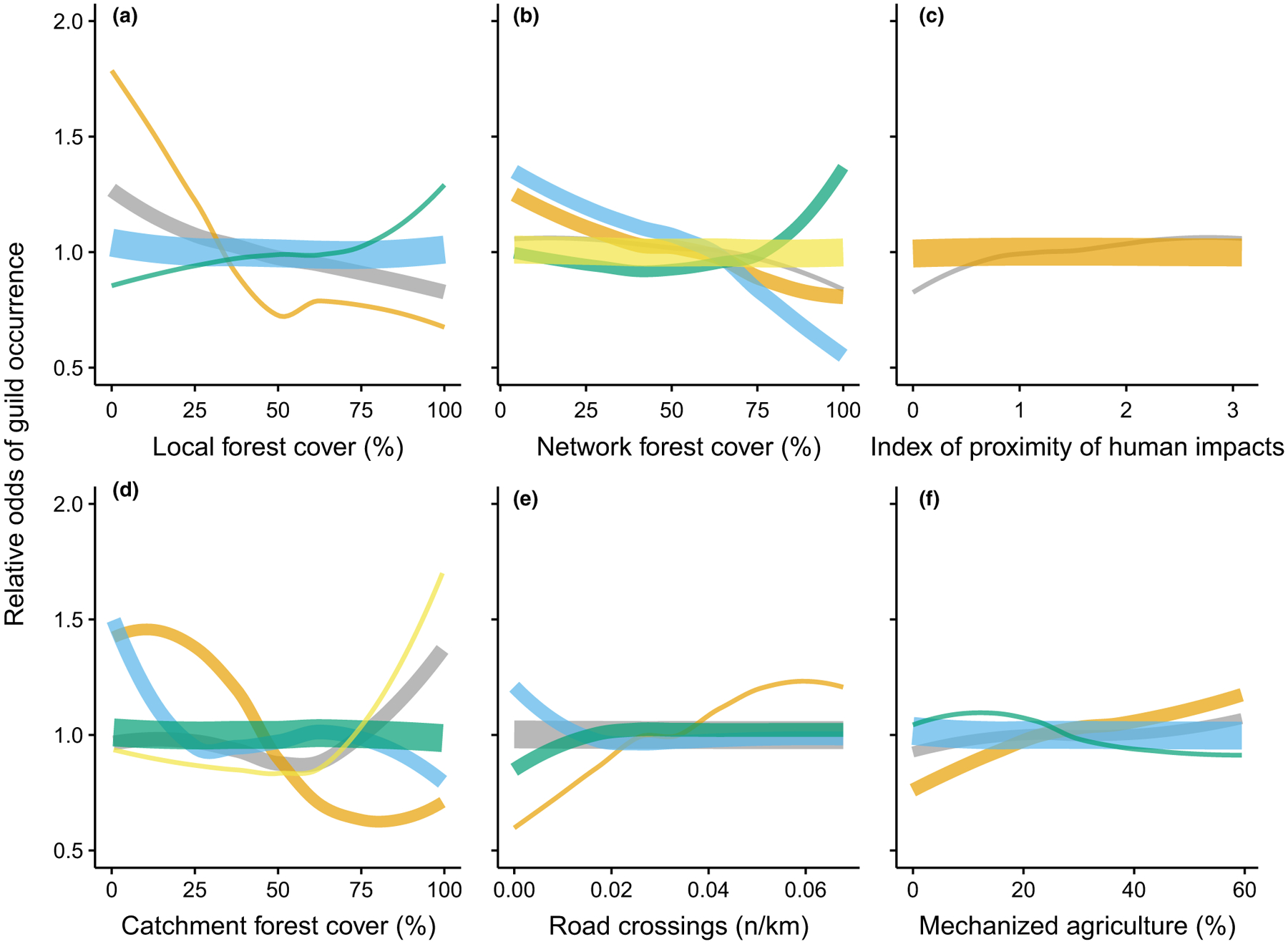
The relative odds of detecting fish functional guilds along gradients of governable management predictor variables. Different coloured lines show classes of guilds with similar responses to human disturbance (see Table S4 for constituent species). Line thickness represents the relative number of guilds in each LTA-defined class
4 |. DISCUSSION
Our large-scale assessment of Amazonian stream fishes provides four sets of insights relevant to the research and management of aquatic diversity in human-dominated landscapes. First, we observed very high levels of species turnover, even within the same river basin, highlighting the importance of conservation measures beyond protected areas. Second, we found that changes in fish abundance were more strongly associated with instream habitat pressures than with the variables more frequently addressed by Brazilian environmental legislation, such as those related to riparian and landscape-scale measurements of forest cover. Third, despite our extensive sampling of environmental features generally thought to affect fish assemblages, our understanding of the relative importance of different impacts was diluted by the amount of unexplained variance, region-specific relationships and the complex interdependent associations among predictor variables. Such challenges are to be expected in biodiversity-rich regions with a diverse mosaic of land uses and natural characteristics and pose particular difficulties for assessments of the most disturbance-sensitive fish species. Lastly, our results underscore a number of priorities for future research on human impacts on tropical stream-fish assemblages, including the assessment of a wide range of impacts at multiple scales, the importance of pre-disturbance information and the relevance of different species traits in determining species’ tolerance to disturbance impacts. We examine these four issues in more detail below.
4.1 |. Conservation of stream fauna beyond protected areas
While high levels of species turnover are typical of many tropical landscapes (Solar et al., 2015), ours is the first study to report such a finding for stream systems in Amazonian agricultural-forest landscapes. This very high level of species turnover in fish assemblages among streams and river basins (Figure 2) lends strong support for legislation, such as the Brazilian FC, which targets the maintenance and rehabilitation of forest cover in private properties throughout agricultural landscapes. However, our results demonstrate that planning needs to consider the scale of entire landscapes and river basins, and cannot be focused on individual private properties or on municipalities, where most environmental legislation (including the FC) is enacted (Viana et al., 2016). Therefore, our results have two important implications for the spatial implementation of FC legislation to conserve aquatic biodiversity.
First, our results provide guidance on forest restoration. The FC offers two alternative mechanisms for landowners to address previous illegal deforestation (the so-called legal reserve deficit; Soares-Filho et al., 2014; Nunes et al., 2016) and come into compliance with the law–landowners can either undertake on-farm rehabilitation or invest in compensation by renting or purchasing forest in other regions. However, the FC does not specify which action should occur, and any compensation only needs to occur within the same biome, that is in the entire Brazilian Amazon (Nunes et al., 2016). By demonstrating the high turnover in species composition, our results provide strong empirical support for the recommendations of Nunes et al. (2016) to encourage compliance efforts to take place locally, either by focusing on rehabilitation in landscapes that are heavily deforested or by undertaking off-farm compensation within the same river basin.
Second, our results show that the FC focus on land use in the riparian zone to protect streams should not undermine the necessity to maintain and restore forest cover elsewhere in the catchment. In some cases, catchment-scale pressures were of comparable importance to riparian-scale pressures in shaping fish assemblages (Figures 3 and 4), which supports other studies that show how management practices in the riparian zone are insufficient for restoring biodiversity unless incorporated with improved catchment and channel network management (Fausch, Torgersen, Baxter, & Li, 2002; Mantyka-Pringle et al., 2016). Within the Brazilian Amazon, this is particularly important in areas that have been designated as “consolidated zones” for agriculture as part of ecological–economic zoning plans, where properties that have cleared more than 50% of their forest cover only have to restore (or compensate) back to 50%. However, our results show that even 50% forest cover in catchments risks altering the abundance and composition of fish functional guilds (Figure 6). More work is needed to identify thresholds in the abundance of species of the highest conservation concern (e.g. de Oliveira-Junior et al., 2015; Leitão et al., 2016).
4.2 |. The importance of local stream condition
Our findings show that fish assemblages are influenced by changes in local stream condition, which includes a suite of factors that are not currently addressed by any environmental legislation. This is important because it implies that disregarding changes in local stream condition can lead to an underestimation of the effects of human disturbances at the catchment and riparian scales, given that many such impacts are only observable through changes in instream habitat condition (Leal et al., 2016). The question remains as to whether management can address such impacts.
First, it is important to examine to what extent these changes in instream condition are an outcome of indirect interactions with broader-scale human pressures, such as forest cover, that are already being addressed by existing legislation. For example, while our results were statistically independent of our catchment and riparian-scale variables, linkages between landscape change and instream condition can be complex and diverse (Leal et al., 2016), and it is unlikely that they were fully represented by our explanatory variables. It is highly probable that the human alterations at riparian and catchment scales play indirect roles in influencing fish assemblages by, say, regulating channel morphology, bed substrate composition, wood and leaf litter inputs, shade and water quality (Kaufmann & Hughes, 2006; Leal et al., 2016; Leitão et al., 2017). These linkages between human disturbances and instream habitat conditions are further complicated by interactions with factors such as the degree of basin disturbance (Sály et al., 2011; Wang et al., 2006), type of disturbance (USEPA, 2016), biotic group (Marzin et al., 2012) and the intrinsic geomorphological characteristics of the systems (Kaufmann & Hughes, 2006); all of these factors may have contributed to low levels of explained variation in our models. Given these complexities, there is a genuine risk that monitoring and assessment programmes that focus only on instream habitat or riparian zones are likely to underestimate the effects of cumulative human disturbances on streams (e.g. Schinegger, Trautwein, Melcher, & Schmutz, 2012; USEPA, 2016).
A second argument against legislating for instream condition relates to evidence from other systems. Although management practices in temperate and tropical nations are often restricted to reach or riparian scales (Bernhardt & Palmer, 2011; Giling, Mac Nally, & Thompson, 2015), there is growing recognition of the importance of implementing catchment- or basin-scale management (Abell, Allan, & Lehner, 2007). Moreover, there is a lack of evidence supporting the effectiveness of reach-scale interventions (e.g. channel re-configuration or the addition of boulders and logs) or point-source pollution treatment for restoring aquatic biodiversity in Europe and the United States (Hughes et al., 2014; Palmer et al., 2010). Most aspects of instream habitat are difficult and costly to manage directly, and it would be nearly impossible to monitor effectively across very large spatial scales such as the Amazon Basin (Castello et al., 2013).
Although there are many challenges to developing management strategies that focus on changes in instream condition in complex tropical landscapes, our results do nevertheless highlight the importance of these changes for stream-fish assemblages. Perhaps a more effective approach would be to develop a better understanding of the linkages between landscape-scale changes and instream condition, through assessing key indicators (e.g. volume of wood, water temperature, discharge, measures of sedimentation) as part of a wider approach to monitor and improve the effectiveness of riparian and catchment-scale interventions. Such monitoring programmes have been established in developed countries, and incorporate multiple biotic and abiotic indicators, catchment and riparian conditions, and relative risk assessments for linking instream conditions with multiple pressures. The results of such assessments have been effective in providing the scientific evidence for mitigating or preventing further reductions in instream biotic condition in a cost-effective manner (Davies, Harris, Hillman, & Walker, 2010; Hughes & Peck, 2008; USEPA, 2016).
Developing these assessments in the Amazon would be challenging, particularly given the current changes in environmental laws in Brazil (e.g. Azevedo-Santos et al., 2017; Fearnside, 2016; Ferreira et al., 2014). One option would be to use demonstration studies at ecoregion (McCormick et al., 2001) or basin (Jiménez-Valencia, Kaufmann, Sattamini, Mugnai, & Baptista, 2014) scales to develop these schemes–effective protocols could then be rolled out to other regions.
4.3 |. The challenge of unexplained variance and region-specific relationships
Among river basins, fish assemblages often showed different responses to the partial effects of the predictors (Figure 4), further illustrating the heterogeneity of Amazonian streams. For example, we found no substantial effects of riparian-scale pressures on Curuá-Una fish assemblages (Figure 4a), but these were as important as instream habitat variables in structuring Capim fish assemblages (Figure 4b). Although road crossings and the extent of mechanized agriculture were unrelated to the composition of fish functional guilds (Figure 6) and had limited effects on fish assemblages (Figure 5), both are known to affect instream habitat and fish functional structure of Amazonian streams in agricultural landscapes (Leal et al., 2016; Leitão et al., 2017; Macedo et al., 2013)–and have impacts on stream condition that are both cumulative and potentially multiplicative. Without clear empirical evidence, it is even harder to translate these findings into guidance for decision-makers, and current legislation may miss some of the key impacts by focusing on a limited number of management variables (e.g. the FC focuses only on forest cover).
Despite including detailed trophic and habitat-use information that is considered to be ecologically relevant to Amazonian stream-fish assemblages, we found few clear associations between fish and gradients of human pressures or specific impacts. Up to 22.5% of the variation in insectivorous fish was explained by riparian and catchment pressures (Table S3), yet partial effects from single predictor variables were mostly small (partial R2 values <5%). However, the best explained guild, nektonic herbivores, increased with decreased forest cover at all three spatial scales (Table S3, Figure S1). Deforestation increases insolation and aquatic vegetation, which favours herbivores. However, the lack of expected guild associations with forest cover, road crossings, mechanized agriculture and the index of proximity of human impact highlights the complex nature of linking multiple human disturbances to aspects of aquatic condition. This seems to be a nearly ubiquitous problem because researchers developing multimetric indices of fish assemblage condition in Europe, the USA and Brazil have had to reject the majority of candidate metrics because of low range, insensitivity to disturbance or poor reproducibility (de Carvalho et al., 2017; Esselman et al., 2013; Pont et al., 2006).
4.4 |. Implications for understanding fish distributions in tropical streams
Results from this study provide the basis for four recommendations for future applied research on fish–environment relationships. First, the importance of regional context suggests we need more multiscale studies in other river basins to understand the factors that underpin this context specificity. This would allow us to scale up these results to the rest of the Amazon and to other tropical systems and would assist with regional conservation planning. Future work should also address the specific design parameters of existing environmental legislation and current management and conservation strategies from other Amazonian countries to identify and help address potential inadequacies.
Second, we recommend that studies account for the full range of potential human disturbances. Both of our study regions have relatively high levels of catchment forest cover (60%–69%) and a recent history of intensified agricultural land use (i.e. mechanized agriculture was established in the early 2000s), so that we did not sample the most heavily disturbed catchments affected by mining, oil and gas drilling or urbanization. Allan (2004) noted that temperate streams may show little change in biota until reaching 30%–50% of agriculture extension in the catchment, although Fitzpatrick, Scudder, Lenz, and Sullivan (2001) reported thresholds at 10%–20% agriculture in the riparian zone. We did not account for degradation of the riparian forest (e.g. fire or logging), which can affect functioning in agricultural landscapes (Ferraz et al., 2014). Similarly, the recent spread of mechanized agriculture in Amazonia means it is important to investigate the effects of pesticides and fertilizers that result in high levels of contamination in surface and groundwater supplies, soil and biota (Schiesari & Grillitsch, 2011).
Third, we encourage more monitoring to investigate how time lags and shifting baselines in undisturbed forests influence stream condition responses to human disturbances. Our study was a temporal snapshot, which has two shortcomings. First, we have no information on pre-disturbance conditions, which is important because there is evidence that space-for-time approaches may lack the statistical power to detect changes identified by before-and-after studies (França et al., 2016; Larsen, Kaufmann, Kincaid, & Urquhart, 2004). Second, lag effects mean the full effects of disturbance may only become evident over longer times (Harding, Benfield, Bolstad, Helfman, & Jones, 1998; Hylander & Ehrlén, 2013).
Last, further studies are needed to relate fish ecophysiology (e.g. tolerance to pollutants and hypoxia), life-history traits (e.g. reproduction strategy, dispersal ability) and finer-tuned information on energetic sources (e.g. isotopic analysis revealing the real interdependence between terrestrial and aquatic food webs) to predict their tolerance to human impacts (de Carvalho, de Castro, Callisto, Moreira, & Pompeu, 2017; Leitão et al., 2017). Such information is scarce for the majority of Amazonian stream-fish species and would be of great value for improving our understanding of fish responses to human disturbances and the FC effectiveness.
Supplementary Material
ACKNOWLEDGEMENTS
We are grateful to the invaluable support of our field assistants and the farmers and communities of all surveyed municipalities. We also appreciate financial support from INCT-Biodiversidade e Uso da Terra na Amazônia (CNPq; 574008/2008-0), Embrapa (SEG: 02.08.06.005.00), the UK government Darwin Initiative (17-023), The Nature Conservancy and NERC (NE/F01614X/1 and NE/G000816/1). Individual funding included scholarships from CAPES in Brazil and a Science without Borders grant in the UK (PDSE-2943/13-1), and a Programa de Capacitação Institucional (MPEG/MCTIC, CNPq 300231/2016-4) to C.G.L; a CNPq productivity grant (306325/2011-0) and a FAPEMIG researcher grant (PPM-00237/13) to P.S.P.; a CNPq (156915/2011-1) and CAPES Science Without Borders grant in France (PDSE-1914/13-8) to R.P.L.; a CNPq award (400640/2012-0) to J.B.; a Fulbright Brasil grant to R.M.H; and a CNPq productivity grant (313183/2014-7) to J.Z. Our manuscript benefitted greatly from reviews by Kirk Winemiller, the associate editor, and three anonymous reviewers. It also was subjected to review by the U.S. Environmental Protection Agency National Health and Environmental Effects Research Laboratory’s Western Ecology Division and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use. This paper is #60 in the Sustainable Amazon Network (http://www.redeamazoniasustentavel.org/) and #52 in the Projeto Igarapés (http://www.igarapes.bio.br) publication series.
Funding information
The UK government Darwin Initiative, Grant/Award Number: 17-023; Natural Environment Research Council, Grant/Award Number: NE/F01614X/1 and NE/G000816/1; The Nature Conservancy; Instituto Nacional de Ciência e Tecnologia Biodiversidade e Uso da Terra na Amazônia, Grant/Award Number: 574008/2008-0; Empresa Brasileira de Pesquisa Agropecuária, Grant/Award Number: SEG: 02.08.06.005.00
Footnotes
DATA ACCESSIBILITY
All relevant data used in this manuscript are publicly available in the Dryad Digital Repository https://doi.org/10.5061/dryad.d5k7p (Leal et al., 2017).
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- Abell R, Allan J, & Lehner B (2007). Unlocking the potential of protected areas for freshwaters. Biological Conservation, 134, 48–63. 10.1016/j.biocon.2006.08.017 [DOI] [Google Scholar]
- Allan JD (2004). Landscapes and riverscapes: The influence of land use on stream ecosystems. Annual Review of Ecology, Evolution, and Systematics, 35, 257–284. 10.1146/annurev.ecolsys.35.120202.110122 [DOI] [Google Scholar]
- Allan JD, Erickson DL, & Fay J (1997). The influence of catchment land use on stream integrity across multiple spatial scales. Freshwater Biology, 37, 149–161. 10.1046/j.1365-2427.1997.d01-546.x [DOI] [Google Scholar]
- Almeida CAD, Coutinho AC, César J, Mora D, Adami M, Venturieri A, … Gomes AR (2016). High spatial resolution land use and land cover mapping of the Brazilian Legal Amazon in 2008 using Landsat-5/TM and MODIS data. Acta Amazonica, 46, 291–302. 10.1590/1809-4392201505504 [DOI] [Google Scholar]
- Arroyo-Rodríguez V, Rös M, Escobar F, Melo FPL, Santos BA, Tabarelli M, & Chazdon R (2013). Plant β-diversity in fragmented rain forests: Testing floristic homogenization and differentiation hypotheses. Journal of Ecology, 101, 1449–1458. 10.1111/1365-2745.12153 [DOI] [Google Scholar]
- Azevedo-Santos VM, Fearnside PM, Oliveira CS, Padial AA, Pelicice FM, Lima DP, … Vitule JRS (2017). Removing the abyss between conservation science and policy decisions in Brazil. Biodiversity and Conservation, 26, 1745–1752. 10.1007/s10531-017-1316-x [DOI] [Google Scholar]
- Barlow J, Lennox GD, Ferreira J, Berenguer E, Lees AC, Mac Nally R, … Gardner TA (2016). Anthropogenic disturbance in tropical forests can double biodiversity loss from deforestation. Nature, 535, 144–147. 10.1038/nature18326 [DOI] [PubMed] [Google Scholar]
- Barthem RB, de Brito Ribeiro MCL, & Petrere M (1991). Life strategies of some long-distance migratory catfish in relation to hydroelectric dams in the Amazon Basin. Biological Conservation, 55, 339–345. 10.1016/0006-3207(91)90037-A [DOI] [Google Scholar]
- Baselga A (2010). Partitioning the turnover and nestedness components of beta diversity. Global Ecology and Biogeography, 19, 134–143. 10.1111/j.1466-8238.2009.00490.x [DOI] [Google Scholar]
- Beighley RE, & Gummadi V (2011). Developing channel and floodplain dimensions with limited data: A case study in the Amazon Basin. Earth Surface Processes and Landforms, 36, 1059–1071. 10.1002/esp.v36.8 [DOI] [Google Scholar]
- Bernhardt ES, & Palmer MA (2011). River restoration: The fuzzy logic of repairing reaches to reverse catchment scale degradation. Ecological Applications, 21, 1926–1931. 10.1890/10-1574.1 [DOI] [PubMed] [Google Scholar]
- Borcard D, Legendre P, & Drapeau P (1992). Partialling out the spatial component of ecological variation. Ecology, 73, 1045–1055. 10.2307/1940179 [DOI] [Google Scholar]
- Brasil. (1997). Lei No 9.433 de 8 de Janeiro de 1997.
- Brasil. (2009). Lei No 11.959 de 29 de Junho de 2009.
- Brasil. (2012). Lei No 12.651 de 12 de Maio de 2012.
- Breiman L (2001). Random forests. Machine Learning, 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Castello L, & Macedo MN (2016). Large-scale degradation of Amazonian freshwater ecosystems. Global Change Biology, 3, 990–1007. 10.1111/gcb.13173 [DOI] [PubMed] [Google Scholar]
- Castello L, McGrath DG, Hess LL, Coe MT, Lefebvre PA, Petry P, … Arantes CC (2013). The vulnerability of Amazon freshwater ecosystems. Conservation Letters, 6, 217–229. 10.1111/conl.12008 [DOI] [Google Scholar]
- CBD. (2010). The Strategic Plan for Biodiversity 2011–2020 and the Aichi Biodiversity Targets. Nagoya. [Google Scholar]
- Davies PEA, Harris JHB, Hillman TJC, & Walker KFD (2010). The sustainable rivers audit: Assessing river ecosystem health in the Murray Darling Basin, Australia. Marine and Freshwater Research, 61, 764–777. 10.1071/MF09043 [DOI] [Google Scholar]
- de Carvalho DR, de Castro DMP, Callisto M, Moreira MZ, & Pompeu PS (2017). The trophic structure of fish communities from streams in the Brazilian Cerrado under different land uses: An approach using stable isotopes. Hydrobiologia, 795, 199–217. 10.1007/s10750-017-3130-6 [DOI] [Google Scholar]
- de Carvalho DR, Leal CG, Junqueira NT, de Castro MA, Fagundes DC, Alves CBM, … Pompeu PS (2017). A fish-based multimetric index for Brazilian savanna streams. Ecological Indicators, 77, 386–396. 10.1016/j.ecolind.2017.02.032 [DOI] [Google Scholar]
- de Oliveira-Junior JMB, Shimano Y, Gardner TA, Hughes RM, de Marco Júnior P, & Juen L (2015). Neotropical dragonflies (Insecta: Odonata) as indicators of ecological condition of small streams in the eastern Amazon. Austral Ecology, 40, 733–744. 10.1111/aec.12242 [DOI] [Google Scholar]
- Di Luzio M, Srinivasan R, & Arnold JG (2004). A GIS-coupled hydrological model system for the watershed assessment of agricultural non-point and point sources of pollution. Transactions in GIS, 8, 113–136. 10.1111/j.1467-9671.2004.00170.x [DOI] [Google Scholar]
- Dias MS, Magnusson WE, & Zuanon J (2010). Effects of reduced-impact logging on fish assemblages in central Amazonia. Conservation Biology, 24, 278–286. 10.1111/j.1523-1739.2009.01299.x [DOI] [PubMed] [Google Scholar]
- Dolezsai A, Sály P, Takács P, Hermoso V, & Erős T (2015). Restricted by borders: Trade-offs in transboundary conservation planning for large river systems. Biodiversity and Conservation, 24, 1403–1421. 10.1007/s10531-015-0864-1 [DOI] [Google Scholar]
- Ellis N, Smith SJ, & Roland Pitcher C (2012). Gradient forests: Calculating importance gradients on physical predictors. Ecology, 93, 156–168. 10.1890/11-0252.1 [DOI] [PubMed] [Google Scholar]
- Esselman PC, Infante DM, Wang L, Cooper AR, Wieferich D, Tsang YP, … Taylor WW (2013). Regional fish community indicators of landscape disturbance to catchments of the conterminous United States. Ecological Indicators, 26, 163–173. 10.1016/j.ecolind.2012.10.028 [DOI] [Google Scholar]
- Fausch KD, Torgersen CE, Baxter CV, & Li HW (2002). Landscapes to Riverscapes: Bridging the gap between research and conservation of stream fishes. BioScience, 52, 483 10.1641/0006-3568(2002)052[0483:LTRBTG]2.0.CO;2 [DOI] [Google Scholar]
- Fearnside PM (2016). Brazilian politics threaten environmental policies. Science, 353, 746–748. 10.1126/science.aag0254 [DOI] [PubMed] [Google Scholar]
- Ferraz SFB, Ferraz KMPMB, Cassiano CC, Brancalion PHS, da Luz DTA, Azevedo TN, … Metzger JP (2014). How good are tropical forest patches for ecosystem services provisioning? Landscape Ecology, 29, 187–200. 10.1007/s10980-014-9988-z [DOI] [Google Scholar]
- Ferreira J, Barlow J, Barreto P, Berenguer E, Bustamante M, Gardner TA, … Zuanon J (2014). Brazil’s environmental leadership at risk. Science, 346, 706–707. 10.1126/science.1260194 [DOI] [PubMed] [Google Scholar]
- Ferreira J, Pardini R, Metzger JP, Fonseca CR, Pompeu PS, Sparovek G, & Louzada J (2012). Towards environmentally sustainable agriculture in Brazil: Challenges and opportunities for applied ecological research. Journal of Applied Ecology, 49, 535–541. [Google Scholar]
- Fitzpatrick FA, Scudder BC, Lenz BN, & Sullivan DJ (2001). Effects of multi-scale environmental characteristics on agricultural stream biota in Eastern Wisconsin. Journal of the American Water Resources Association, 37, 1489–1507. https://doi.org/10.1111j.1752-1688.2001.tb03655.x [Google Scholar]
- França F, Louzada J, Korasaki V, Griffiths H, Silveira J, & Barlow J (2016). Do space-for-time assessments underestimate the impacts of logging on tropical biodiversity? An Amazonian case study using dung beetles. Journal of Applied Ecology, 53, 1098–1105. 10.1111/1365-2664.12657 [DOI] [Google Scholar]
- Gardner TA, Ferreira J, Barlow J, Lees AC, Parry L, Guimarães IC, … Zuanon J (2013). A social and ecological assessment of tropical land uses at multiple scales: The Sustainable Amazon Network. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20120166 10.1098/rstb.2012.0166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giling DP, Mac Nally R, & Thompson RM (2015). How sensitive are invertebrates to riparian-zone replanting in stream ecosystems? Marine and Freshwater Research, 67, 1500–1511. [Google Scholar]
- Harding JS, Benfield EF, Bolstad PV, Helfman GS, & Jones EB (1998). Stream biodiversity: The ghost of land use past. Proceedings of the National Academy of Sciences of the United States of America, 95, 14843–14847. 10.1073/pnas.95.25.14843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes RM, Dunham S, Maas-Hebner KG, Yeakley JA, Harte M, Molina N, … Kaczynski VW (2014). A review of urban water body challenges and approaches: (2) Mitigating effects of future urbanization. Fisheries, 39, 30–40. 10.1080/03632415.2014.866507 [DOI] [Google Scholar]
- Hughes RM, & Peck DV (2008). Acquiring data for large aquatic resource surveys: The art of compromise among science, logistics, and reality. Journal of the North American Benthological Society, 27, 837–859. 10.1899/08-028.1 [DOI] [Google Scholar]
- Hughes RM, Wang L, & Seelbach PW (2006). Landscape influences on stream habitats and biological assemblages. Bethesda, MD: American Fisheries Society. [Google Scholar]
- Hurd LE, Sousa RGC, Siqueira-Souza FK, Cooper GJ, Kahn JR, & Freitas CEC (2016). Amazon floodplain fish communities: Habitat connectivity and conservation in a rapidly deteriorating environment. Biological Conservation, 195, 118–127. 10.1016/j.biocon.2016.01.005 [DOI] [Google Scholar]
- Hylander K, & Ehrlén J (2013). The mechanisms causing extinction debts. Trends in Ecology & Evolution, 28, 341–346. 10.1016/j.tree.2013.01.010 [DOI] [PubMed] [Google Scholar]
- Issues A (2002). Effects of deforestation on fish community structure in Ecuadorian Amazon streams. Freshwater Biology, 47, 2246–2260. [Google Scholar]
- Jiménez-Valencia J, Kaufmann PR, Sattamini A, Mugnai R, & Baptista DF (2014). Assessing the ecological condition of streams in a southeastern Brazilian basin using a probabilistic monitoring design. Environmental Monitoring and Assessment, 186, 4685–4695. 10.1007/s10661-014-3730-9 [DOI] [PubMed] [Google Scholar]
- Kaufmann PR, Faustini JM, Larsen DP, & Shirazi MA (2008). A roughness-corrected index of relative bed stability for regional stream surveys. Geomorphology, 99, 150–170. [Google Scholar]
- Kaufmann PR, & Hughes RM (2006). Geomorphic and anthropogenic influences on fish and amphibians in Pacific northwest coastal streams In Hughes RM, Wang L, & Seelbach PW (Eds.), Landscape influences on stream habitats and biological assemblages (pp. 429–455). Bethesda, MD: American Fisheries Society. [Google Scholar]
- Kaufmann PR, Larsen DP, & Faustini JM (2009). Bed stability and sedimentation associated with human disturbances in Pacific Northwest streams. Journal of the American Water Resources Association, 45, 434–459. [Google Scholar]
- Kaufmann PR, Levine P, Robison EG, Seeliger C, & Peck DV (1999). Quantifying physical habitat in wadeable streams. Washington, DC: U.S. Environmental Protection Agency. [Google Scholar]
- Larsen DP, Kaufmann PR, Kincaid TM, & Urquhart NS (2004). Detecting persistent change in the habitat of salmon-bearing streams in the Pacific Northwest. Canadian Journal of Fisheries and Aquatic Sciences, 61, 283–291. 10.1139/f03-157 [DOI] [Google Scholar]
- Laurance WF, Sayer J, & Cassman KG (2014). Agricultural expansion and its impacts on tropical nature. Trends in Ecology & Evolution, 29, 107–116. 10.1016/j.tree.2013.12.001 [DOI] [PubMed] [Google Scholar]
- Leal CG, Barlow J, Gardner T, Hughes RM, Leitão RP, Mac Nally R, … Pompeu PS (2017). Data from: Is environmental legislation conserving tropical stream faunas? A large-scale assessment of local, riparian and catchment-scale influences on Amazonian stream fish. Dryad Digital Repository, 10.5061/dryad.d5k7p [DOI] [PMC free article] [PubMed]
- Leal CG, Pompeu PS, Gardner TA, Leitão RP, Hughes RM, Kaufmann PR, … Barlow J (2016). Multi-scale assessment of human-induced changes to Amazonian instream habitats. Landscape Ecology, 31, 1725–1745. 10.1007/s10980-016-0358-x [DOI] [Google Scholar]
- Lee P, Smyth C, & Boutin S (2004). Quantitative review of riparian buffer width guidelines from Canada and the United States. Journal of Environmental Management, 70, 165–180. 10.1016/j.jenvman.2003.11.009 [DOI] [PubMed] [Google Scholar]
- Legendre P (2008). Studying beta diversity: Ecological variation partitioning by multiple regression and canonical analysis. Journal of Plant Ecology, 1, 3–8. 10.1093/jpe/rtm001 [DOI] [Google Scholar]
- Leitão RP, Zuanon J, Mouillot D, Leal CG, Hughes RM, Kaufmann PR, … Gardner TA (2017). Disentangling the pathways of land use impacts on the functional structure of fish assemblages in Amazon streams. Ecography, 10.1111/ec [DOI] [PMC free article] [PubMed]
- Leitão RP, Zuanon J, Villéger S, Williams SE, Baraloto C, Fortunel C, … Mouillot D (2016). Rare species contribute disproportionately to the functional structure of species assemblages. Proceedings of the Royal Society B: Biological Sciences, 283, 20160084 10.1098/rspb.2016.0084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo MN, Coe MT, De Fries R, Uriarte M, Brando PM, Neill C, & Walker WS (2013). Land-use-driven stream warming in southeastern Amazonia. Philosophical transactions of the Royal Society of London. Series B, Biological Sciences, 368, 20120153 10.1098/rstb.2012.0153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macedo DR, Hughes RM, Ligeiro R, Ferreira WR, Castro MA, Junqueira NT, … Callisto M (2014). The relative influence of catchment and site variables on fish and macroinvertebrate richness in cerrado biome streams. Landscape Ecology, 29, 1001–1016. 10.1007/s10980-014-0036-9 [DOI] [Google Scholar]
- Mantyka-Pringle CS, Martin TG, Moffatt DB, Udy J, Olley J, Saxton N, … Rhodes JR (2016). Prioritizing management actions for the conservation of freshwater biodiversity under changing climate and land-cover. Biological Conservation, 197, 80–89. 10.1016/j.biocon.2016.02.033 [DOI] [Google Scholar]
- Marzin A, Archaimbault V, Belliard J, Chauvin C, Delmas F, & Pont D (2012). Ecological assessment of running waters: Do macrophytes, macroinvertebrates, diatoms and fish show similar responses to human pressures? Ecological Indicators, 23, 56–65. 10.1016/j.ecolind.2012.03.010 [DOI] [Google Scholar]
- Marzin A, Verdonschot PFM, & Pont D (2013). The relative influence of catchment, riparian corridor, and reach-scale anthropogenic pressures on fish and macroinvertebrate assemblages in French rivers. Hydrobiologia, 704, 375–388. 10.1007/s10750-012-1254-2 [DOI] [Google Scholar]
- McClain ME, & Elsenbeer H (2001). Terrestrial inputs to amazon streams and internal biogeochemical processing In McClain ME, Victoria RL, & Riche JE (Eds.), The biogeochemistry of the Amazon Basin (pp. 185–208). New York, NY: Oxford University Press. [Google Scholar]
- McCormick FH, Hughes RM, Kaufmann PR, Peck DV, Stoddard JL, & Herlihy AT (2001). Development of an index of biotic integrity for the mid-Atlantic highlands region. Transactions of the American Fisheries Society, 130, 857–877. [DOI] [Google Scholar]
- Mojica JI, Castellanos C, & Lobón-Cerviá J (2009). High temporal species turnover enhances the complexity of fish assemblages in Amazonian Terra firme streams. Ecology of Freshwater Fish, 18, 520–526. 10.1111/j.1600-0633.2009.00382.x [DOI] [Google Scholar]
- Mouillot D, Graham NAJ, Villéger S, Mason NWH, & Bellwood DR (2013). A functional approach reveals community responses to disturbances. Trends in Ecology and Evolution, 28, 167–177. 10.1016/j.tree.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Nunes S, Gardner T, Barlow J, Martins H, Salomão R, Monteiro D, & Souza C (2016). Compensating for past deforestation: Assessing the legal forest surplus and deficit of the state of Pará, eastern Amazonia. Land Use Policy, 57, 749–758. 10.1016/j.landusepol.2016.04.022 [DOI] [Google Scholar]
- Oksanen J, Blanchet F, Kindt R, Legendre P, Minchin P, O’Hara R, … Wagner H (2013). vegan: Community Ecology Package. R package version 2.0-7.
- Palmer MA, Menninger HL, & Bernhardt E (2010). River restoration, habitat heterogeneity and biodiversity: A failure of theory or practice? Freshwater Biology, 55, 205–222. 10.1111/j.1365-2427.2009.02372.x [DOI] [Google Scholar]
- Peres CA, Gardner TA, Barlow J, Zuanon J, Michalski F, Lees AC, … Feeley KJ (2010). Biodiversity conservation in human-modified Amazonian forest landscapes. Biological Conservation, 143, 2314–2327. 10.1016/j.biocon.2010.01.021 [DOI] [Google Scholar]
- Peres-Neto PR, Legendre P, Dray S, & Borcard D (2006). Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology, 87, 2614–2625. 10.1890/0012-9658(2006)87[2614:VPOSDM]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pont D, Hugueny B, Beier U, Goffaux D, Melcher A, Noble R, … Schmutz S (2006). Assessing river biotic condition at a continental scale: A European approach using functional metrics and fish assemblages. Journal of Applied Ecology, 43, 70–80. 10.1111/j.1365-2664.2005.01126.x [DOI] [Google Scholar]
- Proust-Lima C, Philipps V, Amadou D, & Liquet B (2016). Package’lcmm’, https://cran.r-project.org/web/packages/lcmm/lcmm.pdf
- Prudente BS, Pompeu PS, Juen L, & Montag LFA (2017). Effects of reduced-impact logging on physical habitat and fish assemblages in streams of Eastern Amazonia. Freshwater Biology, 62, 303–316. 10.1111/fwb.12868 [DOI] [Google Scholar]
- R Core Team. (2013). R: A language and environment for statistical computing.
- Reis RE, Kullander SO, & Ferraris CJJ (2003). Check list of the freshwater fishes of South and Central America. Porto Alegre, Brazil: EDIPUCRS. [Google Scholar]
- Roth N, Allan J, & Erickson D (1996). Landscape influences on stream biotic integrity assessed at multiple spatial scales. Landscape Ecology, 11, 141–156. 10.1007/BF02447513 [DOI] [Google Scholar]
- Sály P, Takács P, Kiss I, Bíró P, & Eros T (2011). The relative influence of spatial context and catchment- and site-scale environmental factors on stream fish assemblages in a human-modified landscape. Ecology of Freshwater Fish, 20, 251–262. 10.1111/j.1600-0633.2011.00490.x [DOI] [Google Scholar]
- Schiesari L, & Grillitsch B (2011). Pesticides meet megadiversity in the expansion of biofuel crops. Frontiers in Ecology and the Environment, 9, 215–221. 10.1890/090139 [DOI] [Google Scholar]
- Schinegger R, Trautwein C, Melcher A, & Schmutz S (2012). Multiple human pressures and their spatial patterns in European running waters. Water and Environment Journal, 26, 261–273. 10.1111/j.1747-6593.2011.00285.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SJ, Ellis N, & Pitcher CR (2011). Conditional variable importance in R package extendedForest, http://gradientforest.r-forge.r-project.org/Conditional-importance.pdf.
- Soares-Filho B, Rajão R, Macedo M, Carneiro A, Costa W, Coe M, … Alencar A (2014). Cracking Brazil’s forest code. Science, 344, 363–364. 10.1126/science.1246663 [DOI] [PubMed] [Google Scholar]
- Solar RR, Barlow J, Ferreira J, Berenguer E, Lees AC, Thomson JR, … Gardner TA (2015). How pervasive is biotic homogenization in human-modified tropical forest landscapes? Ecology Letters, 18, 1108–1118. 10.1111/ele.12494 [DOI] [PubMed] [Google Scholar]
- Strayer DL, & Dudgeon D (2010). Freshwater biodiversity conservation: Recent progress and future challenges. Journal of the North American Benthological Society, 29, 344–358. 10.1899/08-171.1 [DOI] [Google Scholar]
- Terra B, Hughes RM, & Araújo FG (2016). Fish assemblages in Atlantic Forest streams: The relative influence of local and catchment environments on taxonomic and functional species. Ecology of Freshwater Fish, 25, 527–544. 10.1111/eff.12231 [DOI] [Google Scholar]
- Tregidgo DJ, Barlow J, Pompeu PS, de Almeida Rocha M, & Parry P (2017). Rainforest metropolis casts 1,000-km defaunation shadow. Proceedings of the National Academy of Sciences of the United States of America, 114, 8655–8659. 10.1073/pnas.1614499114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA. (2016). Office of water and office of research and development national rivers and streams assessment 2008–2009: A collaborative survey (EPA/841/R-16/007), Washington, DC. [Google Scholar]
- Van Sickle J, Baker J, Herlihy A, Bayley P, Gregory S, Haggerty P, … Li J (2004). Projecting the biological condition of streams under alternative scenarios of human land use. Ecological Applications, 14, 368–380. 10.1890/02-5009 [DOI] [Google Scholar]
- Viana C, Coudel E, Barlow J, Ferreira J, Gardner T, & Parry L (2016). How does hybrid governance emerge? Role of the elite in building a green municipality in the Eastern Brazilian Amazon. Environmental Policy and Governance, 26, 337–350. 10.1002/eet.v26.5 [DOI] [Google Scholar]
- Wang L, Lyons J, Rasmussen P, Seelbach P, Simon T, Wiley M, … Stewart PM (2003). Watershed, reach, and riparian influences on stream fish assemblages in the Northern Lakes and Forest Ecoregion, U.S.A. Canadian Journal of Fisheries and Aquatic Sciences, 60, 491–505. 10.1139/f03-043 [DOI] [Google Scholar]
- Wang L, Seelbach PW, & Lyons J (2006). Effects of levels of human disturbance on the influence of catchment, riparian, and reach-scale factors on fish assemblages In Hughes RM, Wang L, & Seelbach PW (Eds.), Landscape influences on stream habitats and biological assemblages (pp. 199–219). Bethesda, MD: American Fisheries Society. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


