Abstract
BACKGROUND
Neonates receiving extracorporeal membrane oxygenation (ECMO) support are transfused large volumes of red blood cells (RBCs) and platelets (PLTs). Transfusions are often administered in response to specific, but largely unstudied thresholds. The aim of this study is to examine the relationship between RBC and PLT transfusion rates and mortality in neonates receiving ECMO support.
STUDY DESIGN AND METHODS
We retrospectively examined outcomes of neonates receiving ECMO support in the neonatal intensive care unit (NICU) for respiratory failure between 2010 and 2016 at a single quaternary-referral NICU. We examined the association between RBC and PLT transfusion rate (mL per kg per day) and in-hospital mortality, adjusting for confounding by using a validated composite baseline risk score (Neo-RESCUERS).
RESULTS
Among the 110 neonates receiving ECMO support, in-hospital mortality was 28%. The median RBC transfusion rate (mL/kg/d) after cannulation was greater among non-survivors, compared to survivors: 12.4 (IQR 9.3–16.2) versus 7.3 (IQR 5.1–10.3), p < 0.001. Similarly, PLT transfusion rate was greater among non-survivors: 22.9 (9.3–16.2) versus 12.1 (8.4–20.1), p = 0.02. After adjusting for baseline mortality risk, both RBC transfusion (adjusted relative risk per 5 mL/kg/d increase: 1.33; 95% Cl 1.05–1.69, p = 0.02) and PLT transfusion (adjusted relative risk per 5 mL/kg/d increase: 1.12; 95% Cl 1.02–1.23, p = 0.02) were both associated with in-hospital mortality.
CONCLUSIONS
RBC and PLT transfusion rates are associated with in-hospital mortality among neonates receiving ECMO. These data provide a basis for future studies evaluating more restrictive transfusion practices for neonates receiving ECMO support.
Blood transfusion is a frequent and necessary practice in neonates receiving extracorporeal membrane oxygenation (ECMO) due to frequent laboratory sampling, clinical bleeding, and the need to support tissue oxygen delivery.1,2 Guidelines provided by the Extracorporeal Life Support Organization (ELSO) recommend the maintenance of a target hematocrit (Hct) of 40% for neonatal ECMO patients, which typically leads to recurring red blood cell (RBC) transfusions.3 Overall, there exists an absence of data from clinical trials examining transfusion practices in critically ill term neonates, including those supported by ECMO. Thus, appropriate thresholds for RBC transfusion are uncertain, with many ECMO centers using center-specific thresholds for transfusions in response to studies reporting associations between a greater transfusion volume and increased morbidity and mortality in this population.4,5 In addition, recent studies suggest that a more restrictive approach may be safe and adequately support neonates undergoing ECMO support5,6
Similarly, platelet transfusions are commonly administered due to persistent consumption of platelets in the neonatal ECMO circuits coupled with the patient’s underlying disease process.7–11 Bleeding complications on ECMO are an important and frequent source of mortality and this leads to higher thresholds for platelet transfusion for patients undergoing ECMO support as compared to other critically ill patients.12 ELSO recommends maintenance of a platelet threshold of 100,000/μL, which is above common thresholds for other critically ill populations. However, a recently published clinical trial in preterm infants showed that a lower platelet transfusion of 25,000/μL in preterm infants, compared to 50,000/μL, improved survival without increased bleeding, suggesting that more restrictive platelet transfusion practices could be used safely in critically-ill neonates.13 Unfortunately, data regarding platelet transfusion thresholds and bleeding risk in neonatal ECMO is poorly defined.12,14
Overall, the lack of data to support RBC and platelet transfusions during neonatal ECMO has led to variability in clinical practice and uncertainty surrounding best practices.15 Thus, we examined the relationship between RBC and platelet transfusions and in-hospital mortality in a population of neonates undergoing ECMO for hypoxic respiratory failure, while controlling for baseline risk of the population using a validated risk score.
MATERIALS AND METHODS
Neonates (less than 28 days) requiring ECMO for respiratory failure in the Level IV NICU at Children’s Healthcare of Atlanta at Egleston between January 1, 2010 and December 1, 2016 were identified through a retrospective chart review of the institutional ECMO database; supplemental information was gathered from individual medical records. Patients undergoing ECMO support met our institution specific criteria for ECMO (Table S1, available as supporting information in the online version of this paper), and all patients treated during the time period were included in the study.16 The institutional review boards at Emory University School of Medicine and Children’s Healthcare of Atlanta approved this study. For neonatal ECMO, we primarily utilize veno- venous (VV) cannulation for hypoxic respiratory failure and routinely insert a cephalic drain (+V) at cannulation. In priming the ECMO circuit, we provide 1 or 2 units of packed RBCs (to a volume of 350 mL); albumin, calcium gluconate, sodium bicarbonate and heparin (100 units) are also added. A quarter of an apheresis platelet unit is given immediately after initiation of ECMO flow, fresh frozen plasma (FFP) is not routinely given as part of ECMO initiation. RBC used for ECMO are leukoreduced, irradiated, and less than 5 days old. Platelets used are irradiated and apheresis. For subjects undergoing ECMO during the study period, “traditional” thresholds for reflexive transfusion of blood products were utilized including maintenance of an Hct > 40%, platelet count >100,000/μL, Fibrinogen level > 100 mg/dL and these were included in routine ECMO order sets. Transfusion of FFP (as for an elevated PT/INR) is not protocol-based and occurs at the discretion of the attending physician. These institutional guidelines and order sets were consistent during the study period. Transfused volumes of RBCs, platelets, FFP and cryoprecipitate during ECMO support, excluding the volume needed to prime the ECMO circuit, were collected from medical records. A substantial portion of the patients had congenital diaphragmatic hernia (CDH) and these patients are not typically repaired on ECMO at our institution.
Neo-RESCUERS score, a validated illness-severity index for neonatal respiratory failure patients receiving ECMO, was calculated as described (http://www.neo-rescuers.com).17 Briefly, the score is comprised of the following pre-ECMO factors: pH, PaO2, birth weight, gestational age, postnatal age, sex, primary diagnosis (e.g., meconium aspiration syndrome, CDH), presence of comorbidity, pre-ECMO renal failure and pre-ECMO inhaled nitric oxide. This allowed multiple important risk factors that influence neonatal ECMO survival to be included into a single composite, validated risk score.
We specified the primary exposure of interest as transfusion volume after the first 24 hours of ECMO, to assess transfusions that were given once the patient was at a steady state and typically for pre-defined ECMO thresholds. We normalized volume to birth weight and days of ECMO, as these two variables, in addition to the Neo-RESCUERS score, are important potential confounders as longer duration of ECMO is associated with increased blood product exposure and increased mortality.1 The primary outcome was defined as death before hospital discharge.
Statistical analysis
Continuous variables were described using median and interquartile ranges and compared using the Wilcoxon rank-sum test. Categorical variables were compared using the χ2 or Fisher’s exact tests for event rates <5. Violin plots were used to depict the distribution of the primary exposures of interest by outcome groups (survivors vs. non-survivors) and the probability density of the data. Poisson regression with robust standard errors was used to estimate relative risks of mortality.18 Multivariable analysis was conducted to adjust for confounding by illness severity by using a single continuous composite risk score (Neo-RESCUERS), which was specified as a continuous variable in the model with each individual study exposure of interest (RBC transfusion rate and platelet transfusion rate after 24 hours of ECMO). An additional model combined both RBC and platelet transfusion rates to determine the independent contribution of each blood component. Correlation between both RBC and platelet transfusion rates was assessed using the Spearman correlation coefficient and linear regression.
RESULTS
During the study period, 110 neonates received ECMO support for primary respiratory failure. Cohort demographics are described in Table 1. Seventy-nine (72%) subjects survived to hospital discharge. Non-survivors were more likely to have a diagnosis of CDH, longer duration of ECMO, and a higher Neo-RESCUERS score. Consistent with prior studies, the Neo-RESCUERS score had a high discrimination for mortality in this cohort (AUC area under the receiver operating curve for the Neo-RESCUERS score was 0.74 (95 CI 0.64–0.85), highlighting its utility as a tool for risk adjustment.17
TABLE 1.
Patient characteristics
| Characteristic | Survivors (n = 79) | Non-survivors (n = 31) | p |
|---|---|---|---|
| Gestational Age, median weeks (IQR) | 39 (38–40) | 38 (37–39) | 0.18 |
| Birthweight, median kg (IQR) | 3.32 (2.91–3.64) | 2.87 (2.72–3.49) | 0.12 |
| Female sex | 29 (37%) | 14 (45%) | 0.41 |
| Race/ethnicity | 0.78 | ||
| Black | 41 (52%) | 14 (45%) | |
| White | 29 (37%) | 14 (45%) | |
| Hispanic | 4 (5%) | 2 (7%) | |
| Other | 5 (6%) | 1 (3%) | |
| 1 Minute Apgar, median (IQR) | 2 (4–6) | 2 (4–6) | 0.55 |
| 5 Minute Apgar, median (IQR) | 5 (7–8) | 5 (7–8) | 0.94 |
| Diagnosis | 0.002 | ||
| CDH | 22 (28%) | 21 (68%) | |
| MAS | 30 (38%) | 3 (10%) | |
| PPHN | 22 (38%) | 5 (16%) | |
| Sepsis | 3 (4%) | 2 (7%) | |
| Other | 2 (3%) | 0 | |
| OI, median (IQR) | 43 (33–59) | 52 (38–75) | 0.11 |
| Neo-RESCUERS Score, median (IQR) | −1.6 (−2.5, −0.3) | −0.1 (−0.8, −0.2) | <0.01 |
| VIS pre cannulation, median (IQR) | 26 (20–40) | 33 (20–50) | 0.23 |
| ECMO Mode | 0.32 | ||
| VV | 70 (89%) | 26 (84%) | |
| VA | 8 (10%) | 3 (10%) | |
| VV to VA | 1 (1%) | 2 (6%) | |
| Duration of ECMO run, median d (IQR) | 6 (5–8) | 11 (6–15) | 0.001 |
| ECMO Complications | |||
| Mechanical - Clot | 4 (5%) | 7 (23%) | 0.01 |
| Cannula site bleeding | 5 (5%) | 2 (7%) | >0.99 |
| Circuit change | 6 (8%) | 12 (39%) | <0.001 |
| CNS bleed | 5 (6%) | 8 (26%) | 0.008 |
| CNS infarct | 0 (0%) | 2 (7%) | 0.08 |
Data are n (%), unless indicated otherwise.
Abbreviations: IQR = interquartile range; CDH = congenital diaphragmatic hernia; MAS = meconium aspiration syndrome; PPHN = persistent pulmonary hypertension of the newborn; OI = oxygenation index; VIS = vasoactive inotrope score; VV = veno-venous; VA = veno-arterial; ECMO = extracorporeal membrane oxygenation; CNS = central nervous system.
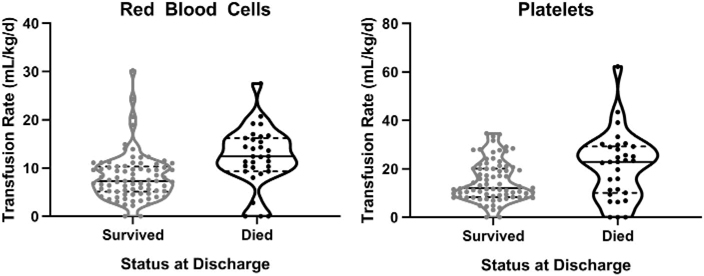
RBC transfusion volume after the first 24 hours and during the ECMO run was significantly higher in non-survivors (Table 2). Further, transfused platelet and fresh frozen plasma volumes, but not cryoprecipitate were significantly higher in non-survivors (Table 2 and Fig. 1a, b). Platelet transfusion volume was more than twice that of RBC transfused in both groups. Relevant complications such as CNS bleeding and ECMO circuit change were also more common in non-survivors. Documented cannula site bleeding was associated with a higher rate of RBC transfusion. Likewise, changing of the ECMO circuit was accompanied by exposure to a larger volume of transfused blood products to prime the circuit; however, statistically significant associations between RBC transfusion rate and mortality were maintained after removing subjects who required a circuit change from the analysis (survivors: median mL/kg/d [interquartile range] 7.1 [4.7–10.1] vs. non-survivors 11.2 [8.0–14.0]; p = 0.02, n = 92).
TABLE 2.
Transfusion volume by survival status
| Blood product | Survivor (n = 79) | Non-survivor (n = 31) | p |
|---|---|---|---|
| RBC in first 24 hours* | 0 (0–44) | 45 (0–74) | 0.03 |
| RBC after 24 hours | 117 (74–236) | 411 (208–624) | <0.001 |
| Fresh frozen plasma in first 24 hours | 30 (0–68) | 59 (33–72) | 0.01 |
| Fresh frozen plasma after 24 hours | 0 (0–55) | 70 (0–158) | 0.002 |
| Platelet in first 24 hours† | 96 (60–120) | 88 (79–125) | 0.66 |
| Platelet after 24 hours | 252 (151–492) | 805 (286–1383) | 0.001 |
| Cryoprecipitate after 24 hours | 0 (0–0) | 0 (0–7) | 0.28 |
| RBC rate in mL/kg/d‡ | 7.3 (5.1–10.3) | 12.4 (9.3–16.2) | <0.001 |
| Platelet rate in mL/kg/d‡ | 12.1 (8.4–20.1) | 22.9 (10.1–29.2) | 0.02 |
| Fresh frozen plasma rate in mL/kg/d‡ | 0 (0–2.5) | 2.1 (0–5.5) | 0.006 |
| Cryoprecipitate rate in mL/kg/d‡ | 0 (0–0.4) | 0 (0–0) | 0.34 |
Data are reported as median mL (interquartile range), unless otherwise noted.
Does not include blood volume needed to prime ECMO circuit.
includes volume of platelets given per protocol after ECMO initiation.
After 24 hours.
Abbreviation: RBC = red blood cell.
Fig. 1.
Red blood cell and platelet transfusion rate after 24 hours by survival status. Violin plots depict the distribution of transfusion rates, by blood component and survival status. Each dot represents data from one infant, with the dotted lines indicating 25th and 75th percentiles and the solid line the median value for each group. The probability density of the data is reflected by the curved kernel density around the data points.
After controlling for illness severity at ECMO initiation using the Neo-RESCUERS score, we identified an association between RBC transfusion rate and mortality: Adjusted relative risk per 5 mL/kg/day increase: 1.33 (95% CI 1.05–1.69) (Table 3). Also, platelet transfusion rate was associated with mortality risk: adjusted relative risk per 5 mL/kg/d increase 1.12 (95% CI 1.02–1.23). Findings were consistent in additional analyses that adjusted for cannula site bleeding and central nervous system bleeding (p = 0.047), suggesting these sources of bleeding were not the sole mediators of the relationship between transfusion rates and mortality. Estimates of association of each exposure of interest (RBC and platelet transfusion rate) with mortality were diminished when considering both together in multivariable analysis, possibly due to a high correlation between RBC and platelet transfusion rates (Spearman correlation coefficient 0.60, Fig. S1, available as supporting information in the online version of this paper).
TABLE 3.
Risk factors associated with mortality
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Risk factor | RR (95% CI) for mortality | RR (95% CI) for mortality | RR (95% CI) for mortality | RR (95% CI) for mortality |
| Neo-RESCUERS score (per 1 point íncrease) | 1.81 (1.32–2.49) | 1.65 (1.18–2.29) | 1.72 (1.23–2.39) | 1.63 (1.16–2.28) |
| RBC transfusion rate after 24 hours (per 5 mL/kg/day increase) | 1.46 (1.17–1.84) | 1.33 (1.05–1.69) | 1.25 (0.97–1.61) | |
| PLT transfusion rate after 24 hours (per 5 mL/kg/day increase) | 1.19 (1.08–1.31) | 1.12 (1.02–1.23) | 1.06 (0.96–1.17) | |
Abbreviations: RBC = red blood cell; PLT = platelet.
Correction added on Dec 27, after first online publication: Column headings have been revised. “Univariable” column heading applies to the first column only. “Multivariable” column heading applies to the remaining three columns.
DISCUSSION
The primary goal of ECMO is to support oxygen delivery to vital organs. Maintaining an adequate hemoglobin content is essential to achieve this goal, and RBC transfusion is thought to improve oxygen carrying capacity and delivery to tissues. However, emerging evidence suggests that more liberal transfusion of blood products, particularly RBCs and platelets, may increase morbidity and mortality. Along the same lines, moderate to severe thrombocytopenia increases bleeding risk, including intracranial hemorrhage, but there is little evidence to support a specific platelet transfusion threshold during ECMO support where the potential risks of transfusion out-weigh the benefits.13,19–21
In adults, evidence supports restrictive transfusion practices in critically ill patients and hemoglobin thresholds for RBC transfusion of 7 or 8 g/dL appear safe and may be associated with improved outcomes.22 Application to adults undergoing ECMO suggests that restrictive transfusion practices are safe, although substantial variability in practice exists.11,23,24 Similar results have been observed in critically ill pediatric patients, where restrictive transfusion strategies are now routinely employed.25,26 Less data is available for pediatric and neonatal ECMO populations.
A number of non-randomized studies have reported an association between RBC transfusion and mortality in pediatric and neonatal ECMO patients.2,5,27 The observational study designs make causal inference challenging as it is difficult to determine whether the mortality risk associated with blood product administration is direcdy attributable, reflects greater disease burden, or indirectly relates to complications of fluid overload, pulmonary edema, and inflammation.28,29 As expected, non-survivors in our study had a higher pre-ECMO mortality risk as indicated by a higher baseline Neo-RESCUERS scores; however the association between RBC or platelet transfusion rate and mortality persisted after risk adjustment. Further, our reported transfusion volumes were lower than previous reports suggesting that the association of RBC transfusion with mortality may occur regardless of the approaches to RBC transfusion utilized.2,27
Limited data suggests that liberal transfusions do not result in a clear benefit and that restrictive transfusion approaches do not lead to increased harm.6 Fiser et al. demonstrated that less than 10% of RBC transfusions lead to an increase in SVO2 or cerebral perfusion in pediatric ECMO patients (for a median Hct of 36%).30 These observations suggest that RBC transfusions do not improve cerebral oxygenation. A recent report demonstrated that targeting a lower threshold for transfusion (35% instead of 40%) was not associated with adverse events in a group of 35 neonatal ECMO patients, but did result in fewer transfusions.6
Bleeding complications on ECMO are common and underscore the need for RBC transfusion and maintenance of clotting and platelet function. ELSO and the American Association of Blood Banking (AABB) recommend maintenance of platelets above 100,000/μL, which is thought to provide adequate primary hemostatis.1,3,4,31 Platelet loss and/or consumption is common in ECMO patients. Christensen et al. described a population of more than 47,000 NICU patients of which only 45 received more than 20 platelet transfusions and 21 (47%) of these patients were on ECMO.7,20
Within our cohort, platelet transfusion volume was substantially higher than RBC or other blood products and was also associated with increased mortality. Intracranial hemorrhage (ICH) was more common in this group; however, the incidence of ICH did not correlate with the severity of thrombocytopenia as only one of these patients had a platelet count <50,000/μL, which occurred 4 days prior to the ICH. As per protocol, the thrombocytopenia was treated with several platelet transfusions, which did not prevent a bleeding complication in this patient.
Platelet count alone is an unreliable indicator of bleeding risk.32 In critically ill neonates with severe thrombocytopenia (<50,000/μL), the degree of thrombocytopenia was not associated with mortality or the occurrence of central nervous system, gastrointestinal, or pulmonary hemorrhage; however, the number of platelet transfusions predicted mortality and hemorrhage.20 An increasing appreciation for the immunomodulatory and pro-inflammatory effects of platelets may underscore the increased morbidity and mortality associated with donor platelets.28,29 Much like RBC transfusions, the benefits of reflexive platelet transfusion are unclear, and determining the number of platelets needed to limit unwanted bleeding has not been established and likely varies between patients.12,14
The retrospective nature of this study presents several limitations, not the least of which is that indications for transfusion beyond the set transfusion threshold are not documented. The Neo-RESCUERS score includes factors such as gestational age, that affect the risk for bleeding, but does not account for ongoing coagulopathy or baseline hematologic parameters. Patients with complications known to effect outcome, such as documented bleeding and the need for a circuit change, both received a higher transfusion volume and were more likely to die. Because we could not account for all confounding factors in our multivariable analysis, residual confounding is possible. Based on our study we cannot conclude that more liberal RBC or platelet transfusion is harmful, rather that there is not clear evidence of benefit, supporting the rationale for additional evaluation of more restrictive transfusion strategies. Further, analysis of models including both RBC transfusion rate and platelet rate should be viewed cautiously given the collinearity in these variables. Finally, we were unable to determine adherence to default transfusion order sets and therefore, cannot exclude the possibility of deviation from default thresholds in select patients.
CONCLUSIONS
In summary, our data suggests that the rate of RBC and platelet transfusions administered during neonatal ECMO is associated with increased mortality. These findings provide a basis for additional studies, including clinical trials, to determine optimal transfusion practices in this critically ill population.
Supplementary Material
Table S1. Inclusion Criteria for Neonatal Respiratory ECMO at CHOA-E.
Figure S1. Correlation between red blood cell and platelet transfusion rate. The Spearman correlation coefficient was 0.60 (p < 0.001). Model R2 0.32.
ACKNOWLEDGMENTS
We would like to thank our ECMO team at Children’s Healthcare of Atlanta for their excellent care of these patients and assistance with detailed data collection.
Funding: We received no specific financial support for this work.
Grant Support: R.M.P. received salary support from the National Heart Lung Blood Institute (NHLBI) under award K23 HL128942 and C.D.J. received support from the NHLBI under award P01 HL086773. The NIH had no role in: 1) study design; 2) the collection, analysis, and interpretation of data; 3) the writing of the report; and 4) the decision to submit the paper for publication.
Work was performed at Emory University School of Medicine and Children’s Healthcare of Adanta—Egleston campus.
Footnotes
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Disclosures: The authors declare that they have no conflicts of interest relevant to the manuscript submitted to TRANSFUSION. Dr. Annie Winkler is an employee at Instrumentation Laboratory but there is no direct relationship to any of the data presented. The findings and conclusions in this abstract are those of the authors and do not necessarily represent the views of the NIH.
We have no disclaimers.
SUPPORTING INFORMATION
Additional Supporting Information may be found in the online version of this article.
REFERENCES
- 1.Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors associated with bleeding and thrombosis in children receiving extracorporeal membrane oxygenation (ECMO). Am J Respir Crit Care Med 2017; 196:762–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson HT, Oyetunji TA, Thomas A, et al. The impact of leukoreduced red blood cell transfusion on mortality of neonates undergoing extracorporeal membrane oxygenation. J Surg Res 2014;192:6–11. [DOI] [PubMed] [Google Scholar]
- 3.Winkler AM. Transfusion management during extracorporeal support In: Brogan TV, Lequier L, Lorusso R et al. , Extracorporeal life support: the ELSO red book. 5th ed. Ann Arbor, Michigan: Extracorporeal Life Support Organization; 2017, p. 105–16. [Google Scholar]
- 4.Dalton HJ, Garcia-Filion P, Holubkov R, et al. Association of bleeding and thrombosis with outcome in extracorporeal life support. Pediatr Crit Care Med 2015;16:167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith A, Hardison D, Bridges B, et ai. Red blood cell transfusion volume and mortality among patients receiving extracorporeal membrane oxygenation. Perfusion 2013;28:54–60. [DOI] [PubMed] [Google Scholar]
- 6.Sawyer AA, Wise L, Ghosh S, et al. Comparison of transfusion thresholds during neonatal extracorporeal membrane oxygenation. Transfusion 2017;57:2115–20. [DOI] [PubMed] [Google Scholar]
- 7.Christensen RD, Henry E, Del Vecchio A. Thrombocytosis and thrombocytopenia in the NICU: incidence, mechanisms and treatments. J Matem Fetal Neonatal Med 2012;25(Suppl 4): 15–7. [DOI] [PubMed] [Google Scholar]
- 8.Henriquez-Henriquez M, Kattan J, Chang M, et al. Blood component usage during extracorporeal membrane oxygenation: experience in 98 patients at a Latin-American tertiary hospital. Int J Artif Organs 2014;37:233–40. [DOI] [PubMed] [Google Scholar]
- 9.Abrams D, Baldwin MR, Champion M, et al. Thrombocytopenia and extracorporeal membrane oxygenation in adults with acute respiratory failure: a cohort study. Intensive Care Med 2016;42:844–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs G, Berg N, Broman LM, et al. Flow-induced platelet activation in components of the extracorporeal membrane oxygenation circuit. Sci Rep 2018;8:13985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esper SA, Weisby IJ, Subramaniam K, et al. Adult extracorporeal membrane oxygenation: an international survey of transfusion and anticoagulation techniques. Vox Sang 2017;112: 443–52. [DOI] [PubMed] [Google Scholar]
- 12.Murphy DA, Hockings LE, Andrews RK, et al. Extracorporeal membrane oxygenation-hemostatic complications. Transfus Med Rev 2015;29:90–101. [DOI] [PubMed] [Google Scholar]
- 13.Curley A, Stanworth SJ, Willoughby K, et ai. Randomized trial of platelet-transfusion thresholds in neonates. N Engl J Med 2019;380:242–51. [DOI] [PubMed] [Google Scholar]
- 14.Josephson CD, Su LL, Christensen RD, et al. Platelet transfusion practices among neonatologists in the United States and Canada: results of a survey. Pediatrics 2009;123:278–85. [DOI] [PubMed] [Google Scholar]
- 15.Andrews J, Winkler AM. Challenges with navigating the precarious hemostatic balance during extracorporeal life support: implications for coagulation and transfusion management. Transfus Med Rev 2016;30:223–9. [DOI] [PubMed] [Google Scholar]
- 16.Roberts J, Keene S, Heard M, et al. Successful primary use of VVDL+V ECMO with cephalic drain in neonatal respiratory failure. J Perinatol 2016;36:126–31. [DOI] [PubMed] [Google Scholar]
- 17.Barbaro RP, Bartlett RH, Chapman RL, et al. Development and validation of the neonatal risk estimate score for children using extracorporeal respiratory support. J Pediatr 2016;173: 56–61. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knol MJ, Le Cessie S, Algra A, et al. Overestimation of risk ratios by odds ratios in trials and cohort studies: alternatives to logistic regression. CMAJ 2012;184:895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Estcourt LJ, Malouf R, Doree C, et al. Prophylactic platelet transfusions prior to surgery for people with a low platelet count Cochrane Database Syst Rev 2018. [cited 2008 Dec 11]. Available from: https://www.cochrane.org. [DOI] [PMC free article] [PubMed]
- 20.Baer VL, Lambert DK, Henry E, et al. Severe thrombocytopenia in the NICU. Pediatrics 2009;124:el095–100. [DOI] [PubMed] [Google Scholar]
- 21.Doymaz S, Zinger M, Sweberg T. Risk factors associated with intracranial hemorrhage in neonates with persistent pulmonary hypertension on ECMO. J Intensive Care 2015;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueller MM, Van Remoortel H, Meybohm P, et al. Patient Blood management: recommendations from the 2018 Frankfurt Consensus Conference. JAMA 2019;321:983–97. [DOI] [PubMed] [Google Scholar]
- 23.Bembea MM, Cheifetz IM, Fortenberry JD, et al. Recommendations on the indications for RBC transfusion for the critically ill child receiving support from extracorporeal membrane oxygenation, ventricular assist, and renal replacement therapy devices from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 2018;19(9S Suppl l):S157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carson JL, Guyatt G, Heddle NM, et al. Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 2016;316:2025–35. [DOI] [PubMed] [Google Scholar]
- 25.Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med 2007;356:1609–19. [DOI] [PubMed] [Google Scholar]
- 26.Valentine SL, Bembea MM, Muszynski JA, et al. Consensus recommendations for RBC transfusion practice in critically ill children from the pediatric critical care transfusion and anemia expertise initiative. Pediatr Crit Care Med 2018;19:884–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muszynski JA, Reeder RW, Hall MW, et al. RBC transfusion practice in pediatric extracorporeal membrane oxygenation support. Crit Care Med 2018;46:e552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sut C, Tariket S, Aubron C, et al. The non-hemostatic aspects of transfused platelets. Front Med 2018;5:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomas MR, Storey RF. The role of platelets in inflammation. Thromb Haemost 2015;114:449–58. [DOI] [PubMed] [Google Scholar]
- 30.Fiser RT, Irby K, Ward RM, et al. RBC transfusion in pediatric patients supported with extracorporeal membrane oxygenation: is there an impact on tissue oxygenation? Pediatr Crit Care Med 2014;n15:806–13. [DOI] [PubMed] [Google Scholar]
- 31.Bembea MM, Schwartz JM, Shah N, et al. Anticoagulation monitoring during pediatric extracorporeal membrane oxygenation. ASAIO J 2013;59:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deschmann E, Saxonhouse MA, Feldman HA, et al. Association between in vitro bleeding time and bleeding in preterm infants with thrombocytopenia. JAMA Pediatr 2019;173;393–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Inclusion Criteria for Neonatal Respiratory ECMO at CHOA-E.
Figure S1. Correlation between red blood cell and platelet transfusion rate. The Spearman correlation coefficient was 0.60 (p < 0.001). Model R2 0.32.