Introduction
Given the global climate warming and the intensive genetic selection for high milk production, it seems that reduced fertility will become a limiting factor for the dairy industry in the coming years. The term “global warming” expresses various climate changes, such as rising sea levels, increased heat content of the oceans, decreased snow and ice surface coverage, and very intense rains. A prominent feature which directly affects the animal is the increase in the environmental temperature, expressed by an increase in the number of consecutive hot days (i.e., daily temperature higher from the average annual temperature) and increased frequency of extremely hot days (i.e., daily temperature higher from the average temperature of the hot season) (IPCC, 2014). For instance, an increase in summer temperatures, hot days, and heat waves have been recorded in Europe (Zampieri et al., 2016). Heat waves are defined as a sequence of at least 6 d with air temperature higher than the reference values for the period and the geographical area. The waves are classified according to their duration and/or their intensity and are known to cause extreme losses to the dairy industry (for review, see Pasqui and Giuseppe, 2019).
Among the climate changes, extreme events have a prominent impact on the lactating cow, most likely due to the lack of acclimatization to sudden environmental change and/or an absence of efficient cooling management. For instance, data from the Israeli herd book show a clear seasonal effect on conception rate over the last 18 yr, with 27.7 versus 42.6% during the summer and winter, respectively. In the years 2010, 2012, and 2015, the air temperature during the summer was higher by 1.5 °C than the average summer temperature, and an additional decrease in conception rate of 5% units was recorded (Wolfenson and Roth, 2019). Nevertheless, it has been estimated that the impact of metabolic heat production for lactating cows, due to increased productivity, is about 2- to 4-fold higher relative to that of global warming (St-Pierre et al., 2003; Collier et al., 2019). Given the genetic direction for improving milk production, an increased sensitivity and reduced tolerance to heat are expected. Therefore, efficient strategies to cope with elevated temperatures are required. This statement is relevant for both the cow (i.e., physiological response) and the farmer (cooling management).
Physiological and Behavioral Responses
The purpose of this section is to introduce the processes by which dairy cows respond to heat load (i.e., elevated temperature and high humidity). The author believes that being aware of the cow’s physiological limitations in maintaining normothermia might encourage the practitioner to implant some of the existing cooling methods. In addition, recognizing the physiological responses of the cow to elevated temperature, it is highly important to handle an efficient cooling management, as discussed below. With that respect, the animal stress response depends on the severity and the duration of heat exposure. Acute stress responses last for a short time, from a mint to a few days (Horowitz, 2001). It is activated by the autonomic nervous system and characterized by the release of catecholamine and glucocorticoid, metabolic changes, and activation of transcription factor. The chronic response is associated with long-term heat exposure. It is activated by the endocrine system and characterized by the homeostasis signaling and formation of new physiological status (for review, see Collier et al., 2017).
Thermal regulation by the cow is based on the following three physical mechanisms of heat loss: conduction, convection, and radiation (i.e., sensible routes). The severity of the stress response is related to body heat production, which associated with, the level of milk production, dry matter intake, and metabolic heat production. Lactating cows has significantly more heat to drive away than nonlactating cows (West et al., 2003). For instance, heat production of cows yielding 18.5 kg/d milk is 1.3 times higher, and that of cows yielding 31.6 kg/d is about 1.5 times higher than the maintenance heat production of nonlactating cows (Purwanto et al., 1990). When the body temperature is above the ambient temperature, thermal gradient is formed, which in turn enables a heat exchange from the body to the environment. However, when the environmental temperature meets or exceeds the body temperature, these routes are lost, that is, the sensible routes are not sufficient to maintain normothermia and the cow responds to elevated temperature by additional physiological, behavioral, and metabolic changes (Collier and Gebremehin, 2015).
The cardiovascular system provides a major thermoregulatory effector mechanism as it allows rapid and efficient control of body temperature. At high ambient temperature, the blood flow to the superficial body tissue and the evaporative tissues of tongue and nose are significantly enhanced in dog (Krönert and Pleschka, 1976) but lack in sheep (Pleschka et al., 1982). To avoid a fall in arterial blood pressure, compensatory cardiovascular changes are required. These include an increase in cardiac output and heart rate, redistribution of blood flow between the body region as well as an increase in blood volume (Rubsamen and Hales, 1985). Interestingly, the volume of both plasma and whole blood are higher in Bos indicus than in Bos taurus cattle (Howes et al., 1963). These physiological changes are also characterized by the increased respiratory rate and oxygen consumption and massive skin vasodilation (Rubsamen and Hales, 1985).
Another route for heat exchange is via evaporation (sweating and panting). In cattle, at high environmental temperature, the respiratory rate increases but the volume decreases, that is, rapid shallow panting (Riek and Lee, 1948). In severity hot environment, when body temperature rises continuously, the panting is changed to a slow and deeper pattern (Findlay, 1957), which characterized by increased oxygen consumption (Whittow and Findlay, 1968) and respiratory alkalosis (Hales, 1967). The efficiency of these routes is highly dependent upon the relative humidity, which controls the vapor pressure gradient. In cattle, the major evaporative heat loss is by sweating rather than respiration (i.e., panting) (Collier and Gebremedhin, 2015). For instance, steers in natural environment (15 °C) lose 10% of their heat via respiratory evaporation. At a higher temperature (35 °C), sweating increases most and respiratory evaporation accounts for 32% of the total heat loss (McLean, 1972).
With respect to the behavioral responses, the most prominent are reduced lying- and increased in standing time, and reduced in rumination time (Honig et al., 2012). One of the rapid behavioral responses of high lactating cows to heat load, is reducing the feed intake, that is, metabolic strategies to reduce metabolic heat production. Reduced feed intake caused by heat stress has traditionally been assumed to be primarily responsible for the decrease in milk yield (Fuquay, 1981; West, 2003). However, reduced feed intake accounts for only about 35% of the heat stress-induced decrease in milk synthesis (Rhoads et al., 2009). Heat induced alteration in postabsorptive charbohydrate, lipid, and protein metabolism, independently of reduced feed intake, have been suggested to be involved in the reduction in milk production upon elevated temperature (for review, see Baumgard and Roads, 2013). Water intake requirements are increased in thermal stress to balance the loss of water via evaporation. It should be pointed out that the impact of heat stress on feed intake and water consumption are related to the level of milk produced by individual cow (Collier et al., 2019). This cascade of events highlights the need to implant nutritional management to keep feed and water consumption as high as possible during the summer.
One of the first steps that should be taken to moderate the effects of hot climate is to protect the cow from direct solar radiation (for review, see West, 2003). Providing fresh food a few times a day, providing shade above the feed line and troughs to keep the feed and water fresh, and to eliminate feed fermentation should be also implanted in dairy herd management during the summer. Furthermore, the ration composition should be adjusted to summer conditions (i.e., reformulate the rations) with the aim of increasing dry matter intake while reducing metabolic heat production. For instance, concentrate the energy, considering mineral losses (Na, K) via sweating, using high-quality forage, and reducing its proportion in the ration, replacement of roughage components with by-products enriched with digestible neutral detergent fiber, such as soy hulls. Efficiency feeding management to control the time of metabolic heat production might include feeding at the evening or night. Utilizing these approaches has shown to increase dry matter intake by 8.3% and milk production by 6.2%. This was associated with decrease in respiratory rate by 13% and rectal temperature by 0.3 °C (Adin et al., 2008; Miron et al., 2008).
Effect of Heat Stress on Endocrinology and Reproductive Physiology
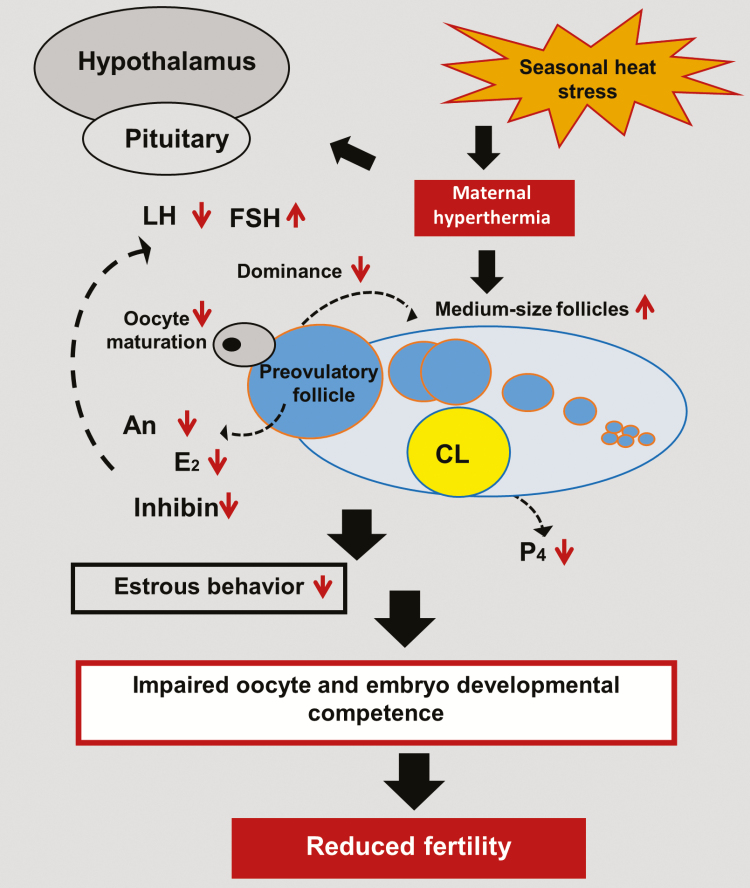
Heat stress during the summer disrupts several reproductive processes, resulting in a pronounced depression of conception rate. Increased body temperature during the summer, has direct and indirect effects on the reproductive tract, in particular, on the hypothalamus–pituitary–ovarian axis. This section summarizes the main alterations that underlie the low fertility of dairy cows during the summer (Figure 1).
Figure 1.
Diagram illustrating the long-term effects of seasonal heat stress on the hypothalamus–pituitary–ovarian axis and its involvement in reducing fertility of lactating cows. Reduced LH secretion is associated with reduced follicular estradiol (E2) secretion. Reduced dominance of the preovulatory follicle is reflected by reduced androstenedione (An) and E2 concentrations and is associated with reduced estrous behavior. Increased number of medium-sized follicles (6–9 mm in diameter), most likely due to reduced dominance, is associated with reduced inhibin and increased FSH concentrations. Reduced oocyte and embryo developmental competence is associated with disruption of nuclear and cytoplasmic maturation. Reduced plasma progesterone (P4) concentration is related to impaired function of the CL. Reduced fertility in heat-stressed cows is presumed to result from additive effects. Adapted from Wolfenson and Roth, 2019.
Gonadotrophins
Luteinizing hormone (LH) and follicle-stimulating hormone (FSH) play important roles in the regulation of follicular growth, ovulation, and corpus luteum (CL) development. Thus, heat induced alteration in the secretion of the gonadotropins, might impair ovarian function. Decreased expression of LH receptor in the ovarian follicles has been reported in heat-stressed goats (Ozawa et al., 2005). Following gonadotropin stimulation, lower concentrations of LH (Gilad et al., 1993) and steroids (Bridges et al., 2005) were detected in the plasma of heat-stressed cows. Heat-induced depression in LH secretion and function might impair the cascade of events leading to ovulation, oocyte maturation, and formation of a functional CL.
FSH secretion has been found to increase under heat stress and is associated with the development of a larger number of follicles (Wolfenson et al., 1995), most likely due to the decreased inhibin concentration in heat-stressed cows (Roth et al., 2000). Increased FSH concentration is suggested to underlie the rise of double ovulations and calving of twins during the summer.
Ovarian follicles
Heat stress alters follicular growth dynamics, in particular that of the preovulatory follicles. Two physiologically significant impairments are associated with attenuation of dominance: a rise in the number of medium and large follicles in a follicular wave, and extended duration of dominance of the preovulatory follicle (Wolfenson et al., 1995). Both might depress reproductive performance. With respect to the follicular function, a reduction in the steroidogenic capacity of follicles has been documented under thermal stress (Badinga et al., 1993; Wolfenson et al., 1997), characterized by low aromatase activity in granulosa cells and decreased estradiol concentration in the follicular fluid of the dominant follicle. Low estradiol production might include impaired estrus duration and intensity; suppression of LH secretion (discussed above), which in turn, might impair events associated with ovulation and the development of ovarian cysts (Wolfenson et al., 2000). These alterations have been reported to affect steroid production (Roth et al., 2001b). In light of these findings, treatment with exogenous gonadotrophin releasing hormone (GnRH) is suggested, as discussed below (Approach 1).
The oocyte
The ovarian pool of oocytes is also sensitive to elevated temperatures. Oocytes collected from Holstein cows during the summer have a reduced developmental competence, expressed by a delay in the two first embryonic divisions and a reduced proportion of embryos developed to the blastocyst stage (Gendelman et al., 2010). A period of two to three estrous cycles is required for recovery from summer heat damage and the appearance of competent oocytes in the subsequent autumn (Roth et al., 2001a). This long-lasting effect of heat stress on the ovarian pool of oocytes might explain the reduced fertility during the autumn, when cows are not exposed to environmental thermal stress. On the other hand, the spontaneous recovery of oocyte developmental competence, during the autumn and subsequent winter, suggests that only a subpopulation of the ovarian antral follicles, rather than the entire follicular reservoir, is damaged upon maternal hyperthermia. Nevertheless, the follicular stages that are susceptible to thermal stress have not been precisely defined mostly due to the complexity of the process and the absence of accurate experimental models (Silva et al., 2016). Early antral follicles of approximately 0.5 to 1 mm in diameter have been suggested to be sensitive to heat stress (Roth et al., 2000). In Gir cows, a 28-d period of heat stress reduced oocyte competence for 105 d (de S Torres-Júnior et al., 2008), indicating that alterations occur at the small antral follicle stage. These findings indicate a carryover effect on follicular growth and development (Roth et al., 2001a, 2001b). Using in vitro culture of ovarian fragments or isolated follicles, it was shown that the exposure to 41 °C does not affect the viability of preantral follicles, presumably because follicles at these stages are less sensitive to heat shock (Paes et al., 2016). In light of this, enhanced removal of impaired follicles has been suggested to improve fertility (Roth et al., 2001a), as discussed below (Approach 3).
The corpus luteum
Heat-induced alteration in luteal function and reduced progesterone concentration in the circulation is suggested to reduce embryo survival and to increase early embryonic loss. Plasma progesterone concentrations during the summer are lower than those during the spring or winter (Howell et al., 1994, Wolfenson et al., 2002). Chronic seasonal heat exposure, but not short-term acute heat stress, leads to the formation of a suboptimal corpus luteum and low progesterone concentration in the plasma (Wolfenson et al., 2000). In support of this, an in vitro study suggested that luteal insufficiency is the result, at least in part, of previous exposure of the preovulatory follicle to thermal stress and decreased production of progesterone by follicular cells, in particular theca cells (Wolfenson et al., 2002). Supplement of exogenous progesterone to support the development of the embryo preimplantation is suggested (see Approach 4 below).
The embryo
While much of the effect of heat stress involves alterations in the follicle and its enclosed oocyte, preimplantation embryos are also sensitive to elevated temperatures, in a stage-dependent manner. Two-cell-stage embryos are more sensitive to heat stress than those at 16-cell stages. Embryos at later developmental stages (i.e., morula, blastocyst) are more resistant to heat stress (Hansen, 2007). While the mechanism underlying the embryo’s acquisition of thermotolerance is not clear, it was suggested that after the 8-cell to 16-cell stage (i.e., embryonic genome activation), an acquisition of transcription capacity allows the embryo a better resistance upon stress (for review, see Hansen, 2013). The embryo capacity to undergo apoptosis is one of the mechanisms that protect embryos from heat shock. Pro apoptotic signals are inhibited in two-cell sage embryos but activated after embryonic genome activation and facilitates embryonic survival upon heat exposure (Paula-Lopes and Hansen, 2002). Another mechanism might be a change in the balance between free-radical generation and antioxidant protection through embryonic development. Exposing in-vitro derived preimplantation embryo to heat shock increased free radical production at days 0 and 2 but not at days 4 and 6 postfertilization (Sakatani et al., 2004). On the other hand, glutathione, a cytoplasmic antioxidant, found to be lower in two- to eight-cell stage embryos and increases in advanced stages of embryonic development (Lim et al, 1996). In light of these, treatments with antioxidants have been suggested to improve fertility during the summer. For example, treatment of dairy cows with melatonin, a potent reactive oxygen species scavenger, in the summer before calving, improved their reproductive performance in the subsequent lactation (Garcia-Ispierto et al., 2013).
Given that preimplantation embryos at early stages of development are highly sensitive to heat stress, embryo transfer at day 8, to bypass the thermosensitive developmental stages, has been suggested (Hansen, 2013). By that time, embryo acquired resistance to heat stress. It is worth noting that the pregnancy rate following embryo transfer can be compromised when the recipient cows cannot maintain normothermia (Vasconcelos et al., 2006), suggesting that the extent of the blastocyst’s thermotolerance is limited. Nonetheless, accumulated findings indicate that embryo transfer is an effective tool to improve fertility during the summer. Embryo transfer during the summer increased pregnancy rate to those achieved with artificial insemination or embryo transfer in the winter. A summary of eight studies in which seasonal variations in pregnancy rate revealed that the seasonal variation in embryo transfer success is lower than that of artificial insemination. In light of these, it was suggested that survival of 7 d embryo is not dependent to any large extent on maternal hyperthermia (for review, see Hansen, 2019). Nonetheless, utilizing cooling methods to maintain recipient cows at normothermia, before and after embryo transfer, is highly recommended by the author.
Cooling Management and Hormonal Treatment
In the current section, some means to improve conception the summer are reported. These include cooling systems and various hormonal treatments. Worth noting is that the use of cooling to prevent severe hyperthermia is a prerequisite for successful hormonal treatment.
Cooling systems
Given the limitation of the physiological responses of the cow (discussed earlier), an intensive management has been developed in order to alleviate the effects of thermal stress during the summer. Different cooling strategies have been developed, for different climate (dry vs. humid) and levels of heat stress (severe vs. moderate). The most common strategy to alleviate the effect of heat stress is providing shade to eliminate direct solar radiation, fan systems, and evaporative cooling.
Three main methods are utilized in supplementing natural ventilation in the housing area. These include high-volume low-speed fans, low-volume high-speed fans, and tunnel ventilation (Smith and Harner, 2012). In the United States, many dairy farms are using tunnel-ventilate free stall buildings, which required closing the sidewalls and moving air the length of the building. Evaporative cooling can be used to cool the air around the cow by using tunnel or cross ventilation with evaporative pads and combinations of fans and high-pressure sprayers to cool the air around cow (Smith and Harner, 2012). These methods work well in arid climate. Another intensive cooling system used is the low-profile cross-ventilated free stall building (Smith et al., 2008; Smith and Harner, 2012). Evaporative cooling pads are placed along one side of the building and fans are placed along the opposite side. This system provides a better control over the cow’s environment and enables the animal to keep a stable core temperature, that is, lower than 38.9 °C (Smith et al., 2008).
In Israel, evaporative cooling was successfully implemented 22 yr ago. The approach is based on sprinkling and ventilation installed in both the holding pen and the feeding area. The cooling management is based on the few 30 to 45 min cooling periods a day. The goal is to maximize the number of wet and dry cycles per hour. Management which based on three cooling periods in the holding pen enables to maintain normal body temperature (<39.0 °C) in cows producing 30 kg milk/d with conception rates similar to those in the winter (Wolfenson et al., 1988). However, for high lactating cows (45 to 50 kg milk/d), intensive cooling consisting of 10 periods for a total of 5 to 7 cumulative h/d are required.
The efficiency of cooling implanted in commercial farms can be evaluated by calculating the ratios between summer and winter milk production and conception rates. The summer-to-winter milk production ratio in Israel is 0.985, indicating that intensive cooling can prevent the decline in milk production even in extremely high-yielding cows (>13,000 kg/yr). However, although intensive cooling is utilized the conception rate is lower by 22 percentage units in the summer versus winter (Flamenbaum and Golan, 2010). Another practical way of evaluating the efficiency of the cooling system is by continuously measuring the body temperature of arbitrarily selected cows. Analyzing the vaginal temperature curve throughout an entire day can assist in developing an efficient cooling management (Figure 2). In addition, the accumulated hours of vaginal temperature above 39 °C have been shown to correlate with conception rate (Gershon et al., unpublished data). This includes the number and the duration of cooling periods and the interval between periods. Comparing the effect of five- versus eight cooling period, 45 min each, revealed a significant change or rise in efficiency, while providing an intensive cooling, that is, accumulation of 6 h of cooling (Honig et al., 2012). In particular, the dry matter intake was higher (27.0 vs. 24.7 kg/d, respectively); the milk production was higher (40.1. vs. 36.6 kg/d, respectively) for eight versus five cooling periods, respectively. In addition, eight cooling periods improved the behavioral responses, expressed by increased lying- and rumination time and physiological responses, expressed by reduced respiratory rate and lower rectal temperature.
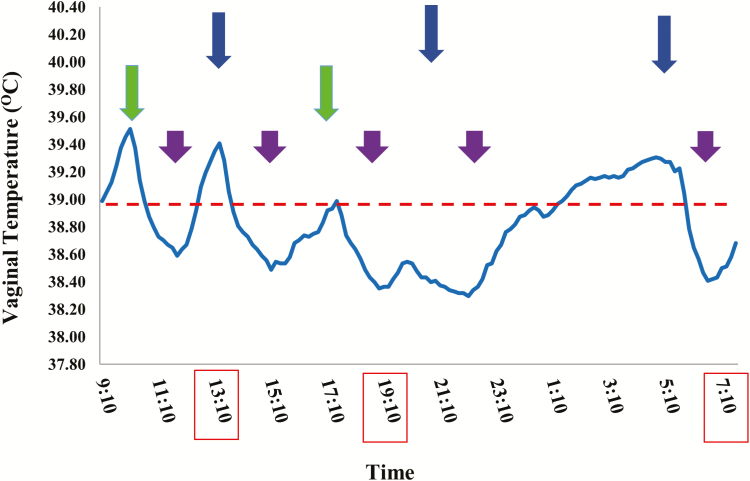
Figure 2.
Cooling management and vaginal temperature of lactating cows (Kaim, unpublished). Cows were cooled five times a day, 45 min each cooling period; three times before milking (05:10, 13:10, and 19:10; blue arrows); and two times between milking (10:10 and 15:10; green arrows). In addition, cows were cooled while on the feeding line (purple arrows). Data indicated that the vaginal temperature was below 39 °C most of the day. At some points, the vaginal temperature was above the red line, that is, 39 °C. Providing additional cooling period at night (01:10) might eliminate the increase throughout the night, and will most likely improve the response of the cows on the subsequent morning.
Taken together, environmental modification combined with intensive and efficient cooling of animal itself, is a practical management approach to enhance both milk production and reproductive efficiencies during the summer. Nevertheless, with the high producing cows, a restoration of pregnancy rate to that of the winter is limited to some extent. Other strategies have been suggested to be combined with cooling.
Hormonal treatment
Approach 1
Administration of a single dose of GnRH at the onset of estrus, that is, coinciding with the secretion of the endogenous LH surge has been suggested to form a preovulatory LH surge. A single dose of GnRH analogue was administered 2 to 3 h after the onset of estrus increased the conception rates in heat-stressed cows (Kaim et al., 2003).
Approach 2
Reduced estradiol production and secretion by the preovulatory follicle might lead to depression of estrous behavior during the summer. Using of Ovsynch and timed AI protocols have been shown to improve the overall pregnancy rate of cows in summer, most likely because all of the cows are inseminated (de la Sota et al., 1998).
Approach 3
Accumulative findings indicate that the ovarian pool of follicles and their enclosed oocyte are damaged by heat exposure. Various hormonal treatments to enhance the removal of impaired follicles have been examined (Roth et al., 2002). For example, the induction of three consecutive follicular waves by GnRH and PGF2α improved the conception rate during the summer and autumn. The most prominent effect was in first-calving cows and cows with high body condition score postpartum (Friedman et al., 2011).
Approach 4
Various studies have attempted to moderate the deleterious effects of heat stress on luteal function by inducing an accessory corpus luteum and excess luteal tissue. Administration of GnRH between days 5 and 15 post-AI increased conception rate by approximately 15 percentage units (Willard et al., 2003; López-Gatius et al., 2006). Administration of human chorionic gonadotropin on day 5 or 6 post-AI, did not affect conception rate (Schmitt et al., 1996) or increased it by 13 percentage units (Beltran and Vasconcelos, 2008).
Approach 5
Supplementation of exogenous progesterone at the very early stages of pregnancy has the potential to increase embryo survival and to improve conception in cows. Friedman et al., (2012) reported that administration of a controlled intravaginal drug-releasing (CIDR) device on day 5 ± 1 post-AI, for 13 d, had a substantial effect on conception rate in the summer when the device was inserted on day 4, relative to days 5 or 6 (43, 39, and 34%, respectively). CIDR significantly increased the conception rate in subgroups of cows with low body condition after calving and in cows that exhibited uterine disorders at parturition. In a follow-up study (Shiff et al., 2018), CIDR was inserted only in cows with low body condition after calving, or in cows diagnosed with uterine disease postpartum. Results confirmed the findings of the earlier study, showing improved conception rate in subgroups of treated cows in the summer.
Summary
The author believes that using an efficient cooling system to maintain normothermia is a prerequisite to any additional remedial approaches. Hormonal treatments to support corpus luteum functioning and embryonic survival are more efficient if the cow maintains normal body temperature. Accumulated findings indicate that embryo transfer is an effective tool to improve fertility during the summer, most likely because the 7-d embryo already enquired thermotolerance. Given that the effect of heat stress on fertility is multifactorial in nature, a combination of treatment approaches might be most effective.
Glossary
Abbreviations
- CIDR
controlled intravaginal drug-releasing
- CL
corpus luteum
- FSH
follicle-stimulating hormone
- GnRH
gonadotrophin releasing hormone
- LH
luteinizing hormone
Conflict of interest statement
The authors declare no real or perceived conflicts of interest.
Literature Cited
- Adin G., Solomon R., Shoshani E., Flamenbaum I., Nikbachat M., Yosef E., Zenou A., Halachmi I., Shamaya A., Brosh A., et al. 2008. Heat production, eating behavior and milk yield of lactating cows fed two rations differing in roughage content and digestibility under heat load conditions. Livest. Sci. 119:145–153. doi: 10.1016/j.livsci.2008.03.012 [DOI] [Google Scholar]
- Badinga L., Thatcher W. W., Diaz T., Drost M., and Wolfenson D.. 1993. Effect of environmental heat stress on follicular development and steroidogenesis in lactating Holstein cows. Theriogenology 39:797–810. doi: 10.1016/0093-691x(93)90419-6 [DOI] [PubMed] [Google Scholar]
- Baumgard L. H., and Roads R. P.. 2013. Effect of heat stress on postabsorptive metabolism and energetics Annu. Rev. Biosci. 1:311–37. doi: 10.1146/annurev-animal-031412-103644 [DOI] [PubMed] [Google Scholar]
- Beltran M.P., and Vasconcelos J. L. M.. 2008. Conception rate in Holstein cows treated with GnRH or hCG on the fifth day post artificial insemination during summer. Arq. Bras. Med. Vet. Zootec. 60:580–586. doi: 10.1590/S0102-09352008000300009 [DOI] [Google Scholar]
- Bridges P. J., Brusie M. A., and Fortune J. E.. 2005. Elevated temperature (heat stress) in vitro reduces androstenedione and estradiol and increases progesterone secretion by follicular cells from bovine dominant follicles. Domest. Anim. Endocrinol. 29:508–522. doi: 10.1016/j.domaniend.2005.02.017 [DOI] [PubMed] [Google Scholar]
- Collier R. J., and Gebremedhin K. G.. 2015. Thermal biology of domestic animals. Annu. Rev. Anim. Biosci. 3:513–532. doi: 10.1146/annurev-animal-022114-110659 [DOI] [PubMed] [Google Scholar]
- Collier R. J., Baumgard L. H., Zimbelman R. B., and Xiao Y.. 2019. Heat stress: physiology of acclimation and adaptation. Anim. Front. 9:12–19. doi: 10.1093/af/vfy031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Renquist B. J., and Xiao Y.. 2017. A 100-year review: stress physiology including heat stress. J. Dairy Sci. 100:10367–10380. doi: 10.3168/jds.2017-13676 [DOI] [PubMed] [Google Scholar]
- de la Sota R. L., Risco C. A., Moreira F., Burke J. M., and Thatcher W. W.. 1998. Evaluation of a timed insemination during summer heat stress in lactating dairy cows. Theriogenology. 49:761–770. doi: 10.1016/S0093-691X(98)00025-9 [DOI] [PubMed] [Google Scholar]
- de S Torres-Júnior J. R., de F A Pires M., de Sá W. F., de M Ferreira A., Viana J. H., Camargo L. S., Ramos A. A., et al. 2008. Effect of maternal heat stress on follicular growth and oocyte competence in Bos indicus cattle. Theriogenology. 69:155–66. doi: 10.1016/j.theriogenology.2007.06.023 [DOI] [PubMed] [Google Scholar]
- Findlay J. D. 1957. The respiratory activity of calves subjected to thermal stress. J. Physiol. 136:300–309. doi: 10.1113/jphysiol.1957.sp005761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flamenbaum I., and Galon N.. 2010. Management of heat stress to improve fertility in dairy cows in Israel. J. Reprod. Dev. 56 Suppl:S36–S41. doi: 10.1262/jrd.1056s36 [DOI] [PubMed] [Google Scholar]
- Friedman E., Voet H., Reznikov D., Dagoni I., and Roth Z.. 2011. Induction of successive follicular waves by gonadotropin-releasing hormone and prostaglandin F(2α) to improve fertility of high-producing cows during the summer and autumn. J. Dairy Sci. 94:2393–2402. doi: 10.3168/jds.2010-3939 [DOI] [PubMed] [Google Scholar]
- Friedman E., Roth Z., Voet H., Lavon Y., and Wolfenson D.. 2012. Progesterone supplementation postinsemination improves fertility of cooled dairy cows during the summer. J. Dairy Sci. 95:3092–3099. doi: 10.3168/jds.2011-5017 [DOI] [PubMed] [Google Scholar]
- Fuquay J. W. 1981. Heat stress as it affects animal production. J. Anim. Sci. 52:164–174. doi: 10.2527/jas1981.521164x [DOI] [PubMed] [Google Scholar]
- García-Ispierto I., Almería S., Serrano B., de Sousa N. M., Beckers J. F., and López-Gatius F.. 2013. Plasma concentrations of pregnancy-associated glycoproteins measured using anti-bovine PAG-2 antibodies on day 120 of gestation predict abortion in dairy cows naturally infected with Neospora caninum. Reprod. Domest. Anim. 48:613–618. doi: 10.1111/rda.12134 [DOI] [PubMed] [Google Scholar]
- Gendelman M., Aroyo A., Yavin S., and Roth Z.. 2010. Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 140:73–82. doi: 10.1530/REP-10-0055 [DOI] [PubMed] [Google Scholar]
- Gilad E., Meidan R., Berman A., Graber Y., and Wolfenson D.. 1993. Effect of heat stress on tonic and GnRH-induced gonadotrophin secretion in relation to concentration of oestradiol in plasma of cyclic cows. J. Reprod. Fertil. 99:315–321. doi: 10.1530/jrf.0.0990315 [DOI] [PubMed] [Google Scholar]
- Hales J. R., Findlay J. D., and Mabon R. M.. 1967. Tissue hypoxia in oxen exposed to severe heat. Respir. Physiol. 3:43–46. doi: 10.1016/0034-5687(67)90022-9 [DOI] [PubMed] [Google Scholar]
- Hansen P. J. 2007. Exploitation of genetic and physiological determinants of embryonic resistance to elevated temperature to improve embryonic survival in dairy cattle during heat stress. Theriogenology 68 Suppl 1:S242–S249. doi: 10.1016/j.theriogenology.2007.04.008 [DOI] [PubMed] [Google Scholar]
- Hansen P. J. 2013. Cellular and molecular basis of therapies to ameliorate effects of heat stress on embryonic development in cattle. Anim. Reprod. 10:322–333 [Google Scholar]
- Hansen P. J. 2019. Reproductive physiology of heat-stressed dairy cows: implications for fertility and assisted reproduction. Anim. Repro 16:497–507. doi: 10.21451/1984-3143-ar2019-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig H., Miron J., Lehrer H., Jackoby S., Zachut M., Zinou A., Portnick Y., and Moallem U.. 2012. Performance and welfare of high-yielding dairy cows subjected to 5 or 8 cooling sessions daily under hot and humid climate. J. Dairy Sci. 95:3736–3742. doi: 10.3168/jds.2011-5054 [DOI] [PubMed] [Google Scholar]
- Horowitz M. 2001. Heat acclimation: phenotypic plasticity and cues underlying the molecular mechanism. J. Term. Boil. 26:357–363. doi: 10.1016/S0306-4565(01)00044-4 [DOI] [Google Scholar]
- Howell J. L., Fuquay J. W., and Smith A. E.. 1994. Corpus luteum growth and function in lactating Holstein cows during spring and summer. J. Dairy Sci. 77:735–739. doi: 10.3168/jds.S0022-0302(94)77007-7 [DOI] [PubMed] [Google Scholar]
- Howes J. R., Hentges J. F., and Feaster J. P.. 1963. Blood volume of Brahman and Herford cattle as measured by injected radioiodinated bovine serum albumin. J Anim. Sci. 22:183–187. doi: 10.2527/jas1963.221183x [DOI] [Google Scholar]
- IPCC, 2014. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, Pachauri R. K. and Meyer L. A. (eds.)]. IPCC, Geneva, Switzerland, p. 151. [Google Scholar]
- Kaim M., Bloch A., Wolfenson D., Braw-Tal R., Rosenberg M., Voet H., and Folman Y.. 2003. Effects of GnRH administered to cows at the onset of estrus on timing of ovulation, endocrine responses, and conception. J. Dairy Sci. 86:2012–2021. doi: 10.3168/jds.S0022-0302(03)73790-4 [DOI] [PubMed] [Google Scholar]
- Krönert H., and Pleschka K.. 1976. Lingual blood flow and hypothalamic control in the dog during panting. Pflugers. Arch. 367:25–31. doi: 10.1007/bf00583652 [DOI] [PubMed] [Google Scholar]
- Lim J. M., Liou S. S., and Hansel W.. 1996. Intracytoplasmic glutathione concentration and the role of beta-mercaptoethanol in preimplantation development of bovine embryos. Theriogenology 46:429–439. doi: 10.1016/0093-691x(96)00165-3 [DOI] [PubMed] [Google Scholar]
- López-Gatius F., Santolaria P., Martino A., Delétang F., and De Rensis F.. 2006. The effects of GnRH treatment at the time of AI and 12 days later on reproductive performance of high producing dairy cows during the warm season in northeastern Spain. Theriogenology 65:820–830. doi: 10.1016/j.theriogenology.2005.07.002 [DOI] [PubMed] [Google Scholar]
- McLean J. A., and Calvert D. T.. 1972. Influence of air humidity on the partition of heat exchange of cattle. J. Agric. Sci. (Cambrige). 78:303–307. doi: 10.1017/S0021859600069148 [DOI] [Google Scholar]
- Miron J., Adin G., Solomon R., Nikbachat M., Zenou A., Shamay A., Brosh A., and Mabjeesh S. Y.. 2008. Heat production and retained energy in lactating cows held under hot summer conditions with evaporative cooling and fed two rations differing in roughage content and in vitro digestibility. Animal 2:843–848. doi: 10.1017/S1751731108001900 [DOI] [PubMed] [Google Scholar]
- Ozawa M., Tabayashi D., Latief T. A., Shimizu T., Oshima I., and Kanai Y.. 2005. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction. 129:621–630. doi: 10.1530/rep.1.00456 [DOI] [PubMed] [Google Scholar]
- Paes V. M., Vieira L. A., Correia H. H. V., Sa N. A. R., Moura A. A. A., Sales A. D., Rodrigues A. P. R., Magalhães-Padilha D. M., Santos F. W., Apgar G. A., et al. 2016. Effect of heat stress on the survival and development of in vitro cultured bovine preantral follicles and on in vitro maturation of cumulus-oocyte complex. Theriogenology. 86:994–1003. doi: 10.1016/j.theriogenology.2016.03.027 [DOI] [PubMed] [Google Scholar]
- Pasqui M., and Di Giuseppe E.. 2019. Climate change, future warming, and adaptation in Europe. Anim. Front. 9:6–11. doi: 10.1093/af/vfy036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula-Lopes F. F., and Hansen P. J.. 2002. Apoptosis is an adaptive response in bovine preimplantation embryos that facilitates survival after heat shock. Biochem. Biophys. Res. Commun. 295:37–42. doi: 10.1016/s0006-291x(02)00619-8 [DOI] [PubMed] [Google Scholar]
- Pleschka K., Hales J. R. S., King R. B., and Fawcett A. A.. 1982. Lack of lingual heat loss in the sheep during panting in hot environments. Pfluger. Arch. 394:R39. doi: 10.1007/BF02580732 [DOI] [Google Scholar]
- Purwanto B. P., Abo Y., Sakamoto R., Furumoto F., and Yamamoto S.. 1990. Diurnal patterns of heat production and heart rate under thermoneutral conditions in Holstein Friesian cows differing in milk production. J. Agric Sci. (Camb.). 114:139–142. doi: 10.1017/S0021859600072117 [DOI] [Google Scholar]
- Rhoads M. L., Rhoads R. P., VanBaale M. J., Collier R. J., Sanders S. R., Weber W. J., Crooker B. A., and Baumgard L. H.. 2009. Effects of heat stress and plane of nutrition on lactating Holstein cows: I. Production, metabolism, and aspects of circulating somatotropin. J. Dairy Sci. 92:1986–1997. doi: 10.3168/jds.2008-1641 [DOI] [PubMed] [Google Scholar]
- Riek R. F., and Lee D. H. K.. 1948. Reaction of hot atmospheres to the milk of Jersey cows. J. Dairy. Res. (Cambridge). 15:219–226. doi: 10.1017/S0022029900005069 [DOI] [Google Scholar]
- Roth Z., Arav A., Bor A., Zeron Y., Braw-Tal R., and Wolfenson D.. 2001a. Improvement of quality of oocytes collected in the autumn by enhanced removal of impaired follicles from previously heat-stressed cows. Reproduction 122:737–744 [PubMed] [Google Scholar]
- Roth Z., Arav A., Braw-Tai R., Bor A., and Wolfenson D.. 2002. Effect of treatment with follicle-stimulating hormone or bovine somatotropin on the quality of oocytes aspirated in the autumn from previously heat-stressed cows. J. Dairy Sci. 85:1398–1405. doi: 10.3168/jds.s0022-0302(02)74207-0 [DOI] [PubMed] [Google Scholar]
- Roth Z., Meidan R., Braw-Tal R., and Wolfenson D.. 2000. Immediate and delayed effects of heat stress on follicular development and its association with plasma FSH and inhibin concentration in cows. J. Reprod. Fertil. 120:83–90 [PubMed] [Google Scholar]
- Roth Z., Meidan R., Shaham-Albalancy A., Braw-Tal R., and Wolfenson D.. 2001b. Delayed effect of heat stress on steroid production in medium-sized and preovulatory bovine follicles. Reproduction 121:745–751 [PubMed] [Google Scholar]
- Rubsamen K., and Hales J. R. S.. 1985. Stress physiology in livestock. In: Yousef M. K., editor, Basic principles, Volume 1. CRC Press, Boca Raton, FL: p. 143–154. [Google Scholar]
- Sakatani M., Kobayashi S., and Takahashi M.. 2004. Effects of heat shock on in vitro development and intracellular oxidative state of bovine preimplantation embryos. Mol. Reprod. Dev. 67:77–82. doi: 10.1002/mrd.20014 [DOI] [PubMed] [Google Scholar]
- Schmitt E. J., Diaz T., Drost M., and Thatcher W. W.. 1996. Use of a gonadotropin-releasing hormone agonist or human chorionic gonadotropin for timed insemination in cattle. J. Anim. Sci. 74:1084–1091. doi: 10.2527/1996.7451084x [DOI] [PubMed] [Google Scholar]
- Shiff O., Lavon Y., Wolfenson D., and Roth Z.. 2018. Effect of exogenous progesterone supplementation on conception rate of lactating cows in the summer. The 30th Annual Cattle Production Conference, Jerusalem, Israel, p. 125–126
- Silva J. R., van den Hurk R., and Figueiredo J. R.. 2016. Ovarian follicle development in vitro and oocyte competence: advances and challenges for farm animals. Domest. Anim. Endocrinol. 55:123–135. doi: 10.1016/j.domaniend.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Smith J. F., and Harner J. P.. 2012Strategies to reduce the impact of heat and cold stress in dairy cattle facilities. In: Collier R. J. and Collier J. L., editors, Environmental physiology of livestock. 1st ed. West Sussex (UK) John Wiley and Sons, Inc.ch15, p. 267–288 [Google Scholar]
- Smith J. F., Harner J. P., Bardford B. J., Overton M. W., and Dhuyvetter K. C.. 2008. Opportunities with low profile cross ventilated freestall facilities. In: Harner J. and Smith J. F., editors, Dairy housing of the future, 10–11 September 2008. Sioux Fall, South Dakota, Kansas State University, Manhattan, KS: p. 1–20 [Google Scholar]
- St-Pierre N. R., Cobanov B., and Schnitkey G.. 2003. Economic loss from heat stress by U.S. livestock industries. J. Dairy. Sci. 86:E52–E77. doi: 10.3168/jds.S0022-0302(03)74040-5 [DOI] [Google Scholar]
- Vasconcelos D. G. B., Demétrio R. M., Santos J. R., Chiari C. A., Rodrigues O. G., and Sá Filho J. L. M.. 2006. Factors potentially affecting fertility of lactating dairy cow recipients. Theriogenology. 65:192–200. doi: 10.1016/j.theriogenology.2005.09.030 [DOI] [PubMed] [Google Scholar]
- West J. W. 2003. Effect of heat stress on production in dairy cattle. J. Dairy. Sci. 86:3131–3144. doi: 10.3168/jds.S0022-0302(03)73803-X [DOI] [PubMed] [Google Scholar]
- Whittow G. C., and Findlay J. D.. 1968. Oxygen cost of thermal panting. Am. J. Physiol. 214:94–99. doi: 10.1152/ajplegacy.1968.214.1.94 [DOI] [PubMed] [Google Scholar]
- Willard S., Gandy S., Bowers S., Graves K., Elias A., and Whisnant C.. 2003. The effects of GnRH administration postinsemination on serum concentrations of progesterone and pregnancy rates in dairy cattle exposed to mild summer heat stress. Theriogenology 59:1799–1810. doi: 10.1016/s0093-691x(02)01232-3 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., and Roth Z.. 2019. Impact of heat stress on cow reproduction and fertility. Anim. Front. 9:32–38. doi: 10.1093/af/vfy027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson D., Flamenbaum I., and Berman A.. 1988. Hyperthermia and body energy store effects on estrous behavior, conception rate, and corpus luteum function in dairy cows. J. Dairy Sci. 71:3497–3504. doi: 10.3168/jds.S0022-0302(88)79956-7 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Lew B. J., Thatcher W. W., Graber Y., and Meidan R.. 1997. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 47:9–19. doi: 10.1016/s0378-4320(96)01638-7 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Roth Z., and Meidan R.. 2000. Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim. Reprod. Sci. 60-61:535–547. doi: 10.1016/s0378-4320(00)00102-0 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Sonego H., Bloch A., Shaham-Albalancy A., Kaim M., Folman Y., and Meidan R.. 2002. Seasonal differences in progesterone production by luteinized bovine thecal and granulosa cells. Domest. Anim. Endocrinol. 22:81–90. doi: 10.1016/s0739-7240(01)00127-8 [DOI] [PubMed] [Google Scholar]
- Wolfenson D., Thatcher W. W., Badinga L., Savio J. D., Meidan R., Lew B. J., Braw-Tal R., and Berman A.. 1995. Effect of heat stress on follicular development during the estrous cycle in lactating dairy cattle. Biol. Reprod. 52:1106–1113. doi: 10.1095/biolreprod52.5.1106 [DOI] [PubMed] [Google Scholar]
- Zampieri M., Russo S., di Sabatino S., Michetti M., Scoccimarro E., and Gualdi S.. 2016. Global assessment of heat wave magnitudes from 1901 to 2010 and implications for the river discharge of the Alps. Sci. Total Environ. 571:1330–1339. doi: 10.1016/j.scitotenv.2016.07.008 [DOI] [PubMed] [Google Scholar]