Introduction
Postparturient disorders in sows represent an economically important disease complex in modern piglet production worldwide. Possible findings affecting the mammary glands and/or the reproductive tract and the sow’s general condition are summarized as a syndrome consisting of a set of symptoms such as mastitis and/or metritis. The term postpartum dysgalactia syndrome (PDS) is most commonly used (Klopfenstein et al., 2006), but other names such as mastitis–metritis–agalactia (MMA) (Martin et al., 1967), agalactia toxemica (Ringarp, 1960), agalactia complex (Penny, 1970), agalactia postpartum (Hermannson et al., 1978), lactation failure (Elmore and Martin, 1980), periparturient hypogalactia syndrome (Smith et al., 1992) or puerperal septicemia and toxemia (Bostedt et al., 1998) have been suggested, too. The predominant symptom is reduced milk production with or without apparent mastitis during the first days after farrowing (Ross et al., 1981; Wegmann et al., 1986; Heinritzi and Hagn, 1999). Increased piglet losses due to high mortality rates caused by a lack of sufficient milk production lead to a significantly reduced number of weaned piglets. Possible causes of PDS vary, and the etiology of the syndrome is clearly multifactorial (Gerjets and Kemper, 2009). In most cases, PDS is limited to a few animals and may only be sporadic. Nonetheless, almost epidemic cases in affected herds were described with documented incidence in sow herds of up to 60% (Hirsch et al., 2004). The average incidence thereof was described as being approximately 13% (Hermannson et al., 1978; Jorsal, 1983; Bäckström et al., 1984; Madec and Leon, 1992; Papadopoulos et al., 2008a). However, these epidemiological studies on incidence at herd level are not directly comparable because of different trait definitions of PDS with no commonly defined phenotype. Sows in herds of different hygienic practices and standards may show PDS. It even occurs on excellently managed farms with optimized disinfection practices. Most research on this topic was conducted between the 1970s and 1990s (Gerjets and Kemper, 2009). Despite continued research activities, no real breakthrough has been made, and no single pathway has been identified. This either confirms the multifactorial etiology or indicates that if there is one single pathway, it may be masked by other overlaying factors. Both can be expected in a syndrome defined as a set of symptoms. However, PDS is still regularly diagnosed on farms. In practice, most clinical cases can be treated effectively with anti-inflammatory drugs, hormones, or antibiotics. Nevertheless, because of the evolving need for the prudent use of antimicrobials in the last decade, a more detailed look into pathoetiology and possible prevention of PDS has become necessary. As clinical signs are as varying as possible etiologies, this review aims to summarize recent insights into PDS gained in the last decade, with a main emphasis on mastitis and its prevention by considering the respective contributory factors.
Etiology
One widely accepted theoretical model of etiopathogenesis of PDS is that bacteria of the sows’ environment infect the mammary gland via the galactogenous or the endogenous route. Via the galactogenous route, bacteria can enter the teat duct and the two glands systems behind it, whose orifices are, in contrast to those of cows, not closed by muscular sphincters (Klopfenstein et al., 2006). Several experiments confirm this hypothesis of a galactogenous route of infection and show a reduction in mastitis incidence after protecting the mammary glands against fecal contamination (Middleton Williams et al., 1977; Bertschinger et al., 1990). In an experimental setting, the time of galactogenous infection was determined to happen in more than 50% of the cases before birth, but only before the 108th day of gestation, and then again in the first 2 d after farrowing before the teat order of the piglets was finally established (Bertschinger et al., 1990). In infection experiments inoculating sows intramammarily with Escherichia coli to provoke mastitis, the infection dose was very low with less than 100 bacteria (Österlundh et al., 2002). Concerning the endogenous route of infection, it was hypothesized that bacteria colonize the mammary gland after passing the barriers in the gut or the uterus. However, the uterine origin of bacteria isolated from the mammary gland does not seem likely. Indeed, in a previous study comparing the bacterial flora in different gut sections, the uterus, and the mammary gland, Gram-negative bacteria were only isolated from the latter and the ileum (Morkoc et al., 1983). These findings are supported by earlier studies (Armstrong et al., 1968; Bertschinger et al., 1977a). A variety of bacterial species have been isolated from the milk of PDS-affected sows and their contribution to pathoetiology has been the subject of controversial discussion (Awad Masalmeh et al., 1990; Kobera, 2000; Kemper et al., 2013). Bacteria of the bacterial genera Escherichia, Citrobacter, Enterobacter, and Klebsiella, belonging to the class of coliforms were the most frequent species isolated from mastitis-affected sows (Ross et al., 1981; Awad Masalmeh et al., 1990; Hirsch et al., 2003; Angjelovski et al., 2016). Infection experiments demonstrated the role of these bacteria in the etiology of sows’ mastitis (Bertschinger et al., 1977b; Ross et al., 1981; Wegmann and Bertschinger, 1984; Pedersen Mörner et al., 1998; Österlundh et al., 2002). However, other species, such as Streptococcus species (spp.), Staphylococcus spp., Pseudomonas spp., Clostridium spp., Proteus spp., or Corynebacterium spp., were isolated from milk samples of diseased sows, too (Baer and Bilkei, 2005; Angjelovski et al., 2016). As discussed in detail later in the section on bacteria as an influencing factor, the exact determination of the role of bacteria in pathogenesis is challenging. First, bacteria were also isolated in colostrum and milk of sows showing no signs of clinical PDS (Preissler and Kemper, 2011; Kemper et al., 2013; Angjelovski et al., 2016), and second, not all sows inoculated with defined amounts of infectious strains developed clinical PDS in infection experiments (Österlundh et al., 1998). This indicates that even though bacteria cause inflammation, the development of PDS is related to other factors, too. In a recent commentary, it was suggested to define PDS as “subclinical coliform mastitis” similar to coliform mastitis in cows and to take advantage of this similarity in further research approaches (Pospischil and Bertschinger, 2018).
Another hypothesis for etiology of PDS is the involvement of lipopolysaccharides (LPS) endotoxins triggering various endogenous mediators causing a strong pathological reaction in sows (Elmore et al., 1978). As a major part of the outer membrane in Gram-negative bacteria, especially those located in the gut, LPS are set free when these bacteria decay. They show a strong systemic effect on the general condition, but with regard to PDS, their impact on the hormonal regulation of colostrum and milk secretion is of special importance; already in 1985, Smith and Wagner (1984) showed that LPS suppresses prolactin release by the anterior pituitary. In this way, cortisol concentration increases and the circulating thyroid hormone decreases, resulting in decreased milk production and secretion (Smith and Wagner, 1984; Reiner et al., 2009). After experimentally administering LPS intravenously, subcutaneously, intramammarily or via the uterus, clinical, and also blood chemical changes similar to on-farm PDS cases were observed (Nachreiner and Ginther, 1974; Elmore et al., 1978).
Summarizing the known risk factors and putting them in a new context, namely homeorhesis, Martineau et al. (2013) proposed that the clinical approach to PDS also has to consider physiology, endocrinology, innate immunology, and ethology in the critical time around parturition with the critical shift from anabolic to catabolic state. First studies comparing hormonal and metabolic alterations in PDS-affected and healthy sows every 14 h in the period from 60 h before to 36 h after parturition showed significant differences in several of the examined hormones and metabolic indicators before birth indicating an imbalance in later diseased animals (Kaiser et al., 2018a, 2018b), supporting the theory of Martineau et al. (2013). This seems to be true especially in modern hyperprolific sows, with large litters and longer birth durations (Peltoniemi et al., 2016), and possible relations to the development of PDS should be investigated in further research.
Diseased Sow-Affected Piglets
Even though PDS is primarily a disease occurring in sows, mainly the piglets suffer as a consequence because of the restricted access to milk. PDS therefore affects both sows’ and piglets’ health and welfare.
In the first weeks of life, the access to colostrum and milk is vital, and the piglets are totally reliant on the sow. Milk yield and composition are critical factors in determining the growth rate in suckling piglets (Gruen et al., 1993). PDS-affected sows fail to meet the needs of their piglets due to dysgalactia in combination with an increased soreness of the mammary gland. Affected sows tend to lie on their mammary glands, therefore refusing piglets access to their painful teats. The lack of milk provision leads to an increased mortality rate and growth retardation in piglets (Ringarp, 1960; Penny, 1970). The combination of weak, undersupplied piglets and the sow’s raised tendency to lie down in lateral recumbency leads to increased incidence of crushing (Hellbrügge et al., 2008). In litters of PDS-affected sows, piglet mortalities within the first-week postpartum were reported to increase by 5.0% (Hühn and Rehbock, 1999) to 38.6% (Bäckström et al., 1984).
As PDS is characterized by the occurrence of reduced milk production during the first days after farrowing, it is obvious that the colostrum period within the first 24 h after birth (Farmer et al., 2019) is affected. The special importance of sufficient colostrum intake in neonatal piglets has been well established. For the survival of piglets, the first days after birth are the most critical period, and inadequate intake of colostrum is the underlying cause for the majority of piglet deaths during this period (Quesnel et al., 2012). It is essential to fill the low glycogen stores in new-born piglets because they are not able to perform sufficient glyconeogenesis, and hypoglycemia may be induced by the rapid decrease in glycogen (Farmer et al., 2019). Besides its energy content, colostrum is essential for the healthy development of piglets because of the maternal transfer of immunoglobulins and lymphocytes (Chase and Lunney, 2019). Primarily, inadequate colostrum intake can result in inanition, poor growth, and even deaths due to starvation and hypothermia. Secondly, it can also lead to secondary infections such as diarrhea if the piglets are not protected by maternal antibodies (Rooke and Bland, 2002).
Even in circumstances with no evidence of PDS in a sow herd, the provision of milk, and especially of colostrum, can be critical in hyperprolific sows. It was shown that with increasing litter sizes over the last decades, milk production has risen, too. However, this is not proportional to the number of piglets (Prunier et al., 2010). Even more important, increasing litter sizes lead to a decrease in the colostrum amount per piglet in modern hyperprolific sows (Devillers et al., 2007). As the cited studies were conducted around 10 years ago, it can be assumed that the proportions might be worse today, but this requires further verification. However, it is clear that this situation exacerbates with the occurrence of PDS.
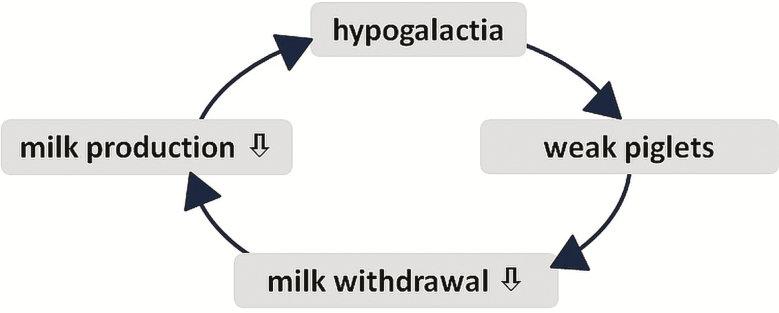
In this context, the vicious circle of lacking milk withdrawal (Figure 1) worsens the situation. Only milk withdrawn by the piglets is reproduced, and only vital and healthy pigs are capable of adequately suckling on a healthy sow allowing them to do so. If the sow’s willingness and her ability to provide sufficient milk are impaired, the piglets become weaker and suckle even less, which can result not only in hypoagalactia but also in agalactia. To break this vicious circle, not only lactation has to be stimulated again, but also adequate provision for the pigs is essential to ensure their vitality.
Figure 1.
The vicious circle of lacking milk withdrawal.
Diagnosis
The main clinical signs of PDS in sows are mastitis, dysgalactia, and fever above 39.5 °C in sows within 12 to 48 h postpartum (Furniss, 1987; Gerjets and Kemper, 2009). However, due to a highly variable clinical picture, diagnosis can be difficult. The most common practice for early diagnosis on commercial farms is to measure the rectal temperature after farrowing. Nevertheless, this should be performed ideally in combination with an examination of mammary gland changes, and consideration of signs of decreased milk production or reduced appetite. The range of critical temperature thresholds varies between 39.3 °C and 40.5 °C (Waldmann and Wendt, 2001), and treatment, mainly with antibiotics, is administered as soon as these thresholds are exceeded. However, physiological hyperthermia is often observed in postparturient sows, especially gilts, leading to misinterpretations (Klopfenstein et al., 2006; Gerjets et al., 2008; Stiehler et al., 2015). Stiehler et al. (2015) stress that temperature measurement should be standardized and measured at the same time of the day.
Investigation of mammary glands, and especially of behavioral changes in sows and piglets, allows a more precise diagnosis. Mammary glands can appear normal or pathologically altered, varying from swollen, firm, and warm to the touch to changes in color. Changes can be limited to only a few teats with variable localization or generalized. Concerning the location of affected mammary glands, the abdominal glands are more prone to pathological changes compared with the pectoral ones (Baer and Bilkei, 2005). Another criterion related to a high risk of developing PDS can be any sign of constipation, such as solid and dry feces, or no defecation at all (Pendl et al., 2017).
As already mentioned, the sows’ behavior can be altered with regard to their general condition and their will to allow the piglets access to the udder by lying permanently. The teats can become traumatized and show superficial or deeper lesions caused by the piglets in their desire for milk after strenuous nursing efforts. On the one hand, behavioral changes in sows have to be considered in diagnosis; on the other hand, conspicuous behavior of piglets can give hints of sows developing PDS. For instance, vigorous nursing efforts might be noticed. The reduced or absent milk ejection leads to decreased nursing intervals and an increase in the piglets’ activity, often with agonistic behavior, also followed by skin lesions (Prunier et al., 2010). Subsequently, with diminishing energy reserves, the piglets’ attempt to nurse weakens, and they often retreat to the warmest parts of the farrowing pen and show signs of isolation behavior (Klopfenstein et al., 2006). Moreover, they try to intake other liquids, which, in combination with the lack of colostrum, might cause diarrhea (Sărăndan et al., 2009).
As the measurement of body temperature is nonspecific, with an increase only indicating alterations in the physiological state of warm-blooded animals, and the other aforementioned behavioral factors indicating PDS are not necessarily assessed on the farm, it can be supposed that a significant percentage of PDS is wrongly diagnosed. Therefore, special emphasis should be laid on correctly diagnosing PDS.
One further clinical diagnostic approach was the use of ultrasonography as a precautionary measure for differentiating sows having suffered recurrent PDS and showing hyperechogenic images from healthy animals (Baer and Bilkei, 2005). However, it has not been implemented in sow management due to additional costs and impractical handling.
Instead, a number of laboratory indicators have been proposed to diagnose PDS more precisely (Mirko and Bilkei, 2004; Zhu et al., 2004, 2007a, 2007b). However, these have not been put into practice on farms due to different reasons. All approaches to diagnose PDS based on changes in the blood, whether inflammatory markers or hormones, are not feasible on farms because the taking of blood samples is much more laborious than measuring sows’ body temperature. These practical limitations also hold true for attempts to diagnose PDS according to changed parameters in the urine of diseased sows (Petersen, 1983; Papadopoulos et al., 2008b) or in their milk. Parallel to the use of mastitis indicators in cow milk, cell count (Persson et al., 1996) and milk pH (Waldmann and Wendt, 2001) were suggested as indicators. Nonetheless, due to difficulties in gaining adequate amounts of milk from sows in practice, their use is very limited, too. Besides the problems in acquiring suitable sample material, another shortcoming of the suggested parameters is the nonspecific information they provide. For instance, similar to fever, levels of plasma concentration of acute phase proteins increase in stressful situations such as PDS, and therefore were reported to change in diseased sows (Mirko and Bilkei, 2004). However, these levels are nonspecific and can also, as fever, vary substantially around birth (Magnusson and Fossum, 1992). More recently, saliva has been proposed as a promising matrix to identify PDS-affected sows at an early stage, with increased concentrations of salivary chromogranin A and cortisol in affected animals (Kaiser et al., 2018b). In addition, serum 8-epi-PGF2 was shown to be increased in PDS-affected sows before parturition, indicating oxidative stress (Kaiser et al., 2018b).
All these restrictions of alternative diagnostic parameters illustrate the importance of carefully monitoring the sows and their piglets after farrowing, recording any changes in their behavior in order to correctly diagnose actual cases of PDS.
Influencing Factors
The factors shown to have an influence on the clinical development of PDS are numerous and are summarized in Figure 2. In research, PDS has been only a minor issue over the last years in published research articles. This can be related to the fact that with its multifactorial etiology, most probably not only a single pathway is responsible for the outcome of the disease, or, if a single, hitherto unidentified pathway is relevant, it might be masked by other influencing factors. Thus, so far, the single factors can be categorized into those decreasing the immune defense, increasing the infection pressure, or extending the duration of birth. In the following, the factors “bacteria,” “husbandry and management,” and “sows’ predisposition” are elucidated in more detail.
Figure 2.
Scheme of potential influencing factors contributing to PDS, which can be summarized as factors decreasing the immune defense, increasing the infection pressure and extending the birth duration.
Bacteria
Bacteria isolated from sow’s milk are only of informative value in combination with the clinical picture. The occurrence of bacteria in sow’s milk alone does not lead to clinical signs, and in milk samples of healthy sows, various bacteria can be found regularly (Kemper et al., 2013; Angjelovski et al., 2016). One reason can be the relatively open teat ductus with possibly ascending bacteria from the sow’s environment. The other reason might be related to the sampling procedure, because milking a sow after the first hours postpartum is all but easy, and often only realizable after oxytocin injection. The whole procedure may lead to contamination and false-positive results.
Escherichia coli is the pathogen most frequently isolated in association with PDS (Armstrong et al., 1968; Ross et al., 1981; Awad Masalmeh et al., 1990). Further studies on the virulence profiles of E. coli did not reveal differences in the prevalence or in specific virulence gene profiles of isolates from either diseased or healthy sows (Gerjets et al., 2011b). In this study, a variety of virulence genes were detected in E. coli isolates both from PDS-positive and -negative sows, with most virulence genes belonging to the large group of genes related to extraintestinal pathogenic Escherichia coli (ExPEC). However, a categorization into the pathotype ExPEC only by virulence gene typing was not possible (Gerjets et al., 2011b). There is no single E. coli-strain causing PDS; rather, any given E. coli strain, even if considered to be nonpathogenic, can cause PDS in sows if further adversely environmental, genetic, or other influencing factors promoting infection are present.
Besides E. coli, other bacteria such as strains of Staphylococcaceae, Streptococcaceae and other bacteria families have been isolated from milk of affected sows with an unclear contribution to actual pathogenesis (Awad Masalmeh et al., 1990; Hirsch et al., 2004; Kemper et al., 2013). Most of the isolated species are ubiquitous in the sows’ environment and can originate from both fecal, urine and other contamination. As in the case of E. coli, other unfavorable influencing factors play a significant role in the development of clinical disease. These factors can be attributed to the host or the environment.
However, it has to be mentioned that also other pathogens can cause clinical pictures similar to PDS. For instance, Mycoplasma suis has recently been described to cause dysgalactia in a Belgian sow herd (Laitat et al., 2019). Ju et al. (2019) detected Pseudomonas spp. to be the most frequently isolated bacteria from mammary lesions in South Korean slaughterhouse sows and suggested that there may be a higher prevalence of cases of these agents causing mastitis than previously estimated. The occurrence of mycotoxins, originating from moldy feed or other material, is also discussed as impacting lactation negatively (Heinritzi et al., 2006).
Husbandry and Management
Environmental factors such as husbandry and hygiene management as well as feeding can affect the clinical course of PDS. Overviews of risk factors increasing PDS prevalence, as studied so far, are discussed in detail elsewhere (Papadopoulos et al., 2008a; Gerjets and Kemper, 2009). Regarding the likelihood of a sow becoming affected by PDS, existing data on the impact of parity number are inconsistent (Bertschinger et al., 1977b; Baer and Bilkei, 2005). Gerjets et al. (2011a) detected an increased risk for developing PDS in sows of the first parity, with litters of more than 13 piglets born alive and more than one piglet born dead, and after birth intervention. These results were confirmed by Bardehle et al. (2012), revealing significant relations between PDS, birth induction, and obstetrics. Sows affected by PDS often show delayed or extended parturition over 4 h (Tummaruk and Sang-Gassanee, 2013), and therewith birth assistance is more likely (Berg et al., 2001). Another strongly correlated influencing factor is nutrition, for instance, lack of crude fiber in the ration (Plonait and Bickhart, 1997), and diet-related conditions such as obstipation (Bostedt et al., 1998), which is also often caused by physical inactivity of periparturient sows, whether voluntarily or due to confinement in the farrowing crate. In this context, sufficient water intake also plays an important role (Krüger et al., 2002; Jenny et al., 2015). Nutrition can influence the hypothalamo-hypophysical gonadal axis, and therefore also lactogenesis itself, as reviewed by Cosgrove and Foxcroft (1996). Other documented factors increasing the risk of developing PDS are gestation duration exceeding 116 days (Awad Masalmeh et al., 1990), prolonged births (Petersen, 1983; Bostedt et al., 1998), occurrence of urinary tract infections (Bilkei et al., 1994), high ambient temperatures (Quiniou and Noblet, 1999), and late introduction into the farrowing pen after the 110th day of gestation (Valenčak et al., 2006). Peltoniemi et al. (2016) reviewed the data on space provision for sows and offering of nest-building material before parturition, showing the potential to reduce farrowing duration, stillbirth rate, and potential stress also during the early nursing period. As stress is a risk factor for PDS, both space allowance and provision of nesting material can have beneficial effects.
Sows’ predisposition
As one key factor for the clinical outcome of disease, the host defense status is of major importance (Burvenich et al., 2003). The individual skills of sows to cope with possible pathogenic bacteria is an important factor in the etiology of PDS, being influenced by many factors, such as parity number, birth duration, and genetic variation.
A potential genetic predisposition to PDS has been discussed for some time (Ringarp, 1960; Preissler et al., 2012b). Based on various definitions of phenotypes, heritability ranging from 2% to 20% has been estimated (Lingaas and Ronningen, 1991; Berg et al., 2001; Krieter and Presuhn, 2009; Preissler et al., 2012a). In the most recent study, an estimated heritability of approximately 9% in modern sow lines was assessed, and with that, a genetic background cannot be excluded (Preissler et al., 2012a). On the one hand, even with this relatively low heritability percentage, breeding success is possible as shown for mastitis in dairy cows (Heringstad et al., 2003). On the other hand, a heritability in this range clearly shows the importance of other influencing factors and the need for an optimized environment. With modern technologies enabling whole-genome studies, a first study to detect genetic variation in PDS was conducted in five commercial pig breeding lines (Preissler et al., 2013). The genetic susceptibility to PDS was confirmed in this previous study. However, no single causative gene or genomic regions for PDS were identified, but several contributing genes in different regions: Three significant SNPs were located on Sus Scrofa Chromosome (SSC) 13, SSC 15, and SSC 17 (Preissler et al., 2013). These first findings are promising for potential genetic improvement, even of low heritable traits. Nonetheless, further studies are necessary to validate these results in other lines and larger data sets.
Treatment and Prevention
The main treatment of PDS includes the administration of nonsteroidal anti-inflammatory drugs (NSAIDs) or other analgetic drugs to help the sows and enable continued suckling in piglets (Pendl et al., 2017, Farmer et al., 2019). To maintain milk production, oxytocin can be additionally administered (Hirsch et al., 2003; Gerjets and Kemper, 2009; Pendl et al., 2017). Maintenance of milk production, or milk provision by other sources, is essential for the piglets’ health. If they are already weakened, additional heating should also be provided.
Considering prudent use of antibiotics, as demanded in the EU Guidelines on the Prudent Use of Antimicrobials in Veterinary Medicine (2015), temperature alone should not be used as a single criterion to treat PDS with antibiotics in sows postpartum. The temperature threshold is defined rather subjectively, and its use might be regarded critically, since increases and decreases in temperature can appear physiologically, especially in the peripartal period. It is essential to perform a diagnosis of PDS not only due to temperature increase, but also based on a combination of the appropriate criteria mentioned above. Wrongly diagnosed PDS cases should be avoided to reduce the use of antimicrobials and the occurrence of bacterial strains resistant to one or more antibiotics (Silley and Stephan, 2017). Moreover, antibiotics should only be administered if the treatment with NSAIDs and Oxytocin was not successful.
In Swedish (Clemensson Lindell et al., 2019) and Swiss sow herds (Hartmann, 2016), PDS was shown to be the most frequent reason for antibiotic treatment. One finding of the latter and another Swiss study was the high percentage (23% to 33%) of “Highest Priority Critically Important Antimicrobials” for human medicine (Jenny et al., 2015; Hartmann, 2016), as defined by the World Health Organization (2019), such as fluorochinolones or third and fourth generation cephalosporines used for PDS treatment. Moreover, Jenny et al. (2015) showed that in 54% of antibiotic treatments, treatment duration was too short, and in 19% thereof the dosage was too low, which both can have triggering effects on antibiotic resistance.
The reduction in antibiotic administration in lactating sows is not only strived for because of antibiotic resistance prevention, but also because of other disadvantages related to excessive use. Hartmann (2016) showed that antibiotic treatments in sows in the peripartal period are followed by increased antimicrobial treatment of diarrhea, polyarthritis, and runting in suckling piglets and weaners. Since antibiotics can be partially excreted via milk and affect the development of a physiological intestinal flora in piglets, antibiotics should only be administered after a confirmed diagnosis (Oliel, 1995).
As the economic impact of PDS is significant, prevention is most important. Indeed, a model eliminating the risk of PDS showed that the value of sow space by €279 compared with the baseline scenario (Niemi et al., 2017). Economic losses are mainly related to increased piglet losses, lower weight gain in piglets, and a reduced sow lifetime performance by one parity (Hühn and Rehbock, 1999; Hoy, 2003). Prevention is, therefore, the best way to cope with PDS in a population, but difficult to accomplish due to etiology. Even though various studies have been conducted to elucidate causative factors and the potential genetic background including individual resistance (Preissler et al., 2013), the reason for only some sows developing clinical signs of infection after contact with ubiquitous bacteria remains unknown. However, providing a suitable environment and adapted feeding strategies for preparturient sows accounts for a large proportion of successful long-term prevention. It was shown that the use of prepartal transition feed is an effective tool for reducing PDS prevalence (Pendl et al., 2017). An overview on recent literature on feeding the sow for ease of farrowing, and with that, for PDS prevention, can be found elsewhere (Peltoniemi et al., 2016). It has to be stressed that the provision of roughage is recommended both as nest-building material and as source of fiber to prevent constipation.
Special care should be taken for sows at risk such as primiparous sows and sows with a prolonged duration of farrowing over 4 h, because those have a high risk of developing postparturient disorders (Tummaruk and Pearodwong, 2015). As stated by Peltoniemi et al. (2016), it can be assumed that prolonged parturition affects uterine health. Especially in contemporary, highly prolific sows, the duration of farrowing is increased due to the high number of piglets, and with that, often also the need for birth induction and obstetrics, being further risks factors for PDS. The effect of larger litter size on PDS has not been investigated in detail yet.
All measures to reduce the risk factors mentioned in the sections above are beneficial. The correlations between physiology and behavior around farrowing and the influence of prolonged farrowing, retained placenta, development of PDS and impaired involution of the uterus on colostrum yield, and subsequent fertility are presented in a review by Peltoniemi et al. (2016). In this review, several recommendations for successful farrowing and the following lactation are given, for instance, the provision of suitable nest-building material, freedom of movement, and a diet to reduce the risk of constipation and obesity. Moreover, the importance of a short parturition duration is emphasized (Peltoniemi et al., 2016). To enable easy births and to provide obstetrics if needed, regular controls, at least hourly, in the time around expected parturitions are beneficial, also during nightshifts. In outdoor sows, which were allowed to move freely, a lower incidence of PDS was detected (Mortensen et al., 1994). Sufficient water intake is essential to avoid obstipation, and may be supported by an open water source. A general high level of farm hygiene management and specific measures to reduce the infection risk, that is, washing the sows before entering the farrowing compartment, decrease PDS risk, too (Hulten et al., 2004). During parturition, a high level of hygiene should be realized. Soiling has to be avoided in the critical period around birth and lactation, and in farrowing pens or crates, the sow should not lie in her own excreta, which requires regular removal of feces.
Summary and Conclusions
As a multifactorial syndrome, PDS is not only variable in its clinical picture but also in its underlying causes. Accordingly, a comprehensive knowledge of all potential influences and their optimization is a prerequisite for raising healthy sows and piglets. With mainly ubiquitous bacteria as pathogens, emphasis has to be laid on noninfectious risk factors. PDS is not so much a challenge in treatment, but more so in prevention and early diagnosis. As is the case for almost all on-farm situations, and especially for multifactorial diseases, the human factor is of utmost importance: careful and close animal monitoring is the key to early identification of sows and litters at risk of developing PDS. The often practiced administration of drugs just based on a detected increase in body temperature postpartum is not justified professionally. The prudent use of antibiotics demands a proper trait definition, and therefore other traits should be included in diagnosis. An increased body temperature above 39.5 °C within 12 to 48 h postpartum indicates PDS. However, additional clinical alterations on sows’ teats (reddening, swelling, hardening, etc.) and/or noticeable behavioral changes in sows and piglets have to be present to confirm the diagnosis. Sow’s and piglet’s behavior should be the focus of each routine observation of farmers or farm personnel when checking the farrowing compartments, and even slight changes should be followed up. This is of special importance particularly the higher the demands on management are if litter size is large. Increasing litter size has been one goal in pig breeding over the last decades, but modern sow lines with large litters represent a challenge in pig farming, especially during farrowing and lactation. Uneven litters and prolonged duration of birth are associated with increasing litter sizes (Farmer et al., 2019), and the latter is a known risk factor for PDS. Other risk factors, which might be related to the etiology of PDS such as physiological challenges facing highly prolific sows during this period still remain unknown. Even with these unknown correlations, documentation of birth duration and possible problems such as need for obstetrics or subsequent PDS represents a useful tool for prevention when sows are selected based on these traits. Moreover, there is still potential for adaptations and improvements in existing farrowing systems to increase the general immune defense of sows, reduce infection pressure, and decrease birth duration, this way preventing PDS.
Glossary
Abbreviations
- ExPEC
extraintestinal pathogenic Escherichia coli
- LPS
lipopolysaccharides
- MMA
mastitis–metritis–agalactia
- PDS
postpartum dysgalactia syndrome
- SPP
species
Conflict of interest statement
The author declares no real or perceived conflicts of interest.
Literature Cited
- Angjelovski B., Cvetkovikj A., Mrenoshki S., Radeski M., Cvetkovikj I., Ratkova M., and Dovenski T.. 2016. Bacteria associated with clinical postpartum dysgalactia syndrome in farmed sows in the Republic of Macedonia. Turk. J. Vet. Anim. Sci. 40(6):776–781. doi: 10.3906/vet-1602-102 [DOI] [Google Scholar]
- Armstrong C. H., Hooper B. E., and Martin C. E.. 1968. Microflora associated with agalactia syndrome of sows. Am. J. Vet. Res. 29:1401–1407. [PubMed] [Google Scholar]
- Awad Masalmeh M., Baumgartner W., Passering A., Silber R., and Hinterdorfer F.. 1990. Bacteriological studies in sows with puerperal mastitis in different herds in Austria. Tierärztl. Umsch. 45:526–535. [Google Scholar]
- Bäckström L., Morkoç A. C., Connor J., Larson R., and Price W.. 1984. Clinical study of mastitis-metritis-agalactia in sows in Illinois. J. Am. Vet. Med. Assoc. 185:70–73. [PubMed] [Google Scholar]
- Baer C., and Bilkei G.. 2005. Ultrasonographic and gross pathological findings in the mammary glands of weaned sows having suffered recidiving mastitis metritis agalactia. Reprod. Domest. Anim. 40:544–547. doi: 10.1111/j.1439-0531.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- Bardehle D., Preissler R., Lehmann J., Looft H., and Kemper N.. 2012. Analysis of fertility and performance parameters in piglet production considering partus induction, birth assistance and mastitis-metritis-agalactia (MMA). Züchtungskd. 84(4):293–306. [Google Scholar]
- Berg P., Andersen S., Henryon M., and Nielsen J.. 2001. Genetic variation for birth assistance and MMA in sows and diarrhoea in their litters. In: EAAP, editor. Annual Meeting of European Association for Animal Production; August 26 to 29, 2001; Budapest, Hungary; p. 315.
- Bertschinger H. U., Bürgi E., Eng V., and Wegmann P.. 1990. Lowering of the incidence of puerperal mastitis in the sow by protection of the mammae from contamination. Schweiz. Arch. Tierheilkd. 132:557–566. [PubMed] [Google Scholar]
- Bertschinger H. U., Pohlenz J., and Hemlep I.. 1977a. Studies on the mastitis-metritis-agalactia syndrome (milk fever) in sows. II. Bacteriological findings in spontaneous cases. Schweiz. Arch. Tierheilkd. 119:223–233. [PubMed] [Google Scholar]
- Bertschinger H. U., Pohlenz J., and Middleton-Williams D. M.. 1977b. Studies on the mastitis-metritis-agalactia syndrome (milk fever) in sows. III. Galactogenic induction of Klebsiella-induced mastitis. Schweiz. Arch. Tierheilkd. 119:265–275. [PubMed] [Google Scholar]
- Bilkei G., Bolcskei A., Clavadetscher E., Goos T., Hofmann C., Bilkei H., and Szenci O.. 1994. The periparturient disease complex of the sow, 1. Evaluation of the course of the bacteriuria of urine samples of old sows during the periparturient period. Berl. Münch. Tierärztl. Wochenschr. 107(10):327–330. [PubMed] [Google Scholar]
- Bostedt H., Maier G., Herfen K., and Hospen R.. 1998. Clinical examinations on gilts with puerperal septicaemia and toxaemia. Tierärztl. Prax. 26:332–338. [PubMed] [Google Scholar]
- Burvenich C., Van Merris V., Mehrzad J., Diez-Fraile A., and Duchateau L.. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet. Res. 34:521–564. doi: 10.1051/vetres:2003023. [DOI] [PubMed] [Google Scholar]
- Chase C., and Lunney J. K.. 2019. Immune system. In: Zimmerman J. J., Karriker L., Ramirez A., Schwartz K. J., Stevenson G. W., and Zhang J., editors. Diseases of swine. 11th ed. Hoboke (NJ): Wiley-Blackwell; p. 264–291. [Google Scholar]
- Clemensson Lindell I., Lindahl E., Lundeheim N., and Eliasson-Selig L.. 2019. Antibiotic treatment in swedish pig herds as number of treated disease cases in relation to herd production data. In: ECPHM, editor. Proceedings of the 11th European Symposium Porcine Health Management; May 22 to 24, 2019. Utrecht, The Netherlands; p. HHM-PP-47.
- Cosgrove J. R., and Foxcroft G. R.. 1996. Nutrition and reproduction in the pig: ovarian aetiology. Animal. Repr. Sci. 42(1–4):131–141. [Google Scholar]
- Devillers N., Farmer C., Le Dividich J., and Prunier A.. 2007. Variability of colostrum yield and colostrum intake in pigs. Animal 1:1033–1041. doi: 10.1017/S175173110700016X. [DOI] [PubMed] [Google Scholar]
- Elmore R. G., and Martin C. E.. 1980. Agalactia in current therapy. In: Morrow D. A., editor. Theriogenology 2: diagnosis, treatment and prevention of reproductive diseases in animals. Philadelphia (PA): WB Saunders Co; p. 1083–1086. [Google Scholar]
- Elmore R. G., Martin C. E., and Berg P.. 1978. Absorption of Escherichia coli endotoxin from the mammary glands and uteri of early postpartum sows and gilts. Theriogenology 10:439–445. [Google Scholar]
- Farmer C., Maes D. G. D., and Peltoniemi O. A. T.. 2019. Mammary System. In: Zimmerman J. J., Karriker L., Ramirez A., Schwartz K. J., Stevenson G. W., and Zhang J., editors, Diseases of swine. 11th ed. Hoboke (NJ): Wiley-Blackwell; p. 313–338. [Google Scholar]
- Furniss S. J. 1987. Measurement of rectal temperature to predict mastitis, metritis and agalactia (MMA) in sows after farrowing. Prev. Vet. Med. 5:133–139. [Google Scholar]
- Gerjets I., and Kemper N.. 2009. Coliform mastitis in sows: a review. J. Swine Health Prod. 17(2):97–105. [Google Scholar]
- Gerjets I., Kruse S., Krieter J., and Kemper N.. 2008. Diagnosis of MMA affected sows: bacteriological differentiation, temperature measurement and water intake. In: IPVS, editor. Proceedings of the 20th International Pig Veterinary Society Congress; June 22 to 26, 2008; Durban, South Africa; p. 236.
- Gerjets I., Traulsen I., Reiners K., and Kemper N.. 2011a. Assessing individual sow risk factors for coliform mastitis: a case–control study. Prev. Vet. Med. 100:248–251. doi: 10.1016/j.prevetmed.2011.04.012 [DOI] [PubMed] [Google Scholar]
- Gerjets I., Traulsen I., Reiners K., and Kemper N.. 2011b. Comparison of virulence gene profiles of Escherichia coli isolates from sows with coliform mastitis and healthy sows. Vet. Microbiol. 152:361–367. doi: 10.1016/j.vetmic.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Gruen D., Reiner G., and Dzabo V.. 1993. Investigations on breed differences in milk yield of swine. II. Commitment: milk composition and its relation towards piglet development during suckling period. Repr. Dom. Animal 28:22–27. [Google Scholar]
- Hartmann S. 2016. Erhebung von Risikofaktoren für einen Antibiotikumverbrauch unter Berücksichtigung von Biosicherheit, Tiergesundheit, Management und Transport [doctoral thesis]. Zürich (Switzerland): University of Zürich. [Google Scholar]
- Heinritzi K., Gindele H. R., Gerald R., and Schnurrbusch U.. 2006. Schweinekrankheiten. Stuttgart (Germany): Verlag Eugen Ulmer. [Google Scholar]
- Heinritzi K., and Hagn J.. 1999. Comparison of therapeutic performance of the new Cephalosporin Cefquinome with other treatment regimes in gilts with puerperal septicaemia and toxaemia syndrome. Tierärztl. Prax. 27:114–121. [PubMed] [Google Scholar]
- Hellbrügge B., Tölle K.-H., Bennewitz J., Presuhn U., Henze C., and Krieter J.. 2008. Genetic aspects regarding piglet losses and the maternal behaviour of sows. Part 1. Genetic analysis of piglet mortality and fertility traits in pigs. Animal 2(9): 1273–1280. doi: 10.1017/S1751731108002504 [DOI] [PubMed] [Google Scholar]
- Heringstad B., Klemetsdal G., and Steine T.. 2003. Selection responses for clinical mastitis and protein yield in two Norwegian dairy cattle selection experiments. J. Dairy Sci. 86:2990–2999. doi: 10.3168/jds.S0022-0302(03)73897-1 [DOI] [PubMed] [Google Scholar]
- Hermannson I., Einarsson S., Larsson K., and Bäckström L.. 1978. On the agalactia postpartum in the sows: a clinical study. Nor. Vet. Med. 30:465–473. [PubMed] [Google Scholar]
- Hirsch A. C., Philipp H., and Kleemann R.. 2003. Investigation on the efficacy of meloxicam in sows with mastitis-metritis-agalactia syndrome. J. Vet. Pharmacol. Ther. 26:355–360. doi: 10.1046/j.1365-2885.2003.00524.x [DOI] [PubMed] [Google Scholar]
- Hirsch A. C., Philipp H., and Kleemann R.. 2004. Efficacy of meloxicam for treatment of the mastitis-metritis-agalactia syndrome in sows. Prakt. Tierarzt 85(11):842–848. [Google Scholar]
- Hoy S. 2003. Auswirkungen der Puerperalerkrankungen bei Sauen auf die Fruchtbarkeitsleistung. Arch. Tierz. 46 (4):341–346. [Google Scholar]
- Hühn U., and Rehbock F.. 1999. Prostaglandine contra Umrauschen. Krankhaftem Scheidenausfluss nach der Geburt Paroli bieten. dlz agrarmagazin 50:192–196. [Google Scholar]
- Hultén F., Persson A., Eliasson-Selling L., Heldmer E., Lindberg M., Sjögren U., Kugelberg C., and Ehlorsson C. J.. 2004. Evaluation of environmental and management-related risk factors associated with chronic mastitis in sows. Am. J. Vet. Res. 65:1398–1403. doi: 10.2460/ajvr.2004.65.1398 [DOI] [PubMed] [Google Scholar]
- Jenny B., Vidondo B., Pendl W., Kümmerlen D., and Sidler X.. 2015. Evaluation of risk factors for mastitis-metritis-agalactia in pig farms in Switzerland. Schweiz. Arch. Tierheilkd. 157:689–696. doi: 10.17236/sat00047 [DOI] [PubMed] [Google Scholar]
- Jorsal S. E. 1983. Epidemiological and statistical analysis of disease records from pig testing stations (PhD thesis). Copenhagen (Denmark): University of Copenhagen. [Google Scholar]
- Ju J. H., Shin J. I., Lim H. Y., Kim H. W., Seung B. J., Cho S. H., Kim S. H., and Sur J. H.. 2019. Classification, bacteriological findings, and analysis of sex hormone receptors and cytokine expression in mammary lesions of abattoir sows. J. Vet. Sci. 20:e11. doi: 10.4142/jvs.2019.20.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M., Jacobson M., Andersen P. H., Bækbo P., Cerón J. J., Dahl J., Escribano D., and Jacobsen S.. 2018a. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 14(1):83. doi: 10.1186/s12917-018-1382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M., Jacobsen S., Andersen P. H., Bækbo P., Cerón J. J., Dahl J., Escribano D., Theil P. K., and Jacobson M.. 2018b. Hormonal and metabolic indicators before and after farrowing in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 14:334. doi: 10.1186/s12917-018-1649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper N., Bardehle D., Lehmann J., Gerjets I., Looft H., and Preissler R.. 2013. The role of bacterial pathogens in coliform mastitis in sows. Berl. Munch. Tierarztl. Wochenschr. 126:130–136. [PubMed] [Google Scholar]
- Klopfenstein C., Farmer C., and Martineau G. P.. 2006. Diseases of the mammary glands and lactation problems. In: Straw B. E., Zimmermann J. J., and Taylor D. J., editors. Diseases of swine. Iowa City (IA): Iowa State University Press; p. 833–860. [Google Scholar]
- Kobera R. 2000. Comparative analysis of the efficacy of Cefquinome and Enrofloxacine in the treatment of the MMA-complex in sows (doctoral thesis). Leipzig (Germany): University of Leipzig. [Google Scholar]
- Krieter J., and Presuhn U.. 2009. Genetic variation for MMA treatment. Züchtungskd 81:149–154. [Google Scholar]
- Krueger M., Schroedl W., Isik W., Lange W., and Hagemann L.. 2002. Effects of lactulose on the intestinal microflora of periparturient sows and their piglets. Eur. J. Nutr. 41 (Suppl 1):I26–I31. doi: 10.1007/s00394-002-1104-5 [DOI] [PubMed] [Google Scholar]
- Laitat M., Vandenbruaene J., Dalle S., and Vyt P.. 2019. Mycoplasma suis associated with dysgalactia and anemia in a sow herd. In: ECPHM, editor. Proceedings of the 11th European Symposium Porcine Health Management; May 22 to 24, 2019; Utrecht, The Netherlands; p. HHM-PP-92.
- Lingaas F., and Rønningen K.. 1991. Epidemiological and genetical studies in Norwegian pig herds. V. Estimates of heritability and phenotypic correlations of the most common diseases in Norwegian pigs. Acta Vet. Scand. 32:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madec F., and Leon E.. 1992. Farrowing disorders in the sow: a field study. Zentralbl. Veterinarmed. A 39:433–444. doi: 10.1111/j.1439-0442.1992.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Magnusson U., and Fossum C.. 1992. Effect of estradiol-17 beta treatment of gilts on blood mononuclear cell functions in vitro. Am. J. Vet. Res. 53:1427–1430. [PubMed] [Google Scholar]
- Martin C. E., Hooper B. E., and Armstrong C. H.. 1967. A clinical and pathological study of the mastitis-metritis-agalactia syndrome of sows. J. Am. Vet. Med. Assoc. 151:1629–1634. [Google Scholar]
- Martineau G. P., Le T. Y., and Guillou D.. 2013. Postpartum dysgalactia syndrome: a simple change in homeorhesis? J. Swine Health. Prod. 21(2):85–93. [Google Scholar]
- Middleton-Williams D. M., Pohlenz J., Lott-Stolz G., and Bertschinger H. U.. 1977. Investigations on the mastitis metritis agalactia syndrome (milk fever) in sows. Schweiz. Arch. Tierheilkd. 119:213–222. [PubMed] [Google Scholar]
- Mirko C. P., and Bilkei G.. 2004. Acute phase proteins, serum cortisol and preweaning litter performance in sows suffering from periparturient disease. Acta Vet. 54(2–3):153–161. [Google Scholar]
- Morkoç A., Bäckström L., Lund L., and Smith A. R.. 1983. Bacterial endotoxin in blood of dysgalactic sows in relation to microbial status of uterus, milk, and intestine. J. Am. Vet. Med. Assoc. 183:786–789. [PubMed] [Google Scholar]
- Mortensen B., Ruby V., Pedersen B. K., Smidth J., and Larsen V. A.. 1994. Outdoor pig production in Denmark. Pig News Info 15:117N–120N. [Google Scholar]
- Nachreiner R. F., and Ginther O. J.. 1974. Induction of agalactia by administration of endotoxin (Escherichia coli) in swine. Am. J. Vet. Res. 35:619–622. [PubMed] [Google Scholar]
- Niemi J. K., Bergman P., Ovaska S., Sevón-Aimonen M. L., and Heinonen M.. 2017. Modeling the costs of postpartum dysgalactia syndrome and locomotory disorders on sow productivity and replacement. Front. Vet. Sci. 4:181. doi: 10.3389/fvets.2017.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliel N. 1995. Chemoprophylaxe von puerperaler Mastitis (Milchfieber) bei der Sau mit Enrofloxacin (BAYTRIL®) (doctoral thesis). Zürich (Switzerland): University of Zürich. [Google Scholar]
- Osterlundh I., Holst H., and Magnusson U.. 1998. Hormonal and immunological changes in blood and mammary secretion in the sow at parturition. Theriogenology 50:465–477. doi: 10.1016/s0093-691x(98)00153-8 [DOI] [PubMed] [Google Scholar]
- Österlundh I., Hulten F., Johannison A., and Magnusson U.. 2002. Sows intrammarily inoculated with Escherichia coli at parturition: i. Functional capacity of granulocytes in sows affected or non-affected by clinical mastitis. Vet. Imm. Immunopath. 90:35–44. doi: 10.1016/S0165-2427(02)00204-0 [DOI] [PubMed] [Google Scholar]
- Papadopoulos G. A., Janssens G. P. J., Dewulf J., Vanderhaeghe C., and Maes D. G. D.. 2008. Risk factors implicated in the occurrence of post partum dysgalactia syndrome in sows. In: IPVS, editor. Proceedings of the 20th International Pig Veterinary Society Congress; June 22 to 26, 2008.; Durban, South Africa; p. 229.
- Papadopoulos G. A., Maes D. G., Van Weyenberg S., Verheyen A., and Janssens G. P.. 2008b. Selected parameters in urine as indicators of milk production in lactating sows: a pilot study. Vet. J. 177:104–109. doi: 10.1016/j.tvjl.2007.03.007 [DOI] [PubMed] [Google Scholar]
- Pedersen Mörner A., Faris A., and Krovacek K.. 1998. Virulence determinants of Escherichia coli isolated from the milk of sows with coliform mastitis. J. Vet. Med. Ser. B 45(5):287–295. [DOI] [PubMed] [Google Scholar]
- Peltoniemi O., Björkman S., and Oliviero C.. 2016. Parturition effects on reproductive health in the gilt and sow. Reprod. Domest. Anim. 51 (Suppl 2):36–47. doi: 10.1111/rda.12798 [DOI] [PubMed] [Google Scholar]
- Pendl W., Jenny B., Torgerson P. R., Spring P., Kümmerlen D., and Sidler X.. 2017. Effect of herd health management on the prevalence of postpartum dysgalaktie syndrome (PPDS) and the treatment incidence. Schweiz. Arch. Tierheilkd. 159:109–116. doi: 10.17236/sat00105 [DOI] [PubMed] [Google Scholar]
- Penny R. H. C. 1970. The agalactia complex in the sow: a review. Aust. Vet. J. 46(4):153–159. [DOI] [PubMed] [Google Scholar]
- Persson A., Pedersen Mörner A., and Kuhl W.. 1996. A long-term study on the health status and performance of sows on different feed allowances during late pregnancy. II. The total cell content and its percentage of polymorphonuclear leucocytes in pathogen-free colostrum and milk collected from clinically healthy sows. Acta Vet. Scand. 37:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen B. 1983. Methods of early recognition of puerperal and fertility disorders in the sow. Livest. Prod. Sci. 10:253–264. [Google Scholar]
- Plonait H., and Bickhart K.. 1997. Geburt, Puerperium und perinatale Verluste. In: Plonait H. and Bickhart K., editors. Lehrbuch der Schweinekrankheiten. Berlin/Hamburg: Parey; p. 472–512. [Google Scholar]
- Pospischil A., and Bertschinger H. U.. 2018. Evident similarity of porcine postparturient dysgalactia to subclinical porcine coliform mastitis. J. Swine Health Prod. 26(6):316–322. [Google Scholar]
- Preissler R., Hinrichs D., Reiners K., Looft H., and Kemper N.. 2012a. Estimation of variance components for postpartum dysgalactia syndrome in sows. J. Anim. Breed. Genet. 129:98–102. doi: 10.1111/j.1439-0388.2011.00969.x [DOI] [PubMed] [Google Scholar]
- Preissler R., and Kemper N.. 2011. Bacterial flora on the mammary gland skin of sows and in their colostrum. J. Swine Health. Prod. 19(2):112–115. [Google Scholar]
- Preissler R., Looft H., and Kemper N.. 2012b. The postpartum dysgalactia syndrome: an overview of the genetic predisposition. Tierärztl. Umsch. 67:520–527. doi: 10.1111/j.1439-0388.2011.00969.x [DOI] [Google Scholar]
- Preissler R., Tetens J., Reiners K., Looft H., and Kemper N.. 2013. A genome-wide association study to detect genetic variation for postpartum dysgalactia syndrome in five commercial pig breeding lines. Anim. Genet. 44:502–508. doi: 10.1111/age.12047 [DOI] [PubMed] [Google Scholar]
- Prunier A., Heinonen M., and Quesnel H.. 2010. High physiological demands in intensively raised pigs: impact on health and welfare. Animal 4:886–898. doi: 10.1017/S175173111000008X [DOI] [PubMed] [Google Scholar]
- Quesnel H., Farmer C., and Devillers N.. 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146(2–3):105–114. doi: 10.1016/j.livsci.2012.03.010 [DOI] [Google Scholar]
- Quiniou N., and Noblet J.. 1999. Influence of high ambient temperatures on performance of multiparous lactating sows. J. Anim. Sci. 77:2124–2134. doi: 10.2527/1999.7782124x. [DOI] [PubMed] [Google Scholar]
- Reiner G., Hertrampf B., and Richard H. R.. 2009. Dysgalactia in the sow post partum—a review with special emphasis on pathogenesis. Tierärztl. Prax. Ausg. G 37(5):305–318. [Google Scholar]
- Ringarp N. 1960. A post-parturient syndrome with agalactia in sows. Acta Agric. Scand. (Suppl 7):1–166. [Google Scholar]
- Rooke J. A., and Bland I. M.. 2002. The acquisition of passive immunity in the new-born piglet. Livest. Prod. Sci. 78(1):13–23. doi: 10.1016/S0301-6226(02)00182-3 [DOI] [Google Scholar]
- Ross R. F., Orning A. P., Woods R. D., Zimmermann B. J., Cox D. F., and Harris D. L.. 1981. Bacteriologic study of sow agalactia. Am. J. Vet. Res. 42:949–955. [PubMed] [Google Scholar]
- Sărăndan H., Sărăndan R., Petroman I., Ognean L., Sărăndan M., Balint A., and Faur B.. 2009. Growth rate and mortality in suckling piglets and their correlation to the sows’milk yield. Zooteh. Biotehnol. 42:277–283. [Google Scholar]
- Silley P., and Stephan B.. 2017. Prudent use and regulatory guidelines for veterinary antibiotics—politics or science? J. Appl. Microbiol. 123(6):1373–1380. doi: 10.1111/jam.13553 [DOI] [PubMed] [Google Scholar]
- Smith B. B., Martineau G. P., and Bisaillon A.. 1992. Mammary glands and lactation problem. In: Leman A. D., Straw B. E., Mengeling W. L., D’Allaire S., and Taylor D. J., editors. Diseases of swine. Ames (IA): Iowa State University Press; p. 523–634. [Google Scholar]
- Smith B. B., and Wagner W. C.. 1984. Suppression of prolactin in pigs by Escherichia coli endotoxin. Science 224:605–607. doi: 10.1126/science.6369541 [DOI] [PubMed] [Google Scholar]
- Stiehler T., Heuwieser W., Pfutzner A., and Burfeind O.. 2015. The course of rectal and vaginal temperature in early postpartum sows. J. Swine Health Prod. 23(2):72–83. [DOI] [PubMed] [Google Scholar]
- Tummaruk P., and Pearodwong P.. 2015. Postparturient disorders and backfat loss in tropical sows associated with parity, farrowing duration and type of antibiotic. Trop. Anim. Health Prod. 47:1457–1464. doi: 10.1007/s11250-015-0883-7 [DOI] [PubMed] [Google Scholar]
- Tummaruk P., and Sang-Gassanee K.. 2013. Effect of farrowing duration, parity number and the type of anti-inflammatory drug on postparturient disorders in sows: a clinical study. Trop. Anim. Health Prod. 45:1071–1077. doi: 10.1007/s11250-012-0315-x [DOI] [PubMed] [Google Scholar]
- Valenčak Z., Štukelj M., and Ščuka L.. 2006. Therapeutic effects of enrofloxacin in mastitis-metritis-agalactia syndrome: a review. Acta Vet. Brno 75:515–522. doi: 10.2754/avb200675040515 [DOI] [Google Scholar]
- Waldmann K.-H., and Wendt M.. 2001. Lehrbuch der Schweinekrankheiten. 4th ed. Stuttgart (Germany): Parey Verlag. [Google Scholar]
- Wegmann P., and Bertschinger H. U.. 1984. Sequential cytological and bacteriological examination of the secretions from sucked and unsucked mammary glands with and without mastitis. In: IPVS, editor. Proceedings of the 8th International Pig Veterinary Society Congress; August 27 to 31, 1984; Ghent, Belgium; p. 287.
- Wegmann P., Bertschinger H. U., and Jecklin H.. 1986. A field study on the prevalence of coliform mastitis (MMA syndrome) in Switzerland and the antimicrobial susceptibility of the coliform bacteria from the milk. In: IPVS, editor. Proceedings of the 9th International Pig Veterinary Society Congress; July 15 to 18, 1986; Barcelona, Spain; p. 92.
- World Health Organization 2019. Critically important antimicrobials for human medicine, 6th revision. Geneva (Switzerland): World Health Organization. [Google Scholar]
- Zhu Y., Berg M., Fossum C., and Magnusson U.. 2007a. Proinflammatory cytokine mRNA expression in mammary tissue of following intramammary inoculation with Escherichia coli. Vet. Imm. Immunopath. 116:98–103. doi: 10.1016/j.vetimm.2006.12.003 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Fossum C., Berg M., and Magnusson U.. 2007b. Morphometric analysis of proinflammatory cytokines in mammary glands of sows suggests an association between clinical mastitis and local production of IL-1beta, IL-6 and TNF-alpha. Vet. Res. 38:871–882. doi: 10.1051/vetres:2007035 [DOI] [PubMed] [Google Scholar]
- Zhu Y., Osterlundh I., Hultén F., and Magnusson U.. 2004. Tumor necrosis factor-alpha, interleukin-6, serum amyloid A, haptoglobin, and cortisol concentrations in sows following intramammary inoculation of Escherichia coli. Am. J. Vet. Res. 65:1434–1439. doi: 10.2460/ajvr.2004.65.1434 [DOI] [PubMed] [Google Scholar]