Introduction
Both the uterus and oviduct are functionally adapted to facilitate sperm transport, oocyte fertilization, embryonic/fetal development, and deliver a calf. However, the mucosa of the uterus and oviduct is exposed to ascending infections at coitus, during pregnancy, and after parturition. Therefore, the mucosal immune system of the uterus and oviduct should exhibit a large degree of flexibility to potentially regulate maternal immune reactions to allogeneic spermatozoa and semi-allogeneic embryos without hindering the effective immune defense responses against infectious agents (Ellington, 1991; Yaniz et al., 2000; Bauersachs and Wolf, 2013).
Immunologically, sperm are allogeneic to the maternal immune system (Gaunt, 1983; Lander et al., 1990). Therefore, once sperm is deposited into the uterus during artificial insemination (AI), a sequence of cellular dynamics and immunological inflammatory reactions toward sperm is anticipated. These inflammatory responses are regarded as the rapid and transient leukocytic infiltration, mostly polymorphonuclear neutrophils (PMNs), into the uterine lumen which remove the excess or dead sperm (Katila, 2012). Very few sperm can escape from PMNs’ phagocytosis in the uterus and reach the oviduct where sperm reservoirs are formed to induce capacitation and to support sperm survival until fertilization (Hunter, 2012).
Little is known about the immunological crosstalk between bovine sperm and the uterus and oviduct when compared with other animal species and humans. In this review based upon our and other recent findings, more focus will be given to the bovine species. In particular, novel interactions between sperm and the uterine and oviductal mucosa under physiological conditions will be highlighted.
General Aspects of Mucosal Innate Immunity of the Uterus and Oviduct
The mucosal epithelial cells lining the uterus and oviduct have the capacity to recognize pathogen-associated molecular pattern molecules and damage-associated molecular patterns through a variety of pattern recognition receptors. Bovine endometrial epithelial cells (BEECs) express transcripts of Toll-like receptor (TLR)-1–7 and 9 genes, while endometrial stromal cells expressed detectable transcripts of the TLR 1–4, 6, 7, 9, and 10 genes (Davies et al., 2008). Additionally, by means of immunohistochemistry, it was shown that TLR2 and TLR4 proteins were expressed in the bovine uterus as well as oviduct with intensive staining of epithelial cells (Kowsar et al., 2013; Ezz et al., 2019). Bovine endometrial cells use TLR4 to sense lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, and TLR1, TLR2, and TLR6 to detect bacterial lipopeptides. The binding of LPS to TLR4 leads to the activation of nuclear factor-kappa B, and consequently the transcription of pro-inflammatory cytokines such as interleukin (IL)-6 and chemokines IL-8, and prostaglandin E2 (Swangchan-Uthai et al., 2012; Fu et al., 2013). Likewise, the challenging of bovine oviduct epithelial cells (BOECs) with LPS increased pro-inflammatory cytokine secretions (Kowsar et al., 2013; Ibrahim et al., 2015).
The mucosal immune systems of the uterus and oviduct may act not only as immune sensors and effectors, but also as regulators of immune cell functions through the secretion of some cytokines and chemokines. Chemokines such as IL-8 (encoded by the CXCL8 gene) act as potent neutrophil chemo-attractants (MacKintosh et al., 2013), and granulocyte-macrophage colony stimulating factor secreted by epithelial cells is required for macrophage migration (de Moraes et al., 1999) to the site of infection or tissue damage, where these hematopoietic cells are regulated by IL-6 (Zerbe et al., 2003). The PMNs and macrophages represent the majority of circulating leukocytes and are often the first cells to migrate toward inflammatory lesions where they exert host defence functions through the phagocytosis of invading pathogens and cell debris, the generation of oxygen-derived reactive agents, and the release of proteolytic enzymes (Tapper, 1996).
Another important component of the mucosal innate immunity of the female genital tract is the release of local and hepatic-derived antimicrobial proteins, including the alpha 1-acid glycoprotein (AGP), an acute-phase protein in response to uterine infection (Sheldon et al., 2001). Acute phase proteins are produced in the liver in response to increased concentrations of cytokines such as IL-6 and tumor necrosis factor-alpha (TNFα) (Lecchi et al., 2009). The bovine oviduct tissue expresses AGP protein in epithelial cells and the smooth muscle layer and the stimulation of BOECs with LPS induced the mRNA expression of AGP (Kowsar et al., 2014).
It has been observed that ovarian steroids partially regulate the maternal innate immune responses to infection. However, the precise mechanisms underlying these observations are still unclear. High progesterone concentrations suppress the uterine innate immune responses that makes the uterus more susceptible to bacterial infection, while high estradiol around estrus is associated with the elimination of infection (Rowson et al., 1953; Lewis, 2003). Similarly, in postpartum beef cows, intrauterine infusions of Trueperella pyogenes and Escherichia coli developed infections only at the onset of luteal stage where progesterone concentrations had begun to increase (Del Vecchio et al., 1994). In oviduct, ovarian steroids and luteinizing hormone (LH) were involved in maintaining an immunological homeostasis through inhibition of LPS-induced pro-inflammatory responses in BOECs in vitro (Kowsar et al., 2013).
Together, the uterine and oviductal mucosa exerts their innate immune responses via efficient recognition and reaction to pathogens (mainly via TLRs), regulation of immune cell function (mainly PMNs and macrophages), and release of antimicrobial proteins (such as AGP).
Sperm–Uterus Immune Crosstalk
During AI in cows, millions of sperm are deposited into the uterus to favor the chances of fertilization. However, most of these sperm are eliminated by either backflow, degradation, or phagocytosis by PMNs, and only few thousands are transported to the oviduct (Rath et al., 2008). After the coitus, sperm need approximately 6–8 h to move to the oviduct in sufficient numbers for oocyte fertilization (Wilmut and Hunter, 1984). Recently, we conducted a series of in vivo experiments to investigate the spatio-temporal changes in sperm transport and distribution in the uterus and vagina after AI in cows. Several uterine lavages of the uterine body and horn (ipsilateral to ovulatory follicle) and vaginal smears in multiparous Japanese black cows were performed at different time points after AI. The results showed that most of sperm are rapidly transported simultaneously either forward into the uterine horn or backward into vagina within 1 h after AI. Most of sperm were eliminated from the uterus or vagina 6 h after AI and only a scant number was left in the uterus or vagina. The data also showed that the uterine lavage or vaginal smears were completely free from sperm within 10 h after AI (Marey et al., 2019b). This rapid transport of sperm is thought to be through the aid of the contractions of uterine smooth muscles which start immediately after the natural mating or AI by mechanical stimulation of the vagina and cervix, which mediate neurogenic oxytocin release (Madill et al., 2000). These myometrial contractions carry sperm in both directions, either in the forward direction to the oviduct, or back-flowed and drained through the cervix (Hawk, 1987; Bourke and Lindsay 1988; Katila et al., 2000; Sumransap et al., 2007).
Most recently, the detailed molecular interactions and inflammatory responses to bovine sperm on the uterine epithelium were intensively investigated using either a simplified in vitro co-culture model of BEECs and frozen–thawed sperm, or an ex vivo bovine uterine explant culture model with fresh sperm. The results of in vitro studies showed that only live sperm stimulated an inflammatory cascade in BEECs which was characterized by increased expressions of IL8, TNFα, IL1B, and Prostaglandin E synthase (PGES) mRNA, and suppression of the anti-inflammatory cytokine, TGFB1 mRNA, (Elweza et al., 2018; Figure 1a). Furthermore, addition of TLR2/4 blockers to sperm–BEECs co-culture significantly suppressed sperm-inflammatory responses as well as the sperm-induced phosphorylation of p38MAPK and JNK as downstream targets of signaling pathways of TLR2/4 in BEECs. These findings indicated that BEECs respond to sperm via TLR2/4 signal transduction (Ezz et al., 2019). In an ex vivo model, the findings revealed that active sperm glided over the endometrial surface epithelium until they encountered and entered uterine glands, and consequently induced a uterine inflammatory process. Scanning electron microscopy (SEM) observations showed that the entrance of sperm into endometrial glands stimulated PMNs assembly most probably from the endometrial stroma in order to attack sperm and hinder their motility. These observations suggest that uterine glands serve as sensors for sperm signals which trigger the maternal innate immune responses (Akthar et al., 2019).
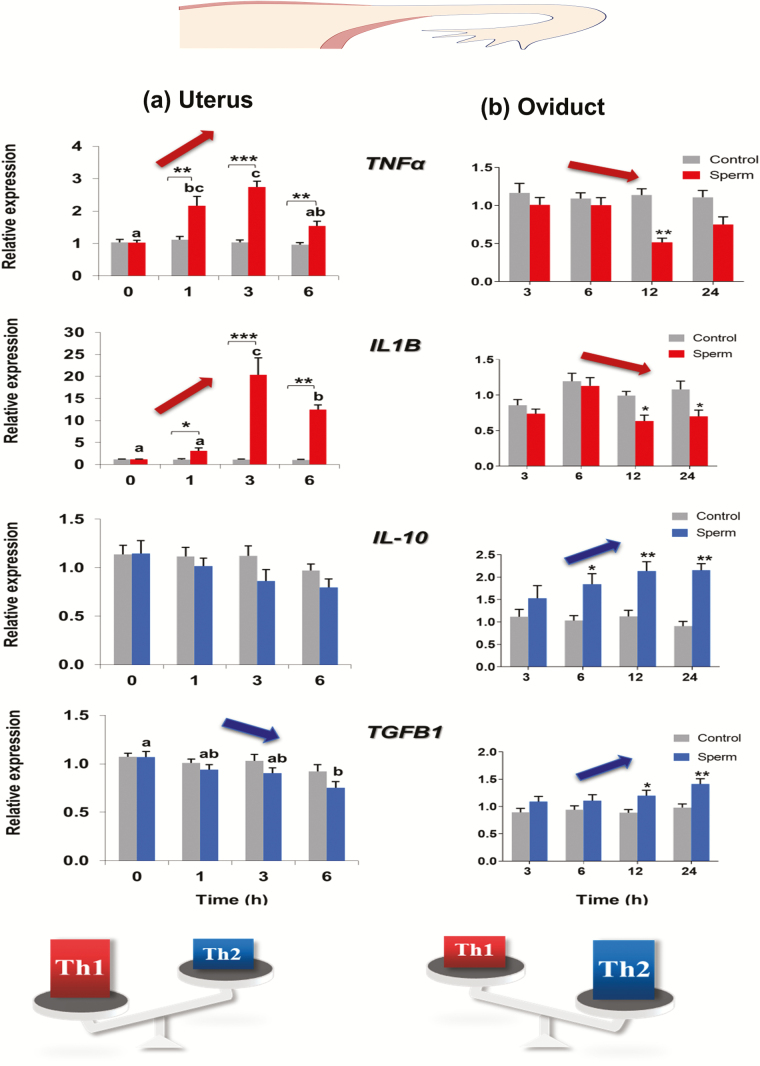
Figure 1.
Effects of sperm on the mRNA expression of pro- and anti-inflammatory cytokines in the monolayers of (a) BEECs and (b) BOECs. BEECs monolayers were co-cultured with washed sperm (106/ml) for 0, 1, 3, or 6 h. BOECs monolayers were co-cultured with swim-up sperm (2 × 105 sperm/mL) for 3, 6, 12, or 24 h. The mRNA expressions of TNFα, IL1B, IL-10, and TGFB1 were measured by a real-time PCR. Different letters denote a significant difference (P ˂ 0.05) between different time points. Asterisks denote statistical differences: *P < 0.05, **P < 0.01, ***P < 0.001, when compared with control effect at the same time point. The data clearly show the opposite effect of sperm on BEECs (a) and BOECs (b) monolayers. Sperm-induced pro-inflammatory (Th1) responses in BEECs through up-regulation of the expression of TNFα and IL1B mRNA and down-regulation of TGFB1 mRNA. On the contrary, sperm-induced anti-inflammatory (Th2) responses in BOECs through down-regulation of the expression of TNFα and IL1B mRNA and up-regulation of IL-10 and TGFB1 mRNA. The findings show that sperm sensing system to generate the optimal maternal immune crosstalk is strictly regulated through the local balance between the pro-inflammatory and anti-inflammatory immune responses in the bovine uterus and oviduct. The maintenance of this immune balance is required for fertilization. Adapted from Yousef et al. (2016) and Elweza et al. (2018).
In most animal species, the presence of sperm in the uterus induces a rapid chemotaxis of PMNs as reviewed by Katila (2012). Sperm are not inherently chemotactic, but they activate a complement cascade in uterine secretions which in turn mediates chemotaxis of PMNs (Tizard, 1996). PMNs are the first cells in blood that are rapidly recruited into inflamed tissues because of the kinetics of expression of leuco-endothelial adhesion molecules on leucocytes and endothelial cells (Kaplanski et al., 2003). In cows, PMNs are always detected in the uterine mucosa, but their infiltration into the endometrium was greatly increased from proestrus through metestrus (Ohtani et al., 1993). In 1963, Howe and Black reported that PMNs peaked around 8–16 h after insemination in cattle, whereas more recently, it was proposed that PMNs infiltrations peaked within 3 h after insemination (Alghamdi et al., 2009). Our recent findings showed that PMNs start to appear in the uterus 3 h after AI and reach their maximum 6 h after AI, and completely disappear 10 h after AI (Marey et al., 2019b). This rapid and relatively short duration of PMN infiltration ensures effective removal of excess sperm and bacteria and subsequent return of the endometrium to a normal state before receiving the embryo. Activated PMNs phagocytize spermatozoa either by direct cell membrane attachment or by neutrophil extracellular traps (NETs) entanglement. Activated PMNs extrude their nuclear DNA and histones to form NETs that ensnare sperm and hinder their motility (Alghamdi and Foster, 2005).
Sperm–Oviduct Immune Crosstalk
Unlike the uterus, the bovine oviduct is considered as a safe refuge for sperm, and it does not respond to insemination with an influx of leucocytes (Rodriguez-Martinez et al., 1990). The oviduct is the main site for sperm capacitation and storage until fertilization (Mitchell et al., 1985; Sostaric et al., 2008). Very few sperm (several hundreds or thousands) can escape from PMNs phagocytosis in the uterus, overcome the physical and biochemical barriers in the utero-tubal junction, and bypass to the oviduct. In the oviduct, sperm colonize at the oviduct epithelium, and thus forming sperm reservoirs where sperm undergo capacitation and display characteristics of hyperactivation (Ellington, 1991; Hunter, 2012).
Recently, more considerations were given for better understanding of the local innate immune system of the bovine oviduct and the immunological consequences of sperm–oviduct immune crosstalk. Basically, by means of immunohistochemistry, it was shown that the anti-inflammatory cytokines, TGFB1 and IL10, are strongly and constantly expressed in the bovine oviduct epithelial and endothelial cells of the surrounding vasculature (in both isthmus and ampulla) throughout the estrous cycle under physiological conditions (Yousef et al., 2016). This constant expression of both epithelial-derived T-helper type 2-driving (Th2, anti-inflammatory) cytokines suppressed the immune system, thereby inducing a state of immune-tolerance toward sperm and early embryo in the oviduct. Importantly, the BOEC–sperm binding further stimulated the gene expression for TGFB1 and IL10 and slightly decreased the epithelial-derived T-helper type 1-driving (Th1, pro-inflammatory), TNFα and IL-1B, in vitro (Yousef et al., 2016; Figure 1b). Additionally, it has been shown that sperm dose- and time-dependently stimulated BOECs to secrete PGE2 (Kodithuwakku et al., 2007) which in turn suppressed the expression of pro-inflammatory cytokines (TNFα and IL1B) in BOECs in vitro (Yousef et al., 2016). These findings suggest that under physiological conditions, the oviduct mucosal epithelium provides a strong and stable anti-inflammatory environment and the sperm–epithelium binding further strengthens this milieu.
Likewise, recent investigations clearly demonstrated that the oviduct epithelium is capable of regulating the immunological activity and phagocytic behavior of PMNs normally present in the bovine oviductal fluid during the pre-ovulatory stage (Marey et al., 2014). Further, the BOEC media changed PMNs gene expressions in vitro, such as a decrease in TNFα and an increase in PGES, but the conditioned media after BOEC–sperm binding induced mRNA expressions of anti-inflammatory cytokines (IL-10 and TGFB1) in PMNs (Yousef et al., 2016). Furthermore, a stimulation of PMNs with the BOEC-conditioned medium resulted in suppression of their phagocytic activity for sperm (Marey et al., 2014). The SEM analysis demonstrated that LH-stimulated BOEC-conditioned medium completely blocked NETs formation for sperm entanglement (Marey et al., 2014). In this study, LH enhanced BOECs to release PGE2 which can suppress the phagocytic activity of PMNs for sperm.
BOECs secrete the vasoactive peptides, angiotensin II (ANG II) and endothelin-1 (EDN-1), which are involved in regulating the oviduct contractile activity (Wijayagunawardane et al., 2001a, 2001b). Interestingly, Ang II up-regulated the phagocytic activity and superoxide production of PMNs against sperm (Marey et al., 2016c), whereas EDN-1 suppressed them (Marey et al., 2016b). Therefore, it was suggested that the endothelin-angiotensin-PGE2 system is involved in the fine regulation of sperm survival in the bovine oviduct (Marey et al., 2016a). This means that during the preovulatory stage, sperm induce their own protection from the maternal immune response and PMNs attack and elicit a state of immune tolerance against these allogeneic paternal cells in the oviduct. Most recently, a new visual evidence confirmed that a BOEC conditioned medium clearly suppressed PMNs phagocytosis of sperm but not of bacteria such as Escherichia coli (Marey et al., 2019a). All these findings prompted us to state that the bovine oviduct has a highly specialized local immune system that can differentially recognize and therefore efficiently respond to different arrays of foreign entities to which it is exposed.
After ovulation, the luminal surface of the oviduct epithelium is temporarily exposed to follicular fluid (FF; de los Santos et al., 2013). Most recently, the immunological impact of FF on sperm phagocytosis by neutrophils was investigated in the buffalo oviduct in vitro. Pre-incubation of neutrophils with FF collected from the pre-ovulatory follicle-enhanced sperm phagocytosis through the formation of NETs together with hydrogen peroxide production, while the oviductal fluid suppressed this activity. The oviductal environment seems to minimize the inflammatory effect of FF released at the time of ovulation (Yousef et al., 2019). Thus, the oviduct appears to support sperm survival until ovulation in order to enhance fertilization. After ovulation, FF flows into the oviductal lumen and stimulates PMNs phagocytosis of sperm in order to remove excess and remaining sperm after fertilization. These biphasic and contradictory phagocytic activities of oviductal PMNs toward sperm (rescues sperm before ovulation and attacks sperm after ovulation) are complementary, and it is likely that both mechanisms are essential to maintain optimal conditions for fertilization.
This tissue homeostasis and delicate immunological balance in the bovine oviduct can be easily disrupted by various risk factors including those derived from undesirable nutrition and environment, that may negatively affect fertility of dairy cows. Urea, obtained from excessive dietary protein intake in early lactating dairy cows, can influence the expression of some immune-related genes toward Th2 type and prostaglandin E2 secretion in vitro, leading to disruption in the local environment for fertilization and early embryonic development (Kowsar et al., 2016). Zearalenone (ZEN) is a non-steroidal estrogenic Fusarium mycotoxin, found in contaminated foods (Placinta et al., 1999). ZEN can reach the bovine oviduct via FF or the blood circulation (Winkler et al., 2015) and consequently may disturb the immunological environment of the oviduct during the peri-ovulatory stage. ZEN induced the expression of the pro-inflammatory cytokines, TNFα and IL-1B, as well as the chemokine, IL-8, in BOECs in a dose-dependent manner. Most importantly, the addition of ZEN into sperm–BOEC co-culture disrupted the typical responses of BOECs to sperm through suppressing the up-regulation of PGES and the down-regulation of IL-1B in BOECs generated by sperm (Yousef et al., 2017). All these findings reveal a complex regulation of the local innate immune system of the bovine oviduct through the expressions of pro- and anti-inflammatory cytokines when exposed to the different physiological and patho-physiological conditions.
Conclusion
Accumulating information provides compelling evidence that the bovine uterus and oviduct possess a well-developed innate immune system that can efficiently recognize, differentiate, and react to pathogens and sperm signals. It is evident that the sperm–uterine crosstalk generates Th1 immune responses which are required to remove excess and dead sperm as well as invading pathogens during insemination. Meanwhile, the sperm–oviduct interaction generates Th2 immune responses which support sperm survival until fertilization. Together, it can be concluded that the sperm sensing system generating necessary maternal immune crosstalk (attack or tolerance) is strictly regulated based on the local homeostatic balance of Th1/Th2 in the uterus and oviduct (Figure 2). This immune balance could be easily disrupted by various risk factors including those derived from undesirable nutrition and environment, and thus may damage reproductive performances of dairy cows. Together, the maintenance of this immune balance is required for fertilization and subsequent pregnancy to proceed. Clearly further investigations are needed for better understanding of the molecular mechanisms behind various deleterious factors that threaten the maternal immune homeostasis and thus interfere with fertility.
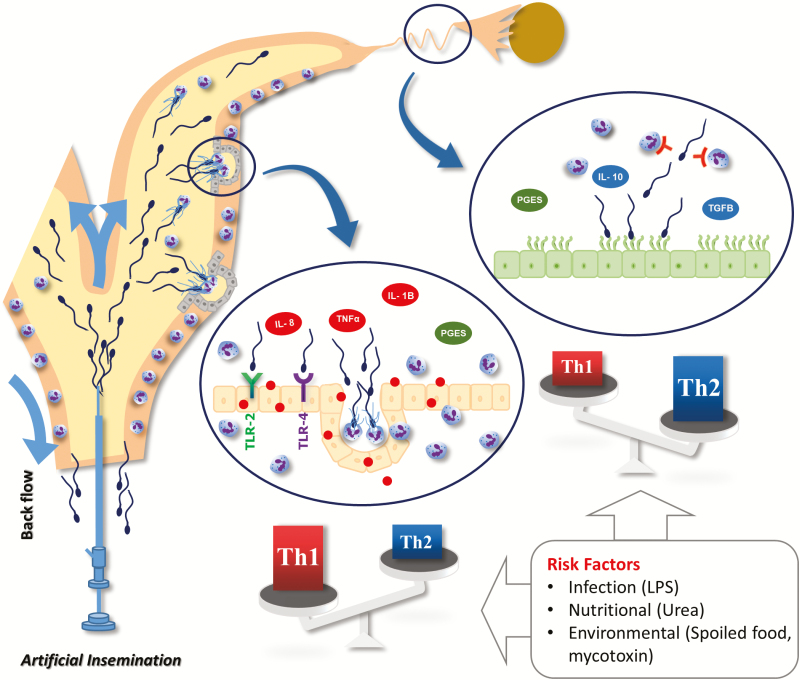
Figure 2.
A schematic representation of the impact of bovine sperm interaction with the uterine and oviductal mucosa for physiological changes in the local immunity during the period from insemination until ovulation. Once active sperm arrive the uterine milieu, they glided over the endometrial surface epithelium until they encountered and entered uterine glands and consequently induced PMNs migration probably from the endometrial stroma. The sperm–uterine interaction induces pro-inflammatory responses through inducing the mRNA expressions of IL-8, TNFα, IL-1B, and PGES. In the oviduct during the pre-ovulatory stage, the sperm–BOEC binding stimulates epithelial cells to secrete high levels of PGE2 that strongly suppresses the PMN phagocytic behavior to sperm. Moreover, the sperm–BOEC binding induces the mRNA expressions of major anti-inflammatory cytokines, IL-10, and TGFB1 in BOEC. Overall, the sperm–uterine crosstalk induces pro-inflammatory responses which are required to remove excess and dead sperm as well as invading pathogens during insemination. Meanwhile, the sperm–BOEC binding induces an anti-inflammatory immune environment and suppresses PMN phagocytosis for sperm and induce a state of immune-tolerance that supports sperm survival until fertilization. This homeostatic balance can be easily disrupted by various risk factors such as infection (LPS), nutritional stress (urea), or environmental factors that induce food spoilage and contamination by mycotoxins.
Funding
This work was supported by Grant-in Aid for Scientific Research (16H05013, 17F17407, and 18K19259) of Japan Society for the Promotion of Science (JSPS), Kieikai Research Foundation (2017C015 and 2019C055), and in part by Livestock Promotional Funds of Japan Racing Association (JRA). Mohamed Ali Marey received a post-doctoral fellowship from JSPS.
Conflict of Interest Statement
The authors declare that there is no conflict of interest that could be preserved as prejudicing the impartiality of the research reported.
Literature Cited
- Akthar I., Suarez S., Morillo V., Sasaki M., Ezz M., Takahashi K., Shimada M., Marey M. A., and Miyamoto A.. 2019. Sperm enter glands of preovulatory bovine endometrial explants and initiate inflammation. Reproduction 159:181–192. doi: 10.1530/REP-19-0414 [DOI] [PubMed] [Google Scholar]
- Alghamdi A. S., and Foster D. N.. 2005. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol. Reprod. 73:1174–1181. doi: 10.1095/biolreprod.105.045666 [DOI] [PubMed] [Google Scholar]
- Alghamdi A. S., Lovaas B. J., Bird S. L., Lamb G. C., Rendahl A. K., Taube P. C., and Foster D. N.. 2009. Species-specific interaction of seminal plasma on sperm-neutrophil binding. Anim. Reprod. Sci. 114:331–344. doi: 10.1016/j.anireprosci.2008.10.015 [DOI] [PubMed] [Google Scholar]
- Bauersachs S., and Wolf E.. 2013. Immune aspects of embryo-maternal cross-talk in the bovine uterus. J. Reprod. Immunol. 97:20–26. doi: 10.1016/j.jri.2012.11.002 [DOI] [PubMed] [Google Scholar]
- Bourke D. A., and Lindsay F. E. F.. 1988. Uterine contractions in the cow and associated movement of inseminate and other substances. 11th International Congress on Animal Reproducion, June 26 to 30, 1988; Dublin, Ireland.
- Davies D., Meade K. G., Herath S., Eckersall P. D., Gonzalez D., White J. O., Conlan R. S., O’Farrelly C., and Sheldon I. M.. 2008. Toll-like receptor and antimicrobial peptide expression in the bovine endometrium. Reprod. Biol. Endocrinol. 6:53. doi: 10.1186/1477-7827-6-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de los Santos M. J., García-Laez V., Beltrán D., Labarta E., Zuzuarregui J. L., Alamá P., Gámiz P., Crespo J., Bosch E., and Pellicer A.. 2013. The follicular hormonal profile in low-responder patients undergoing unstimulated cycles: is it hypoandrogenic? Hum. Reprod. 28:224–229. doi: 10.1093/humrep/des349 [DOI] [PubMed] [Google Scholar]
- de Moraes A. A., Paula-Lopes F. F., Chegini N., and Hansen P. J.. 1999. Localization of granulocyte-macrophage colony-stimulating factor in the bovine reproductive tract. J. Reprod. Immunol. 42:135–145. doi: 10.1016/s0165-0378(98)00075-8 [DOI] [PubMed] [Google Scholar]
- Del Vecchio R. P., Matsas D. J., Fortin S., Sponenberg D. P., and Lewis G. S.. 1994. Spontaneous uterine infections are associated with elevated prostaglandin F(2)alpha metabolite concentrations in postpartum dairy cows. Theriogenology 41:413–421. doi: 10.1016/0093-691x(94)90077-v [DOI] [PubMed] [Google Scholar]
- Ellington J. E. 1991. The bovine oviduct and its role in reproduction: a review of the literature. Cornell Vet. 81:313–328. [PubMed] [Google Scholar]
- Elweza A. E., Ezz M. A., Acosta T. J., Talukder A. K., Shimizu T., Hayakawa H., Shimada M., Imakawa K., Zaghloul A. H., and Miyamoto A.. 2018. A proinflammatory response of bovine endometrial epithelial cells to active sperm in vitro. Mol. Reprod. Dev. 85:215–226. doi: 10.1002/mrd.22955 [DOI] [PubMed] [Google Scholar]
- Ezz M. A., Marey M. A., Elweza A. E., Kawai T., Heppelmann M., Pfarrer C., Balboula A. Z., Montaser A., Imakawa K., Zaabel S. M., et al. 2019. TLR2/4 signaling pathway mediates sperm-induced inflammation in bovine endometrial epithelial cells in vitro. Plos One 14:e0214516. doi: 10.1371/journal.pone.0214516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Liu B., Feng X., Liu Z., Liang D., Li F., Li D., Cao Y., Feng S., Zhang X., et al. 2013. Lipopolysaccharide increases Toll-like receptor 4 and downstream Toll-like receptor signaling molecules expression in bovine endometrial epithelial cells. Vet. Immunol. Immunopathol. 151(1–2):20–27. doi: 10.1016/j.vetimm.2012.09.039 [DOI] [PubMed] [Google Scholar]
- Gaunt S. J. 1983. Spreading of a sperm surface antigen within the plasma membrane of the egg after fertilization in the rat. J. Embryol. Exp. Morphol. 75:259–270. [PubMed] [Google Scholar]
- Hawk H. W. 1987. Transport and fate of spermatozoa after insemination of cattle. J. Dairy Sci. 70:1487–1503. doi: 10.3168/jds.S0022-0302(87)80173-X [DOI] [PubMed] [Google Scholar]
- Howe G. R., and Black D. L.. 1963. Spermatozoan transport and leucocytic responses in the reproductive tract of calves. J. Reprod. Fertil. 6:305–311. doi: 10.1530/jrf.0.0060305 [DOI] [PubMed] [Google Scholar]
- Hunter R. H. 2012. Components of oviduct physiology in eutherian mammals. Biol. Rev. Camb. Philos. Soc. 87:244–255. doi: 10.1111/j.1469-185X.2011.00196.x [DOI] [PubMed] [Google Scholar]
- Ibrahim S., Salilew-Wondim D., Rings F., Hoelker M., Neuhoff C., Tholen E., Looft C., Schellander K., and Tesfaye D.. 2015. Expression pattern of inflammatory response genes and their regulatory micrornas in bovine oviductal cells in response to lipopolysaccharide: implication for early embryonic development. Plos One 10:e0119388. doi: 10.1371/journal.pone.0119388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G., Marin V., Montero-Julian F., Mantovani A., and Farnarier C.. 2003. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24:25–29. doi: 10.1016/s1471-4906(02)00013-3 [DOI] [PubMed] [Google Scholar]
- Katila T. 2012. Post-mating inflammatory responses of the uterus. Reprod. Domest. Anim. 47Suppl 5:31–41. doi: 10.1111/j.1439-0531.2012.02120.x [DOI] [PubMed] [Google Scholar]
- Katila T., Sankari S., and Makela O.. 2000. Transport of spermatozoa in the reproductive tracts of mares. J. Reprod. Fertil. Suppl. 56:571–578. [PubMed] [Google Scholar]
- Kodithuwakku S. P., Miyamoto A., and Wijayagunawardane M. P.. 2007. Spermatozoa stimulate prostaglandin synthesis and secretion in bovine oviductal epithelial cells. Reproduction 133:1087–1094. doi: 10.1530/REP-06-0201 [DOI] [PubMed] [Google Scholar]
- Kowsar R., Hambruch N., Liu J., Shimizu T., Pfarrer C., and Miyamoto A.. 2013. Regulation of innate immune function in bovine oviduct epithelial cells in culture: the homeostatic role of epithelial cells in balancing Th1/Th2 response. J. Reprod. Dev. 59:470–478. doi: 10.1262/jrd.2013-036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowsar R., Hambruch N., Marey M. A., Liu J., Shimizu T., Pfarrer C., and Miyamoto A.. 2014. Evidence for a novel, local acute-phase response in the bovine oviduct: progesterone and lipopolysaccharide up-regulate alpha 1-acid-glycoprotein expression in epithelial cells in vitro. Mol. Reprod. Dev. 81:861–870. doi: 10.1002/mrd.22355 [DOI] [PubMed] [Google Scholar]
- Kowsar R., Marey M. A., Shimizu T., and Miyamoto A.. 2016. Short communication: urea induces T helper 2 (Th2) type environment at transcriptional level and prostaglandin E2 secretion in bovine oviduct epithelial cells in culture. J. Dairy Sci. 99:5844–5850. doi: 10.3168/jds.2016-10874 [DOI] [PubMed] [Google Scholar]
- Lander M. F., Hansen P. J., and Drost M.. 1990. Antisperm antibodies in cows after subcutaneous and intra-uterine immunisation. Vet. Rec. 126:461–462. [PubMed] [Google Scholar]
- Lecchi C., Avallone G., Giurovich M., Roccabianca P., and Ceciliani F.. 2009. Extra hepatic expression of the acute phase protein alpha 1-acid glycoprotein in normal bovine tissues. Vet. J. 180:256–258. doi: 10.1016/j.tvjl.2007.12.027 [DOI] [PubMed] [Google Scholar]
- Lewis G. S. 2003. Steroidal regulation of uterine resistance to bacterial infection in livestock. Reprod. Biol. Endocrinol. 1:117. doi: 10.1186/1477-7827-1-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh S. B., Schuberth H. J., Healy L. L., and Sheldon I. M.. 2013. Polarised bovine endometrial epithelial cells vectorially secrete prostaglandins and chemotactic factors under physiological and pathological conditions. Reproduction 145:57–72. doi: 10.1530/REP-12-0253 [DOI] [PubMed] [Google Scholar]
- Madill S., Troedsson M. H., Alexander S. L., Shand N., Santschi E. M., and Irvine C. H.. 2000. Simultaneous recording of pituitary oxytocin secretion and myometrial activity in oestrous mares exposed to various breeding stimuli. J. Reprod. Fertil. Suppl. 56:351–361. [PubMed] [Google Scholar]
- Marey M. A., Liu J., Kowsar R., Haneda S., Matsui M., Sasaki M., Takashi S., Hayakawa H., Wijayagunawardane M. P., Hussein F. M., et al. 2014. Bovine oviduct epithelial cells downregulate phagocytosis of sperm by neutrophils: prostaglandin E2 as a major physiological regulator. Reproduction 147:211–219. doi: 10.1530/REP-13-0375 [DOI] [PubMed] [Google Scholar]
- Marey M.A., Matsukawa H., Sasaki M., Ezz M. A., Yousef M. S., Takahashi K., and Miyamoto A.. 2019a. Bovine oviduct epithelial cells suppress the phagocytic activity of neutrophils towards sperm but not for bacteria in vitro: immunofluorescence and electron microscopic observations. Histol Histopathol 17:18172. doi: 10.14670/HH-18-172 [DOI] [PubMed] [Google Scholar]
- Marey M. A., Yoshino H., Elesh I., Moriyasu S., and Miyamoto A.. 2019b. Real-time investigation in vivo of sperm and neutrophils distribution in the bovine uterus after artificial insemination. The 112th Meeting of the Society for Reproduction and Development September 2 to 5, 2019; Japan.
- Marey M. A., Yousef M. S., Kowsar R., Hambruch N., Shimizu T., Pfarrer C., and Miyamoto A.. 2016a. Local immune system in oviduct physiology and pathophysiology: attack or tolerance? Domest. Anim. Endocrinol. 56Suppl:S204–S211. doi: 10.1016/j.domaniend.2016.02.005 [DOI] [PubMed] [Google Scholar]
- Marey M. A., Yousef M. S., Liu J., Morita K., Sasaki M., Hayakawa H., Shimizu T., Elshahawy I. I., and Miyamoto A.. 2016b. Endothelin-1 downregulates sperm phagocytosis by neutrophils in vitro: a physiological implication in bovine oviduct immunity. J. Reprod. Dev. 62:151–157. doi: 10.1262/jrd.2015-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marey M. A., Yousef M. S., Liu J., Morita K., Sasaki M., Hayakawa H., Shimizu T., and Miyamoto A.. 2016c. Angiotensin II increases sperm phagocytosis by neutrophils in vitro: a possible physiological role in the bovine oviduct. Mol. Reprod. Dev. 83:630–639. doi: 10.1002/mrd.22672 [DOI] [PubMed] [Google Scholar]
- Mitchell J. R., Senger P. L., and Rosenberger J. L.. 1985. Distribution and retention of spermatozoa with acrosomal and nuclear abnormalities in the cow genital tract. J. Anim. Sci. 61:956–967. doi: 10.2527/jas1985.614956x [DOI] [PubMed] [Google Scholar]
- Ohtani S., Okuda K., Nishimura K., and Mohri S.. 1993. Histological changes in bovine endometrium during the estrous cycle. Theriogenology 39:1033–1042. doi: 10.1016/0093-691x(93)90004-o [DOI] [PubMed] [Google Scholar]
- Placinta C. M., Felix D’mello J. P., and Macdonald A. M. C.. 1999. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 78:21–37. doi: 10.1016/S0377-8401(98)00278-8 [DOI] [Google Scholar]
- Rath D., Schuberth H. J., Coy P., and Taylor U.. 2008. Sperm interactions from insemination to fertilization. Reprod. Domest. Anim. 43Suppl 5:2–11. doi: 10.1111/j.1439-0531.2008.01250.x [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez H., Nicander L., Viring S., Einarsson S., and Larsson K.. 1990. Ultrastructure of the uterotubal junction in preovulatory pigs. Anat. Histol. Embryol. 19:16–36. doi: 10.1111/j.1439-0264.1990.tb00875.x [DOI] [PubMed] [Google Scholar]
- Rowson L. E., Lamming G. E., and Fry R. M.. 1953. Influence of ovarian hormones on uterine infection. Nature 171:749–750. doi: 10.1038/171749a0 [DOI] [PubMed] [Google Scholar]
- Sheldon I. M., Noakes D. E., Rycroft A., and Dobson H.. 2001. Acute phase protein response to postpartum uterine bacterial contamination in cattle. Veterinary Record 148:172–175. [DOI] [PubMed] [Google Scholar]
- Sostaric E., Dieleman S. J., van de Lest C. H., Colenbrander B., Vos P. L., Garcia-Gil N., and Gadella B. M.. 2008. Sperm binding properties and secretory activity of the bovine oviduct immediately before and after ovulation. Mol. Reprod. Dev. 75:60–74. doi: 10.1002/mrd.20766 [DOI] [PubMed] [Google Scholar]
- Sumransap P., Tummaruk P., and Kunavongkrit A.. 2007. Sperm distribution in the reproductive tract of sows after intrauterine insemination. Reprod. Domest. Anim. 42:113–117. doi: 10.1111/j.1439-0531.2006.00696.x [DOI] [PubMed] [Google Scholar]
- Swangchan-Uthai T., Lavender C. R., Cheng Z., Fouladi-Nashta A. A., and Wathes D. C.. 2012. Time course of defense mechanisms in bovine endometrium in response to lipopolysaccharide. Biol. Reprod. 87:135. doi: 10.1095/biolreprod.112.102376 [DOI] [PubMed] [Google Scholar]
- Tapper H. 1996. The secretion of preformed granules by macrophages and neutrophils. J. Leukoc. Biol. 59:613–622. doi: 10.1002/jlb.59.5.613 [DOI] [PubMed] [Google Scholar]
- Tizard I. R. 1996. Veterinary immunology. An introduction. Philadelphia (PA): WB Saunders Company. [Google Scholar]
- Wijayagunawardane M. P., Miyamoto A., Taquahashi Y., Acosta T. J., Nishimura M., and Sato K.. 2001a. Angiotensin II and atrial natriuretic peptide in the cow oviductal contraction in vitro: direct effect and local secretion of prostaglandins, endothelin-1, and angiotensin II. Biol. Reprod. 65:799–804. doi: 10.1095/biolreprod65.3.799 [DOI] [PubMed] [Google Scholar]
- Wijayagunawardane M. P., Miyamoto A., Taquahashi Y., Gabler C., Acosta T. J., Nishimura M., Killian G., and Sato K.. 2001b. In vitro regulation of local secretion and contraction of the bovine oviduct: stimulation by luteinizing hormone, endothelin-1 and prostaglandins, and inhibition by oxytocin. J. Endocrinol. 168:117–130. doi: 10.1677/joe.0.1680117 [DOI] [PubMed] [Google Scholar]
- Wilmut I., and Hunter R. H.. 1984. Sperm transport into the oviducts of heifers mated early in oestrus. Reprod. Nutr. Dev. 24:461–468. doi: 10.1051/rnd:19840411 [DOI] [PubMed] [Google Scholar]
- Winkler J., Kersten S., Meyer U., Stinshoff H., Locher L., Rehage J., Wrenzycki C., Engelhardt U. H., and Dänicke S.. 2015. Diagnostic opportunities for evaluation of the exposure of dairy cows to the mycotoxins deoxynivalenol (DON) and zearalenone (ZEN): reliability of blood plasma, bile and follicular fluid as indicators. J. Anim. Physiol. Anim. Nutr. (Berl). 99:847–855. doi: 10.1111/jpn.12285 [DOI] [PubMed] [Google Scholar]
- Yániz J. L., Lopez-Gatius F., Santolaria P., and Mullins K. J.. 2000. Study of the functional anatomy of bovine oviductal mucosa. Anat. Rec. 260:268–278. doi: [DOI] [PubMed] [Google Scholar]
- Yousef M. S., Abd-Elhafeez H. H., Talukder A. K., and Miyamoto A.. 2019. Ovulatory follicular fluid induces sperm phagocytosis by neutrophils, but oviductal fluid around oestrus suppresses its inflammatory effect in the buffalo oviduct in vitro. Mol. Reprod. Dev. 86:835–846. doi: 10.1002/mrd.23164 [DOI] [PubMed] [Google Scholar]
- Yousef M. S., Marey M. A., Hambruch N., Hayakawa H., Shimizu T., Hussien H. A., Abdel-Razek A. K., Pfarrer C., and Miyamoto A.. 2016. Sperm binding to oviduct epithelial cells enhances TGFB1 and IL10 expressions in epithelial cells as well as neutrophils in vitro: prostaglandin E2 as a main regulator of anti-inflammatory response in the bovine oviduct. Plos One 11:e0162309. doi: 10.1371/journal.pone.0162309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef M. S., Takagi M., Talukder A. K., Marey M. A., Kowsar R., Abdel-Razek A. K., Shimizu T., Fink-Gremmels J., and Miyamoto A.. 2017. Zearalenone (ZEN) disrupts the anti-inflammatory response of bovine oviductal epithelial cells to sperm in vitro. Reprod. Toxicol. 74:158–163. doi: 10.1016/j.reprotox.2017.09.012 [DOI] [PubMed] [Google Scholar]
- Zerbe H., Schuberth H. J., Engelke F., Frank J., Klug E., and Leibold W.. 2003. Development and comparison of in vivo and in vitro models for endometritis in cows and mares. Theriogenology 60:209–223. doi: 10.1016/s0093-691x(02)01376-6 [DOI] [PubMed] [Google Scholar]