Introduction
Colostrum is a vital component to the raising of nearly all mammalian newborns, especially those reared domestically for agricultural purposes. Colostrum contains multiple immunoglobulins (Ig; IgA, IgM, IgG, etc.), with the most abundant Ig in colostrum generally being IgG. The various Ig molecules serve different purposes within the neonate. Immunoglobulin M is primarily made during the primary immune response and is usually found in higher quantities than IgA, which congregates on epithelial surfaces and is found in high content in saliva (Tizard, 2013). Immunoglobulin G, a large globular protein with a molecular weight of roughly 150 kDa, is the most commonly discussed Ig in calf-raising and is measured in calf serum around 24-h post-colostrum feeding to evaluate passive transfer of immunity (PTI). Passive transfer of immunity rates on a given operation are a critical benchmark to determine how well colostrum is managed. Producers, veterinarians, and consultants must understand how PTI is achieved to ensure this critical objective is achieved consistently by calves on farm. The following review is intended to help summarize the understanding of how PTI is achieved, and what factors in maternal colostrum (MC) influence PTI.
Passive Transfer of Immunity
Placental transfer of Ig does not occur in many mammalian species, including cattle (Tizard, 2013). Therefore, newborn calves must receive immunity from their dams via transfer of Ig, specifically IgG, from colostrum. This intake of a large mass of colostral IgG in the few hours shortly after birth assures calves receive PTI, which generally last for 2–3 wk (Heinrichs and Elizondo-Salazar, 2009), until the calf’s active immunity can take over. Sufficient PTI is assumed as a serum IgG content 24-h post-feeding of 10 g IgG/liter of serum (Quigley, 2002). When measuring PTI, IgG is generally used as IgG makes up roughly 90% of the Ig present in MC (Godden et al., 2009). It should be mentioned that two isotypes of IgG exist in maternal, bovine colostrum: IgG1 and IgG2. Immunoglobulin G1 exists in a much higher quantity in MC than IgG2. Immunoglobulin G1 and IgG2 are absorbed in the small intestine of the calf, via nonselective pinocytosis (Heinrichs and Elizondo-Salazar, 2009). The difference between IgG1 and IgG2 is that IgG1 is resecreted back into the lumen of the gastro-intestinal tract to provide local immunity at the gut level, whereas IgG2 is not resecreted (Godden et al., 2009). Maternal colostrum and colostrum replacers (CR) would contain similar ratios of IgG1:IgG2 (roughly 95% vs. 5%), with the exception of CR based off of animal plasma, where the ratio of IgG1:IgG2 is closer to 50:50 (Godden et al., 2009).
As of 2011, 100% of U.S. dairy operations were feeding colostrum to calves, with 64.3% of farms feeding colostrum originating from their dairy, and 53.8% of farms also utilizing a colostrum replacer. However, only 40.3% of all operations were monitoring serum IgG in calves post-colostrum feeding (NAHMS, 2011). Monitoring serum IgG for PTI rates can be cumbersome, as serum IgG is not easily measured on farm and often must be sent to a lab for analysis via radial immunodiffusion or ELISA. Therefore, almost every farm would measure serum total protein (STP), which is known to correlate with serum IgG. It is generally accepted that a value of 5.5 g/dL for STP equals 10 g IgG/liter of serum (McGuirk and Collins, 2004). Because of this correlation, benchmarks for PTI on farm are also created using STP thresholds (i.e. 95% of calves with STP > 5.2 g/dL and 90% of calves with STP > 5.5 g/dL). Serum total protein can be measured on farm using an optical refractometer or a brix refractometer. Refractometers are a useful tool to estimate PTI on farm for a group of calves, but it should be noted, however, that it is merely an estimation. Refractometers do not directly measure IgG, and, therefore, should be used more as a general indicator of calf and colostrum management on farm, and not directly used to assume passive transfer for a given calf. When in doubt, serum samples can always be sent to a lab for IgG determination. Brix refractometers can be optical or digital. Digital brix refractometers are generally more expensive, but more durable. In the author’s opinion, if affordable, a digital brix refractometer is preferred due to its durability and versatility. It should be noted, however, that not all brix refractometers directly measure STP. Some brix refractometers will only provide a brix value (%), and the cut-points to measure PTI using a brix cut-point are still a subject of debate in the industry. Regardless, benchmarking PTI on farm is critical to the success of an operation. Data indicate an increase in death loss of roughly 5% can be expected in calves that do not achieve PTI (Donovan et al., 1998; Virtala et al., 1999). Additionally, long-lasting benefits have been observed for calves fed greater quantities of colostrum in a number of studies (Kühne et al., 2000; Hammon et al., 2002; Faber et al., 2005). For example, Faber et al. (2005) fed Brown Swiss calves either 2 or 4 liters of colostrum at birth. All calves were managed similarly from that point forward. Calves fed 4 liters of colostrum at birth compared with 2 liters of colostrum produced 2,607 kg more milk through 2 lactations (21,201 vs. 18,594 kg of 305-d mature equivalent milk; P < 0.05). Achieving PTI consistently on farm should be a high priority to all dairy producers. However, achieving PTI is not always easy, as factors associated with MC may make it difficult.
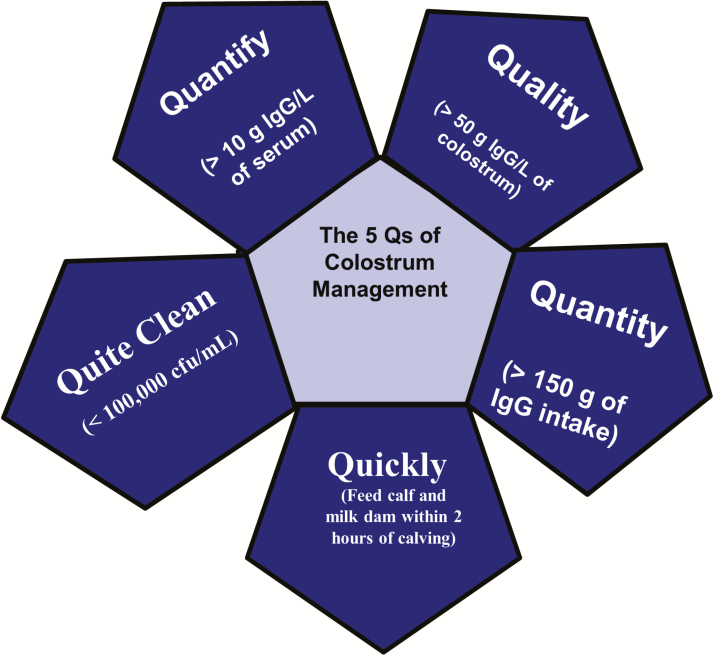
When Maternal Colostrum Falls Short: The 5 Q’s of Colostrum Management
Maternal colostrum is often called “nature’s perfect food” for good reason. Maternal colostrum can have nearly double the fat content of whole milk, greater than 4× the protein content, and contains well over 250× the Ig content of mature milk (Blum and Hammon, 2000). However, MC is not always perfect. This may be due to cow or management factors, but it is important to know the reasons which cause MC to fall short. It is common to assess MC using 3 to 5 criteria which help to determine its acceptability as a feedstuff for newborn calves. For the sake of this review, this will be discussed as the 5 Q’s of Colostrum Management: Quality, Quantity, Quickness, Quite Clean, and Quantification (Figure 1).
Figure 1.
The 5 Q’s of colostrum management. The 5 Q’s consist of 1) Quality (>50 g of Immunoglobulin G (IgG)/liter of maternal colostrum), 2) Quantity (>150 g IgG fed per calf immediately after birth), 3) Quickness (feed calves and milk dam within 2 h of calving), 4) Quite Clean (maternal colostrum should have a total bacteria count <100,000 cfu/mL and a total coliform count <10,000 cfu/mL), and 5) Quantification (at 24 h of life, serum IgG in calves should be >10 g of IgG/liter of serum).
Quality
High quality colostrum is a term that is used often but rarely given meaning or context. It is generally accepted that “good” quality colostrum contains 50 g IgG/liter of colostrum, and, therefore, quality of colostrum is often assumed to mean a certain content of IgG. However, not all cows will produce good quality colostrum by this definition. A survey conducted across multiple farms in the state of Pennsylvania in the United States found that the average quality of colostrum across farms was 41 g/liter (lower than the threshold of 50 g/liter). What was really telling, however, was the range of colostrum in that study, which ranged from close to 10 g/liter to nearly 100 g/liter (Kehoe et al., 2007). A second study was conducted on a single large farm in the United States. This farm had an average colostral IgG content of 71.7 g/liter, but the range told the same story as the Pennsylvania study, ranging from close to 10 g/liter to much greater than 100 g/liter (Godden, 2008). Therefore, not only does colostrum quality vary across farms, it also varies within farm. The objective of dairy producers should be to determine colostrum quality in order to ensure that colostrum with a quality of less than 50 g/liter is not fed to any calf. Colostrometers measure colostrum quality using specific gravity and are therefore impacted by temperature of the MC. Additionally, dry matter content of colostrum impacts the reading given by a colostrometer. Colostrum with a higher solids content or more fat will yield a higher specific gravity reading. When using a colostrometer, colostrum should be measured at temperatures around 20–21 °C. Brix refractometers are not temperature dependent and are, therefore, preferred.
The question then often arises: “What impacts colostrum quality?” In general, there are not a lot of factors that can improve colostrum quality to a large degree, but there are some factors that may have minor impacts. In general, dietary modifications in the dry period (specifically the close-up period, i.e. day 30 to day 1 pre-calving) have little impact if metabolizable protein (1,100–1,300 g/d) and metabolizable energy requirements of the cow are met (Davis and Drackley, 1998). However, trace mineral supplementation has been shown to have an impact on colostrum quality. Kincaid and Socha (2004) supplemented cows beginning 21-d prepartum with 360 mg Zn, 200 mg Mn, 125 mg Cu, and 25 mg Co. Compared with control cows supplemented with similar levels of inorganic minerals, cows supplemented with the amino acid complexed minerals produced colostrum with 32.3% greater IgG content (P < 0.05). Similarly, researchers in Poland (Kinal et al., 2005) supplemented cows with 315 mg Zn, 315 mg Mn, and 63 mg Cu beginning 6 wk pre-partum with amino acid complexed minerals. Compared with inorganic minerals, cows fed the treatment diet produced colostrum with 26.6% greater IgG content. Aside from nutrition modifications to the close-up dry cow diet, it has been hypothesized that other factors may play a role in colostrum quality. Limiting stress (pen movements, heat stress, etc.) has been hypothesized as a tactic to ameliorate any reductions of colostral IgG that may occur in the dry period (Nardone et al., 1997). Excessive dry period length modifications have also been proposed (i.e. dry period length of <30 d or >90 d; Annen, 2004). Allowing calves to suckle their dams (Edwards and Broom, 1979; Morin et al., 1997) as well as delays in harvesting of MC have also been evaluated although the first does not truly decrease colostrum quality (Moore et al., 2005), and the second is not the fault of the cow. Perhaps most commonly discussed is the impact of parity and breed. Many believe that Jersey cows to produce higher quality colostrum than Holsteins, however, based on a recent U.S. survey, that appears to no longer be accurate (Morrill et al., 2012). Data also indicate that multiparous cows produce greater quality colostrum than primiparous cows (Morrill et al., 2012). It should never be assumed, however, that just because a cow is primiparous, that her colostrum quality should be low, as more recent data does show a considerable amount of variation and that primiparous cows can produce very high-quality colostrum (Kessler et al., 2014; Gross et al., 2017). Finally, health status of the cow can impact colostrum. Illness during the dry period has been hypothesized to decrease colostrum quality/quantity (Maunsell et al., 1999) from an IgG content standpoint, whereas specific vaccinations in the dry period may alter the antibody profile of colostrum (Kasonta et al., 2014). However, quality of colostrum is not always objective. Colostrum from cows exposed to particular disease or given certain vaccinations will have a different antibody profile while still potentially having the same IgG content (Kasonta et al., 2014), which would result in a higher quality colostrum with the same IgG content. Colostrum antibody profiles that are specific to pathogens on a particular farm would be of high value to the young calf. Therefore, it should never be assumed that colostrum quality is solely a factor of IgG content, but is multi-faceted, with antibody profile (i.e. specificity against certain pathogens) being equally as important as actual IgG content.
Quantity
In many areas of the world, colostrum quantity is defined as liters of colostrum fed to a calf at birth. It should be noted that calves do not have a specific requirement for volume of colostrum, but a requirement for the IgG within the colostrum. A simple equation can be used to determine the amount of colostral IgG required from MC or CR to achieve PTI levels of IgG of 10 g/liter. For example, 9% of the calf’s birthweight is plasma (or serum; Quigley, 2002). Therefore, a calf that is 45 kg at birth has an initial serum volume of 4.05 liters. To achieve 10 g/liter of IgG in the serum, the calf must then see 40.5 g of IgG enter into circulation (4.05 liters × 10 g/liter). However, not all IgG consumed will enter circulation. Instead only part of the IgG will pass into circulation. This percentage is often referred to as the apparent efficiency of absorption (AEA). Apparent efficiency of absorption values in the literature can range from less than 20% to greater than 40%. For the sake of this example, assume an AEA of 25%. To achieve 10 g/liter of IgG circulating in the serum, a 45-kg calf must consume more than 160 g of IgG (assuming AEA of 25%). If we assume colostrum quality to be 50 g/liter that means the calf must consume 3.2 liters of colostrum to consume 160 g of IgG. Thinking about quantity in terms of IgG instead of liquid volume helps to better meet the animals needs and ensure PTI is achieved. A common suggestion is to feed 4 liters of good quality MC to calves as soon as possible after birth. In the above outlined scenario, that would result in a serum IgG of 12.34 g/liter at 24-h of age. A common industry recommendation is to feed 150 to 200 g of IgG shortly after birth. Previous recommendations of 100 g of IgG (Davis and Drackley, 1998) shortly after birth should be used cautiously, especially in larger calves. A common misconception is that when a high volume of colostrum is produced (“the rule of 18-lbs”; Pritchett et al., 1991), colostrum quality is compromised. However, more recent data indicates that this most likely no longer the case (Jardon et al., 1998; Kessler et al., 2014; Gross et al., 2017)
Quickness
Quickness as it relates to colostrum management involves two factors: 1) how quickly the calf is fed, and 2) how quickly the dam is milked. The ability of the calf to absorb IgG (AEA) begins to decline shortly after birth, and this decline is accelerated by the introduction of macromolecules into the gastro-intestinal tract (Davis and Drackley, 1998). Even in near-sterile environments, the gastrointestinal tract of calves is quickly colonized by bacteria or other microorganisms and closure of the gut begins (Alipour et al., 2018). Therefore, it should be of paramount importance to feed colostrum quickly after birth, as even a delay of 1 d can have dramatic impacts on metabolite profiles in the young calf (Blum et a1., 1997). It has been suggested that the AEA in newborn calves can decrease from nearly 50% at birth to less than 30% by 6 h (Quigley et al., 2002).
It is just as important to feed the calf quickly as it is to milk the cow. Recent work (Moore et al., 2005) with fresh cows milked at 2, 6, 10, and 14 h post-calving shows that if milked at 2 h, cows produced colostrum that contained 100% of the IgG available to the calf (these cows were called controls). By 6 h post-calving, only 83.2% of colostral IgG concentration (compared to the 2-h controls) was found and that number decreased to 72.6% by 10 h. It is clear that the ability of the calf to absorb colostral IgG and the quality of MC declines sharply post-calving. Therefore, colostrum feeding on farm should be treated with a sense of urgency.
Quite clean
As mentioned previously, introduction of bacteria into the gut have the ability to accelerate the closing of the gut lining. Additionally, data indicate that as bacterial content of the colostrum increases, serum IgG at 24 h of life decreases (James et al., 1981). This negative correlation has led to the recommendation that colostrum should have a total bacteria count of less than 100,000 cfu/mL and a total coliform count of less than 10,000 cfu/mL (McGuirk and Collins, 2004; Stewart et al., 2005). The understanding that bacteria load in colostrum decreases the likelihood of PTI has led to the adoption of pasteurization of colostrum on many farms globally.
Bacterial contamination can come from a host of sources. Stewart et al. (2005) measured contamination on a variety sources related to colostrum management. These researchers found that if the udder of the cow was properly prepped, very little contamination comes from the udder. Collection and feeding equipment can result in very high levels of colostrum contamination (97,724 and 45,709 cfu/mL from a fresh cow bucket and esophageal feeder, respectively). Of extreme importance was the fact that allowing colostrum to cool in a refrigerator for 24 h resulted in colostrum with a bacteria reading of 562,341 cfu/mL. These findings illustrate the need for well-defined colostrum management protocols and appropriate sanitation practices. Cleanliness of equipment can be measured on farm with equipment such as ATP meters (Renaud et al., 2017), but these machines can be expensive and not an option for all farms. However, regular measurements of colostrum bacterial levels (via a milk processor, for example), can help determine the extent of contamination on a given farm.
Quantification
Quantification is simply the benchmarking of PTI on a given farm. If PTI is not consistently reached on farm, it is more than likely one of the 5 Q’s of colostrum management is not being met and further auditing should be conducted. If meeting all 5 Q’s of colostrum management is a consistent issue, a CR may be appropriate.
Colostrum Replacers
Colostrum replacers are a tool available to producers in many parts of the world. Although often expensive, the strategic use of CR on farm can be economically advantageous. Three main types of CR exist: dried MC, whey-based CR, and plasma-based CR. As mentioned previously, the ratio of IgG1:IgG2 is anticipated to be similar in dried MC and whey-based CR, as both are manufactured from colostrum collected from dairy cows. However, plasma-based CR would have a higher content of IgG2 compared to other CR (Godden et al., 2009). It is important to understand that subtle, yet important differences exist between dried MC and whey-based CR. Dried MC is simply high-quality MC that is dried and guaranteed to meet a certain content of IgG in the dried powder. Whey-based CR, however, is MC that goes through an enzymatic set prior to being dried (casein and fat removed). Because of this, it would be anticipated that for a similar mass of dried MC and whey-based CR, a greater IgG content would exist in whey-based CR (IgG concentrated when casein and fat removed). However, whey-based CR does not contain fat. Although there is no published evidence to indicate that colostral fat is superior to fat from whole milk or milk replacer, calves fed whey-based CR would not receive fat until their second feeding (all necessary IgG fed at first feeding). Although research has indicated the capacity of calves to absorb fatty acids from colostrum within the first 24 h of life (Blum et al., 1997), it is unclear if this results in lasting impacts on the calf, and if a 6–8 h delay in fat feeding (i.e. receiving fat at the second feeding) is biologically significant to the calf. Furthermore, data does not exist on the impact of a short-term delay of fat feeding on the neonatal calf (above study was a 24-h delay), but it should still be a consideration for producers. A recent study using a whey-based CR (Lago et al., 2017) fed 1,220 Jersey and Jersey×Holstein calves either CR or MC. A subset of calves were analyzed for passive transfer, and only one calf from each treatment (of 300 measured) experienced failure of PTI. Total IgG consumed was 150 g for calves fed CR and approximately 180 g for calves fed MC. Furthermore, it was found that bacterial load was lower in CR (897 cfu/mL) compared with heat-treated MC (2,476 cfu/mL).
A recent study utilizing whey-based CR from Pennsylvania State University (Lopez et al., 2020) found that calves fed 150 g of IgG from the CR (40% IgG powder) had 24-h AEA levels of 40.09% compared to high quality (106 g/liter) MC levels of 24.38% (both resulted in PTI in all calves fed). This may be due to a saturation-type mechanism of absorption, but also may be due to the CR being a more concentrated source of IgG lacking casein. Casein would be found in high quantities in MC and dried-MC replacers. Casein is critical in protein absorption in the neonatal calf, as it forms a clot in the presence of chymosin (rennet) in the abomasum of the calf, and results in more prolonged nutrient release (Miyazaki et al., 2019). However, previous data (Davenport et al., 2000) has shown that the addition of casein to CR can actually decrease serum IgG and AEA in calves. This may be due to the fact that the casein clot may trap a portion of IgG in the abomasum (IgG located in the whey fraction of colostrum; Al-Mashikhi and Nakai, 1987), making it unavailable to the calf until a later point when AEA has decreased. This is also an important differentiation between dried MC and whey-based CR and may explain the high levels of AEA observed in the Penn State study. Additionally, a portion of calves in the previous mentioned study were fed a treatment of low-quality MC (41 g IgG/liter) plus an additional 40 g of IgG from a whey-based CR. These calves had an average AEA value of 54.28% (Lopez et al., 2019). This is one of the highest reported AEA value in the literature and the reason for such a high value is not completely understood. However, this data does indicate that supplementing of low-quality colostrum with whey-based CR may be a good strategy to improve PTI rates on farm. This option may not be viable with dried MC due to the associated higher feeding rates. Finally, a current topic of interest is the impact of other factors associated with colostrum. Although not the topic of this review, how these factors are present in various CR is of interest. Whey-based factors such as lactoferrin and insulin-like growth factor I would be anticipated to be higher in whey-based CR, but more research is needed to confirm.
There are a host of features associated with various CR that may make them a desirable option. Aside from the differences between CR, CR may complement MC in a colostrum management program as they help to eliminate the variability associated with the 5 Q’s of colostrum management: 1) Quality of a CR should be guaranteed in each bag, 2) A CR should be mixed with a defined amount of water making quantity consistent, 3) CR is not dependent on milk time of the dam and does not need to pasteurized/thawed/warmed, 4) CR should have bacterial guarantees and come into contact with less equipment on farm that can cause contamination, and 5) Greater consistency associated with a CR may result in more consistent PTI results on farm.
Prolonged Feeding of Colostrum
Recently, much emphasis has been placed on the concept of feeding “transition milk” to calves. This concept arises from the fact that colostrum does not become mature milk immediately, but rather, over the course of roughly 1 wk. This transition results in a nutritious food that is higher in fat and protein (and Ig) than whole milk. As continued public pressure encourages producers to find alternatives to antibiotics on farm, an opportunity may exist for producers to feed this source of nutrients to calves to potentially help improve animal health and performance. It is clear that more research is needed on this topic moving forward.
The idea associated with transition milk feeding is to provide Ig at the local gut level (Geiger et al., 2019a, b). Although some Ig will still enter into circulation, due to the rapid decline in AEA in the days after birth, a majority of IgG will have a local impact in the gastrointestinal tract. A majority of work associated with transition milk has been done with CR, to improve treatment consistency. Early work (Blättler et al., 2001) supplemented calves with varying diets for the first week of life. Calves were either supplemented with 1st milking MC for 3 d before being transitioned to milk replacer by day 8, supplemented milking directly correlated to their dam’s production (i.e. 1st and 2nd milkings on day 1, 3rd and 4th milkings on day 2, 5th and 6th milkings on day 3, milk replacer on days 4 through 7), or a formula diet lacking Ig on days 1 through 3 followed by milk replacer on days 4 through 7. Calves fed 1st milking MC for the first 3 d before transitioning to milk replacer over the next 4 d had improved development of the duodenal villi (villi circumference, villi area, villi height, and villi height:crypt depth). Since, similar studies have shown positive impacts on growth, and nutrient availability and metabolism when colostrum is fed post-gut closure as opposed to milk replacer (Kühne et al., 2000; Hammon et al., 2002). Subsequently, Berge et al. (2009) fed calves a control milk replacer diet, a milk replacer diet supplemented with 20 g IgG/d (140 g of dried MC CR powder/d), or a nutritional supplement fed at the same rate as the CR for the first 14 d of life. Calves fed 20 g IgG/d from the CR experienced greater growth through day 28 (280 vs. 220 and 230 g/d, respectively; P < 0.05), decreased days with scours (6.1% vs. 9.7% and 10.7%, respectively; P < 0.05), and decreased treatment days (8.2% vs. 10.6% and 12.3%, respectively, P < 0.05) compared with calves fed the control and nutritional supplement treatments. It should be understood that in this particular study, failure of PTI rates for all treatments averaged over 60%, making interpretation of results difficult.
A more recent study (Chamorro et al., 2017) assessed the impact of feeding a dried MC CR to calves for the first 14 d of life. Calves fed the CR received 150 g of CR twice daily for the first 14 d of life (21% IgG, 64 g IgG fed daily). Calves fed the CR did not experience an improvement in growth rate. However, calves fed the CR were less likely to be treated with antibiotics during the trial (18.8% vs. 76.5%, respectively). The amount of CR fed in this study is much greater than in other reported studies and may make implementation financially difficult for many producers.
A final study supplemented calves (n = 1,037) with a whey-based CR for the first 14 d of life (Geiger et al., 2019a, b). Calves received either no supplemental IgG per day, 10 g IgG per day, or 20 g IgG per day. Unlike the Berge et al. (2009) study, PTI rates were between 75% and 80% for each treatment. In this study, supplementing increasing amounts of IgG linearly increased ADG through 14 d, but growth advantages were lost by weaning. Of most interest, however, is the fact that feeding 20 g of IgG reduced antibiotic treatments for scours compared with the control (35.3% vs. 44.4%; P = 0.06). Additionally, supplementing calves with 20 g of IgG/d for the first 14 d reduced mortality by roughly 70% (2.0% vs. 6.6%; P < 0.01). Immune status at the start of the trial did not impact antibiotic treatments or mortality. Supplementing IgG to calves post-gut closure appears to be a viable means to reduce antibiotic use on farm. However, more, large-scale studies (similar to Geiger et al., 2019a, b) should be conducted to determine the economic ramifications for producers and the impact of the immune status of the calf may have. Some results are promising (Chamorro et al., 2017; Geiger et al., 2019a, b) as long as they are not too expensive (Chamorro et al., 2017) and we can be confident they represent typical dairy conditions.
Conclusions
Colostrum continues to be a subject of discussion throughout the dairy industry. Granted much of recent conversation focuses on the impacts of other factors aside from IgG in colostrum, the importance of colostral Ig cannot be overlooked or forgotten. Additionally, if PTI benchmarks are continually left unmet, a CR may be a viable option to complement a high-quality MC. Finally, the value of transition milk or supplementation of colostral IgG post-gut closure may be a viable means to reduce antibiotic use on farm and improve calf health and performance, but this emerging area of research requires more focus.
Conflict of interest statement
The author works for a company that manufactures a colostrum replacer. All data presented herein however is based off of published literature.
Literature Cited
- Alipour M. J., Jalanka J., Pessa-Morikawa T., Kokkonen T., Satokari R., Hynönen U., Iivanainen A., and Niku M.. 2018. The composition of the perinatal intestinal microbiota in cattle. Sci. Rep. 8:10437. doi: 10.1038/s41598-018-28733-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mashikhi S. A., and Nakai S.. 1987. Isolation of bovine immunoglobulins and lactoferrin from whey proteins by gel filtration techniques. J. Dairy Sci. 70:2486–2492. doi: 10.3168/jds.S0022-0302(87)80315-6 [DOI] [PubMed] [Google Scholar]
- Annen E. L., Collier R. J., McGuire M. A., Vicini J. L., Ballam J. M., and Lormore M. J.. 2004. Effect of modified dry period lengths and bovine somatotropin on yield and composition of milk from dairy cows. J. Dairy Sci. 87:3746–3761. doi: 10.3168/jds.S0022-0302(04)73513-4 [DOI] [PubMed] [Google Scholar]
- Berge A. C., Besser T. E., Moore D. A., and Sischo W. M.. 2009. Evaluation of the effects of oral colostrum supplementation during the first fourteen days on the health and performance of preweaned calves. J. Dairy Sci. 92:286–295. doi: 10.3168/jds.2008-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blättler U., Hammon H. M., Morel C., Philipona C., Rauprich A., Romé V., Le Huërou-Luron I., Guilloteau P., and Blum J. W.. 2001. Feeding colostrum, its composition and feeding duration variably modify proliferation and morphology of the intestine and digestive enzyme activities of neonatal calves. J. Nutr. 131:1256–1263. doi: 10.1093/jn/131.4.1256 [DOI] [PubMed] [Google Scholar]
- Blum J. W., and Hammon H.. 2000. Colostrum effects on the gastrointestinal tract, and on nutritional, endocrine, and metabolic parameters in neonatal calves. Livest. Prod. Sci. 66:151–159. doi: 10.1093/jn/10.1016/S0301-6226(00)00222-0 [DOI] [Google Scholar]
- Blum J. W., Hadorn U., Sallmann H. P., and Schuep W.. 1997. Delaying colostrum intake by one day impairs plasma lipid, essential fatty acid, carotene, retinol and alpha-tocopherol status in neonatal calves. J. Nutr. 127:2024–2029. doi: 10.1093/jn/127.10.2024 [DOI] [PubMed] [Google Scholar]
- Chamorro M. F., Cernicchiaro N., and Haines D. M.. 2017. Evaluation of the effects of colostrum replacer supplementation of the milk replacer ration on the occurrence of disease, antibiotic therapy, and performance of pre-weaned dairy calves. J. Dairy Sci. 100:1378–1387. doi: 10.3168/jds.2016-11652 [DOI] [PubMed] [Google Scholar]
- Davenport D. F., Quigley J. D. III, Martin J. E., Holt J. A., and Arthington J. D.. 2000. Addition of casein or whey protein to colostrum or a colostrum supplement product on absorption of IgG in neonatal calves. J. Dairy Sci. 83:2813–2819. doi: 10.3168/jds.S0022-0302(00)75180-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. L., and Drackley J. K.. 1998. The development, nutrition, and management of the young calf. Ames (IA): Iowa State University Press. [Google Scholar]
- Donovan G. A., Dohoo I. R., Montgomery D. M., and Bennett F. L.. 1998. Associations between passive immunity and morbidity and mortality in dairy heifers in Florida, USA. Prev. Vet. Med. 34:31–46. doi: 10.1016/s0167-5877(97)00060-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S. A., and Broom D. M.. 1979. The period between birth and first suckling in dairy calves. Res. Vet. Sci. 26:255–256. doi: 10.1016/s0167-5877(97)00060-3 [DOI] [PubMed] [Google Scholar]
- Faber S. N., Faber N. E., Mccauley T. C., and Ax R. L.. 2005. Case study: effects of colostrum ingestion on lactation performance. Prof. Anim. Sci. 21:420–425. doi: 10.15232/S1080-7446(15)31240-7 [DOI] [Google Scholar]
- Geiger A. J., Leonardi C., and Lago A.. 2019a. Feeding whey-based colostrum replacer for the first 14 days of life improves dairy calf performance. J. Dairy Sci. 102:324 (Abstr.) [Google Scholar]
- Geiger A. J., Leonardi C., and Lago A.. 2019b. Supplementing dairy calves with colostral immunoglobulins for 14 days reduces death loss and antibiotic usage. J. Dairy Sci. 102:182 (Abstr.) [Google Scholar]
- Godden S. 2008. Colostrum management for dairy calves. Vet. Clin. North Am. Food Anim. Pract. 24:19–39. doi: 10.1016/j.cvfa.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godden S. M., Haines D. M., and Hagman D.. 2009. Improving passive transfer of immunoglobulins in calves. I: dose effect of feeding a commercial colostrum replacer. J. Dairy Sci. 92:1750–1757. doi: 10.3168/jds.2008-1846 [DOI] [PubMed] [Google Scholar]
- Gross J. J., Kessler E. C., and Bruckmaier R. M.. 2017Quarter vs. composite colostrum composition assessed by Brix refractometry, specific gravity, and visual color appearance in primiparous and multiparous dairy cows. Transl. Anim. Sci. 1:26–35. doi: 10.2527/tas2016.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammon H. M., Schiessler G., Nussbaum A., and Blum J. W.. 2002. Feed intake patterns, growth performance, and metabolic and endocrine traits in calves fed unlimited amounts of colostrum and milk by automate, starting in the neonatal period. J. Dairy Sci. 85:3352–3362. doi: 10.3168/jds.S0022-0302(02)74423-8 [DOI] [PubMed] [Google Scholar]
- Heinrichs A. J., and Elizondo-Salazar J. S.. 2009. Reducing failure of passive immunoglobulin transfer in dairy calves. Rev. Méd. Vét. 160:436–440 [Google Scholar]
- James R. E., Polan C. E., and Cummins K. A.. 1981. Influence of administered indigenous microorganisms on uptake of iodine125 gamma-globulin in vivo by intestinal segments of neonatal calves. J. Dairy Sci. 64:52–61. doi: 10.3168/jds.S0022-0302(81)82528-3 [DOI] [PubMed] [Google Scholar]
- Jardon P. W., Robinson J., and Myake J.. 1998. Evaluation of specific gravity as a screening test for colostrum. In: Proceedings of American Association of Bovine Practitioners; Spokane, WA; 196 [Google Scholar]
- Kasonta R., Holsteg M., Duchow K., Dekker J. W., Cussler K., Bendall J. G., and Bastian M.. 2014. Colostrum from cows immunized with a vaccine associated with bovine neonatal pancytopenia contains allo-antibodies that cross-react with human MHC-I molecules. Plos One 9:e109239. doi: 10.1371/journal.pone.0109239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe S. I., Jayarao B. M., and Heinrichs A. J.. 2007. A survey of bovine colostrum composition and colostrum management practices on Pennsylvania dairy farms. J. Dairy Sci. 90:4108–4116. doi: 10.3168/jds.2007-0040 [DOI] [PubMed] [Google Scholar]
- Kessler E. C., Bruckmaier R. M., and Gross J. J.. 2014. Milk production during the colostral period is not related to the later lactational performance in dairy cows. J. Dairy Sci. 97:2186–2192. doi: 10.3168/jds.2013-7573 [DOI] [PubMed] [Google Scholar]
- Kinal S., Korniewicz A., Jamroz D., Zieminski R., and Slupczynska M.. 2005. Dietary effects of zinc, copper, and manganese chelates and sulphates on dairy cows. J. Food Ag. Environ. 3:168–172 [Google Scholar]
- Kincaid R. L., and Socha M. T.. 2004. Inorganic versus complexed trace mineral supplements on performance of dairy cows. Prof. Anim. Sci. 20:66–73. doi: 10.15232/S1080-7446(15)31274-2 [DOI] [Google Scholar]
- Kühne S., Hammon H. M., Bruckmaier R. M., Morel C., Zbinden Y., and Blum J. W.. 2000. Growth performance, metabolic and endocrine traits, and absorptive capacity in neonatal calves fed either colostrum or milk replacer at two levels. J. Anim. Sci. 78:609–620. doi: 10.2527/2000.783609x [DOI] [PubMed] [Google Scholar]
- Lago A., Socha M., Geiger A., Cook D., Silva-Del-Río N., Blanc C., Quesnell R., and Leonardi C.. 2017. Efficacy of colostrum replacer versus maternal colostrum on immunological status, health, and growth of preweaned dairy calves. J. Dairy Sci. 101:1344–1354. doi: 10.3168/jds.2017-13032 [DOI] [PubMed] [Google Scholar]
- Lopez A. J., Jones C. M., Geiger A. J., and Heinrichs A. J.. 2020. Comparison of IgG absorption in calves fed a commercial colostrum replacer or supplemented maternal colostrum. J Dairy. Sci. 103(5):4838–4845. doi: 10.3168/jds.2019-17949 [DOI] [PubMed] [Google Scholar]
- Maunsell F. P., Morin D. E., Constable P. D., Hurley W. L., and McCoy G. C.. 1999. Use of mammary gland and colostral characteristics for prediction of colostral IgG1 concentration and intramammary infection in Holstein cows. J. Am. Vet. Med. Assoc. 214:1817–1823 [PubMed] [Google Scholar]
- McGuirk S. M., and Collins M.. 2004. Managing the production, storage, and delivery of colostrum. Vet. Clin. North Am. Food Anim. Pract. 20:593–603. doi: 10.1016/j.cvfa.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Miyazaki T., Okada K., Yamashita T., and Miyazaki M.. 2019. Temporal changes of abomasal contents and volumes in calves fed milk diluted with oral rehydration salt solution. J. Vet. Med. Sci. 81:256–262. doi: 10.1292/jvms.18-0466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore M., Tyler J. W., Chigerwe M., Dawes M. E., and Middleton J. R.. 2005. Effect of delayed colostrum collection on colostral IgG concentration in dairy cows. J. Am. Vet. Med. Assoc. 226:1375–1377. doi: 10.2460/javma.2005.226.1375 [DOI] [PubMed] [Google Scholar]
- Morin D. E., McCoy G. C., and Hurley W. L.. 1997. Effects of quality, quantity, and timing of colostrum feeding and addition of a dried colostrum supplement on immunoglobulin G1 absorption in Holstein bull calves. J. Dairy Sci. 80:747–753. doi: 10.3168/jds.S0022-0302(97)75994-0 [DOI] [PubMed] [Google Scholar]
- Morrill K. M., Conrad E., Lago A., Campbell J., Quigley J., and Tyler H.. 2012. Nationwide evaluation of quality and composition of colostrum on dairy farms in the United States. J. Dairy Sci. 95:3997–4005. doi: 10.3168/jds.2011-5174 [DOI] [PubMed] [Google Scholar]
- Nardone A., Lacetera N., Bernabucci U., and Ronchi B.. 1997. Composition of colostrum from dairy heifers exposed to high air temperatures during late pregnancy and the early postpartum period. J. Dairy Sci. 80:838–844. doi: 10.3168/jds.S0022-0302(97)76005-3 [DOI] [PubMed] [Google Scholar]
- NAHMS (National Animal Health Monitoring System). US Department of Agriculture:Animal and Plant Health Inspection Service:Veterinary Services:Centers for Epidemiology and Animal Health (USDA:APHIS:VS:CEAH), Fort Collins, CO: 2011. [Google Scholar]
- Pritchett L. C., Gay C. C., Besser T. E., and Hancock D. D.. 1991. Management and production factors influencing immunoglobulin G1 concentration in colostrum from Holstein cows. J. Dairy Sci. 74:2336–2341. doi: 10.3168/jds.S0022-0302(91)78406-3 [DOI] [PubMed] [Google Scholar]
- Quigley J. D. 2002. Passive immunity in newborn calves. Adv. Dairy Tech. 14:273–292 [Google Scholar]
- Renaud D. L., Kelton D. F., LeBlanc S. J., Haley D. B., Jalbert A. B., and Duffield T. F.. 2017. Validation of commercial luminometry swabs for total bacteria and coliform counts in colostrum-feeding equipment. J. Dairy Sci. 100:9459–9465. doi: 10.3168/jds.2017-13228 [DOI] [PubMed] [Google Scholar]
- Stewart S., Godden S., Bey R., Rapnicki P., Fetrow J., Farnsworth R., Scanlon M., Arnold Y., Clow L., Mueller K., et al. . 2005. Preventing bacterial contamination and proliferation during the harvest, storage, and feeding of fresh bovine colostrum. J. Dairy Sci. 88:2571–2578. doi: 10.3168/jds.S0022-0302(05)72933-7 [DOI] [PubMed] [Google Scholar]
- Tizard I. R. 2013. Veterinary immunology. 9th ed. St. Louis (MO): Elsevier, Inc. [Google Scholar]
- Virtala A. M., Grohn Y. T., Mechor G. D., and Erb H. N.. 1999. The effect of maternally derived immunoglobulin G on the risk of respiratory diseases in heifers during the first 3 months of life. Prevent. Vet. Med. 29:9–19. doi: 10.3168/jds.S0022-0302(05)72933-7 [DOI] [PubMed] [Google Scholar]