Consequences of Increased Litter Size
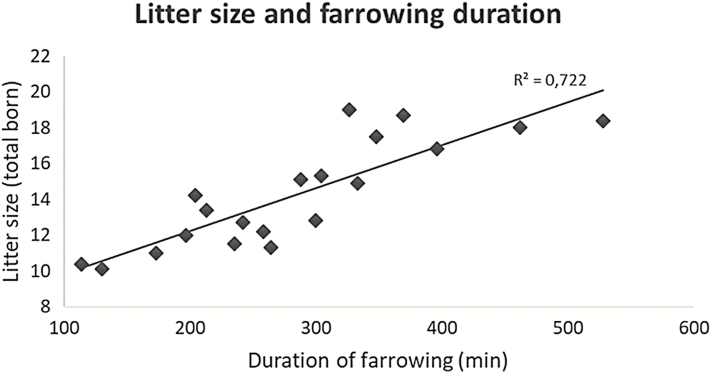
Over the past three decades, the litter size of most European domestic pig breeds has approximately doubled (Oliviero et al., 2019). In the same period, the average duration of farrowing has extended considerably in those breeds, from a bit more than 2 h per 12 piglets (Madec and Leon, 1992) to 6 h 40 min per 19 piglets born (Figure 1; Yun et al., 2019). The difference is even larger if the point of reference is the wild pig with only five piglets and a duration of farrowing of just 1 h 20 min (Harris et al., 2001). Therefore, there is good reason to question the implications that such an extended duration of parturition holds in terms of physiology, pathology, immunity, behavior, welfare (extended time in pain), resilience, and performance of the pig. All of these essential aspects are profoundly affected by the present increase in litter size. A prevailing question is what could be done at the farm level in terms of management of parturition in order to alleviate the current problems. On the other hand, crossbreeding with less productive but more robust breeds may actually be a better alternative to improve the survival and immune state of newborn piglets and the health and welfare of the sow during the postpartum period.
Figure 1.
Relationship between litter size and duration of farrowing in 20 studies from 1992 to 2018 (Oliviero et al., 2019).
An uncomplicated vaginal delivery of fetuses involves timely contractions of the uterine structures (Oscarsson et al., 2006; Berglund et al., 2008; Taverne and van den Weijden, 2008; Senger, 2012). While contractions are good in terms of the actual expulsion of the fetuses, they result in vasoconstriction of placental circulation, and ultimately rupture of the umbilical cord, exposing fetuses still within the uterus to oxidative stress. In the longer run, this leads to hypoxia (Oscarsson et al., 2006; Berglund et al., 2008; Taverne and van den Weijden, 2008; Boksa et al., 2015). If fetuses are subjected to hypoxia during the birth process, they are much more likely to be hypoglycemic, less alert, and under increased risk of being crushed by the dam (Oliviero, 2013). This may indeed present a problem when very large litters are in the process of being born. Furthermore, these extra-large litters also hold the risk of decreased piglet birth weight (Akdag et al., 2009; Beaulieu et al., 2010) and increased rate of intra-uterine growth retardation (IUGR) (Matheson et al., 2018; Oliviero et al., 2019). Such IUGR piglets are not only immature in terms of their immunity, but they are less active and need more time to achieve the first suckle. Furthermore, the ability of the sow to provide a reliable source of colostrum for all piglets may reach a limit, as the number of piglets approaches or even surpasses the number of functional teats (Spinka and Illmann, 2015). The window for access to colostrum is also shortened through prolonged farrowing in the hyperprolific sows (Oliviero et al., 2019). Therefore, colostrum intake per piglet decreases with increasing litter size. An estimated 35% of sows do not produce enough colostrum to adequately supply all of their piglets (Quesnel et al., 2012). As a result, low birth weight piglets in large litters are at a greater risk of not obtaining at least 200–250 g of colostrum (Quesnel et al., 2012; Hasan et al., 2019), which is the amount needed for adequate immunoglobulin levels and minimum growth (Spinka and Illmann, 2015).
Another major problem that arises from extra-large litters is an increased incidence of diseases of the udder and the uterus, resulting in decreased reproductive performance of the sow. Prior to parturition, hyperprolific sows need to be fed according to the number of developing fetuses. This led to increases in the amounts of energy and feed provided to gestating sows. However, a greater volume of feed ingested in gestation is known to be a risk factor for the metabolism of periparturient sows, likely creating a negative energy balance (Feyera et al., 2018; Farmer et al., 2019). Increasing the volume of feed, and thus energy, is also a risk factor for constipation and poor mammary gland development (Farmer and Quesnel, 2009; Oliviero et al., 2010; Farmer and Hurley, 2015; Farmer et al., 2019), leading to insufficient colostrum and milk production and, therefore, low piglet survival and development (Edwards and Baxter, 2015).
After parturition, prolonged farrowing may be manifested as adverse development of microbiota in the sow (Hasan et al., 2018b). This may be due to the birth canal staying open for extended periods of time (Peltoniemi et al., 2019a). It is, therefore, not surprising that in hyperprolific sows a greater incidence of uterine problems and placental expulsion have been reported (Björkman et al., 2017b) as well as increased rate of uterine inflammation giving rise to postpartum dysgalactia (PDS, Björkman et al., 2018c). It was recently reported that the incidence of PDS has increased along with litter size, reaching the current value of 34% in the DanBred breed (Kaiser et al., 2018a, b).
Therefore, the problems related to prolonged farrowing, which are highly associated with the hyperprolific sow (Oliviero et al., 2019), warrant careful consideration in future management and breeding. It is hypothesized that with optimal management the duration of farrowing may be decreased, improving the prospects for newborn piglets. However, cross breeding of the modern highly prolific sow lines back with the less productive but more robust breeds may be needed to cope with the evolving challenges. The following text will cover the management practices currently available for successful farrowing, reduction of duration of farrowing, optimization of colostrum production and intake by piglets, favorable changes in microbiota in piglets and sows, and a decrease in diseases such as PDS that affect the udder and the uterus.
Diagnosis of Abnormal Parturition
Behavior during the first and second stages of parturition
The first stage of parturition overlaps with the time period of nest-building behavior, which is a highly expressed, intrinsic behavior of the pig occurring during the last 24 h prior to the onset of the expulsion stage (Jensen, 1986; Algers and Uvnäs-Moberg, 2007). Nest-building activity is at its peak between 6 and 12 h preceding the expulsion of the first piglet (for reviews, see Lawrence et al., 1997; Wischner et al., 2009). If space and materials for nest-building are lacking, sows may redirect the need for nest-building to other types of activities, giving rise to greater overall activity level during parturition. Thodberg et al. (2002) found that sows housed in pens without nesting materials expressed less nest-building behavior and an increased frequency of attempts to express nest-building prior to the onset of expulsion of piglets. However, more nest-building-like behavior was observed in pens without nesting material during the expulsion (second) stage of parturition compared with pens provided with straw (Thodberg et al., 2002). Alternatively, inhibition of the expression of nest-building behavior prior to the second stage of parturition by lack of space and substrates may provoke a stress response in sows. The stress response may then result in increased behavioral activities during parturition, thus delaying the farrowing process (for review, see Yun and Valros, 2015). The occurrence of prepartum nest-building behavior was shown to be positively correlated with increased litter size (Pedersen et al., 2006). Therefore, providing optimal farrowing environment with adequate space and nesting materials appears particularly important for the hyperprolific sow. It can encourage the performance of nest-building during the first stage of parturition. A supportive environment may, therefore, decrease stress and shorten the second stage of parturition.
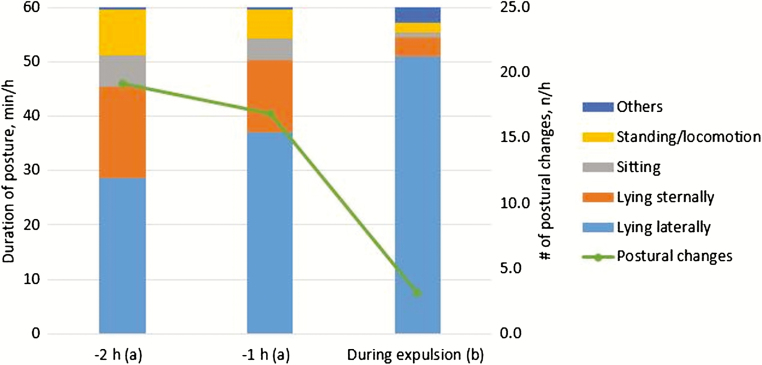
After entering the second stage of parturition, postural changes decrease and duration of lying gradually increases (Figure 2). Harris and Gonyou (1998) proposed that frequent postural changes or restlessness can be indicative of the state of discomfort in peripartum gilts. Furthermore, our previous findings (Yun et al., 2019) suggested that suboptimal farrowing environments for sows with a large litter may cause considerable stress. The increased stress appears to induce a greater frequency of postural changes and longer duration of standing position of sows during the expulsion stage of parturition (Yun et al., 2019). Stress and discomfort of sows can therefore be suspected when there are no signs of cessation of activities such as nest building in the beginning of the second stage of parturition.
Figure 2.
Mean duration of standing or locomotion, sitting, and sternal and lateral lying-down postures and mean number of postural changes per hour in 31 sows during the 2 h prior to onset of expulsion stage of parturition (a) and during the expulsion stage of parturition (b) for sows housed in closed or open farrowing crates (modified from Yun et al., 2019).
Prolonged second and third stages of farrowing
Only a decade ago, a farrowing duration of more than 5 h and a piglet birth interval longer than 25 min were considered excessively long farrowing (Oliviero, 2010). With the avenue of hyperprolific sows, the average farrowing duration has considerably increased. In fact, in Danish sows, the average duration of farrowing was recently reported to be 7 to 7.5 h (Hales et al., 2015; Thorsen et al., 2017). Hence, it is important to recognize the signs of a prolonged farrowing both during the process and immediately after it. It is often recommended that the sow should be supervised and, if necessary, assisted during farrowing (Kirkden et al., 2013). Signs of prolonged farrowing could include restless behavior, as discussed above, and strong abdominal straining due to acute obstructive dystocia (Cowart, 2007). On the other hand, weak or absent abdominal contractions in a calm sow most likely refer to uterine inertia. Dystocia in sows is relatively rare compared with other livestock species, but this condition has increased over the years from 0.5% to 5%, with an even higher incidence in modern hyperprolific sow lines (Jones, 1966; Randall, 1972; Peltoniemi et al., 2019a). In the past, when litter size was not so large, uterine inertia was considered mainly to be secondary due to fatigue of dystocia (Almond et al., 2006). On the other hand, large litters are nowadays the likely primary cause of uterine inertia due to a heavy and overstretched uterus and hormonal disturbances in the sow. Thus, prevention of prolonged parturition in hyperprolific sows is essential and will be discussed later in more detail.
If preventive measures fail to be successful, sows need more intense monitoring at farrowing. For instance, sows that are obese or show constipation during the days before expected farrowing should be monitored more carefully. Constipation and presence of excessive fecal material in the colon and rectum may result in partial occlusion of the birth canal (Cowart, 2007). One study reported an increase in farrowing duration associated with clinical constipation during the 5 d prior to farrowing (Oliviero et al., 2010). Another sign of prolonged farrowing is expulsion of the placenta during the second stage of birth. If the placenta is expelled before the last piglet, it is likely that parturition has been ongoing for more than 5 h (Björkman et al., 2017b). On the other hand, expulsion of minor parts of the placenta or absence of placental expulsion after the last piglet is born may also be a sign of prolonged parturition. This may indicate a substantial slowing of the birth process (Björkman et al., 2017b). In summary, while litter size has doubled in the past three decades, duration of farrowing has increased by 4 to 5 times, hence presenting a great challenge for the pig industry.
Colostrum quality
Several studies report that 200 to 250 g of colostrum per piglet is the minimum requirement to induce sufficient immune protection (Devillers et al., 2007; Quesnel, 2011; Hasan et al., 2019). Colostrum production lasts only 16 to 24 h after parturition starts, and already after the first 6 h the IgG content in colostrum is halved (Le Dividich et al., 2005). However, by the sixth hour, there is still an average of 20% piglets still within the uterus in the hyperprolific sow (Oliviero et al., 2019) Those piglets miss out on access to colostrum of good quality. Therefore, especially when managing large litters, determination of the level of immunoglobulins in colostrum could be a useful tool. Hasan et al. (2016) proposed the use of the Brix refractometer to evaluate sow colostrum content of IgG on the farm. The IgG content in sows peaks shortly after the onset of farrowing, with reported concentrations 60 to 70 mg/mL at this time (Hurley, 2015; Quesnel, 2015). However, concentrations decrease to around 10 mg/mL at the end of colostrogenesis (24 h after the onset of farrowing). Colostrum should be tested with the Brix refractometer at the beginning of farrowing (0 to 3 h) when it is easiest to collect and when concentrations of IgG are expected to be near the peak values for this time period (Hasan et al., 2016). The authors considered an IgG content of 50 mg/mL as a fairly safe cutoff point to provide adequate amounts of IgG to newborn piglets. A table for evaluation of the Brix refractometer results is shown in Table 1. When Brix values are <20%, they reflect very low levels of IgG, while values from 25% upwards are considered to correspond to adequate or very good concentrations of IgG in colostrum. Values between 20% and 24% are defined as borderline, yet they should only be considered critical if within the lowest range of this category (20% to 21%). Therefore, with borderline results, the authors suggest taking another sample within 1 to 2 h to determine whether the estimated IgG content is stable, increasing, or decreasing compared with the initial value. Using colostral Brix measurement at farm level may help to identify sows with impaired IgG concentrations in early parturition. In conclusion, the Brix refractometer could be used to increase stockmanship with large litters by routinely screening the colostrum quality of sows in the herd in order to detect unfavorable low levels of IgG as result of feeding, diseases, or management issues.
Table 1.
Colostrum IgG content based on two methods of evaluation and the categories of estimation
| Brix % | ELISA IgG 0–3 h, mg/mL, average ± SEM | IgG estimation categories |
|---|---|---|
| < 20 | 14.5 ± 1.8 | Poor |
| 20–24 | 43.8 ± 2.3 | Borderline |
| 25–29 | 50.7 ± 2.1 | Adequate |
| ≥ 30 | 78.6 ± 8.4 | Very good |
From Hasan et al. (2016).
Ultrasonography and other biomarkers
A prolonged process of parturition is a high risk situation for impaired uterine health (Björkman, 2017; Björkman et al., 2017b; 2018a). Therefore, expertise in diagnostic imaging can be used peripartum to diagnose the causes of dystocia as well as postpartum to diagnose diseases of the reproductive tract. Whenever a sow shows signs of dystocia, an obstetric examination should be performed before any interventions are conducted. It is recommended to include ultrasound in the obstetric examination in order to visualize piglets located in the birth canal and other parts of the uterus. If a piglet is located in the birth canal, it is more likely that the cause of dystocia is an obstructive one. If there are piglets deep in the uterus and not in the birth canal, it is likely that the cause is a failure of expulsive forces. Ultrasound can also be used to see whether piglets are still present inside the uterus or whether the second stage of parturition is completed.
After parturition is completed, ultrasound is beneficial to determine uterine health. The timely and correct diagnosis of postpartum uterine disease is essential to prevent postpartum dysgalactia syndrome, hence, decreased neonatal development and survival as well as decreased subsequent fertility of the sow (Kauffold and Wehrend, 2014). Ultrasonography is considered to be the best tool for diagnosis of endometritis and retained placenta (Björkman et al., 2018c; Kauffold et al., 2019). Examination of uterine structures currently utilizes three criteria: fluid echogenicity, echotexture, and size (Kauffold and Althouse, 2007; Peltoniemi et al., 2016; Björkman 2017). Increased size and echotexture are a reflection of edema changes in the endometrium, which is usually abnormal. Nevertheless, the parity of the sow and the postpartum day on which the examination is performed need to be taken into consideration when interpreting uterine size. Furthermore, any fluid causing echogenicity must be considered abnormal and indicative of an exudative inflammation of an acute or acute-chronic type (Kauffold and Althouse, 2007). Fluid echogenicity is often associated with increased echotexture and size of uterine cross-sections (Björkman et al., 2018c), therefore, sharing similar risk factors. These risk factors are prolonged parturition, obstetric intervention, placental retention, and birth of two or more stillborn piglets (Björkman et al., 2018c). Postpartum ultrasound can be used to diagnose retention of the placenta (Peltoniemi et al., 2016; Björkman 2017; Björkman et al., 2017b; Kauffold et al., 2019).
Besides ultrasonography, other biomarkers have been extensively used to evaluate health status, establish a diagnosis or prognosis of the disease, predict and/or monitor response to therapy, and assess reproductive failure (Myers et al., 2017). Characteristics related to clinical signs such as vaginal discharge, total amount, color, number of cells, and cell characteristics, are considered as biomarkers that are used to evaluate uterine health, but different variables are needed to strengthen a presumptive diagnosis of endometritis in sows after birth (Grahofer et al., 2019). As cystitis is one of the main risk factors for sows showing sustained uterine infections (Biksi et al., 2002), several useful biomarkers are described in the literature (Kauffold et al., 2010; Bellino et al., 2013; Grahofer et al., 2014; Sipos et al., 2014). Midstream urine samples are often contaminated through the environment, and therefore sterile urine or swabs from the bladder of culled sows should be tested and a culture performed if there are problems with the urinary tract in a sow herd (Grahofer et al., 2014; Sipos et al., 2014). Ultrasonography of the urinary bladder is inappropriate for the diagnosis of urinary tract infections in sows because the variables are dependent on filling of the urinary bladder. Only moderate to high amounts of sediment seem to be indicative of cystitis (Kauffold et al., 2010). Hence, a combination of several variables is necessary to diagnose infections of the urogenital tract in sows. In conclusion, ultrasonography with modern equipment appears to be a good tool to efficiently follow-up on the health status of the birth canal, uterus, and urinary tract in postpartum sows.
Treatment of abnormal parturition
Such new developments as the tendency towards free farrowing and the increased likelihood of heat stress with climate change require that researchers and the industry have a critical look at the breeding targets. It is likely that these new perspectives, e.g. free farrowing and thermal resilience, will need to be taken into consideration in creating new breeding goals (for review, see Peltoniemi et al., 2019b). Recent developments in reproductive technology should be helpful for international transfer of germ cells and embryos of more robust and resilient sow lines across borders that may be more suitable in free farrowing type of production and in areas where sows are suffering from heat stress (Hansen, 2019; Peltoniemi et al., 2019b). These developments include ovarian biopsy and ovum pick-up (Brüssow et al., 1997; Björkman et al., 2017c; Peltoniemi et al., 2019b), cryopreservation of germ cells and embryos (Cuello et al., 2016), and development of embryo transfer technology (Martinez et al., 2016).
Treatment of prolonged parturition
The role of biosecurity and expert level stockmanship are of highest value in obstetric interventions. Appropriate and prompt treatment of the sow during prolonged parturition is important to avoid negative effects on the sow’s reproductive health and the piglets’ health and survival. This can be achieved through continuous farrowing supervision (Holyoake et al., 1995). In general, obstetric intervention is indicated if more than 45 min have passed since the last piglet was expelled (Peltoniemi et al., 2019a). This especially applies if the sow is restless and has strong abdominal contractions or if parturition is prolonged beyond 300 min (Oliviero et al., 2008). If the sow is still at the beginning of parturition and shows no signs of discomfort or strong abdominal straining, obstetric intervention is usually not indicated before 1 h has passed since the last piglet was born (Peltoniemi et al., 2019a). Most often maternal causes, such as secondary uterine inertia, lead to dystocia in sows. Nevertheless, general and obstetric examinations, including palpation and ultrasonography of the birth canal, are necessary to rule out other causes of dystocia, such as obstruction of the birth canal, ventral deviation of the uterine horns, or fetal malposition, before treating for uterine inertia (Peltoniemi et al., 2019a). Oxytocin provokes uterine contractions and it is frequently used during farrowing to treat dystocia (Straw et al., 2000). Use of oxytocin is only indicated when there is no obstruction of the birth canal, the piglet is well positioned, and the sow has poor uterine contractions (Gilbert,1999). Before administration of any exogenous oxytocin, it is recommended to try means of releasing endogenous oxytocin, e.g. manual induction of the Ferguson reflex through stimulation of the cervix or encouraging the sow to move, especially if the sow is still at the beginning of the second phase of parturition. Nevertheless, if secondary uterine inertia is diagnosed towards the end of the second phase of parturition, immediate injection of oxytocin is indicated (Peltoniemi et al., 2019a). Several studies were conducted to prove the effect of oxytocin on the birth process and piglet survivability and to evaluate the proper dosage of oxytocin in dystotic sows (Mota-Rojas et al., 2002; 2005a,b; 2007). An intramuscular administration of 10 IU of oxytocin did not cause any side effects. However, higher dosages (20 to 50 IU) led to an increase in stillborn piglets, changes in the umbilical cord, and greater meconium scoring (Mota-Rojas et al., 2002; 2005a,b; 2007; Kaeoket, 2006). Furthermore, improper use of oxytocin can increase uterine inertia (Dial et al., 1987). Hence, oxytocin should be only restrictively administered, e.g. a maximum of 10 IU one to two times during parturition. Recently, two studies investigated the use of carbetocin, a long-acting analogue of oxytocin, administered routinely after expulsion of the first piglet (Jiarpinitnun et al.,2019; Ward et al., 2019). Their findings indicated that while use of carbetocin may reduce duration of farrowing, it can also reduce colostrum yield and increase still born rate. Another study reported severe undesirable side effects after routine administration of 35 µg of carbetocin during the farrowing process in a free farrowing system (Grahofer et al.,2019). A prolonged piglet-to-piglet birth interval directly after application, loss of colostrum, and increased number of weak and stillborn piglets were also detected. Therefore, administration of carbetocin is not currently recommended to improve the birth process. In conclusion, timely application of birth assistance, considering behavior of the sow and the time elapsed since start of parturition, has become more important than ever in hyperprolific sows. Hyper-stimulation of the uterus with excessive oxytocin must, however, be avoided. In addition, breeding goals of pig production should be reexamined for robustness and resilience.
Improving colostrum uptake
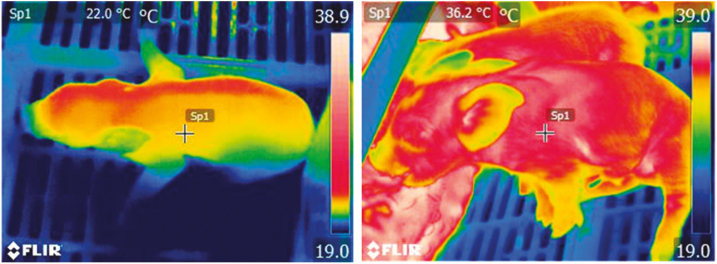
Once piglets are born, farmers need to quickly implement a strategy to reduce piglet mortality. This is important especially in large litters which have greater incidences of low-viability piglets and where competition for colostrum and milk access are increased (Lund et al., 2002; Devillers et al., 2011; Hasan et al., 2019). To provide all piglets with sufficient individual colostrum intake (200 to 250 g) within 12 to 16 h from the beginning of parturition, different strategies can be adopted. When possible, small and low-viable piglets should be assisted to suckle, helping them to attach to the teat, and ensuring that they are able to ingest colostrum. Baxter et al. (2008) investigated behavioral and physiological indicators of neonatal survival and provided data that could be used to identify piglets in need of assistance (Table 2). Besides birth weight and crown-rump length, survival of piglets was positively related with rectal temperature between birth and 24 h postpartum and with vitality score, rooting response, and latency to access a teat and suckle. These traits were also correlated with each other, showing the importance of prevention of the hypothermia–starvation–crushing complex in neonatal piglets, as summarized by Edwards and Baxter (2015). Those authors concluded that a piglet needs to be assisted to suckle if the following criteria are met: vitality of less than 2, meaning no movements within 15 s of birth, latency to access and suckle a teat of more than 30 min, and rectal temperature of less than 37 oC during the first hour after birth. This may, however, be difficult to put into practice and it may be helpful to make use of thermal images to overcome these difficulties (Alexopoulos et al., 2018). As is the case with body temperature, skin temperature is linked to birth weight, vitality, and colostrum ingestion and can be used to determine whether a piglet has reached a teat, suckled, and ingested colostrum within 30 min of birth (Santiago et al., 2019; Zhang et al., 2019a). As a piglet begins to suck and ingest colostrum, energy and warmth are provided, increasing rectal and skin temperatures (Figure 3; Alexopoulos et al., 2018). If skin temperature drops below 30oC, the piglet has not been successful in suckling (Figure 3; Alexopoulos et al., 2018) and needs to be assisted to attach to the teat and ensure that it ingests colostrum. The digital measurements such as digital skin temperature in Figure 3 may be part of automated supervision of farrowing, which appears as an important and cost-effective part of future management of parturition. It is important to remember that small piglets have difficulties in suckling from teats with big nipples, and therefore, the smallest functioning nipples should be preferred when assisting suckling. This procedure should be repeated 3 to 4 times within the first few hours if these piglets are not seen to actively suckle at the udder. Additionally, low-viable piglets can be hand-fed with colostrum collected from their mother or other sows within 6 to 12 h from the beginning of farrowing. Assisted suckling and hand feeding work well in small to normal litters with only one or two small piglets. In large litters and litters with more than two low-viable piglets, a split-suckling strategy could be more effective (Oliviero, 2013). To minimize sibling competition for colostrum intake, the litter is split into two groups. The more vigorous piglets with a full stomach are kept in the creep area or in a separate box, allowing the other piglets to suckle for 60 to 90 min, and then the groups are switched (Oliviero, 2013). This process is repeated as many times as possible. When separating the piglets, both groups should always have free access to a warm creep area. If some small piglets are still unable to successfully suckle, assisted suckling should be combined with split-suckling. In conclusion, litters from hyperprolific sows require close attention and assistance must be provided to late-born piglets, piglets without successful access to a teat, as well as underweight and less active piglets. New technology, such as the use of infrared cameras, may be utilized to assess the status of the newborn piglets.
Table 2.
Significant postnatal survival indicators (means and SE) comparing surviving piglets with those dying during the neonatal period (adapted from Baxter et al., 2008)
| Traits | Surviving (±SE) | Dying (±SE) | P-value |
|---|---|---|---|
| Piglet traits | |||
| Crown-rump length, cm | 28 (±0.26) | 25 (±0.90) | <0.001 |
| Birth weight, g | 1485 (±30.25) | 1176 (±79.35) | <0.001 |
| 24 h weight, g | 1584 (±34.1) | 1035 (±70.2) | <0.001 |
| Birth temperature, oC | 37.70 (±0.13) | 36.47 (±0.61) | 0.012 |
| 1 h temperature, oC | 37.94 (±0.10) | 36.70 (±0.48) | 0.002 |
| 2 h temperature, oC | 38.02 (±0.07) | 37.54 (±0.33) | 0.047 |
| 3 h temperature, oC | 38.02 (±0.06) | 37.53 (±0.13) | 0.010 |
| 24 h temperature, oC | 38.29 (±0.07) | 37.58 (±0.26) | 0.004 |
| Piglet behavioral traits | |||
| Vitality score | 2.28 (±0.06) | 1.77 (±0.20) | 0.017 |
| Rooting response, m | 1.42 (±0.10) | 0.47 (±0.17) | <0.001 |
| Latency to teat, min | 22 (±1.24) | 34 (±6.52) | 0.025 |
| Latency to suckle, min | 29 (±1.67) | 53 (±8.32) | <0.001 |
Figure 3.
Thermal image detecting skin temperature of newborn piglets receiving colostrum (right: 36.2 °C) or failing to reach the udder and ingest colostrum (left: 22.0 °C). Image taken by Jena G. Alexopoulos (Alexopoulos et al., 2018).
Prevention
Feeding and mammary gland development
Adequate mammary gland development is important for optimal colostrum and milk production and this topic has been recently reviewed by Farmer and Hurley (2015). In terms of mammary gland development and ease of farrowing, ad-libitum feeding with low fiber high energy feed in the last third of gestation should be avoided and more attention should be directed to feed composition. Providing the right amount of crude fiber and an adequate uptake of crude protein and certain essential amino acids are important (Farmer and Hurley, 2015). Interestingly, restricted feeding (about 50%) during the last third of gestation seems to be beneficial since backfat loss in that period has been positively associated with colostrum yield (Decaluwe et al., 2013). Yet, this can only be recommended for sows in good body condition in order to achieve optimal body condition (Oliviero et al., 2010) and mammary development (Farmer et al., 2016) at parturition. These studies suggest that more than 16 mm of backfat should be achieved, while also avoiding over conditioning. However, one must ensure that gestating sows receive sufficient energy to satisfy the demands of the forthcoming lactation, hence, sows are usually fed with high-energy concentrated lactation diets during late gestation (Einarsson and Rojkittikhun, 1993). Such concentrated diets, which contain less fiber than standard pregnancy diets (at least 7% crude fiber), can promote obesity and constipation. These two conditions are associated with prolonged farrowings and an increased stillbirth rate (Oliviero et al., 2010). Late pregnancy diets should contain up to 7% to 10% crude fiber in order to reduce constipation and excessive fat depots (Oliviero et al., 2009). If the diet cannot be easily modified, a good fiber source can be provided by offering different types of roughage (straw, hay) or adding any other feedstuffs with high levels of fiber, such as sugar beet pulp (Quesnel et al., 2009). The provision of roughage may not only be a way of increasing fiber intake and alleviating constipation and obesity, but can also serve as an appropriate material for nest-building. Proper nest-building behavior is linked to lower concentrations of progesterone and greater concentrations of prolactin prepartum (Algers and Uvnäs-Moberg, 2007). A high prepartum prolactin to progesterone ratio is also needed for adequate colostrum production (Foisnet et al., 2010). In addition, the use of dietary fiber can promote a better colostrum yield (Foisnet et al., 2010). Feeding fiber to gestating sows can also influence their gut microbiota, increasing the number of bacteria that are able to breakdown and ferment complex carbohydrates into short-chain fatty acids (SCFAs, Jha et al., 2019). The SCFAs produced by bacteria are thought to provide up to 30% of the maintenance energy requirement of gestating sows (Varel and Yen, 1997). Moreover, an increase in the concentration of SCFAs, more specifically butyrate, can improve gut mucosal health and the immune system of pigs. A recent study reported that an increased amount of cellulolytic bacteria such as Paraprevotella and Roseburia in the gut of sows was correlated with greater colostrum production (Hasan et al., 2018a). Moreover, the mother can influence the gut microbiota of her piglets, which was shown to improve piglet growth performance (Hasan et al., 2018a; Cheng et al., 2018). Another avenue that was recently investigated is the sow glucose metabolism shortly before and during the expulsion stage of farrowing. More specifically, Feyera et al. (2018) found that a short time lapse (less than 3 h) between the last meal and the onset of the expulsion stage shortened the duration of farrowing. Therefore, it appears that modifying the sow’s late gestation diet as well as providing the sow with frequent access to feed prior to farrowing can have beneficial effects on colostrum production and health of the sow, and on piglet growth.
In addition to improving mammary gland development for optimal colostrum and milk production, health of the mammary gland should be assessed before and after parturition. Before parturition, it is important to assess the number and morphology of functional teats and the degree of edema. After parturition, it is important to assess the mammary gland for any injuries or inflammation. The number of functional teats available per piglet is positively associated with piglet survival. If piglets have access to less than one functional teat, mortality increases by more than 14%. However, if more than one teat is available, mortality may be reduced to below 8% (Vasdal and Andersen, 2012; Alexopoulos et al., 2018) instead of the commonly reported mortality of 16% to 20% (Edwards and Baxter, 2015). Besides the number of functional teats, morphology of the udder is important. Piglets tend to suckle first from teats that are close to the abdominal midline and have longer inter-teat distances (Alexopoulos et al., 2018). Thus, a functional teat with short inter-teat distance and/or long distance between teat base and abdominal midline may be unusable for the piglet (Balzani et al., 2016). Furthermore, severe edema of the mammary gland before parturition will have a negative impact on teat accessibility, reduce colostrum quality, and increase the risk of subsequent mastitis (Björkman et al., 2017a; 2018a). The degree of mammary gland edema can be graded visually or with the aid of ultrasound, and a tissue sample by biopsy is also possible (Björkman et al., 2017a; 2018a,b; Han et al., 2018). It is important to keep in mind that the number of teats and teat morphology are factors that should be considered in farrowing management.
The immune system and microbiota
With regard to colostrum quality, there is a strong relationship between the process of parturition and maternal or piglet immunity. When the process of parturition is prolonged, the endocrine and immune systems are disturbed. Maternal nutrition plays a vital role in fetal development, early development of neonates, and lactation performance, and it regulates the lifetime productivity of the offspring (Zhang et al., 2019b). Nutritional strategies include feed additives such as organic acids, short- and medium-chain fatty acids, probiotics, prebiotics, and certain specific carbohydrates. After parturition, maternal nutrition can also affect development of the immune system in piglets (Salmon et al., 2009). Many studies have reported that supplementation with specific essential fatty acids (conjugated linolenic, linolenic and oleic acids, and resin acid-enriched composition) in gestating and lactating diets can improve colostral immunoglobulin concentrations, average daily gain, and weaning weight (Bontempo et al., 2004; Corino et al., 2009; Yao et al., 2012; Hasan et al., 2018b). The exact mechanisms underlying how these dietary components can increase different colostral immunoglobulins are not yet fully understood. However, their use in specific conditions of large litters and reduced colostrum quality could be beneficial (Oliviero et al., 2019). A recent study investigating late pregnancy diet supplementation with resin acid-enriched composition (RAC) reported that sows fed this compound had a relatively lower abundance of Proteobacteria in the hind gut (Hasan et al., 2018b). This can be considered beneficial for the sow because a high prevalence of Proteobacteria, representative of an unstable microbial community (dysbiosis), is a potential diagnostic criterion for diseases in humans (Shin et al., 2015). Proteobacteria are also linked to intestinal inflammation (Mukhopadhya et al., 2012). Bacteria belonging to the Proteobacteria phylum are known to cause intestinal diseases in humans and animals (Salyers and Whitt, 2002) indicating that RAC might contribute to the balance of intestinal microbiota. Sows fed RAC had more stabilized gut microbiota and reduced risk of pathogen colonization. Barnesiella, Sporobacter, Intestinimonas, and Campylobacter decreased in the hindgut of the RAC group, while Romboutsia and Clostridium sensu stricto significantly increased. Barnesiella, Sporobacter, Intestinimonas, and Campylobacter are well-known initiators of inflammatory diseases and gastrointestinal disorders in humans and animals (Weijtens et al., 1999; Zhang et al., 2017). On the other hand, Romboutsia and Clostridium sensu stricto, the main energy source for colonocytes, produce SCFAs by anaerobic fermentation of dietary components and protect from inflammation (Lopetuso et al., 2013). Clostridium sensu stricto is reported to promote the intestinal mucus barrier, and thus to inhibit adherence of pathogenic microbes (Wlodarska et al., 2015). In another study, supplementation with a hydrolysate yeast derivative (YD) in the late gestation diet increased colostrum yield and microbiota in sows (Hasan et al., 2018a). More beneficial and fermentative bacteria (Roseburia, Paraprevotella, Eubacterium) were found in the YD-fed group, while some opportunistic pathogens, including Proteobacteria, especially the genera Desulfovibrio, Escherichia/Shigella, and Helicobacter, were suppressed. At 1 wk of age, piglets born from YD-fed sows had better microbial populations with significant diversity and fewer opportunistic pathogens (Hasan et al., 2018a). Interestingly, the increased abundance of families of Proteobacteria (Enterobacteriaceae, Desulfovibrionaceae, Desulfovibrionaceae) in the control group was associated with low colostrum yield, low colostrum proteins, low colostrum IgM, and high stillbirth rate (Hasan et al., 2018a). In conclusion, targeted feeding strategies could favorably modulate colostrum production, colostrum immunoglobulin content, and gut microbiota of sows, hence indirectly benefiting piglets.
Conclusions
This review outlined current management and nutritional strategies arising from the large increase in sow litter size. Breeding goals should be reconsidered, addressing the ever-increasing duration of parturition in this species, which is not sustainable. In addition, attention should be paid to improving the international trade of germ cells and embryos in order to better cope with the challenges of the large litter. Other challenges await, including free farrowing housing and better resilience of sows as they approach farrowing to allow them to cope with the potential heat stress brought about by climate change. Behavioral traits can be useful for diagnosis of abnormal parturition. Sows should be allowed to express nest-building behavior and deviations from normal behavior just before and during the expulsion phase of parturition may indicate problematic cases. In addition, ultrasound technology is very useful, especially during the last third of pregnancy and postpartum, so that the most appropriate actions can be taken with regard to uterine health. Proper feeding management during the last third of pregnancy is crucial for mammary development and appropriate colostrum production. Prevention of constipation through adequate fiber provision and frequent meals prior to the onset of farrowing are important in the hyperprolific sow. Feeding management can be used to promote the immunity of the sow and the newborn. Feeding components, such short-chain fatty acids and yeast derivate, also appears to promote a favorable microbiota of the sow. Neonatal care and management become critical with large litters. The focus should be on situations where the number of piglets is greater than the number of teats. Applications of new technology, e.g. infrared cameras, may be useful in detecting piglets in need of assistance. In addition, such practices as cross fostering and split suckling are necessary when trying to handle the increasing litter size. The on-farm use of a Brix refractometer permits estimation of the quality of colostrum at the individual sow level, thereby allowing the farmer to target actions at specific sows that produce low-quality colostrum.
Funding
This study was funded by the Finnish Ministry of Agriculture (grants 1788/312/2014 and 1487/03.01.02/2016), the Mercedes-Zacharias Research Foundation, Hankkija, Vetcare, Figen, Atria, and the Finnish Foundation of Veterinary Research.
References
- Akdag F., Arslan S., and Demir H.. 2009. The effect of parity and litter size on birth weight and the effect of birth weight variations on weaning weight and pre-weaning survival in piglet. J. Anim. Vet. Adv. 8:2133–2138. [Google Scholar]
- Alexopoulos J., Lines D., Hallett S., and Plush K.. 2018. A review of success factors for piglet fostering in lactation. Animals. 8:38. doi: 10.3390/ani8030038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algers B., and Uvnäs-Moberg K.. 2007. Maternal behavior in pigs. Horm. Behav. 52:78–85. doi: 10.1016/j.yhbeh.2007.03.022 [DOI] [PubMed] [Google Scholar]
- Almond G. W., Flowers W. L., Batista L., and D’Allaire S.. 2006. Diseases of the reproductive system. In: Straw B. E., Zimmerman J.J ., D’Allaire S., Taylor D. J., editors. Diseases of swine. 9th ed. Ames (IA): Blackwell; p. 125. [Google Scholar]
- Balzani A., Cordell H. J., and Edwards S. A.. 2016. Relationship of sow udder morphology with piglet suckling behavior and teat access. Theriogenology. 86:1913–1920. doi: 10.1016/j.theriogenology.2016.06.007 [DOI] [PubMed] [Google Scholar]
- Baxter E. M., Jarvis S., D’Eath R. B., Ross D. W., Robson S. K., Farish M., Nevison I. M., Lawrence A. B., and Edwards S. A.. 2008. Investigating the behavioural and physiological indicators of neonatal survival in pigs. Theriogenology. 69:773–783. doi: 10.1016/j.theriogenology.2007.12.007 [DOI] [PubMed] [Google Scholar]
- Beaulieu A. D., Aalhus J. L., Williams N. H., and Patience J. F.. 2010. Impact of piglet birth weight, birth order, and litter size on subsequent growth performance, carcass quality, muscle composition, and eating quality of pork. J. Anim. Sci. 88:2767–2778. doi: 10.2527/jas.2009-2222 [DOI] [PubMed] [Google Scholar]
- Bellino C., Gianella P., Grattarola C., Miniscalco B., Tursi M., Dondo A., D’Angelo A., and Cagnasso A.. 2013. Urinary tract infections in sows in Italy: accuracy of urinalysis and urine culture against histological findings. Vet. Rec. 172:183. doi: 10.1136/vr.101219 [DOI] [PubMed] [Google Scholar]
- Berglund S., Grunewald C., Pettersson H., and Cnattingius S.. 2008. Severe asphyxia due to delivery-related malpractice in Sweden 1990–2005. B.J.O.G. 115:316–323. doi: 10.1111/j.1471-0528.2007.01602.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biksi I., Takács N., Vetési F., Fodor L., Szenci O., and Fenyö E.. 2002. Association between endometritis and urocystitis in culled sows. Acta Vet. Hung. 50:413–423. doi: 10.1556/AVet.50.2002.4.4 [DOI] [PubMed] [Google Scholar]
- Björkman S. 2017. Parturition and subsequent uterine health and fertility in sows [PhD thesis]. Helsinki (Finland): University Helsinki; https://helda.helsinki.fi/handle/10138/220927. [Google Scholar]
- Björkman S., Oliviero C., Hasan S., and Peltoniemi O. A. T.. 2017a. Mammary gland edema as a cause of postpartum dysgalactia in the sow-a case report. Reprod. Dom. Anim. 52:72 (Abstr.). [Google Scholar]
- Björkman S., Oliviero C., Rajala-Schultz P. J., Soede N. M., Peltoniemi O. A. T.. 2017b. The effect of litter size, parity, farrowing duration on placenta expulsion and retention in sows. Theriogenol. 92:36–44. doi: 10.1016/j.theriogenology.2017.01.003 [DOI] [PubMed] [Google Scholar]
- Björkman S., Yun J., Niku M., Oliviero C., Soede N. M., and Peltoniemi O. A. T.. 2017c. Serial transvaginal ultrasound-guided biopsy of the porcine corpus luteum in vivo. Reprod. Fertil. Dev. 29:931–939. doi: 10.1071/RD15435 [DOI] [PubMed] [Google Scholar]
- Björkman S., Grahofer A., Han T., Oliviero C., and Peltoniemi O. A. T.. 2018a. Severe udder edema as a cause of reduced colostrum quality and milk production in sows – a case report. Proceedings of the 10th European Symposium of Porcine Health Management; Barcelona, Spain; p. 110–111.
- Björkman S., Han T., Oliviero C., and Peltoniemi O. A. T.. 2018b. Biopsy of mammary gland in sows: a tool for studying colostrum production. Reprod. Dom. Anim. 53:111–112. doi: 10.1071/RD15435 [DOI] [Google Scholar]
- Björkman S., Oliviero C., Kauffold J., Soede N. M., and Peltoniemi O. A. T.. 2018c. Prolonged parturition and impaired placenta expulsion increase the risk of postpartum metritis and delay uterine involution in sows. Theriogenology. 106:87–92. doi: 10.1016/j.theriogenology.2017.10.003 [DOI] [PubMed] [Google Scholar]
- Boksa P., Zhang Y., and Nouel D.. 2015. Maternal oxytocin administration before birth influences the effects of birth anoxia on the neonatal rat brain. Neurochem. Res. 40:1631–1643. doi: 10.1007/s11064-015-1645-7 [DOI] [PubMed] [Google Scholar]
- Bontempo V., Sciannimanico D., Pastorelli G., Rossi R., Rosi F., and Corino C.. 2004. Dietary conjugated linoleic acid positively affects immunologic variables in lactating sows and piglets. J. Nutr. 134:817–824. doi: 10.1093/jn/134.4.817 [DOI] [PubMed] [Google Scholar]
- Brüssow K. P., Torner H., Rátky J., Hunter M. G., and Nürnberg G.. 1997. Ovum pick up in swine: the influence of aspiration vacuum pressure on oocyte recovery from preovulatory follicles. Acta Vet. Hung. 45:189–196. [PubMed] [Google Scholar]
- Cheng C., Wei H., Xu C., Xie X., Jiang S., and Peng J.. 2018. Maternal soluble fiber diet during pregnancy changes intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets. Appl. Environ. Microbiol. 84:e01047–18. doi: 10.1128/AEM.01047-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corino C., Pastorelli G., Rosi F., Bontempo V., and Rossi R.. 2009. Effect of dietary conjugated linoleic acid supplementation in sows on performance and immunoglobulin concentration in piglets. J. Anim. Sci. 87:2299–2305. doi: 10.2527/jas.2008-1232 [DOI] [PubMed] [Google Scholar]
- Cowart R. P. 2007. Parturition and dystocia in swine. In: Youngquist R. S. and Threlfall W. R., editors. Large animal theriogenology. St. Louis (MI): Saunders; p. 778–784. [Google Scholar]
- Cuello C., Martinez C. A., Nohalez A., Parrilla I., Roca J., Gil M. A., and Martinez E. A.. 2016. Effective vitrification and warming of porcine embryos using a pH-stable, chemically defined medium. Sci. Rep. 6:33915. doi: 10.1038/srep33915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaluwé R., Maes D., Declerck I., Cools A., Wuyts B., De Smet S., and Janssens G. P.. 2013. Changes in back fat thickness during late gestation predict colostrum yield in sows. Animal. 7:1999–2007. doi: 10.1017/S1751731113001791 [DOI] [PubMed] [Google Scholar]
- De Cock H. E., Marks S. L., Stacy B. A., Zabka T. S., Burkitt J., Lu G., Steffen D. J., and Duhamel G. E.. 2004. Ileocolitis associated with Anaerobiospirillum in cats. J. Clin. Microbiol. 42:2752–2758. doi: 10.1128/JCM.42.6.2752-2758.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers N., Farmer C., Le Dividich J., and Prunier A.. 2007. Variability of colostrum yield and colostrum intake in pigs. Animal. 1:1033–1041. doi: 10.1017/S175173110700016X [DOI] [PubMed] [Google Scholar]
- Devillers N., Le Dividich J., and Prunier A.. 2011. Influence of colostrum intake on piglet survival and immunity. Animal. 5:1605–1612. doi: 10.1017/S175173111100067X [DOI] [PubMed] [Google Scholar]
- Dial G. D., Almond G. W., Hilley H. D., Repasky R. R., and Hagan J.. 1987. Oxytocin precipitation of prostaglandin-induced farrowing in swine: determination of the optimal dose of oxytocin and optimal interval between prostaglandin F2 alpha and oxytocin. Am. J. Vet. Res. 48:966–970. [PubMed] [Google Scholar]
- Edwards S. A., and Baxter E. M.. 2015. Piglet mortality: causes and prevention. In: Farmer C., editor. The gestating and lactating sow. Wageningen (the Netherlands): Wageningen Academic Publishers; p. 649–653. [Google Scholar]
- Einarsson S., and Rojkittikhun T.. 1993. Effects of nutrition on pregnant and lactating sows. J. Reprod. Fertil. Suppl. 48:229–239. [PubMed] [Google Scholar]
- Farmer C., and Hurley W. L.. 2015. Mammary development. In: Farmer C., editor. The gestating and lactating sow. Wageningen (the Netherlands): Wageningen Academic Publishers; p. 193–216. [Google Scholar]
- Farmer C., Duarte C. R., Vignola M., and Palin M. F.. 2016. Body condition of gilts at the end of gestation affects their mammary development. J. Anim. Sci. 94:1897–1905. doi: 10.2527/jas.2016-0336 [DOI] [PubMed] [Google Scholar]
- Farmer C., Mae D., and Peltoniemi O. A. T.. 2019. Mammary system. In: Zimmerman J. A., Karriker L. A., Ramirez A., Schwartz K. J., Stevenson G. W. and Zhang J., editors. Diseases of swine. 11th ed. Hoboken (NJ): Wiley Blackwell; p. 313–338. [Google Scholar]
- Farmer C., and Quesnel H.. 2009. Nutritional, hormonal, and environmental effects on colostrum in sows. J. Anim. Sci. 87(13 Suppl):56–64. doi: 10.2527/jas.2008-1203 [DOI] [PubMed] [Google Scholar]
- Feyera T., Pedersen T. F., Krogh U., Foldager L., and Theil P. K.. 2018. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 96:2320–2331. doi: 10.1093/jas/sky141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisnet A., Farmer C., David C., and Quesnel H.. 2010. Relationships between colostrum production by primiparous sows and sow physiology around parturition. J. Anim. Sci. 88:1672–1683. doi: 10.2527/jas.2009-2562 [DOI] [PubMed] [Google Scholar]
- Gilbert C. L. 1999. Oxytocin secretion and management of parturition in the pig. Reprod. Dom. Anim. 34:193–200. doi: 10.1111/j.1439-0531.1999.tb01240.x [DOI] [Google Scholar]
- Grahofer A., Dettwiller M., and Nathues H.. 2019. Uterotonic agent during farrowing caused an increases stillborn rate in a free farrowing system. First Symposium of the European College of Animal Reproduction, Vienna, Austria.
- Grahofer A., Sipos S., Fischer L., Entenfellner F., and Sipos W.. 2014. Relationship between bacteriological and chemic-analytical urinalysis from sows with reproductive disorders. The 6th European Symposium of Porcine Health Management. 126. [Google Scholar]
- Gu Z., Gao Y., Lin B., Zhong Z., Liu Z., Wang C., and Li B.. 2011. Impacts of a freedom farrowing pen design on sow behaviours and performance. Prev. Vet. Med. 102:296–303. doi: 10.1016/j.prevetmed.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Hales J., Moustsen V. A., Devreese A. M., Nielsen M. B. F., and Hansen C. F.. 2015. Comparable farrowing progress in confined and loose housed hyper-prolific sows. Livest. Sci. 171:64–72. doi: 10.1016/j.livsci.2014.11.009 [DOI] [Google Scholar]
- Han T., Björkman S., Oliviero C., and Peltoniemi O. A. T.. 2018. Mammary gland biopsy does not affect colostrum yield of sows: a pilot study. Reprod. Domest. Anim. 53:144. [Google Scholar]
- Hansen P. J. 2019. Reproductive physiology of the heat-stressed dairy cow: implications for fertility and assisted reproduction. Anim. Reprod. 16: 497–507. doi: 10.21451/1984-3143-AR2019-0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. J., Bergeron R. A., and Gonyou H. W.. 2001. Parturient behaviour and offspring-directed aggression in farmed wild boar of three genetic lines. Appl. Anim. Behav. Sci. 74:153–163. doi: 10.1016/S0168-1591(01)00160-5 [DOI] [Google Scholar]
- Harris M. J., and Gonyou H. W.. 1998. Increasing available space in a farrowing crate does not facilitate postural changes or maternal responses in gilts. Appl. Anim. Behav. Sci. 59:285–296. doi: 10.1016/S0168-1591(98)00142-7 [DOI] [Google Scholar]
- Hasan S. M., Junnikkala S., Valros A., Peltoniemi O., and Oliviero C.. 2016. Validation of Brix refractometer to estimate colostrum immunoglobulin G content and composition in the sow. Animal. 10:1728–1733. doi: 10.1017/S1751731116000896 [DOI] [PubMed] [Google Scholar]
- Hasan S., Junnikkala S., Peltoniemi O., Paulin L., Lyyski A., Vuorenmaa J., and Oliviero C.. 2018a. Dietary supplementation with yeast hydrolysate in pregnancy influences colostrum yield and gut microbiota of sows and piglets after birth. Plos One. 13:e0197586. doi: 10.1371/journal.pone.0197586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan S., Saha S., Junnikkala S., Orro T., Peltoniemi O. A. T., and Oliviero C.. 2018b. Late gestation diet supplementation of resin acid-enriched composition increases sow colostrum immunoglobulin G content, piglet colostrum intake and improve sow gut microbiota. Animal. 1–8. doi: 10.1017/S1751731118003518 [DOI] [PubMed] [Google Scholar]
- Hasan S., Orro T., Junnikkala S., Valros A., Peltoniemi O. A. T., and Oliviero C.. 2019. Factors affecting sow colostrum yield and composition, and their impact on piglet growth and health. Livest. Sci. 227:60–67. doi: 10.1016/j.livsci.2019.07.004 [DOI] [Google Scholar]
- Herpin P., Hulin J. C., Le Dividich J., and Fillaut M.. 2001. Effect of oxygen inhalation at birth on the reduction of early postnatal mortality in pigs. J. Anim. Sci. 79:5–10. doi: 10.2527/2001.7915 [DOI] [PubMed] [Google Scholar]
- Holyoake P. K., Dial G. D., Trigg T., and King V. L.. 1995. Reducing pig mortality through supervision during the perinatal period. J. Anim. Sci. 73:3543–3551. doi: 10.2527/1995.73123543x [DOI] [PubMed] [Google Scholar]
- Hurley W. 2015. Composition of sow colostrum and milk. In: Farmer C., editor. The gestating and lactating sow; p. 193–229. Wageningen (The Netherlands): Wageningen Academic Publishers. [Google Scholar]
- Jensen P. 1986. Observations of the maternal behaviour of free-ranging domestic pigs. Appl. Anim. Behav. Sci. 16:131–142. doi: 10.1016/0168-1591(86)90105-X [DOI] [Google Scholar]
- Jha R., Fouhse J. M., Tiwari U. P., Li L., and Willing B. P.. 2019. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6:48. doi: 10.3389/fvets.2019.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiarpinitnun P., Loyawatananan S., Sangratkanjanasin P., Kompong K., Nuntapaitoon M., Muns R., De Rensis F., and Tummaruk P.. 2019. Administration of carbetocin after the first piglet was born reduced farrowing duration but compromised colostrum intake in newborn piglets. Theriogenology. 128:23–30. doi: 10.1016/j.theriogenology.2019.01.021 [DOI] [PubMed] [Google Scholar]
- Jones J. E. T. 1966. Observations on parturition in the Sow: part II: the parturient and post-parturient phases. Brit. Vet. J. 122:471–478. doi: 10.1016/S0007-1935(17)40303-4 [DOI] [Google Scholar]
- Kaeoket K. 2006. The effect of dose and route of administration of R-cloprostenol on the parturient response of sows. Reprod. Domest. Anim. 41:472–476. doi: 10.1111/j.1439-0531.2006.00674.x [DOI] [PubMed] [Google Scholar]
- Kaiser M., Jacobson M., Andersen P. H., Bækbo P., Cerón J. J., Dahl J., Escribano D., and Jacobsen S.. 2018a. Inflammatory markers before and after farrowing in healthy sows and in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 14:83. doi: 10.1186/s12917-018-1382-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser M., Jacobsen S., Andersen P. H., Bækbo P., Cerón J. J., Dahl J., Escribano D., Theil P. K., and Jacobson M.. 2018b. Hormonal and metabolic indicators before and after farrowing in sows affected with postpartum dysgalactia syndrome. BMC Vet. Res. 14:334. doi: 10.1186/s12917-018-1649-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffold J., and Althouse G. C.. 2007. An update on the use of B-mode ultrasonography in female pig reproduction. Theriogenology. 67:901–911. doi: 10.1016/j.theriogenology.2006.12.005 [DOI] [PubMed] [Google Scholar]
- Kauffold J., von dem Bussche B., Failing K., Wehrend A., and Wendt M.. 2010. Use of B-mode ultrasound and grey-scale analysis to study uterine echogenicity in the pig. J. Reprod. Dev. 56:444–448. doi: 10.1262/jrd.09-220t [DOI] [PubMed] [Google Scholar]
- Kauffold J., Peltoniemi O. A. T., Wehrend A., and Althouse G. C.. 2019. Principles and clinical uses of real time ultrasonography in female swine reproduction. Animal. 9(11):950. doi: 10.3390/ani9110950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffold J., and Wehrend A.. 2014. Fertilitätsstörungen beim weiblichen Schwein. Tierärztl. Praxis Ausgabe G: Großtiere/Nutztiere. 42:179–186. doi: 10.1055/s-0038-1623223 [DOI] [PubMed] [Google Scholar]
- Kirkden R. D., Broom D. M., and Andersen I. L.. 2013. Invited review: piglet mortality: management solutions. J. Anim. Sci. 7:3361–33 89. doi: 10.2527/jas.2012-5637 [DOI] [PubMed] [Google Scholar]
- Klobasa F., and Butler J. E.. 1987. Absolute and relative concentrations of immunoglobulins G, M, and A, and albumin in the lacteal secretion of sows of different lactation numbers. Am. J. Vet. Res. 48:176–182. [PubMed] [Google Scholar]
- Lawrence A. B., McLean K. A., Jarvis S., Gilbert C. L., and Petherick J. C.. 1997. Stress and parturition in the pig. Reprod. Dom. Anim. 32:231–236. doi: 10.1111/j.1439-0531.1997.tb01287.x [DOI] [Google Scholar]
- Le Dividich J., Rooke J., and Herpin P.. 2005. Review: nutritional and immunological importance of colostrum for the newborn pig. J. Agric. Sci. 143:469–485. doi: 10.1017/S0021859605005642 [DOI] [Google Scholar]
- Lopetuso L. R., Scaldaferri F., Petito V., and Gasbarrini A.. 2013. Commensal Clostridia: leading players in the maintenance of gut homeostasis. Gut Pathog. 5:23. doi: 10.1186/1757-4749-5-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund M., Puonti M., Rydhmer L., and Jensen J.. 2002. Relationship between litter size and perinatal and pre-weaning survival in pigs. Anim. Sci. 74:217–222. doi: 10.1017/S1357729800052383 [DOI] [Google Scholar]
- Madec F., and Leon E.. 1992. Farrowing disorders in the sow: a field study. Zentralbl. Veterinarmed. A. 39:433–444. doi: 10.1111/j.1439-0442.1992.tb00202.x [DOI] [PubMed] [Google Scholar]
- Martinez E. A., Nohalez A., Martinez C. A., Parrilla I., Vila J., Colina I., Diaz M., Reixach J., Vazquez J. L., Roca J., et al. 2016. The recipients’ parity does not influence their reproductive performance following non-surgical deep uterine porcine embryo transfer. Reprod. Domest. Anim. 51:123–129. doi: 10.1111/rda.12654 [DOI] [PubMed] [Google Scholar]
- Matheson S. M., Walling G. A., and Edwards S. A.. 2018. Genetic selection against intrauterine growth retardation in piglets: a problem at the piglet level with a solution at the sow level. Genet. Sel. Evol. 50:46. doi: 10.1186/s12711-018-0417-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota-Rojas D., Martínez-Burnes J., Trujillo M. E., López A., Rosales A. M., Ramírez R., Orozco H., Merino A., and Alonso-Spilsbury M.. 2005. Uterine and fetal asphyxia monitoring in parturient sows treated with oxytocin. Anim. Reprod. Sci. 86:131–141. doi: 10.1016/j.anireprosci.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Mota-Rojas D., Martínez-Burnes J., Trujillo-Ortega M. E., Alonso-Spilsbury M. L., Ramírez-Necoechea R., and López A.. 2002. Effect of oxytocin treatment in sows on umbilical cord morphology, meconium staining, and neonatal mortality of piglets. Am. J. Vet. Res. 63:1571–1574. doi: 10.2460/ajvr.2002.63.1571 [DOI] [PubMed] [Google Scholar]
- Mota-Rojas D., Rosales A. M., Trujillo M. E., Orozco H., Ramírez R., and Alonso-Spilsbury M.. 2005. The effects of vetrabutin chlorhydrate and oxytocin on stillbirth rate and asphyxia in swine. Theriogenology. 64:1889–1897. doi: 10.1016/j.theriogenology.2004.12.018 [DOI] [PubMed] [Google Scholar]
- Mota-Rojas D., Villanueva-García D., Velazquez-Armenta E. Y., Nava-Ocampo A. A., Ramírez-Necoechea R., Alonso-Spilsbury M., and Trujillo M. E.. 2007. Influence of time at which oxytocin is administered during labor on uterine activity and perinatal death in pigs. Biol. Res. 40:55–63. doi: 10.4067/s0716-97602007000100006 [DOI] [PubMed] [Google Scholar]
- Mukhopadhya I., Hansen R., El-Omar E. M., Hold G. L.. 2012. IBD—what role do Proteobacteria play? Nat. Rev. Gastroenterol. Hepatol. 9:219–230. doi: 10.1038/nrgastro.2012.14 [DOI] [PubMed] [Google Scholar]
- Myers M. J., Smith E. R., and Turfle P. G.. 2017. Biomarkers in veterinary medicine. Annu. Rev. Anim. Biosci. 8:65–87. doi: 10.1146/annurev-animal-021815-111431 [DOI] [PubMed] [Google Scholar]
- Oliviero C.2010. http://urn.fi/URN:ISBN:978-952-92-7637-0 Successful farrowing [PhD thesis]. University Helsinki.
- Oliviero C. 2013. Management to improve neonate piglet survival. In: Rodriqez-Martinez H., Soede N., and Flowers W., editors, Control of pig reproduction IX, Leicestershire (UK): Context; pp. 203–210. [Google Scholar]
- Oliviero C., Heinonen M., Valros A., Hälli O., and Peltoniemi O. A.. 2008. Effect of the environment on the physiology of the sow during late pregnancy, farrowing and early lactation. Anim. Reprod. Sci. 105:365–377. doi: 10.1016/j.anireprosci.2007.03.015 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Heinonen M., Valros A., and Peltoniemi O.. 2010. Environmental and sow-related factors affecting the duration of farrowing. Anim. Reprod. Sci. 119:85–91. doi: 10.1016/j.anireprosci.2009.12.009 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Junnikkala S., and Peltoniemi O.. 2019. The challenge of large litters on the immune system of the sow and the piglets. Reprod. Domest. Anim. 54(Suppl 3):12–21. doi: 10.1111/rda.13463 [DOI] [PubMed] [Google Scholar]
- Oliviero C., Kokkonen T., Heinonen M., Sankari S., and Peltoniemi O.. 2009. Feeding sows with high fibre diet around farrowing and early lactation: impact on intestinal activity, energy balance related parameters and litter performance. Res. Vet. Sci. 86:314–319. doi: 10.1016/j.rvsc.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Oscarsson M. E., Amer-Wåhlin I., Rydhstroem H., and Källén K.. 2006. Outcome in obstetric care related to oxytocin use. A population-based study. Acta Obstet. Gynecol. Scand. 85:1094–1098. doi: 10.1080/00016340600804530 [DOI] [PubMed] [Google Scholar]
- Pedersen L. J., Jørgensen E., Heiskanen T., and Damm B. I.. 2006. Early piglet mortality in loose-housed sows related to sow and piglet behaviour and to the progress of parturition. Appl. Anim. Behav. Sci. 96:215–232. doi: 10.1016/j.applanim.2005.06.016 [DOI] [Google Scholar]
- Peltoniemi O., Björkman S., and Oliviero C.. 2016. Parturition effects on reproductive health in the gilt and sow. Reprod. Domest. Anim. 51(Suppl 2):36–47. doi: 10.1111/rda.12798 [DOI] [PubMed] [Google Scholar]
- Peltoniemi O. A. T., Björkman S., and Oliviero C.. 2019a. Disorders of parturition and the puerperium in the gilt and sow. In: Noakes D. E., Parkinson T., England G. C. W., editors. Veterinary reproduction and obstetrics. 10th ed. China: Elsevier, Veterinary reproduction and obstetrics; p. 315–325. [Google Scholar]
- Peltoniemi O. A. T., Björkman S., Oropeza-Moe M., and Oliviero C.. 2019b. Developments of reproductive management and biotechnology in the pig. Anim. Reprod. 16:524–538. doi: 10.21451/1984-3143-AR2019-0055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesnel H. 2011. Colostrum production by sows: variability of colostrum yield and immunoglobulin G concentrations. Animal. 5:1546–1553. doi: 10.1017/S175173111100070X [DOI] [PubMed] [Google Scholar]
- Quesnel H., Brossard L., Valancogne A., and Quiniou N.. 2008. Influence of some sow characteristics on within‐litter variation of piglet birth weight. Animal. 2:1842–1849. doi: 10.1017/S175173110800308X [DOI] [PubMed] [Google Scholar]
- Quesnel H., Farmer C., and Devillers N.. 2012. Colostrum intake: influence on piglet performance and factors of variation. Livest. Sci. 146:105–114. doi: 10.1016/j.livsci.2012.03.010 [DOI] [Google Scholar]
- Quesnel H., Farmer C., and Theil P. K.. 2015. Colostrum and milk production. In: Farmer C., editor. The gestating and lactating sow. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 173–192. [Google Scholar]
- Quesnel H., Meunier-Salaün M. C., Hamard A., Guillemet R., Etienne M., Farmer C., Dourmad J. Y., and Père M. C.. 2009. Dietary fiber for pregnant sows: influence on sow physiology and performance during lactation. J. Anim. Sci. 87:532–543. doi: 10.2527/jas.2008-1231 [DOI] [PubMed] [Google Scholar]
- Randall G. C. 1972. Observations on parturition in the sow. I. Factors associated with the delivery of the piglets and their subsequent behaviour. Vet. Rec. 90:178–182. doi: 10.1136/vr.90.7.178 [DOI] [PubMed] [Google Scholar]
- Salmon H., Berri M., Gerdts V., and Meurens F.. 2009. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 33:384–393. doi: 10.1016/j.dci.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Salyers A. A., and Whitt D. D.. 2002. Bacterial pathogenesis: a molecular approach. 2nd ed. Washington, DC: ASM Press. [Google Scholar]
- Santiago P. R., Martínez-Burnes J., Mayagoitia A. L., Ramírez-Necoechea R., and Mota-Rojas D.. 2019. Relationship of vitality and weight with the temperature of newborn piglets born to sows of different parity. Livest. Sci. 220:26–31. doi: 10.1016/j.livsci.2018.12.011 [DOI] [Google Scholar]
- Senger P. L. 2012. The organization & function of the female reproductive tract. In: Senger P. L., editor. Pathways to pregnancy & parturition. 3rd ed. Redmond (OR): Current Conceptions Inc.; p. 10–43. [Google Scholar]
- Shin N. R., Whon T. W., and Bae J. W.. 2015. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33:496–503. doi: 10.1016/j.tibtech.2015.06.011 [DOI] [PubMed] [Google Scholar]
- Sipos W., Grahofer A., Fischer L., Entenfellner F., and Sipos S.. 2014. Keimspektrum des Urogenitaltraktes von Sauen mit Fertilitätsstörungen. Wien Tierarztl Monat. 101:214–220. [Google Scholar]
- Špinka M., and Illmann G.. 2015. Nursing behavior. In: Farmer C, editor. The gestating and lactating sow. Wageningen (The Netherlands): Wageningen Academic Publishers; p. 149–150. [Google Scholar]
- Straw B. E., Bush E. J., and Dewey C. E.. 2000. Types and doses of injectable medications given to periparturient sows. J. Am. Vet. Med. Assoc. 216:510–515. doi: 10.2460/javma.2000.216.510 [DOI] [PubMed] [Google Scholar]
- Taverne M. A. M., and van den Weijden G. C.. 2008. Parturition in domestic animals: targets for future research. Reprod. Dom. Anim. 43(Suppl.):36–42. doi: 10.1111/j.1439-0531.2008.01219.x [DOI] [PubMed] [Google Scholar]
- Thodberg K., Jensen K. H., and Herskin M. S.. 2002. Nest building and farrowing in sows: relation to the reaction pattern during stress, farrowing environment and experience. Appl. Anim. Behav. Sci. 77:21–42. doi: 10.1016/S0168-1591(02)00026-6 [DOI] [Google Scholar]
- Thorsen C. K., Aagaard Schild S. L., Rangstrup-Christensen L., Bilde T., and Pedersen J. L.. 2017. The effect of farrowing duration on maternal behavior of hyperprolific sows in organic outdoor production. Livest. Sci. 204:92–97. doi: 10.1016/j.livsci.2017.08.015 [DOI] [Google Scholar]
- Varel V. H., and Yen J. T.. 1997. Microbial perspective on fiber utilization by swine. J. Anim. Sci. 75:2715–2722. doi: 10.2527/1997.75102715x [DOI] [PubMed] [Google Scholar]
- Vasdal G., and Andersen I. L.. 2012. A note on teat accessibility and sow parity—consequences for newborn piglets. Livest. Sci. 146:91–94. doi: 10.1016/j.livsci.2012.02.005 [DOI] [Google Scholar]
- Ward S. A., Kirkwood R. N., and Plush K. L.. 2019. Effects of oxytocin and carbetocin on farrowing performance. Anim. Reprod. Sci. 205:88–93. doi: 10.1016/j.anireprosci.2019.04.007 [DOI] [PubMed] [Google Scholar]
- Weijtens M. J., Reinders R. D., Urlings H. A., and Van der Plas J.. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. J. Appl. Microbiol. 86:63–70. doi: 10.1046/j.1365-2672.1999.00636.x [DOI] [PubMed] [Google Scholar]
- Wischner D., Kemper N., and Krieter J.. 2009. Nest-building behaviour in sows and consequences for pig husbandry. Livest. Sci. 124:1–8. doi: 10.1016/j.livsci.2009.01.015 [DOI] [Google Scholar]
- Wlodarska M., Willing B. P., Bravo D. M., and Finlay B. B.. 2015. Phytonutrient diet supplementation promotes beneficial Clostridia species and intestinal mucus secretion resulting in protection against enteric infection. Sci. Rep. 5:9253. doi: 10.1038/srep09253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao W., Li J., Wang J. J., Zhou W., Wang Q., Zhu R., Wang F., and Thacker P.. 2012. Effects of dietary ratio of n-6 to n-3 polyunsaturated fatty acids on immunoglobulins, cytokines, fatty acid composition, and performance of lactating sows and suckling piglets. J. Anim. Sci. Biotechnol. 3:43. doi: 10.1186/2049-1891-3-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J., Han T., Björkman S., Nystén M., Hasan S., Valros A., Oliviero C., Kim Y., and Peltoniemi O.. 2019. Factors affecting piglet mortality during the first 24 h after the onset of parturition in large litters: effects of farrowing housing on behaviour of postpartum sows. Animal. 13:1045–1053. doi: 10.1017/S1751731118002549 [DOI] [PubMed] [Google Scholar]
- Yun J., and Valros A.. 2015. Benefits of prepartum nest-building behaviour on parturition and lactation in sows—a review. Asian-Australas J. Anim. Sci. 28:1519. doi: 10.5713/ajas.15.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Zhang H., and Liu T.. 2019a. Study on body temperature detection of pig based on infrared technology: a review. Artif. Intell. Agric. 1:14–26. doi: 10.1016/j.aiia.2019.02.002 [DOI] [Google Scholar]
- Zhang S., Heng J., Song H., Zhang Y., Lin X., Tian M., Chen F., and Guan W.. 2019b. Role of maternal dietary protein and amino acids on fetal programming, early neonatal development, and lactation in swine. Animal. 13:9. doi: 10.3390/ani9010019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Zhu Y., Zhou D., Wu Q., Song D., Dicksved J., and Wang J.. 2017. Oral administration of a select mixture of Bacillus probiotics affects the gut microbiota and goblet cell function following Escherichia coli challenge in newly weaned pigs of genotype MUC4 that are supposed to be enterotoxigenic E. coli F4ab/ac receptor negative. Appl. Environm. Microbiol. 83:2747. doi: 10.1128/AEM.02747-16 [DOI] [PMC free article] [PubMed] [Google Scholar]