Introduction
The periparturient period is one of the most challenging periods in dairy cows and encompasses the 3 wk prior to and 3 wk after parturition. The nutrient requirements of dairy cows change greatly during this time, largely due to the exponential growth of the gravid uterus and fetus, followed by the demands of lactation (NRC, 2001). Inflammation, oxidative stress, and adipose tissue mobilization lead to a reduction in dry matter intake (DMI) during the periparturient period. This reduction in DMI leads to a negative nutrient balance (NNB), with a shortfall in the nutrient availability for the cow and fetus (Ingvartsen and Andersen, 2000). Additionally, this reduction in DMI also increases the risk of metabolic (ketosis, fatty liver, milk fever) and immune-related disorders. The risk of these diseases, poor reproduction, and low efficiency is greatly impacted by the degree and length of time during which these systems (metabolism and immune response) remain out of balance (Loor et al., 2013a). Much of the research over the last decade have examined these biological interactions to identify the mechanisms behind the metabolic, physiologic, and immune adaptations that occur during the periparturient period (Loor et al., 2013a, 2013b; Roche et al., 2013; Bradford et al., 2015).
It is now known that nutrients, such as amino acids (AA), serve functional roles outside of their use as building blocks for proteins and have immunomodulatory properties and interact through common biochemical pathways (e.g., 1-carbon metabolism; Figure 1). This concept has been well explored in nonruminant species (Li et al., 2007; Sikalidis, 2015). With nutritional management during the periparturient period continuing to be an active area of research, it is important to develop a system understanding the potential immunometabolic role that dietary AA may play during this period. Thus, the objective of this review is to provide an overview of physiological adaptations during the periparturient period, the immune system, and methods to assess immune function and oxidative stress. This will be followed by a more specific discussion of the immunometabolic roles of specific AA and their potential effects in dairy cow during the periparturient period. The potential effects of enhanced AA supply during the preweaning period will also be discussed briefly.
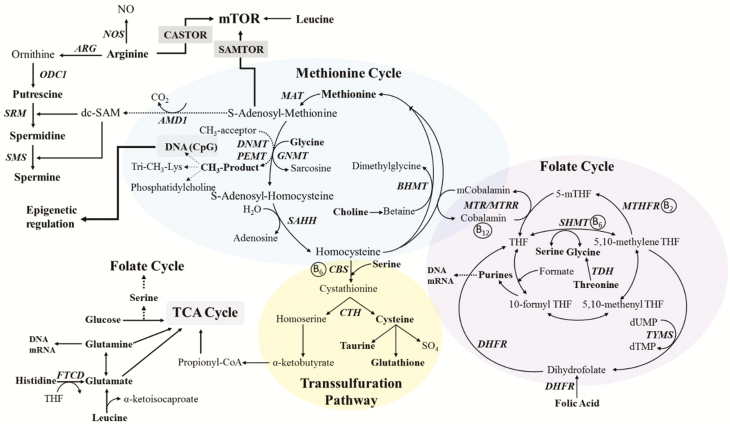
Figure 1.
Interrelationships among components of the 1-carbon metabolism pathway (Methionine cycle, Folate cycle, and Transsulfuration pathway). 5-Mthf, 5-methyl-tetrahydrofolate; AMD1, adenosylmethionine decarboxylase 1; ARG, arginase; B12, cobalamin; B2, riboflavin; B6, Pyridoxal 5′-phosphate; CBS, cystathionine beta-synthase; dc-SAM, decarboxylated S-adenosylmethionine; DHFR, dihydrofolate reductase; DNMT, DNA methyltransferase; dTMP, thymidine monophosphate; dUMP, deoxyuridine monophosphate; FTCD, formimidoyltransferase cyclodeaminase; GNMT, glycine N-methyltransferase; MAT, methionine adenosyltransferase; MTHFR, methylenetetrahydrofolate reductase; MTRR, 5-methyltetrahydrofolate-homocysteine methyltransferase reductase; ODC1, ornithine decarboxylase 1; PEMT, phosphatidylethanolamine N-methyltransferase; SAHH, S-adenosyl-homocysteine hydrolase; SHMT, serine hydroxymethyltransferase; SMS, spermine synthase; SRM, spermidine synthase; TDH, threonine dehydrogenase; THF, tetrahydrofolate; TYMS, thymidylate synthetase.
Biological Adaptations in the Transition Cow—a Brief Overview
During the NNB associated with the periparturient period, biological mechanisms coordinate the mobilization of body reserves in order to support fetal growth and milk production (Bauman and Currie, 1980; Ingvartsen, 2006); insulin concentrations are reduced and the response of hormone-sensitive lipase in adipose tissue (e.g., low insulin, high growth hormone and catecholamines, or high glucocorticoid concentrations) is greater to facilitate lipid mobilization. This periparturient period is also characterized by a state of inflammation encompassing an increase in hepatic production of positive acute-phase proteins (APP), such as haptoglobin and serum amyloid A (SAA), and a decrease in the production of negative APP, such as albumin (Bertoni et al., 2008). It has been well established that these responses are mediated by the pro-inflammatory cytokines interleukin (IL)-6, IL-1β, and tumor necrosis factor-α (TNF-α) (Kindt et al., 2007). Additionally, oxidative stress also occurs during this period and is driven by the imbalance between the production of reactive oxygen metabolites (ROM), reactive nitrogen species (RNS), and the neutralizing capacity of antioxidant mechanisms in tissues and blood. Some of the well-established cellular antioxidants include glutathione (GSH), taurine, superoxide dismutase (SOD), and vitamins A and E (Bernabucci et al., 2005). When oxidative stress overwhelms cellular antioxidant capacity, ROM induce an inflammatory response that is controlled via changes in mRNA abundance of transcription regulators (e.g., signal transducer and activator of transcription 3 [STAT3], nuclear factor-kappa b [NF-κB]). The increase in oxidative stress and inflammation during this period is also negatively associated with a reduction in liver functionality, and measurement of APP can provide a useful tool to assess liver function as well as inflammation (Bertoni and Trevisi, 2013).
During the periparturient period, AA metabolism is also altered, with moderate carcass protein losses reported even when animals are fed to their predicted metabolizable protein requirements (Bell et al., 2000). Additionally, circulating AA concentrations change dramatically, with favorable circulating profiles of many AA not being restored until 28 d postpartum (Zhou et al., 2016b). Furthermore, the total concentration of AA in plasma reaches a nadir around day 1 postpartum (Zhou et al., 2016b), which corresponds to a peak in total disease incidence during early lactation (Ingvartsen, 2006). This decrease in circulating AA is likely associated with the increased use of AA for gluconeogenesis as well as for hepatic production of APP. Thus, it is important to investigate how supplemental AA during the periparturient period may modulate metabolism and immune responses to promote production and reduce susceptibility to disease.
General Overview of the Immune System
The immune system consists of both the adaptive and innate immune responses, which are linked together through signaling molecules such as cytokines. The innate immune system is the first line of defense and includes physical barriers such as epithelial cell layers that express tight cell junctions and the mucus layer of the respiratory, gastrointestinal, and genitourinary tracts (Chaplin, 2010). Cells involved in the innate immune response include: macrophages, polymorphonuclear neutrophils (PMN), dendritic cells, mast cells, eosinophils, natural killer cells, and natural killer T cells (Turvey and Broide, 2010). The focus of the present review is on the effects of AA on phagocytic cells, that is, cells that engulf and kill such as macrophages and PMN. Macrophages not only phagocytose invading pathogens but also produce cytokines such as ILs and TNF-α, which initiate innate and adaptive immune responses and recruit PMN to the site of infection (Chaplin, 2010).
The adaptive immune response consists of T lymphocytes, B Lymphocytes, and humoral factors (Marshall et al., 2018). There are two types of T lymphocytes: cytotoxic T cells (CD8+ cells) and T-helper (Th) cells (CD4+ cells) (Marshall et al., 2018). Cytotoxic T cells detect and eliminate infected cells, while Th cells produce ILs and interferon-γ (Ingvartsen and Moyes, 2013). The B lymphocytes are cells that produce antibodies that bind to antigens on the surface of pathogens to mark them for destruction (Marshall et al., 2018).
AA, Immune Function, and Oxidative Stress
During the periparturient period, the metabolic status is associated with the inflammatory regulation of peripheral blood mononuclear cells, with a more pronounced inflammatory response in those cells during the NNB immediately postpartum (Agrawal et al., 2017; Mann et al., 2019). This period is also associated with altered signaling of nutrient-sensing kinases in immune cells, such as the protein kinase B (AKT) and mechanistic target of rapamycin (MTOR) pathway, which may modulate cytokine production (Mann et al., 2018). AA can directly and indirectly alter the immune system. Besides being used in energy metabolism reactions and the synthesis of proteins, AA are critical for the synthesis of other functional molecules such as the antioxidants GSH and taurine, NO, histamine, and hydrogen peroxide (Li et al., 2007). Thus, this section of the review will focus on the role that AA play in modulating immune function and oxidative stress in dairy cows during the periparturient period. A special focus will be placed on AA involved in 1-carbon metabolism, as this represents an interconnected route through which AA could impact molecular events such as epigenetic regulation, protein synthesis via MTOR, energy metabolism, and antioxidant synthesis. A summary of studies investigating the effects of AA on immune function and oxidative stress in dairy cows is provided in Table 1. A summary model of how AA might alter immune function and oxidant status is depicted in Figure 2.
Table 1.
Summary of studies in dairy cows investigating the effects of supplemental AA on immune function, oxidative stress, and inflammation
| Tissue/cells | Treatment | Main outcome | Reference |
|---|---|---|---|
| Plasma | RPM for 28 d prepartum and 60 d postpartum | Improvements in plasma biomarkers indicating reduced oxidative stress and inflammation and enhanced liver function. Increased neutrophil phagocytosis and oxidative burst. | Batistel et al. (2018) |
| Plasma | Abomasal infusion of Glu for first 21 d postpartum | Infusions of Gln increased the abundance of CD4 T-cells on day 4 postpartum and increased the abundance of monocytes. | Doepel et al. (2006) |
| Mammary gland | RPM for 28 d prepartum and 60 d postpartum | Methionine supply upregulated expression of genes involved in antioxidant metabolism and increased activation of NFE2L2. | Han et al. (2018) |
| Plasma | Intravenous infusions of Gln for 5 d post-calving | Glutamine infusion decreased plasma haptoglobin and increased LPS-binding protein and SAA. | Jafari et al. (2006) |
| Subcutaneous adipose | RPM for 28 d prepartum and 60 d postpartum | Enhanced Met supply increased mRNA and protein abundance of enzymes related to GSH metabolism. | Liang et al. (2019a) |
| PMNL | Incubation with Met and/or choline | Supplemental Met coupled with adequate choline enhanced gene expression of pathogen recognition mechanisms. Methionine ameliorated the increased inflammation and oxidative stress observed when cells were incubated without choline. | Lopreiato et al. (2019) |
| Plasma and milk | Protected Gln for 21 d postpartum | Increased total blood protein and albumin, decreased plasma aspartate aminotransferase, and milk somatic cell count. | Nemati et al. (2018) |
| Whole blood | RPM for 21 d prepartum and 30 d postpartum | Increased whole blood neutrophil phagocytosis on day 21 postpartum with supplemental Met. | Osorio et al. (2013a) |
| Liver | RPM for 21 d prepartum and 30 d postpartum | Methionine supply altered flux through 1-carbon metabolism via changes in mRNA to support antioxidant and Met synthesis. | Osorio et al. (2014a) |
| Liver and plasma | RPM for 21 d prepartum and 30 d postpartum | Methionine increased liver GSH and decreased concentrations of plasma biomarkers of inflammation. | Osorio et al. (2014b) |
| Plasma | RPM for 28 d during mid-lactation | Increased proliferative ability of peripheral blood T lymphocytes with supplemental Met. | Soder and Holden (1999) |
| Plasma | RPM for 21 d prepartum and postpartum | Increased antioxidant capacity of plasma and CD4+/CD8+ T lymphocyte ratio with Met supply. | Sun et al. (2016) |
| Whole blood | RPM for 21 d prepartum and 30 d postpartum | Methionine damped the hyper-response of IL-1β during an LPS challenge through improvements in oxidative stress. | Vailati-Riboni et al. (2017) |
| Plasma | Jugular infusion of Arg and LPS in mid-lactation cows | Arginine alleviated LPS-triggered inflammation by decreasing IL-6, inducible NOS, and LPS-binding protein | Zhao et al. (2018a) |
| Serum | Jugular infusion of Arg and LPS in mid-lactation cows | Infusion of Arg promoted antioxidant mechanisms during LPS-triggered inflammation by increasing total antioxidant capacity and GSH peroxidase activity and decreasing malondialdehyde. | Zhao et al. (2018b) |
| Liver and plasma | RPM for 21 d prepartum and 30 d postpartum | Increased hepatic GSH and improved plasma biomarkers of liver function and inflammation with Met. Neutrophil phagocytosis capacity and oxidative burst were also increased with Met. | Zhou et al. (2016a) |
| Liver | RPM for 21 d prepartum and 30 d postpartum | Enhanced Met supply increased mRNA expression of genes associated with PC and antioxidant synthesis. | Zhou et al. (2017b) |
| PMNL | RPM for 21 d prepartum and 30 d postpartum | Decreased expression of genes related to inflammation and oxidative stress. | Zhou et al. (2018b) |
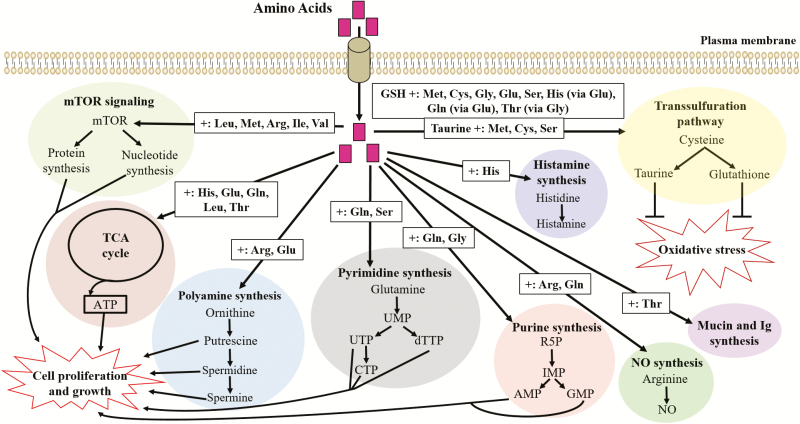
Figure 2.
The theoretical model of cellular AA utilization. AMP, adenosine monophosphate; ATP, adenosine triphosphate; CTP, cytidine triphosphate; dTTP, deoxythymidine triphosphate; GMP, guanosine monophosphate; IMP, inosine monophosphate; NO, nitric oxide; R5P, ribose 5-phosphate; TCA cycle, tricarboxylic acid cycle; UMP, uridine monophosphate; UTP, uridine-5′-triphosphate.
Methionine
Methionine not only is essential for protein synthesis but also serves as a functional nutrient that is needed for the production of the antioxidants GSH and taurine (Atmaca, 2004) and provision of methyl groups (Finkelstein, 1990). These features are exemplified by the central role of Met in 1-carbon metabolism (Figure 1). In these pathways, Met is used to synthesize S-adenosyl methionine (SAM), which can be used to methylate DNA and to support phosphatidylcholine (PC) and carnitine synthesis for fatty acid metabolism (Vance et al., 1997). During the periparturient period, triacyglycerol (TAG) accumulate in the liver leading to mitochondrial dysfunction, inflammation, and reduced liver function (Li et al., 2015; Du et al., 2018). PC is the main phospholipid component of very-low-density lipoproteins (VLDL) (Vance, 2002). Thus, enhancing PC synthesis through greater Met supply may help improve VLDL synthesis and reduce hepatic TAG accumulation.
As part of 1-carbon metabolism, homocysteine can be remethylated to Met using betaine or folate as methyl donors via betaine homocysteine methyltransferase (BHMT) and 5-methyltetrahydrofolate-homocysteine methyltransferase (MTR), respectively. Homocysteine is also used to synthesize cystathionine via cystathionine β-synthase (CBS) in the first reaction of the transsulfuration pathway (Banerjee et al., 2003). Cystathionine is used to make Cys, which is utilized to synthesize the antioxidants taurine or GSH. CBS is allosterically activated by SAM (Banerjee et al., 2003). Therefore, enhanced SAM production with increased Met supply can help enhance the flux of the transsulfuration pathway, increasing taurine and GSH production to help reduce oxidative stress.
In dairy cattle, low levels of serum Met postpartum are associated with severe hepatic lipidosis (Shibano and Kawamura, 2006) and other than His, Met is the only AA for which net uptake by the liver increased pre- and postpartum (Larsen and Kristensen, 2013). Therefore, enhancing post-ruminal Met supply during the periparturient period is of interest to increase circulating concentrations of Met for its functional roles in the body. The work from Dalbach et al. (2011) demonstrated that rumen-protected Met (RPM) can be used to increase serum concentrations of Met in the first 2-wk postpartum, which will enhance the availability of Met for protein synthesis and metabolism via the 1-carbon pathways. In terms of production, supplementation of Met during the peripartal period concomitantly increases milk yield, milk protein, and milk fat soon after calving (Ordway et al., 2009; Osorio et al., 2013). These responses are in large part driven by enhancing Met availability and by the additional flux of Met through the Met cycle in the liver, which consequently increases the production of downstream compounds such as SAM, PC, GSH, and taurine. Additionally, work feeding RPM during the periparturient period has detected positive responses in maintaining consistent rates of DMI prepartum (last 21 d) and faster and greater rates of DMI during the first 30 to 60 d after calving (Osorio et al., 2013; Zhou et al., 2016c; Batistel et al., 2017). The same milk production response was also observed when Met was supplemented postpartum as the isopropyl ester of 2-Hydroxy-4-(methylthio)-butanoic acid (St-Pierre and Sylvester, 2005).
As described earlier, the transient inflammatory-like status around parturition appears to be a “normal” aspect of the adaptations to lactation. As cows approach parturition, those with greater (but still subclinical) concentrations of circulating cytokines have greater inflammation and oxidative stress, and lower liver function, along with lower milk yield and lower postpartum DMI (Bertoni et al., 2008). In addition to their function in the immune system, cytokines, interferons, and TNF-α also elicit pathophysiological effects, leading to “sickness behaviors,” whose primary manifestation is satiety (Larson and Dunn, 2001). An example of this behavior in dairy cows is the reduction in DMI around calving. In mice, these cytokines have been shown to reduce meal size and duration as well as decrease meal frequency and prolong inter-meal intervals (Plata-Salamán, 1995). Furthermore, cytokines directly affect the hypothalamus. IL-1β and IFN act directly and specifically on the glucose-sensitive neurons in the brain “satiety” and “hunger” sites (Plata-Salamán, 1995).
In addition to increases in DMI and milk production, RPM supplementation during the periparturient period has been associated with positive health responses. Across four studies (Osorio et al., 2014b; Sun et al., 2016; Zhou et al., 2016a; Batistel et al., 2018), there have been consistent improvements in the concentrations of plasma biomarkers of inflammation, where IL-1β and haptoglobin have decreased and albumin has increased (summarized in Table 2). Improvements in biomarkers of oxidative stress have also been observed with enhanced Met supply during the periparturient period. In the study by Batistel et al. (2018), plasma concentrations of ferric-reducing antioxidant power, β-carotene, tocopherol, and total and reduced GSH were increased with RPM, while ROM were lower. Sun et al. (2016) also observed an improvement in blood antioxidant status, with RPM increasing total antioxidant capacity, glutathione peroxidase (GPX) activity, and vitamin E. A similar effect was observed in liver tissue by Osorio et al. (2014b), where cows fed RPM had greater hepatic concentrations of total and reduced GSH.
Table 2.
Summary of additional beneficial effects of feeding RPM during the transition period and early lactation
| Biomarker | Response1,2 | Site | Biological function |
|---|---|---|---|
| Metabolism | |||
| Carnitine | ↑ | Liver | β-oxidation of fatty acids |
| Cholesterol | ↑↑ | Plasma | Lipoprotein metabolism |
| One-carbon metabolism | |||
| Cystathionine beta-synthase activity | ↑ | Liver | Antioxidant synthesis |
| Cystathionine | ↑ | Plasma | H2S biosynthesis, redox status |
| Cystine | ↑ | Plasma | Redox status |
| Homocysteine | ↑ | Plasma | Methylation reactions |
| Inflammation | |||
| IL-1beta | ↓ | Plasma | Pro-inflammatory cytokine |
| Haptoglobin | ↓↓ | Plasma | Inflammation signal |
| Albumin | ↑↑ | Plasma | Acute-phase response |
| Oxidative stress | |||
| ROM | ↔/↓ | Plasma | Peroxides, superoxide, OH-radicals |
| GSH | ↑↑ | Liver, blood | Antioxidant |
| Taurine | ↔/↑ | Plasma | Antioxidant |
| Antioxidant capacity | ↔/↑ | Plasma | Total antioxidants in blood |
| Paraoxonase | ↑↑ | Plasma | Antioxidant enzyme |
1↑, beneficial increase; ↓, beneficial decrease; ↔, no change in concentration.
2Relative to a control or rumen-protected choline supplemented diet (Osorio et al., 2014b; Zhou et al., 2016a; Sun et al., 2016; Batistel et al., 2018); Vailati-Riboni et al., 2019).
From a mechanistic standpoint, changes in the mRNA abundance of S-adenosyl homocysteine hydrolase (SAHH), MTR, SOD1, glutamate cysteine ligase catalytic (GCLC) subunit, and DNA methyltransferase 3A suggested alterations in flux through 1-carbon metabolism (Osorio et al., 2014a). Importantly, SAHH, the enzyme that makes homocysteine from S-adenosylhomocysteine, was upregulated with Met, which would support a supply of homocysteine to be used for antioxidant and Met synthesis. Furthermore, in the study by Zhou et al. (2016a), greater Met supply compared with rumen-protected choline increased antioxidant concentration in liver tissue, despite a lower concentration of PC. Those responses were due to the greater abundance of phosphatidylethanolamine methyltransferase (the enzyme that utilizes SAM and phosphatidylethanolamine to make PC) and CBS (Zhou et al., 2017b). Additionally, enhanced Met supply during the periparturient period was observed to increase mRNA and protein abundance of enzymes related to GSH metabolism in subcutaneous adipose tissue, suggesting greater activation of those pathways (Liang et al., 2019a). A greater dietary supply of choline did not change the mRNA abundance of BHMT and MTR in cows (Zhou et al., 2017b).
A positive effect of Met supplementation on mammary gland antioxidant mechanisms was observed by Han et al. (2018); mRNA abundance of GPX1, GCLC, glutamate cysteine ligase (GCL) modifier subunit, malic enzyme 1, ferrochelatase and ferritin heavy chain 1, and genes involved in GSH and iron metabolism were upregulated. Protein abundance of phosphorylated nuclear factor erythroid 2-like-2 (NFE2L2):total NFE2L2 was also increased (Han et al., 2018). NFE2L2is a regulator of transcription of antioxidant genes; hence, an increase in phosphorylated NFE2L2 suggested greater activation of antioxidant systems and is likely one of the mechanisms behind the changes in mRNA abundance. Lastly, across studies, there has also been a consistent improvement in the concentrations of plasma biomarkers of liver function such as increases in paraoxonase and cholesterol with RPM (Osorio et al., 2014b; Zhou et al., 2016a; Batistel et al., 2018), which is likely linked to the reduction in inflammation and oxidative stress. Thus, the consistent changes across studies in metabolites and plasma biomarkers, as well as mRNA abundance across tissues, indicate that enhanced Met supply during the periparturient period reduces oxidative stress and inflammation. However, more work is needed to verify the exact mechanisms behind the observed changes.
Enhanced post-ruminal supply in the form of RPM during the periparturient period has been associated with improvements in immune cell function. When RPM was provided for 21 d prepartum and 30 d postpartum, whole blood neutrophil phagocytosis was increased compared with control cows at 21 d postpartum (Osorio et al., 2013). In the study by Zhou et al. (2016c), RPM supplementation from day −21 prepartum to day 30 postpartum increased neutrophil phagocytosis capacity and oxidative burst activity (Zhou et al., 2016a). This same improvement in neutrophil immune function was observed when RPM was supplied from day −28 prepartum to day 60 postpartum (Batistel et al., 2018). Furthermore, an increase in the proliferative ability of peripheral blood T lymphocytes was observed when RPM was supplemented to mid-lactation cows (Soder and Holden, 1999).
Zhou et al. (2018b) isolated polymorphonuclear leukocytes (PMNL) and observed lower abundance of genes related to inflammation (IL1B, TLR2, NF-κB, and STAT3) and oxidative stress (CBS, GPX1, glutathione synthase [GSS], and SOD2) as well as an increase in plasma taurine with supplemental Met, suggesting a better redox and inflammatory status of those cells. Additionally, those same cows were used for an ex vivo whole blood challenge with lipopolysaccharide (LPS) to further investigate immune cell responses. During this challenge, a hyper-response in IL-1β was observed around parturition, which likely arose from oxidative stress (Vailati-Riboni et al., 2017). However, RPM supplementation dampened this hyper-response, likely through improvements in oxidative stress (Vailati-Riboni et al., 2017).
The recent work by Lopreiato et al. (2019) investigated the effects of incubating bovine PMNL with Met and/or choline and observed that supplemental Met coupled with adequate choline enhanced gene expression of TLR2 and L-selectin (SELL), which are pathogen recognition mechanisms. In the same experiment, cells incubated without choline had greater mRNA abundance of IL1B, IL6, IL10, and myeloperoxidase (MPO), glutathione reductase (GSR), GSS, cystathionine gamma-lyase (CTH), and cysteine sulfinic acid decarboxylase (CSAD), indicating greater inflammation and oxidative stress; this effect, however, was ameliorated by supplementing additional Met (Lopreiato et al., 2019). Thus, the increased DMI and milk production observed when feeding RPM can be partly explained by a reduction in inflammation as it directly (at the hepatic level and by dampening the immune cell overresponse) and indirectly (reducing oxidative stress) decreases circulating pro-inflammatory cytokines.
Enhanced Met supply during the periparturient period has also been associated with changes in MTOR signaling. MTOR is a serine/threonine kinase that plays a central role in integrating environmental cues from growth factors, nutrients (particularly AA), and energy (Powell et al., 2012). In dairy cattle, MTOR has traditionally been studied in the context of its role in regulating protein synthesis; however, work from humans and nonruminant species has indicated that MTOR is an important regulator of immune responses (Powell et al., 2012; Jones and Pearce, 2017). When activated by AA, MTOR directs an activation of anabolic metabolism, which allows growth, proliferation, and development. This makes the activation of MTOR in immune cells particularly important for maintaining proliferation and without proper activation, cells may enter periods of growth arrest (Jones and Pearce, 2017). Methionine specifically may interact with MTOR via the production of SAM. Specifically, SAM can bind to S-adenosylmethionine sensor upstream of MTOR (SAMTOR), a protein that inhibits MTOR complex 1 (MTORC1) by interacting with GTPase-activating protein activity towards Rags 1 (GATOR1) (Gu et al., 2017). When SAM binds to SAMTOR, it inhibits the association of SAMTOR and GATOR1, allowing MTORC1 to be activated (Gu et al., 2017).
To our knowledge, there is only one study investigating the expression of MTOR signaling proteins in immune cells in dairy cattle, and work is also lacking in nonruminant species. In periparturient cows, the activation of AKT/MTOR signaling in immune cells was reduced postpartum compared with prepartum (Mann et al., 2018). Importantly, Agrawal et al. (2017) also identified the expression of AA transporters and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways related to 1-carbon metabolism and MTOR in PMNL from peripartal cows. A list of these transporters and KEGG pathways related to AA and 1-carbon metabolism are summarized in Table 3. Together, these studies support the potential importance of AA for nutrient signaling in immune cells.
Table 3.
Nutrient transporters and KEGG pathways related to AA and 1-carbon metabolism detected in dairy cow immune cells via transcriptomics analysis (Agrawal et al., 2017; Mann et al., 2018)
| Transporter and gene symbol | KEGG pathways |
|---|---|
| Neutral AA (SLC1A4) | Metabolic pathways |
| Neutral AA (SLC1A5) | Nucleotide metabolism |
| Glu/Asp (SLC1A6) | Energy metabolism |
| Heavy chain AA (SLC3A2) | Carbohydrate metabolism |
| Taurine (SLC6A6) | Amino acid metabolism |
| Gly (SLC6A9) | Immune-related pathways |
| Branched-chain and aromatic AA (SLC7A5) | NO and ROS production in macrophages |
| Dibasic and neutral AA (SLC7A6) | iNOS signaling |
| Neutral AA (SLC7A7) | Acute-phase response signaling |
| Branched-chain and aromatic AA (SLC7A8) | Genetic information processing |
| His, di-, and tri-peptides (SLC15A4) | Transcription |
| Trp, Pro, and Ala (SLC36A4) | Translation |
| Neutral AA (SLC38A1) | Folding, sorting, and degradation |
| Neutral AA (SLC38A2) | Replication and repair |
| Gln, His, Asn, and Ala (SLC38A3) | Organismal systems |
| Sodium-dependent AA (SLC38A6) | Immune system |
| Glu. Gln, His, Ser, Ala, and Asn (SLC38A7) | Endocrine system |
| Sodium-dependent AA, Ala, Gln, Glu, and Asp (SLC38A10) | Cellular processes |
| Neutral AA (SLC43A1) | Cell growth and death |
| Sodium-independent, Leu, Phe, Val, and Met (SLC43A2) | Cell communication |
| Glucose, galactose, mannose (SLC2A1) | Cell transport and catabolism |
| Glucose, galactose, mannose, maltose (SLC2A3) | |
| Glucose/fructose (SLC2A4) | |
| Glucose (SLC2A6) | |
| Glucose and fructose (SLC2A8) | |
| Glucose (SLC2A10) | |
| Glucose cotransporter (SLC2A13) | |
| Glucose, fructose, and mannose (SLC5A9) | |
| Long-chain fatty acid (SLC27A1) | |
| Long-chain fatty acid (SLC27A2) | |
| Long-chain fatty acid (SLC27A3) | |
| Long-chain fatty acid (SLC27A4) | |
| Carnitine (SLC22A5) | |
| Thiamine (SLC19A2) | |
| Choline (SLC44A1) | |
| Choline (SLC44A2) | |
| Choline (SLC44A4) | |
| Betaine (SLC6A12) | |
| Folate (SLC19A1) | |
| Multivitamin (SLC5A6) |
Recent work indicating that enhanced Met supply activates MTOR signaling in the mammary gland supports its role in enhancing protein synthesis. For example, in vitro, enhancing Met supply to immortalized bovine mammary epithelial cells (BMEC) by varying the ratio of Lys to Met increased the concentration and utilization of essential AA, particularly branched-chain AA (BCAA; Dong et al., 2018). This change was potentially mediated by alterations in AA transporters controlled by MTOR (Dong et al., 2018). Other studies with BMEC have also revealed a potential for greater MTOR activation when Met supply is enhanced (Nan et al., 2014; Sala ma et al., 2019).
In vivo, the effects of supplemental Met during the periparturient period on MTOR signaling were explored using cows from the study by Batistel et al. (2017). In the mammary gland, cows receiving RPM had lower protein abundance of total mTOR and phosphorylated MTOR (p-MTOR) compared with control cows on day 21 postpartum, but the ratio of p-MTOR:total MTOR was not different, suggesting that there was no difference in MTOR activation between treatments (Ma et al., 2019). However, changes in AA transporters and insulin signaling indicated that insulin sensitivity of the mammary gland was enhanced with supplemental Met (Ma et al., 2019). In subcutaneous adipose tissue, the mRNA and protein abundance of some AA transporters and p-MTOR were upregulated with enhanced Met supply, while changes in insulin signaling and plasma glucose also indicated that Met helped improve insulin sensitivity (Liang et al., 2019b). Thus, enhancing Met supply during the periparturient period may lead to MTOR activation in immune cells, as well as improved nutrient uptake, which could help to support proliferation and development. Additional work in ruminants and nonruminants is needed to understand whether Met modulates MTOR signaling in immune cells.
Cysteine
Cysteine can be synthesized endogenously from homocysteine (as described earlier) and is needed to synthesize GSH and taurine. GSH is synthesized via two enzymes, GCL and GSS (Lushchak, 2012). To synthesize taurine, cysteine sulfinic acid is first synthesized from cysteine by cysteine dioxygenase and can then be utilized by CSAD to produce taurine (Park et al., 2017). Dietary Cys has been explored as a way to improve health in nonruminant species and humans under a variety of conditions, including type-2 diabetes, cardiovascular disease, and liver disease to name a few (Yin et al., 2016). Across these studies, increased dietary Cys increased the concentrations of taurine and GSH, helping reduce oxidative stress as well as decrease the concentrations of proinflammatory cytokines (Yin et al., 2016). Work with rodents and humans has also identified Cys as a limiting factor for growth and proliferation of lymphocytes (Sikalidis, 2015). However, to our knowledge, there are no published studies with dairy cows investigating the role of Cys supply on oxidative stress and immune function. In growing pigs, stimulation of the immune system with LPS has recently been observed to increase GSH synthesis and plasma Cys flux (Rakhshandeh et al., 2019), indicating that there is an increased need for Cys during periods of immune activation. In a similar study with growing pigs, immune activation by LPS was also observed to increase the amount of dietary Cys needed (Litvak et al., 2013). In dairy cows, plasma concentrations of cystine were decreased around parturition, while taurine and GSH increased (Zhou et al., 2017c). Together, these data suggest that the need for Cys may also be increased in dairy cattle during periods of immune activation such as the periparturient period.
Arginine
Arginine (Arg) can be synthesized from citrulline in nearly all cell types via arginosuccinate synthase and lyase (Wu and Morris, 1998). One of the pathways of Arg catabolism is the arginase pathway, which produces polyamines (Wu et al., 2009). In this pathway, decarboxylated SAM is needed to synthesize two polyamines, spermidine and spermine (Wu et al., 2009), providing a link between Met and Arg metabolism. The polyamines that are produced in Arg catabolism are involved in cell proliferation and death, DNA replication, and can activate toll-like receptors (TLR) that detect microbes and activate innate immunity (Handa et al., 2018). One of the other major pathways of Arg catabolism is in the production of nitric oxide (NO) via nitric oxide synthase (NOS) (Wu et al., 2009). There are three isoforms of NOS: NOS1, NOS2 or inducible NOS (iNOS), and NOS3 (Wu et al., 2009). All three of these forms of NOS are sensitive to Arg concentrations, but most pertinent to this review is iNOS, which can be activated by cytokines and is particularly important in immune cells where it is used to kill pathogens and promotes their proliferation (Bogdan, 2015). The availability of nutrients such as glucose and AA to immune cells can be regulated by Arg through its role as a secretagogue for insulin, growth hormone, prolactin, and insulin-like growth factor 1 (Newsholme et al., 2005). Additionally, Arg can activate MTOR by binding to cytosolic arginine sensor for mTORC1 subunit 1 (Chantranupong et al., 2016). As described earlier, MTOR is an important regulator of immune cell responses, providing another link between Arg and immune cell function.
During periods of inflammation and immune system activation, Arg catabolism is increased, while supplies are decreased (Flynn et al., 2002). When the immune system of sheep was activated by an infusion of LPS, the plasma concentrations of Arg decreased (Hoskin et al., 2016; McNeil et al., 2016). Infusion with LPS in sheep also increased both net hepatic removal of Arg and net total splanchnic appearance of Arg (McNeil et al., 2016). Thus, despite Arg being synthesized endogenously, it is critical to provide it via diet to maintain production and health.
In nonruminants, there is evidence that enhanced Arg supply attenuates inflammation and enhances immune function during periods of infection such as an LPS challenge, for example, in weaned pigs (Zhu et al., 2013), broiler chickens (Tan et al., 2014), fish (Jiang et al., 2015), and mice (Calkins et al., 2001). However, to date, there are no studies in periparturient dairy cows investigating the potential effects of enhanced dietary Arg supply on immune function and oxidative stress. Evidence of the beneficial role for Arg in dairy cows was first reported in a study with BMEC incubated with or without LPS and treated with or without Arg (Wu et al., 2016); Arg attenuated the inflammatory response induced by LPS by reducing the abundance of NF-κB and decreasing the secretion of IL-6, IL-1β, and TNF-α, while also enhancing MTOR signaling (Wu et al., 2016). It is noteworthy that after 12 h of incubation, the abundance of iNOS and NO content in BMEC was lower with Arg, further suggesting that it may be able to alleviate inflammatory injury during LPS-induced inflammation (Wu et al., 2016). While this change would be unexpected due to Arg fueling NO production, it has previously been observed in rodent and human cells that supplying NO either exogenously via NO donors or synthesized endogenously can downregulate iNOS abundance through a negative feedback loop (Griscavage et al., 1993; Hinz et al., 2000). Furthermore, in human microglial cells NO inhibited LPS-induced iNOS abundance (Colasanti et al., 1995). Thus, despite the lower concentration of NO and abundance of iNOS, it is possible that in the experiment of Wu et al. (2016), the concentrations of NO increased due to Arg supplementation during the LPS challenge.
In an in vivo study, mid-lactation cows received jugular infusions of Arg alone and/or LPS and Arg alleviated LPS-triggered inflammation by decreasing IL-6, iNOS, and LPS-binding protein (Zhao et al., 2018a). Compared with cows challenged with LPS, Arg attenuated the drop in DMI associated with LPS, while also numerically increasing milk yield (Zhao et al., 2018a). Infusions of Arg to dairy cows during an LPS infusion also increased the total antioxidant capacity and activity of GPX, while malondialdehyde concentrations were decreased, indicating an improvement in antioxidant mechanisms (Zhao et al., 2018b). Thus, supplying Arg during periods of inflammation and oxidative stress, such as the periparturient period, may be a viable option to help support immune and antioxidant responses.
Heat stress is also known to induce inflammation and reduce immune function (Bradford et al., 2015). These responses have been observed to be associated with changes at the transcriptome level in immune cells. Contreras-Jodar et al. (2018) exposed lactating dairy goats to chronic heat stress and detected a downregulation in the abundance of genes associated with hematopoiesis, leukocyte diapedesis, and lipid metabolism in whole blood cells, while nucleotide metabolism was activated. These changes provided a mechanistic link between heat stress and its reduction in immune function. When Arg was supplied to heat-stressed BMEC, mammary cell proliferation was increased and there was an increase in the abundance of genes related to transcription and translation (Sala ma et al., 2019). Thus, it is possible that providing AA, such as Arg, could help to alter these responses to heat stress in immune cells.
Vicini et al. (1988) performed abomasal and intravenous infusions of Arg in lactating cows and observed an increase in somatotropin, prolactin, insulin, and placental lactogen compared with cows receiving infusions of saline. Furthermore, work with BMEC has also shown that enhanced Arg supply upregulated mRNA abundance of MTOR and the signaling proteins STAT 5, and ribosomal protein S6 kinase and Janus kinase 2, suggesting greater activation of MTOR signaling (Wang et al., 2014). While these studies did not directly examine immune function or oxidative stress, these observed responses under “non-stressful” conditions may help to promote a better response to an immune challenge. More studies are needed that specifically examine immune responses and whether changes observed in BMEC correspond to changes in immune cell mRNA abundance. Overall, available data suggest that Arg supplementation during the periparturient period may be beneficial to promote production, reduce inflammation, and enhance immune function.
Glutamine and glutamate
Glutamine (Gln) and glutamate (Glu) are two nonessential AA whose metabolism is interrelated due to the ability of Gln to be catabolized via glutaminolysis (Newsholme et al., 1999). Gln has a variety of roles beyond protein synthesis: it is a precursor of purines and pyrimidines, it is needed for NO and cytokine production, lymphocyte cell division, production of NADPH for superoxide production in macrophages, and it may also be one of the main sources of fuel for immune cells (Yassad et al., 1997; Newsholme et al., 1999; Furukawa et al., 2000). The fact that purines and pyrimidines are essential for cell proliferation renders Gln supply critical for immune cells. Glutamine has also been observed to protect neutrophils from events associated with triggering and executing apoptosis (Pithon-Curi et al., 2003). Lastly, Gln is well known as a modulator of the transcription and production of cytokines and transcription of genes responsible for heat shock proteins and respiratory burst (see reviews by Newsholme (2001) and Curi et al. (2007)). Because of these critical roles related to promoting immune function, the uptake of Gln by immune cells and tissues increases during the periods of immune challenge (Holtenius et al., 2004).
Glu is needed for the conversion of cysteine to γ-glutamyl cysteine during the synthesis of GSH (Wu et al., 2004) and is also used by glutamate dehydrogenase to synthesize alpha ketoglutarate. Furthermore, Glu is also needed for the synthesis of gamma-aminobutyrate, the primary inhibitory neurotransmitter (Newsholme et al., 2003). In immune cells, Glu is needed for AA transamination, NADPH production, and is a precursor for ornithine (Newsholme et al., 2003). Ornithine, as mentioned in a previous section, is needed for the synthesis of polyamines, underscoring the potential role of Glu in promoting cell proliferation and division. Lastly, work with alanine-serine-cysteine transporter 2 (ASCT2), a transporter of Gln, has indicated an important role of Gln and Glu with regard to oxidative stress and inflammation, particularly during cancer (Liu et al., 2018). For example, in a study with human gastric cancer cells, knockdown for ASCT2 reduced Gln uptake and GSH production, increased the concentration of reactive oxygen species (ROS), and induced apoptosis (Wang et al., 2019). Together, these functions of Gln and Glu point at their role in reducing oxidative stress and inflammation and promoting energy metabolism and proliferation in immune cells.
To our knowledge, the effects of Gln on immune cell function and oxidative stress in dairy cows during the periparturient period have not been studied. However, several studies have observed that circulating concentrations of Gln decrease during the periparturient period (Doepel et al., 2006; Zhou et al., 2017c), likely due to a combination of the increased metabolic demands and the transient inflammatory-like status that occurs. Thus, increasing the supply of Gln during the periparturient period may be beneficial for improving immune function and antioxidant responses to reduce inflammation and the risk of disease in early lactation. Intravenous infusions of Gln in dairy cows at three doses (0, 106, or 212 g/d) starting 1 d post-calving for 7 d decreased plasma haptoglobin concentrations while increasing LPS-binding protein and SAA (Jafari et al., 2006). While there were no changes in DMI or milk yield with Gln, the changes in APP suggested a potential priming effect of Gln; however, the mechanisms behind these effects could not be determined (Jafari et al., 2006). In a recent study, supplying rumen-protected Gln for the first 21 d postpartum seemed to induce beneficial effects on health; total blood protein and plasma albumin concentrations were increased while plasma aspartate aminotransferase and milk somatic cell counts decreased (Nemati et al., 2018). Additionally, abomasal infusions of Gln during the first 21 d of lactation increased the abundance of CD4 T-cells on day 4, suggesting a short-term improvement in immunocompetence (Doepel et al., 2006). Gln also increased the abundance of monocytes but did not alter T lymphocyte proliferation, abundance of CD8 T-cells, DMI, or milk yields (Doepel et al., 2006). Therefore, Gln may play a role in promoting immune cell proliferation in dairy cattle.
With regard to Glu, there are no studies in periparturient dairy cows investigating its effects on oxidative stress and immune function. However, in weaned pigs, Glu supplementation decreased plasma concentration of malondialdehyde during an intraperitoneal challenge with hydrogen peroxide (Duan et al., 2016). Similarly, in piglets challenged with deoxynivalenol (a mycotoxin), Glu supplementation reduced oxidative stress and attenuated the inhibition of MTOR signaling that was induced by a mycotoxin challenge (Wu et al., 2014). Lin et al. (1999) reported that dietary Glu enhanced lymphocyte proliferation in rats recovering from a period of immunosuppression. In fish, Glu supplementation during an LPS challenge reduced oxidative damage in the intestine via increased GSH concentration and increased activity of SOD, GPX, and GSR, while also reducing mRNA abundance of cytokines and NF-κB p65 (Jiang et al., 2017). Thus, there is potential for enhanced dietary Glu supply to elicit beneficial effects, particularly on oxidative stress, in dairy cattle.
Histidine
Histidine (His) is another AA with a variety of roles related to the immune system. One of the major routes of His catabolism is conversion to histamine via histidine decarboxylase (Grohmann and Bronte, 2010). Histamine is a mediator of inflammatory reactions that are produced by many immune cells, including mast cells, basophils, macrophages, and T lymphocytes, but can only be stored in mast cells and basophils (Dy and Schneider, 2004). The effects of histamine include increased dendritic cell migration, increased pro-inflammatory cytokine production, and promotion of Th2 cell activity (Branco et al., 2018). Trans-urocanic acid can also be made from His and can be used to synthesize Glu, which can then be used to synthesize alpha-ketoglutarate or GSH as described earlier (Glinton et al., 2018). This renders His as a potentially important in promoting energy metabolism and antioxidant responses. Additionally, His has the ability to scavenge hydroxyl radicals and singlet oxygen (Wade and Tucker, 1998), which is another way that His may enhance antioxidant responses. Lastly, one of the important functions of His is the synthesis of His-rich glycoproteins. These proteins are abundant in plasma and are involved in a variety of processes related to the immune system, including clearance of antibody complexes, phagocytosis, and cell migration and adhesion (Wakabayashi, 2013).
Most studies in dairy cows have focused on the supply of His related to the changes in metabolizable protein and the effects on production. However, in one study with late-lactating cows receiving a mastitis challenge, a jugular injection of His led to the lower antioxidant capacity of plasma from 6 to 12 h after the induction of mastitis (Chaiyotwittayakun et al., 2002). Unlike ruminants, there have been a number of studies in nonruminants exploring the effects of His on immune response and oxidative stress. In the gills of fish, His deficiency inhibited NFE2L2 signaling, upregulated pro-inflammatory cytokines, and reduced antioxidant capacity with less GSH and lower antioxidant enzyme activity (Jiang et al., 2016). However, an excess supply of dietary His resulted in similar effects with an impairment of the structural integrity of the gills of fish (Jiang et al., 2016). In broiler chickens, supplementation with His increased the activity of GPX in plasma and SOD activity of erythrocytes, suggesting an improvement in antioxidant capacity (Kopeć et al., 2013). Using mice, Andou et al. (2009) induced colitis and observed a reduction histologic damage of the colon and decreased TNF-α expression when dietary His supply was enhanced. Additionally, in the same experiment, they isolated mouse peritoneal macrophages and incubated them with or without His and LPS; His attenuated the production of TNF-α and IL-6 and the activation of NF-kB by LPS (Andou et al., 2009). Together, these studies suggest a potential for enhanced His supply to attenuate oxidative stress and inflammation. Due to the potential pro-inflammatory effects of histamine, it would be important to ascertain how much histamine is produced in response to dietary His supply, and the potential effects of increased histamine on immune responses.
BCAA: leucine, isoleucine, and valine
BCAA such as Leu, Ile, and Val play critical roles in the regulation of energy homeostasis, nutrition metabolism, gut health, immunity, and disease in humans and animals. As the most abundant of essential AA, BCAA serve as substrates for the synthesis of nitrogenous compounds, as well as signaling molecules regulating the metabolism of glucose, lipid, and protein synthesis, intestinal health, and immunity via special signaling networks, especially phosphoinositide 3-kinase/AKT/mammalian target of rapamycin (PI3K/AKT/mTOR) signal pathway (Neinast et al., 2019). In mammals, the BCAAs are initially transaminated by branched-chain aminotransferases (BCAT) to form branched-chain α-ketoacids (BCKA), representing the first step of BCAA catabolism. The last step of BCAA catabolism provides carbon skeletons that are either lost as CO2 or enter the TCA cycle.
Interest on the effects of AA on immune function is continuously growing and little is known about the impact of changes in BCAA availability on immune system functionality both in ruminants and in nonruminants. However, in human studies, it has been reported that immune cells incorporate BCAA into proteins; incorporation of Ile is the greatest into lymphocytes, followed by eosinophils and then neutrophils (Skaper et al., 1976), which implies cell-specific differences in protein-synthetic rates and in the types of proteins synthetized. BCAA deficiency is known to impair innate immune function in mammals due to a shortage of lymphocytes and white blood cells, which increases susceptibility to pathogens (Ma et al., 2018); however, this has primarily been studied in monogastric species. In cirrhotic patients, the supplementation of BCAA led to a restoration of host defense mechanisms such as the phagocytic function of neutrophils and natural killer cell activity (Nakamura, 2014). In addition, BCAAs have been observed to stimulate IgA secretion to enhance the mucosal surface defense in monogastric species and can also inhibit pathogen invasion into the lamina propria to improve host immunity (Ma et al., 2018). Studies in rodents reported that the amount of BCAA in the diet can affect killer-cell activities, mainly those involved in the elimination of virus or tumor cells. Thus, both human and rodent studies have been able to enlighten the relationship between BCAA availability and the immune function response with similar outcomes (Jose and Good, 1973; Petro and Bhattacharjee, 1981; Tsukishiro et al., 2000; Bassit et al., 2002).
Leucine regulates the immune system mainly through the MTOR signaling pathway. As already described in this review (see the Methionine section), MTOR plays a key role in linking the development of immune response, the surrounding environment, and cellular metabolism (Powell et al., 2012), regulating the innate and adaptive immune responses and also several immune functions such as promoting differentiation, activation, and function in T cells, B cells, and antigen-presenting cells. Particularly in T cells, a primary role of MTOR signaling is to sense and integrate for Th1 and Th17 differentiation from native T cells upon T cell receptor engagement (Lo et al., 2014). For instance, supplying Leu (40 mg/mL) to mice enhanced protective immunity against mucosal infection with herpes simplex virus type 1 (Uyangaa et al., 2012). In grass carp (Ctenopharyngodon idella), it has also been demonstrated that deficiency of Leu could impair immune status, upregulate pro-inflammatory cytokines, and downregulate anti-inflammatory cytokines of grass carp by the NF-κB and MTOR signaling pathways (Deng et al., 2016). These effects were reversed by optimum Leu supplementation (Deng et al., 2016).
In contrast to Leu, Ile has a strong correlation with the excretion of β-defensin. Indeed, 25 or 50 μg/mL Ile increases the mRNA and protein expressions of β-defensin 1, 2, and 3 in intestinal porcine enterocyte J2 cell line (Mao et al., 2013). Furthermore, in mice infected with tuberculosis, treatment with 250 μg of intratracheal L-Ile every 48 h induced a significant increase of β-defensins 3 and 4 associated with decreased bacillary loads and tissue damage (Rivas-Santiago et al., 2011).
Regarding the effect of Val, several studies underscored a regulatory effect of this AA on immune function pathways. In fact, Val supplementation improved dendritic cell function in cirrhotic patients (Kakazu et al., 2007), whereas a deficiency of Val in fish led to decreased growth and intestinal immune status by increasing pro-inflammatory cytokines (IL-8 and TNF-α) and decreasing anti-inflammatory cytokines (IL-10 and TGF-β1), which might be caused by changes in the NF-κB and MTOR signaling pathways (Luo et al., 2014). However, it is noteworthy that some studies reported the little effect of Val on innate or adaptive immunity in broilers (Thornton et al., 2006).
Nutritional modulation of intestinal health represents a novel approach for disease control and prevention, especially in those animals (particularly piglets and young calves) with the high risk of disorders when facing the transition from a nutritional phase to another (e.g., weaning). Supplementing BCAA (0.1% l-Leu, 0.34% l-Val, and 0.19% l-Ile) in a protein-restricted diet (17%) was shown to improve intestinal immune defense functions with an increase of intestinal immunoglobulins (IgA and sIgA) in weaned piglets (Ren et al., 2015).
There is one study in dairy cattle investigating the potential for rumen-protected BCAA supplementation during the transition period to alter nutrient signaling and inflammatory responses (Mann et al., 2018). However, no measurable effects of BCAA on the AKT and MTOR signaling pathway or cytokine mRNA expression were detected (Mann et al., 2018). Although still unclear, overall, these studies support the use of BCAA in improving health and preventing infectious diseases in animals and humans by regulating the immune system. In our opinion, the similar responses reported for laboratory (rodents) and nonruminant livestock underscore the need for studies with dairy cows involving the use of BCAA during the periparturient period.
Glycine and serine
Gly and Ser are two nonessential AA whose metabolism is interconnected due to their ability to be interconverted. Serine is derived endogenously from glucose and Glu (Wang et al., 2013), while Gly can be synthesized from several precursors, including Ser, Thr, and choline (Wang et al., 2013). Serine is converted to Gly via the enzyme serine hydroxymethyltransferase (SHMT) using tetrahydrofolate. This reaction is reversible and also represents a route of catabolism of both Gly and Ser.
The functions of Gly include protein synthesis, bile acid conjugation, purine synthesis, and heme synthesis (Wang et al., 2013). With relation to immune cells, Gly is needed for Gly-gated chloride channels in leukocytes and macrophages; the activation of these channels by Gly reduces cellular calcium, which is needed for the production of cytokines and superoxide, leading to potential anti-inflammatory effects (Zhong et al., 2003). Its need for purine synthesis also underscores the importance of Gly for promoting cell proliferation and differentiation. Glycine also plays a role in regulating oxidative stress due to its need for GSH synthesis; it is combined with γ-glutamylcysteine to GSH via GSS (Chen et al., 2013).
While the effects of Gly on immune function and oxidative stress have not been widely studied in ruminant species, it has been investigated as a therapeutic agent in nonruminant species and humans. In LPS-stimulated macrophages from rats, Gly activated Gly-gated chloride channels to reduce the influx of calcium ion, thereby blunting the production of IL-1, superoxide, and TNF-α (Wheeler and Thurman, 1999). Additionally, in rat lymphocytes stimulated with anti-CD3 antibody, Gly supply did not alter the production of IL-2, but by attenuating the increase in calcium levels it inhibited proliferation; however, the latter was a dose-dependent effect (Stachlewitz et al., 2000). In chickens treated with LPS, a deficiency of dietary Gly was associated with impaired immune responses (Konashi et al., 2000). Furthermore, in rats, feeding diet containing Gly improved survival to an endotoxin shock (lethal injections of LPS) through a reduction in TNF-α and a reduction in liver damage (Ikejima et al., 1996). Glycine has also been observed to help ameliorate metabolic syndrome in obesity and diabetes, with growing evidence that Gly supplementation may have therapeutic benefits through improving insulin sensitivity, enhancing antioxidant capacity, and reducing pro-inflammatory cytokines (reviewed previously by Wang et al., 2013). For example, when sucrose feeding was used to induce insulin resistance, rats supplemented with Gly had improved insulin sensitivity and increased hepatic concentrations of GSH, Cys, and γ-glutamylcysteine, which could help reduce oxidative stress (El-Hafidi et al., 2018).
Besides its need for protein synthesis and synthesis of Gly, Ser is catabolized in the production of Cys; Ser is needed by CBS to convert homocysteine to cystathionine (Zhu et al., 2018). This function gives Ser a role in promoting the entry of homocysteine into the transsulfuration pathway to enhance GSH and taurine production. Serine is also utilized in the production of phosphatidylserine, ceramide, and glucose (Li et al., 2007). This is important in terms of immune function since glucose is a major fuel of lymphocytes and macrophages (Newsholme et al., 1999). Additionally, in the conversion of Ser to Gly, tetrahydrofolate is converted to methylene-tetrahydrofolate, which is subsequently used to make methyl-tetrahydrofolate. The use of tetrahydrofolate in this reaction gives Ser a role in fueling the folate cycle, to provide methyl groups for the remethylation of homocysteine to Met via MTR (Figure 1).
In mouse and human T cells, Ser has been identified as an essential metabolite, with Ser metabolism being rapidly increased upon activation to supply glycine and maintain 1-carbon metabolism to support proliferation (Ma et al., 2017). Furthermore, restriction of Ser impairs pathogen-driven expansion of T cells (Ma et al., 2017), and in broiler chickens, there is also evidence that dietary restriction of Ser reduces immune responses (Konashi et al., 2000). In humans, stimulation of immune cells increased sharply the activity of SHMT just before a rise in cell number (Thorndike et al., 1979), hence, underscoring both the importance of this enzyme and the increased need of Ser during an immune response. Additionally, when lymphocytes are cultured with Ser, apoptosis was reduced, while cell growth and antibody production were stimulated (Duval et al., 1991; Franěk and Šrámková, 1996). In mice, Ser supplementation attenuated the effects of oxidative stress induced by treatment with diquat, a bipyridyl herbicide which generates ROS, by supporting GSH synthesis and the methionine cycle (Zhou et al., 2017a). In the same experiment, mouse hepatocytes were cultured with diquat and Ser alone, or with diquat and Ser, while CBS was inhibited via RNA interference. Compared with incubation with diquat and Ser alone, when CBS was inhibited, Ser supply did not alleviate oxidative stress and GSH synthesis was inhibited, indicating that the effects of Ser on oxidative stress are mediated by its promotion of CBS activity (Zhou et al., 2017a). Additionally, in mice, Ser supplementation helped reduce oxidative stress and steatosis induced by feeding a high-fat diet through epigenetically modulating the abundance of genes for GSH synthesis to promote synthesis and reduce ROS (Zhou et al., 2018a).
Overall, studies in rodents and humans have indicated the potential benefits of Gly and Ser supplementation with regard to immune function and oxidative stress, with effects linked to their involvement in 1-carbon metabolism. While studies are lacking in both nonruminant and ruminant livestock species, available data suggest that there may be potential benefits in livestock species as well when the dietary supply of Gly or Ser are enhanced. Thus, future studies on the effects of these two AA in both ruminant and nonruminant species are encouraged.
Threonine
Outside of protein synthesis, Thr is another essential AA with a link to 1-carbon metabolism, through its role as a precursor for Gly as described earlier. By promoting Gly synthesis, Thr may alter GSH synthesis as well as exert effects on immune function. Threonine can also be catabolized via Thr dehydratase to α-ketobutyrate, which can subsequently be used to produce propionyl-CoA and fuel the TCA cycle (House et al., 2001). Additionally, Thr is needed for mucin (Corfield, 2015) and immunoglobulin synthesis (Li et al., 2007). While mucin is a component of the mucus that covers many of the surfaces in the body, much of the research with Thr have focused on the effects of Thr in promoting gut health (Ruth and Field, 2013). This is partly due to evidence that Thr has a high retention rate in the intestine, suggesting an important role there (Stoll et al., 1998). In fact, dietary Thr restriction has been observed to decrease mucin synthesis, particularly in the intestine, in a variety of species (Faure et al., 2005; Law et al., 2007; Horn et al., 2009).
Studies on Thr in ruminants are lacking; however, there have been studies in a variety of nonruminant species on the effects of Thr on immune responses. In young pigs challenged with Escherichia coli, increasing dietary Thr increased antibody concentrations in serum and in the jejunal mucosa (Wang et al., 2006). In fish (Megalobrama amblycephala) fed deficient, optimal or excess amounts of Thr, the optimal amount increased plasma immunoglobulin M, SOD, GPX, CAT and total iron binding capacity (Habte-Tsion et al., 2016). Furthermore, in the hepatopancreas of those fish, optimal dietary Thr not only upregulated the expression of NFE2L2, CAT, SOD, and GPX1 but also upregulated TNF-α (Habte-Tsion et al., 2016). Similar effects of optimal Thr on immune function and health were also observed in the fish intestine in another study by the same group (Habte-Tsion et al., 2015).
In broiler chicks challenged with feed withdrawal and a coccidial vaccine, a Thr-deficient diet increased oocyst shedding, impaired gut integrity, decreased immunoglobulin A production, and reduced lymphocyte numbers in the cecum, suggesting an important role of Thr in the response to vaccines (Zhang et al., 2016). In growing pigs fed increasing levels of Thr, serum immunoglobulin G increased, but there were no differences in the circulating levels of Gly (Defa et al., 1999). Due to its use as antigen in immunological tests to assess humoral immunity, Defa et al. (1999) also injected bovine serum albumin (BSA) in pigs prior to feeding increasing levels of Thr. They observed that the enhanced supply of Thr increased concentration of anti-BSA antibody 7 d after the injection of BSA, suggesting an improvement in the humoral immune response. In another study, Pekin ducks were fed graded levels of Thr from hatch to 21 d of age and were challenged with BSA on day 11; increasing Thr improved mucin-2 gene expression in the ileum on day 21 and increased serum immunoglobulin A concentration; however, there were no effects of Thr on the response to BSA (Bi et al., 2018). Additionally, when feeding laying hens low crude protein diets, Thr was observed to have beneficial effects on immune responses, decreasing ileal expression of IL-2, IL-6, and IL-1β and increasing mucin-2 gene expression (Azzam et al., 2017). Lastly, in one study with dairy calves, adding three levels of Thr to milk replacer for 5 wk did not improve average daily gains or efficiency, but no measures of immune function or oxidative stress were measured in this study (Hill et al., 2008).
Overall, there is evidence that dietary Thr levels are important for maintaining immune responses, particularly in the intestine where Thr deficiency reduces mucin production and impairs intestinal immune responses in nonruminant species. However, more work is needed in ruminant species to understand the effects of Thr on intestinal immune responses. Work in both ruminants and nonruminants on the potential regulation of oxidative stress by Thr is also encouraged.
AA and Dairy Calves
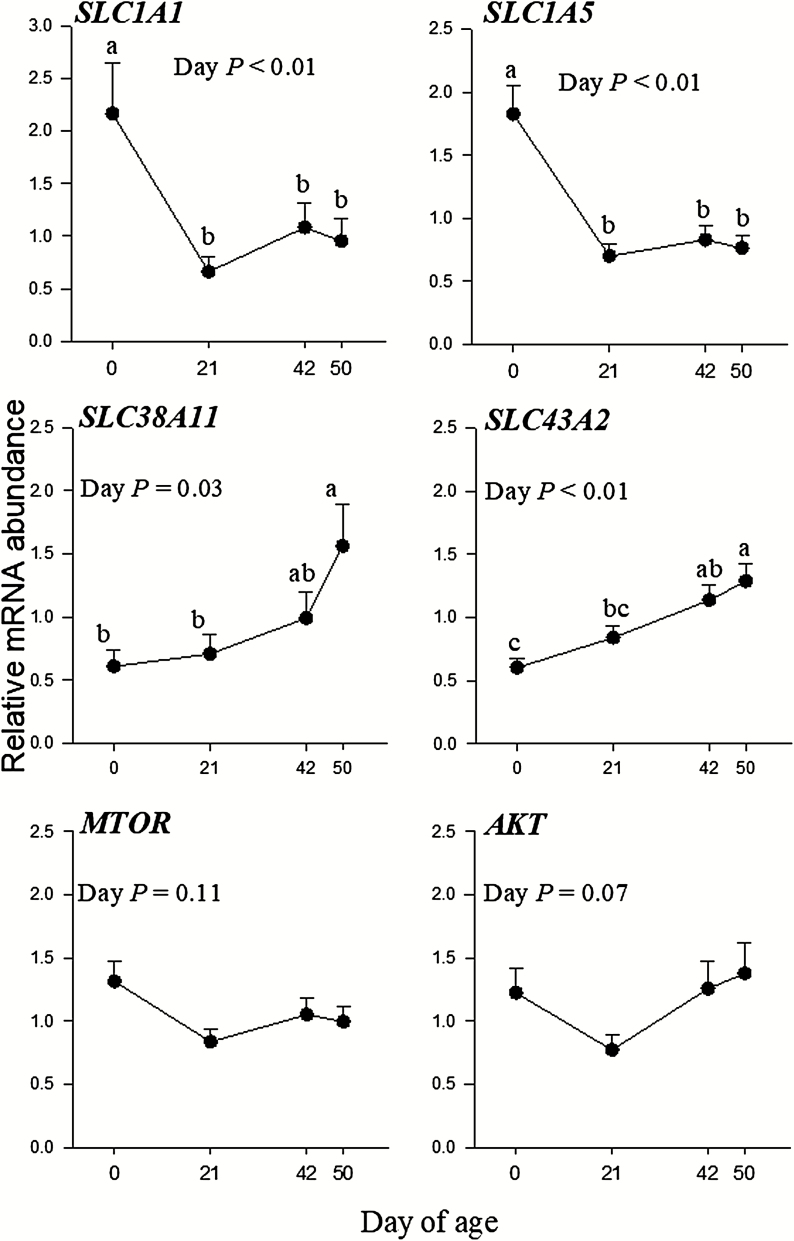
Calves are characterized by an immune system with a slow development process, including functions such as phagocytosis and respiratory burst, which requires several weeks to months to reach a mature state (Kampen et al., 2006). Thus, it is worth noting that AA may have beneficial effects on calf health as well. In a recent study from our laboratory (Alharthi et al., unpublished), we detected the abundance of various AA transporters, AKT and MTOR in PMNL isolated from whole blood at birth (before colostrum), 21, 42 (weaning), and 50 d of age (Figure 3). The abundance of AA transporters changed over time, with solute carrier family 1 member 1 (SLC1A1) and solute carrier family 1 member 5 (SLC1A5) decreasing from birth to 21 d of age, while solute carrier family 38 member 11 and solute carrier family 43 member 2 increased over time. Importantly, these calves were all born to multiparous dams that were fed the same diet during the last 45 d of gestation. Of particular interest are the changes in SLC1A1 and SLC1A5; SLC1A1 is a transporter of Glu, while SLC1A5 (also known as ASCT2) is a transporter of Gln. Therefore, the fact that the expression of these two transporters is greatest in PMN at birth suggests a reliance on Gln and Glu for their roles in promoting immune function and reducing oxidative stress as described earlier. Furthermore, we speculate that the decrease in SLC1A1 and SLC1A5 over time indicates a reduction in the reliance of calf PMN on Gln and Glu. This change is likely due to the greater dietary supply of these and other AA as the calf grows and the rumen becomes functional. Thus, changes in the expression of AA transporters are likely indicative of the developing immune system of calves and the importance of AA for their function. Additionally, the detection of AKT and MTOR also suggests that AA and their signaling pathways play a role in the regulation of immune function in neonatal calves. While more work is needed to understand the mechanisms behind changing the expression of AA transporters and signaling over time, these data suggest that AA supply during late pregnancy or during the preweaning period may alter immune function and oxidative stress to help improve calf health and reduce stress during the neonatal and weaning periods.
Figure 3.
Relative mRNA abundance of amino acid transporters and AKT in blood PMNL isolated from calves born to multiparous Holstein cows fed the same diet during the last 45 d of pregnancy. Data are presented as means ± SEM. a,bMeans differ P < 0.05 between time points. SLC1A1, high-affinity Glu transporter; SLC1A5, Na+-dependent neutral amino acid transporter SLC38A11, Gln transporter; SLC43A2, transport of l-isomers of neutral AA, including leucine, phenylalanine, valine, and methionine.
With regard to maternal AA supply and calf development, a nutritional strategy that our group has been investigating is the enhancement of maternal Met supply during the last 3 to 4 wk prepartum. Throughout the preweaning period, calves born to dams supplemented with RPM during late gestation had greater in vitro neutrophil phagocytosis, suggesting a priming effect of maternal Met supplementation on calf immune function (Alharthi et al., 2019b). Maternal Met supply also increased the hepatic activity of CBS and the concentrations of taurine compared with control calves, suggesting a greater flux of the transsulfuration pathways and a better antioxidant response with maternal Met supply (Alharthi et al., 2019a). Additionally, in another study, maternal Met supply during late gestation led to lower expression of TNF-α and MPO in PMNL at birth, while TLR2, NOS 2, and cell adhesion molecule 1 were lower throughout the preweaning period (Jacometo et al., 2018). During a whole blood challenge with LPS, a lower basal concentration of IL-1β was detected in RPM calves (Jacometo et al., 2018), indicating that Met attenuates the proinflammatory response and confirming data obtained from Vailati-Riboni et al. (2017) in peripartal dairy cows. Lastly, there were also changes in blood biomarkers with maternal Met, such as lower ROM, MPO, and ceruloplasmin, that suggested a lower oxidative stress and better health status of those calves compared with controls (Jacometo et al., 2016). Thus, maternal Met supply during late gestation may enhance calf immune function and antioxidant responses during the preweaning period.
When looking at the preweaning period, there is limited information on the effects of supplemental AA on dairy calf immune function and oxidative stress. However, Hill et al. (2008) reported that Lys and Met are limiting AA for the newborn animal. On this basis, to improve calf performance, it is crucial to supplement milk protein-based milk replacers with those AA, particularly Met. As detailed earlier, it is often reported the great involvement of Met to drive the regulation of the immune system of periparturient cows. Methionine has also been linked with the regulation of monocytes and neutrophils in dairy calves. Abdelmegeid et al. (2017) supplied Met to isolated PMNL from neonatal calves and observed a modulatory effect of Met on decreasing the expression of NF-κB1, TNF-α, IL-10, and SELL, indicating that Met supply during the preweaning period may reduce inflammation and promote immune function. Furthermore, the enhanced supply of Met to bovine neonatal hepatocytes decreased mRNA abundance of BHMT and MTR, suggesting less remethylation of homocysteine to Met (Chandler and White, 2017), which could increase the flux of homocysteine to the transsulfuration pathway for antioxidant synthesis. Additionally, Fligger et al. (1997) provided supplemental Arg in colostrum and then in milk replacer for the first 4 wk of age, while also immunizing calves against keyhole limpet hemocyanin (KLH). While calves receiving Arg had increased average daily gain, they had lower total and KLH-specific immunoglobulins in plasma, as well as decreased circulating leukocytes (Fligger et al., 1997). While this suggests a potential decrease in adaptive immune responses, no measures of NO production, phagocytosis, or oxidative burst were performed to better asses innate or antioxidant responses.
Glutamine has also been observed to be beneficial in calves that were weaned at 41 d of age; intravenous infusions of Gln increased CD2+ and CD4+ lymphocytes and the ratio of CD4+:CD8+ lymphocytes, as well as serum concentrations of immunoglobulins A and G (Zhou et al., 2012). Similarly, infusing three doses of Gln to dairy calves for 2 wk following weaning quadratically increased the abundance of CD4+ cells, monocytes, and the ratio of CD4+:CD8+ (Hu et al., 2013). Thus, while more work is needed, available data suggest that enhanced AA supply to dairy calves may promote a better basal inflammatory status (e.g., lower pro-inflammatory signaling), which could greatly help these animals that possess an underdeveloped immunity, especially in a period of high susceptibility to environmental pathogens (i.e., the postnatal stage).
Conclusions
In addition to their need for protein synthesis, AA serve as functional nutrients with a variety of roles in regulating key metabolic and immunological pathways such as one-carbon metabolism. These unique functions render the modulation of dietary AA supply during periods of stress such as the periparturient and preweaning periods a viable option for reducing oxidative stress and improving immune function. The data supporting the use of supplemental AA, particularly Met, Arg, and Gln, to enhance immune function and antioxidant responses during the periparturient period are strong. However, there is a paucity of information on the molecular mechanisms by which AA may alter immune responses in dairy cattle. To understand these mechanisms and their benefits to the animal, a systems biology approach will be needed, combining production data with high-throughput “omics” techniques such as transcriptomics, proteomics, and metabolomics. The development of robust “protection” technologies that limit the ruminal metabolism of the target AA, but allow high bioavailability in the small intestine, will also be key to furthering this research. Emphasis on nutritional programming, either during pregnancy or early life, is continuing to grow in the dairy industry, with evidence that maternal supply of nutrients (including AA) plays an important role in modulating calf growth and development. While alterations in maternal Met supply during late-gestation have been shown to alter dairy calf growth and immune response, more work is needed to uncover the effects of other AA and the underlying mechanisms. Integrating nutritional programming research with periparturient cow research in the context of functional AA will be key as we seek to offer diets that are formulated for the dual purpose of promoting the health and performance of both cow and calf.
Glossary
Abbreviations
- AA
amino acids
- AKT
protein kinase B
- APP
acute-phase proteins
- ASCT2
alanine-serine-cysteine transporter 2
- BCAA
branched-chain amino acids
- BCAT
branched-chain amino transferases
- BCKA
branched-chain α-ketoacids
- BHMT
betaine homocysteine methyltransferase
- BMEC
bovine mammary epithelial cells
- BSA
bovine serum albumin
- CBS
cystathionine β-synthase
- CSAD
cysteine sulfinic acid decarboxylase
- CTH
cystathionine gamma-lyase
- DMI
dry matter intake
- GATOR1
GTPase-activating protein activity towards Rags 1
- GCL
glutamate cysteine ligase
- GCLC
glutamate cysteine ligase catalytic
- GPX
glutathione peroxidase
- GSH
glutathione
- GSR
glutathione reductase
- GSS
glutathione synthase
- IL
interleukin
- iNOS
inducible NOS
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- KLH
keyhole limpet hemocyanin
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- MTOR
mechanistic target of rapamycin
- MTORC1
MTOR complex 1
- MTR
5-methyltetrahydrofolate-homocysteine methyltransferase
- NFE2L2
nuclear factor erythroid 2-like-2
- NF-κB
nuclear factor kappa b
- NNB
negative nutrient balance
- NOS
nitric oxide synthase
- PC
phosphatidylcholine
- PMN
polymorphonuclear neutrophils
- PMNL
polymorphonuclear leukocytes
- p-MTOR
phosphorylated MTOR
- RNS
reactive nitrogen species
- ROM
reactive oxygen metabolites
- ROS
reactive oxygen species
- RPM
rumen-protected Met
- SAA
serum amyloid A
- SAHH
S-adenosyl homocysteine hydrolase
- SAM
S-adenosyl methionine
- SAMTOR
S-adenosylmethionine sensor upstream of MTOR
- SELL
L-selectin
- SHMT
serine hydoxymethyltransferase
- SLC1A1
solute carrier family 1 member 1
- SLC1A5
solute carrier family 1 member 5
- SOD
superoxide dismutase
- STAT
signal transducer and activator of transcription
- TAG
triacyglycerol
- Th
T-helper
- TLR
toll-like receptors
- TNF-α
tumor necrosis factor-α
- VLDL
very-low-density lipoproteins
Conflict of interest statement
The authors declare that no conflicts of interest exist.
Literature Cited
- Abdelmegeid M. K., Vailati-Riboni M., Alharthi A., Batistel F., and Loor J. J.. 2017. Supplemental methionine, choline, or taurine alter in vitro gene network expression of polymorphonuclear leukocytes from neonatal Holstein calves. J. Dairy Sci. 100:3155–3165. doi: 10.3168/jds.2016-12025 [DOI] [PubMed] [Google Scholar]
- Agrawal A., Khan M. J., Graugnard D. E., Vailati-Riboni M., Rodriguez-Zas S. L., Osorio J. S., and Loor J. J.. 2017. Prepartal energy intake alters blood polymorphonuclear leukocyte transcriptome during the peripartal period in Holstein cows. Bioinform. Biol. Insights 11:1177932217704667. doi: 10.1177/1177932217704667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alharthi A. S., Coleman D. N., Liang Y., Batistel F., Elolimy A. A., Yambao R. C., Abdel-Hamied E., Pan Y. X., Parys C., Alhidary I. A., et al. . 2019a. Hepatic 1-carbon metabolism enzyme activity, intermediate metabolites, and growth in neonatal Holstein dairy calves are altered by maternal supply of methionine during late pregnancy. J. Dairy Sci. 102:10291–10303. doi: 10.3168/jds.2019-16562 [DOI] [PubMed] [Google Scholar]
- Alharthi A. S., Lopreiato V., Dai H., Bucktrout R., Abdelmegeid M., Batistel F., Parys C., Shen X., Ballou M. A., Trevisi E., et al. . 2019b. Short Communication: Supply of methionine during late pregnancy enhances whole-blood innate immune response of Holstein calves partly through changes in mRNA abundance in polymorphonuclear leukocytes. J. Dairy Sci. 102:10599–10605. doi: 10.3168/jds.2018-15676 [DOI] [PubMed] [Google Scholar]
- Andou A., Hisamatsu T., Okamoto S., Chinen H., Kamada N., Kobayashi T., Hashimoto M., Okutsu T., Shimbo K., Takeda T., et al. . 2009. Dietary histidine ameliorates murine colitis by inhibition of proinflammatory cytokine production from macrophages. Gastroenterology 136:564–74.e2. doi: 10.1053/j.gastro.2008.09.062 [DOI] [PubMed] [Google Scholar]
- Atmaca G. 2004. Antioxidant effects of sulfur-containing amino acids. Yonsei Med. J. 45:776–788. doi: 10.3349/ymj.2004.45.5.776 [DOI] [PubMed] [Google Scholar]
- Azzam M. M. M., Dong X. Y., and Zou X. T.. 2017. Effect of dietary threonine on laying performance and intestinal immunity of laying hens fed low-crude-protein diets during the peak production period. J. Anim. Physiol. Anim. Nutr. (Berl). 101:e55–e66. doi: 10.1111/jpn.12559 [DOI] [PubMed] [Google Scholar]
- Banerjee R., Evande R., Kabil O., Ojha S., and Taoka S.. 2003. Reaction mechanism and regulation of cystathionine beta-synthase. Biochim. Biophys. Acta 1647:30–35. doi: 10.1016/s1570-9639(03)00044-x [DOI] [PubMed] [Google Scholar]
- Bassit R. A., Sawada L. A., Bacurau R. F., Navarro F., Martins E. Jr, Santos R. V., Caperuto E. C., Rogeri P., and Costa Rosa L. F.. 2002. Branched-chain amino acid supplementation and the immune response of long-distance athletes. Nutrition 18:376–379. doi: 10.1016/s0899-9007(02)00753-0 [DOI] [PubMed] [Google Scholar]
- Batistel F., Arroyo J. M., Bellingeri A., Wang L., Saremi B., Parys C., Trevisi E., Cardoso F. C., and Loor J. J.. 2017. Ethyl-cellulose rumen-protected methionine enhances performance during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 100:7455–7467. doi: 10.3168/jds.2017-12689 [DOI] [PubMed] [Google Scholar]
- Batistel F., Arroyo J. M., Garces C. I. M., Trevisi E., Parys C., Ballou M. A., Cardoso F. C., and Loor J. J.. 2018. Ethyl-cellulose rumen-protected methionine alleviates inflammation and oxidative stress and improves neutrophil function during the periparturient period and early lactation in Holstein dairy cows. J. Dairy Sci. 101:480–490. doi: 10.3168/jds.2017-13185 [DOI] [PubMed] [Google Scholar]
- Bauman D. E., and Currie W. B.. 1980. Partitioning of nutrients during pregnancy and lactation: a review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 63:1514–1529. doi: 10.3168/jds.s0022-0302(80)83111-0 [DOI] [PubMed] [Google Scholar]
- Bell A. W., Burhans W. S., and Overton T. R.. 2000. Protein nutrition in late pregnancy, maternal protein reserves and lactation performance in dairy cows. Proc. Nutr. Soc. 59:119–126. doi: 10.1017/s0029665100000148 [DOI] [PubMed] [Google Scholar]
- Bernabucci U., Ronchi B., Lacetera N., and Nardone A.. 2005. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 88:2017–2026. doi: 10.3168/jds.S0022-0302(05)72878-2 [DOI] [PubMed] [Google Scholar]
- Bertoni G., and Trevisi E.. 2013. Use of the liver activity index and other metabolic variables in the assessment of metabolic health in dairy herds. Vet. Clin. North Am. Food Anim. Pract. 29:413–431. doi: 10.1016/j.cvfa.2013.04.004 [DOI] [PubMed] [Google Scholar]
- Bertoni G., Trevisi E., Han X., and Bionaz M.. 2008. Effects of inflammatory conditions on liver activity in puerperium period and consequences for performance in dairy cows. J. Dairy Sci. 91:3300–3310. doi: 10.3168/jds.2008-0995 [DOI] [PubMed] [Google Scholar]
- Bi Y., Nan X. M., Zheng S. S., Jiang L. S., and Xiong B. H.. 2018. Effects of dietary threonine and immune stress on growth performance, carcass trait, serum immune parameters, and intestinal muc2 and NF-κb gene expression in Pekin ducks from hatch to 21 days. Poult. Sci. 97:177–187. doi: 10.3382/ps/pex283 [DOI] [PubMed] [Google Scholar]
- Bogdan C. 2015. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 36:161–178. doi: 10.1016/j.it.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Bradford B. J., Yuan K., Farney J. K., Mamedova L. K., and Carpenter A. J.. 2015. Invited Review: Inflammation during the transition to lactation: new adventures with an old flame. J. Dairy Sci. 98:6631–6650. doi: 10.3168/jds.2015-9683 [DOI] [PubMed] [Google Scholar]
- Branco A. C. C. C., Yoshikawa F. S. Y., Pietrobon A. J., and Sato M. N.. 2018. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018:9524075. doi: 10.1155/2018/9524075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins C. M., Bensard D. D., Heimbach J. K., Meng X., Shames B. D., Pulido E. J., and McIntyre R. C. Jr. 2001. l-Arginine attenuates lipopolysaccharide-induced lung chemokine production. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L400–L408. doi: 10.1152/ajplung.2001.280.3.L400 [DOI] [PubMed] [Google Scholar]
- Chaiyotwittayakun A., Erskine R. J., Bartlett P. C., Herd T. H., Sears P. M., and Harmont R. J.. 2002. The effect of ascorbic acid and l-histidine therapy on acute mammary inflammation in dairy cattle. J. Dairy Sci. 85:60–67. doi: 10.3168/jds.s0022-0302(02)74053-8 [DOI] [PubMed] [Google Scholar]
- Chandler T. L., and White H. M.. 2017. Choline and methionine differentially alter methyl carbon metabolism in bovine neonatal hepatocytes. PLoS One. 12:e0171080. doi: 10.1371/journal.pone.0171080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L., Scaria S. M., Saxton R. A., Gygi M. P., Shen K., Wyant G. A., Wang T., Harper J. W., Gygi S. P., and Sabatini D. M.. 2016. The CASTOR proteins are arginine sensors for the mTORC1 pathway. Cell 165:153–164. doi: 10.1016/j.cell.2016.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplin D. D. 2010. Overview of the immune response. J. Allergy Clin. Immunol. 125(2 Suppl 2):S3–S23. doi: 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Dong H., Thompson D. C., Shertzer H. G., Nebert D. W., and Vasiliou V.. 2013. Glutathione defense mechanism in liver injury: insights from animal models. Food Chem. Toxicol. 60:38–44. doi: 10.1016/j.fct.2013.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti M., Persichini T., Menegazzi M., Mariotto S., Giordano E., Caldarera C. M., Sogos V., Lauro G. M., and Suzuki H.. 1995. Induction of nitric oxide synthase mRNA expression. Suppression by exogenous nitric oxide. J. Biol. Chem. 270:26731–26733. doi: 10.1074/jbc.270.45.26731 [DOI] [PubMed] [Google Scholar]
- Contreras-Jodar A., Salama A. A., Hamzaoui S., Vailati-Riboni M., Caja G., and Loor J. J.. 2018. Effects of chronic heat stress on lactational performance and the transcriptomic profile of blood cells in lactating dairy goats. J. Dairy Res. 85:423–430. doi: 10.1017/S0022029918000705 [DOI] [PubMed] [Google Scholar]
- Corfield A. P. 2015. Mucins: a biologically relevant glycan barrier in mucosal protection. Biochim. Biophys. Acta 1850:236–252. doi: 10.1016/j.bbagen.2014.05.003 [DOI] [PubMed] [Google Scholar]
- Curi R., Newsholme P., Procopio J., Lagranha C., Gorjão R., and Pithon-Curi T. C.. 2007. Glutamine, gene expression, and cell function. Front. Biosci. 12:344–357. doi: 10.2741/2068 [DOI] [PubMed] [Google Scholar]
- Dalbach K. F., Larsen M., Raun B. M., and Kristensen N. B.. 2011. Effects of supplementation with 2-hydroxy-4-(methylthio)-butanoic acid isopropyl ester on splanchnic amino acid metabolism and essential amino acid mobilization in postpartum transition Holstein cows. J. Dairy Sci. 94:3913–3927. doi: 10.3168/jds.2010-3724 [DOI] [PubMed] [Google Scholar]
- Defa L., X Changting, Q. Shiyan, Z. Jinhui, E. W. Johnson, and P. A. Thacker. 1999. Effects of dietary threonine on performance, plasma parameters and immune function of growing pigs. Anim. Feed Sci. Technol. 78: 179–188. doi: 10.1016/S0377-8401(99)00005-X [DOI] [Google Scholar]
- Deng Y.-P.,, W.-d. Jiang,, Y. Liu,, B. Qu,, J. Jiang,, S.-Y. Kuang,, L. Tang,, W.-N. Tang,, P. Wu,, W.-A. Zhang, et al. 2016. Dietary leucine improves flesh quality and alters mRNA expressions of Nrf2-mediated antioxidant enzymes in the muscle of grass carp (Ctenopharyngodon idella). Aquaculture 452:380–387. doi: 10.1016/j.aquaculture.2015.11.007 [DOI] [Google Scholar]
- Doepel L., Lessard M., Gagnon N., Lobley G. E., Bernier J. F., Dubreuil P., and Lapierre H.. 2006. Effect of postruminal glutamine supplementation on immune response and milk production in dairy cows. J. Dairy Sci. 89:3107–3121. doi: 10.3168/jds.S0022-0302(06)72585-1 [DOI] [PubMed] [Google Scholar]
- Dong X., Zhou Z., Saremi B., Helmbrecht A., Wang Z., and Loor J. J.. 2018. Varying the ratio of Lys:Met while maintaining the ratios of Thr:Phe, Lys:Thr, Lys:His, and Lys:Val alters mammary cellular metabolites, mammalian target of rapamycin signaling, and gene transcription. J. Dairy Sci. 101:1708–1718. doi: 10.3168/jds.2017-13351 [DOI] [PubMed] [Google Scholar]
- Du X., Shen T., Wang H., Qin X., Xing D., Ye Q., Shi Z., Fang Z., Zhu Y., Yang Y., et al. . 2018. Adaptations of hepatic lipid metabolism and mitochondria in dairy cows with mild fatty liver. J. Dairy Sci. 101:9544–9558. doi: 10.3168/jds.2018-14546 [DOI] [PubMed] [Google Scholar]
- Duan J., Yin J., Ren W., Liu T., Cui Z., Huang X., Wu L., Kim S. W., Liu G., Wu X., et al. . 2016. Dietary supplementation with l-glutamate and l-aspartate alleviates oxidative stress in weaned piglets challenged with hydrogen peroxide. Amino Acids 48:53–64. doi: 10.1007/s00726-015-2065-3 [DOI] [Google Scholar]
- Duval D., Demangel C., Munier-Jolain K., Miossec S., and Geahel I.. 1991. Factors controlling cell proliferation and antibody production in mouse hybridoma cells: I. Influence of the amino acid supply. Biotechnol. Bioeng. 38:561–570. doi: 10.1002/bit.260380602 [DOI] [PubMed] [Google Scholar]
- Dy M., and Schneider E.. 2004. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 15:393–410. doi: 10.1016/j.cytogfr.2004.06.003 [DOI] [PubMed] [Google Scholar]
- El-Hafidi M., Franco M., Ramírez A. R., Sosa J. S., Flores J. A. P., Acosta O. L., Salgado M. C., and Cardoso-Saldaña G.. 2018. Glycine increases insulin sensitivity and glutathione biosynthesis and protects against oxidative stress in a model of sucrose-induced insulin resistance. Oxid. Med. Cell. Longev. 2018:2101562. doi: 10.1155/2018/2101562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M., Moënnoz D., Montigon F., Mettraux C., Breuillé D., and Ballèvre O.. 2005. Dietary threonine restriction specifically reduces intestinal mucin synthesis in rats. J. Nutr. 135:486–491. doi: 10.1093/jn/135.3.486 [DOI] [PubMed] [Google Scholar]
- Finkelstein J. D. 1990. Methionine metabolism in mammals. J. Nutr. Biochem. 1:228–237. doi: 10.1016/0955-2863(90)90070-2 [DOI] [PubMed] [Google Scholar]
- Fligger J. M., Gibson C. A., Sordillo L. M., and Baumrucker C. R.. 1997. Arginine supplementation increases weight gain, depresses antibody production, and alters circulating leukocyte profiles in preruminant calves without affecting plasma growth hormone concentrations. J. Anim. Sci. 75:3019–3025. doi: 10.2527/1997.75113019x [DOI] [PubMed] [Google Scholar]
- Flynn N. E., Meininger C. J., Haynes T. E., and Wu G.. 2002. The metabolic basis of arginine nutrition and pharmacotherapy. Biomed. Pharmacother. 56:427–438. doi: 10.1016/s0753-3322(02)00273-1 [DOI] [PubMed] [Google Scholar]
- Franĕk F., and Srámková K.. 1996. Protection of B lymphocyte hybridoma against starvation-induced apoptosis: survival-signal role of some amino acids. Immunol. Lett. 52:139–144. doi: 10.1016/0165-2478(96)02591-6 [DOI] [PubMed] [Google Scholar]
- Furukawa S., Saito H., Inoue T., Matsuda T., Fukatsu K., Han I., Ikeda S., and Hidemura A.. 2000. Supplemental glutamine augments phagocytosis and reactive oxygen intermediate production by neutrophils and monocytes from postoperative patients in vitro. Nutrition 16:323–329. doi: 10.1016/s0899-9007(00)00228-8 [DOI] [PubMed] [Google Scholar]
- Glinton K. E., Levy H. L., Kennedy A. D., Pappan K. L., and Elsea S. H.. 2018. Untargeted metabolomics identifies unique though benign biochemical changes in patients with pathogenic variants in UROC1. Mol. Genet. Metab. Rep. 18:14–18. doi: 10.1016/j.ymgmr.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griscavage J. M., Rogers N. E., Sherman M. P., and Ignarro L. J.. 1993. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J. Immunol. 151:6329–6337. [PubMed] [Google Scholar]
- Grohmann U., and Bronte V.. 2010. Control of immune response by amino acid metabolism. Immunol. Rev. 236:243–264. doi: 10.1111/j.1600-065X.2010.00915.x [DOI] [PubMed] [Google Scholar]
- Gu X., Orozco J. M., Saxton R. A., Condon K. J., Liu G. Y., Krawczyk P. A., Scaria S. M., Harper J. W., Gygi S. P., and Sabatini D. M.. 2017. SAMTOR is an S-adenosylmethionine sensor for the mTORC1 pathway. Science 358:813–818. doi: 10.1126/science.aao3265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habte-Tsion H. M., Ge X., Liu B., Xie J., Ren M., Zhou Q., Miao L., Pan L., and Chen R.. 2015. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 42:439–446. doi: 10.1016/j.fsi.2014.11.021 [DOI] [PubMed] [Google Scholar]
- Habte-Tsion H. M., Ren M., Liu B., Ge X., Xie J., and Chen R.. 2016. Threonine modulates immune response, antioxidant status and gene expressions of antioxidant enzymes and antioxidant-immune-cytokine-related signaling molecules in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 51:189–199. doi: 10.1016/j.fsi.2015.11.033 [DOI] [PubMed] [Google Scholar]
- Han L., Batistel F., Ma Y., Alharthi A. S. M., Parys C., and Loor J. J.. 2018. Methionine supply alters mammary gland antioxidant gene networks via phosphorylation of nuclear factor erythroid 2-like 2 (NFE2L2) protein in dairy cows during the periparturient period. J. Dairy Sci. 101:8505–8512. doi: 10.3168/jds.2017-14206 [DOI] [PubMed] [Google Scholar]
- Handa A. K., Fatima T., and Mattoo A. K.. 2018. Polyamines: bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 6:10. doi: 10.3389/fchem.2018.00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Bateman H. G. 2nd, Aldrich J. M., Schlotterbeck R. L., and Tanan K. G.. 2008. Optimal concentrations of lysine, methionine, and threonine in milk replacers for calves less than five weeks of age. J. Dairy Sci. 91:2433–2442. doi: 10.3168/jds.2007-0610 [DOI] [PubMed] [Google Scholar]
- Hinz B., Brune K., and Pahl A.. 2000. Nitric oxide inhibits inducible nitric oxide synthase mRNA expression in RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 271:353–357. doi: 10.1006/bbrc.2000.2632 [DOI] [PubMed] [Google Scholar]
- Holtenius K., Persson Waller K., Essén-Gustavsson B., Holtenius P., and Hallén Sandgren C.. 2004. Metabolic parameters and blood leukocyte profiles in cows from herds with high or low mastitis incidence. Vet. J. 168:65–73. doi: 10.1016/j.tvjl.2003.09.015 [DOI] [PubMed] [Google Scholar]
- Horn N. L., Donkin S. S., Applegate T. J., and Adeola O.. 2009. Intestinal mucin dynamics: response of broiler chicks and White Pekin ducklings to dietary threonine. Poult. Sci. 88:1906–1914. doi: 10.3382/ps.2009-00009 [DOI] [PubMed] [Google Scholar]
- Hoskin S. O., Bremner D. M., Holtrop G., and Lobley G. E.. 2016. Responses in whole-body amino acid kinetics to an acute, sub-clinical endotoxin challenge in lambs. Br. J. Nutr. 115:576–584. doi: 10.1017/S0007114515004894 [DOI] [PubMed] [Google Scholar]
- House J. D., Hall B. N., and Brosnan J. T.. 2001. Threonine metabolism in isolated rat hepatocytes. Am. J. Physiol. Endocrinol. Metab. 281:E1300–E1307. doi: 10.1152/ajpendo.2001.281.6.E1300 [DOI] [PubMed] [Google Scholar]
- Hu Z. Y., Su H. W., Li S. L., and Cao Z. J.. 2013. Effect of parenteral administration of glutamine on autophagy of liver cell and immune responses in weaned calves. J. Anim. Physiol. Anim. Nutr. (Berl). 97:1007–1014. doi: 10.1111/jpn.12002 [DOI] [PubMed] [Google Scholar]
- Ikejima K., Iimuro Y., Forman D. T., and Thurman R. G.. 1996. A diet containing glycine improves survival in endotoxin shock in the rat. Am. J. Physiol. 271(1 Pt 1):G97–103. doi: 10.1152/ajpgi.1996.271.1.G97 [DOI] [PubMed] [Google Scholar]
- Ingvartsen K. L. 2006. Feeding- and management-related diseases in the transition cow: Physiological adaptations around calving and strategies to reduce feeding-related diseases. Anim. Feed Sci. Technol. 126: 175–213. doi: 10.1016/j.anifeedsci.2005.08.003 [DOI] [Google Scholar]
- Ingvartsen K. L., and Andersen J. B.. 2000. Integration of metabolism and intake regulation: a review focusing on periparturient animals. J. Dairy Sci. 83:1573–1597. doi: 10.3168/jds.S0022-0302(00)75029-6 [DOI] [PubMed] [Google Scholar]
- Ingvartsen K. L., and Moyes K.. 2013. Nutrition, immune function and health of dairy cattle. Animal 7 (Suppl 1):112–122. doi: 10.1017/S175173111200170X [DOI] [PubMed] [Google Scholar]
- Jacometo C. B., Alharthi A. S., Zhou Z., Luchini D., and Loor J. J.. 2018. Maternal supply of methionine during late pregnancy is associated with changes in immune function and abundance of microRNA and mRNA in Holstein calf polymorphonuclear leukocytes. J. Dairy Sci. 101:8146–8158. doi: 10.3168/jds.2018-14428 [DOI] [PubMed] [Google Scholar]
- Jacometo C. B., Zhou Z., Luchini D., Trevisi E., Corrêa M. N., and Loor J. J.. 2016. Maternal rumen-protected methionine supplementation and its effect on blood and liver biomarkers of energy metabolism, inflammation, and oxidative stress in neonatal Holstein calves. J. Dairy Sci. 99:6753–6763. doi: 10.3168/jds.2016-11018 [DOI] [PubMed] [Google Scholar]
- Jafari A., Emmanuel D. G., Christopherson R. J., Thompson J. R., Murdoch G. K., Woodward J., Field C. J., and Ametaj B. N.. 2006. Parenteral administration of glutamine modulates acute phase response in postparturient dairy cows. J. Dairy Sci. 89:4660–4668. doi: 10.3168/jds.S0022-0302(06)72516-4 [DOI] [PubMed] [Google Scholar]
- Jiang W. D., Feng L., Qu B., Wu P., Kuang S. Y., Jiang J., Tang L., Tang W. N., Zhang Y. A., Zhou X. Q., et al. . 2016. Changes in integrity of the gill during histidine deficiency or excess due to depression of cellular anti-oxidative ability, induction of apoptosis, inflammation and impair of cell-cell tight junctions related to Nrf2, TOR and NF-κB signaling in fish. Fish Shellfish Immunol. 56:111–122. doi: 10.1016/j.fsi.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Jiang J., Shi D., Zhou X. Q., Hu Y., Feng L., Liu Y., Jiang W. D., and Zhao Y.. 2015. In vitro and in vivo protective effect of arginine against lipopolysaccharide induced inflammatory response in the intestine of juvenile Jian carp (Cyprinus carpio var. Jian). Fish Shellfish Immunol. 42:457–464. doi: 10.1016/j.fsi.2014.11.030 [DOI] [PubMed] [Google Scholar]
- Jiang J., Yin L., Li J. Y., Li Q., Shi D., Feng L., Liu Y., Jiang W. D., Wu P., Zhao Y., et al. . 2017. Glutamate attenuates lipopolysaccharide-induced oxidative damage and mRNA expression changes of tight junction and defensin proteins, inflammatory and apoptosis response signaling molecules in the intestine of fish. Fish Shellfish Immunol. 70:473–484. doi: 10.1016/j.fsi.2017.09.035 [DOI] [PubMed] [Google Scholar]
- Jones R. G., and Pearce E. J.. 2017. MenTORing immunity: mTOR signaling in the development and function of tissue-resident immune cells. Immunity 46:730–742. doi: 10.1016/j.immuni.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jose D. G., and Good R. A.. 1973. Quantitative effects of nutritional essential amino acid deficiency upon immune responses to tumors in mice. J. Exp. Med. 137:1–9. doi: 10.1084/jem.137.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakazu E., Kanno N., Ueno Y., and Shimosegawa T.. 2007. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J. Immunol. 179:7137–7146. doi: 10.4049/jimmunol.179.10.7137 [DOI] [PubMed] [Google Scholar]
- Kampen A. H., Olsen I., Tollersrud T., Storset A. K., and Lund A.. 2006. Lymphocyte subpopulations and neutrophil function in calves during the first 6 months of life. Vet. Immunol. Immunopathol. 113:53–63. doi: 10.1016/j.vetimm.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Kindt T. J., Goldsby R. A., Osborne B. A., and Kuby J.. 2007. Kuby immunology. 6th ed. New York (NY): W.H. Freeman. [Google Scholar]
- Konashi S., Takahashi K., and Akiba Y.. 2000. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 83:449–456. doi: 10.1017/S0007114500000556 [DOI] [PubMed] [Google Scholar]
- Kopeć W., Jamroz D., Wiliczkiewicz A., Biazik E., Pudlo A., Hikawczuk T., Skiba T., and Korzeniowska M.. 2013. Influence of different histidine sources and zinc supplementation of broiler diets on dipeptide content and antioxidant status of blood and meat. Br. Poult. Sci. 54:454–465. doi: 10.1080/00071668.2013.793295 [DOI] [PubMed] [Google Scholar]
- Larsen M., and Kristensen N. B.. 2013. Precursors for liver gluconeogenesis in periparturient dairy cows. Animal 7:1640–1650. doi: 10.1017/S1751731113001171 [DOI] [PubMed] [Google Scholar]
- Larson S. J., and Dunn A. J.. 2001. Behavioral effects of cytokines. Brain. Behav. Immun. 15:371–387. doi: 10.1006/brbi.2001.0643 [DOI] [PubMed] [Google Scholar]
- Law G. K., Bertolo R. F., Adjiri-Awere A., Pencharz P. B., and Ball R. O.. 2007. Adequate oral threonine is critical for mucin production and gut function in neonatal piglets. Am. J. Physiol. Gastrointest. Liver Physiol. 292:G1293–G1301. doi: 10.1152/ajpgi.00221.2006 [DOI] [PubMed] [Google Scholar]
- Li H., Wang H., Yu L., Wang M., Liu S., Sun L., and Chen Q.. 2015. Effects of supplementation of rumen-protected choline on growth performance, meat quality and gene expression in longissimus dorsi muscle of lambs. Arch. Anim. Nutr. 69:340–350. doi: 10.1080/1745039X.2015.1073001 [DOI] [PubMed] [Google Scholar]
- Li P., Yin Y. L., Li D., Kim S. W., and Wu G.. 2007. Amino acids and immune function. Br. J. Nutr. 98:237–252. doi: 10.1017/S000711450769936X [DOI] [PubMed] [Google Scholar]
- Liang Y., Batistel F., Parys C., and Loor J. J.. 2019a. Glutathione metabolism and nuclear factor erythroid 2-like 2 (NFE2L2)-related proteins in adipose tissue are altered by supply of ethyl-cellulose rumen-protected methionine in peripartal Holstein cows. J. Dairy Sci. 102:5530–5541. doi: 10.3168/jds.2018-15687 [DOI] [PubMed] [Google Scholar]
- Liang Y., Batistel F., Parys C., and Loor J. J.. 2019b. Methionine supply during the periparturient period enhances insulin signaling, amino acid transporters, and mechanistic target of rapamycin pathway proteins in adipose tissue of Holstein cows. J. Dairy Sci. 102:4403–4414. doi: 10.3168/jds.2018-15738 [DOI] [PubMed] [Google Scholar]
- Lin C. M., Abcouwer S. F., and Souba W. W.. 1999. Effect of dietary glutamate on chemotherapy-induced immunosuppression. Nutrition 15:687–696. doi: 10.1016/s0899-9007(99)00153-7 [DOI] [PubMed] [Google Scholar]
- Litvak N., Rakhshandeh A., Htoo J. K., and de Lange C. F. M.. 2013. Immune system stimulation increases the optimal dietary methionine to methionine plus cysteine ratio in growing pigs1. J. Anim. Sci. 91: 4188–4196. doi: 10.2527/jas.2012-6160 [DOI] [PubMed] [Google Scholar]
- Liu Y., Zhao T., Li Z., Wang L., Yuan S., and Sun L.. 2018. The role of ASCT2 in cancer: A review. Eur. J. Pharmacol. 837:81–87. doi: 10.1016/j.ejphar.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Lo Y. C., Lee C. F., and Powell J. D.. 2014. Insight into the role of mTOR and metabolism in T cells reveals new potential approaches to preventing graft rejection. Curr. Opin. Organ Transplant. 19:363–371. doi: 10.1097/MOT.0000000000000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loor J. J., Bertoni G., Hosseini A., Roche J. R., and Trevisi E.. 2013a. Functional welfare – using biochemical and molecular technologies to understand better the welfare state of peripartal dairy cattle. Anim. Prod. Sci. 53:931–953. doi: 10.1071/AN12344 [DOI] [Google Scholar]
- Loor J. J., Bionaz M., and Drackley J. K.. 2013b. Systems physiology in dairy cattle: nutritional genomics and beyond. Annu. Rev. Anim. Biosci. 1:365–392. doi: 10.1146/annurev-animal-031412-103728 [DOI] [PubMed] [Google Scholar]
- Lopreiato V., Vailati-Riboni M., Bellingeri A., Khan I., Farina G., Parys C., and Loor J. J.. 2019. Inflammation and oxidative stress transcription profiles due to in vitro supply of methionine with or without choline in unstimulated blood polymorphonuclear leukocytes from lactating Holstein cows. J. Dairy Sci. 102:10395–10410. doi: 10.3168/jds.2019-16413 [DOI] [PubMed] [Google Scholar]
- Luo J. B., Feng L., Jiang W. D., Liu Y., Wu P., Jiang J., Kuang S. Y., Tang L., Zhang Y. A., and Zhou X. Q.. 2014. The impaired intestinal mucosal immune system by valine deficiency for young grass carp (Ctenopharyngodon idella) is associated with decreasing immune status and regulating tight junction proteins transcript abundance in the intestine. Fish Shellfish Immunol. 40:197–207. doi: 10.1016/j.fsi.2014.07.003 [DOI] [PubMed] [Google Scholar]
- Lushchak V. I. 2012. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids 2012:736837. doi: 10.1155/2012/736837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E. H., Bantug G., Griss T., Condotta S., Johnson R. M., Samborska B., Mainolfi N., Suri V., Guak H., Balmer M. L., et al. . 2017. Serine is an essential metabolite for effector T cell expansion. Cell Metab. 25:345–357. doi: 10.1016/j.cmet.2016.12.011 [DOI] [PubMed] [Google Scholar]
- Ma Y. F., Batistel F., Xu T. L., Han L. Q., Bucktrout R., Liang Y., Coleman D. N., Parys C., and Loor J. J.. 2019. Phosphorylation of AKT serine/threonine kinase and abundance of milk protein synthesis gene networks in mammary tissue in response to supply of methionine in periparturient Holstein cows. J. Dairy Sci. 102:4264–4274. doi: 10.3168/jds.2018-15451 [DOI] [PubMed] [Google Scholar]
- Ma N., Guo P., Zhang J., He T., Kim S. W., Zhang G., and Ma X.. 2018. Nutrients mediate intestinal bacteria-mucosal immune crosstalk. Front. Immunol. 9:5. doi: 10.3389/fimmu.2018.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann S., Sipka A. S., and Grenier J. K.. 2019. The degree of postpartum metabolic challenge in dairy cows is associated with peripheral blood mononuclear cell transcriptome changes of the innate immune system. Dev. Comp. Immunol. 93:28–36. doi: 10.1016/j.dci.2018.11.021 [DOI] [PubMed] [Google Scholar]
- Mann S., Sipka A., Leal Yepes F. A., Nydam D. V., Overton T. R., and Wakshlag J. J.. 2018. Nutrient-sensing kinase signaling in bovine immune cells is altered during the postpartum nutrient deficit: a possible role in transition cow inflammatory response. J. Dairy Sci. 101:9360–9370. doi: 10.3168/jds.2018-14549 [DOI] [PubMed] [Google Scholar]
- Mao X., Qi S., Yu B., He J., Yu J., and Chen D.. 2013. Zn(2+) and l-isoleucine induce the expressions of porcine β-defensins in IPEC-J2 cells. Mol. Biol. Rep. 40:1547–1552. doi: 10.1007/s11033-012-2200-0 [DOI] [PubMed] [Google Scholar]
- Marshall J. S., Warrington R., Watson W., and Kim H. L.. 2018. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 14(Suppl 2):49. doi: 10.1186/s13223-018-0278-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil C. J., Hoskin S. O., Bremner D. M., Holtrop G., and Lobley G. E.. 2016. Whole-body and splanchnic amino acid metabolism in sheep during an acute endotoxin challenge. Br. J. Nutr. 116:211–222. doi: 10.1017/S0007114516001860 [DOI] [PubMed] [Google Scholar]
- Nakamura I. 2014. Impairment of innate immune responses in cirrhotic patients and treatment by branched-chain amino acids. World J. Gastroenterol. 20:7298–7305. doi: 10.3748/wjg.v20.i23.7298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nan X., Bu D., Li X., Wang J., Wei H., Hu H., Zhou L., and Loor J. J.. 2014. Ratio of lysine to methionine alters expression of genes involved in milk protein transcription and translation and mTOR phosphorylation in bovine mammary cells. Physiol. Genomics 46:268–275. doi: 10.1152/physiolgenomics.00119.2013 [DOI] [PubMed] [Google Scholar]
- Neinast M., Murashige D., and Arany Z.. 2019. Branched chain amino acids. Annu. Rev. Physiol. 81:139–164. doi: 10.1146/annurev-physiol-020518-114455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemati M., Menatian S., Joz Ghasemi S., Hooshmandfar R., Taheri M., and Saifi T.. 2018. Effect of protected-glutamine supplementation on performance, milk composition and some blood metabolites in fresh Holstein cows. Iran. J. Vet. Res. 19:225–228. [PMC free article] [PubMed] [Google Scholar]
- Newsholme P. 2001. Why is l-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection? J. Nutr. 131(9 Suppl):2515S–22S; discussion 2523S. doi: 10.1093/jn/131.9.2515S [DOI] [PubMed] [Google Scholar]
- Newsholme P., Brennan L., Rubi B., and Maechler P.. 2005. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. (Lond). 108:185–194. doi: 10.1042/CS20040290 [DOI] [PubMed] [Google Scholar]
- Newsholme P., Curi R., Pithon Curi T. C., Murphy C. J., Garcia C., and Pires de Melo M.. 1999. Glutamine metabolism by lymphocytes, macrophages, and neutrophils: its importance in health and disease. J. Nutr. Biochem. 10:316–324. doi: 10.1016/s0955-2863(99)00022-4 [DOI] [PubMed] [Google Scholar]
- Newsholme P., Procopio J., Lima M. M., Pithon-Curi T. C., and Curi R.. 2003. Glutamine and glutamate–their central role in cell metabolism and function. Cell Biochem. Funct. 21:1–9. doi: 10.1002/cbf.1003 [DOI] [PubMed] [Google Scholar]
- NRC 2001. Nutrient requirements of dairy cattle. 7th ed. Washington (DC): The National Academies Press. [PubMed] [Google Scholar]
- Ordway R. S., Boucher S. E., Whitehouse N. L., Schwab C. G., and Sloan B. K.. 2009. Effects of providing two forms of supplemental methionine to periparturient Holstein dairy cows on feed intake and lactational performance. J. Dairy Sci. 92:5154–5166. doi: 10.3168/jds.2009-2259 [DOI] [PubMed] [Google Scholar]
- Osorio J. S., Ji P., Drackley J. K., Luchini D., and Loor J. J.. 2013. Supplemental Smartamine M or MetaSmart during the transition period benefits postpartal cow performance and blood neutrophil function. J. Dairy Sci. 96:6248–6263. doi: 10.3168/jds.2012-5790 [DOI] [PubMed] [Google Scholar]
- Osorio J. S., Ji P., Drackley J. K., Luchini D., and Loor J. J.. 2014a. Smartamine M and MetaSmart supplementation during the peripartal period alter hepatic expression of gene networks in 1-carbon metabolism, inflammation, oxidative stress, and the growth hormone-insulin-like growth factor 1 axis pathways. J. Dairy Sci. 97:7451–7464. doi: 10.3168/jds.2014-8680 [DOI] [PubMed] [Google Scholar]
- Osorio J. S., Trevisi E., Ji P., Drackley J. K., Luchini D., Bertoni G., and Loor J. J.. 2014b. Biomarkers of inflammation, metabolism, and oxidative stress in blood, liver, and milk reveal a better immunometabolic status in peripartal cows supplemented with Smartamine M or MetaSmart. J. Dairy Sci. 97:7437–7450. doi: 10.3168/jds.2013-7679 [DOI] [PubMed] [Google Scholar]
- Park E., Park S. Y., Cho I. S., Kim B. S., and Schuller-Levis G.. 2017. A novel cysteine sulfinic acid decarboxylase knock-out mouse: taurine distribution in various tissues with and without taurine supplementation. Adv. Exp. Med. Biol. 975 (Pt 1):461–474. doi: 10.1007/978-94-024-1079-2_37 [DOI] [PubMed] [Google Scholar]
- Petro T. M., and Bhattacharjee J. K.. 1981. Effect of dietary essential amino acid limitations upon the susceptibility to Salmonella typhimurium and the effect upon humoral and cellular immune responses in mice. Infect. Immun. 32:251–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pithon-Curi T. C., Schumacher R. I., Freitas J. J., Lagranha C., Newsholme P., Palanch A. C., Doi S. Q., and Curi R.. 2003. Glutamine delays spontaneous apoptosis in neutrophils. Am. J. Physiol. Cell Physiol. 284:C1355–C1361. doi: 10.1152/ajpcell.00224.2002 [DOI] [PubMed] [Google Scholar]
- Plata-Salamán C. R. 1995. Cytokines and feeding suppression: an integrative view from neurologic to molecular levels. Nutrition 11(5 Suppl):674–677. [PubMed] [Google Scholar]
- Powell J. D., Pollizzi K. N., Heikamp E. B., and Horton M. R.. 2012. Regulation of immune responses by mTOR. Annu. Rev. Immunol. 30:39–68. doi: 10.1146/annurev-immunol-020711-075024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakhshandeh A., de Lange C. F. M., Htoo J. K., Gheisari A., and Rakhshandeh A. R.. 2019. Immune system stimulation increases the plasma cysteine flux and whole-body glutathione synthesis rate in starter pigs1. J. Anim. Sci. 97:3871–3881. doi: 10.1093/jas/skz211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M., Zhang S. H., Zeng X. F., Liu H., and Qiao S. Y.. 2015. Branched-chain amino acids are beneficial to maintain growth performance and intestinal immune-related function in weaned piglets fed protein restricted diet. Asian-Australas. J. Anim. Sci. 28:1742–1750. doi: 10.5713/ajas.14.0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Santiago C. E., Rivas-Santiago B., León D. A., Castañeda-Delgado J., and Hernández Pando R.. 2011. Induction of β-defensins by l-isoleucine as novel immunotherapy in experimental murine tuberculosis. Clin. Exp. Immunol. 164:80–89. doi: 10.1111/j.1365-2249.2010.04313.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche J. R., Bell A. W., Overton T. R., and Loor J. J.. 2013. Nutritional management of the transition cow in the 21st century – a paradigm shift in thinking. Anim. Prod. Sci. 53:1000–1023. doi: 10.1071/AN12293 [DOI] [Google Scholar]
- Ruth M. R., and Field C. J.. 2013. The immune modifying effects of amino acids on gut-associated lymphoid tissue. J. Anim. Sci. Biotechnol. 4:27. doi: 10.1186/2049-1891-4-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama A. A. K., Duque M., Wang L., Shahzad K., Olivera M., and Loor J. J.. 2019. Enhanced supply of methionine or arginine alters mechanistic target of rapamycin signaling proteins, messenger RNA, and microRNA abundance in heat-stressed bovine mammary epithelial cells in vitro. J. Dairy Sci. 102:2469–2480. doi: 10.3168/jds.2018-15219 [DOI] [PubMed] [Google Scholar]
- Shibano K., and Kawamura S.. 2006. Serum free amino acid concentration in hepatic lipidosis of dairy cows in the periparturient period. J. Vet. Med. Sci. 68:393–396. doi: 10.1292/jvms.68.393 [DOI] [PubMed] [Google Scholar]
- Sikalidis A. K. 2015. Amino acids and immune response: a role for cysteine, glutamine, phenylalanine, tryptophan and arginine in T-cell function and cancer? Pathol. Oncol. Res. 21:9–17. doi: 10.1007/s12253-014-9860-0 [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Molden D. P., and Seegmiller J. E.. 1976. Maple syrup urine disease: branched-chain amino acid concentrations and metabolism in cultured human lymphoblasts. Biochem. Genet. 14:527–539. doi: 10.1007/bf00485832 [DOI] [PubMed] [Google Scholar]
- Soder K. J., and Holden L. A.. 1999. Lymphocyte proliferation response of lactating dairy cows fed varying concentrations of rumen-protected methionine. J. Dairy Sci. 82:1935–1942. doi: 10.3168/jds.S0022-0302(99)75429-9 [DOI] [PubMed] [Google Scholar]
- Stachlewitz R. F., Li X., Smith S., Bunzendahl H., Graves L. M., and Thurman R. G.. 2000. Glycine inhibits growth of T lymphocytes by an IL-2-independent mechanism. J. Immunol. 164:176–182. doi: 10.4049/jimmunol.164.1.176 [DOI] [PubMed] [Google Scholar]
- Stoll B., Henry J., Reeds P. J., Yu H., Jahoor F., and Burrin D. G.. 1998. Catabolism dominates the first-pass intestinal metabolism of dietary essential amino acids in milk protein-fed piglets. J. Nutr. 128:606–614. doi: 10.1093/jn/128.3.606 [DOI] [PubMed] [Google Scholar]
- St-Pierre N. R., and Sylvester J. T.. 2005. Effects of 2-hydroxy-4-(methylthio) butanoic acid (HMB) and its isopropyl ester on milk production and composition by Holstein cows. J. Dairy Sci. 88:2487–2497. doi: 10.3168/jds.S0022-0302(05)72926-X [DOI] [PubMed] [Google Scholar]
- Sun F., Cao Y., Cai C., Li S., Yu C., and Yao J.. 2016. Regulation of nutritional metabolism in transition dairy cows: energy homeostasis and health in response to post-ruminal choline and methionine. PLoS One. 11:e0160659. doi: 10.1371/journal.pone.0160659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J., Liu S., Guo Y., Applegate T. J., and Eicher S. D.. 2014. Dietary l-arginine supplementation attenuates lipopolysaccharide-induced inflammatory response in broiler chickens. Br. J. Nutr. 111:1394–1404. doi: 10.1017/S0007114513003863 [DOI] [PubMed] [Google Scholar]
- Thorndike J., Pelliniemi T. T., and Beck W. S.. 1979. Serine hydroxymethyltransferase activity and serine incorporation in leukocytes. Cancer Res. 39:3435–3440. [PubMed] [Google Scholar]
- Thornton S. A., Corzo A., Pharr G. T., Dozier Iii W. A., Miles D. M., and Kidd M. T.. 2006. Valine requirements for immune and growth responses in broilers from 3 to 6 weeks of age. Br. Poult. Sci. 47:190–199. doi: 10.1080/00071660600610989 [DOI] [PubMed] [Google Scholar]
- Tsukishiro T., Shimizu Y., Higuchi K., and Watanabe A.. 2000. Effect of branched-chain amino acids on the composition and cytolytic activity of liver-associated lymphocytes in rats. J. Gastroenterol. Hepatol. 15:849–859. doi: 10.1046/j.1440-1746.2000.02220.x [DOI] [PubMed] [Google Scholar]
- Turvey S. E., and Broide D. H.. 2010. Innate immunity. J. Allergy Clin. Immunol. 125(2 Suppl 2):S24–S32. doi: 10.1016/j.jaci.2009.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyangaa E., Lee H. K., and Eo S. K.. 2012. Glutamine and leucine provide enhanced protective immunity against mucosal infection with herpes simplex virus type 1. Immune Netw. 12:196–206. doi: 10.4110/in.2012.12.5.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vailati-Riboni M. F. Batistel, R. R. C. S. Yambao, C. Parys, Y. X. Pan, and J. J. Loor. 2019. Hepatic cystathionine β-synthase activity is increased by greater postruminal supply of Met during the periparturient period in dairy cows. Curr. Dev. Nutr. 3: nzz128. doi: 10.1093/cdn/nzz128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vailati-Riboni M., Zhou Z., Jacometo C. B., Minuti A., Trevisi E., Luchini D. N., and Loor J. J.. 2017. Supplementation with rumen-protected methionine or choline during the transition period influences whole-blood immune response in periparturient dairy cows. J. Dairy Sci. 100:3958–3968. doi: 10.3168/jds.2016-11812 [DOI] [PubMed] [Google Scholar]
- Vance J. E. 2002. Assembly and secretion of lipoproteins New Comprehensive Biochemistry No. 36. Amsterdam (The Netherlands): Elsevier; p 505–526. [Google Scholar]
- Vance D. E., Walkey C. J., and Cui Z.. 1997. Phosphatidylethanolamine N-methyltransferase from liver. Biochim. Biophys. Acta 1348:142–150. doi: 10.1016/s0005-2760(97)00108-2 [DOI] [PubMed] [Google Scholar]
- Vicini J. L., Clark J. H., Hurley W. L., and Bahr J. M.. 1988. Effects of abomasal or intravenous administration of arginine on milk production, milk composition, and concentrations of somatotropin and insulin in plasma of dairy cows. J. Dairy Sci. 71:658–665. doi: 10.3168/jds.S0022-0302(88)79604-6 [DOI] [PubMed] [Google Scholar]
- Wade A. M., and Tucker H. N.. 1998. Antioxidant characteristics of l-histidine. J. Nutr. Biochem. 9:308–315. doi: 10.1016/S0955-2863(98)00022-9 [DOI] [Google Scholar]
- Wakabayashi S. 2013. New insights into the functions of histidine-rich glycoprotein. Int. Rev. Cell Mol. Biol. 304:467–493. doi: 10.1016/B978-0-12-407696-9.00009-9 [DOI] [PubMed] [Google Scholar]
- Wang L., Liu Y., Zhao T. L., Li Z. Z., He J. Y., Zhang B. J., Du H. Z., Jiang J. W., Yuan S. T., and Sun L.. 2019. Topotecan induces apoptosis via ASCT2 mediated oxidative stress in gastric cancer. Phytomedicine 57:117–128. doi: 10.1016/j.phymed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Wang X., Qiao S. Y., Liu M., and Ma Y. X.. 2006. Effects of graded levels of true ileal digestible threonine on performance, serum parameters and immune function of 10–25kg pigs. Anim. Feed Sci. Technol. 129:264–278. doi: 10.1016/j.anifeedsci.2006.01.003 [DOI] [Google Scholar]
- Wang W., Wu Z., Dai Z., Yang Y., Wang J., and Wu G.. 2013. Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477. doi: 10.1007/s00726-013-1493-1 [DOI] [PubMed] [Google Scholar]
- Wang M., Xu B., Wang H., Bu D., Wang J., and Loor J. J.. 2014. Effects of arginine concentration on the in vitro expression of casein and mTOR pathway related genes in mammary epithelial cells from dairy cattle. PLoS One. 9:e95985. doi: 10.1371/journal.pone.0095985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. D., and Thurman R. G.. 1999. Production of superoxide and TNF-alpha from alveolar macrophages is blunted by glycine. Am. J. Physiol. 277:L952–L959. doi: 10.1152/ajplung.1999.277.5.L952 [DOI] [PubMed] [Google Scholar]
- Wu G., Bazer F. W., Davis T. A., Kim S. W., Li P., Marc Rhoads J., Carey Satterfield M., Smith S. B., Spencer T. E., and Yin Y.. 2009. Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168. doi: 10.1007/s00726-008-0210-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Fang Y. Z., Yang S., Lupton J. R., and Turner N. D.. 2004. Glutathione metabolism and its implications for health. J. Nutr. 134:489–492. doi: 10.1093/jn/134.3.489 [DOI] [PubMed] [Google Scholar]
- Wu G., and Morris S. M. Jr. 1998. Arginine metabolism: nitric oxide and beyond. Biochem. J. 336 (Pt 1):1–17. doi: 10.1042/bj3360001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Wang C., Ding L., Shen Y., Cui H., Wang M., and Wang H.. 2016. Arginine relieves the inflammatory response and enhances the casein expression in bovine mammary epithelial cells induced by lipopolysaccharide. Mediators Inflamm. 2016:9618795. doi: 10.1155/2016/9618795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Xiao H., Ren W., Yin J., Tan B., Liu G., Li L., Nyachoti C. M., Xiong X., and Wu G.. 2014. Therapeutic effects of glutamic acid in piglets challenged with deoxynivalenol. PLoS One. 9:e100591. doi: 10.1371/journal.pone.0100591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassad A., Lavoinne A., Bion A., Daveau M., and Husson A.. 1997. Glutamine accelerates interleukin-6 production by rat peritoneal macrophages in culture. FEBS Lett. 413:81–84. doi: 10.1016/s0014-5793(97)00881-8 [DOI] [PubMed] [Google Scholar]
- Yin J., Ren W., Yang G., Duan J., Huang X., Fang R., Li C., Li T., Yin Y., Hou Y., et al. . 2016. l-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 60:134–146. doi: 10.1002/mnfr.201500031 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Chen X., Eicher S. D., Ajuwon K. M., and Applegate T. J.. 2016. Effect of threonine deficiency on intestinal integrity and immune response to feed withdrawal combined with coccidial vaccine challenge in broiler chicks. Br. J. Nutr. 116:2030–2043. doi: 10.1017/S0007114516003238 [DOI] [PubMed] [Google Scholar]
- Zhao F. F., Wu T. Y., Wang H. R., Ding L. Y., Ahmed G., Li H. W., Tian W., and Shen Y. Z.. 2018a. Jugular arginine infusion relieves lipopolysaccharide-triggered inflammatory stress and improves immunity status of lactating dairy cows. J. Dairy Sci. 101:5961–5970. doi: 10.3168/jds.2017-13850 [DOI] [PubMed] [Google Scholar]
- Zhao F., Wu T., Zhang H., Loor J. J., Wang M., Peng A., and Wang H.. 2018b. Jugular infusion of arginine has a positive effect on antioxidant mechanisms in lactating dairy cows challenged intravenously with lipopolysaccharide1. J. Anim. Sci. 96:3850–3855. doi: 10.1093/jas/sky250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z., Wheeler M. D., Li X., Froh M., Schemmer P., Yin M., Bunzendaul H., Bradford B., and Lemasters J. J.. 2003. l-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr. Opin. Clin. Nutr. Metab. Care 6:229–240. doi: 10.1097/00075197-200303000-00013 [DOI] [PubMed] [Google Scholar]
- Zhou X., L. He, C. Wu, Y. Zhang, X. Wu, and Y. Yin. 2017a. Serine alleviates oxidative stress via supporting glutathione synthesis and methionine cycle in mice. Mol. Nutr. Food Res. 61. doi: 10.1002/mnfr.201700262 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Bulgari O., Vailati-Riboni M., Trevisi E., Ballou M. A., Cardoso F. C., Luchini D. N., and Loor J. J.. 2016a. Rumen-protected methionine compared with rumen-protected choline improves immunometabolic status in dairy cows during the peripartal period. J. Dairy Sci. 99:8956–8969. doi: 10.3168/jds.2016-10986 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Ferdous F., Montagner P., Luchini D. N., Corrêa M. N., and Loor J. J.. 2018b. Methionine and choline supply during the peripartal period alter polymorphonuclear leukocyte immune response and immunometabolic gene expression in Holstein cows. J. Dairy Sci. 101:10374–10382. doi: 10.3168/jds.2018-14972 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Garrow T. A., Dong X., Luchini D. N., and Loor J. J.. 2017b. Hepatic activity and transcription of betaine-homocysteine methyltransferase, methionine synthase, and cystathionine synthase in periparturient dairy cows are altered to different extents by supply of methionine and choline. J. Nutr. 147:11–19. doi: 10.3945/jn.116.240234 [DOI] [PubMed] [Google Scholar]
- Zhou X., He L., Zuo S., Zhang Y., Wan D., Long C., Huang P., Wu X., Wu C., Liu G., et al. . 2018a. Serine prevented high-fat diet-induced oxidative stress by activating AMPK and epigenetically modulating the expression of glutathione synthesis-related genes. Biochim. Biophys. Acta. Mol. Basis Dis. 1864:488–498. doi: 10.1016/j.bbadis.2017.11.009 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Loor J. J., Piccioli-Cappelli F., Librandi F., Lobley G. E., and Trevisi E.. 2016b. Circulating amino acids in blood plasma during the peripartal period in dairy cows with different liver functionality index. J. Dairy Sci. 99:2257–2267. doi: 10.3168/jds.2015-9805 [DOI] [PubMed] [Google Scholar]
- Zhou Z., Vailati-Riboni M., Luchini D. N., and Loor J. J.. 2017c. Methionine and choline supply during the periparturient period alter plasma amino acid and one-carbon metabolism profiles to various extents: potential role in hepatic metabolism and antioxidant status. Nutrients 9:10. doi: 10.3390/nu9010010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z., Vailati-Riboni M., Trevisi E., Drackley J. K., Luchini D. N., and Loor J. J.. 2016c. Better postpartal performance in dairy cows supplemented with rumen-protected methionine compared with choline during the peripartal period. J. Dairy Sci. 99:8716–8732. doi: 10.3168/jds.2015-10525 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Zhang P., Deng G., Liu X., and Lu D.. 2012. Improvements of immune status, intestinal integrity and gain performance in the early-weaned calves parenterally supplemented with l-alanyl-l-glutamine dipeptide. Vet. Immunol. Immunopathol. 145:134–142. doi: 10.1016/j.vetimm.2011.10.020 [DOI] [PubMed] [Google Scholar]
- Zhu H., Blake S., Chan K. T., Pearson R. B., and Kang J.. 2018. Cystathionine β-synthase in physiology and cancer. Biomed Res. Int. 2018:11. doi: 10.1155/2018/3205125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H. L., Liu Y. L., Xie X. L., Huang J. J., and Hou Y. Q.. 2013. Effect of l-arginine on intestinal mucosal immune barrier function in weaned pigs after Escherichia coli LPS challenge. Innate Immun. 19:242–252. doi: 10.1177/1753425912456223 [DOI] [PubMed] [Google Scholar]