Introduction
According to the British Farm Animal Welfare Council (FAWC), keel bone fractures (KBF) are considered one of the most important welfare problems in commercial laying hens (FAWC, 2010, 2013). Besides the likely association of KBF with impaired welfare (reviewed by Riber et al., 2018), the high prevalence reported across bird strains in many countries highlights the global relevance of this topic. Two of the most frequently cited publications (Google Scholar, September 2019) dealing with KBF incidence present end-of-lay prevalence rates of 97% and 86% among commercial flocks in Belgium and the Netherlands (Rodenburg et al., 2008; 98 citations) and the UK (Wilkins et al., 2011; 102 citations). Despite other studies reporting relatively low prevalence for similarly aged birds housed under similar commercial conditions (11.6%; Riber and Hinrichsen, 2016; 11 citations), KBF prevalence is often described using phrases such as “over 85%” (Casey-Trott and Widowski, 2016), “up to 85%” (Hardin et al., 2019), “up to 90%” (Richards et al., 2011), “up to 80%” (Nasr et al., 2015), “greater than 50%” (Toscano et al., 2018), or “52% to 73%” (Lay et al., 2011). The variation in the way in which KBF prevalence is reported makes it difficult to identify the actual extent of the problem.
The challenge of accurately describing the extent of KBF prevalence reflects the multifactorial nature of the problem (Harlander-Matauschek et al., 2015). Besides nutrition and genetics, the housing system and design are assumed to be a key contributing factor. For example, perches have been identified as a hazardous element in both cage and cage-free housing systems (Sandilands et al., 2009). In addition, vertically complex housing environments such as multi-tier aviaries have been associated with falls and collisions (Stratmann et al., 2015a), which are likely to cause KBF (Makagon et al., 2017). Overall, there is a general consensus that the “complexity” of the housing system—despite not being clearly defined—is related with increased KBF prevalence. In other words, it is often assumed that hens housed in aviaries are more likely to obtain fractures than hens housed in single-tier or floor housing systems, whereas hens housed in cages are associated with the lowest KBF prevalence.
Despite the general consensus regarding the effect of housing system on KBF, there is—to our knowledge—no systematic review of KBF prevalence across housing systems, or hen ages and strains. To address this void, we compiled the wealth of existing literature from the past 30 yr with the goals of 1) summarizing published data on KBF prevalence with regard to hen age, strain, and features of the rearing and laying housing system and 2) conducting a meta-analysis to confirm the link between housing systems and KBF prevalence. In this review, we present the summarized data and discuss the numerous sources of bias and issues related to the way in which the study details are reported that prohibited us from conducting a meta-analysis. We begin by detailing our literature review search, screening, and inclusion criteria. We highlight global publication trends on the topic before discussing our key findings in the context of KBF assessment methodology, bird age and strain considerations, the role of adult and rearing housing systems, and other management practices. We conclude this review by summarizing and providing several recommendations for how to address the existing knowledge gaps.
Literature Review Methods
We conducted a literature search following the PRISMA guidelines (Moher et al., 2009). The publication search targeted four scientific databases (PubMed, BIOSIS, AGRICOLA, and CAB abstracts) using a combination of the keywords “laying hen” AND (“keel” OR “sternum” OR “bone”) AND (“fracture” OR “break”). Publications from the last 30 yr (1989 to 2018) were included. The database searches identified 351 publications matching the search criteria. This number was reduced to 210 after duplicate publications were removed. A total of 72 publications failed to meet the initial screening criterion, which required that keel bone assessment be mentioned in the Materials and Methods section. As only original, peer-reviewed research articles published in English were targeted for this review (second screening criterion), 60 additional publications were removed. Of the 88 remaining articles, 32 manuscripts were not eligible as they did not report actual prevalence data. For instance, some articles used a multi-score system to assess KBF and presented average scores rather than prevalence (e.g., Abrahamsson et al., 1996; Rufener et al., 2019b). Others assessed KBF and a selection of hens with a specific keel bone condition for controlled experiments (e.g., Casey-Trott and Widowski, 2016; LeBlanc et al., 2016) without reporting the KBF prevalence of the initial population. Another eight articles were not subject to review for other reasons (e.g., KBF prevalence was intertwined with other data).
The remaining 49 articles were reviewed in random order by a single individual (C.R.) who extracted information about a total of 84 variables (see E-Supplementary Material). In brief, we collected information about the study (four variables; e.g., country, longitudinal vs. cross-sectional, applied treatments), the birds (four variables; e.g., strain, age, production level), the rearing environment (30 variables; e.g., experimental vs. commercial facility, housing system, access to perches, height of the system, access to outdoor area), the laying environment (the same 30 variables as for the rearing environment), and KBF assessment methodologies and prevalence (16 variables; e.g., fracture prevalence, assessment method, training of observers, reliability testing). If KBF prevalence was not given, the overall damage prevalence (i.e., deviations or a combination of fractures and deviations) was recorded as a separate variable. Studies using the four-point scale developed by Scholz et al. (2008) score overall damage, but scores 1 and 2 are related to fractures based on histological evidence. Therefore, the prevalence of scores 1 and 2 was included as KBF prevalence scores. Modeled prevalence data (e.g., estimated means or model coefficients) were discarded. When prevalence was not reported in the text or in a table format, values were estimated or calculated based on the information provided in the paper. As an example, when prevalence was shown in graphs, the prevalence was estimated by reading the values directly from the graphs. To increase precision, the total axis length was measured with a ruler and the relative distance of the data point from zero (e.g., length of bars in bar plots) was used to calculate the numerical values.
Many of the studies reported KBF prevalence as part of general welfare assessments in the context of experimental treatments, e.g., housing conditions, diet, or bird characteristics such as age or strain. As a result, it was possible to extract multiple KBF prevalence data points from a single study, especially if data for multiple ages within the same flock were provided. As an example, Eusemann et al. (2018a) presented KBF data separately for pens differing by housing system (cage vs. floor) and strain (five strains) in a 2 × 5 factorial design resulting in 10 housing system by strain combinations. Within each treatment, KBF prevalence was reported at three time points. Therefore, we extracted 30 entries of KBF prevalence from this one publication, with each entry representing a different combination of age, strain, rearing, and layer housing conditions. On the other hand, when prevalence was reported separately for replicates of the same conditions, we collapsed the data into a single entry by averaging KBF prevalence. For example, Blatchford et al. (2016) reported prevalence separately for two consecutive flocks of the same strain in the same housing environment at the same age and under the same management conditions, thus prevalence was averaged over the two flocks. If data were collected over a range of ages, the average age was used. If data were collected at the end of lay but no specific age was given, 70 wk of age was assumed to be the end of lay (e.g., Wilkins et al., 2004).
Importantly, only prevalence values for flocks housed under conditions that could be considered commercially standard were included. If prevalence was reported separately for multiple commercially standard treatments, we averaged the reported values as long as age, strain, and housing conditions combinations were identical. As an example, Riber and Hinrichsen (2017) reported KBF prevalence in flocks being beak trimmed vs. non-beak trimmed. Both treatments are commercially applied management practices, housing conditions did not differ, and no statistical difference between treatments was found; hence, the average prevalence was used.
In all, the evaluation of the 49 articles (Table 1) yielded 283 individual literature review entries (data provided in E-Supplementary Material). Thirty-three studies (67.3%) reported prevalence for fractures specifically, whereas overall damage, i.e., deviations or fractures and deviations combined, was given in 16 studies (32.7%). As most studies reported multiple prevalence values or data points, the studies reporting fracture prevalence specifically accounted for 206 of the 283 entries (72.8% of total entries). The majority of the research was conducted in Europe. Over 70% of the studies were published in the last 10 yr.
Table 1.
Overview of studies reviewed, sorted by year
| Fracture prevalence | Damage prevalence | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Authors | Year | Country | Strain | Age, wk | Rearing system(s) | Laying system(s) | Min | Max | Min | Max | Assessment method | Times cited1 |
| Abrahamsson and Tauson | 1993 | Sweden | Mixed | 80 | Conventional Cage | Conventional cage Furnished cage |
12.6 | 39.8 | — | 88 | ||
| Appleby et al. | 1993 | UK | Brown | 71 | Floor | Conventional cage Furnished cage |
4 | 43 | — | 154 | ||
| Abrahamsson and Tauson | 1995 | Sweden | White | 80 | Single-tier Aviary |
Conventional cage Aviary |
4.2 | 39.9 | — | 163 | ||
| Appleby | 1998 | UK | Brown | 38 to 67 | Floor | Conventional cage Furnished cage |
13 | 63.5 | Palpation | 41 | ||
| Budgell and Silversides | 2004 | Canada | Mixed | 77 | Floor | Conventional cage | 0 | 0.07 | Dissection | 32 | ||
| Wilkins et al. | 2004 | UK | — | 70 | — | Mixed information | 38.7 | 58.7 | Dissection | 88 | ||
| Nicol et al. | 2006 | UK | — | 70 | Floor | Single-tier | 57 | 58 | Dissection | 96 | ||
| Clark et al. | 2008 | Canada | — | 47 to 65 | Conventional cage | Conventional cage | 0 | 5.5 | Radiography | 21 | ||
| Rodenburg et al. | 2008 | The Netherlands | Mixed | 60 to 64 | — | Furnished cage Floor Aviary |
62 | 97 | Dissection | 98 | ||
| Sherwin et al. | 2010 | UK | — | 70 | Mixed | Conventional cage Furnished cage Single-tier |
17.7 | 59.8 | Dissection | 147 | ||
| Käppeli et al. | 2011a | Switzerland | Mixed | 22 to 65 | Aviary | Floor Aviary |
0 | 53 | Palpation | 32 | ||
| Käppeli et al. | 2011b | Switzerland | Mixed | 70 to 71 | — | Mixed | 17 | 27.4 | Palpation | 50 | ||
| Wilkins et al. | 2011 | UK | Brown | 70 | — | Furnished cage Floor Single-tier |
32 | 87 | Palpation, dissection | 102 | ||
| Donaldson et al. | 2012 | UK | Brown | 20 to 72 | — | Single-tier | 10 | 86.3 | Palpation, dissection | 27 | ||
| Habig and Distl | 2013 | Germany | Mixed | — | Mixed | Furnished cage | 28 | Palpation | 17 | |||
| Hester et al. | 2013 | United States | White | 71 | Conventional cage Furnished cage |
Conventional cage Furnished cage |
82 | 93 | Dissection | 52 | ||
| Nasr et al. | 2012 | UK | Brown | 35 to 38 | — | — | 10 | 20 | Palpation | 32 | ||
| Petrik et al. | 2013 | Canada | Brown | 68 | — | Single-tier | 62 | Dissection | 15 | |||
| Tarlton et al. | 2013 | UK | — | 30 to 70 | — | — | 4 | 64 | Palpation, dissection | 44 | ||
| Bestman and Wagenaar | 2014 | The Netherlands | Mixed | 55 | Mixed | Mixed | 21 | Palpation | 29 | |||
| Scholz et al. | 2014 | Germany | Mixed | 21 | Floor | Floor | 0 | 0.1 | Palpation | 13 | ||
| Petrik et al. | 2015 | Canada | Brown | 20 to 65 | Conventional cage Single-tier |
Conventional cage Single-tier |
4 | 63 | Palpation | 47 | ||
| Stratmann et al. | 2015a | Switzerland | White | 18 to 66 | Aviary | Aviary | 0 | 90.5 | Palpation, dissection | 52 | ||
| Stratmann et al. | 2015b | Switzerland | Mixed | 18 to 64 | Aviary | Aviary | 2 | 38 | Palpation | 37 | ||
| Blatchford et al. | 2016 | United States | White | 52 to 72 | Conventional cage Aviary |
Conventional cage Furnished cage Aviary |
6 | 49 | Palpation | 33 | ||
| Heerkens et al. | 2016a | Belgium | Mixed | 29 to 49 | Aviary | Single-tier | 60 | 86.5 | Palpation | 16 | ||
| Heerkens et al. | 2016b | Belgium | Brown | 60 | — | Aviary | 82.5 | Palpation | 22 | |||
| Hinrichsen et al. | 2016 | Denmark | Mixed | 34 to 70 | — | Mixed | 3.7 | 18.7 | Palpation | 7 | ||
| Kajlich et al. | 2016 | United States | Mixed | 25 to 70 | — | Single-tier Aviary |
29 | 64.7 | — | 10 | ||
| Riber and Hinrichsen | 2016 | Denmark | Mixed | 32 to 62 | — | Single-tier Aviary |
2.9 | 11.6 | Palpation | 11 | ||
| Regmi et al. | 2016 | United States | Mixed | 78 | Conventional cage Floor |
Mixed | 37.3 | 63 | CT | 11 | ||
| Stratmann et al. | 2016 | Switzerland | White | 17 to 63 | Aviary | Aviary | 1 | 37 | Palpation | 18 | ||
| Campbell et al. | 2017 | Australia | Brown | 20 to 36 | Floor | Floor | 0.8 | 15.2 | Palpation | 15 | ||
| Candelotto et al. | 2017 | Switzerland | Mixed | 29 | — | Single-tier | 9.0 | 71 | Dissection | 6 | ||
| Casey-Trott et al. | 2017a | Canada | White | 16 to 70 | Conventional cage Aviary |
Furnished cage | 0 | 73 | Palpation | 15 | ||
| Gebhardt-Henrich et al. | 20172 | Switzerland | Mixed | 64 to 96 | — | Floor Aviary |
0.06 | 46 | Palpation | 3 | ||
| Grafl et al. | 2017 | Austria | Mixed | 75 | — | — | 22.9 | 25.8 | — | 9 | ||
| Riber and Hinrichsen | 2017 | Denmark | Mixed | 32 to 62 | — | Mixed | 4.2 | 9.8 | Palpation | 5 | ||
| Stojcic et al. | 2017 | Serbia | Mixed | 45 | — | Mixed information | 3 | 39 | Palpation | 0 | ||
| Widowski et al. | 2017 | Canada | White | 30 to 70 | Conventional cage | Conventional cage Furnished cage |
12 | 80 | Palpation | 9 | ||
| Buijs et al. | 2018 | Unknown | Brown | 75 | — | Aviary | 100 | Dissection | 2 | |||
| Chargo et al. | 20183 | United States | White | 55 to 80 | — | Furnished cage | 54.2 | 97.5 | CT | 2 | ||
| Eusemann et al. | 2018b | Germany | White | 20 to 35 | — | Floor | 0 | 0.4 | Radiography | 0 | ||
| Eusemann et al. | 2018a | Germany | Mixed | 35 to 72 | Floor | Furnished cage Single-tier |
0 | 100 | Radiography | 2 | ||
| Larsen et al. | 2018 | Australia | Brown | 44 to 66 | — | Single-tier | 45 | 62 | Palpation | 3 | ||
| Regmi et al. | 2018 | United States | White | 37 to 68 | — | Aviary | 30 | 92 | Palpation | 2 | ||
| Riddle et al. | 2018 | United States | Mixed | 28 | Floor | Aviary | 3 | 9 | Palpation | 5 | ||
| Rorvang et al. | 2019 | Denmark | White | 32 to 77 | — | Furnished cage | 2.4 | 19.8 | Palpation | 2 | ||
| Sirovnik et al. | 2018 | Switzerland | Mixed | 29 to 65 | Aviary | Aviary | 25.6 | 87 | Palpation | 2 | ||
1According to Google Scholar, September 2019.
2Using a subset of data from Käppeli et al. (2011b).
3Published online in 2018.
Growing Interest in Keel Bone Damage
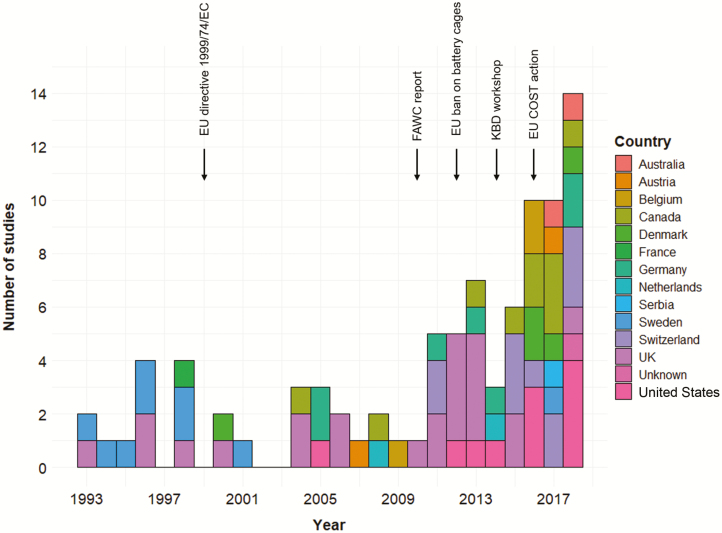
The growing interest in the topic has often coincided with regulatory and scientific milestones which sparked discussions about improving poultry welfare (Figure 1). For instance, the EU directive 1999/74/EC banning battery cages was passed in 1999. This directive aimed to improve housing conditions of laying hens but did not discuss the issue of KBF specifically. It is, therefore, not surprising that its passing was not associated with an increased interest in KBF. The number of studies that included keel bone assessment rose markedly after 2010, which is when the FAWC’s “Opinion on Osteoporosis and Bone Fractures in Laying Hens” acknowledging KBF as a welfare issue was published (FAWC, 2010). Two years later, in 2012, the battery cage ban in the EU was enforced after a 13-yr phase-out. In 2015, two key review articles on the causes (Harlander-Matauschek et al., 2015) and the assessment (Casey-Trott et al., 2015) of KBF were published as the outcomes of the first International Keel Bone Damage Workshop held in Switzerland (2014). These activities brought the issue to the attention of the global scientific community and resulted in the formation of a long-term international initiative focused on coordinating keel bone damage research and dissemination activities (EU COST Action CA15224). Accordingly, 34 of the 88 articles that met our second screening criterion were published between 2016 and 2018.
Figure 1.
The number of studies assessing KBF (total of 88 studies) published over the last 30 yr (1989 to 2018). Scientific and regulatory milestones discussing the improvement of poultry welfare are indicated with arrows.
Assessment Methods Impede Comparability
The reviewed studies relied on a number of keel bone assessment methods, including palpation, dissection, radiography, and computed tomography (CT) scanning (reviewed by Casey-Trott et al., 2015; see Chargo et al., 2018, 2019; Eusemann et al., 2018a; Rufener et al., 2018; Tracy et al., 2019). These methods differ in reliability, sensitivity, and in the type of assessor training needed to accurately detect damage to the keel. If KBF prevalence results are to be compared across studies (or combined for a meta-analysis), the utilized methods of damage detection must all be accurate, sufficient methodological details must be reported for each study, and the way in which KBF prevalence is reported must be standardized. As discussed below, we found that a large proportion of reviewed papers did not fulfill these criteria. In fact, and surprisingly, the specific method of assessment was not reported in 5 of the 49 reviewed studies (10.2%).
Palpation is the most frequently used method as it is cost-effective, does not require specialized equipment, and is, therefore, relatively easy to conduct in farm and research settings. Accordingly, palpation was used in 30 of the 49 reviewed studies (61.3%), including four (8.2%) that used it in combination with dissections. The accuracy of palpation to detect fractures is limited (Casey-Trott et al., 2015) as the method relies on the tactile perception of callus material indicative for old fractures and lacks sensitivity for fresh or hairline fractures as well as fractures on the dorsal site of the keel (Richards et al., 2011). Fractures located at the caudal third of the keel are especially difficult to detect using palpation, and this region is associated with particularly low palpation accuracy and reliability (Buijs et al., 2018). Using radiography, 62% of fractures have been shown to be located in this area (Baur et al., 2020), thus a valid assessment of KBF is unlikely when palpation is used. Because the validity of palpation is closely tied to the accuracy with which the individual researcher or assessor can detect fractures (Martin and Bateson, 1993), reporting an accuracy metric is key for data interpretation. We found that only three (10%) of the studies that used palpation and four (8.2%) of all reviewed studies mentioned that accuracy was tested, and only one reported a specific metric. The proportion of studies that reported on intra- or inter-rater reliability testing when using palpation was also low (11 of 30 studies; 36.7%), with only 5 studies (16.7%) providing actual reliability test outcomes. While reporting of reliability has its own value (ensuring the repeatability of results, confirming relative skills of multiple assessors), it does not indicate whether the palpation values reflect the actual state of the keel (Martin and Bateson, 1993). It is possible to have high intra- and inter-rater reliability but low accuracy, in which case the validity of results is low. For this reason, both accuracy and reliability should be reported. Training can improve both accuracy and reliability of palpation as a fracture assessment method (Petrik et al., 2013; Buijs et al., 2018; Gebhardt-Henrich et al., 2019), but training information was provided in only 8 of the 30 reviewed studies that used palpation. The lack of reporting makes it difficult to determine whether or not the researchers involved in the remaining 18 studies received training. In all, given that comparability among studies is dependent on accurate and reliable reporting of KBF prevalence, it remains difficult to generalize conclusions based on the palpation data.
Dissection, which was used as the sole method of keel assessment in nine (18.4%) of the reviewed studies and in tandem with palpation in another four studies, allows for visual inspection of the keel bone. The location and number of new and old fractures on the entirety of the bone can be detected using this method. Given that clinical assessment of bone morphology is contingent on training, researchers assessing fracture prevalence using visual examination of the bone should be trained. Only 2 of the 13 studies that assessed bones using dissection (15.4%) reported that observers received training. Similarly, only two studies using dissection reported that the reliability of fracture assessment was tested. Irrespective of training and reliability, measuring the accuracy of visual inspection of the keel bone is problematic as it is not clear which metric should be used for comparison. Casey-Trott et al. (2015) pointed out that histological and radiographic examinations of the bone provide more detail than gross visual inspection. However, only a few studies have compared dissection outcomes to these methods (e.g., Scholz et al., 2008; Tracy et al., 2019). Because it is unclear which types of bone damage are relevant to the hen’s welfare, it is difficult to determine which of these methods should be used as the “gold standard.” An additional limitation of gross visual keel bone evaluation following dissection is that it is, by definition, conducted postmortem and, therefore, cannot be used to study the bone development of individual hens. Accordingly, dissections tend to be conducted at the end of lay, whereas prevalence values obtained during the laying phase are most often based on palpation (Figure 2). While this is not problematic when the dissections are used as an opportunity to confirm palpation accuracy (keeping in mind the limitations described above), it does pose a problem when used as the sole method for a single time point of a longitudinal study. As an example, Stratmann et al. (2015a) used palpation to assess KBF prevalence repeatedly throughout lay until 60 wk of age, but dissected hens at depopulation at 66 wk of age without palpating the birds immediately before dissection. The authors concluded that they might have underestimated the true prevalence of KBF using palpation and pointed out that their conclusions would have been strengthened by conducting palpation and dissection at the same time point.
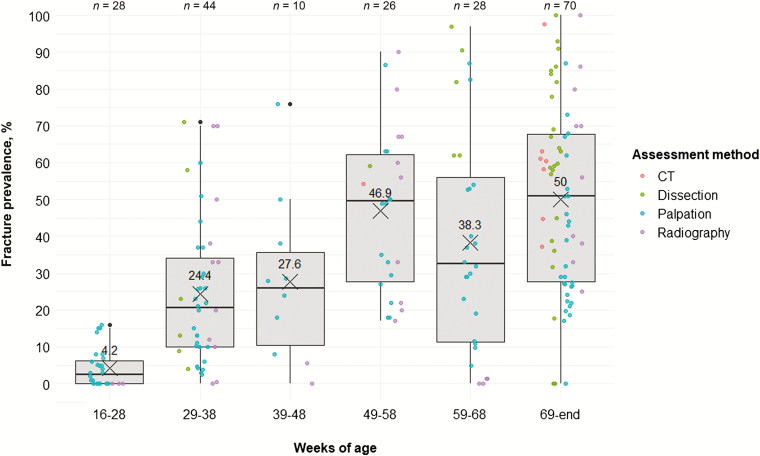
Figure 2.
Keel bone fracture prevalence (%) across age categories. Colors indicate the assessment method used. Boxplots show medians and interquartile and absolute ranges of raw data plus outliers. The cross and numbers indicate mean prevalence. n indicates the number of entries within one category.
Details of keel bone integrity including the presence of fractures can be obtained using imaging technologies. Radiography (Clark et al., 2008; Richards et al., 2011; Eusemann et al., 2018a; Rufener et al., 2018), ultrasound (Tracy et al., 2019), and CT scanning (Baker et al., 2020; Chargo et al., 2018, 2019) have all been used for keel bone assessment. These methods require specialized equipment and training and are, therefore, not practical for on-farm use. Only five of the reviewed studies used imaging technologies (radiography: three [6.1%] studies, CT: two [4.1%] studies), all under experimental settings. A single study reported that assessors were trained, and none reported on accuracy or reliability testing. Training is especially relevant for studies using imaging technologies due to the great amount of detail these methods provide (e.g., Loughran, 1994). For instance, an increased radiographic density on an x-ray image does not necessarily indicate a fracture but could be due to sclerosis (Baur et al., 2020), and a lack of training could result in an overestimation of fracture prevalence. On the other hand, with appropriate training, it is possible to quantify and describe a wide range of fracture characteristics in great detail. While this provides a better estimate of true KBF prevalence, the relevance of these fracture characteristics has to be considered when imaging technologies are used for welfare assessments. As an example, it remains unclear whether a measure for overall fracture severity, the location or type of fractures on the keel bone, or specific measures such as the diameter of a callus or an angle between two fracture segments are most relevant regarding individual hen welfare.
Overall, the wide array of methods used for KBF assessment makes it difficult to compare KBF prevalence across studies. The use of multiple scoring systems within each method further complicates the matter. For instance, at least five different palpation scoring systems were used within the reviewed studies, each providing slightly different information. The popularly used Welfare Quality Protocol (Welfare Quality, 2009) uses a binary system that differentiates straight from “deformed” bones, thus lumping fractures with other forms of keel bone damage. Studies using the SKAP palpation system (Casey-Trott et al., 2015) report fractures and deviations as binary, mutually exclusive variables. We found it surprising that some studies did not specify a scoring system or provided a very limited description hindering our ability to interpret the presented results. For example, Riber and Hinrichsen (2016) reported a relatively low prevalence of KBF among hens housed in single- and multi-tiered systems in Denmark, concluding that the low prevalence may reflect superior management practices. The authors reported that they used the HealthyHen system without providing further information within the manuscript. A later study conducted by the same group (Rørvang et al., 2019) included details about the scoring system, noting that tip fractures were not included as part of the KBF assessment and raising the possibility that this may have led to an under-estimation of actual KBF prevalence. This second study specified that the same scoring protocol and observers were used as by Riber and Hinrichsen (2016), bringing into question whether the low prevalence reported by Riber and Hinrichsen (2016) may have also been an underestimation. While disregarding tip fractures may improve inter- and intra-rater reliability and, therefore, the quality of the obtained data, this example highlights the importance of reporting the details of the scoring system used.
Taken together, comparing KBF prevalence across studies is difficult due to the differences in the information provided by the various assessment methodologies. In order to make direct comparisons possible, we must develop strategies to 1) calibrate sensitivity among assessment methods, 2) standardize the way of reporting prevalence, 3) report accuracy and reliability for all assessors, and 4) determine which features of fractures are relevant for hen welfare.
Bird Age and Strain Matter
KBF are closely linked to hen age (Eusemann et al., 2018b, 2020), with the most dramatic increase in prevalence reported to occur between the onset and peak of lay (i.e., 25 to 35 wk of age; Harlander-Matauschek et al., 2015). In order to cover the high calcium demand for egg production, laying hens mobilize 40% to 60% of their daily calcium requirements from the skeleton (Johnson, 2015). With ongoing lay, structural bone decreases continuously, resulting in a progressive weakening of bones and thus increased fracture susceptibility (Whitehead, 2004). Given that egg production in commercial strains remains relatively high throughout lay and structural bone decreases continuously, it is reasonable to assume that KBF prevalence would increase with hen age. Indeed, such a trend has been reported by Heerkens et al. (2016a). However, Toscano et al. (2018) found that susceptibility for experimentally induced postmortem fractures stabilized after approximately 49 wk of age. Stratmann et al. (2015b) reported a similar developmental trend in fracture prevalence in aviary-housed hens. Fracture severity has also been reported to follow the same kind of age-related pattern (Rufener et al., 2019a, 2019b). An increase in average fracture prevalence from the onset of lay to late lay with KBF prevalence leveling out after 49 wk of age was also evident in the graphical representation of the data we compiled through the literature review process (Figure 2).
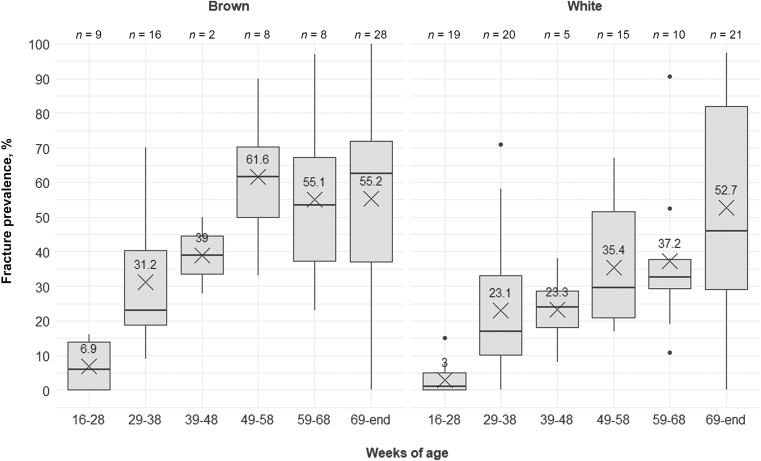
Keel bone fracture susceptibility as related to hen age may vary by strain. Laying hen strains differ regarding their laying performance, with brown birds typically having slightly lower egg production than white birds (e.g., Lohmann Selected Leghorn: 94% to 96% maximal performance vs. Lohmann Brown: 92% to 94% maximal performance; Lohmann Tierzucht GmbH). If egg production was directly related to KBF prevalence (i.e., higher productivity = higher prevalence), we would expect white hens to have more fractures than brown hens. Indeed, this relationship has been demonstrated by Stratmann et al. (2015b). However, other studies examining KBF prevalence across strains have reported the opposite trend (more fractures in brown vs. white strains; Habig and Distl, 2013; Heerkens et al., 2016a; Candelotto et al., 2017; Gebhardt-Henrich et al., 2017; Eusemann et al., 2018a), perhaps due to the higher body weights of brown hens (Gebhardt-Henrich et al., 2017). Summarizing results across the reviewed studies, the average KBF prevalence seems to be higher in brown hens than in white hens for all age categories (Figure 3). The increase in KBF prevalence is more pronounced in brown than in white hens until 49 to 58 wk of age. From 49 wk of age, KBF prevalence in brown hens seems to remain constant until the end of lay. Keel bone fracture prevalence in white hens, on the other hand, seems to develop in a more linear pattern, with the highest prevalence at the end of lay. Rufener et al. (2019a, 2019b) explored the development of KBF severity in individual hens from 21 to 62 wk of age and found similar strain-dependent patterns.
Figure 3.
Keel bone fracture prevalence (%) across age categories and strains. Boxplots show medians and interquartile and absolute ranges of raw data plus outliers. The cross and numbers indicate mean prevalence. n indicates the number of entries within one category.
At this point in time, it is not possible to determine whether the identified age by strain patterns are reflective of the true development of KBF prevalence, strain differences, or other confounding factors. The majority of the reviewed studies have focused on early to peak lay (up to 38 wk of age) and/or end of lay, while information on KBF during the critical age range where prevalence seems to level out is sparse (Figure 3). Therefore, it is possible that the age by strain differences in KBF development presented might be reflective of a sampling bias. Overall, there is a clear need for studies to sample birds at these underrepresented time points.
Laying and Rearing Environments: the Higher, the Better, or Worse?
It is generally thought that KBF prevalence increases with the spatial “complexity” of the housing system. Accordingly, hens housed in non-cage systems and especially in multi-tier aviaries are frequently cited as having more fractures than hens kept in single-tier or cage systems (e.g., Rodenburg et al., 2008; Riber and Hinrichsen, 2016). The presence of perches and the height of system components available to hens are often discussed as two important risk factors that may account for this difference.
The strongest evidence linking perches to KBF development comes from studies conducted under experimental conditions in controlled settings that directly test the effect of perch presence on KBF. Whereas perch presence affects KBF prevalence, properties of the perch such as material and shape can mitigate these effects (reviewed by Sandilands et al., 2009; Pickel et al., 2011). The majority of the reviewed studies provided perch access during the lay period to hens in all (26 studies) or some (12 studies) of the study treatments or housing systems used. No perches were provided in only three of the studies. We were unable to isolate and further explore the role of perches on KBF prevalence as perch access was intertwined with the housing system (e.g., aviaries always offer perches, whereas commercial conventional cages do not). Notably, it was not possible to determine whether perches were provided in eight of the reviewed studies, reiterating the importance of reporting methodological study details known to impact KBF prevalence in order to enable future cross-study comparisons.
Whereas the effect of perches on bone integrity can be investigated experimentally as an isolated factor, the effect of height cannot. Comparing two housing systems that exclusively differ in height is not possible because the height of the systems to be examined is limited by the hens’ ability to reach these areas. To test commercially relevant system heights, it would be necessary to introduce intermediate transitional areas (e.g., perches, platforms, ramps), which would effectively change the other aspects of housing “complexity.” In other words, height is intrinsically intertwined with other “complexity”-related aspects of the housing system. As a result, the link between system height and KBF prevalence is often inferred from studies that compare prevalence values across multiple housing systems (e.g., Rodenburg et al., 2008; Blatchford et al., 2016; Riber and Hinrichsen, 2016). This inference is supported by a single study (Wilkins et al., 2011) that modeled the correlation between height and KBF prevalence irrespective of the housing system type, recognizing that this relationship is not necessarily linear. A main objective of our review was to conduct a meta-analysis to further explore this relationship using the wealth of data available across published studies. However, only 29.3% of the entries compiled from the 33 reviewed studies that provided KBF prevalence data included sufficient detail for us to extract information on system height. This includes entries where the system height was noted as well as entries providing details about system name and manufacturers, which allowed us to look up system height. Due to the lack of information, we are limited to exploring the relationship of housing system types with KBF prevalence.
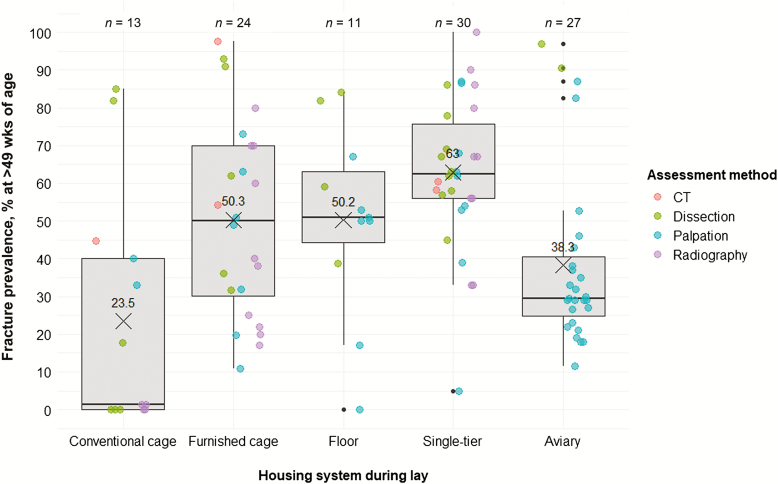
In the reviewed studies, hens were housed in conventional cages (11 studies), furnished cages (14), floor systems (7), single-tier systems (11), and aviaries (15). Three studies did not provide information about the housing system. Acknowledging that KBF prevalence depends on age until at least 49 wk of age (discussed above), we summarize KBF data provided for hens at 49 wk of age and older (Figure 4). The average KBF prevalence was lowest in conventional cages, and prevalence in aviaries was intermediate, whereas furnished cages, floor systems, and single-tier systems were associated with the highest average KBF prevalence. This trend is surprising as it seems to contradict the general consensus that the overall “complexity” of the housing system is related to increased KBF prevalence in a linear fashion. In addition to—and likely in interaction with—housing “complexity” factors such as bird genetics, nutrition, or other aspects of management contribute to KBF susceptibility and may be reflected in the distribution of compiled data. It is also possible that the KBF prevalence distributions are skewed as a result of the way in which prevalence values were obtained. For example, given that assessment methods differ regarding their sensitivity to detect fractures (see discussion above), an under- or over-representation of an assessment method within a specific housing system type could shift KBF prevalence data for that housing system. Indeed, we found that studies conducted in multi-tier aviaries almost exclusively used palpation for fracture assessment (Figure 4), which could have led to an underestimation of KBF prevalence in these systems.
Figure 4.
Keel bone fracture prevalence (%) in hens older than 49 wk of age across housing system types used during lay. Colors indicate the assessment method used. Boxplots show medians and interquartile and absolute ranges of raw data plus outliers. The cross and numbers indicate mean prevalence. n indicates the number of entries within one category.
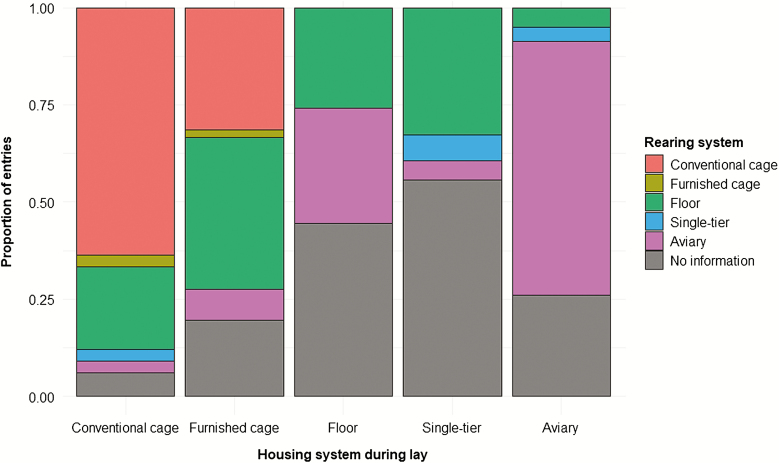
Most research investigating KBF prevalence and its association with housing has focused on the housing environment that the birds experience during lay despite the well-known fact that the rearing system is associated with skeletal integrity (Enneking et al., 2012; Regmi et al., 2015; Casey-Trott et al., 2017a, 2017b). Rearing in complex systems is linked to increased bone strength and improved cognitive abilities resulting in a lower susceptibility and risk of fractures during lay (reviewed by Rufener and Toscano, in press). Of the reviewed studies, 23 (46.9%) did not provide information about the rearing system. When information was provided, the rearing system tended to match layer housing (Figure 5), as is considered best practice (Janczak and Riber, 2015). Given that hens housed in less complex systems such as furnished cages or single-tier systems were often reared in floor pens or conventional cages, these hens might be more susceptible to KBF than hens reared and housed in aviaries. This could explain the relatively lower average prevalence of KBF reported among aviary-housed birds (Figure 4), which we found were typically reared in aviaries (Figure 5).
Figure 5.
Within each layer housing type, the proportion of keel bone fracture prevalence entries that reference each type of rearing system.
Overall, the compiled data do not support the general consensus that multi-tier aviaries are associated with higher KBF prevalence than less complex systems. However, underreporting of methodological details in publications, particularly those popularly regarded as affecting KBF prevalence, prohibited us from conducting a formal analysis.
Management Goes Beyond Housing System
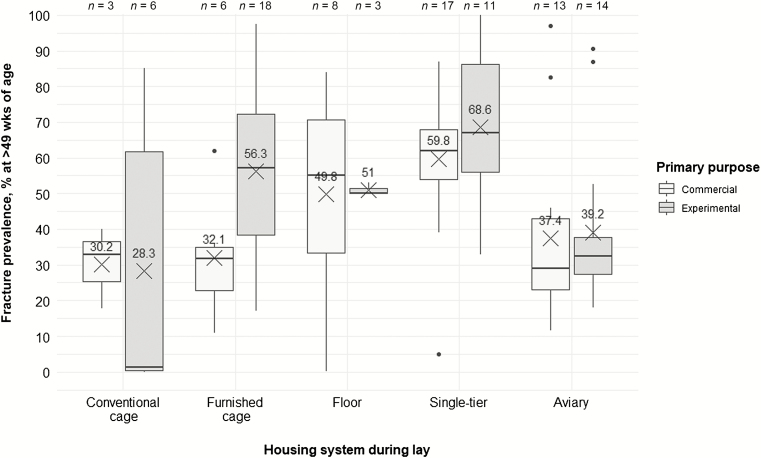
Management goes beyond simple housing systems. The review process allowed us to explore factors that are likely to affect KBF prevalence but are not often discussed. One such set of factors is the quality and quantity of husbandry-related activities that the birds are exposed to, which may depend on whether the birds are kept primarily for commercial or experimental use. Animals kept under experimental conditions are likely to repeatedly experience a suite of procedures that deviate from daily commercial husbandry protocols (e.g., catching, weighing, keel bone assessment, treatment). Additionally, they are likely to be kept in smaller groupings. Sudden escape reactions or panic, which have been suggested to be a source for KBF (Harlander-Matauschek et al., 2015), often happen in response to procedures deviating from the daily care protocols (Richards et al., 2012). For birds in conventional cages, floor, and aviary systems, we found that average KBF prevalence in hens older than 49 wk of age seemed to be similar regardless of whether the primary use of the facility was commercial or experimental/research. However, more hens seemed to be affected by KBF in experimental furnished cages and single-tier systems than in their commercial equivalents (Figure 6). Moreover, the range of values collected under experimental settings tended to be wider, and the maximum prevalence seems to be higher than in commercial conditions for most systems. In all, it is possible that average KBF prevalence in furnished cages—which was among the highest across all housing systems (Figure 4)—may be an overestimation driven by sampling bias toward experimental settings (18 entries for experimental and 6 entries for commercial furnished cages).
Figure 6.
Keel bone fracture prevalence (%) in hens older than 49 wk of age across adult housing system types depending on primary purpose of the facilities (commercial vs. experimental). Boxplots show medians and interquartile and absolute ranges of raw data plus outliers. The cross and numbers indicate mean prevalence. n indicates the number of entries within one category.
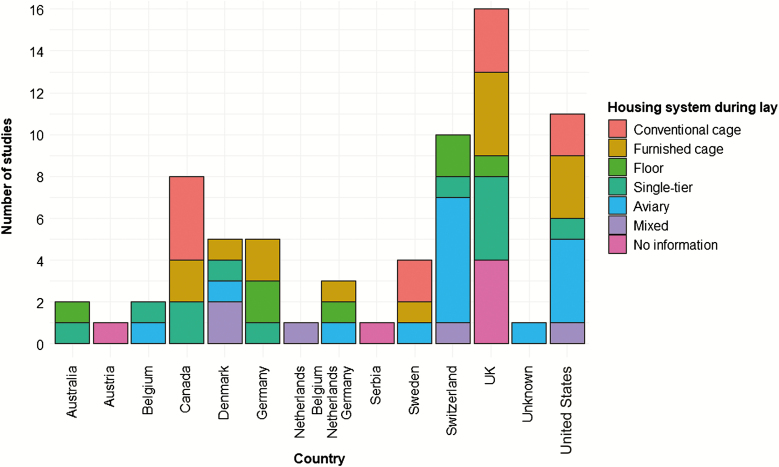
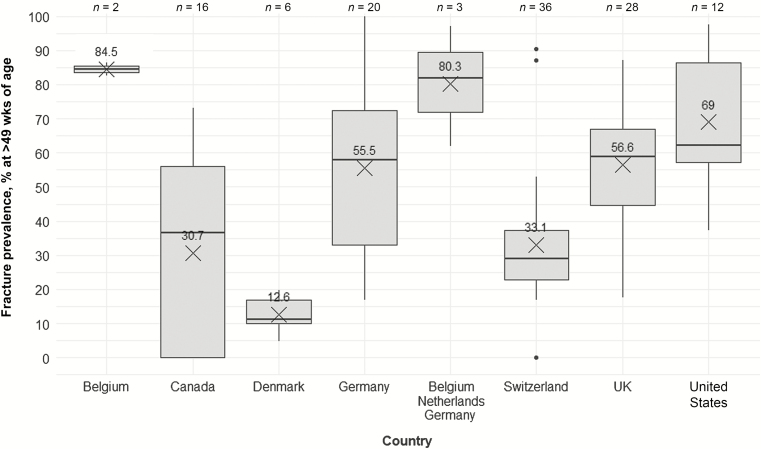
Management and data collection protocols are not only related to the primary purpose of a facility but presumably differ between countries and research groups. Housing systems were not evenly distributed across countries (Figure 7), revealing that associations between housing system and KBF prevalence are at least partly biased by country and thus management practice and environmental factors. One cannot assume that an aviary in a country such as Switzerland follows the same management protocol as one in the United States. Whereas Switzerland has been cage-free since 1992 and has capped flock sizes at a maximum of 18,000 hens, aviary systems are just now starting to gain traction in the United States, where flock sizes can be in the 100,000s. In addition to country-related differences in management, a “herding effect” can occur at the research group level, where members are trained the same way and design experiments to support a similar point of view (Lazic, 2016). With regard to KBF assessment, this phenomenon might manifest when researchers from a single research group are training each other using protocols differing from those used by other groups. For instance, most Swiss researchers publishing data on KBF prevalence used the palpation scoring system developed by Scholz et al. (2008). Danish studies are mostly based on the HealthyHens palpation protocol, and most studies from the UK used the scheme from Wilkins et al. (2004). As discussed previously, it is likely that these different schemes result in different outcomes. Taken together, herding effects in combination with uneven distribution of housing systems may contribute to the high variation in KBF prevalence we found when comparing data across countries (Figure 8). In other words, variation in KBF prevalence across countries may reflect methodological issues rather than differences in the true KBF prevalence.
Figure 7.
The number of studies published across countries in relation to the type of housing system the hens were placed in during lay.
Figure 8.
Keel bone fracture prevalence (%) in hens older than 49 wk of age across countries. Boxplots show medians and interquartile and absolute ranges of raw data plus outliers. The cross and numbers indicate mean prevalence. n indicates the number of entries within one category.
In summary, there are numerous possible sources of bias and management factors other than the housing system that should be given consideration when interpreting KBF prevalence data. Continued collaborations and cross-training across research groups, particularly ones in different geographical regions of the world, are encouraged.
Key Take-Aways
Increased interest in KBF as a welfare issue has initiated discussions about its causes and has resulted in coordinated global research efforts to find solutions. Research meetings and symposia centered on the topic of KBF have been held worldwide. In parallel, training workshops have been organized in an attempt to improve the quality and comparability of KBF prevalence data. New methods and protocols aiming to improve the accuracy and reliability of KBF assessment have been developed. Despite these efforts, our review demonstrates that multiple issues continue to hamper the comparability and interpretation of the data and, thus, the overall goal of reducing KBF prevalence.
First, we found underreporting of key methodological and study design details to be a common issue. Indeed, numerous studies failed to provide information about which KBF assessment method was used (10.2%) or the type of system the hens were housed in (12.2%). This information is absolutely critical for studies to be replicated. We additionally recommend that the authors include sufficient information so that details about the aspects of housing assumed to affect KBF prevalence can be extracted. For example, when commercial housing is used, we recommend that system name and manufacturer be provided, along with a link to a schematic or brochure. If this is not possible, information about the height of the highest accessible structure above floor level should be provided, in addition to information about perch presence, and the type of rearing system used.
Second, currently, there is no standardized way of reporting KBF prevalence. Studies focusing on the general welfare assessment of hens usually evaluate the prevalence of overall damage. This is problematic, as the welfare consequences of KBF vs. deviations are not the same. Additionally, the different types of damage are likely related to different causal factors (reviewed by Riber et al., 2018). Hence, it is important for the reader to be able to extract information about fracture prevalence specifically. Scoring fractures and deviations as mutually exclusive variables (Casey-Trott et al., 2015) can address this issue, but only if prevalence is subsequently reported separately for all of the resulting combinations (no fracture, deviation, fracture, or deviation and fracture). We recognize that researchers select and adapt scoring systems to match their specific research needs. Regardless of the scoring system used, sufficient details must be provided to enable replication of the study and appropriate data interpretation. For example, assessments that target specific parts of the keel bone should be reported as such.
Third, missing information about the training of the observers and accuracy of the results make it impossible to evaluate the validity, and thus the scientific rigor and comparability, of published studies. Achieving valid results with cost-effective and practical assessment methods, such as palpation, is a challenge. It is important that anyone conducting palpations (or other KBF assessments) be trained, and that their accuracy for detecting KBF be evaluated and reported. This can be done by reporting an accuracy value for each assessor or by reporting an inter-rater reliability measured against a trainer with documented high accuracy. Although not currently standard practice, intra-rater reliability should be assessed and reported for all assessors and trainers. We encourage efforts to train the palpation skills of researchers across countries and research groups to facilitate more direct outcome comparisons.
Fourth, data are lacking about the relative sensitivities of assessment methods. It is likely that palpation is underestimating the true fracture prevalence (Wilkins et al., 2011; Petrik et al., 2013; Stratmann et al., 2015a), but to date, only a handful of studies have compared the outcomes of multiple assessment methods applied to the same birds. Investing in this research is critical for evaluating how results obtained using one method (e.g., palpation) relate to the results obtained using another method (e.g., radiographs). Such information will move forward discussion about what the “gold standard” for accuracy testing should be.
Fifth, a renewed focus on longitudinal data collection targeting individual birds can help alleviate the shortcomings of flock-level approaches. Thus far, the majority of research has been conducted at the flock level yielding a wealth of information about the overall KBF prevalence and possible risk factors. However, the limitation of this approach is that flock level information may be subject to ecological bias. Ecological bias occurs when conclusions about an individual phenomenon—in this case, KBF—are drawn based on group-level data such as flock prevalence (Siegford et al., 2016; Bushby et al., 2018). For instance, all hens in a given housing system may have access to perches but drawing the conclusion that perch use affects KBF prevalence is only valid if we can prove that the hens that used the perches are the ones who developed fractures. Longitudinal observations in small increments would further allow internal controls (i.e., pre- vs. post-fracture data) as well as the identification of “key time points” in a hen’s life that might be linked with KBF development.
Sixth, we encourage deeper consideration about the impact of study design decisions on KBF prevalence outcomes and conclusions. For example, experimental studies allow us to investigate the isolated effect of specific elements of housing “complexity” as a proof of concept (e.g., the effect of perch presence on KBF prevalence). However, we cannot assume that prevalence data stemming from experimental work are necessarily representative of the prevalence on commercial farms. Management bias should similarly be considered when collecting data on multiple commercial farms, particularly ones that use different housing systems (i.e., when farm A using housing system A is compared with farm B using housing system B). We recommend taking into account these study design decisions before drawing conclusions about the link between housing systems and KBF prevalence.
Finally, it is still not clear which aspects of KBF are most relevant to hen welfare. We need to better understand whether it is the presence or absence of a fracture, the overall state of the keel bone (e.g., number of fractures, overall fracture severity, overall damage), healing status (e.g., fresh vs. healed fracture), and/or some other fracture characteristic (e.g., location on the bone, fracture type) that matter most to an individual bird. To do so, we need to link these aspects and their combinations to known welfare indicators such as the experience of pain or other negative affective states. Only then, we can determine which aspects of bone damage our assessment protocols should actually measure.
Concluding Remarks
Although fractures of the keel bone have been identified as a key welfare issue, the extent of the problem has yet to be defined. Instead, KBF prevalence is often described using nonspecific phrases such as “over 85%” or “up to 90%.” As highlighted in our review, KBF development is affected by a multitude of factors, making it difficult to estimate the average KBF prevalence. The impacts of hen age, strain, housing system, and other management factors were explored here. A main finding was that the general consensus regarding the link between housing system and KBF prevalence may not be supported when the whole body of knowledge is compiled and examined. We further discuss how the aspects of study design and reporting habits, including those related to assessment methods and assessor training, prevent integration of data across studies. In order to ensure comparability of data and facilitate interpretation in the future, we recommend that attention be given to: reporting of study details, providing KBF prevalence separate from other forms of damage, documenting the accuracy and reliability of the assessment, comparing the relative sensitivities of assessment methods, and focusing on longitudinal data collection in individual birds. We further highlight the need for consideration of how study design impacts the conclusions that can be drawn. The last 30 yr of research efforts have yielded important information about the possible causes and likely scope of KBF as a welfare problem and laid the foundations for an in-depth exploration of underlying mechanisms. There is no doubt that KBF is a prevalent problem in the laying hen industry. However, the full scope of its impact on the global industry and the welfare of the individual hens are still to be determined.
Supplementary Material
Glossary
Abbreviations
- FAWC
Farm Animal Welfare Council
- KBF
keel bone fractures
Acknowledgments
Dr. Rufener’s salary was supported by the Foundation for Food and Agriculture Research under award number - Grant ID 550830. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the Foundation.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Literature Cited
- Abrahamsson P., and Tauson R.. 1993. Effect of perches at different positions in conventional cages for laying hens of two different strains. Acta Agric. Scand. A–Anim. Sci. 43:228–235. doi: 10.1080/09064709309410171 [DOI] [Google Scholar]
- Abrahamsson P., and Tauson R.. 1995. Aviary systems and conventional cages for laying hens: effects on production, egg quality, health and bird location in three hybrids. Acta Agric. Scand. Sect. A–Anim. Sci. 45:191–203. doi: 10.1080/09064709509415851 [DOI] [Google Scholar]
- Abrahamsson P., Tauson R., and Appleby M. C.. 1996. Behaviour, health and integument of four hybrids of laying hens in modified and conventional cages. Br. Poult. Sci. 37:521–540. doi: 10.1080/00071669608417882 [DOI] [PubMed] [Google Scholar]
- Appleby M. C. 1998. The Edinburgh modified cage: effects of group size and space allowance on brown laying hens. J. Appl. Poult. Res. 7:152–161. doi: 10.1080/00071669308417644 [DOI] [Google Scholar]
- Appleby M. C., Smith S. F., and Hughes B. O.. 1993. Nesting, dust bathing and perching by laying hens in cages: effects of design on behaviour and welfare. Br. Poult. Sci. 34:835–847. doi: 10.1080/00071669308417644 [DOI] [PubMed] [Google Scholar]
- Baker S. L., Robison C. I., Karcher D. M., Toscano M. J., and Makagon M.. 2020. Identification of keel impacts and associated behaviors in laying hens. Appl. Anim. Behav. Sci. 222:104886. doi: 10.1016/j.applanim.2019.104886 [DOI] [Google Scholar]
- Baur S., Rufener C., Toscano M. J., and Geissbühler U.. 2020. Radiographic evaluation of keel bone damage in laying hens – morphologic and temporal observations in a longitudinal study. Front. Vet. Sci.12:129. doi: 10.3389/fvets.2020.00129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman M., and Wagenaar J. P.. 2014. Health and welfare in dutch organic laying hens. Animals (Basel). 4:374–390. doi: 10.3390/ani4020374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatchford R. A., Fulton R. M., and Mench J. A.. 2016. The utilization of the Welfare Quality® assessment for determining laying hen condition across three housing systems. Poult. Sci. 95:154–163. doi: 10.3382/ps/pev227 [DOI] [PubMed] [Google Scholar]
- Budgell K. L., and Silversides F. G.. 2004. Bone breakage in three strains of end-of-lay hens. Can. J. Anim. Sci. 84:745–747. doi: 10.4141/A04-040 [DOI] [Google Scholar]
- Buijs S., Heerkens J. L. T., Ampe B., Delezie E., Rodenburg T. B., and Tuyttens F. A. M.. 2018. Assessing keel bone damage in laying hens by palpation: effects of assessor experience on accuracy, inter-rater agreement and intra-rater consistency. Poult. Sci. 98:1–8. doi: 10.3382/ps/pey326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushby E. V., Friel M., Goold C., Gray H., Smith L., and Collins L. M.. 2018. Factors influencing individual variation in farm animal cognition and how to account for these statistically. Front. Vet. Sci. 5:193. doi: 10.3389/fvets.2018.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell D. L. M., Hinch G. N., Downing J. A., and Lee C.. 2017. Outdoor stocking density in free-range laying hens: effects on behaviour and welfare. Animal 11:1036–1045. doi: 10.1017/S1751731116002342 [DOI] [PubMed] [Google Scholar]
- Candelotto L., Stratmann A., Gebhardt-Henrich S. G., Rufener C., van de Braak T., and Toscano M. J.. 2017. Susceptibility to keel bone fractures in laying hens and the role of genetic variation. Poult. Sci. 96:3517–3528. doi: 10.3382/ps/pex146 [DOI] [PubMed] [Google Scholar]
- Casey-Trott T. M., Guerin M. T., Sandilands V., Torrey S., and Widowski T. M.. 2017a. Rearing system affects prevalence of keel-bone damage in laying hens: a longitudinal study of four consecutive flocks. Poult. Sci. 96:2029–2039. doi: 10.3382/ps/pex026 [DOI] [PubMed] [Google Scholar]
- Casey-Trott T., Heerkens J. L., Petrik M., Regmi P., Schrader L., Toscano M. J., and Widowski T.. 2015. Methods for assessment of keel bone damage in poultry. Poult. Sci. 94:2339–2350. doi: 10.3382/ps/pev223 [DOI] [PubMed] [Google Scholar]
- Casey-Trott T. M., Korver D. R., Guerin M. T., Sandilands V., Torrey S., and Widowski T. M.. 2017b. Opportunities for exercise during pullet rearing, Part II: long-term effects on bone characteristics of adult laying hens at the end-of-lay. Poult. Sci. 96:2518–2527. doi: 10.3382/ps/pex060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey-Trott T. M., and Widowski T. M.. 2016. Behavioral differences of laying hens with fractured keel bones within furnished cages. Front. Vet. Sci. 3:42. doi: 10.3389/fvets.2016.00042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargo N. J., Robison C. I., Akaeze H. O., Baker S. L., Toscano M. J., Makagon M. M., and Karcher D. M.. 2019. Keel bone differences in laying hens housed in enriched colony cages. Poult. Sci. 98:1031–1036. doi: 10.3382/ps/pey421 [DOI] [PubMed] [Google Scholar]
- Chargo N. J., Robison C. I., Baker S. L., Toscano M. J., Makagon M. M., and Karcher D. M.. 2018. Keel bone damage assessment: consistency in enriched colony laying hens. Poult. Sci. 98:1–6. doi: 10.3382/ps/pey373 [DOI] [PubMed] [Google Scholar]
- Clark W. D., Cox W. R., and Silversides F. G.. 2008. Bone fracture incidence in end-of-lay high-producing, noncommercial laying hens identified using radiographs. Poult. Sci. 87:1964–1970. doi: 10.3382/ps.2008-00115 [DOI] [PubMed] [Google Scholar]
- Donaldson C. J., Ball M. E., and O’Connell N. E.. 2012. Aerial perches and free-range laying hens: the effect of access to aerial perches and of individual bird parameters on keel bone injuries in commercial free-range laying hens. Poult. Sci. 91:304–315. doi: 10.3382/ps.2011-01774 [DOI] [PubMed] [Google Scholar]
- Enneking S. A., Cheng H. W., Jefferson-Moore K. Y., Einstein M. E., Rubin D. A., and Hester P. Y.. 2012. Early access to perches in caged White Leghorn pullets. Poult. Sci. 91:2114–2120. doi: 10.3382/ps.2012-02328 [DOI] [PubMed] [Google Scholar]
- Eusemann B. K., Baulain U., Schrader L., Thöne-Reineke C., Patt A., and Petow S.. 2018a. Radiographic examination of keel bone damage in living laying hens of different strains kept in two housing systems. PLoS One. 13:e0194974. doi: 10.1371/journal.pone.0194974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusemann B. K., Sharifi A. R., Patt A., Reinhard A. K., Schrader L., Thöne-Reineke C., and Petow S.. 2018b. Influence of a sustained release deslorelin acetate implant on reproductive physiology and associated traits in laying hens. Front. Physiol. 9:1–11. doi: 10.3389/fphys.2018.01846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eusemann, B. K., A. Patt, L. Schrader, S. Weigend, C. Thöne-Reineke, and S. Petow. 2020. The role of egg production in the etiology of keel bone damage in laying hens. Front. Vet. Sci. 7:81. doi: 10.3389/fvets.2020.00081 [DOI] [PMC free article] [PubMed]
- FAWC 2010. FAWC opinion on osteoporosis and bone fractures in laying hens. London: Farm Animal Welfare Council; Available from https://www.gov.uk/government/publications/fawc-opinion-on-osteoporosis-and-bone-fractures-in-laying-hens [accessed September 22, 2019]. [Google Scholar]
- FAWC 2013. An open letter to Great Britain Governments: keel bone fracture in laying hens. London: Farm Animal Welfare Council; Available from https://www.gov.uk/government/publications/fawc-advice-on-keel-bone-fractures-in-laying-hens [accessed September 22, 2019]. [Google Scholar]
- Gebhardt-Henrich S. G., Pfulg A., Fröhlich E. K. F., Käppeli S., Guggisberg D., Liesegang A., and Stoffel M. H.. 2017. Limited associations between keel bone damage and bone properties measured with computer tomography, three-point bending test, and analysis of minerals in swiss laying hens. Front. Vet. Sci. 4:128. doi: 10.3389/fvets.2017.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhardt-Henrich S. G., Rufener C., and Stratmann A.. 2019. Improving intra- and inter-observer repeatability and accuracy of keel bone assessment by training with radiographs. Poult. Sci. 98:5234–5240. doi: 10.3382/ps/pez410 [DOI] [PubMed] [Google Scholar]
- Grafl B., Polster S., Sulejmanovic T., Pürrer B., Guggenberger B., and Hess M.. 2017. Assessment of health and welfare of Austrian laying hens at slaughter demonstrates influence of husbandry system and season. Br. Poult. Sci. 58:209–215. doi: 10.1080/00071668.2017.1280723 [DOI] [PubMed] [Google Scholar]
- Habig C., and Distl O.. 2013. Evaluation of bone strength, keel bone status, plumage condition and egg quality of two layer lines kept in small group housing systems. Br. Poult. Sci. 54:413–424. doi: 10.1080/00071668.2013.792405 [DOI] [PubMed] [Google Scholar]
- Hardin E., Castro F. L. S., and Kim W. K.. 2019. Keel bone injury in laying hens: the prevalence of injuries in relation to different housing systems, implications, and potential solutions. World Poult. Sci. J. 75:285–291. doi: 10.1017/S0043933919000011 [DOI] [Google Scholar]
- Harlander-Matauschek A., Rodenburg T. B., Sandilands V., Tobalske B. W., and Toscano M. J.. 2015. Causes of keel bone damage and their solutions in laying hens. Worlds. Poult. Sci. J. 71:461–472. doi: 10.1017/S0043933915002135 [DOI] [Google Scholar]
- Heerkens J. L., Delezie E., Ampe B., Rodenburg T. B., and Tuyttens F. A.. 2016a. Ramps and hybrid effects on keel bone and foot pad disorders in modified aviaries for laying hens. Poult. Sci. 95:2479–2488. doi: 10.3382/ps/pew157 [DOI] [PubMed] [Google Scholar]
- Heerkens J. L. T., Delezie E., Rodenburg T. B., Kempen I., Zoons J., Ampe B., and Tuyttens F. A. M.. 2016b. Risk factors associated with keel bone and foot pad disorders in laying hens housed in aviary systems: table 1. Poult. Sci. 95:482–488. doi: 10.3382/ps/pev339 [DOI] [PubMed] [Google Scholar]
- Hester P. Y., Enneking S. A., Haley B. K., Cheng H. W., Einstein M. E., and Rubin D. A.. 2013. The effect of perch availability during pullet rearing and egg laying on musculoskeletal health of caged White Leghorn hens. Poult. Sci. 92:1972–1980. doi: 10.3382/ps.2013-03008 [DOI] [PubMed] [Google Scholar]
- Hinrichsen L. K., Riber A. B., and Labouriau R.. 2016. Associations between and development of welfare indicators in organic layers. Animal 10:953–960. doi: 10.1017/S1751731115003018 [DOI] [PubMed] [Google Scholar]
- Janczak A. M., and Riber A. B.. 2015. Review of rearing-related factors affecting the welfare of laying hens. Poult. Sci. 94:1454–1469. doi: 10.3382/ps/pev123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. L. 2015. Reproduction in the Female. In: Scanes C. G., editor. Sturkie’s avian physiology. 6th ed. London: Elsevier Academic Press; p. 635–665. [Google Scholar]
- Kajlich A. S., Shivaprasad H. L., Trampel D. W., Hill A. E., Parsons R. L., Millman S. T., and Mench J. A.. 2016. Incidence, severity, and welfare implications of lesions observed postmortem in laying hens from commercial noncage farms in California and Iowa. Avian Dis. 60:8–15. doi: 10.1637/11247-080415-Reg.1 [DOI] [PubMed] [Google Scholar]
- Käppeli S., Gebhardt-Henrich S. G., Fröhlich E., Pfulg A., Schäublin H., and Stoffel M. H.. 2011a. Effects of housing, perches, genetics, and 25-hydroxycholecalciferol on keel bone deformities in laying hens. Poult. Sci. 90:1637–1644. doi: 10.3382/ps.2011-01379 [DOI] [PubMed] [Google Scholar]
- Käppeli S., Gebhardt-Henrich S. G., Fröhlich E., Pfulg A., and Stoffel M. H.. 2011b. Prevalence of keel bone deformities in Swiss laying hens. Br. Poult. Sci. 52:531–536. doi: 10.1080/00071668.2011.615059 [DOI] [PubMed] [Google Scholar]
- Larsen H., Hemsworth P. H., Cronin G. M., Gebhardt-Henrich S. G., Smith C. L., and Rault J. L.. 2018. Relationship between welfare and individual ranging behaviour in commercial free-range laying hens. Animal 12:2356–2364. doi: 10.1017/S1751731118000022 [DOI] [PubMed] [Google Scholar]
- Lay D. C. Jr, Fulton R. M., Hester P. Y., Karcher D. M., Kjaer J. B., Mench J. A., Mullens B. A., Newberry R. C., Nicol C. J., O’Sullivan N. P., et al. 2011. Hen welfare in different housing systems. Poult. Sci. 90:278–294. doi: 10.3382/ps.2010-00962 [DOI] [PubMed] [Google Scholar]
- Lazic S. E. 2016. Experimental design for laboratory biologists: maximising information and improving reproducibility. Cambridge: Cambridge University Press. [Google Scholar]
- LeBlanc S., Tobalske B., Quinton M., Springthorpe D., Szkotnicki B., Wuerbel H., and Harlander-Matauschek A.. 2016. Physical health problems and environmental challenges influence balancing behaviour in laying hens. PLoS One. 11:e0153477. doi: 10.1371/journal.pone.0153477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann Tierzucht GmbH Management guide alternative systems. Available from https://www.ltz.de/de/layers/alternative-housing/lohmann-lsl-classic.php [accessed September 22, 2019]. [Google Scholar]
- Loughran C. F. 1994. Reporting of fracture radiographs by radiographers: the impact of a training programme. Br. J. Radiol. 67:945–950. doi: 10.1259/0007-1285-67-802-945 [DOI] [PubMed] [Google Scholar]
- Makagon M. M., Baker S. L., Robison C. I., Karcher D. M., and Toscano M. J.. 2017. Keel bone damage: the role of behavior and impacts experienced at the keel. Poult. Sci. 96(e-supplement 1):101 [Google Scholar]
- Martin P., and Bateson P.. 1993. Measuring behaviour. An introductory guide. 2nd ed. Cambridge (UK): Cambridge University Press [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., and Altman D. G.; PRISMA Group 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasr M. A. F., Murrell J., Wilkins L. J., and Nicol C.. 2012. The effect of keel fractures on egg-production parameters, mobility and behaviour in individual laying hens. Anim. Welf. 21:127–135. doi: 10.7120/096272812799129376 [DOI] [Google Scholar]
- Nasr M. A., Nicol C. J., Wilkins L., and Murrell J. C.. 2015. The effects of two non-steroidal anti-inflammatory drugs on the mobility of laying hens with keel bone fractures. Vet. Anaesth. Analg. 42:197–204. doi: 10.1111/vaa.12175 [DOI] [PubMed] [Google Scholar]
- Nicol C. J., Brown S. N., Glen E., Pope S. J., Short F. J., Warriss P. D., Zimmerman P. H., and Wilkins L. J.. 2006. Effects of stocking density, flock size and management on the welfare of laying hens in single-tier aviaries. Br. Poult. Sci. 47:135–146. doi: 10.1080/00071660600610609 [DOI] [PubMed] [Google Scholar]
- Petrik M. T., Guerin M. T., and Widowski T. M.. 2013. Keel fracture assessment of laying hens by palpation: inter-observer reliability and accuracy. Vet. Rec. 173:500. doi: 10.1136/vr.101934 [DOI] [PubMed] [Google Scholar]
- Petrik M. T., Guerin M. T., and Widowski T. M.. 2015. On-farm comparison of keel fracture prevalence and other welfare indicators in conventional cage and floor-housed laying hens in Ontario, Canada. Poult. Sci. 94:579–585. doi: 10.3382/ps/pev039 [DOI] [PubMed] [Google Scholar]
- Pickel T., Schrader L., and Scholz B.. 2011. Pressure load on keel bone and foot pads in perching laying hens in relation to perch design. Poult. Sci. 90:715–724. doi: 10.3382/ps.2010-01025 [DOI] [PubMed] [Google Scholar]
- Regmi P., Deland T. S., Steibel J. P., Robison C. I., Haut R. C., Orth M. W., and Karcher D. M.. 2015. Effect of rearing environment on bone growth of pullets. Poult. Sci. 94:502–511. doi: 10.3382/ps/peu041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regmi P., Nelson N., Steibel J. P., Anderson K. E., and Karcher D. M.. 2016. Comparisons of bone properties and keel deformities between strains and housing systems in end-of-lay hens. Poult. Sci. 95:2225–2234. doi: 10.3382/ps/pew199 [DOI] [PubMed] [Google Scholar]
- Regmi P., Robison C. I., Jones D. R., Gast R. K., Tempelman R. J., and Karcher D. M.. 2018. Effects of different litter substrates and induced molt on production performance and welfare quality parameters of white Leghorn hens housed in multi-tiered aviary system. Poult. Sci. 97:3397–3404. doi: 10.3382/ps/pey211 [DOI] [PubMed] [Google Scholar]
- Riber A. B., Casey-Trott T. M., and Herskin M. S.. 2018. The influence of keel bone damage on welfare of laying hens. Front. Vet. Sci. 5:6. doi: 10.3389/fvets.2018.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber A. B., and Hinrichsen L. K.. 2016. Keel-bone damage and foot injuries in commercial laying hens in Denmark. Anim. Welf. 25:179–184. doi: 10.7120/09627286.25.2.179 [DOI] [Google Scholar]
- Riber A. B., and Hinrichsen L. K.. 2017. Welfare consequences of omitting beak trimming in barn layers. Front. Vet. Sci. 4:222. doi: 10.3389/fvets.2017.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards G. J., Brown S. N., Booth F., Toscano M. J., and Wilkins L. J.. 2012. Panic in free-range laying hens. Vet. Rec. 170. doi: 10.1136/vr.100685 [DOI] [PubMed] [Google Scholar]
- Richards G. J., Nasr M. A., Brown S. N., Szamocki E. M. G., Murrell J., Barr F., and Wilkins L. J.. 2011. Use of radiography to identify keel bone fractures in laying hens and assess healing in live birds. Vet. Rec. 169:279. doi: 10.1136/vr.d4404 [DOI] [PubMed] [Google Scholar]
- Riddle E. R., Ali A. B. A., Campbell D. L. M., and Siegford J. M.. 2018. Space use by 4 strains of laying hens to perch, wing flap, dust bathe, stand and lie down. PLoS One. 13:1–16. doi: 10.1371/journal.pone.0190532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenburg T. B., Tuyttens F. A. M., de Reu K., Herman L., Zoons J., Sonck B., de Reu K., Herman L., Zoons J., and Sonck B.. 2008. Welfare assessment of laying hens in furnished cages and non-cage systems: an on-farm comparison. Anim. Welf. 17:355–361. [Google Scholar]
- Rørvang M. V., Hinrichsen L. K., and Riber A. B.. 2019. Welfare of layers housed in small furnished cages on Danish commercial farms: the condition of keel bone, feet, plumage and skin. Br. Poult. Sci. 60:1–7. doi: 10.1080/00071668.2018.1533632 [DOI] [PubMed] [Google Scholar]
- Rufener, C., S. Baur, A. Stratmann, and M. J. Toscano. 2018. A reliable method to assess keel bone fractures in laying hens from radiographs using a tagged visual analogue scale. Front. Vet. Sci. 5:124. doi: 10.3389/fvets.2018.00124 [DOI] [PMC free article] [PubMed]
- Rufener C., Abreu Y., Asher L., Berezowski J. A., Maximiano F., Stratmann A., and Toscano M. J.. 2019a. Keel bone fractures are associated with individual mobility of laying hens in an aviary system. Appl. Anim. Behav. Sci. 217: 48–56. doi: 10.1016/j.applanim.2019.05.007 [DOI] [Google Scholar]
- Rufener C., Baur S., Stratmann A., and Toscano M. J.. 2019b. Keel bone fractures affect egg laying performance but not egg quality in laying hens housed in a commercial aviary system. Poult. Sci. 98:1589–1600. doi: 10.3382/ps/pey544 [DOI] [PubMed] [Google Scholar]
- Rufener C., and Toscano M. J.. Poultry health monitoring and management: bone health in layers. In: Nicol C. J., editor. Understanding the behaviour and improving the welfare of chickens. Cambridge: Burleigh Dodds Science Publisher. (in press). [Google Scholar]
- Sandilands V., Moinard C., and Sparks N. H.. 2009. Providing laying hens with perches: fulfilling behavioural needs but causing injury? Br. Poult. Sci. 50:395–406. doi: 10.1080/00071660903110844 [DOI] [PubMed] [Google Scholar]
- Scholz B., Kjaer J. B., and Schrader L.. 2014. Analysis of landing behaviour of three layer lines on different perch designs. Br. Poult. Sci. 1668:37–41. doi: 10.1080/00071668.2014.933175 [DOI] [PubMed] [Google Scholar]
- Scholz B., Rönchen S., Hamann H., Hewicker-Trautwein M., and Distl O.. 2008. Keel bone condition in laying hens: a histological evaluation of macroscopically assessed keel bones. Berl. Munch. Tierarztl. Wochenschr. 121:89–94. [PubMed] [Google Scholar]
- Sherwin C. M., Richards G. J., and Nicol C. J.. 2010. Comparison of the welfare of layer hens in 4 housing systems in the UK. Br. Poult. Sci. 51:488–499. doi: 10.1080/00071668.2010.502518 [DOI] [PubMed] [Google Scholar]
- Siegford J. M., Berezowski J., Biswas S. K., Daigle C. L., Gebhardt-Henrich S. G., Hernandez C. E., Thurner S., and Toscano M. J.. 2016. Assessing activity and location of individual laying hens in large groups using modern technology. Animals 6. doi: 10.3390/ani6020010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirovnik J., Stratmann A., Gebhardt-Henrich S. G., Würbel H., and Toscano M. J.. 2018. Feeding from perches in an aviary system reduces aggression and mortality in laying hens. Appl. Anim. Behav. Sci. 202:53–62. doi: 10.1016/j.applanim.2018.01.005 [DOI] [Google Scholar]
- Stojcic M., Peric L., Relic R., Bozickovic I., Rodic V., and Rezar V.. 2017. Keel bone damage in laying hens reared in different production systems in Serbia. Biotechnol. Anim. Husb. 33:487–492. doi: 10.2298/BAH1704487D [DOI] [Google Scholar]
- Stratmann A., Fröhlich E. K. F., Gebhardt-Henrich S. G., Harlander-Matauschek A., Würbel H., and Toscano M. J.. 2015a. Modification of aviary design reduces incidence of falls, collisions and keel bone damage in laying hens. Appl. Anim. Behav. Sci. 165:112–123. doi: 10.1016/j.applanim.2015.01.012 [DOI] [Google Scholar]
- Stratmann A., Fröhlich E. K., Gebhardt-Henrich S. G., Harlander-Matauschek A., Würbel H., and Toscano M. J.. 2016. Genetic selection to increase bone strength affects prevalence of keel bone damage and egg parameters in commercially housed laying hens. Poult. Sci. 95:975–984. doi: 10.3382/ps/pew026 [DOI] [PubMed] [Google Scholar]
- Stratmann A., Fröhlich E. K., Harlander-Matauschek A., Schrader L., Toscano M. J., Würbel H., and Gebhardt-Henrich S. G.. 2015b. Soft perches in an aviary system reduce incidence of keel bone damage in laying hens. PLoS One. 10:e0122568. doi: 10.1371/journal.pone.0122568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlton J. F., Wilkins L. J., Toscano M. J., Avery N. C., and Knott L.. 2013. Reduced bone breakage and increased bone strength in free range laying hens fed omega-3 polyunsaturated fatty acid supplemented diets. Bone 52:578–586. doi: 10.1016/j.bone.2012.11.003 [DOI] [PubMed] [Google Scholar]
- Toscano M., Booth F., Richards G., Brown S., Karcher D., and Tarlton J.. 2018. Modeling collisions in laying hens as a tool to identify causative factors for keel bone fractures and means to reduce their occurrence and severity. PLoS One. 13:e0200025. doi: 10.1371/journal.pone.0200025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy L. M., Temple S. M., Bennett D. C., Sprayberry K. A., Makagon M. M., and Blatchford R. A.. 2019. The reliability and accuracy of palpation, radiography, and sonography for the detection of keel bone damage. Animals 9. doi: 10.3390/ani9110894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead C. C. 2004. Overview of bone biology in the egg-laying hen. Poult. Sci. 83:193–199. doi: 10.1093/ps/83.2.193 [DOI] [PubMed] [Google Scholar]
- Widowski T. M., Caston L. J., Casey-Trott T. M., and Hunniford M. E.. 2017. The effect of space allowance and cage size on laying hens housed in furnished cages, Part II: behavior at the feeder. Poult. Sci. 96:3805–3815. doi: 10.3382/ps/pex198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins L. J., Brown S. N., Zimmerman P. H., Leeb C., and Nicol C. J.. 2004. Investigation of palpation as a method for determining the prevalence of keel and furculum damage in laying hens. Vet. Rec. 155:547–549. doi: 10.1136/vr.155.18.547 [DOI] [PubMed] [Google Scholar]
- Wilkins L. J., McKinstry J. L., Avery N. C., Knowles T. G., Brown S. N., Tarlton J., and Nicol C. J.. 2011. Influence of housing system and design on bone strength and keel bone fractures in laying hens. Vet. Rec. 169:414. doi: 10.1136/vr.d4831 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.