Abstract
Human lung transplant recipients undergoing rejection induce circulatory exosomes with lung self-antigens (SAgs), K-alpha 1 Tubulin and Collagen V, and immunization of C57BL/6 mice with exosomes induced obliterative airway disease (HEI-OAD). We analyzed whether exosomes with SAgs induced immunity in microRNA-155 knockout mice (miR-155KO), as microRNA-155 is an immune regulator. C57BL/6 and miR-155KO were immunized with exosomes from stable or chronic rejection (bronchiolitis obliterans syndrome (BOS) and on day 30, induction of exosomes, antibodies (Abs) to SAgs and cellular immunity were determined. C57BL/6 immunized with exosomes from BOS developed OAD. These immunized animals also developed Abs to SAgs and increased frequency of SAg-specific IFNγ and IL17- producing cells. In contrast, Abs to SAgs did not develop in miR-155KO and there was reduction in frequency of cells producing IL10. Upregulation of suppressor of cytokine signaling for lung inflammation was also noted resulting in abrogation of induction of exosomes with SAgs OAD.
Keywords: miRNA 155, Bronchiolitis Obliterans Syndrome, Obliterans Airway disease, Immunization, Exosomes
1. Introduction
MicroRNAs (miRNAs) are single-strand RNAs that are 18 to 25 nucleotides in length. In the genome, miRNAs suppress newly synthesized proteins either by causing mRNA degradation or by inhibiting the translation of mRNA into proteins [1]. More than 500 miRNAs have been identified in humans, but their exact functions have yet to be fully elucidated [2, 3]. MicroRNA-155 (miR-155) was originally identified as a gene in B-cell integration cluster in chickens; however, its homolog was subsequently found in mice and in humans [4–6]. miR-155 is abundantly expressed in many types of cells, including macrophages, B cells, T cells, and dendritic cells. It appears to be essential for cell-mediated immune responses [6] and it is involved in T cell differentiation and activation. miR-155 also plays an important role in regulatory CD4 T cell generation and function, which includes maintaining suppression of respiratory diseases such as asthma and allergies [4, 6, 7] In dendritic cells, miR-155 has also been implicated as a positive regulator of inflammatory cytokine production, similar to macrophages.
Exosomes are vesicles smaller than 200 nm in diameter, and are released by most cells; however, the exosome composition differs based on the cell origin [8]. The state of the cells (eg, cancer, infection, transplantation) also affects exosomal composition [9]. Exosomes also contain biomolecules, including carbohydrates, proteins, lipids, nucleic acids (ie, DNA and different types of RNAs, miRNAs), and metabolites [10].
In a previous report from our laboratory, we demonstrated that miR-155 is present in higher amounts in exosomes isolated from serum samples and bronchoalveolar lavage (BAL) fluid from patients experiencing chronic rejection after lung transplant (LTx) [11]. Furthermore, exosomes from LTx recipients (LTxRs) undergoing rejection induced both cellular and humoral immune responses in C57BL/6 animals (HEI-OAD), whereas stable LTxRs did not. In the current study, we demonstrate an important role for miR-155 in regulating immune responses induced by circulating exosomes isolated from human LTxRs with chronic lung allograft dysfunction (ie, bronchiolitis obliterans syndrome [BOS]). Furthermore, the suppressor of cytokine signaling (SOCS) was identified as a direct target of miR-155.
2. Materials and Methods
2.1. Mice
Age-matched 8–12 week-old male or female C57BL/6 and miR-155 knockout (KO) mice were obtained from a commercial laboratory (Jackson Laboratories). All animal studies were performed with sterile precautions and approved by the Animal Studies Committee at St. Joseph’s Hospital and Medical Center, Phoenix, Arizona.
2.2. Exosome isolation from human lung transplant recipients
Plasma was collected from blood samples from human LTxRs at St. Joseph’s Hospital and Medical Center and stored at −20°C. Ten samples from stable LTxRs and 10 from LTxRs with chronic lung allograft dysfunction, i.e: BOS were pooled separately and used for exosome isolation. 3.0 mL of each plasma sample was used to isolate exosomes using the ultracentrifugation method [11]. All samples were centrifuged at 3000 rpm for 30 minutes at 4°C, followed by 2 cycles of centrifugation at 10,000 rpm at 4°C for 30 minutes. Supernatants from all tubes were collected and small samples were left at the bottom to avoid inadvertent inclusion of precipitates in the samples. The supernatant was diluted with phosphate-buffered saline (PBS) and ultracentrifuged at 42,000 rpm at 4°C for 120 minutes. The supernatant was removed and vortexed with fresh PBS to suspend exosomes. The ultracentrifugation step was repeated, the supernatant was discarded, and the exosomes were recovered for further experimentation. Exosome purity was monitored by NanoSight (<200 nm particle size).
2.3. Exosome quantitation
Isolated exosomes were subjected to protein estimation using a BCA protein estimation kit (Thermo Fisher) prior to injection in mice.
2.4. Immunization of mice with exosomes
Mice were divided into 3 groups: (1) A control group with no exosome immunization (C57BL/6 and miR-155KO), (2) a group immunized with exosomes isolated from stable LTxRs (C57BL/6 and miR-155KO), and (3) a group immunized with exosomes isolated from LTxRs diagnosed with BOS (C57BL/6 and miR-155KO). Each group consisted of 5 mice (weighing 20–25 g and 8 weeks old). The mice that received exosome immunization did not require any adjuvants, and immunizations were carried out subcutaneously, with 20 ug per injection on days 1, 3, 7, 14, and 21. Nine days after final injection, mice were euthanized. Blood samples were collected at alternate time points to facilitate the study of kinetics and development of immune response in mice. Age-matched mice without exosome immunization were used as controls.
2.5. Antibodies (Abs) to K-alpha 1 Tubulin (Kα1T) and Collagen V (Col-V)
Rabbit polyclonal immunoglobulin G Abs to Kα1T and Col-V were produced using purified Kα1T and Col-V proteins, as previously described [12]. Specificity of the Abs was studied using enzyme-linked immunosorbent assay (ELISA). Plates were coated with purified proteins Col-V, Col-I, and Col-II (optical densities for Col-V, Col-I, and Col-II are 0.863, 0.124, and 0.109, respectively).
2.6. ELISA
Development of Abs to Kα1T, Col-V, and Col-II (control) was determined by ELISA, as previously described [13, 14]. In brief, 1μg/mL of Col-V and Col-II suspended in PBS were coated onto an ELISA plate (Col-II and Col-V were obtained from Chemicon/Millipore; recombinant Kα1T was produced in our laboratory) and incubated overnight at 4°C. Diluted patient and normal sera samples were then added to these plates.
Detection was done using an anti-mouse immunoglobulin G-horseradish peroxidase (1:10,000) and developed using tetramethylbenzidine substrate and read at 450 nm. Antibody concentration was calculated using a standard curve from known concentrations of respective Abs (Santa Cruz Biotechnology).
2.7. Enzyme-linked immunospot
Cellular immune responses to Kα1T, Col-V, and Col-II were enumerated using enzyme-linked immunospot (ELISPOT). Splenocytes were isolated by Ficoll-Hypaque gradient centrifugation, following sacrifice of exosome-immunized animals, and used in ELISPOT analyses, as described earlier [15]. All antigens used in this study were endotoxin-free. Briefly, multiscreen 96-well filtration plates were coated overnight at 4°C with 5.0μg/ml of human cytokine-specific monoclonal Abs: IFN-g, IL-10, IL-2, IL-4 and IL-17 (BD Biosciences). The plates were then blocked with 1% BSA for 2 hours. Subsequently, 3×105 C57BL/6 and miR-155 KO splenocytes were seeded into the wells in triplicate as responders, and Col-V (5 mg/mL) or Kα1T (5 mg/mL) were subsequently added as a stimulator. The plates were then incubated in a humidified CO2 incubator for 48 hours. The plates were washed with PBS three times and PBST three times. The splenocytes were pulsed with the respective antigen overnight. After 48 hours, the plates were washed and 2.0μg/mL of biotinylated cytokine-specific monoclonal antibody (BD Biosciences) was added to the wells. After an overnight incubation at 4°C, the plates were developed using horseradish peroxidase-labeled streptavidin (BD Biosciences), and 3-amino-9-ethylcarbazole substrate reagent (BD Biosciences). The spots were analyzed in an ImmunoSpot Series I analyzer (Cellular Technology).
2.8. Exosome isolation from mice immunized with exosomes isolated from lung transplant recipients
At day 30, all mice in each group were euthanized. Blood and serum samples were collected from these mice. Exosomes were isolated from mice serum samples using an Invitrogen exosome isolation kit. The purity of exosomes was determined by Nanosight (<200 nm particle size). Protein estimation was performed following lysis of exosomes with RIPA buffer with protease inhibitor cocktail (Sigma). Estimation of protein concentration was done using BCA protein estimation kit (Thermo Fisher).
2.9. Western blot
Fifteen ugs of protein was loaded onto SDS-PAGE and transferred to a polyvinylidene fluoride membrane. Membranes were probed for Abs to lung self-antigens (SAgs) Col-V (anti-rabbit antibody, Abcam) and Kα1T (anti-mouse antibody, Santa Cruz), SOCS (anti-rabbit, cell signaling) and CD9 antibody (anti-mouse antibody, BioLegend) and H2Kb (Bioexcel) as exosome markers.
2.10. Histology and immunohistochemistry
Mice were euthanized and lungs were collected for histochemistry. Lung specimens were fixed in neutralized 10% formalin and embedded in paraffin blocks. 4– 5mm-thick sections were cut from each paraffin block and mounted on slides (Leica) for hematoxylin and eosin staining and trichrome staining. Sections were stained with anti-CD3, anti-CD4, anti-CD8, anti-C4D (Cell Signaling Technology, Abcam) Abs to identify cellular filtration. Images were obtained on a Leica microscope at 40X and morphometric analysis was performed using Aperio ImageScope software (Leica). Analysis was performed by looking at 5 different areas on the same slide and identifying alveolar lesions under different treatment conditions (ie, exosome administration from stable LTxRs and from LTxRs with BOS).
2.11. Statistical analysis
Student’s t-test was used to compare antibody development and antibody levels between miR-155KO and C57BL/6 mice that received BOS-LTxRs immunization. Statistical analysis for antibody development, SAg-specific cytokines, and serum cytokines was performed using GraphPad Prism (GraphPad Software). Data were analyzed in SPSS v.19 (IBM Inc.) and GraphPad Prism 5.0 and represented as mean (SEM), with at least 5 mice in each cohort. ANOVA and Student’s t-tests were used as appropriate. Results were considered significant if the p-value was <0.05. Optical density of the immunoblot was quantified using ImageJ software.
3. Results
3.1. Exosome isolation and characterization
We isolated exosomes from plasma samples from human LTxRs and the sizes of these exosomes were measured using NanoSight. Representative figures of NanoSight analysis for exosomes isolated from miR-155 KO and C57BL/6 mice are shown in Figure 1A. The average exosome size ranged from 100 to 200 nm.
Figure 1:
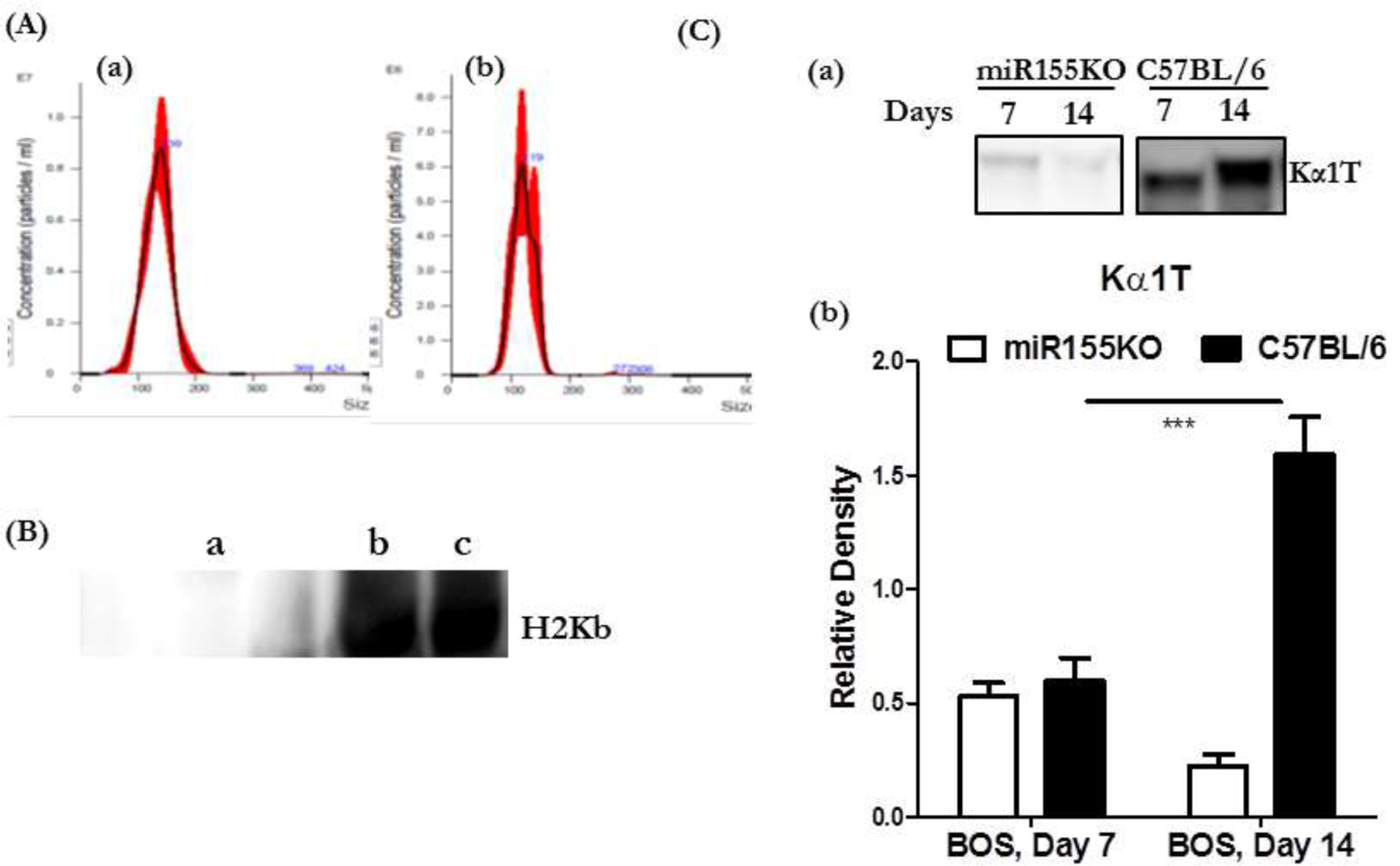
(A) Analysis of circulating exosomes using Nanosight (A) <200nm size of exosomes isolated from miR155KO mice and (B) <200 nm size of exosomes isolated from C57BL/6. (B) Western blot analysis using Abs specific to H2Kb of circulating exosomes isolated from LTxRs diagnosed with BOS used for immunization of mice and exosomes isolated from C57B/6 mice serum following immunization. (a): Exosomes from human LTxRs did not react to anti-H2Kb. Exosomes from C57B/6 mice (b) and miR155KO mice (c) after immunization with exosomes from LTxRs with BOS induced murine exosomes reactive to anti-H2Kb. (C) Kinetics of exosome induction on days 7 and 14 (a) Western blots for lung SAg, Kα1T, demonstrate that exosomes isolated from C57BL/6 following immunization induced exosomes with lung SAg, Kα1T, whereas miRNA155KO mice failed to induce exosomes with lung SAgs. (b) Densitometry of Western blots showing increased levels of exosomes with lung SAgs on day 14 in C57BL/6 but not in miRNA155KO animals following immunization with exosomes from LTxRs diagnosed with BOS (***p< 0.001).
The mean size of exosomes isolated from serum of miR-155KO mice was 134.0 ± 1.7 nm, and the concentration was 4.93×108 particles per mL. The mean size of exosomes isolated from serum of C57BL/6 mice was 120.9 ± 1.9nm, and the concentration was 2.9×108 particles per mL.
To determine whether exosomes used for immunization resulted in persistence of the human exosomes or induced murine exosomes from the native lungs, we analyzed the exosomes isolated from both miR-155KO and C57BL/6 mice immunized with exosomes isolated from LTxRs with BOS, using specific Abs to murine MHC (anti-H2Kb) as well as Abs to framework determinants of HLA class I (W632) and class II (L243). As shown in Figure 1B, exosomes isolated from both miR-155KO and C57BL/6 animals after immunization with BOS exosomes were of murine origin. BOS exosomes did not react with anti-mouse H2Kb, whereas they did react with both Abs to HLA class I and II (data not presented).
3.2. Kinetics of exosome induction
We isolated exosomes from mouse serum (both miR-155KO and C57BL/6) following immunization with BOS exosomes on days 7 and 14. Exosomes were analyzed by Western blot, using Abs to lung SAgs to determine the kinetics of induction of exosomes with lung SAgs, Kα1T and Col-V. The results demonstrated that in miR-155KO mice, on days 7 and 14, there were no detectable levels of exosomes with lung SAg, Kα1T; however, in C57BL/6 mice, there was induction of circulating exosomes with lung SAg, Kα1T, which increased in quantity on days 7 and 14 (Figure 1C). A similar trend was also noted for exosomes with the lung SAg, Col-V. This demonstrates that immunization with BOS exosomes in C57BL/6 mice injures the lungs and induces murine exosomes with lung SAgs that persist until day 21, damaging the native lungs. In contrast, miR-155KO mice did not develop any circulating exosomes with lung SAgs at any of the time points, and experienced no lung injury.
3.3. miR-155KO mice immunized with BOS exosomes developed no Abs to lung SAgs, Kα1T and Col-V
In a previous report, we demonstrated that C57BL/6 mice that were immunized with circulating exosomes isolated from LTxRs diagnosed with BOS developed immune responses to the lung SAgs, Kα1T and Col-V [11]. To determine the role of miR-155 in inducing immune responses to lung SAgs, we immunized miR-155KO mice with circulating exosomes isolated from stable LTxRs and from LTxRs diagnosed with BOS. As shown in Figures 2A,B, C57BL/6 animals developed significant levels of Abs to lung SAgs, Kα1T and Col-V, as reported earlier [15] following immunization with exosomes isolated from LTxRs with BOS. In contrast, miR-155KO mice failed to develop significant levels of Abs to either Kα1T (p<o.oo1) or Col-V (p<0.05) (Figures 2A,B) after immunization with exosomes isolated from LTxRs with BOS. These results demonstrate that miR-155KO mice cannot mount humoral immune responses to lung SAgs; therefore, miR-155 is necessary to mount an immune response to lung SAgs.
Figure 2:
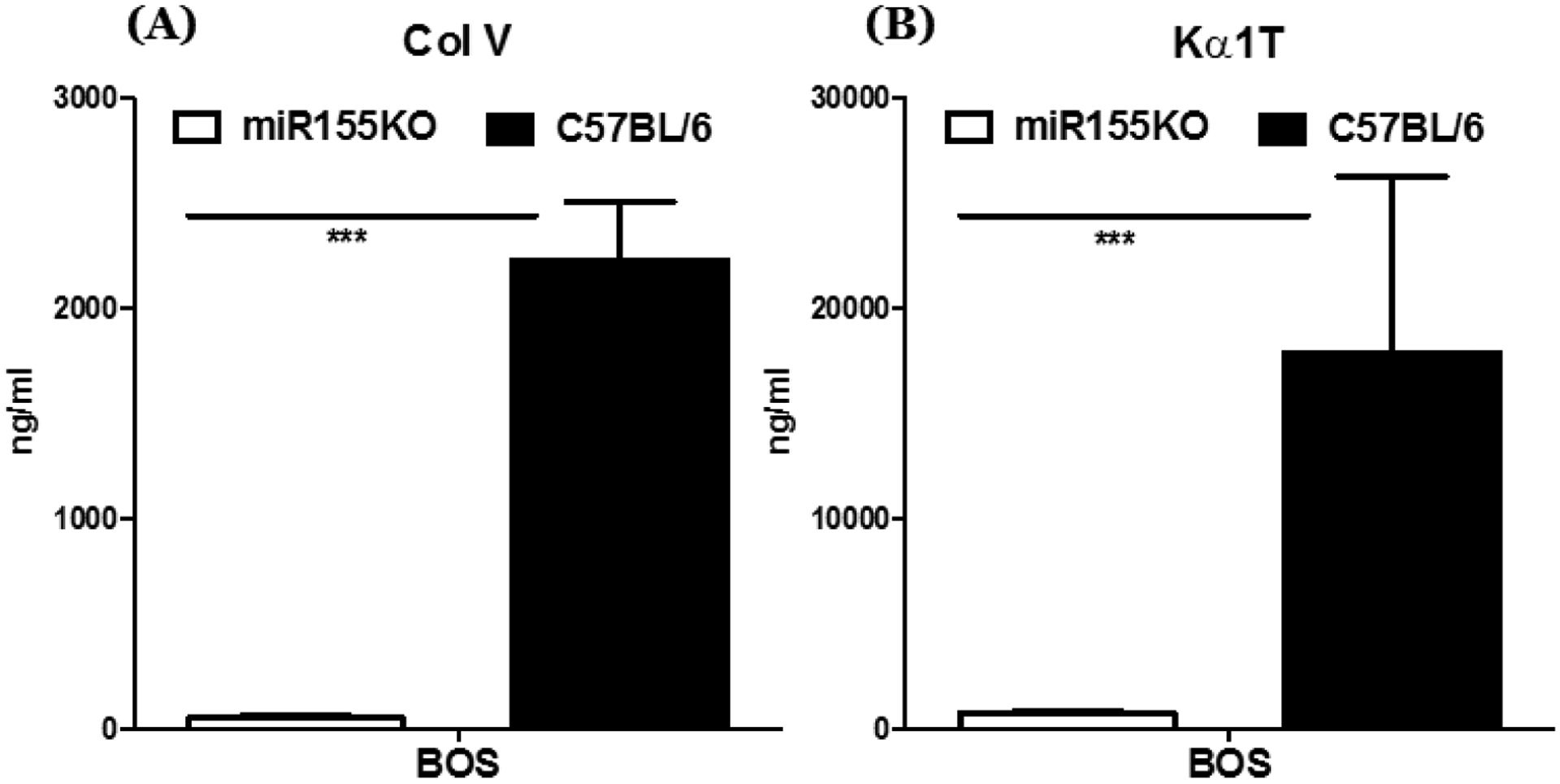
Development of Abs to lung SAgs in C57BL/6 but not in miRNA155KO animals immunized with exosomes from LTxRs diagnosed with BOS. (A) Abs to lung SAg, Col-V. (B) Abs to lung SAg, Kα1T (***p< 0.001).
3.4. Spleen lymphocytes from miR-155KO mice immunized with exosomes isolated from LTxRs with BOS failed to induce pro-inflammatory cytokines (IFN-γ, IL2, IL4, and IL17) or reduce anti-inflammatory cytokine (IL10)
As reported earlier, C57BL/6 mice immunized with exosomes isolated from LTxRs with BOS (HEI-OAD) demonstrated increased frequency of lung-SAg-specific cytokines IFN-ƴ, IL2, IL4, and IL17 (p ≤ 0.05), with significantly reduced frequency of cytokine IL-10 compared with mice immunized with exosomes from stable LTxRs (p<.05, Figure 3A,B,C,D). In contrast, splenocytes isolated on day 30 from miR-155KO mice immunized with exosomes from LTxRs with BOS failed to induce significant levels of SAg-specific cytokines IFNγ, IL2, IL4, and IL17. In addition the decrease in anti-inflammatory cytokine IL10 was insignificant for Col-V and Kα1T (Figure 3A,B,C,D). The difference in the frequency of cells responding to lung SAgs and producing cytokines were statistically significant after immunization of C57BL/6 vs miR-155KO mice with BOS exosomes: IL2 (p=0.027), IFN γ (p=0.05), IL17 (p=0.019), and IL4 (p=0.05) and no significant changes in IL10 for Col-V, and IL2 (p=0.013), IFN γ (p=0.001), IL4 (p=0.007), and IL17, and decreased IL10 (p=0.045) for Kα1T.
Figure 3:
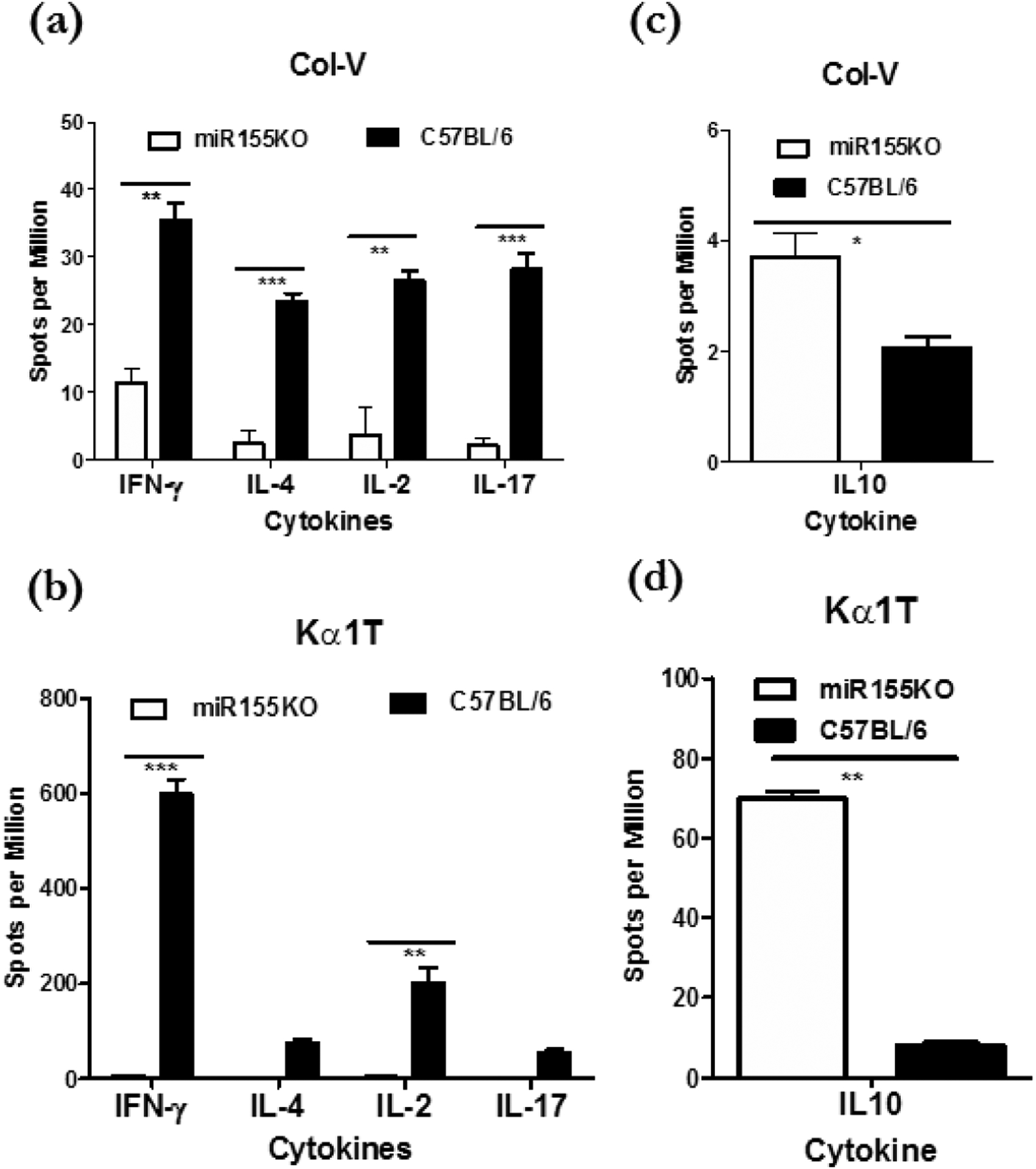
Enumeration of lung SAg specific cytokine producing cells in cells isolated from the spleen following immunization of miRNA155KO and C57BL/6 with exosomes from LTxRs diagnosed with BOS. (a) C57BL/6 but not miRNA155KO following immunization induced high frequency of IFNγ, IL-4, IL-2 and IL17 producing cells specific to lung SAg, Col-V. (b) C57BL/6 but not miRNA155KO following immunization induced high frequency of IFNγ, IL-4, IL-2 and IL17 producing cells specific to lung SAg, Kα1T. In contrast, miRNA155KO but not C57BL/6 induced high frequency of lung SAg, Col-V, specific IL10 producing cells. (c) Lung SAg, Kα1T, specific IL10 producing cells. (d) ***p< 0.001, **p< 0.01 and *p< 0.05.
3.5. miR-155KO mice failed to induce circulating exosomes with lung SAgs after immunization with BOS exosomes
To determine whether induction of murine exosomes with lung SAgs is needed to prompt immune responses to lung SAgs, we analyzed circulating exosomes isolated from miR-155KO and C57BL/6 mice after immunization with exosomes isolated from stable LTxRs and those with BOS. Circulating exosomes were isolated on day 30 after immunization and were analyzed for the presence of lung SAgs Kα1T and Col-V. Exosomes isolated from miR-155KO animals did not have significant levels of either lung SAg in comparison to C57BL/6 animals (Figure 4A,B), suggesting that persistence of lung-SAg-containing exosomes may be necessary for immune activation.
Figure 4:
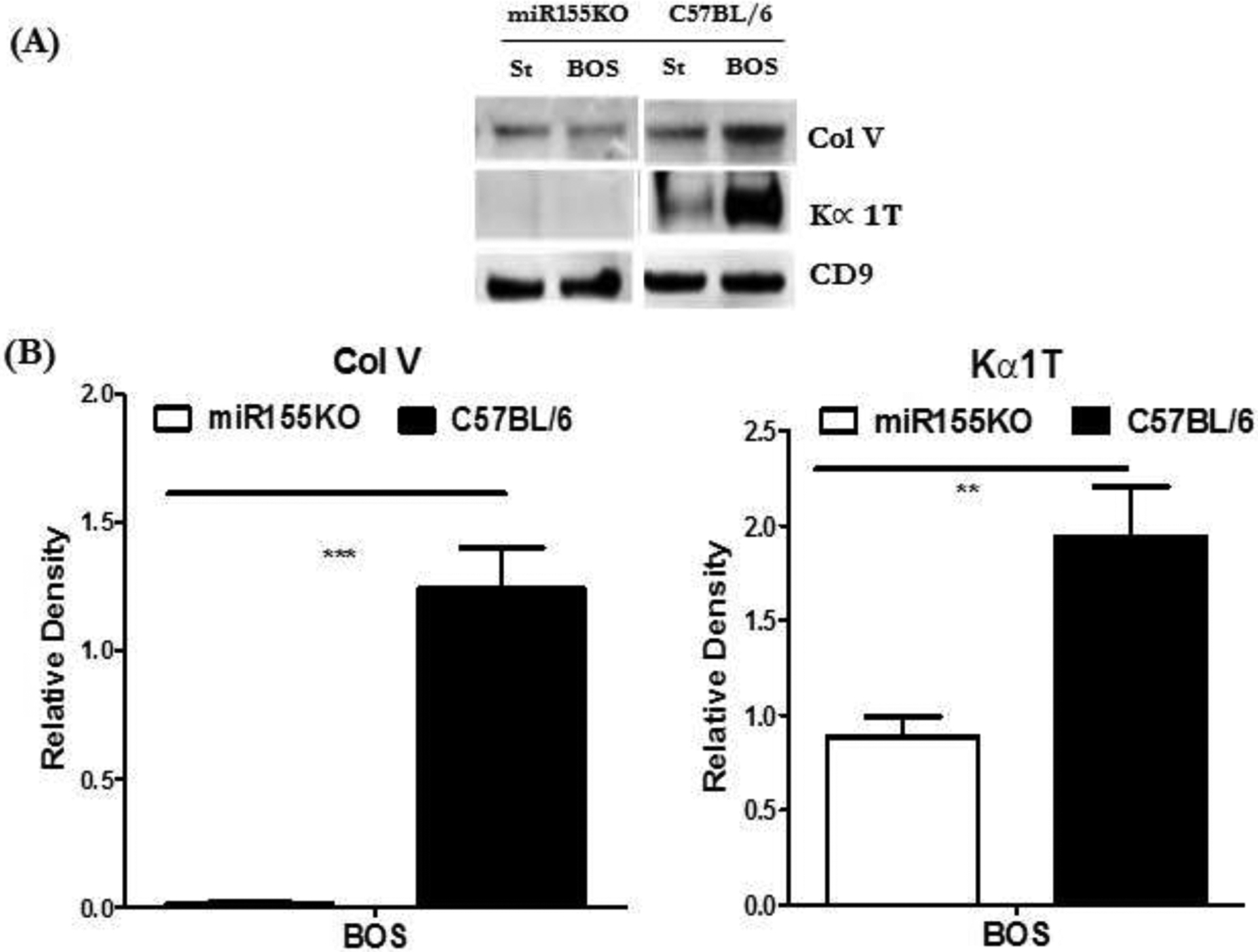
Western blot of proteins isolated from exosomes following immunization with exosomes from LTxRs diagnosed with BOS: St: Mice injected with stable exosomes; BOS: mice injected with BOS exosomes. (A) Exosomes isolated from C57BL/6 but not miRNA155KO contained lung SAgs, Col-V and Kα1T. (B) Densitometry analysis of western blot showing increased levels of exosomes with lung SAgs, Col-V and Kα1T, in C57BL/6 but not miRNA155KO following immunization with exosomes from LTxRs diagnosed with BOS (***p< 0.001 and **p< 0.01).
3.6. Upregulation of SOCS by miR-155KO mice after immunization with BOS exosomes containing lung SAgs
To determine the possible role of SOCS for the inability to induce immune responses to lung SAgs in miR-155KO mice, circulating exosomes isolated on day 30 were analyzed by specific Abs to SOCS using Western blot. We compared those results with results from C57BL/6 animals immunized with exosomes isolated from stable LTxRs or those with BOS. SOCS has been shown to have direct interaction to miR-155, which influences the progress of inflammation in lungs [16]. The results presented in Figure 5A,B demonstrate significant upregulation of SOCS in miR-155KO animals after immunization with exosomes isolated from LTxRs with BOS. These results strongly suggest that SOCS present in the circulating exosomes isolated from miR-155KO animals is likely to be involved in the downregulation of immune responses to lung SAgs by the miR-155KO mice after immunization with exosomes isolated from LTxRs with BOS.
Figure 5:
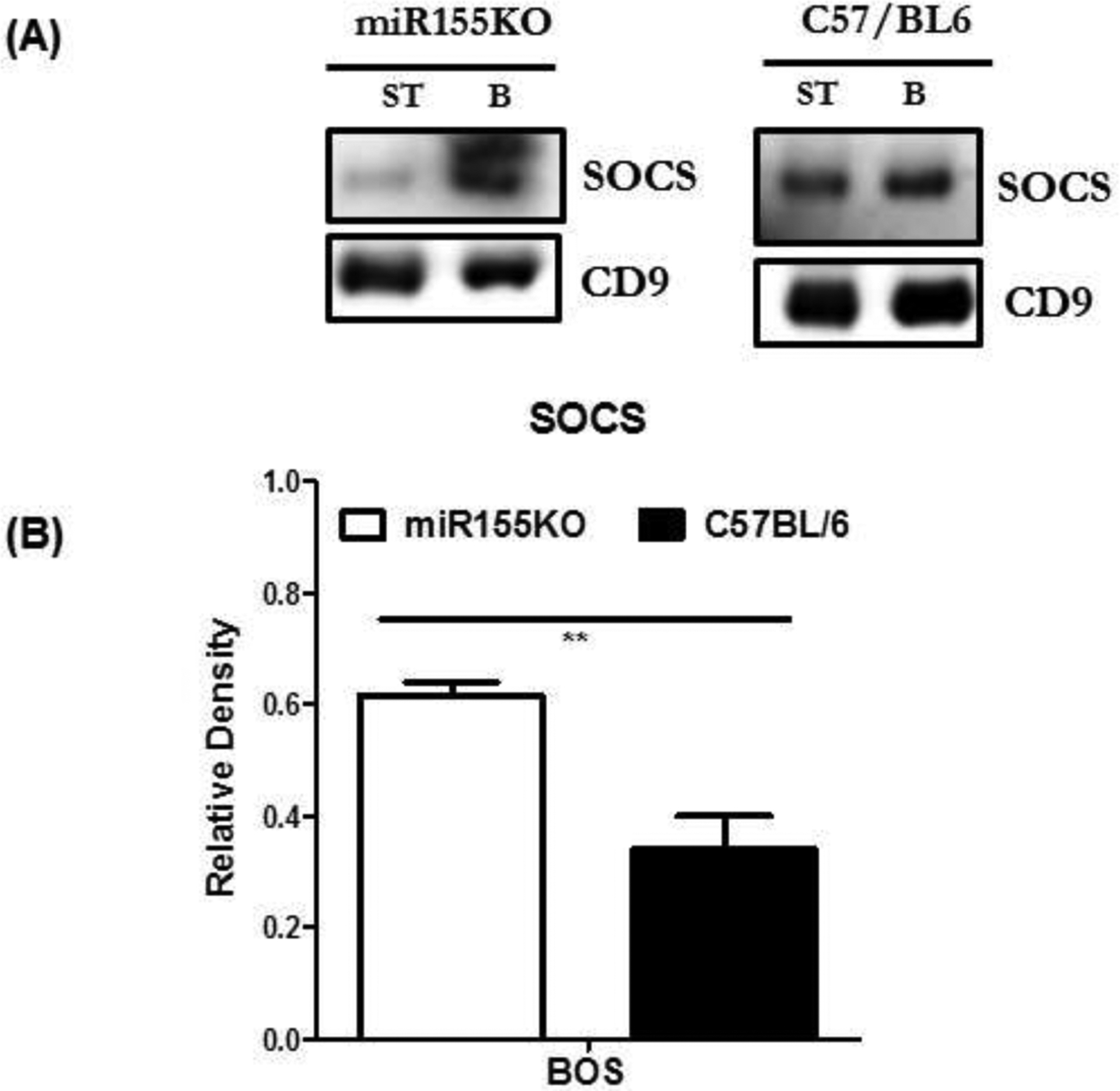
Western blot of proteins isolated from exosomes: St: Mice injected with stable exosomes; BOS: mice injected with BOS exosomes. (A) Exosomes from miR155KO but not C57BL/6 mice demonstrate up regulation of SOCS. (B) Densitometry analysis of western blot showing significantly increased levels of SOCS in miRNA155KO in comparison to C57BL/6 (**p< 0.01).
3.7. miRNA155KO animals immunized with exosomes isolated from LTxRs with BOS failed to induce obliterative airway disease (OAD) lesions
Lungs were harvested on day 30 from miR-155KO and wild-type C57BL/6 animals immunized with exosomes isolated from stable LTxRs and LTxRs with BOS, and the lungs were analyzed using trichrome staining (Figure 6). Cellular infiltration and fibrosis in lung tissue was noted in C57BL/6 animals (Figure 6B blue arrows); in contrast, there was no significant cellular infiltration or fibrosis in lungs from miR-155KO mice that had been immunized with exosomes from LTxRs with BOS (Figure 6B)
Figure 6:
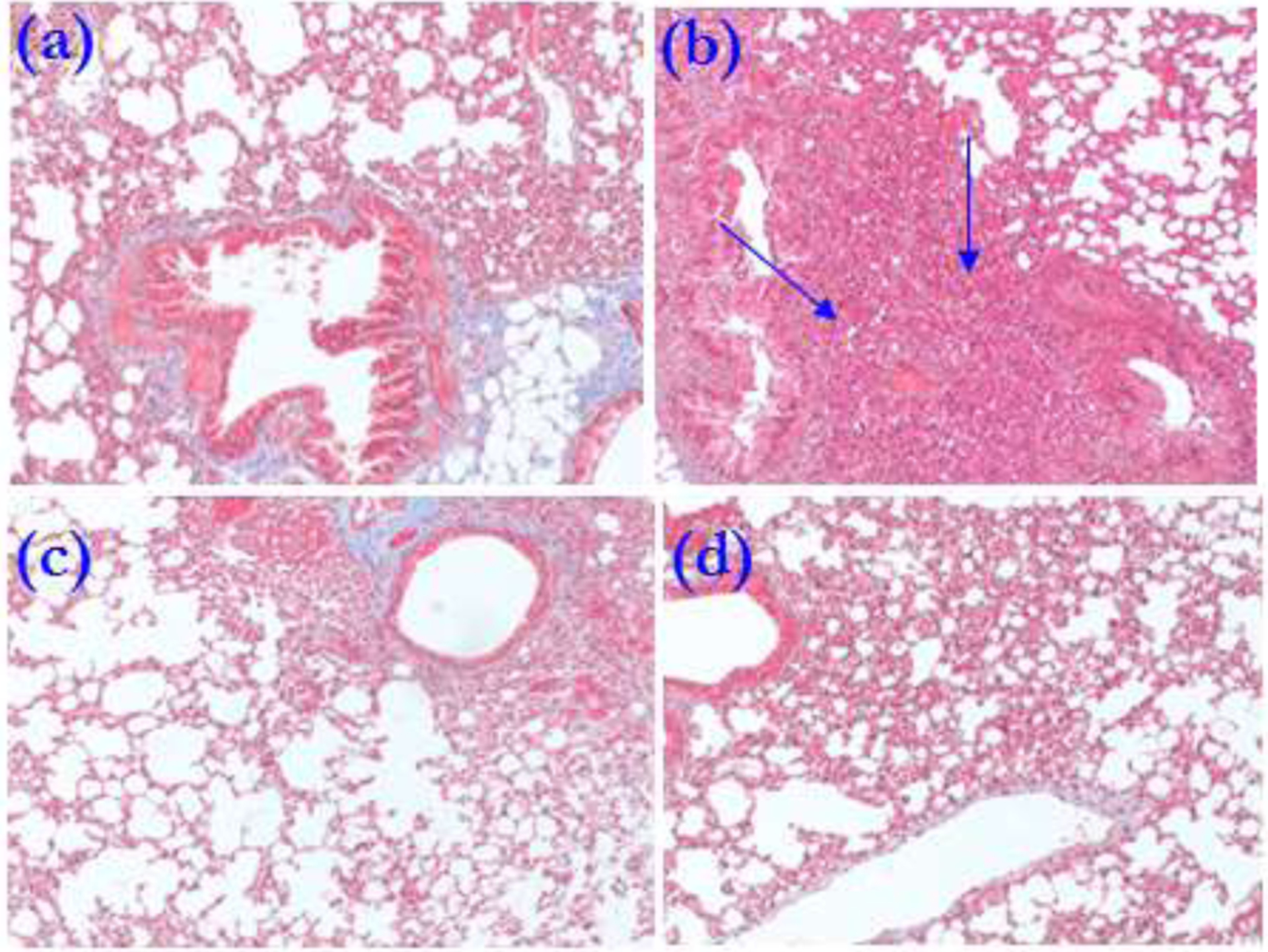
Trichrome staining of lung sections showing increased cellular infiltration in C57BL/6 animals immunized with exosomes from LTxRs diagnosed with BOS (b) but not stable (a). miR155KO did not show cellular infiltration either following immunization with BOS exosomes (d) or exosomes from stable (c) LTxRs. Cellular infiltration is marked with blue arrows.
3.8. Significant reduction in infiltration of CD3, CD4, and CD8 lymphocytes in miR-155KO animals after immunization with exosomes isolated from LTxRs with BOS
Immunohistochemical staining of the lung tissue sections from mice injected with exosomes from stable LTxRs and from LTxRs with BOS with Abs to CD3, CD4, and CD8 was performed, followed by morphometric analysis to quantify cellular filtration (Figure 7A). Significant reduction in CD3, CD4, and CD8 cells infiltrating the lung tissue of miR-155KO animals was observed compared to C57BL/6 mice immunized with exosomes isolated from LTxRs with BOS (p value <0.05; Figure 7B). This finding is in agreement with the lack of immune responses to lung SAgs by miR-155KO animals, as well as lack of OAD lesions in miR-155KO mice.
Figure 7:
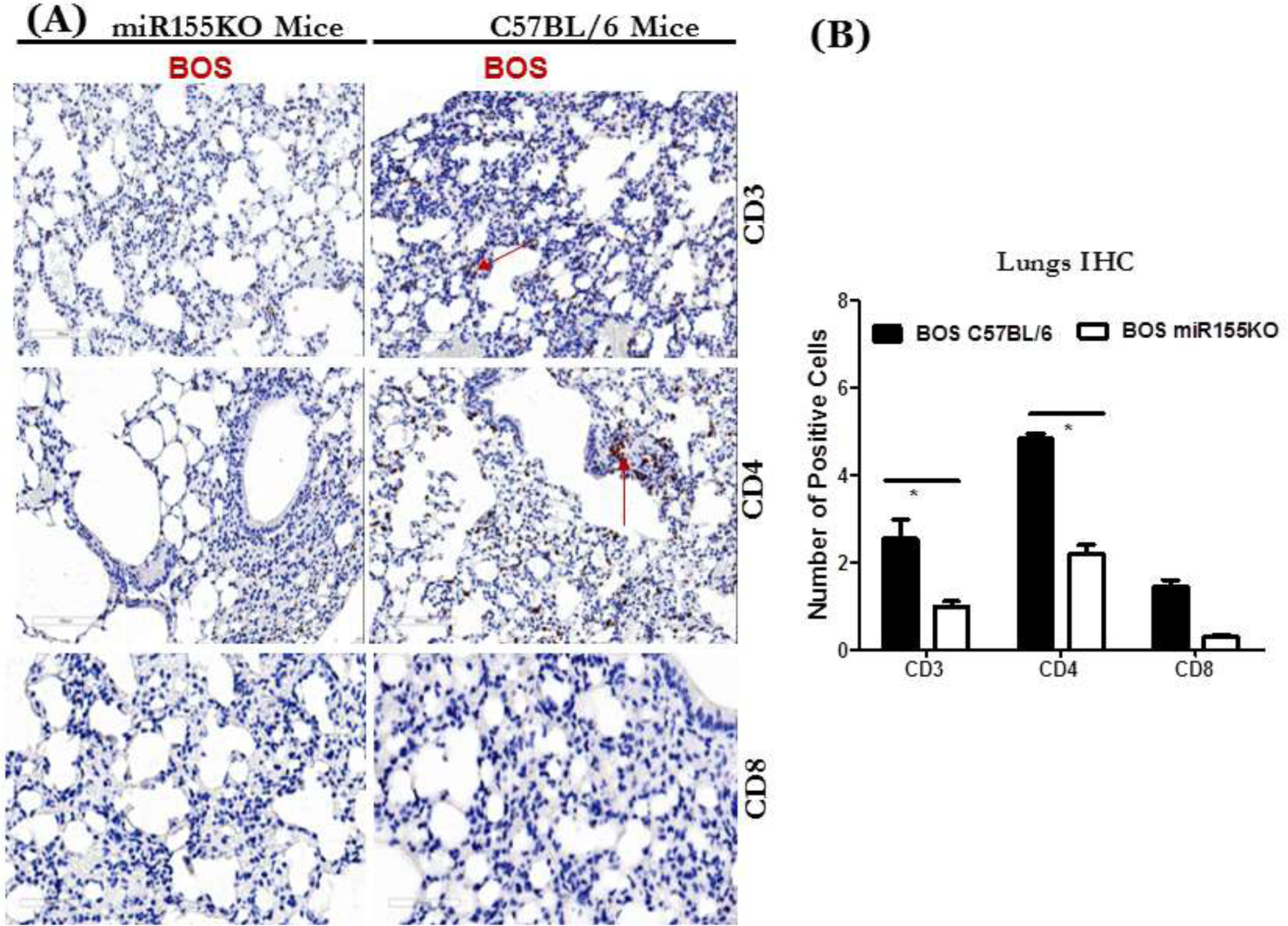
(A) Immunostaining of lung sections at 40x magnification with CD3, CD4 and CD8 Abs of lungs harvested from miR155KO or C57BL/6 immunized with exosomes isolated from LTxRs diagnosed with BOS. (B) Quantitation of lung sections showing increased numbers of CD3, CD4 and CD8 in C57BL/6 but not in miRNA155KO (*p<0.05).
3.9. Lack of C4d deposition in the lungs of miR-155KO animals after immunization with exosomes isolated from LTxRs with BOS
To determine complement activation by Abs and complement deposition in the lung tissue, C4D deposition was analyzed using Abs to C4D. Deposition of C4D in the lung tissue was seen in C57BL/6 animals immunized with exosomes isolated from LTxRs with BOS, whereas there was no detectable C4D depositon in the lungs of miR-155KO animals. This confirms that miR-155KO mice did not develop Abs that can activate complement cascade (p value <0.05; Figure 8).
Figure 8:
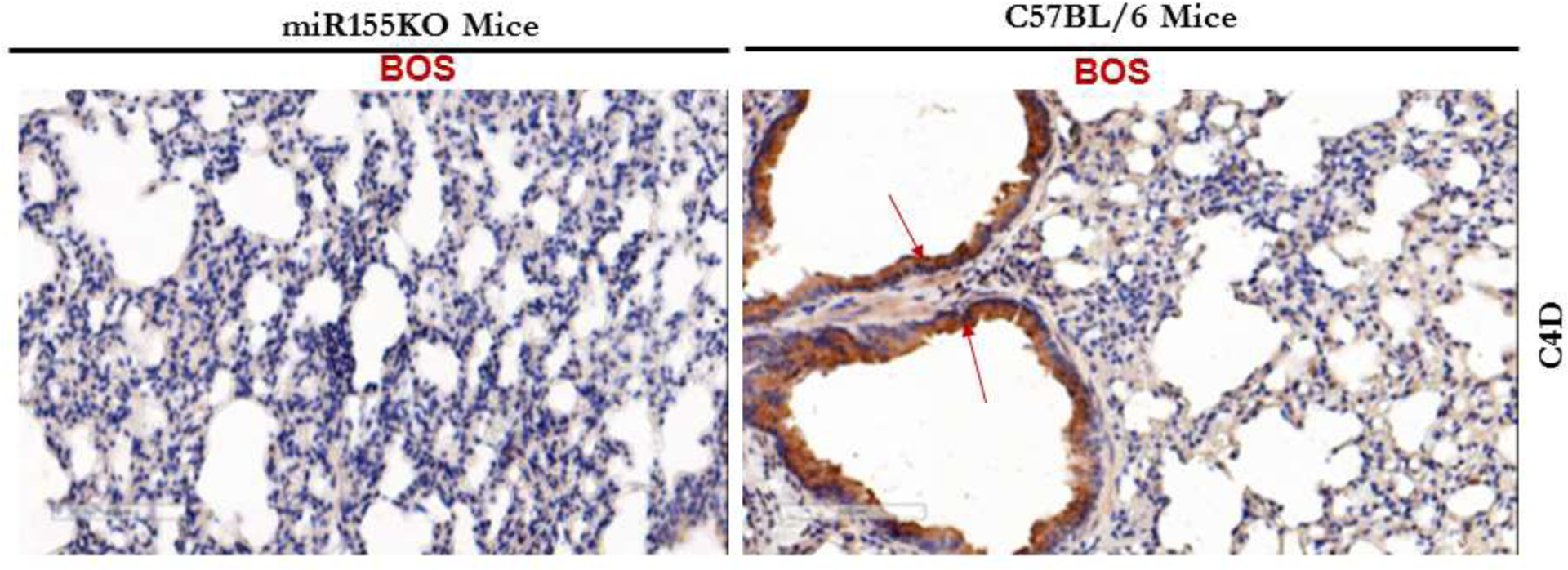
Immunostaining of lung sections at 40x magnification with Abs to C4d demonstrating marked C4d deposition in C57BL/6 animals but not in miRNA155KO immunized with exosomes from LTxRs diagnosed with BOS.
4. Discussion
Circulating exosomes isolated from LTxRs diagnosed with BOS contained mismatched donor HLA, lung SAgs, Col-V and Kα1T, as well as immunomodulatory miRNA involved in inflammation, cytokine upregulation, and endothelial activation [15]. We also demonstrated upregulation of miR-155 in the circulation of LTxRs diagnosed with BOS, suggesting that miR-155 may play an important role in inducing immune responses to donor HLA and lung SAgs [15, 17]. Furthermore, exosomes isolated from LTxRs diagnosed with BOS are immunogenic to C57BL/6 mice, resulting in the development of humoral and cellular immune responses to the lung-SAgs, Col-V and Kα1T and OAD (HEI-OAD), a correlate of BOS in humans after LTx [15]. Based on these findings, we postulated that miR-155 may play a pivotal role in eliciting immune responses to donor allo- and auto-antigens, leading to chronic rejection after human LTx [15]. In this communication, we used an miR-155KO animal model to demonstrate that miR-155 is obligatory for inducing immune responses leading to OAD, and that protection from OAD development is likely mediated by lack of induction of circulating exosomes containing lung SAgs. Lack of immune responses to lung SAgs is likely caused by upregulation of a protein from family of cytokine suppressors (ie, SOCS 1).
We immunized miR-155KO and wild-type C57BL/6 mice with circulating exosomes isolated either from LTxRs with BOS or from stable LTxRs, and compared their ability to mount an immune response to lung SAgs. Unlike wild-type C57BL/6 mice, miR-155-deficient animals failed to develop humoral or cellular immune responses to the lung SAgs, Kα1T and Col-V. Many pro-inflammatory cytokines, including IFNγ IL4, IL2, and IL17, were unchanged after miR-155KO mice were immunized with exosomes containing lung SAgs. Furthermore, circulating exosomes isolated from miR-155KO mice demonstrated not only lack of lung SAgs, but also increased concentration of SOCS, which can suppress synthesis of pro-inflammatory cytokines, resulting in downregulation of the cellular and humoral immune responses responsible for chronic rejection—that is, OAD. This is in agreement with reports that miR-155 plays a pivotal role in lung disease, and confirms that silencing miR-155 can mitigate abnormal cytokine expression and inflammation [18]. It is known that SOCS proteins are negative-feedback inhibitors of the signaling cascade induced by cytokines [19]; therefore, SOCS regulates both innate and adaptive immunity [20]. It is also known that miR-155 is pivotal in regulating immune responses through upregulation of IFNƴ and suppression of SOCS [21]. Taken together, it is not surprising that SOCS is associated with various immune and inflammatory diseases [22]. Immunization of miR-155KO mice with BOS exosomes did not induce pro-inflammatory cytokines or SOCS, indicating that the presence of miR-155 in exosomes isolated from LTxRs with BOS is by itself insufficient to induce an immune response in miR-155KO mice.
To our surprise, after immunizing both types of mice with exosomes isolated from LTxRs with BOS, we saw no significant increase in exosomes containing lung SAgs miR-155KO mice compared with exosomes with lung SAgs isolated from wild-type C57BL/6 animals, even though we found comparable levels of exosomes with specific marker CD9 (Figures 4 and 5). The half-life of human exosomes used for immunization can be 3 to 6 hours [23, 24], and the mouse serum samples used for exosome isolation were collected about 9 days after the final immunization. Therefore, we propose that the exosomes with lung SAgs present in the wild-type C57BL/6 animals are of murine origin and contain lung SAgs, Kα1T and Col-V. Therefore, lung SAgs present in the BOS exosomes are taken up by the murine antigen-presenting cells, and the exosomes generated subsequently result in immune responses against lung parenchyma. This process leads to the induction and release of exosomes from the lungs with murine lung SAgs after immunization with human exosomes. We propose that this process can lead to immune responses, resulting in the lesions seen in wild-type C57BL/6 animals but not in miR-155KO mice. The kinetic analysis presented in Figure 1C, together with the analysis demonstrating that the exosomes with lung SAgs are of murine origin, fully support this conclusion. Our results raise an interesting possibility—that miR-155 could be involved in regulating antigen presentation of SAgs. This theory needs further investigation.
5. Conclusions
As reported earlier, immunizing C57BL/6 mice with exosomes isolated from LTxRs with BOS induced lesions, marked cellular infiltration, occlusion of smaller airways, development of Abs to lung SAgs, fibrosis, and activation of complement, leading to deposition of C4d in the lung parenchyma (HEI-OAD). C57BL/6 mice immunized with exosomes from stable LTxRs did not produce these same reactions. Significant increases in CD3 and CD4 lung-infiltrating cells were also noted in the native lungs from mice with exosomes from LTxR with BOS (Figure 7). Exosomes with lung SAgs were also detected in C57BL/6 mice at day 7, which increased at day 14 and 21. Therefore, we propose that the miR-155 present in the exosomes isolated from LTxRs with BOS leads to activation of a pro-inflammatory response, leading to immune responses against the lungs, which is then perpetuated by release of murine exosomes with lung SAgs from the native murine lungs. This process leads to continued presence of circulating exosomes with lung SAgs in the wild-type C57BL/6 animals, and this process is likely to be involved in the perpetuation of immune responses leading to OAD lesions. In contrast, immunization of miR-155KO mice with exosomes from LTxRs with BOS failed to induce exosomes with lung SAgs from the native murine lungs, as exosomes used for immunization have a relatively short half-life. Therefore, development of both cellular and humoral immune responses to lung SAgs cannot take place, resulting in the lack of development of OAD lesions seen in the native lungs.
There are some limitations for this study. We did not use a lung transplant model of chronic rejection for the studies outlined in this report. We also have not analyzed in detail the possible role of miR-155 in antigen presentation of the lung SAgs. All of the studies were limited to C57BL/6 mice since miR-155KO animals were of this strain. We also need to perform experiments to downregulate SOCS in miR-155KO mice to conclusively demonstrate its role in downregulating the immune response to lung SAgs seen after immunization with exosomes from LTxRs with BOS. In spite of these limitations, our study clearly demonstrates that immunization of miR-155KO mice with exosomes from LTxRs with BOS did not induce circulating exosomes with lung SAgs and did not result in humoral or cellular immune responses to lung SAgs, which is likely mediated by increase seen in the transcription factor SOCS seen in the exosomes after immunization of miR-155KO mice. We conclude that persistence of circulating exosomes with alloantigens and lung SAgs plays a pivotal role in immune activation leading to chronic rejection, and we believe that miR-155 has an important effect on the pathogenesis of chronic rejection after human LTx.
Highlights.
MicroRNA-155 have a potential to play an important role in inducing immune responses to donor HLA and lung SAgs.
Exosomes isolated from LTxRs diagnosed with BOS are immunogenic.
MicroRNA-155 is obligatory for inducing immune responses leading to Obliterans Airway Disease
Lack of immune responses to lung SAgs in miR155KO mice is likely caused by upregulation of a protein from family of cytokine suppressors (SOCS1).
Persistence of circulating exosomes with alloantigens and lung SAgs plays a pivotal role in immune activation leading to chronic rejection
Acknowledgements
The authors wish to acknowledge Billie Glasscock and Clare Sonntag for assistance with preparation and submission of manuscript.
7. Funding
This work was supported by National Institutes of Health grants HL056643 and HL092514 (TM).
Abbreviations:
- BOS
Bronchiolitis obliterans syndrome
- COL-V
Collagen V
- ELISA
Enzyme-linked immunosorbent assay
- HEI-OAD
Human exosome induced - obliterative airway disease
- Kα1T
K-alpha 1 tubulin
- LTx
Lung transplant
- LTxRs
Lung transplant recipients
- miRNA
MicroRNA
- miR-155
MicroRNA-155
- OAD
Obliterative airway disease
- PBS
Phosphate-buffered saline
- SAgs
Self-antigens
- SOCS
Suppressor of cytokine signaling
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. Conflict of Interest
The authors declare that they have no conflict of interest. All authors have approved the final version of this manuscript.
References:
- [1].Engels BM, Hutvagner G, Principles and effects of microRNA-mediated post-transcriptional gene regulation, Oncogene, 25 (2006) 6163–6169. [DOI] [PubMed] [Google Scholar]
- [2].Griffiths-Jones S, Grocock RJ, van Dongen S, Bateman A, Enright AJ, miRBase: microRNA sequences, targets and gene nomenclature, Nucleic Acids Res, 34 (2006) D140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bartel D, MicroRNAs: genomics, biogenesis, mechanism, and function., Cell, 116 (2004) 281–297. [DOI] [PubMed] [Google Scholar]
- [4].Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, van Dongen S, Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A, Requirement of bic/microRNA-155 for normal immune function, Science, 316 (2007) 608–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tam W, Ben-Yehuda D, Hayward WS, bic, a novel gene activated by proviral insertions in avian leukosis virus-induced lymphomas, is likely to function through its noncoding RNA, Mol Cell Biol, 17 (1997) 1490–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y, Lu S, Wang Z, Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response, J Mol Med (Berl), 94 (2016) 1063–1079. [DOI] [PubMed] [Google Scholar]
- [7].Lu LF, Liston A, MicroRNA in the immune system, microRNA as an immune system, Immunology, 127 (2009) 291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Colombo M, Raposo G, Thery C, Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles, Annual review of cell and developmental biology, 30 (2014) 255–289. [DOI] [PubMed] [Google Scholar]
- [9].Ferguson SW, Nguyen J, Exosomes as therapeutics: The implications of molecular composition and exosomal heterogeneity, Journal of controlled release : official journal of the Controlled Release Society, 228 (2016) 179–190. [DOI] [PubMed] [Google Scholar]
- [10].Suryadevara C, Govindugari V, Exosomes and microparticles: The nanosized vesicles released from the cells that act as biomarkers for disease and treatment - riveting on lung diseases., Materials Today: Proceedings, 2(9) (2015) 4626–4631. [Google Scholar]
- [11].Gunasekaran M, Xu Z, Nayak DK, Sharma M, Hachem R, Walia R, Bremner RM, Smith MA, Mohanakumar T, Donor-Derived Exosomes With Lung Self-Antigens in Human Lung Allograft Rejection, Am J Transplant, 17 (2017) 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, Kreisel D, Gelman AE, Mohanakumar T, Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation, Am J Transplant, 14 (2014) 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, Mohanakumar T, Antibodies to MHC class I induce autoimmunity: role in the pathogenesis of chronic rejection, J Immunol, 182 (2009) 309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tiriveedhi V, Gautam B, Sarma NJ, Askar M, Budev M, Aloush A, Hachem R, Trulock E, Myers B, Patterson AG, Mohanakumar T, Pre-transplant antibodies to Kalpha1 tubulin and collagen-V in lung transplantation: clinical correlations, J Heart Lung Transplant, 32 (2013) 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gunasekaran M, Sharma M, Hachem R, Bremner R, Smith MA, Mohanakumar T, Circulating Exosomes with Distinct Properties during Chronic Lung Allograft Rejection, J Immunol, 200 (2018) 2535–2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jiang K, Yang J, Guo S, Zhao G, Wu H, Deng G, Peripheral Circulating Exosome-Mediated Delivery of miR-155 as a Novel Mechanism for Acute Lung Inflammation, Mol Ther, 27 (2019) 1758–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Ladak SS, Ward C, Ali S, The potential role of microRNAs in lung allograft rejection, J Heart Lung Transplant, 35 (2016) 550–559. [DOI] [PubMed] [Google Scholar]
- [18].Alivernini S, Gremese E, McSharry C, Tolusso B, Ferraccioli G, McInnes IB, Kurowska-Stolarska M, MicroRNA-155-at the Critical Interface of Innate and Adaptive Immunity in Arthritis, Front Immunol, 8 (2017) 1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krebs DL, Hilton DJ, SOCS proteins: negative regulators of cytokine signaling, Stem Cells, 19 (2001) 378–387. [DOI] [PubMed] [Google Scholar]
- [20].Yoshimura A, Nishinakamura H, Matsumura Y, Hanada T, Negative regulation of cytokine signaling and immune responses by SOCS proteins, Arthritis Res Ther, 7 (2005) 100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang P, Hou J, Lin L, Wang C, Liu X, Li D, Ma F, Wang Z, Cao X, Inducible microRNA-155 feedback promotes type I IFN signaling in antiviral innate immunity by targeting suppressor of cytokine signaling 1, J Immunol, 185 (2010) 6226–6233. [DOI] [PubMed] [Google Scholar]
- [22].Alexander WS, Suppressors of cytokine signalling (SOCS) in the immune system, Nat Rev Immunol, 2 (2002) 410–416. [DOI] [PubMed] [Google Scholar]
- [23].Lai CP, Mardini O, Ericsson M, Prabhakar S, Maguire C, Chen JW, Tannous BA, Breakefield XO, Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter, ACS nano, 8 (2014) 483–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Riau AK, Ong HS, Yam GHF, Mehta JS, Sustained Delivery System for Stem Cell-Derived Exosomes, Front Pharmacol, 10 (2019) 1368. [DOI] [PMC free article] [PubMed] [Google Scholar]


