Abstract
Although endodontic therapy is typically successful, in approximately 10%–15% of the cases, symptoms can persist or reoccur. Periapical surgery is the preferred treatment of choice in failed root canal therapy, chronic periapical lesion, persistent apical periodontitis, etc., i.e., when conventional treatment modalities fail. Over the past few decades, although the list of indications for endodontic surgery has diminished, there exist definite cases in which the tooth cannot be retained without surgery. This case report, however, sheds light on the incorporation of a novel autologous platelet concentrate-concentrated growth factor (CGF) coupled with an osseograft in surgical endodontic procedure to ensure a swift and successful recovery of the periapical region subjected to extensive lesions. The use of an osseograft combined with CGF has numerous advantages as well due to the formation of sticky bone. There are no articles published in the literature with respect to the potent application of CGF and bone graft (sticky bone) in large periapical lesions to aid in the reparative process. In this case report, the 1-year follow-up radiographs and cone-beam computed tomography showed complete healing of the hard and soft-tissue lesions that conform to achieving repair and regeneration at a rapid rate in extensive periapical lesions.
Keywords: Autologous platelet concentrate, concentrated growth factor, endodontic surgery, sticky bone
INTRODUCTION
The success of endodontic treatment involves an intricate blend of chemical and mechanical debridement techniques. However, in about 10%–15% of treated cases, failure can be attributed to the emergence of uncontrolled symptoms and their long-term persistence in the root canal complexities.[1] Endodontic surgery is implicated when an endodontically treated tooth succumbs to a periradicular pathosis, and nonsurgical endodontic retreatment is not feasible. The aforesaid statement is supported by claims put forward by Natkin et al. 1984 and Nair et al. 1998, wherein they assessed the radiographic size of lesions and finally concluded that if the lesion does not involve the apex with an intact epithelial lining, it may not heal nonsurgically.[2] Over the years, although the list of indications for endodontic surgery has diminished, there however exists definite case reports wherein the tooth could not have been retained without a surgical approach.
The concept of surgical endodontics dates back to 1500 years from the pioneering work initiated by Aetius, based on incision and drainage[3] and has been a major breakthrough ever since. Surgical debridement and resective techniques have been the traditional approaches practiced to enhance periapical healing thereby seizing disease progression.
The incorporation of autologous platelet concentrates in surgical endodontic procedures holds unquestionable promise when dealing with large periapical lesions.[4] Nevertheless, several pitfalls concerning the use of first-generation platelet concentrates are one too many such as the risk of coagulopathies, use of anticoagulants, handling properties, cost and also entails tedious two-step centrifugation and purification process.[5] In order to overcome the aforementioned limitations, concentrated growth factor (CGF), touted as the second-generation platelet concentrate, introduced by Sacco et al., 2006, not only has been suggested as an ideal biomaterial but is also claimed to be better than platelet-rich fibrin (PRF) due to its richer and thicker growth factor content that promotes cell proliferation, migration and also stimulates tissue remodeling at a faster rate.[6] On another note, CGF has been extensively used for implant surgeries and sinus ridge augmentation procedures in the recent past, exhibiting favorable results.[7]
Bioresorbable demineralized bone material has been widely used in varied clinical scenarios, prominently in bony defects, and extensive lesions with histological evidence of regeneration of new bone and its supporting tissues. Bone allograft induces pluripotent stem cells to differentiate into osteoblasts which in turn promotes cellular differentiation and bone formation through osteoinduction.[8] The use of an osseograft combined with CGF has numerous advantages owing to the formation of sticky bone. The product thus formed can be molded, prevent both macro and micro movements, also the network is proven to contain platelets and growth factors thereby contributing to accelerated soft-tissue healing and bone regeneration. Furthermore, the fibrin interconnection prevents the ingrowth of soft tissue into the bone graft as well.[9] A thorough search of the literature disclosed the absence of any publication with reference to the use of sticky bone in endodontic surgical procedures, thus highlighting the uniqueness of this case report in that regard.
CASE REPORT
A 26-year-old male patient reported to the Department of Oral and Maxillofacial surgery with a chief complaint of shift in the normal position of teeth in the right mandibular back tooth region. Following biopsy with respect to the said tooth region, i.e., 43, 44, 45, a provisional diagnosis of odontogenic keratocyst was made. The patient was then referred for endodontic opinion and further management regarding the same before performing elective surgery.
The medical and dental history was non-contributory. Intraoral clinical examination revealed pathological migration of 43 with no debilitating symptoms [Figure 1a]. The electric pulp testing (Gentle Pulse™ Pulp Vitality Tester, Parkell, USA) showed a negative response on 43, 44, 45 when compared with the control teeth. On radiographic examination (VistaScan Mini, UK), periapical radiolucency was evident in 43, 44, 45 [Figure 2a]. Cone-beam computed tomography (CBCT) (Dentsply Sirona, Orthophos XG 3D) was taken at standardized settings (90 kV, 6 mA, 5 cm × 5.5 cm, 160 μm, 14 s) to assess the information on periapical lesion extent and the proximity to anatomic structures. The preoperative measurements of the lesion extent were viewed in different planes; the tangential section measured 14.39 mm × 9.42 mm; cross-sectional measured 14.77 mm × 8.46 mm and axial section measured 12.14 mm × 6.52 mm [Figure 2c–g].
Figure 1.
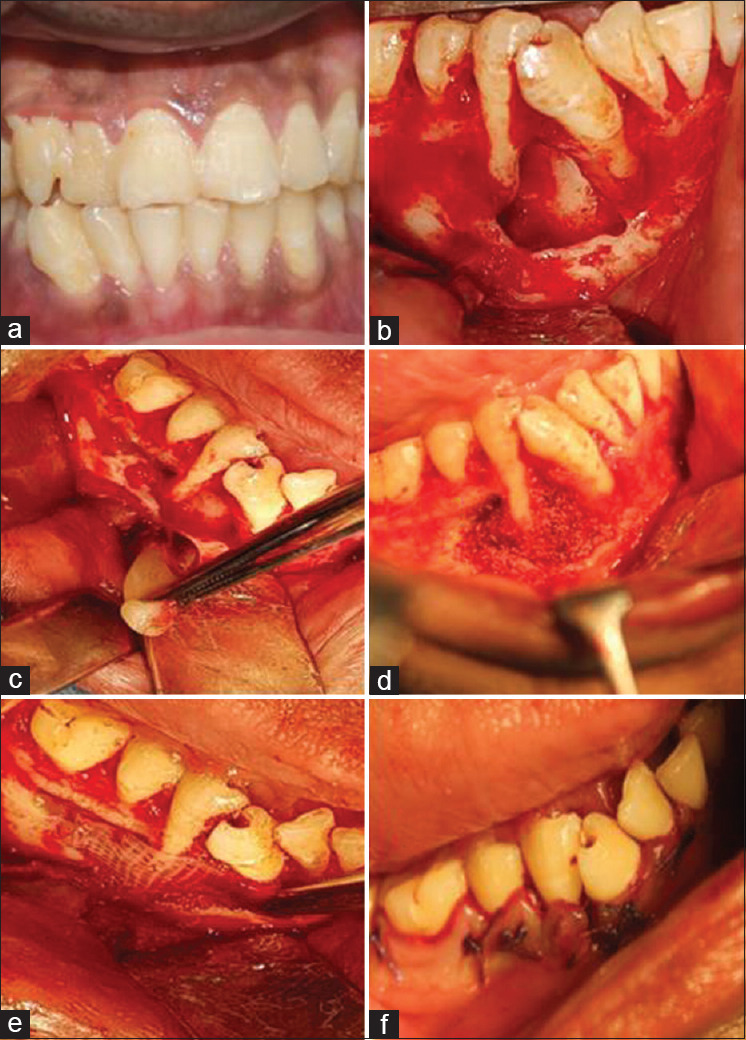
Preoperative clinical and surgical procedure. Preoperative clinical photograph showing drifted 43, 44, 45 (a). Flap elevation and cystic enucleation, apicoectomy, and retrograde filling (b). Placement of concentrated growth factor cystic space (c). Placement of sticky bone (d). Placement of concentrated growth factor membrane (e). Sutures placed (f)
Figure 2.
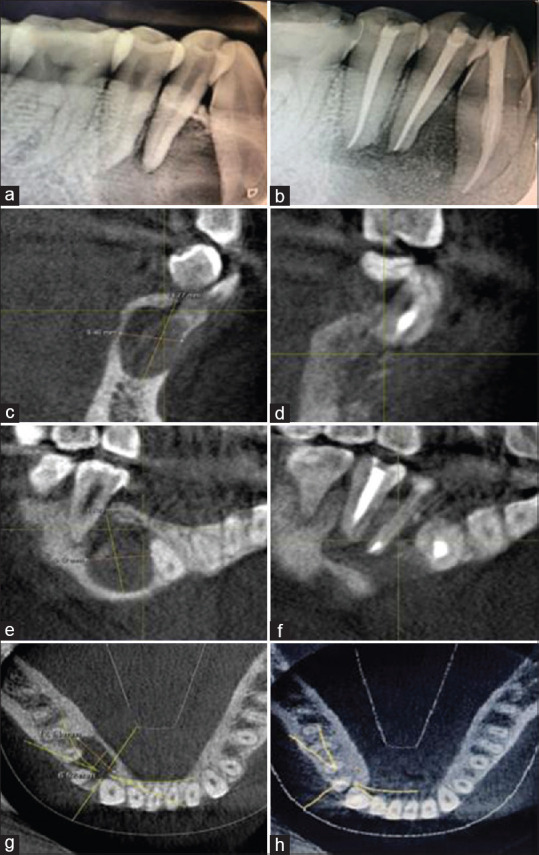
Preoperative radiographic images-intraoral periapical and cone-beam computed tomography images. Preoperative intraoral periapical showing periapical lesion involving the apices of 43, 44, 45 (a). Postoperative intraoral periapical showing healing (b). Preoperative cone-beam computed tomography image showing the extent of lesion in cross-sectional slice (c). Postoperative cone-beam computed tomography image showing the extent of lesion in cross-sectional slice (d). Preoperative cone-beam computed tomography showing the lesion in tangential slice (e). Postoperative cone-beam computed tomography showing the lesion in tangential slice (f). Preoperative cone-beam computed tomography showing the lesion in axial section (g). Postoperative cone-beam computed tomography showing the lesion in axial section (h)
According to the CBCT-periapical index scoring system, the lesion for the present case was graded as 5E. On the basis of the above findings, the diagnosis of pulpal necrosis with a chronic apical abscess in 43, 44, 45 was made. Root canal therapy was proposed as the first line of treatment in relation to 43, 44, 45 prior to periapical surgery followed by the positioning of a combination of CGF and bone graft (sticky bone) over the defective site.
On the first visit, informed consent was obtained from the patient, local anesthesia was administered (2% lignocaine with 1:200,000 dilution adrenaline, Neon laboratories LTD), teeth were isolated with rubber dam, followed by access cavity preparation with endo access bur (DENTSPLY Mallifer, Switzerland). Working length was determined using an electronic apex locator (Propex Pixi, DENTSPLY Mallifer, Switzerland) and confirmed radiographically. The irrigation protocol included thorough rinsing with copious amounts of 3% sodium hypochlorite (VIP, Vensons, India), intermediate flushing with 0.9% saline (acuLIFE, India), and 17% EDTA (Canalarge, Ammdent, India) as the final irrigant. Calcium hydroxide (RC Cal, Prime dental products, India) was placed as intracanal medicament for 3 weeks. Obturation was carried out in 43, 44, 45 [Figure 2b].
A state of unconsciousness was induced by general anesthesia advocating the use of propofol maintained by isoflurane volatile liquid and atracurium as muscle relaxant. Nitrous oxide analgesia was administered followed by neostigmine as a reversal agent. Full-thickness mucoperiosteal flap was elevated with two vertical incisions, complete curettage was carried out, followed by apicectomy and retrograde filling with MTA in 43, 44, 45 [Figure 1b]. Later, the surgical site was prepared for the placement of a combination of CGF with osseograft followed by CGF membrane [Figure 1c and d].
The present case necessitated the use of two 10 mL disposable nonanticoagulant tubes and a centrifuge machine (MEDIFUGE, Silfradentsrl, S. Sofia, Italy). A volume of 20 mL of intravenous blood was drawn from the patient, transferred to centrifuge tubes, accelerated for 30 s; centrifuged at 2700 rpm for 2 min, 2400 rpm for 4 min, 2700 rpm for 4 min, and 3000 rpm for 3 min; decelerated for 36 s to stop. At the end of the automated pre-programmed cycle, the centrifuge tube encompassed a concoction of four different layers; the uppermost or the 1st layer contained serum, the 2nd layer was composed of a fibrin buffy coat, the 3rd layer constituted the much-needed growth factors, finally, the lowermost or the 4th layer was occupied by the red blood cells. The fibrin gel accommodating the CGF was separated from the red blood cells, dispensed onto a metal storage box, compressed using a metal cover to procure the CGF membrane which was effectively used as a substitute for GTR [Figure 1e]. Osseograft was mixed with the fibrin gel to form sticky bone. Subsequent to placement of sticky bone and CGF membrane over the defective site, the flap was approximated with 3-0 vicryl sutures (Ethicon Inc., Piscataway, USA) [Figure 1f]. Postoperative instructions were given to the patient, following which medications were prescribed.
Follow-up
Suture removal was done after a week. No adverse events were reported, at 1 year follow-up, intraoral periapical radiograph [Figure 3], and CBCT revealed a thorough healing at 43, 44, 45 region with bone regeneration at the defective site [Figure 2d, f and h]. Livewire segmentation using OSIRIX Version 9.5 (PIXMEO, Geneva, Switzerland) was done to delineate the lesion from healthy bone. Pre- and post-operative volume calculations were 1.0798 cm3 and 0.2265 cm3, respectively [Figure 4a and b]. The lesion reduction size was found to be 79%.
Figure 3.
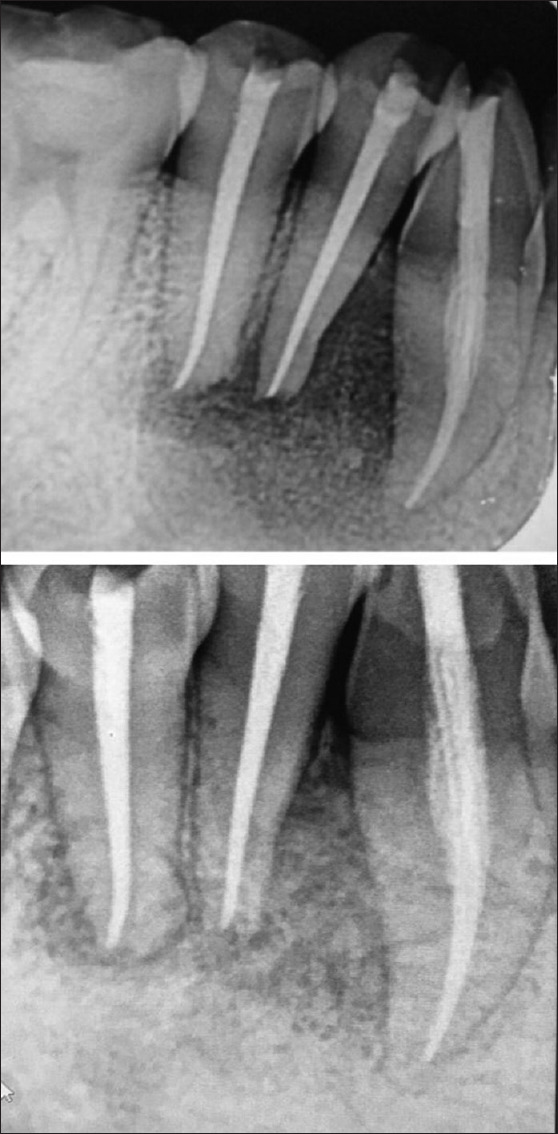
1 and 2 years' follow radiographs showing 90% healing of the periapical lesion
Figure 4.
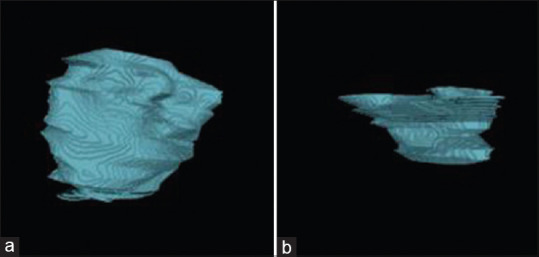
Preoperative and postoperative volume calculations to delineate the lesion from healthy bone. (a and b) Preoperative and postoperative volume calculations, respectively
DISCUSSION
The present case report focuses on the use of CGF combined with bone grafts as a biomaterial that conforms to achieving repair and regeneration following periapical surgery. CGF was prepared in a pre-programmed device, the fibrin rich blocks thus formed were larger and denser encasing innumerable growth factors.[10]
The extensive use of sticky bone in cases of sinus ridge augmentation procedures yielding clinically positive results is discussed in Table 1.
Table 1.
Surgical application of CGF in combination with bone graft – Review of literature
| Author and year | Number of cases and procedure | CGF preparation | CGF and bone graft | Follow-up | Healing assessment method | Results |
|---|---|---|---|---|---|---|
| Torrisi et al., 2011 | 8, sinus augmentation | Medifuge | CGF and Bio-Oss | 5 months | OPG and histology | New bone formation |
| Gheno et al., 2014 | 1, Sinus augmentation | Medifuge | FRB and Xenograft | 4 months | Clinical evaluation and histomorphometric analysis | Presence of NB, together with n-MT and RBG (12 months) |
| Georgakopoul et al., 2014 | 1, Sinus augmentation | Medifuge | FRB and allograft | 8 months | OPG, CBCT, Osstell readings and stability values | ISQ values of 61-69 with new bone formation around implants |
| Dong Seok sohn et al., 2015 | 3, ridge augmentation | Medifuge | FRB, sticky bone and CGF Membrane | 6 months | Histology, OPG, CBCT | Favorable ridge augmentation |
| Chen et al., 2016 | 16, Sinus floor elevation | Medifuge | FRB, BioOss, CGF membrane | 2 weeks, 1, 3, 6, 12 months | Clinical evaluation and CBCT | Predictable clinical result |
| Qiao et al., 2016 | 31, Intrabony defects | Medifuge | FRB, BioOss and CGF membrane | Baseline, 1 year | Clinical evaluation | CGF showed higher clinical positive results compared to PRP |
| Gokmenoglu et al., 2016 | 4, lateral cyst | Medifuge | FRB, Xenografts, CGF membrane | Baseline, 3 months | Clinical and radiographic | Positive clinical impact |
| Doan et al., 2017 | 2, cystic lesions | Medifuge | CGF, Bio Oss, CGF membrane | 1, 7 days; 1, 3 months | Clinically evaluation, Radiography | Mphi laser reduced postoperative pain and yielded wound healing |
| Ying et al., 2017 | 1, bone defect | Medifuge | CGF membrane and autologous bone | 3, 6, 12 months | Clinical evaluation and CBCT | Clinically positive results with bone regeneration evident in CBCT |
CGF: Concentrated growth factor, CBCT: Cone-beam computed tomography, PRP: Platelet-rich plasma, FRB: Fibrin rich block, NB: New bone, ISQ: Implant stability quotient, n-MT: Non-mineralized tissue, OPG: Orthopantomogram, RBG: Residual bone graft
In the literature, two case reports have described the utilization of sticky bone in the treatment of bony defects at which point satisfactory clinical impact was evident in addition to favorable ridge augmentation after 1 year by virtue of lack of macro and micro movements.[5,7] In one case report, there was a lack of evidence on the usage of Medifuge required for CGF preparation, whereas the other laid emphasis on the use of sticky bone along with Mphi laser. However, both did not accentuate an individual parameter. The present case report, on the other hand, is the first of its kind wherein a Medifuge was used as per the required specifications apart from implementing sticky bone in endodontic surgery.
CGF is a distinctive autologous platelet concentrate eminently rich in growth factors, namely transforming growth factor-beta 1 (TGF-ß1) and vasoendothelial growth factor (VEGF). While TGF-ß1 stimulates expression of bone morphogenic proteins, curtails matrix metalloproteinases, and other enzymes to further incite differentiation of osteoblastic cells,[11] VEGF at the same time induces endothelial cell migration and proliferation eventually leading to hyperpermeability of blood vessels.[12] Moreover, in a comparative study, Park et al. reported an escalated level of growth factors, notably VEGF in CGF as opposed to PRF.[13]
Osseograft used in the present case being a demineralized dried xenograft elicits both osteoinductive and osteoconductive properties. Bone grafts containing particle size of 125–1000 μm have greater osteogenic potential. Shortly, after the initial 2 min of centrifugation, autologous fibrin glue was obtained which when combined with particulate bone powder and allowed to polymerize for 5–10 min, led to the acquisition of sticky bone with an inherent capability to not migrate onto the interlinked fibrin matrix.
The present case was graded as 5E, denoting the diameter of periapical radiolucency to be more than 8 mm along with the expansion of periapical cortical bone.[14] However, at 1 year follow-up, CBCT scan with a small field of view, well within the guidelines proposed by the European Society of Endodontology, AAE, and AAOMR joint position statement,[15] helped to determine the extent of healing of the lesion.
All things considered, the present case report showcased exceptional results in terms of periapical healing and is probably ascribable to the inclusion of a dynamic combination of CGF and bone grafts to form sticky bone as a means to induce a rapid repair and regenerative process.
CONCLUSION
In our previous report of two cases, CGF was effectually employed singly as a scaffolding medium, eliciting unprecedented response in terms of periapical healing. However, the present case focuses on a conspicuous coalescence of CGF and bone graft (sticky bone) to intercept a large periapical bony lesion. Considering the pragmatism and relative practicality of this case in conjuncture with the available evidence on the use of sticky bone in varied specialties, CGF and bone grafts in unison can be utilized with great effect so as to yield resounding success to stem the repair and regenerative process. The present case report holds limitations on the grounds that histological evaluation of the bony defect is required to further substantiate the role of sticky bone in regeneration on a long-term basis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge Dr. Sri Prakash for his skills imparted in the preparation of the autologous platelet concentrate (CGF).
REFERENCES
- 1.Tabassum S, Khan FR. Failure of endodontic treatment: The usual suspects. Eur J Dent. 2016;10:144–7. doi: 10.4103/1305-7456.175682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair PN, Sjögren U, Figdor D, Sundqvist G. Persistent periapical radiolucencies of root-filled human teeth, failed endodontic treatments, and periapical scars. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:617–27. doi: 10.1016/s1079-2104(99)70145-9. [DOI] [PubMed] [Google Scholar]
- 3.Leuebke RG, Glick DH, Ingle JI. Indications and contraindications for endodontic surgery. Oral Surg Oral Med Oral Pathol. 1964;18:97–113. doi: 10.1016/0030-4220(64)90264-6. [DOI] [PubMed] [Google Scholar]
- 4.Vaishnavi C, Mohan B, Narayanan LL. Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combination of both: An in vivo study. J Conserv Dent. 2011;14:140–6. doi: 10.4103/0972-0707.82614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, Mouhyi J, et al. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:e37–44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Borsani E, Bonazza V, Buffoli B, Cocchi MA, Castrezzati S, Scarì G, et al. Biological characterization and in vitro effects of human concentrated growth factor preparation: An innovative approach to tissue regeneration. Biol Medicine. 2015;7:1. [Google Scholar]
- 7.Sohn DS, Moon JW, Moon YS, Park JS, Jung HS. The use of concentrated growth factors (CGF) for sinus augmentation. J Oral Implant. 2009;38:25–38. [Google Scholar]
- 8.Mellonig JT. Autogenous and allogeneic bone grafts in periodontal therapy. Crit Rev Oral Biol Med. 1992;3:333–52. doi: 10.1177/10454411920030040201. [DOI] [PubMed] [Google Scholar]
- 9.Doan N, Nguyen-Pham L, Liang C, Duong QT. A review on the application of concentrated growth factors and MPhi laser to regenerate oral defects in the oral and maxillofacial region and a two cases report. Int J Oral Maxillofac Surg. 2017;46:203–4. [Google Scholar]
- 10.Rodella LF, Favero G, Boninsegna R, Buffoli B, Labanca M, Scarì G, et al. Growth factors, CD34 positive cells, and fibrin network analysis in concentrated growth factors fraction. Microsc Res Tech. 2011;74:772–7. doi: 10.1002/jemt.20968. [DOI] [PubMed] [Google Scholar]
- 11.Gosiewska A, Yi CF, Blanc-Brude O, Geesin JC. Characterization of a macrophage-based system for studying the activation of latent TGF-beta. Methods Cell Sci. 1999;21:47–56. doi: 10.1023/a:1009807802589. [DOI] [PubMed] [Google Scholar]
- 12.Lakey MA, Klein MJ, Faye-Petersen OM. A comprehensive clinicopathologic analysis suggests that vascular endothelial growth factor (VEGF) is the most likely mediator of periosteal new bone formation (PNBF) Associated with diverse etiologies. Clin Med Arthrit Musculoskeletal Disorders. 2008;1:CMAMD–S442. [Google Scholar]
- 13.Park HC, Kim SG, Oh JS, You JS, Kim JS, Lim SC, et al. Early bone formation at a femur defect using CGF and PRF grafts in adult dogs: A comparative study. Implant Dent. 2016;25:387–93. doi: 10.1097/ID.0000000000000423. [DOI] [PubMed] [Google Scholar]
- 14.Estrela C, Bueno MR, Leles CR, Azevedo B, Azevedo JR. Accuracy of cone beam computed tomography and panoramic and periapical radiography for detection of apical periodontitis. J Endod. 2008;34:273–9. doi: 10.1016/j.joen.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 15.Fayad MI, Nair M, Levin MD, Benavides E, Rubinstein RA, Barghan S, et al. AAE and AAOMR joint position statement: Use of cone beam computed tomography in endodontics 2015 update. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120:508–12. doi: 10.1016/j.oooo.2015.07.033. [DOI] [PubMed] [Google Scholar]


