Abstract
Purpose:
Frailty and functional status have emerged as significant predictors of morbidity and mortality for patients undergoing cancer surgery. To articulate their impact on value (i.e., quality per cost), we compared perioperative outcomes and expenditures according to patient function for older adults undergoing kidney cancer surgery.
Materials and Methods:
Using linked SEER-Medicare data, we identified 19,129 elderly patients with kidney cancer treated with non-ablative surgery from 2000–2009. We quantified patient function using function-related indicators—claims indicative of dysfunction and disability—and measured 30-day morbidity, mortality, resource use, and cost. Using multivariable, mixed-effects models to adjust for patient and hospital characteristics, we estimated the relationship between patient functionality and both treatment outcomes and expenditures.
Results:
Of 19,129 patients, we identified 5,509 (28.8%) and 3,127 (16.4%) with a function-related indicator count of 1 and ≥2, respectively. While surgical complications did not vary (OR 0.95, 95% CI 0.86–1.05), patients with ≥2 indicators more often experienced a medical (OR 1.22, 95% CI 1.10–1.36) or geriatric (OR 1.55, 95% CI 1.33–1.81) event or died within 30 days of surgery (OR 1.43, 95% CI 1.10–1.86) compared with patients with no baseline dysfunction. These patients utilized significantly more medical resources and amassed higher acute care expenditures (p<0.001).
Conclusion:
During kidney cancer surgery, patients in poor functional health can face a more eventful medical recovery at elevated cost, indicating lower value care. Greater consideration of frailty and functional status during treatment planning and transitions may represent areas for value enhancement in kidney cancer and urology care.
Keywords: frailty, functional health status, kidney cancer, nephrectomy, outcomes, cost
Introduction
Kidney cancer is the 7th most common solid organ malignancy in the US with 63,920 new cases per year.1 With many patients undergoing surgery, nephrectomy has seen the 2nd highest growth among non-orthopedic operations over the past decade.2 Simultaneously, the operative approach has become more advanced with both nephron-sparing and minimally invasive surgery.3
Historically, surgery accounts for a disproportionate percentage of healthcare cost. Although 28% of hospital admissions are for elective operations, they account for nearly 50% of hospital-based expenditures.2 Previous investigations have also established substantial variation in total episodic cost of surgery, suggesting that surgical care may be a ripe target for Accountable Care Organizations or Bundled Payments.4 In these risk-sharing, alternative payment models, it becomes incumbent on the healthcare providers to improve outcomes and save cost. In cancer and surgery, patient function and frailty have been identified as significant predictors of perioperative morbidity and mortality.5-8 Though adverse events presumably add to cost, the total impact of functional disability on value—the quotient of surgical quality over cost—remains poorly defined.
Accordingly, it is crucial to understand the role of patient functionality in determining value in urologic surgery. In this context, we examined concurrently the impact of patient function on outcomes and resource consumption during kidney cancer surgery. In understanding the influence of patient function on quality and cost, we aim to prepare for value-based care.
Materials and Methods
Data Source and Cohort Identification
For this study, we used data from SEER-Medicare. SEER is a population-based US cancer registry that maintains information regarding incidence, treatment, and mortality. This data is linked to Medicare, which provides primary health insurance for 97% of the US population aged ≥65.9, 10
In total, we identified 32,967 subjects aged ≥65 years receiving fee-for-service care diagnosed (while alive) with primary, non-urothelial kidney cancer from 2000–2009. We excluded those without continuous enrollment in the 12 months prior to diagnosis (n=2,496) or in the 6 months following diagnosis or until death (n=313), leaving 30,158 subjects. We restricted our sample to those with complete cancer staging information (n=28,458) and further excluded those with hospice care in the year preceding diagnosis (n=46) or bilateral disease (n=86) to create a preliminary sample of 28,326 subjects.
To identify patients treated with surgery, we applied a validated, claims-based algorithm based on inpatient hospital and physician claims using ICD-9 and Current Procedural Terminology codes.11 In total, we procured an analytic cohort of 19,129 subjects treated with open radical nephrectomy, open partial nephrectomy, minimally invasive radical nephrectomy, and minimally invasive partial nephrectomy.
Primary Measure of Patient Function
To measure patient function, we applied a set of 16 function-related indicators (FRIs) described by Chrischilles et al. to Medicare claims submitted in the 12 months preceding cancer diagnosis.12 FRIs use claims indicative of reduced functional status (e.g., mobility-assist device, falls, fractures, home oxygen, pressure ulcers) or overlying disability (e.g., dementia, depression, malnutrition, respiratory failure, sepsis). Previous assessments have demonstrated a strong correlation with performance status and short-term mortality.12, 13 For kidney cancer specifically, FRI count predicts long-term mortality independent of age and comorbidity.14 For this study, we created a 3-tier categorical variable based on indicator count (i.e., 0, 1, and ≥2).
Patient and Hospital Covariates
From SEER-Medicare, we extracted information on age, gender, marital status, race, year of treatment, and tumor stage (i.e., American Joint Committee on Cancer stages I–IV). We utilized census-tract level estimates of high school education and income to measure socioeconomic position and further identified rural/urban residential status. Comorbidity was assigned using the Klabunde modification of the Charlson Comorbidity index based on inpatient and outpatient claims submitted in the 12 months prior to cancer diagnosis.15
Using the SEER-Medicare hospital file, we classified the treating hospital in terms of ownership (i.e., non-profit versus for-profit versus governmental), academic affiliation, and National Cancer Institute cancer center status. We also ascertained the total number of patient beds and categorized nursing volume based on the number of nursing full-time equivalents per patient bed total. Finally, we calculated the number of kidney cancer surgeries performed by each hospital in each year of study and created a 3-tier categorical variable for hospital volume.
Outcome Measures
For each subject, we assessed outcomes related to morbidity and mortality, resource use, and cost. For the first category, we drew from the Complication Screening Program and Agency for Healthcare Research and Quality Patient Safety Indicators and identified specific ICD-9 codes indicative of potential complications during the index hospitalization or within 30 days from surgery as described previously.16-19 From these codes, we created binary measures for surgical (i.e., accidental puncture or laceration, gastrointestinal complications, genitourinary complications, postoperative hemorrhage, venous thromboembolism, wound complications, and miscellaneous complications) and medical complications (i.e., acute renal failure, cardiac complications, neurologic events, postoperative infection, pulmonary failure, and sepsis). Geriatric events were also identified based on ICD-9 codes indicative of dehydration, delirium, falls/fractures, failure to thrive, and pressure ulcers.20 Next, we defined operative mortality as death during the above-specified time interval. From these above measures, we examined failure to rescue—the case fatality rate among those with a complication.19
Next, to assess resource use, we examined ICU use, LOS, post-acute rehabilitation, ER visits, and rehospitalizations. Based on Medicare billing codes, we defined ICU use as any admission to the intensive, intermediate, or coronary care unit during the index hospitalization or anytime within 30 days of surgery.21 We determined LOS by calculating the interval from hospital admission to discharge inclusive of transfers to acute care facilities. Because LOS varies with the surgical approach, we created an indicator variable for the top decile of hospitalizations according to procedure. We defined post-acute rehabilitation as any claim within 30 days of discharge to a skilled nursing facility or inpatient rehabilitation (i.e., Diagnosis-Related Group 462 before 2008, 945/946 thereafter). ER visits and rehospitalizations were captured by identifying subsequent Medicare claims for ER and inpatient care within 30 days of discharge, respectively.22
Finally, we calculated cost by aggregating Medicare healthcare expenditures from inpatient, outpatient, and physician claims submitted for the index hospitalization or within 30 days from surgery. We treated each subject as his or her own control by subtracting the monthly average of Medicare expenditures reported in the 12 months preceding diagnosis. All costs were adjusted to 2014 dollars using the Annual Report of the Boards of Trustees of the Federal Hospital Insurance and Federal Supplementary Medical Insurance Trust Funds.
Statistical Analysis
First, we compared patient and hospital covariates according to FRI count using chi-square testing. Then, given the hierarchical nature of our data, we built multivariable, mixed-effects models to evaluate the relationship between patient function and our outcomes. These models include the patient (i.e., age, comorbidity, race, gender, marital status, socioeconomic status, surgery type, cancer stage) and hospital (i.e., bed size, nursing volume, hospital case volume, ownership control, cancer center status, academic affiliation) covariates as fixed effects along with a hospital-level random intercept. For our binary outcome measures, we converted the likelihood estimates to risk-adjusted predicted probabilities and obtained 95% CIs using bootstrapping with replacement for 1,000 replications. To evaluate cost, we log transformed our aggregated expenditures for the mixed-effects models and retransformed our predicted log expenditures to obtain the total cost of care according to FRI count. We further determined the marginal cost between patient function categories and obtained 95% CIs for each estimate using bootstrapping with replacement for 1,000 replications.
To assess the robustness of our findings, we performed several sensitivity analyses. As comorbidity and function may be interrelated, we tested models inclusive of an interaction term between Charlson score and FRI count. We further repeated our primary analysis within comorbidity subgroups (i.e., Charlson score 0, 1, and ≥2). Finally, as outcomes and cost can vary by procedure, we refitted separate models for patients undergoing open, nephron-sparing, and minimally invasive surgery.
All statistical testing was 2-sided, completed using computerized software (STATA version 14.1, College Station, TX), and carried out at the 5% significance level. This study was approved by the Institutional Review Board at the UCLA.
Results
Of 19,129 subjects, we identified 5,509 (28.8%) and 3,127 (16.3%) patients with 1 and ≥2 FRIs, respectively, with the most common being history of fall-related injury, malnutrition, depression, pneumonia, syncope, and mobility-assist device (Supplemental Table 1). A higher FRI count was more common among patients who were older, female, unmarried, with lower socioeconomic standing or had greater comorbidity burden (p<0.001). Cancer stage and surgery type also varied with patient function (p≤0.001). We identified no statistically significant relationship between FRI count and our hospital covariates (Tables 1-2).
Table 1:
Patient characteristics according to function-related indicator count^
| Count 0 (n=10,493) |
Count 1 (n=5,509) |
Count ≥2 (n=3,127) |
P-value | |
|---|---|---|---|---|
| Age (years) | ||||
| 65–69 | 2,485 (23.7) | 1,146 (20.8) | 628 (20.1) | <0.001 |
| 70–74 | 3,340 (31.8) | 1,628 (29.6) | 804 (25.7) | |
| 75–79 | 2,611 (24.9) | 1,480 (26.9) | 853 (27.3) | |
| 80–84 | 1,501 (14.3) | 890 (16.2) | 561 (17.9) | |
| 85+ | 556 (5.3) | 365 (6.6) | 281 (9.0) | |
| Female | 4,012 (38.2) | 2,401 (43.6) | 1,585 (50.7) | <0.001 |
| Race/Ethnicity | ||||
| White | 8,618 (82.1) | 4,554 (82.7) | 2,528 (80.8) | 0.091 |
| Black | 844 (8.0) | 430 (7.8) | 290 (9.3) | |
| Hispanic/Latino | 632 (6.0) | 319 (5.8) | 209 (6.7) | |
| Asian | 311 (3.0) | 159 (2.9) | 71 (2.3) | |
| Other | 88 (0.8) | 47 (0.9) | 29 (0.9) | |
| Married | 6,848 (65.3) | 3,334 (60.5) | 1,671 (53.4) | <0.001 |
| Rural Status | 1,234 (11.8) | 599 (10.9) | 359 (11.5) | 0.246 |
| Income* | ||||
| Bottom tercile | 3,162 (30.2) | 1,701 (30.9) | 1,092 (35.0) | <0.001 |
| Middle tercile | 3,516 (33.5) | 1,791 (32.5) | 1,003 (32.1) | |
| Top tercile | 3,805 (36.3) | 2,013 (36.6) | 1,029 (32.9) | |
| Education* | ||||
| Bottom tercile | 3,295 (31.4) | 1,746 (31.7) | 1,093 (35.0) | 0.002 |
| Middle tercile | 3,462 (33.0) | 1,842 (33.5) | 1,015 (32.5) | |
| Top tercile | 3,728 (35.6) | 1,917 (34.8) | 1,016 (32.5) | |
| Charlson Comorbidity Score | ||||
| 0 | 6,600 (62.9) | 2,836 (51.5) | 965 (30.9) | <0.001 |
| 1 | 2,594 (24.7) | 1,510 (27.4) | 863 (27.6) | |
| ≥2 | 1,299 (12.4) | 1,163 (21.1) | 1,299 (41.5) | |
| Tumor Stage† | ||||
| Stage I | 6,412 (61.1) | 3,478 (63.1) | 2,047 (65.5) | <0.001 |
| Stage II | 1,062 (10.1) | 505 (9.2) | 258 (8.3) | |
| Stage III | 2,208 (21.0) | 1,090 (19.8) | 637 (20.4) | |
| Stage IV | 811 (7.7) | 436 (7.9) | 185 (5.9) | |
| Nephrectomy Type | ||||
| Open Radical | 3,522 (33.6) | 1,880 (34.1) | 1,165 (37.3) | 0.001 |
| Laparoscopic Radical | 5,078 (48.4) | 2,566 (46.6) | 1,403 (44.9) | |
| Open Partial | 741 (7.1) | 445 (8.1) | 229 (7.3) | |
| Laparoscopic Partial | 1,152 (11.0) | 618 (11.2) | 330 (10.6) | |
| Year of Treatment | ||||
| 2000 | 812 (7.7) | 403 (7.8) | 240 (7.7) | 0.745 |
| 2001 | 955 (9.1) | 476 (8.6) | 247 (7.9) | |
| 2002 | 963 (9.2) | 488 (8.9) | 307 (9.8) | |
| 2003 | 1,063 (10.1) | 535 (9.7) | 318 (10.2) | |
| 2004 | 1,070 (10.2) | 623 (11.3) | 329 (10.5) | |
| 2005 | 1,141 (10.9) | 588 (10.7) | 346 (11.1) | |
| 2006 | 1,106 (10.5) | 591 (10.7) | 333 (10.7) | |
| 2007 | 1,129 (10.8) | 596 (10.8) | 320 (10.2) | |
| 2008 | 1,108 (10.6) | 579 (10.5) | 322 (10.3) | |
| 2009 | 1,146 (10.9) | 603 (11.0) | 365 (11.7) |
Column percentages may not add up to 100% due to rounding.
Income and education data missing for 17 and 15 patients, respectively.
Based on the American Joint Committee on Cancer Staging Manual, 10th edition.
Table 2:
Hospital characteristics according to function-related indicator count^
| Count 0 (n=10,493) |
Count 1 (n=5,509) |
Count ≥2 (n=3,127) |
P-value | |
|---|---|---|---|---|
| Bed Size | ||||
| Small | 4,715 (44.9) | 2,505 (45.5) | 1,371 (43.8) | 0.167 |
| Medium | 3,274 (31.2) | 1,642 (29.8) | 959 (30.7) | |
| Large | 2,504 (23.9) | 1,362 (24.7) | 797 (25.5) | |
| Nursing Volume | ||||
| Lowest Tertile | 3,525 (33.7) | 1,811 (33.0) | 1,000 (32.4) | 0.446 |
| Middle Tertile | 3,440 (32.9) | 1,823 (33.3) | 1,067 (34.6) | |
| Highest Tertile | 3,485 (33.4) | 1,847 (33.7) | 1,018 (33.0) | |
| Hospital Volume | ||||
| 1-4 per year | 3,537 (33.8) | 1,866 (34.0) | 1,029 (33.4) | 0.970 |
| 5-10 per year | 3,638 (34.8) | 1,899 (34.6) | 1,089 (35.3) | |
| >10 per year | 3,278 (31.4) | 1,717 (31.3) | 967 (31.4) | |
| Cancer Center | 1,338 (12.8) | 690 (12.6) | 382 (12.4) | 0.807 |
| Academic Institution | 3,556 (34.0) | 1,885 (34.4) | 1,090 (35.3) | 0.406 |
| Hospital Type | ||||
| Non-profit | 7,997 (76.6) | 4,228 (77.3) | 2,332 (75.7) | 0.233 |
| For-profit | 937 (9.0) | 510 (9.3) | 305 (9.9) | |
| Government | 1,504 (14.4) | 734 (13.4) | 442 (14.4) |
Hospital characteristics missing for ≤140 subjects, or 0.7% of the analytic sample.
Figures 1 illustrates the relationship between patient function and our morbidity and mortality outcomes. Based on our multivariable, mixed-effects models, surgical complications did not differ according to FRI count. In contrast, patients with 1 FRI had greater likelihoods of medical (OR 1.12, 95% CI 1.02–1.22) and geriatric (OR 1.29, 95% CI 1.14–1.47) events compared with those without any baseline dysfunction. Moreover, patients with at least 2 FRIs exhibited increased likelihoods for medical (OR 1.22, 95% 1.10–1.36) and geriatric (OR 1.55, 95% CI 1.33–1.81) events as well as more failure to rescue (OR 1.41, 95% CI 1.06–1.86) and operative mortality (OR 1.43, 95% CI 1.10–1.86) compared with those with a count of 0. As depicted in Figure 2, patients with reduced function consumed more resources during the perioperative period. The relative increase varied with the healthcare service, ranging from a 10.4% increase in ICU care to a 76.8% increase in post-acute rehabilitation for patients with FRI count ≥2 versus 0. No significant interactions between our measures for comorbidity and patient function were observed.
Figure 1:
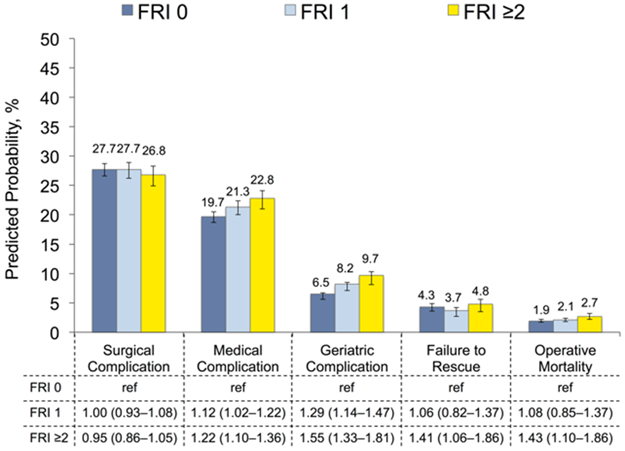
Predicted probability of morbidity and mortality after kidney cancer surgery according to patient function as measured using function-related indicators. Estimates are derived from multivariable, mixed-effects models, adjusted for patient and hospital characteristics. 95% confidence intervals obtained using bootstrapping with replacement for 1000 replications.
Abbreviation: FRI, function-related indicator.
Figure 2:
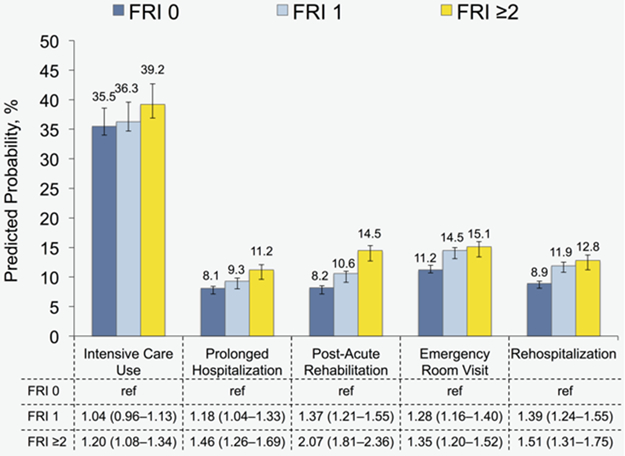
Predicted probability of resource use after kidney cancer surgery according to patient function as measured using function-related indicators. Estimates are derived from multivariable, mixed-effects models, adjusted for patient and hospital characteristics. 95% confidence intervals obtained using bootstrapping with replacement for 1000 replications. Abbreviation: FRI, function-related indicator.
In terms of cost, the predicted expenditures varied significantly with patient function. For patients with no baseline deficit, 30-day expenditures stood at $23,285 (95% CI $22,679–23,957). Patients with 1 and ≥2 FRIs exceeded that figure by $1,335 (95% CI $734–2,100) and $2,120 (95% CI $1,226–3,179), representing a 5.7% (95% CI 2.5–8.7%) and 9.1% (95% CI 4.3–13.0%) increase, respectively.
Regardless of surgical approach or comorbidity burden, patients with ≥2 versus 0 FRIs experienced more geriatric events and generally consumed more resources than their healthier counterparts. Across these subgroups, healthcare expenditures were also consistently higher for patients with baseline dysfunction. Results from these sensitivity analyses are reported in Supplemental Table 2-4.
Discussion
Recently, emphasis in healthcare has shifted from simply improving outcomes to improving value, which is defined as treatment quality per cost. Accordingly, healthcare services that are either costly or yield poor outcomes are lower in value and present opportunities for value enhancement. For older patients undergoing surgery for kidney cancer, we found that patients with reduced function, as evidenced by higher FRI count, experienced a modest elevation in certain complications and a more demonstrable increase in resource use and cost.
Complications following kidney cancer surgery have been previously shown to occur as a function of age and comorbidity.23 In this study, we found that patient function also contributes significantly to postoperative morbidity, consistent with institutional and registry studies surveying a variety of surgical procedures.5-8 However, this relationship appears specific to medical and geriatric adverse events and not for surgical complications that are often more technical in nature. Collectively, this suggests that while surgery is often feasible, recovery for patients with baseline dysfunction may be hampered once out of the operating room.
Likely a reflection of both poorer baseline health and added postoperative morbidity, patients with reduced baseline function consumed significantly more resources than their healthier counterparts. While the relationship between patient function and post-acute rehabilitation has been established,8 our findings highlight the greater use of healthcare services—both in the hospital and following discharge—that carries significant meaning when considering the total cost of care. Intensive, intermediate, and coronary care unit utilization appears to be high across the board but particularly for patients with reduced function. Similar relationships hold true for rehospitalization and post-acute care, two major cost centers in surgery that account for significant variability in total episodic cost.4, 24, 25 When considering these implications on both postoperative morbidity and resource use, kidney cancer surgery in patients with evidence of functional decline stands as an area in need of value enhancement in the current healthcare environment.
These results should be considered in the context of several limitations. First, our analysis depends on a claims-based measure for patient function. As detailed above, the approach described by Chrischilles et al. has a strong relationship with 1-year mortality and incorporates claims correlated with patient-reported performance status and long-term survival.12, 13 Furthermore, the inclusion of claims relating to mobility-assist devices, oxygen, and dementia offers some face validity. Second, given the observational nature of the study, our findings remain subject to potential bias. In particular, the relationship between patient function and outcomes could reflect residual confounding related to comorbidity. However, previous empiric work has shown comorbidity and functionality to be independent components of health,14, 26 and our findings remain largely consistent across comorbidity subgroups. Our findings may also be subject to bias related to omitted variables, such as surgeon volume and other surgeon characteristics. Third, the use of administrative claims to identify complications depends on coding accuracy. To the extent possible, we utilized either validated measures or diagnoses codes used previously in population-based assessments.16-18,20 Even so, misclassification can occur, particularly with preexisting conditions, though these conditions likely carry similar ramifications on resource use. Fourth, because we performed a claims-based analysis, we are unable to examine more granular, patient-reported assessments of function. Fifth, our findings focused on Medicare beneficiaries and may not be generalizable to younger patients.
These limitations notwithstanding, our findings have important implications for urologic surgery. In 2015, the Medicare Access & CHIP Reauthorization Act was signed into law, accelerating the move toward value-based reimbursement.27 Soon, urologists will engage in either next-generation, pay-for-performance system that tracks quality and resource use, or participate in alternative, risk-bearing payment models. With the latter, several care processes could generate value as they relate to patient function. Among them, “prehabilitation” interventions designed to improve patient health and fitness before treatment could be selectively applied.28 Emerging team-based care models that deploy medicine physicians and rehabilitation therapists may offer benefit given the pattern of morbidity and resource use.29 Finally, expectant management could be pursued more readily for patients in poor functional health. In the case of early-stage kidney cancer, patients—including those with baseline disability—have often received surgical treatment despite data supporting an acceptable risk profile with active surveillance.14, 30 As urologist engage in the complexities of Accountable Care Organization and Bundled Payments, the consideration and implementation of care processes geared toward patient function may represent an important opportunity to elevate the value of kidney cancer surgery and urology care more broadly.
Conclusion
With respect to patient function, surgery for kidney cancer can be performed safely, at least from a technical standpoint. However, patients with baseline dysfunction face a more onerous medical recovery at higher cost than those in better functional health. In the setting of risk-sharing payment models, the early identification of at-risk patients coupled with select interventions may represent a potential path to value creation in urology care.
Supplementary Material
Acknowledgments
This work was supported by grants from the American Cancer Society (126217-PF-14-028-01-CPHPS to HT) and the National Institutes of Health Loan Repayment Program (HT and KC). The funding sources had no role in the design, conduct, analysis, or decision to publish the manuscript. The authors have no other financial disclosures or conflicts of interest to report.
Key of Definitions for Abbreviations
- SEER
Surveillance, Epidemiology, and End Results
- OR
Odds Ratio
- CI
Confidence Interval
- ICD-9
International Classification of Diseases, 9th revision, Clinical Modification
- ICU
Intensive Care Unit
- FRI
Functional-related Indicator
- LOS
Length of Stay
- ER
Emergency Room
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: a cancer journal for clinicians. 2016;66(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 2.Fingar KR, Stocks C, Weiss AJ, Steiner CA. Most frequent operating room procedures performed in U.S. hospitals, 2003-2012. HCUP Statistical Brief #186 Agency for Healthcare Research and Quality, Rockville, MD: December 2014. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb186-Operating-Room-Procedures-United-States-2012.pdf [last accessed September 18, 2016] [PubMed] [Google Scholar]
- 3.Smaldone MC, Kutikov A, Egleston B, et al. Assessing performance trends in laparoscopic nephrectomy and nephron-sparing surgery for localized renal tumors. Urology. 2012;80: 286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller DC, Gust C, Dimick JB, Birkmeyer N, Skinner J, Birkmeyer JD. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health aff. 2011;30: 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Audisio RA, Pope D, Ramesh HS, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hemat. 2008;65: 156–163. [DOI] [PubMed] [Google Scholar]
- 6.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210: 901–908. [DOI] [PubMed] [Google Scholar]
- 7.Suskind AM, Walter LC, Jin C, et al. Impact of frailty on complications in patients undergoing common urological procedures: a study from the American College of Surgeons National Surgical Quality Improvement database. BJU international. 2016;117: 836–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TN, Eiseman B, Wallace JI, et al. Redefining geriatric preoperative assessment using frailty, disability and co-morbidity. Ann Surg. 2009;250: 449–455. [DOI] [PubMed] [Google Scholar]
- 9.Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40: IV-3-18. [DOI] [PubMed] [Google Scholar]
- 10.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8: 1117–1121. [PubMed] [Google Scholar]
- 11.Miller DC, Saigal CS, Warren JL, et al. External validation of a claims-based algorithm for classifying kidney-cancer surgeries. BMC Health Serv Res. 2009;9: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chrischilles E, Schneider K, Wilwert J, et al. Beyond comorbidity: expanding the definition and measurement of complexity among older adults using administrative claims data. Med Care. 2014;52 Suppl 3: S75–84. [DOI] [PubMed] [Google Scholar]
- 13.Davidoff AJ, Zuckerman IH, Pandya N, et al. A novel approach to improve health status measurement in observational claims-based studies of cancer treatment and outcomes. J Geriatr Oncol. 2013;4: 157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan HJ, Chamie K, Daskivich TJ, Litwin MS, Hu JC. Patient function, long-term survival, and use of surgery in patients with kidney cancer. Cancer. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45: 613–619. [DOI] [PubMed] [Google Scholar]
- 16.Iezzoni LI, Daley J, Heeren T, et al. Identifying complications of care using administrative data. Med Care. 1994;32: 700–715. [DOI] [PubMed] [Google Scholar]
- 17.AHRQ Quality Indicators – Guide to the Patient Safety Indicators. Agency for Healthcare Research and Quality, Rockville, MD: Version 5.0, March 2015. http://www.qualityindicators.ahrq.gov/Modules/PSI_TechSpec.aspx [accessed June 10, 2016] [Google Scholar]
- 18.Lawthers AG, McCarthy EP, Davis RB, Peterson LE, Palmer RH, Iezzoni LI. Identification of in-hospital complications from claims data. Is it valid? Med Care. 2000;38: 785–795. [DOI] [PubMed] [Google Scholar]
- 19.Tan HJ, Wolf JS Jr., Ye Z, Wei JT, Miller DC. Complications and failure to rescue after laparoscopic versus open radical nephrectomy. J Urol. 2011;186: 1254–1260. [DOI] [PubMed] [Google Scholar]
- 20.Tan HJ, Saliba D, Kwan L, Moore AA, Litwin MS. Burden of Geriatric Events Among Older Adults Undergoing Major Cancer Surgery. J Clin Oncol. 2016;34: 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu W, Ash AS, Levinsky NG, Moskowitz MA. Intensive care unit use and mortality in the elderly. J Gen Intern Med. 2000;15: 97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360: 1418–1428. [DOI] [PubMed] [Google Scholar]
- 23.Tomaszewski JJ, Uzzo RG, Kutikov A, et al. Assessing the burden of complications after surgery for clinically localized kidney cancer by age and comorbidity status. Urology. 2014;83: 843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sacks GD, Lawson EH, Dawes AJ, et al. Variation in Hospital Use of Postacute Care After Surgery and the Association With Care Quality. Med Care. 2016;54: 172–179. [DOI] [PubMed] [Google Scholar]
- 25.Stitzenberg KB, Chang Y, Smith AB, Nielsen ME. Exploring the burden of inpatient readmissions after major cancer surgery. J Clin Oncol. 2015;33: 455–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93: 663–669. [DOI] [PubMed] [Google Scholar]
- 27.Medicare program. Merit-based incentive payment system and alternative payment model incentive under the physician fee schedule, and criteria for physician-focused payment models Proposed Rule. Federal Regist; 2016. May 9; 81:28161–28586. [PubMed] [Google Scholar]
- 28.Mayo NE, Feldman L, Scott S, et al. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150: 505–514. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis. Washington, DC: The National Academies Press, 2013. [PubMed] [Google Scholar]
- 30.Smaldone MC, Kutikov A, Egleston BL, et al. Small renal masses progressing to metastases under active surveillance: a systematic review and pooled analysis. Cancer. 2012;118: 997–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


