Abstract
Background/Aims
A novel medical device based on hyaluronic acid, chondroitin sulfate plus aluminum hydroxide [GERDOFF® (SOFAR S.p.A., Trezzano Rosa, Italy), melt-in-mouth tablets] showed efficacy in reducing gastroesophageal reflux (GER)-related symptoms. This exploratory, open-label study aimed to evaluate symptomatic effects of a 14-day treatment with GERDOFF® in gastroesophageal reflux disease (GERD) patients.
Materials and Methods
GERD Impact Scale (GIS) questionnaire was filled at baseline visit and after 7 and 14 days of treatment; patients’ global satisfaction was evaluated at the final visit. Primary endpoint was the reduction of heartburn episodes per week; secondary endpoints were GERD-related symptoms, patients’ satisfaction, and safety.
Results
A total of 40 patients were included, of which, 22 were on stable therapy with proton pump inhibitor (PPI). Compared with baseline, the days with heartburn episodes and the GIS score progressively decreased during the first (p<0.0001) and the second weeks of treatment (p<0.0001). Heartburn episodes per week (p<0.0001) and the GIS score (p<0.0001) decreased in the first and the last 7 days of the 14-day treatment and did not differ between patients on and off PPI. The treatment was safe and well-tolerated, and it was rated as very good (46.2%) or good (43.6%) on the satisfaction questionnaire.
Conclusion
GERDOFF® could effectively treat GER symptoms in patients not responding to PPI or alginate-based formulation. ISRCTN_15143752.
Keywords: Hyaluronic acid, chondroitin sulfate, aluminum hydroxide, Gastresophageal reflux disease, medical device, heartburn, pump proton inhibitor
INTRODUCTION
Treatment with proton pump inhibitors (PPIs) is pivotal for gastroesophageal reflux disease (GERD). However, despite a reported healing rate of gastroesophageal reflux (GER) esophagitis in more than 80% of patients (1), the symptomatic response to PPI is less satisfactory. About 40% of patients with nonerosive reflux disease (NERD) do not benefit from PPI (2, 3) and, even during PPI treatment, the full symptomatic response is achieved only after four weeks of continuous therapy (4). Furthermore, acid breakthrough symptoms are reported by 30%–60% of PPI-treated patients in primary care and community-based studies (5). Besides acidic reflux, weakly acidic reflux, bile, pepsin, reflux extending up to the proximal part of esophagus, altered esophageal mucosal barrier, and hypersensitivity to reflux constituents are associated with GER symptoms (6, 7). Accordingly, the Rome IV diagnostic criteria for patients without esophagitis and complaining of heartburn with an unsatisfactory symptomatic response to PPI include NERD with abnormal esophageal acidic and nonacidic reflux exposure, “hypersensitive” esophagus, and functional heartburn (8).
Antacids, antireflux, and mucosal protective agents target other mechanisms of GER symptoms and ameliorate clinical benefit. Two controlled trials on efficacy of alginate-antacid complex versus placebo as add-on therapy to PPI for a week-long treatment of GER symptoms reached opposite conclusions (9–10). In clinical practice, we observe many patients who do not benefit from this compound, even as an add-on therapy to PPI.
Novel approaches based on hyaluronic acid and chondroitin sulfate (HYCHS) have been explored to protect the mucosal tissue against aggressive components of refluxate. Hyaluronic acid is involved in epithelial cell turnover, reepithelization, and mucosal hydration in ulcer healing (11, 12); chondroitin sulfate is a glycosaminoglycan secreted by parietal cells that inhibits pepsin-induced damages of gastroduodenal mucosa (13). HYCHS as add-on therapy to PPI improved GER symptoms in NERD patients (14). A trial reported that an oral solution of HYCHS achieved a more favorable response than placebo in symptomatic GER patients unresponsive to PPI or H2 antagonists, however, with the continuous use of antacids at the same dosage (15).
We hypothesized that a device made of HYCHS plus antacid may achieve a more favorable clinical response than HYCHS alone to treat GER symptoms. A recent open-label uncontrolled study with HYCHS plus aluminum hydroxide (HYCHSA) showed a favorable effect in reducing GERD-related clinical symptoms (16). In this study, an almost identical compound of HYCHSA was formulated as a melt-in-mouth tablet (GERDOFF® SOFAR S.p.A., Trezzano Rosa, Italy) to stimulate salivation and deglutition, thus delivering with each swallow the product together with bicarbonate-rich saliva and offering increased buffering effect, protection, and lubrication of esophageal mucosa.
To evaluate how to plan a controlled trial with novel HYCHSA compound, this pilot, open-label study was designed to observe symptomatic effects of GERDOFF® in GERD patients in real life, that is, on and off PPI therapy and after inadequate response to alginates.
MATERIALS AND METHODS
This postmarketing, exploratory, pilot, open-label study was conducted in two Italian sites to investigate the effects of HYCHSA on GERD-associated symptoms in patients with poor response to alginate, having partial or no benefit of PPI.
We included outpatients of both sexes, aged between >18 years, who (i) complained of typical GERD symptoms interfering with normal activities or night-time sleep at least twice a week with heartburn onset at least three months before the study initiation; (ii) were submitted in the last three months to EGDS excluding Los Angeles types 3 and 4 esophagitis and any other esophago-gastroduodenal lesions; (iii) had at least partial symptomatic benefit of PPI treatment for at least eight weeks during six months before the study; (iv) referred at least one episode per day, for at least four days, even nonconsecutive, of heartburn during alginate q.i.d. when either off or on stable dose of PPI in the two weeks before the study; (v) were able to understand and comply with the study procedures; and (vi) signed the informed consent form to participate in the study.
The study was approved by the local ethics committee (Azienda Ospedaliero-Universitaria Policlinico di Modena n.137.14) and was conducted according to the Declaration of Helsinki. All patients signed the informed consent to participate in the study (ISRCTN 15143752, December 19, 2017).
After signing the informed consent, patients underwent the baseline visit (V0) when medical history, with specific questions on GER symptoms, demographics, information about lifestyle and dietary habits, and medical records of vital signs were collected. During this visit, patients were instructed to properly fill a validated Italian translated version of the GERD Impact Scale (GIS) questionnaire (17, 18) and a daily diary. Patients were asked to fill in the GIS questionnaire for symptoms at baseline visit (V0), after seven days of treatment (T7), and at the final visit (V1) and return it together with the daily diary at the final visit (V1) after 14 days of treatment. Patients’ global satisfaction, using a 5-point ordinal semiquantitative scale (Likert score: 0=Bad/Absent, 1=Poor, 2=Discrete, 3=Good, 4=Very Good), was assessed at the final visit.
Patients must take four melt-in-mouth tablets containing HYCHSA (1100 mg, GERDOFF® SOFAR S.p.A., Trezzano Rosa, Italy) after meals and before bedtime for 14 days, up to 6 tablets a day in case of GER symptom occurrence. Patients could continue concomitant treatment with PPI only if dosage, posology, and the product in use were kept constant during the two weeks before enrollment and throughout the study period. Alginate, prokinetics, H2 antagonists, or any other product indicated for GERD treatment were not allowed. Any treatment taken during the study was reported in the daily diary.
The primary outcome was the change in the number of heartburn episodes per week during the last 7 days of the 14-day treatment, compared with baseline. Secondary outcomes were the frequency of GER-related esophageal and extra-esophageal symptoms during the first and the second weeks of treatment, the GIS score, and the patients’ satisfaction.
Patients’ compliance was evaluated in the GIS questionnaire and in the daily diary. Patients who assumed at least 80% of the investigational device were defined as compliant to treatment. All patients who took at least one dose of treatment were included in the intention-to-treat (ITT) population and were considered in the safety analysis. Patients without major violations and who received at least 80% of the investigational device were included in the per protocol (PP) population. Patients who received GERDOFF were included in the safety analysis. Any symptom or event not directly related to GERD and changes in vital signs during the study were assessed for severity and possible relationship with the study product.
Statistical Analysis
Even if a formal calculation of sample size is not required for exploratory studies, we estimated that 33 patients would be enough to observe a decrease of at least 1 day a week of heartburn symptoms compared with baseline, with a standard deviation of 2 days, an 80% power for a two-tailed inferential test, and a probability level of 0.05 (19). After considering a drop-out rate of 15%, 40 patients were enrolled. Continuous variables were reported as mean, standard deviation, median, and range; 95% confidence intervals were provided for mean, when applicable. Changes from baseline for the primary endpoint and in the total score of GIS questionnaire were evaluated by a Student’s test for paired data. Each item of the GIS questionnaire was presented with the most appropriate descriptive statistics, and its changes from baseline were evaluated with a Wilcoxon test for paired data by excluding from the analysis subjects who denied having symptoms at baseline. All statistical analyses were performed using PSPP (psppire.exe 0.8.3-g5f5de6, Free Software Foundation), SAS software version 9.4 (SAS Institute Inc., Cary, NC, USA), and R software (Core Team 2013).
RESULTS
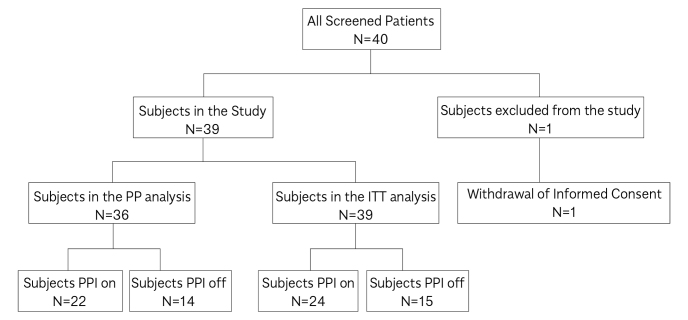
In the study, 40 patients were included: 39 in the ITT population and 36 in the PP population (Figure 1). Demographic features and GERD-related symptoms at baseline are summarized in Table 1. HYCHSA treatment lasted an average of 14±2 days, with an average consumption of 56±14.03 tablets. In total, 38 patients were compliant, having taken at least 4 tablets/day.
Figure 1.
Subjects’ disposition.
Table 1.
Demographic data and baseline clinical data—intention-to-treat (ITT) population.
| Baseline Visit | ITT population |
|---|---|
| Gender (%) | |
| Female | 74.36 |
| Male | 25.64 |
| Age (years), Mean±SD | 52±17 |
| BMI, Mean±SD | 25.40±4.84 |
| Age at diagnosis of GERDa, Mean±SD | 45±17 |
| Duration of GERD symptoms (months)b, Mean±SD | 84.44±93.02 |
| Weekly frequency of GERD symptoms, Mean±SD | 5.54±3.02 |
| Symptoms intensity, Mean±SD | 6.87 ±1.32 |
| Duration of therapy with alginates (days)c, Mean±SD | 45.05±54.14 |
GERD gastroesophageal reflux disease
Age at diagnosis was calculated as a difference between GERD diagnosis date and birth date.
Duration of GERD symptoms (months) was calculated as a difference between baseline visit date and GERD diagnosis date.
Duration of therapy with alginates (days) was calculated as the end date minus start date of alginates therapy.
From baseline to the end of treatment, days with heartburn episode decreased in both ITT [from 5.34 d/w±1.5 (M ± SD) to 1.8 d/w±2.1] and PP [from 5.6 d/w±1.3 to 1.9 d/w±2.1] population. A significant reduction of days with heartburn episode was already evident during the first week of treatment (p<0.0001 vs basal) (Figure 2). The GIS score and each symptom assessed significantly decreased during treatment (Figure 3): at the end of 2-week treatment, 92.3% of patients did not require antacid, whereas many used antacids every day-often (30.7%) or occasionally (43.6%) at baseline.
Figure 2.
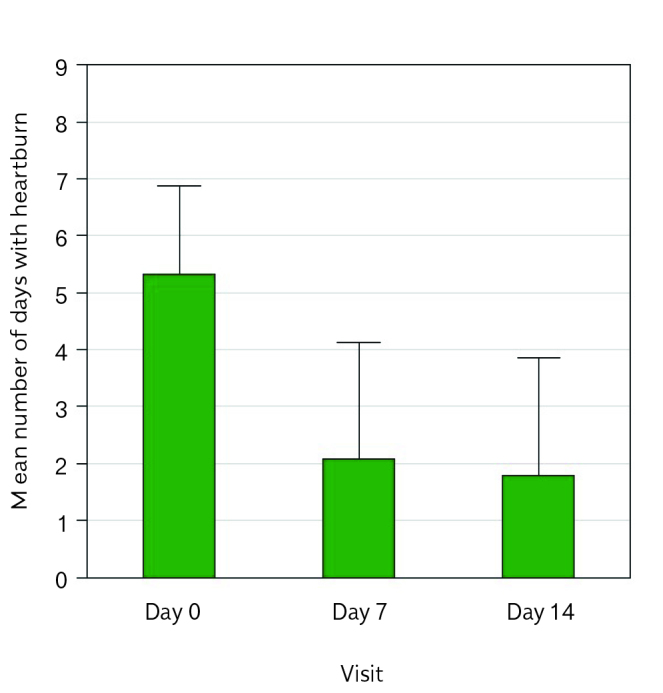
Primary endpoint at day 0, day 7, day 14—ITT population.
Figure 3.
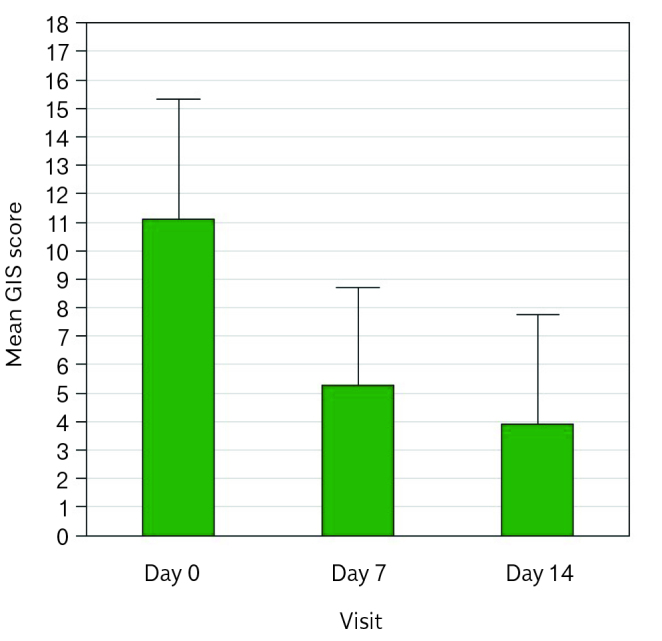
GIS score at day 0, day 7, day 14—ITT population.
During the two weeks before the enrollment, 24/39 patients in the ITT and 22 in the PP population were already using PPIs at constant dose and continued PPI intake in combination with HYCHSA during the study. Off and on PPI populations were similar, except for age (p=0.0132) and age at diagnosis (p=0.0315) (Table 2). In patients off PPI therapy, the number of days with heartburn episodes during the last week of treatment significantly decreased (p<0.0001) (Figure 4). The mean difference was 3.7 d/w±2.7 (p<0.0001; 95% CI 2.2–5.2) for the ITT population and 4.0 d/w±2.6 (p<0.0001, 95% CI 2.5–5.5) for the PP population. The number of days with heartburn episodes was also significantly reduced during the first week of treatment in both ITT (2.5 d/w±2.0) and PP (2.7 d/w±2.0) population.
Table 2.
Demographic data and baseline clinical data in patients off PPI and on PPI therapy.
| Baseline Visit (V0) | Off PPI n=15 | On PPI n=24 |
|---|---|---|
| Gender (%) | ||
| Female | 80.00 | 70.83 |
| Male | 20.00 | 29.17 |
| Age (years), Mean±SD | 44.10±14.93 | 57.77±16.55 |
| BMI, Mean±SD | 25.03±4.85 | 25.64±4.92 |
| Age at diagnosis of GERDa, Mean±SD | 37.81±14.55 | 50.37±18.43 |
| Duration of GERD symptoms (months)b, Mean±SD | 76.17±96.31 | 89.61±92.60 |
| Weekly frequency of GERD symptoms, Mean±SD | 5.40±1.92 | 5.63±3.59 |
| Symptoms intensity, Mean±SD | 6.67±1.35 | 7.00±1.32 |
| Duration of therapy with alginates (days)c, Mean±SD | 61.07±75.16 | 35.04±33.64 |
PPI: proton pump inhibitor; GERD: gastroesophageal reflux disease.
Age at diagnosis was calculated as a difference between GERD diagnosis date and birth date
Duration of GERD symptoms (months) was calculated as a difference between baseline visit date and GERD diagnosis date
Duration of therapy with alginates (days) was calculated as the end date minus start date of alginates therapy
Figure 4.
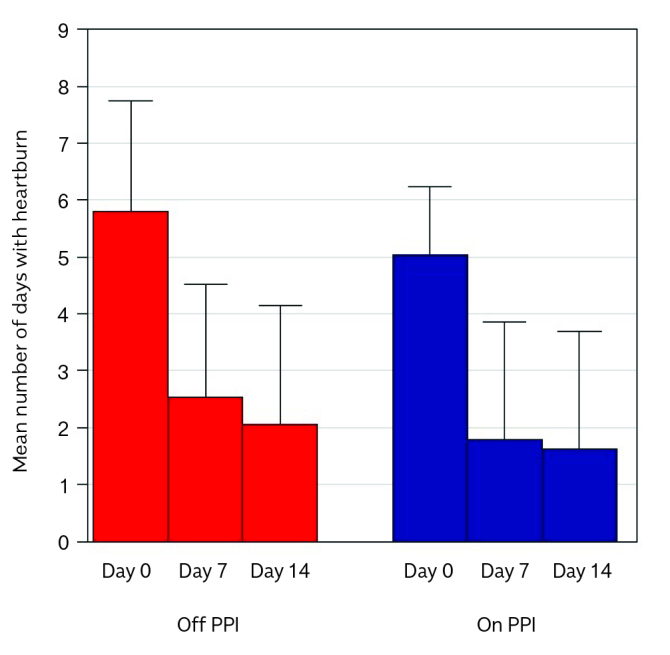
Primary endpoint in PPI off and PPI on subjects.
In PPI off patients, the number of days with heartburn episodes significantly decreased in both the ITT population from 5.8 d/w ± 1.9 (M ± SD) to 2.1 d/w ± 2.0 and in the PP population from 6.2 d/w ± 1.7 to 2.2 d/w ± 2.1. In patients on PPI therapy, the number of days with heartburn episodes decreased in the ITT from 5.0 d/w ± 1.2 (M ± SD) to 1.6 d/w ± 2.0 and in the PP population from 5.1 d/w ± 1.1 to 1.8 d/w ± 2.1. The reduction of heartburn episodes per week in the first and the last 7 days of the 14-day treatment did not differ among off and on PPI therapy (Figure 4). The mean difference was 3.4 d/w ± 2.4 (p<0.0001, 95% CI 2.4–4.4) for the ITT population and 3.4 d/w ± 2.5 (p<0.0001, 95% CI 2.3–4.5) for the PP population. The number of days with heartburn episodes was also significantly reduced during the first week of treatment in both ITT (1.8 d/w ± 2.0) and PP (2.0 d/w ± 2.0) population.
GIS score and all symptoms evaluated (except for the item “How often did you have a disturbed nocturnal sleep because of your symptoms?” in the subgroup off PPI, p=0.0547) significantly decreased after treatment and they did not differ among patients off and on PPI therapy (Figure 5). Sore throat and hoarseness were reported daily or often at V0 in 40% and 45.8% and at V1 in 6.6% and 4.2% of patients off, or on PPI therapy, respectively. Use of antacids was reported daily or often at V0 in 33.3% and 29.1% and at V1 0% and 4.2% of patients off or on PPI therapy, respectively.
Figure 5.
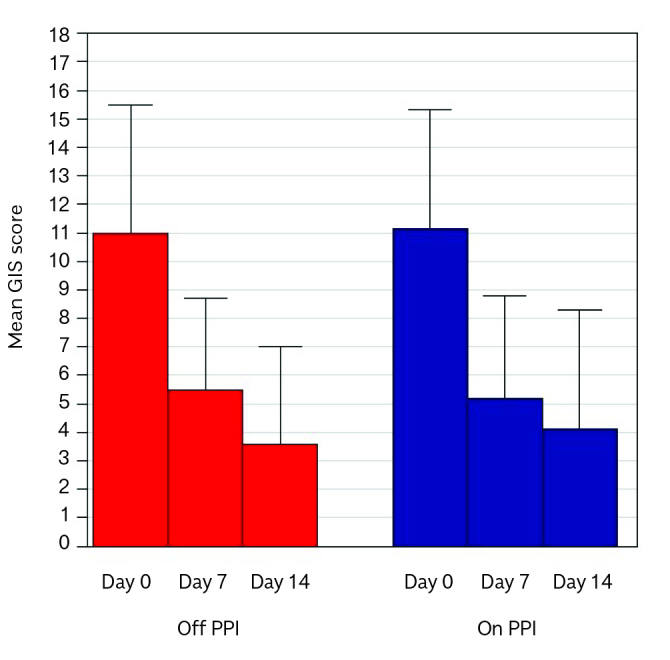
GIS score in PPI off and PPI on subjects.
In the ITT population, 35 patients (89.8%) were highly satisfied with the treatment (very good for 18 patients and good for 17).
No serious adverse events, adverse device effects or device deficiencies occurred during the treatment. In total, 13 adverse events (six cases of migraine and one case each of dysmenorrhea, shoulder pain, abdominal pain, low back pain, headache, otitis, and hypertensive peak) were reported in 11 patients; neither of the events was severe nor related to the study product. No relevant changes in vital signs occurred.
DISCUSSION
This exploratory study showed that a 14-day treatment with a melt-in-mouth formulation of HYCHSA significantly reduced the number of heartburn episodes per week in patients with NERD or Los Angeles type 1 and type 2 esophagitis. This favorable effect was observed with treatment of HYCHSA alone and as add-on therapy to PPI. Similar symptomatic response rate (60%) with HYCHS in a syrup formulation was reported in a previous clinical trial in which the use of antacid as rescue therapy did not change compared with basal period, indicating the need to reinforce the buffering action with antacid addition (15).
The lack of comparative placebo-controlled group does not allow drawing any definitive conclusion on the degree of clinical efficacy of HYCHSA; however, the improvement in days without heartburn compared with baseline in both on and off PPI groups (>60%) exceeds the response rate in GERD previously reported (15% in Savarino et al. [14], 20% in Sigterman et al. [20]). The favorable effect of HYCHSA as add-on therapy to PPI is like 52.6% benefit on GER symptoms reported after two-week treatment with HYCHS as add-on therapy to PPI and superior to the 32.1% reported after placebo in the same study (14).
Such favorable response to HYCHSA is also superior to H2RA response (37%) in NERD patients (20). Furthermore, the progressive GER symptom improvement can be regarded as indirect evidence that the clinical benefit is more likely due to time-related effect of HYCHSA formulation rather than to a placebo effect.
At least in Italy, alginate plus antacid-based formulations are often used for recurrent or occasional GER symptoms in patients off PPI and in those on, partially or totally, ineffective PPI treatment. However, even adding alginate to PPI does not obtain clinical benefit in 43% of GER patients (21).
In our study, the HYCHSA treatment was effective in patients having partial or no benefit with alginate plus antacid. Although we cannot conclude that HYCHSA is superior to the alginate-based product, HYCHSA may be considered as a valid alternative for GERD patients not having benefit from alginate plus antacid-based formulation. The mechanisms of action of these compounds may explain this differential effect. The alginate-based formulations form a gel raft above gastric contents, thus limiting gastric reflux into the esophagus. Compared with antacid alone, an alginate plus antacid formulation reduced significantly distal esophageal acid exposure but not the number of refluxes and proximal reflux events (22). Similarly, PPIs reduce acid reflux events without affecting the number and proximal extension of refluxes that become mainly weakly acid (23). Differently from alginate formulations and PPI that cannot prevent the contact between weakly acidic refluxes and esophageal epithelium, HYCHSA provides a mechanical barrier directly on the esophageal mucosa and prevents the contact between any potentially offensive reflux contents, either acidic or weakly acidic, and the esophageal epithelium.
On the basis of the patients’ self-assessment of symptoms, supraesophageal and esophageal GER-related symptoms such as retrosternal and epigastric pain and burning significantly decreased. Supraesophageal GER-related symptoms are mainly due to the proximal esophageal extension of weakly acidic refluxes and are poorly affected by PPI therapy (24), whereas sore throat and hoarseness decreased remarkably adding HYCHSA to PPI therapy.
The reduction of sore throat and hoarseness occurred already during short-term HYCHSA treatment because relief of supraesophageal GER-related symptoms usually requires higher doses and longer periods of PPI treatment than typical heartburn (25).
The melt-in-mouth formulation requires mastication, suckling, and grinding and stimulates salivation and deglutition; thus, after each swallow, HYCHSA enriched with saliva and saliva bicarbonate adheres to the pharyngeal mucosa, before entering the esophagus. This repetitive process protects the pharyngeal and upper esophageal mucosa and counteracts proximal and weakly acidic refluxes, the main factors underlying supraesophageal GER-related symptoms and PPI unresponsiveness (26,27).
Furthermore, we observed a relevant reduction of antacids as rescue therapy since 92.3% of patients did not require anymore them at the end of treatment. This supports the innovative formulation containing aluminum as mucosal protective agent.
The GIS questionnaire assesses GERD symptoms and how they impact daily life and the general health score by affecting sleep, drinking, and eating habits; improving GIS score, HYCHSA treatment might have a favorable impact on patients’ quality of life.
This study has several limitations due to its exploratory protocol aimed to serve as a pilot experience for future more robust confirmatory studies. Furthermore, a placebo-controlled group is lacking; patients acted as their own controls, thus minimizing the interpatient variability in the evaluation and perception of symptoms. However, although the benefit reported by patients with HYCHSA exceeds any previously reported placebo response for GERD, we cannot exclude the additional benefit reported by patients simply for being included in a clinical trial. Patients were recruited because of typical GER symptoms and only the symptomatic response to therapy was assessed. The lack of esophageal pH-impedance investigation before and after treatment has precluded to differentiate NERD patients from those with hypersensitive esophagus or functional heartburn and verified the presence of any residual reflux after therapy.
A future study should consider assessing HYCHSA efficacy in patients with homogeneous endoscopic findings and heartburn origin properly identified with pH-impedance investigations. Patients on stable therapy with PPI were included for two reasons. First, PPI interruption might have had detrimental effects on symptoms and negatively affected the interpretation of HYCHSA outcomes. Second, the use of additional products is a common practice in symptomatic GERD patients and the protocol enabled to assess the effect of adding HYCHSA to PPI treatment, as it occurs in real life. To avoid that variation of PPI therapy might interfere with HYCHSA treatment, each patient kept as constant type and dosage during the study. Patients off and on PPI had similar symptoms at baseline and both groups equally benefited of HYCHSA treatment.
We acknowledge that PPI therapy represents a potential confounding factor; however, the similar efficacy of HYCHSA in both patients off and on PPI therapy would suggest that the medical device is effective to improve symptoms that did not require or not respond to PPI. HYCHSA might have a complementary effect with PPI, improving therapeutic outcomes in partial PPI responders and the concomitant use of PPIs does not affect HYCHSA efficacy.
Our observations are limited to 14 days of treatment and indication for a longer duration of treatment can be derived from this study; a future trial is warranted to determine the potential effect of HYCHSA in a longer term to determine how sustained the response could be.
In conclusion, GER-related typical esophageal and atypical supraesophageal symptoms in patients not responding or partially responding to alginate-containing formulations improved significantly during the 14-day treatment with GERDOFF®. Clinical improvement was observed also with concomitant stable treatment with PPIs. Furthermore, GERDOFF® was safe and well-tolerated, as indicated by the lack of any severe adverse event and the high degree of patient satisfaction. These preliminary data offer the basis to design an appropriate randomized controlled trial to assess efficacy and safety of GERDOFF® in a more homogeneous population.
MAIN POINTS.
Hyaluronic acid and chondroitin sulfate plus aluminum hydroxide (HYCHSA) provides a mechanical barrier directly on the esophageal mucosa and prevents the contact between any potentially offensive reflux contents and the esophageal epithelium.
A 14-day treatment with a melt-in-mouth formulation of HYCHSA significantly reduced the number of heartburn episodes per week in patients with NERD or Los Angeles type 1 and type 2 esophagitis.
The HYCHSA treatment was effective in patients having partial or no benefit with alginate plus antacid.
Improving the GERD Impact Scale (GIS) score, HYCHSA treatment might have a favorable impact on patients’ quality of life.
Supplementary Data
Supplementary Table 1.
Pre-post statistical difference between any single items of GIS score in patients on and off PPI therapy ( ITT analysis set)
| on PPI subjects | off PPI subjects | |||
|---|---|---|---|---|
|
| ||||
| N* | p ** | N* | p ** | |
| Pain in your chest or behind the breastbone? | 19 | 0.0001 | 9 | 0.0313 |
| Burning sensation in your chest or behind the breastbone? | 20 | 0.0001 | 13 | 0.0020 |
| Regurgitation or acid taste in your mouth? | 20 | <0.0001 | 14 | 0.0005 |
| Pain or burning in your upper stomach? | 23 | <0.0001 | 12 | 0.0010 |
| Sore throat or hoarseness related to your heartburn or acid reflux? | 19 | <0.0001 | 9 | 0.0156 |
| How often have you had difficulty getting a good night’s sleep because of your symptoms? | 19 | 0.0015 | 11 | 0.0547 |
| How often have your symptoms prevented you from eating or drinking any of the food you like? | 19 | 0.0036 | 13 | 0.0049 |
| How frequently have your symptoms kept you from being fully productive in your job or daily activities? | 17 | 0.0002 | 7 | 0.0313 |
| How often do you take additional medication other than what the physician told you to take (such as antacid)? | 17 | <0.0001 | 11 | 0.0010 |
For each item, subjects who reported “Never” at baseline visit have been excluded from the analysis
A Wilcoxon test for paired data comparing time differences (Day 14 versus Day 0) has been performed.
Acknowledgements
We thank Elisa Sala, PhD, professional medical writer from High Research Srl, Italy, for her medical editorial assistance with our report. This assistance was funded by Sofar S.p.a.
Footnotes
Ethics Committee Approval: Ethics committee was received for this study from the Ethics Committee of Azienda Ospedaliero-Universitaria Policlinico di Modena n.137.14.
Informed Consent: Written informed consent was received for this study from the patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - V.B., I.R., M.M., A.M.; Design - V.B., I.R., M.M., A.M; Supervision - A.M; Resource - I.R. A.M.; Materials - V.B., I.R., M.M., A.M; Data Collection and/or Processing - V.B., I.R., M.M., A.M; Analysis and/or Interpretation - V.B., I.R., M.M., A.M; Literature Search - V.B., I.R., M.M., A.M.; Writing - V.B., I.R., M.M., A.M; Critical Reviews - V.B., I.R., M.M., A.M.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The study was funded by SOFAR S.p.A., Trezzano Rosa, Milan, Italy.
REFERENCES
- 1.Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short-term management of reflux esophagitis. Cochrane Database Syst Rev. 2007;2:CD003244. doi: 10.1002/14651858.CD003244.pub2. [DOI] [PubMed] [Google Scholar]
- 2.Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in non-erosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–64. doi: 10.1016/S1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 3.Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease-where next? Aliment Pharmcol Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong D, Talley NJ, Lauritsen K, et al. The role of acid suppression in patients with endoscopy-negative reflux disease: the effect of treatment with esomeprazole or omeprazole. Aliment Pharmacol Ther. 2004;20:413–21. doi: 10.1111/j.1365-2036.2004.02085.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2014;63:871–80. doi: 10.1136/gutjnl-2012-304269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsoukali E, Sifrim D. The role of weakly acidic reflux in proton pump inhibitor failure, has dust settled? J Neurogastroenterol Motil. 2010;16:258–64. doi: 10.5056/jnm.2010.16.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Knowles CH, Aziz Q. Visceral hypersensitivity in non-erosive reflux disease. Gut. 2008;57:674–83. doi: 10.1136/gut.2007.127886. [DOI] [PubMed] [Google Scholar]
- 8.Fass R, Pandolfino JE, Aziz Q, et al. Esophageal disorders. In: Drossman DA, Chang L, Chey WD, et al., editors. Rome IV Functional gastrointestinal disorders. Rome: The Rome Foundation; 2016. pp. 833–902. [DOI] [Google Scholar]
- 9.Reimer C, Lodrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther. 2016;43:899–909. doi: 10.1111/apt.13567. [DOI] [PubMed] [Google Scholar]
- 10.Coyle C, Crawford G, Wilkinson J, Thomas SJ, Bytzer P. Randomised clinical trial: addition of alginate-antacid (Gaviscon Double Action) to proton pump inhibitor therapy in patients with breakthrough symptoms. Aliment Pharmacol Ther. 2017;45:1524–33. doi: 10.1111/apt.14064. [DOI] [PubMed] [Google Scholar]
- 11.Volpi N, Schiller J, Stern P, Soltés L. Role, metabolism, chemical modification and application of hyaluronan. Curr Med Chem. 2009;16:1718–45. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 12.Gaffney J, Matou Nasri S, Grau-Olivares M, Slevin M. Therapeutic applications of hyaluronan. Mol Byosyst. 2010;6:437–43. doi: 10.1039/B910552M. [DOI] [PubMed] [Google Scholar]
- 13.Lauder RM. Chondroitin sulphate a complex molecule with potential impacts on a wide range of biological systems. Complement Ther Med. 2009;17:56–62. doi: 10.1016/j.ctim.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 14.Savarino V, Pace F, Scarpignato C Esoxx Study Group. Randomised clinical trial: mucosal protection combined with acid suppression in the treatment of non-erosive reflux disease - efficacy of Esoxx, a hyaluronic acid-chondroitin sulphate based bioadhesive formulation. Aliment Pharmacol Ther. 2017;45:631–42. doi: 10.1111/apt.13914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmieri B, Merighi A, Corbascio D, Rottigni V, Fistetto G, Esposito A. Fixed combination of hyaluronic acid and chondroitin-sulphate oral formulation in a randomized double blind, placebo-controlled study for the treatment of symptoms in patients with non-erosive gastroesophageal reflux. Eur Rev Med Pharmacol Sci. 2013;17:3272–8. [PubMed] [Google Scholar]
- 16.Iannitti T, Laurino C, Morales-Medina JC, et al. Advances in heath and disease. USA: Nova Science Publisher; 2017. A chondroitin sulfate- and hyaluronic acid-based dietary formulation improves non-erosive gastroesophageal reflux disease-related symptoms: an open-label uncontrolled study. [Google Scholar]
- 17.Jones R, Coyne K, Wiklund I. The gastro-oesophageal reflux disease impact scale: a patient management tool for primary care. Aliment Pharmacol Ther. 2007;25:1451. doi: 10.1111/j.1365-2036.2007.03343.x. [DOI] [PubMed] [Google Scholar]
- 18.Ubaldi E, Innocenti F, Cricelli I, Mazzaglia G. Valutazione del controllo della malattia da reflusso gastroesofageo in Medicina generale attraverso l’utilizzo del questionario “GERD Impact scale”. Rivista della Società italiana di Medicina Generale. 2009;1:29–34. [Google Scholar]
- 19.Jones R, Patrikios T. The effectiveness of esomeprazole 40 mg in patients with persistent symptoms of gastro-oesophageal reflux disease following treatment with a full dose proton pump inhibitor. Int J Clin Pract. 2008;62:1844–50. doi: 10.1111/j.1742-1241.2008.01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sigterman KE, van Pinxteren B, Bonis PA, Lau J, Numans ME. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Coch Dat Syst Rev. 2013;5:CD002095. doi: 10.1002/14651858.CD002095.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manabe N, Haruma K, Ito M, et al. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus. 2012;25:373–80. doi: 10.1111/j.1442-2050.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 22.De Ruigh A, Roman S, Chen J, Pandolfino JE, Kahrilas PJ. Gaviscon Double Action Liquid (antacid & alginate) is more effective than antacid in controlling postprandial esophageal acid exposure in GERD patients; a double-blind crossover study. Aliment Pharmacol Ther. 2014;40:531–7. doi: 10.1111/apt.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemmink GJ, Bredenoord AJ, Weusten BL, Monkelbaan JF, Timmer R, Smout AJ. Esophageal pH-impedance monitoring in patients with therapy-resistant reflux symptoms: ‘on’ or ‘off’ proton pump inhibitor? Am J Gastroenterol. 2008;103:2446–53. doi: 10.1111/j.1572-0241.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 24.Herregods TVK, Pauwels A, Jafari J, et al. Determinants of reflux-induced chronic cough. Gut. 2017;66:2057–62. doi: 10.1136/gutjnl-2017-313721. [DOI] [PubMed] [Google Scholar]
- 25.Katz PO, Castell DO. Medical therapy of supra-esophageal gastroesophageal reflux disease. Am J Med. 2000;108:170S–7S. doi: 10.1016/S0002-9343(99)00359-9. [DOI] [PubMed] [Google Scholar]
- 26.Helm JF, Dodds WJ, Hogan WJ, Soergel KH, Egide MS, Wood CM. Acid neutralizing capacity of human saliva. Gastroenterology. 1982;83:69–74. doi: 10.1016/S0016-5085(82)80286-2. [DOI] [PubMed] [Google Scholar]
- 27.Leone CA, Caruso AA, Allocca V, Barra E, Leone R. Pilot study on the effects of high molecular weight sodium hyaluronate in the treatment of chronic pharyngitis. Int J Immunophatol Pharmacol. 2015;28:532–8. doi: 10.1177/0394632015586497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Pre-post statistical difference between any single items of GIS score in patients on and off PPI therapy ( ITT analysis set)
| on PPI subjects | off PPI subjects | |||
|---|---|---|---|---|
|
| ||||
| N* | p ** | N* | p ** | |
| Pain in your chest or behind the breastbone? | 19 | 0.0001 | 9 | 0.0313 |
| Burning sensation in your chest or behind the breastbone? | 20 | 0.0001 | 13 | 0.0020 |
| Regurgitation or acid taste in your mouth? | 20 | <0.0001 | 14 | 0.0005 |
| Pain or burning in your upper stomach? | 23 | <0.0001 | 12 | 0.0010 |
| Sore throat or hoarseness related to your heartburn or acid reflux? | 19 | <0.0001 | 9 | 0.0156 |
| How often have you had difficulty getting a good night’s sleep because of your symptoms? | 19 | 0.0015 | 11 | 0.0547 |
| How often have your symptoms prevented you from eating or drinking any of the food you like? | 19 | 0.0036 | 13 | 0.0049 |
| How frequently have your symptoms kept you from being fully productive in your job or daily activities? | 17 | 0.0002 | 7 | 0.0313 |
| How often do you take additional medication other than what the physician told you to take (such as antacid)? | 17 | <0.0001 | 11 | 0.0010 |
For each item, subjects who reported “Never” at baseline visit have been excluded from the analysis
A Wilcoxon test for paired data comparing time differences (Day 14 versus Day 0) has been performed.