Abstract
Background and Purpose:
The 2019 novel coronavirus outbreak and its associated disease (coronavirus disease 2019 [COVID-19]) have created a worldwide pandemic. Early data suggest higher rate of ischemic stroke in severe COVID-19 infection. We evaluated whether a relationship exists between emergent large vessel occlusion (ELVO) and the ongoing COVID-19 outbreak.
Methods:
This is a retrospective, observational case series. Data were collected from all patients who presented with ELVO to the Mount Sinai Health System Hospitals across New York City during the peak 3 weeks of hospitalization and death from COVID-19. Patients’ demographic, comorbid conditions, cardiovascular risk factors, COVID-19 disease status, and clinical presentation were extracted from the electronic medical record. Comparison was made between COVID-19 positive and negative cohorts. The incidence of ELVO stroke was compared with the pre-COVID period.
Results:
Forty-five consecutive ELVO patients presented during the observation period. Fifty-three percent of patients tested positive for COVID-19. Total patients’ mean (±SD) age was 66 (±17). Patients with COVID-19 were significantly younger than patients without COVID-19, 59±13 versus 74±17 (odds ratio [95% CI], 0.94 [0.81–0.98]; P=0.004). Seventy-five percent of patients with COVID-19 were male compared with 43% of patients without COVID-19 (odds ratio [95% CI], 3.99 [1.12–14.17]; P=0.032). Patients with COVID-19 were less likely to be White (8% versus 38% [odds ratio (95% CI), 0.15 (0.04–0.81); P=0.027]). In comparison to a similar time duration before the COVID-19 outbreak, a 2-fold increase in the total number of ELVO was observed (estimate: 0.78 [95% CI, 0.47–1.08], P≤0.0001).
Conclusions:
More than half of the ELVO stroke patients during the peak time of the New York City’s COVID-19 outbreak were COVID-19 positive, and those patients with COVID-19 were younger, more likely to be male, and less likely to be White. Our findings also suggest an increase in the incidence of ELVO stroke during the peak of the COVID-19 outbreak.
Keywords: acute stroke, coronavirus disease, hospitalization, incidence, pandemics
The novel coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), and its associated disease, coronavirus disease 2019 (COVID-19), started in December 2019 in Wuhan, China, and rapidly spread to >200 countries. On March 11, 2020, the World Health Organization declared COVID-19 disease a pandemic; and as of June 27, 2020, >9.9 million confirmed cases have been identified worldwide.
COVID-19 is caused by a novel single-stranded enveloped RNA virus called SARS-CoV-2.1 The virus invades cells by adhering to angiotensin-converting enzyme 2 receptors.2 These receptors are prevalent throughout the body, including pulmonary and intestinal epithelia, renal cells, vascular endothelium, and myocardial cells, which may explain the multi-organ dysfunction seen in severe cases of COVID-19.3
The classic COVID-19 presentation includes fever, dry cough, myalgia, and fatigue, although atypical presenting symptoms, such as anosmia or nausea and diarrhea, have been reported.4 A recent report from China demonstrated neurological manifestations in >36% of hospitalized COVID-19 patients with a higher rate among severe patients with COVID-19. The study also suggests an increased rate of stroke among hospitalized patients with severe COVID-19 infection.5 Here, we report our observations of emergent large vessel occlusion (ELVO) acute ischemic stroke across the largest health system in New York City (NYC) during the peak weeks of NYC’s COVID-19 outbreak.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
This retrospective, observational study was conducted across the Mount Sinai Health System encompassing 8 hospitals and receiving patients from all 5 boroughs of NYC. The study was conducted under the auspices of Institutional Review Board approval. The Institutional Review Board waived the need for patient consent. On March 15, 2020, NYC announced it would close public schools, on March 16 all bars and restaurants were closed (except delivery/take-out), and on March 20, all nonessential businesses were closed. The following week marks the beginning of the COVID-19 surge in NYC. We collected data on all ELVO patients presenting to our hospitals over the 3 weeks between March 21 to April 12, 2020, which correlates with the peak number of hospitalizations and deaths from COVID-19 in NYC (https://www1.nyc.gov/site/doh/covid/covid-19-data.page). ELVO diagnosis required vascular imaging confirmation of occlusion of an intracranial internal carotid artery, M1 or M2 segments of a middle cerebral artery, A1 or A2 segments of an anterior cerebral artery, intracranial vertebral artery, basilar artery, or P1 or P2 segments of a posterior cerebral artery, with concomitant acute neurological deficit.
Demographic information, preexisting cardiovascular risk factors (hypertension, diabetes mellitus, hyperlipidemia, atrial fibrillation, and congestive heart failure), initial National Institutes of Health Stroke Scale score, treatments used (alteplase and thrombectomy), and clinical outcome were obtained for every patient. Confirmed COVID-19 cases were defined as positive reverse-transcription polymerase chain reaction analysis of nasal swab specimens. The timing of the SARS-CoV-2 swab test during the hospital course was extracted for each patient. We also reviewed the lung apices on our standard code-stroke neck computed tomography angiography for the presence of abnormal findings in all ELVO patients. Findings in the transthoracic echocardiography, including reduced ejection fraction (defined as ejection fraction <40%) and presence of left ventricular thrombosis, were extracted for each patient. Laboratory findings, including white blood cell and platelet counts, prothrombin time, D-dimer, fibrinogen, blood urea nitrogen, and creatinine, were extracted. Baseline characteristics, imaging findings, and laboratory values were compared between patients with and without COVID-19 who presented to the hospital during the above-mentioned 3-week period. We repeated the statistical analysis after removing patients without COVID-19 diagnostic tests from the study population to ensure their presence did not influence the final findings.
In an exploratory analysis, we compared the prevalence and demographic data of ELVO patients during the COVID-19 outbreak with ELVO patients before COVID-19. For that analysis, we performed 2 separate reviews of our prospectively maintained ELVO database: the first was to obtain the number of ELVO patients during exactly the same 3 weeks period in 2019 (March 21 to April 12) and the second, performed to minimize sample bias, was to obtain the total number of ELVO patients during consecutive 3-week periods from March 20, 2020, backward to include the entire year of 2019. This created 21 consecutive 3-week periods before the outbreak.
The SAS 9.4 software (SAS Institute, Cary, NC) was used for statistical analysis. We used 2 tailed tests for our analysis. For categorical variables, the χ2 test was used to test for a significant difference between the COVID (+) and COVID (−) groups, and the Fisher exact test was used if cell sizes were <5. We used the Wilcoxon Mann-Whitney test for continuous variables. We performed linear regression analysis to detect trends in the number of ELVO patients during 21 consecutive 3-week periods before the COVID-19 outbreak. We then dichotomized the data into pre-COVID time and COVID time and performed Poisson regression analysis to compare the prevalence of ELVO stroke during the 21 consecutive 3-week periods before the COVID-19 pandemic with the 3-week period during the peak time of the outbreak.
Results
Forty-five consecutive patients with ELVO were identified during the 21-day period starting March 21, 2020, of the study. The mean (±SD) age of the patients was 66 (±17); 38% were women, and 38% Black. Twenty-four patients (53% [95% CI, 39–68]) tested positive for COVID-19. All the tested patients were tested for COVID-19 within the first 24 hours from the time of hospital arrival, except for one patient who was tested at day 3. He tested negative and remained asymptomatic. Of note, the COVID-19 diagnostic test was not performed in 7 patients. All of them presented during the first week of the outbreak when testing was not provided to asymptomatic patients. They all remained clinically asymptomatic during the hospital stay.
The mean (±SD) age for COVID-19 ELVO patients was significantly lower than non-COVID-19 ELVO patients: 59±13 versus 74±17 (P=0.004). More COVID-19 ELVO patients were male, 79%, as compared to non-COVID-19 ELVO patients, 43% (odds ratio [95% CI], 3.99 [1.12–14.17]; P=0.032). COVID-19 ELVO patients were also less likely to be White (8% versus 38%, odds ratio [95% CI], 0.14 [0.03–0.81]; P=0.027). The absence of cardiovascular risk factors was seen in 46%, of COVID-19 ELVO patients versus 24% of non-COVID ELVO patients (odds ratio [95% CI], 2.71 [0.75–9.79]; P=0.12). Specifically, the prevalence of atrial fibrillation was substantially lower among patients with COVID-19 (4% versus 24%, P=0.05). Similarly, patients with COVID-19 had a significantly lower prevalence of congestive heart failure in their past medical history (0 versus 19%, P=0.025). Transthoracic echocardiography was performed in 72% of the patients (62% of patients with COVID-19 and 81% of patients without COVID; Table). A total of 4 patients were found to have new-onset reduced ejection fraction; with no history of prior cardiac disease; 3 of them were patients with COVID-19. Notably, one patient was found to have left ventricular thrombus with ejection fraction of 42%. She was a 61-year-old with a history of hypertension; she was found to have COVID-19 infection. No evidence of Central Nervous System vasculopathy was seen among patients with COVID-19 on cerebral digital subtraction angiography or computed tomography angiography of head and neck.
Table.
Demographic and Baseline Characteristics of Patients With Emergent Large Vessel Occlusion Stroke
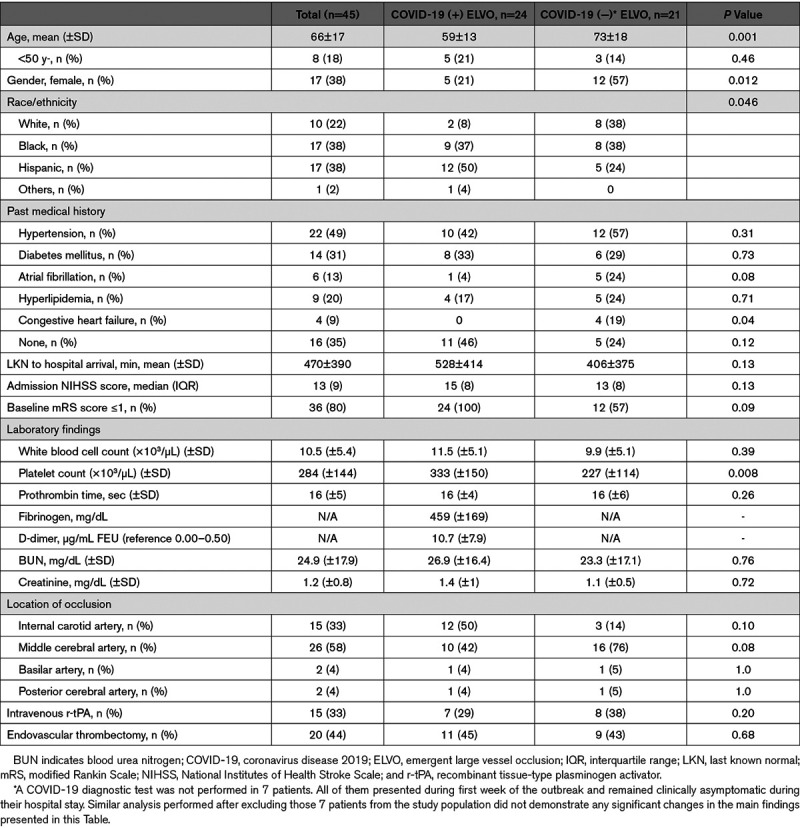
Among the COVID-19 cohort, only 58% had typical COVID-19-related symptoms on presentation. Sixty percent of previously asymptomatic COVID-19 patients developed typical COVID-19 related symptoms during hospitalization, while 4 patients remained asymptomatic beyond stroke symptoms. Cough (37%), fever (29%), and shortness of breath (29%) were the most observed symptoms for patients with COVID-19. Radiographic evidence of apical lung abnormalities was reported in 75% of patients with COVID-19 versus 19% of patients without COVID-19 (odds ratio [95% CI], 12.74 [3.06–53.18]; P=0.0005). One patient who was not tested for COVID-19 had abnormal findings in lung apices. This patient remained asymptomatic during hospital stay and was eventually discharged to home with minimal residual neurological deficits. Forty-six percent of patients with COVID-19 required mechanical ventilation during hospitalization due to respiratory failure. Two (10%) of the patients without COVID-19 required intubation and ventilator support for airway protection; one was intraprocedural (during endovascular thrombectomy); and the other one later during hospital stay.
In an exploratory analysis, we compared the prevalence and demographic data of ELVO patients during the COVID-19 outbreak to ELVO patients that presented during the comparable time the year prior (March/April 2019). The exact 3-week time span in 2019 demonstrated 23 ELVO patients with mean (±SD) age 67 (±15) years, 52% women, and 47% White. We also obtained the number of ELVO strokes during 21 consecutive 3-week periods before the outbreak, which includes a total of 441 days, extending from January 2019 to March 2020, shown in Figure 1. A 3-week average (±SD) of 21 (±4) ELVO patients was identified during this extended period. The mean (±SD) age 71 (±15) years and 49% women and 29% White. The linear regression analysis demonstrated no statistically significant change in the number of ELVO stroke patients during the 21 consecutive 3-week periods from January 2019 to March 2020 (estimated slope of 0.14 [95% CI, −0.14 to 0.42], P=0.33; Figure 2). Finally, comparison of the 3-week period of the COVID-19 outbreak with the prior 21 consecutive 3-week periods revealed a significant increase in the number of ELVO cases (45 patients versus average 21 patients; estimate: 0.78 [95% CI, 0.47–1.08], P≤0.0001).
Figure 1.
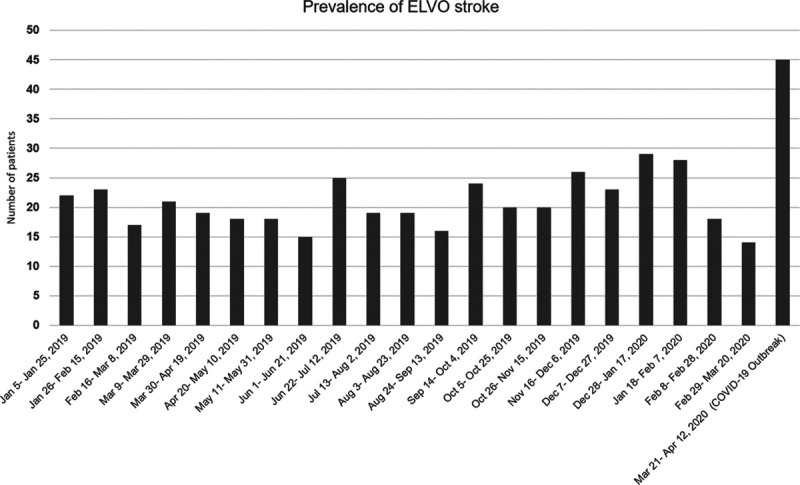
Comparison of the prevalence of acute ischemic stroke from large vessel occlusion in 3-wk during coronavirus disease 2019 (COVID-19) outbreak with consecutive 3-wk periods back to January 2019.
Figure 2.
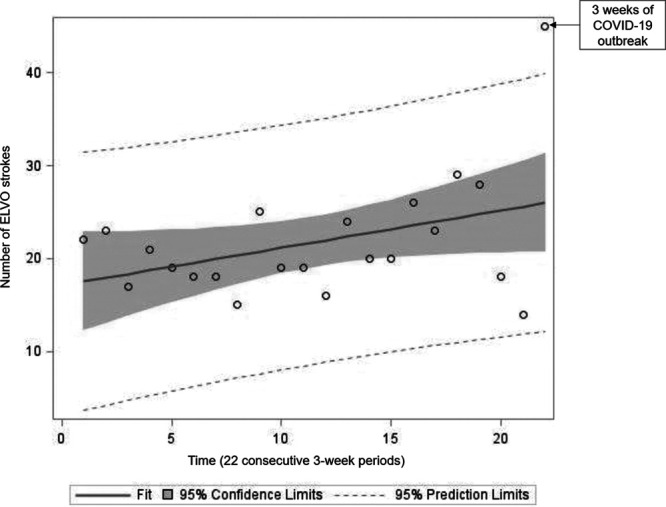
Linear regression analysis demonstrates no change in the trend of the number of emergent large vessel occlusion (ELVO) stroke during the 21 consecutive 3-wk periods before the coronavirus disease 2019 (COVID-19) outbreak (estimated slope of 0.14 [95% CI, −0.14 to 0.42], P=0.33). Poisson regression analysis showed a statistically significant increase in the number of ELVO patients during the COVID outbreak (45 patients vs average 21 patients; estimate: 0.78 [95% CI, 0.47 to 1.08], P≤0.0001).
Of note, the rate of IV r-tPA (intravenous recombinant tissue-type plasminogen activator) treatment for patients with ELVO stroke during the COVID-19 outbreak was 33% which was not statistically different from the rate of treatment before the outbreak (33% versus 24% [difference 11.4, 95% CI, −6.9 to 30.4, P=0.21]). Similarly, the rate of endovascular thrombectomy for patients with ELVO during the COVID-19 outbreak was not different compared with the average treatment rate before the outbreak (66% versus 44% [difference 21.6, 95% CI, −5.3 to 47.8, P=0.11]; Figure 3).
Figure 3.
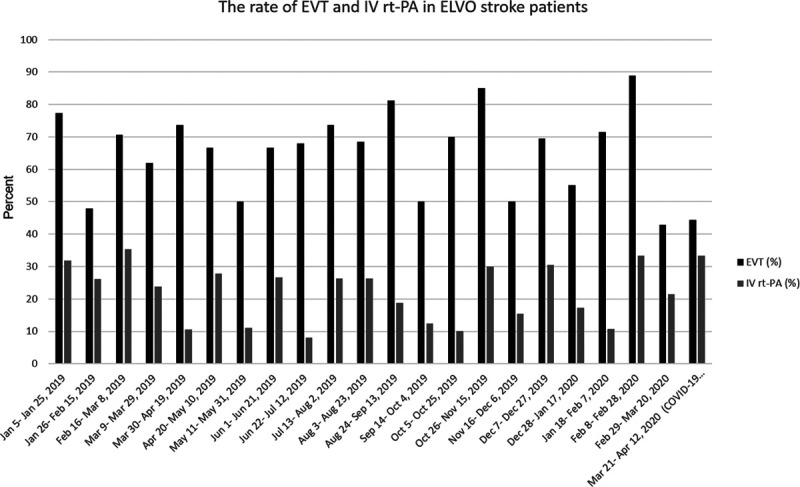
The chart illustrates the rate of endovascular thrombectomy (EVT) and IV r-tPA (intravenous recombinant tissue-type plasminogen activator) treatment during the coronavirus disease 2019 (COVID-19) outbreak and the time before that. The Wilcoxon signed-rank test did not demonstrate statistically significant difference in the rate of IV r-tPA treatment between the COVID-19 outbreak and prior time (33% vs 24% [difference 8.4, 95% CI, −6.9 to 30.4, P=0.21]). Similarly, no significant difference was seen in the rate of EVT in emergent large vessel occlusion (ELVO) patients during COVID-19 outbreak and prior time (66% vs 44% [difference 21.6, 95% CI, −5.3 to 47.8., P=0.11]). (Y axis denotes the percentage of ELVO stroke patients.)
Discussion
Our findings suggest an association between COVID-19 and large vessel ischemic stroke. Over 50% of ELVO patients presenting during the NYC COVID-19 outbreak tested positive, which is significantly higher than 19.9% infection rate in the general population in NYC.6 These patients were younger, more likely to be male, and less likely to be White. ELVO was the first presenting symptom for over 40% of the COVID-19 positive ELVO patients. We observed a 2-fold increase in the number of ELVO patients presenting to our health system during the NYC COVID-19 surge as compared to the same period the year prior and in the past 15 months before the outbreak. Our analysis which includes data on all ELVO stroke in our health system from January 2019 to April 2020 shows that the change in ELVO routing pattern implemented by the Regional Emergency Medical Advisory Committee of NYC in April 2019 (ELVO prehospital diversion to Thrombectomy Stroke Center and Comprehensive Stroke Center)7 did not have a significant impact on the total number of patients seen in our health system. Furthermore, none of the hospitals in Mount Sinai Health System in NYC became a Comprehensive Stroke Center or Thrombectomy Stroke Center since January 2019, which limits the likelihood of the impact of other unrelated confounding factors. Similarly, reviewing the prevalence of ELVO stroke during the COVID-19 outbreak in March and April 2020 and monthly prevalence of ELVO stroke all of 2019, it is unlikely to attribute this increase in the number of ELVO patients during the COVID-19 outbreak to other factors such as increased incidence of ischemic stroke in winter and spring from seasonal variations in biological factors such as blood pressure, lipid profile and hypercoagulable state and influenza epidemics or other respiratory infections which has been reported in the past.8
Notably, 25% of COVID-19 ELVO patients were 50 years old or younger, which is higher than previously observed proportions of ELVO stroke in this age group. The proportion of 50 years old or younger was only 10% among non-COVID ELVO patients, which is consistent with previous epidemiological literature on stroke.9,10
The majority of COVID-19 ELVO patients were men, whereas the non-COVID-19 cohort were more likely to be women. Consistent with our observations, epidemiological studies from China demonstrate a higher prevalence of COVID-19 (ranging from 54% to 62% of cases) and possibly worse outcome among men.4,11–13
In a recently published retrospective observational study from Wuhan, China, Mao et al5 found neurological manifestations in 36% of hospitalized patients with COVID-19. They reported a higher prevalence of neurological symptoms among hospitalized patients with severe disease. In their review of 88 patients with severe COVID-19, 5.7% of patients had ischemic or hemorrhagic stroke. Remarkably, they found that the neurological symptoms can occur early in the course of the disease and may be a presenting symptom. Similarly, in our study, in 42% of the COVID-19 ELVO patients, stroke was their presenting symptom. However, 79% of these patients had radiographic findings consistent with COVID-19 in their lung apices visualized on their code-stroke neck computed tomography angiogram. Moreover, 90% of the COVID-19 cohort had markedly increased D-dimer levels (mean [±SD]: 10.7 [±7.9] μg/mL FEU). Elevated D-dimer has been demonstrated as an indicator of severe COVID-19 disease in previous studies.4,14 It is likely that markedly elevated D-dimer is associated with an increased risk of thromboembolic complications in patients with COVID-19, and ELVO can be the sole clinical presentation in these patients.
The precise mechanism(s) of acute ischemic stroke in patients with COVID-19 is not fully understood. However, a hypercoagulable state, commonly seen in severe forms of the disease, direct cardiac injury and subsequent cardioembolic events, and de novo endothelial injury from direct invasion of the virus via the angiotensin-converting enzyme 2 receptors have been suggested as potential culprits.2,15 Additionally, direct central nervous system invasion by the SARS-CoV-2 virus via a hematogenous or trans-synaptic neuronal route is another important potential mechanism to be considered. Previous versions of coronavirus including SARS-CoV and Middle East respiratory syndrome–related coronavirus have been isolated from the central nervous system.16 Umapathi et al17 reported 5 cases of severe stroke from ELVO in patients with severe SARS infection during the SARS-CoV epidemic in Singapore in 2003. We did not find obvious evidence of Central Nervous System vasculopathy among patients with COVID-19 in cerebral digital subtraction angiography or computed tomography angiography of head and neck in our patients. Of note, 3 patients with COVID-19 were found to have a new-onset reduced ejection fraction on transthoracic echocardiography, and one patient with COVID-19 was found to have a left ventricular thrombus, with no history of prior cardiac disease.
Finally, it is worth mentioning that the total number of ischemic stroke presented to our Comprehensive Stroke Center hospital during the months of March (n=30) and April (n=34) this year which overlaps with the COVID-19 outbreak was significantly lower than the mean (±SD) monthly ischemic stroke patients from January 2019 to February 2020 was 43 (±7.3). There was only one patient in the entire month of April this year who was treated with IV r-tPA, while the median number of IV r-tPA treatment was 3 from January 2019 to March 2020. The decreased number of ischemic stroke and IV r-tPA treatment during the COVID-19 outbreak may be explained by the reported reluctance of the public to seek immediate medical attention for mild symptoms due to the fear of leaving home and coming to the hospital and contracting the virus. It seems likely that mild and moderate stroke patients were more willing to wait it out than to present to emergency departments, given the general fear of COVID-19. Some of these patients potentially improved or slowly recovered without ever presenting to an emergency department. Doubtless some also substantially worsened and then either came to the hospital or possibly passed on at home. In fact, during this same period the Fire Department of the City of New York reported an almost 4-times normal rate of patients being dead on emergency medical service arrival.18 While some of these deaths were likely from COVID-19 many may also have been from reticent myocardial infarction patients, subarachnoid hemorrhage or subdural hemorrhage patients, or large vessel strokes (particularly of the posterior circulation). Last, it is also reasonable that, as emergency departments were overrun with COVID-19 they were less likely to call stroke alerts for patients with mild symptoms.19 Essentially, the threshold likely increased, thereby enriching the population of ELVOs as compared to the general stroke code population. These considerations remain conjecture, but they are certainly plausible considerations.
Our study has several limitations. First, due to relatively small sample size, bias in clinical observation cannot be fully excluded. Due to the retrospective nature of our study, the information regarding COVID-19-related symptoms and cardiovascular risk factors were abstracted from the patients’ electronic medical record, therefore, underestimation of the symptoms and preexisting conditions, due to omissions in reporting, particularly if symptoms were relatively mild, cannot be excluded. Since patients without COVID-19 did not routinely have certain tests such as D-dimer or fibrinogen, a comparison between the 2 cohorts could not be performed. The rate of transfer from peripheral hospitals during the pandemic was slightly higher than prior times. Although there was no report of any thrombectomy capable hospital not accepting stroke patients for thrombectomy during the COVID-19 outbreak in NYC, the possible impact of overburdened hospitals during the pandemic on the pattern of ELVO stroke transfers and subsequently the total number of ELVO received in our health system cannot be completely ruled out. However, as only 2 of the reported ELVO population came from hospitals we do not usually receive strokes from, it seems unlikely that such changes account for the observed effects. Despite the above limitations, this is among the first series of ELVO patients with COVID-19 disease, and it provides valuable insight into the nature of stroke as a presenting manifestation of COVID-19, as well as lending support for COVID-19 initiating a prothrombotic state.
Conclusions
We report an unusual demographic and risk factor profile in patients presenting with ELVO stroke during the peak time of the NYC’s COVID-19 outbreak. More than half of ELVO patients treated in our institution during this period tested positive for COVID-19. COVID-19 ELVO patients were younger, and more likely to be men and less likely to be White. Patients with COVID-19 also were less likely to have past medical history of heart failure than patients without COVID-19. Our findings also suggest an increase in the incidence of ELVO stroke during the peak of the COVID-19 outbreak. Clinicians should consider SARS-CoV-2 infection in the workup of acute ischemic stroke during periods of high SARS-CoV-2 activity, particularly in patients without typical cardiovascular risk factors.
Sources of Funding
None.
Disclosures
Dr Mocco has received research grants from Stryker and Penumbara and Microvention. He is a consultant to Endostream, Viseon, Imperative Care, RIST, Synchron, Viz.ai, Perflow, CVAid, and Cerebrotech. He is investor/Stockholder at Rebound, BlinkTBI, Endostream, Viseon, Imperative Care, Serenity, Cardinal Consulting, NTI, RIST, Synchron, Viz.ai, and Cerebrotech. Dr Oxley has received personal fees from Imperative Care outside the submitted work. The other authors report no conflicts.
Footnotes
Nonstandard Abbreviations and Acronyms
- COVID-19
- coronavirus disease 2019
- ELVO
- emergent large vessel occlusion
- IV r-tPA
- intravenous recombinant tissue-type plasminogen activator
- NYC
- New York City
- SARS-CoV-2
- severe acute respiratory syndrome coronavirus-2
For Sources of Funding and Disclosures, see page 2662.
This manuscript was sent to Marc Fisher, Senior Consulting Editor, for review by expert referees, editorial decision, and final disposition.
References
- 1.Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med 202026450–452doi: 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med 202046586–590doi: 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020181271.e8–280.e8doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 20203231061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020771–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Office of Governor Anrew M. Cuomo. New York City COVID-19 Antibody Test Results. A Guide To Reopenning New York & Building Back Better. https://www.governor.ny.gov/sites/governor.ny.gov/files/atoms/files/NYForwardReopeningGuide.pdf. Accessed July 12, 2020.
- 7.The Regional Emergency Medical Services, Council of New York City. REMAC Advisory Updated Stroke Protocol 2019. https://www.nycremsco.org/2019-remac-advisories. Accessed July 12, 2020.
- 8.Turin TC, Kita Y, Murakami Y, Rumana N, Sugihara H, Morita Y, Tomioka N, Okayama A, Nakamura Y, Abbott RD, et al. Higher stroke incidence in the spring season regardless of conventional risk factors: Takashima Stroke Registry, Japan, 1988-2001. Stroke 200839745–752doi: 10.1161/STROKEAHA.107.495929 [DOI] [PubMed] [Google Scholar]
- 9.Smajlović D. Strokes in young adults: epidemiology and prevention. Vasc Health Risk Manag 201511157–164doi: 10.2147/VHRM.S53203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rennert RC, Wali AR, Steinberg JA, Santiago-Dieppa DR, Olson SE, Pannell JS, Khalessi AA. Epidemiology, natural history, and clinical presentation of large vessel ischemic stroke. Neurosurgery 201985suppl_1S4–S8doi: 10.1093/neuros/nyz042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, et al. ; China Medical Treatment Expert Group for Covid-19 Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 20203821708–1720doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenham C, Smith J, Morgan R; Gender and COVID-19 Working Group COVID-19: the gendered impacts of the outbreak. Lancet 2020395846–848doi: 10.1016/S0140-6736(20)30526-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 20203951054–1062doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 202018844–847doi: 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, Jain SS, Burkhoff D, Kumaraiah D, Rabbani L, et al. COVID-19 and cardiovascular disease. Circulation 20201411648–1655doi: 10.1161/CIRCULATIONAHA.120.046941 [DOI] [PubMed] [Google Scholar]
- 16.Arabi YM, Balkhy HH, Hayden FG, Bouchama A, Luke T, Baillie JK, Al-Omari A, Hajeer AH, Senga M, Denison MR, et al. Middle East respiratory syndrome. N Engl J Med 2017376584–594doi: 10.1056/NEJMsr1408795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Umapathi T, Kor AC, Venketasubramanian N, Lim CC, Pang BC, Yeo TT, Lee CC, Lim PL, Ponnudurai K, Chuah KL, et al. Large artery ischaemic stroke in Severe Acute Respiratory Syndrome (SARS). J Neurol 20042511227–1231doi: 10.1007/s00415-004-0519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan G. Staggering Surge of NYers Dying in Their Homes Suggests City is Undercounting Coronavirus Fatalities. https://gothamist.com/news/surge-number-new-yorkers-dying-home-officials-suspect-undercount-covid-19-related-deaths. Accessed July 12, 2020.
- 19.Montaner J, Barragan-Prieto A, Perez-Sanchez S, Escudero-Martinez I, Moniche F, Sanchez-Miura JA, Ruiz-Bayo L, Gonzalez A. Break in the stroke chain of survival due to COVID-19. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030106. https://www.ahajournals.org/doi/10.1161/STROKEAHA.120.030106. Accessed July 12, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]


