Abstract
A novel infectious disease, coronavirus disease-2019 (COVID-19), spread globally since December 2019. Without effective treatment and vaccination, the strategies to restrain this disease are only keeping social distance, maintaining personal hygiene, quarantine, and isolation. However, thrice-a-week treatment is inevitable for all hemodialysis patients. In addition to the high risk of cluster infection and compromised immunity in patients with end-stage renal disease (ESRD), an atypical disease presentation could also make the medial system neglect these patients during CVOID-19 pandemic. To avoid COVID-19 transmission among patients on dialysis, the major societies of nephrology around the world have provided their guidelines for screening, dialysis facilities adjustment, and health education, respectively. In this review, we summarized the main contents and differences of these guidelines and addressed the prompt management for patients with ESRD to reduce the risk of infection during COVID-19 pandemic.
Keywords: COVID-19, Dialysis facilities, End-stage renal disease, Hemodialysis, Peritoneal dialysis
1. INTRODUCTION
A novel infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been pandemic started from Wuhan, China since December 2019. Without effective treatment and vaccination currently, proper strategies to prevent disease transmission are extremely crucial. In order to break the chain of coronavirus disease-2019 (COVID-19) transmission with limited resources, effective and quick recognition of possible infected patients is very important. However, the variable and non-specific clinical manifestations of COVID-19, including fever, cough, nausea, vomiting, diarrhea, fatigue, and loss of smell, render early recognition a great challenge.1,2 Part of the infected individuals are asymptomatic, but old-aged patients with comorbidities have increased risk of acute respiratory distress syndrome and mortality.3
Center-based hemodialysis (HD) is the main renal replacement modality in most countries, counting near 90% of the total population with end-stage renal disease (ESRD).4 For HD patients, 4-hour restriction in an indoor environment three times a week, frequent contact with medical staff members and other patients are inevitable, and all these factors increase the possibility of cluster infection. Confronting the outbreak of COVID-19 pandemic, nephrologists and medical care providers need to come up with effective strategies to prevent disease transmission.
2. COVID-19 INFECTION IN PATIENTS WITH ESRD
Patients with ESRD consist of more comorbidities4 and immunosuppressive status.5 Infection is the second leading cause of hospitalization and death of ESRD patients, and pneumonia consists 20% of infection. Nevertheless, patients on dialysis also have higher mortality rate and higher medical burden than non-dialysis individuals.4,6,7 Older adults, defined as 65 years and older, are at higher risk for severe illness and death from COVID-19.8 In the USA and many countries, half of the hemodialysis patients are older than 65 years old,9 belonging to the COVID-19 highest risk group. In addition, patients with ESRD usually have other chronic systemic diseases, such as hypertension, diabetes, and cardiovascular diseases, these comorbidities increase the risk of developing severe types and mortality of COVID-19 infection,3 indicating that ESRD patients are more vulnerable to COVID-19.
Unlike SARS, the clinical presentation of SARS-CoV-2 infection varies from asymptomatic, mild symptoms to severe respiratory distress.1,2 Furthermore, fever is not consistently present.2 Based on the experiences of SARS, some hospitals and HD centers use fever as a criterion for isolation and screening policy. However, published data indicate that SARS-CoV-2 can be highly infective 2 days before initial symptoms present.10 In addition, current evidence showed ESRD patients exhibited more gastrointestinal presentation than respiratory symptoms as the initial presentation.11,12 These features indicated that body temperature monitor is not an effective strategy to prevent cluster infection.
It is believed that severe complication of COVID-19 comes from cytokine storm of immunity but not virulence,13,14 even children with very serious medical conditions, like treated with either immunosuppressant or other cancer therapies, are much less affected than adults.15,16 Uremic toxins suppress both cellular and humoral immunity in dialysis patients compared to general population.17,18 An altered immune response might be the reason why ESRD patients have atypical presentation in COVID-19.
3. CHALLENGE OF COVID-19 TREATMENT IN DIALYSIS PATIENTS
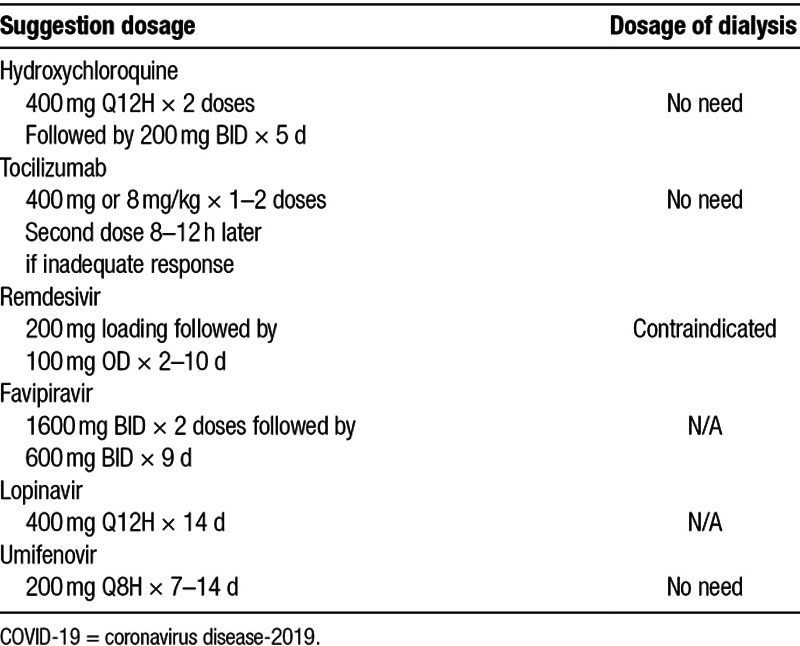
Unlike the SARS patients in 2003,19 some COVID-19 patients did not present prodromal symptoms of upper respiratory tract infection or chest X-ray abnormality. In addition, the incubation period of SARS-CoV-2 infection oscillating between 2 and 14 days makes it remarkably difficult to achieve early diagnosis. Currently, treatment for COVID-19 is still under developing. Potential treatments include hydroxychloroquine, tocilizumab, remdesivir, lopinavir, umifenovir, favipiravir, and convalescent plasma20 (Fig. 1). The efficacy and safety of these medications; however, still need a lot of clinical trials to prove. Even if some regimens are effective, as most of them are eliminated by kidneys; therefore, there is no available recommendation dosage for dialysis patients21 (Table 1).
Fig. 1.
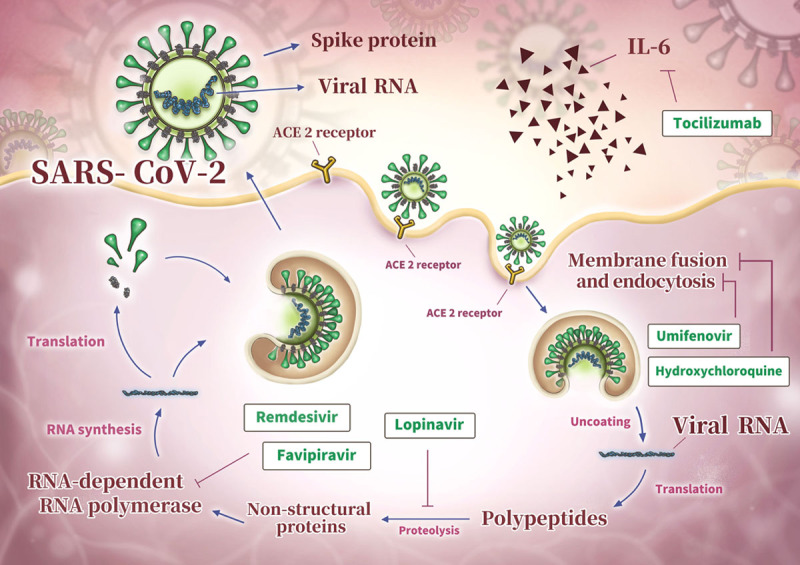
Representative scheme of SARS-CoV-2 life cycle and potential treatment targets.
Table 1.
Potential medications for COVID-19 treatment
As COVID-19 has rapidly spread around the world, there has been a mad scramble to find a vaccine. By April 2020, China, the USA, and the UK have initiated phase I-II safety and efficacy studies in human subjects.22 Because of the compromised host immunity, the vaccine may not exhibit the same efficacy in patients on hemodialysis as it does in immunocompetent individuals. Taking HBV as example, dialysis patients need four, instead of three, doses of vaccination of the hepatitis B virus. Even with increased dosage, approximately one-third of HD patients do not respond to this vaccination schedule.23 With current limited knowledge of this new virus, a serological conversion can be tentatively used as a surrogate of COVID-19 immunity status after vaccine shot in ESRD patients.
4. STRATEGIES TO PREVENT COVID-19 TRANSMISSION IN DIALYSIS FACILITY
Upon now, main nephrology societies have purposed suggestions and guidelines to prevent COVID-19 transmission in dialysis facilities. The guidelines vary because of difference of medical resources, prevalence of COVID-19, and policy of governments in each area.
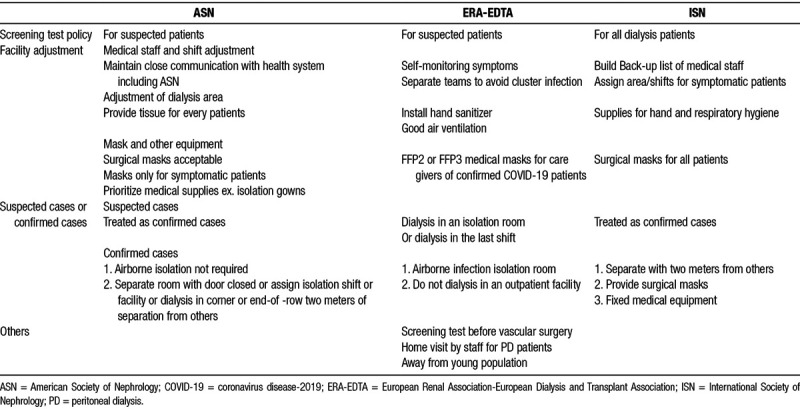
In pandemic areas, the primary goal is to reduce the transmission efficiently under limited medical resources. On the contrary, isolation and tracing every COVID-19 cases is the most important target for areas with relatively few cases of COVID-19. We reviewed suggestions from American Society of Nephrology (ASN),24 European Renal Association-European Dialysis and Transplant Association (ERA-EDTA),25 International Society of Nephrology (ISN),26 and summarized the main content in screening, facility adjustment, and management of confirmed COVID-19 cases here. Difference of each guidelines is also discussed (Table 2).
Table 2.
Guidelines to prevent COVID-19 cluster infection from different nephrology societies
4.1. Screening
Almost every guideline suggested the establishment of a standard screening protocol to prevent cluster infection in dialysis facilities. Before the arrival of dialysis facility, patients should inform the facility if there are suspicious symptoms to allow the preparation before dialysis. Prior to the screening process, medically stable patients are suggested to wait outside the facility or stay at their own vehicles. The whole screening process should be arranged in a separate room. Body temperature, standard history taking, and travel-occupation-contact-cluster (TOCC) history all need to be recorded. The criteria of performing screening test varied between different guidelines, ISN suggested universal test on every dialysis patients,26 while others suggested performing screening test only on symptomatic patients.24,25 A main challenge of contain COVID-19 is that more than half infected individuals are asymptomatic or with minor symptoms;1,11 however, they are still capable of spreading virus. Therefore, performing test on all hemodialysis patients may be an essential way to prevent cluster infection in dialysis facilities. Besides, screening test should also be performed before a vascular access surgery.25
If the tool of screening test could not be fully supported, screening test should at least be performed on patients with symptoms or significant TOCC history, physicians must be alert to the atypical symptoms of COVID-19, like gastrointestinal symptoms, and loss of smell and taste. The screening test should be done in a private room. Anyone with positive results of screening test should be isolated immediately and assigned to a private dialysis room and have proper treatment of COVID-19 based on the clinical condition.
There are mainly three types of screening tests: molecular test, viral antigen detection, and serum antibody detection27 (Table 3). Molecular test exams the presence of viral RNA and RT-PCR is the gold standard of diagnosis currently, a U.S. Food and Drug Administration authorized novel test uses isothermal amplification shorten the reaction time to 5 minutes.28 However, false negative may still occur in inaccurate sampling. Viral antigen detection and serum antibody detection are both under developing. Viral antigen detection is based on the test of viral protein. Serum antibody detection, also known as “immunity tests,” detects the presence of IgM and IgG in patient’s serum, this means they are applied when an immune reaction to the pathogen has already taken place, the presence of antibody usually occurred after 1 week after COVID-19 infection.27 Therefore, it is of value to figure out how many people have been infected with the virus? who may have spread it without knowing it? and could also potentially allow immuned medical care givers to return to work. That could be a huge boost to front-line health workers.
Table 3.
Test methods of COVID-19
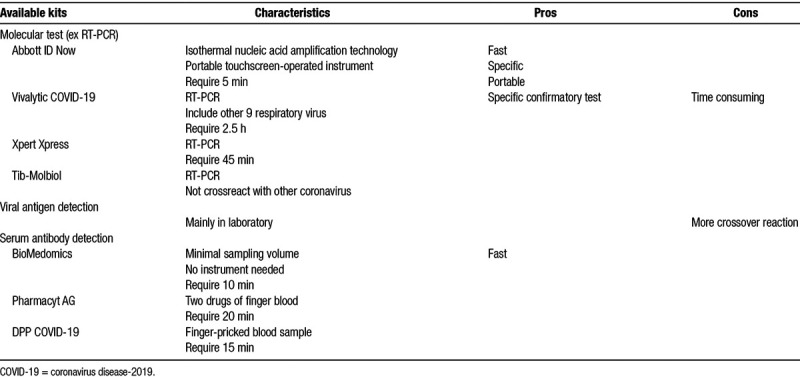
There is debate of which screening test is better for dialysis facilities. To prevent undiagnosed COVID-19 cluster infection within dialysis facility, molecular test for the presence of viral RNA is a reasonable choice. Although the infectivity of COVID-19 exists 2 to 3 days before symptoms onset and last for 2 weeks,1,10 viral RNA shedding can be detected as long as 60 days.29 If patient isolation is only determined by PCR result, it could lead to unnecessary medical cost and staff burn out, eventually paralyze the dialysis center. Besides, although the protectivity of the antibody against COVID-19 is still unclear, the detection of antibody against COVID-19 might be helpful for transmission prevention and patient assignment. HD bed assignment could be based on the serology results of each patient.
4.2. Facility adjustment
4.2.1. Medical staff
Self-monitoring of symptoms by medical staff is suggested in all guidelines. Sick members should stay at home and seek for medical intervention. Besides, medical staff should be grouped based on different shifts and working area. Contact between different groups should be avoided in order to minimized the risk of outbreak of COVID-19 or paralyze the whole dialysis facility. Education about the manipulation of personal protective equipment (PPE) and latest knowledge of COVID-19 should be offered.
4.2.2. Area adjustment
The area adjustment for dialysis facilities different between guidelines. The primary goal is to separate. The dialysis area for suspected or confirmed COVID-19 cases should be assigned to a private room. If private room is not available, corner or end-of row position is the alternative choice and the distance between symptomatic and asymptomatic patients must be longer than 2 meters. For areas with enough airborne infection isolation room, the dialysis should be performed in one of them. Hand sanitizer, and supplies for respiratory hygiene must be fully equipped in dialysis facilities. Good air conditioning and ventilation are also suggested. Regular and frequent disinfection should be conducted.
4.2.3. Personal protective equipment
The medical resources varied between different countries. Prioritized usage of PPE for medical staff need to be established. For example, isolation gowns are suggested throughout procedures with higher risk of transmission, like initiating and terminating dialysis, manipulating access needles and catheters, assisting patients to and from the dialysis station, and cleaning and disinfecting the dialysis station.24 For patients with confirmed COVID-19, visor mask and gloves are suggested by all guidelines.24–26 However, the policy of mask supply varied between different areas. Surgical masks are acceptable if N95 masks are unavailable by ASN suggestion.24 N-95 medical masks (FFP2 or FFP3) are recommended by ERA-EDTA.25 Hand sanitizer should be installed in the facility for both patients and medical staff.
4.3. Management of suspected or confirmed cases of COVID-19
The management of suspected or confirmed cases of COVID-19 with ESRD varied between different countries significantly because the limitation of medical resources and different prevalence of COVID-19. Airborne infection isolation room is not suggested in some countries like America;24 however, others recommend dialysis in airborne infection isolation room.25,30 Overall, the principle is effective distancing. Dialysis should be done in a private room. If no private room is available, dialysis must be arranged in the corner or end-of-row equipment and the distance between the patient and other must be longer than 2 meters. The medical staff should be equipped with full PPE. Disinfection must be done after the dialysis.
4.4. Health education
The risk of COVID-19 infection not only existed in the dialysis facility. The prevention of transmission should be built from the aspect of the patients’ daily life. Health education could be done during the process of hemodialysis. The importance of wearing masks, hand hygiene, social distance and alertness to the atypical symptoms of COVID-19 should be emphasized. ERT-EDTA suggest avoid contact with younger population because they have more asymptomatic infection.25
4.5. Public health
Prevention of COVID-19 is a challenging goal for every country, the policy of government is extremely crucial. For ESRD patients with contact history of COVID-19, the transportation between their home and dialysis facilities should be regulated30 and dialysis facilities for COVID-19 patients are also needed.25,30 For patients under home hemodialysis or peritoneal dialysis (PD), hospital visiting should be avoided, instead, home visit by medical staff could be arranged.25 Getting access of immigration information is also an important information of traveling history for the prevention of COVID-19. In Taiwan, data of immigration are linked to the national health insurance system. Those data can be checked for every patients and their company before dialysis.30
5. INCREASE PERITONEAL DIALYSIS PENETRATION
Screening process and adjustment of dialysis facilities require a great amount of medical resources, medical staff, and reduce the flexibility and capacity of dialysis significantly. Any outbreak of COVID-19 could paralyze a dialysis facility easily. Unlike SARS, the pandemic of COVID-19 may last for a long period. Even after the COVID-19 pandemic, risk of new droplet-spread infectious diseases still exist. Worldwide, humans are interacting with wildlife with increasing frequency, scientists discover six new coronaviruses lurking in bats that are in the same family as the SARS-CoV2 in 2020.31 Under the frequent communication and contact globally. We should also be prepared for the future emerging infectious diseases.
In the pandemic of COVID-19, to reduce every possibility of cluster infection should be took into concern for renal replacement modality selection. Compared with hemodialysis, PD reduces the frequency of hospital visiting, which could significantly reduce the risk of cluster infection. International Society of Peritoneal Society suggest PD patient stay at home, and hospital visits should be minimized for only urgent indications, like suspected peritonitis.32 Isolation of confirmed COVID-19 cases is also easier for PD patients with no need to interrupt renal replacement therapy.
In conclusion, COVID-19 impacts our medical system tremendously. Without effective treatment and vaccination, our temporary goal is to reduce the spreading of COVID-19 and avoid dialysis facility become a reservoir of COVID-19. Increasing evidence emphasizes the role of asymptomatic people in the spread of this virus. SARS-CoV-2 infection present atypical symptoms among patients ESRD. Frequent medical facility visiting increases the risk of cluster infection. Thus, screening protocol and facility adjustment need to be done in every dialysis facility. In order to reduce the risk of cluster infection of COVID-19 and future aerosol/droplet-borne respiratory infection disease, PD might be a reasonable choice for ESRD patients commencing the dialysis therapy.
ACKNOWLEDGMENTS
This work was supported by the grants from the Ministry of Science and Technology (MOST 106-2314-B-010-039-MY3 and 107-2314-B-075-037-MY3) and Taipei Veterans General Hospital (V108D42-003-MY3-2).
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc 2020;83:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY-y, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. https://www.nejm.org/doi/full/10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Q, Yang K, Wang W, Jiang L, Song J. Correction to: Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020 doi: 10.1007/s00134-020-05991-x. 10.1007/s00134-020-06028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US Renal Data System 2019 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis 2020;75(1S1):A6–7. [DOI] [PubMed] [Google Scholar]
- 5.Betjes MGH. Immune cell dysfunction and inflammation in end-stage renal disease. Nat Rev Nephrol 2013;9:255–65.. [DOI] [PubMed] [Google Scholar]
- 6.Slinin Y, Foley RN, Collins AJ. Clinical epidemiology of pneumonia in hemodialysis patients: the USRDS waves 1, 3, and 4 study. Kidney Int 2006;70:1135–41. [DOI] [PubMed] [Google Scholar]
- 7.Sibbel S, Sato R, Hunt A, Turenne W, Brunelli SM. The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: a retrospective, observational cohort study. BMC Nephrol 2016;17:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu L, Wang B, Yuan T, Chen X, Ao Y, Fitzpatrick T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.041. 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.UNITED STATES RENAL DATA SYSTEM. 2019; Available at https://www.usrds.org/.
- 10.He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020 doi: 10.1038/s41591-020-0869-5. 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 11.Ferrey AJ, Choi G, Hanna RM, Chang Y, Tantisattamo E, Ivaturi K, et al. A case of novel coronavirus disease 19 in a chronic hemodialysis patient presenting with gastroenteritis and developing severe pulmonary disease. Am J Nephrol 20201–6. 10.1159/000507417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang R, Liao C, He H, Hu C, Wei Z, Hong Z, et al. COVID-19 in hemodialysis patients: a report of 5 cases. Am J Kidney Dis. 2020 doi: 10.1053/j.ajkd.2020.03.009. 10.1053/j.ajkd.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the ‘Cytokine Storm’ in COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.037. 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hrusak O, Kalina T, Wolf J, Balduzzi A, Provenzi M, Rizzari C, et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Cancer 2020;132:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Antiga L. Coronaviruses and immunosuppressed patients. The facts during the third epidemic. Liver Transpl. 2020 doi: 10.1002/lt.25756. 10.1002/lt.25756. [DOI] [PubMed] [Google Scholar]
- 17.Ifudu O, Tan CC, Dulin AL, Delano BG, Friedman EA. Gouty arthritis in end-stage renal disease: clinical course and rarity of new cases. Am J Kidney Dis 1994;23:347–51. [DOI] [PubMed] [Google Scholar]
- 18.Ohno I, Ichida K, Okabe H, Hikita M, Uetake D, Kimura H, et al. Frequency of gouty arthritis in patients with end-stage renal disease in Japan. Intern Med 2005;44:706–9. [DOI] [PubMed] [Google Scholar]
- 19.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, et al. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003;361:1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.6019. 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 21.Izzedine H, Jhaveri KD, Perazella MA. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020 doi: 10.1016/j.kint.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical trial number NCT04324606 for “A Study of a Candidate COVID-19 Vaccine (COV001)”. Nat Rev Drug Discov 2020. 10.1038/d41573-020-00073-5. [Google Scholar]
- 23.Chaves SS, Daniels D, Cooper BW, Malo-Schlegel S, Macarthur S, Robbins KC, et al. Immunogenicity of hepatitis B vaccine among hemodialysis patients: effect of revaccination of non-responders and duration of protection. Vaccine 2011;29:9618–23. [DOI] [PubMed] [Google Scholar]
- 24.Kliger AS, Silberzweig J. Mitigating risk of COVID-19 in dialysis facilities. Clin J Am Soc Nephrol. 2020 doi: 10.2215/CJN.03340320. 10.2215/cjn.03340320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Basile C, Combe C, Pizzarelli F, Covic A, Davenport A, Kanbay M, et al. Recommendations for the prevention, mitigation and containment of the emerging SARS-CoV-2 (COVID-19) pandemic in haemodialysis centres. Nephrol Dial Transplant. 2020 doi: 10.1093/ndt/gfaa069. 10.1093/ndt/gfaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kliger AS, Cozzolino M, Jha V, Harbert G, Ikizler TA. Managing the COVID-19 pandemic: international comparisons in dialysis patients. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.007. 10.1016/j.kint.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vashist SK. In vitro diagnostic assays for COVID-19: recent advances and emerging trends. Diagnostics (Basel) 2020;10 10.3390/diagnostics10040202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Letter from FDA. March 2020; Available at https://www.fda.gov/media/136522/download.
- 29.Liu WD, Chang SY, Wang JT, Tsai M, Hung CC, Hsu CL, et al. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.063. 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taiwan Society of Nephrology: Guidance of COVID-19 infection prevention in dialysisfacilities_TSN_20200406 [In Chinese] Available at https://www.tsn.org.tw/tsnFile/news//G8D7DB0D9AD62961/20200406-%E9%80%8F%E6%9E%90%E9%86%AB%E7%99%82%E9%99%A2%E6%89%80%E5%9B%A0%E6%87%89%E6%AD%A6%E6%BC%A2%E8%82%BA%E7%82%8ECOVID-19%E6%84%9F%E6%9F%93%E8%99%95%E7%BD%AE%E8%A6%8F%E7%AF%84(%E5%85%AC%E5%91%8A%E7%89%88%E7%AC%AC%E4%B8%89%E7%89%88)-TSN.pdf.
- 31.Valitutto MT, Aung O, Tun KYN, Vodzak ME, Zimmerman D, Yu JH, et al. Detection of novel coronaviruses in bats in Myanmar. PLoS One 2020;15:e0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strategies regarding COVID-19 in PD patients. 2020. Available at https://ispd.org/strategies-covid19/.


