Abstract
The 2019 novel coronavirus (2019-nCoV, later named SARS-CoV-2) is a pandemic disease worldwide. The spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is continuing at a rapid speed. Till May 4, 2020, there have been 3,407,747 confirmed cases and 238,198 deaths globally. The common symptoms in pregnant women are fever, cough, and dyspnea. Angiotensin-converting enzyme 2 (ACE2) has transient overexpression and increased activity during pregnancy, which is now confirmed as the receptor of SARS-CoV-2 and plays essential roles in human infection and transmission. There is no evidence that pregnant women are more susceptible to SARS-CoV-2. To date, there is no valid medication or vaccination. The immune suppression or modulation during pregnancy increases the risk of severe pneumonia. Remdesivir is an antiviral medication targeting ribonucleic acid (RNA) synthesis that has clinical improvement in the treatment of SARS-CoV-2. Chloroquine is controversial in its effectiveness and safety to treat SARS-CoV-2. Remdesivir is safe in pregnancy. Chloroquine has not been formally assigned to a pregnancy category by the Food and Drug Administration (FDA). The management strategy includes monitoring fetal heart rate and uterine contractions; early oxygenation if O2 saturation is less than 95%; empiric antibiotics for prevention of secondary infection; corticosteroid to treat maternal SARS-CoV-2 disease routinely is not suggested, only for fetal lung maturation in selected cases; and consideration of delivery is according to the obstetric indication, gestational age, and severity of the disease. During epidemics, delivery at 32–34 weeks is considered. The indication for the Cesarean section should be flexible to minimize the risk of infection during the delivery. The newborn should be in isolation ward immediately after birth; breastfeeding is not contraindicated but should avoid direct transmission infection.
Keywords: Angiotensin-converting enzyme 2 (ACE2), Antiviral medication, Pregnancy, Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2)
1. INTRODUCTION
In December 2019, a series of pneumonia with unknown origin was noted in Wuhan, Hubei province, China.1–5 The Chinese Academy of Engineering announced the source was a novel coronavirus named 2019-new coronavirus (2019-nCoV) by World Health Organization (WHO), later named severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2).6 According to WHO statistics, there were 3,272,202 people infected with 2019-nCoV and 230,104 people died from the disease globally till May 02, 2020. Coronaviruses (CoVs) are enveloped single-strand RNA viruses. There are four genera, alpha CoV, beta CoV, gamma CoV, and delta CoV.7–9 Before this epidemic of 2019-nCoV, there were six human coronaviruses identified, four of which are in circulation, which causes mild respiratory tract infection. The other two were SARS-CoV and Middle East Respiratory Syndrome coronavirus (MERS-CoV), which had epidemic pneumonia in 2002 and 2012, respectively.10 Pregnant women may be infected with such serious respiratory disease, and we here review a limited cases series of pregnant women with SARS and MERS, which can be helpful for pregnant women who are infected with SARS-CoV-2.11–30
2. SARS AND MERS IN PREGNANCY
Severe acute respiratory syndrome (SARS) caused by SARS coronavirus (SARS-CoV) erupted in November 2002 in Guangdong province, China. The epidemic occurred in China, Hong-Kong, Taiwan, Singapore, and Vietnam. A total of 8098 people were infected, with 774 deaths globally. It was transmitted by respiratory droplets and presented with fever (99%–100%), dry cough (57%–75%), and myalgia (45%–61%).11,12 The mean incubation period was 4–6 days. Overall, case fatality ratio was approximately 15%.13–15
A multicenter observational study in Hong Kong reported outcomes of 12 pregnant women who were infected with SARS-CoV in 2003.15,16 Three pregnant women died. Seven women were in the first trimester, and four had a spontaneous abortion. Five women were more than 24 weeks of gestational age, and four had preterm delivery. Two of the pregnant women recovered from SARS but had intrauterine growth restriction of the ongoing pregnancy. None of the newborn babies had SARS. Fatality rate (25%), intensive care unit (ICU) admission rate (50%), and mechanical ventilation rate (33%) were higher than nonpregnant women. The pregnant women were treated with broad-spectrum antibiotics, and in severe cases, ribavirin was used.16 However, ribavirin has a teratogenic effect in animal.26 Women who are in early pregnancy should consider termination with treating of ribavirin. In a study of placental pathology of patients, there was increased intervillous or subchorionic fibrin and extensive fetal thrombotic vasculopathy, which may result due to the hypoxic respiratory disease.27–29 Nevertheless, villitis, which is the evidence of maternal hematogenous infection that transmit to the fetus, was not found.27,30
MERS is a respiratory disease produced by MERS coronavirus. Since September 2012, 2494 people were diagnosed with MERS coronavirus (MERS-CoV), causing 858 deaths (from WHO).23 Clinical symptoms vary from mild to severe. They usually begin with fever, cough, chills, sore throat, myalgia, and arthralgia, with rapid progress to dyspnea and pneumonia in the first week (epidemic and emerging coronavirus, SARS, and MERS). The mean incubation period was 5.2 days; the fatality rate was 34.4%. A study reviewed 11 pregnant women infected with MERS-CoV; all the pregnant women were symptomatic, six (54%) patients were admitted to ICU, three (27%) patients died during the hospitalization, and three (27%) infants died.24,25
3. SARS-COV-2 AND ANGIOTENSIN-CONVERTING ENZYME 2 IN PREGNANCY
The immunologic change in pregnancy may increase the susceptibility to pathogens.15,31,32 Pneumonia in pregnant women is more frequent and more severe than in nonpregnant women.33 About 25% of pregnant women who have pneumonia need mechanical ventilation.34
A total of 70 pregnant women with SARS-CoV-2 were eligible for the systematic review.15,22,35–40 The common symptoms are fever (84%), cough (28%), and dyspnea (18%). Obstetric complications include preterm birth (39%), intrauterine growth restriction (10%), and miscarriage (2%).22 This study included nine SARS-CoV-2-infected pregnant women in Wuhan, whose amniotic fluid, cord blood, neonatal throat swab, and breast milk were collected. All of the specimens were negative for SARS-CoV-2, which indicates no evidence of vertical transmission.40 But a study indicated that angiotensin-converting enzyme 2 (ACE2), which is the receptor of SARS-CoV-2, was highly expressed in maternal–fetal interface cells, suggesting the possibility of vertical transmission.41 More studies to clarify the role of ACE2 in pregnancy are warranted.42
The renin–angiotensin system (RAS), or renin–angiotensin–aldosterone system (RAAS), is a hormone system that regulates electrolyte balance, as well as systemic vascular resistance.43–45 A gradual increase in the different components of the RAS in pregnancy is a physiological condition for maternal–fetal circulation. The effects of the stimulated RAS in normal pregnancy are incompletely realized.46
ACE2 is the receptor of severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1) and SARS-CoV-2.44,45,47 The ACE cleaves angiotensin I to angiotensin II. ACE2 was first identified in the year 2000, which converts angiotensin I to angiotensin 1–9 and angiotensin II to angiotensin 1–7. ACE2 is a negative regulator in the RAAS. It provides a binding site for the S-protein of coronavirus.48,49 There was structural evidence that the SARS-CoV-2 S protein has a higher affinity to ACE2 than the SARS-CoV S protein.50 Although ACE2 is the receptor of SARS-CoV-2, it plays a protective role in acute lung injury.51 Local activation of the RAAS responses to viral insults in pregnancy may mediate vasoconstriction, acute lung injury, adverse myocardial remodeling, preterm birth, intrauterine growth restriction, and miscarriage.22,52,53 Studies proposed that ACE2 has transient overexpression and increased activity during pregnancy, especially in the placenta.46,54,55 But if pregnant women are more susceptible to SARS-CoV-2 is currently not known. We proposed the deduction effect of ACE2 in pregnancy (Fig. 1).
Fig. 1.
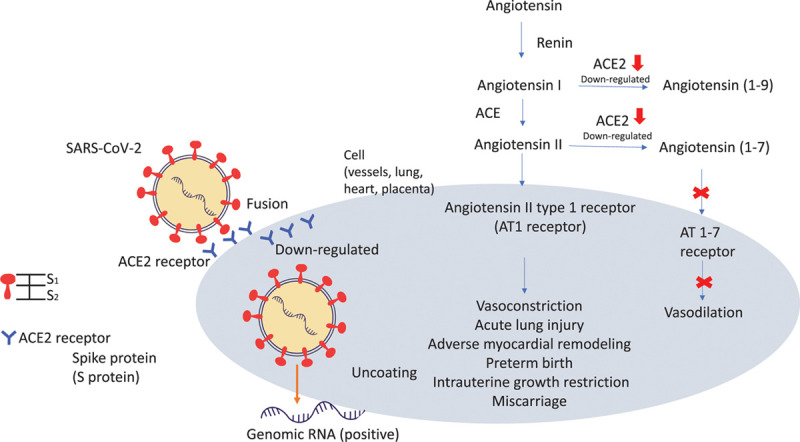
The interaction between SARS-CoV-2 and the renin-angiotensin system. The SARS-CoV-2 spike protein binding to the ACE2 receptor; S1 subunit binds to host cell and S2 subunit triggers the fusion of the viral envelope and target cell. After entry of the virus particle, surface ACE2 is further downregulated, resulting in unrestrained angiotensin II accumulation.
4. TREATMENT AND MANAGEMENT
Antiviral medications remdesivir and chloroquine effectively inhibited SARS-CoV-2 in vitro.56 Remdesivir is an antiviral medication targeting RNA synthesis that is used to treat Ebola, SARS, and MERS. A study had used remdesivir on 53 patients with SARS-CoV-2, of which 68% had clinical improvement.57,58 It is also found to be safe in pregnancy.59 Chloroquine is an antimalarial drug and sometimes used to treat autoimmune disease. Clinical studies on the use chloroquine to treat SARS-CoV-2 in more than 10 hospitals in China found efficacy in treatment pneumonia.60 However, a systemic review study had a conservative attitude.38 Some experts proposed that chloroquine use in SARS-CoV-2 is premature and harmful.61 It is controversial if chloroquine is effective and safe to treat SARS-CoV-2. However, a large-scale study demonstrated the safety of chloroquine in pregnancy.62–64 Pregnant women with SARS-CoV-2 infection are considered relatively high risk. The management strategy is shown below.22,65–68
- Monitoring fetal heart rate and uterine contractions closely.
- Early oxygenation is considered; if O2 saturation is less than 95%, mechanical ventilation may be used.
- Empiric antibiotics for prevention of secondary infection.
- Corticosteroid to treat maternal SARS-CoV-2 infection routinely is not suggested, but for fetal lung, maturation is considered by case.
- Consideration of delivery is according to the obstetric indication, gestational age, and severity of the disease, if there are no obstetric indications, and if the maternal infection is critical, early delivery is considered. During such epidemics, delivery at 32–34 weeks is considered. The indication for the cesarean section should be flexible to minimize the risk of infection during the delivery.
- The newborn should be in isolation ward immediately after delivery; breastfeeding is not contraindicated but should avoid direct infection (Fig. 2).
Fig. 2.
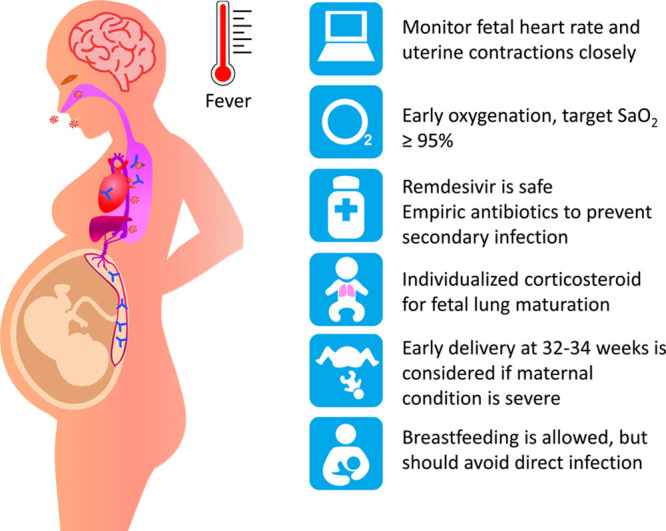
The transmission pathway and the management of SARS-CoV-2 in pregnancy.
SARS-CoV-2 is an ongoing disease. There is no valid medicine and vaccine for the disease; pregnant women have physiologic changes and immunologically susceptible to infectious disease. Besides, consideration of the safety of the fetus makes the treatment more difficult. The experience of pregnant women in SARS-CoV-2 is limited.
ACKNOWLEDGMENTS
This research was funded and supported in part by the following grant from the Tri-Service General Hospital (TSGH-D109-106); the Teh-Tzer Study Group for Human Medical Research Foundation; the Taipei Veterans General Hospital (V109C-108; V109E-005-5; and V109A-022); and the Ministry of Science and Technology, Executive Yuan (MOST: 106-2314-B-075-061-MY3). We would like to thank Hui-Yin Su and Huan-Shuo Chang for figures editing. We also appreciate the financial support from the Female Cancer Foundation, Taipei 104, Taiwan, ROC.
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest related to the subject matter or materials discussed in this article.
REFERENCES
- 1.Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: an overview. J Chin Med Assoc 2020;83:217–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afsar NA. The looming pandemic of COVID-19: What therapeutic options do we have now? J Chin Med Assoc 2020;83:508–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu JY, Chen TJ, Hwang SJ. Analysis of imported cases of COVID-19 in Taiwan: A nationwide study. Int J Environ Res Public Health 2020;17:3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu YC, Chen CS, Chan YJ. Reply of “The outbreak of COVID-19: an overview”. J Chin Med Assoc 2020;83:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng JY. Potential implications of SARS-CoV-2 on pregnancy. Taiwan J Obstet Gynecol 2020;59:464–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol 2020;92:401–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su S, Wong G, Shi W, Liu J, Lai ACK, Zhou J, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol 2016;24:490–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature 2020;581:22–6. [DOI] [PubMed] [Google Scholar]
- 9.Chang TJ, Yang DM, Wang ML, Liang KH, Tsai PH, Chiou SH, et al. Genomic analysis and comparative multiple sequence of SARS-CoV2. J Chin Med Assoc 2020;83:537–43. [DOI] [PubMed] [Google Scholar]
- 10.Kin N, Miszczak F, Lin W, Gouilh MA, Vabret A; EPICOREM Consortium Genomic analysis of 15 human coronaviruses OC43 (HCoV-OC43s) circulating in France from 2001 to 2013 reveals a high intra-specific diversity with new recombinant genotypes. Viruses 2015;7:2358–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hui DSC, Zumla A. Severe acute respiratory syndrome: historical, epidemiologic, and clinical features. Infect Dis Clin North Am 2019;33:869–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu TH, Chang CC, Yang CH, Lin WY, Ee TJ, Lin CW. Hybridization chain reactions targeting the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Int J Mol Sci 2020;21:3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arons MM, Hatfield KM, Reddy SC, Kimball A, James A, Jacobs JR, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lippi G, Mattiuzzi C, Sanchis-Gomar F, Henry BM. Clinical and demographic characteristics of patients dying from COVID-19 in Italy versus China. J Med Virol 2020 Apr 10Doi: 10.1002/jmv.25860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwartz DA, Graham AL. Potential maternal and infant outcomes from (Wuhan) coronavirus 2019-nCoV infecting pregnant women: Lessons from SARS, MERS, and other human coronavirus infections. Viruses 2020;12:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wong SF, Chow KM, Leung TN, Ng WF, Ng TK, Shek CC, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol 2004;191:292–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullins E, Evans D, Viner RM, O’Brien P, Morris E. Coronavirus in pregnancy and delivery: rapid review. Ultrasound Obstet Gynecol 2020;55:586–92. [DOI] [PubMed] [Google Scholar]
- 18.Zimmermann P, Curtis N. COVID-19 in children, pregnancy and neonates: a review of epidemiologic and clinical features. Pediatr Infect Dis J 2020;39:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delfino M, Guida M, Patrì A, Spirito L, Gallo L, Fabbrocini G. SARS-CoV-2 possible contamination of genital area: implications for sexual and vertical transmission routes. J Eur Acad Dermatol Venereol 2020 May 7. Doi: 10.1111/jdv.16591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hantoushzadeh S, Shamshirsaz AA, Aleyasin A, Seferovic MD, Aski SK, Arian SE, et al. Maternal death due to COVID-19 disease. Am J Obstet Gynecol 2020 Apr 28pii:S0002-9378(20)30516-0.Doi: 10.1016/j.ajog.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Mascio D, Khalil A, Saccone G, Rizzo G, Buca D, Liberati M, et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID 1 -19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2020 Mar 25100107.Doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dashraath P, Wong JLJ, Lim MXK, Lim LM, Li S, Biswas A, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol 2020;222:521–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Memish ZA, Perlman S, Van Kerkhove MD, Zumla A. Middle East respiratory syndrome. Lancet 2020;395:1063–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfaraj SH, Al-Tawfiq JA, Memish ZA. Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection during pregnancy: Report of two cases & review of the literature. J Microbiol Immunol Infect 2019;52:501–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stumpfe FM, Titzmann A, Schneider MO, Stelzl P, Kehl S, Fasching PA, et al. SARS-CoV-2 infection in pregnancy - a review of the current literature and possible impact on maternal and neonatal outcome. Geburtshilfe Frauenheilkd 2020;80:380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinclair SM, Jones JK, Miller RK, Greene MF, Kwo PY, Maddrey WC. The Ribavirin Pregnancy Registry: an interim analysis of potential teratogenicity at the mid-point of enrollment. Drug Saf 2017;40:1205–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng WF, Wong SF, Lam A, Mak YF, Yao H, Lee KC, et al. The placentas of patients with severe acute respiratory syndrome: a pathophysiological evaluation. Pathology 2006;38:210–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baergen RN, Heller DS. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr Dev Pathol 2020;23:177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, et al. Detection of SARS-COV-2 in placental and fetal membrane samples. Am J Obstet Gynecol MFM 2020 May 8100133.Doi: 10.1016/j.ajogmf.2020.100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhrt K, McMicking J, Nanda S, Nelson-Piercy C, Shennan A. Placental abruption in a twin pregnancy at 32 weeks’ gestation complicated by COVID-19, without vertical transmission to the babies. Am J Obstet Gynecol MFM 2020 May 8100135.Doi: 10.1016/j.ajogmf.2020.100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yeh CC, Wu KG, Wang PH. High prevalence of IgE sensitization against house dust mites in pregnant women. Medicine (Baltimore) 2018;97:e13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin JH, Hsu YH, Wang PH. Risks for preterm premature labor: Many of them are preventable. J Chin Med Assoc 2020;83:421–2. [DOI] [PubMed] [Google Scholar]
- 33.Inchingolo R, Smargiassi A, Moro F, Buonsenso D, Salvi S, Del Giacomo P, et al. The diagnosis of pneumonia in a pregnant woman with COVID-19 using maternal lung ultrasound. Am J Obstet Gynecol 2020 Apr 28pii:S0002-9378(20)30468-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brito V, Niederman MS. Pneumonia complicating pregnancy. Clin Chest Med 2011;32:121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz DA. The effects of pregnancy on women with COVID-19: maternal and infant outcomes. Clin Infect Dis 2020 May 11pii:ciaa559.Doi: 10.1093/cid/ciaa559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med 2020 Mar 17Doi: 10.5858/arpa.2020-0901-SA. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, et al. Pregnancy and perinatal outcomes of women with coronavirus disease (COVID-19) pneumonia: a preliminary analysis. AJR Am J Roentgenol 2020 Mar 181–6Doi: 10.2214/AJR.20.23072. [DOI] [PubMed] [Google Scholar]
- 38.Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care 2020;57:279–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, Li Q, Zheng D, Jiang H, Wei Y, Zou L, et al. Clinical characteristics of pregnant women with Covid-19 in Wuhan, China. N Engl J Med 2020 Apr 17Doi: 10.1056/NEJMc2009226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020;395:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M, Chen L, Zhang J, Xiong C, Li X. The SARS-CoV-2 receptor ACE2 expression of maternal-fetal interface and fetal organs by single-cell transcriptome study. PLoS One 2020;15:e0230295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen CF, Chien CH, Yang YP, Chou SJ, Wang ML, Huo TI, et al. Role of dipeptidyl peptidase 4 inhibitors in diabetic patients with coronavirus-19 infection J Chin Med Assoc 2020 Apr 27Doi: 10.1097/JCMA.0000000000000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jing Y, Run-Qian L, Hao-Ran W, Hao-Ran C, Ya-Bin L, Yang G, et al. Potential influence of COVID-19/ACE2 on the female reproductive system. Mol Hum Reprod 2020 May 4pii:gaaa030.Doi: 10.1093/molehr/gaaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L, et al. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature 2020 May 7Doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 45.Mali SN, Thorat BR, Chopade AR. A Viewpoint on angiotensin-converting enzyme 2, anti-hypertensives and coronavirus disease 2019 (COVID-19). Infect Disord Drug Targets 2020 May 10Doi: 10.2174/1871526520666200511005546. [DOI] [PubMed] [Google Scholar]
- 46.Valdés G, Neves LA, Anton L, Corthorn J, Chacón C, Germain AM, et al. Distribution of angiotensin-(1-7) and ACE2 in human placentas of normal and pathological pregnancies. Placenta 2006;27:200–7. [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Douglas GC, O’Bryan MK, Hedger MP, Lee DK, Yarski MA, Smith AI, et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology 2004;145:4703–11. [DOI] [PubMed] [Google Scholar]
- 49.Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res 2000;87:E1–9. [DOI] [PubMed] [Google Scholar]
- 50.Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S, et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581:215–20. [DOI] [PubMed] [Google Scholar]
- 51.Kuba K, Imai Y, Rao S, Jiang C, Penninger JM. Lessons from SARS: control of acute lung failure by the SARS receptor ACE2. J Mol Med (Berl) 2006;84:814–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchis-Gomar F, Lavie CJ, Perez-Quilis C, Henry BM, Lippi G. Angiotensin-converting enzyme 2 and antihypertensives (angiotensin receptor blockers and angiotensin-converting enzyme inhibitors) in coronavirus disease 2019. Mayo Clin Proc 2020;95:1222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levy A, Yagil Y, Bursztyn M, Barkalifa R, Scharf S, Yagil C. ACE2 expression and activity are enhanced during pregnancy. Am J Physiol Regul Integr Comp Physiol 2008;295:R1953–61. [DOI] [PubMed] [Google Scholar]
- 55.Brosnihan KB, Neves LA, Joyner J, Averill DB, Chappell MC, Sarao R, et al. Enhanced renal immunocytochemical expression of ANG-(1-7) and ACE2 during pregnancy. Hypertension 2003;42:749–53. [DOI] [PubMed] [Google Scholar]
- 56.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res 2020;30:269–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. Compassionate use of remdesivir for patients with severe Covid-19. N Engl J Med 2020;382:2327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.COVID-19 Investigation Team. Clinical and virologic characteristics of the first 12 patients with coronavirus disease 2019 (COVID-19) in the United States. Nat Med 2020 Apr 23Doi: 10.1038/s41591-020-0877-5. [DOI] [PubMed] [Google Scholar]
- 59.Mulangu S, Dodd LE, Davey RT, Jr, Tshiani Mbaya O, Proschan M, Mukadi D, et al. ; PALM Writing Group; PALM Consortium Study Team A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med 2019;381:2293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao J, Tian Z, Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 2020;14:72–3. [DOI] [PubMed] [Google Scholar]
- 61.Ferner RE, Aronson JK. Chloroquine and hydroxychloroquine in covid-19. BMJ 2020;369:m1432. [DOI] [PubMed] [Google Scholar]
- 62.González R, Pons-Duran C, Piqueras M, Aponte JJ, Ter Kuile FO, Menéndez C. Mefloquine for preventing malaria in pregnant women. Cochrane Database Syst Rev 2018;11:CD011444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Divala TH, Mungwira RG, Mawindo PM, Nyirenda OM, Kanjala M, Ndaferankhande M, et al. Chloroquine as weekly chemoprophylaxis or intermittent treatment to prevent malaria in pregnancy in Malawi: a randomised controlled trial. Lancet Infect Dis 2018;18:1097–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chico RM, Ter Kuile FO. Back to chloroquine for malaria prophylaxis in pregnancy? Lancet Infect Dis 2018;18:1051–2. [DOI] [PubMed] [Google Scholar]
- 65.Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus Disease 2019 (COVID-19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol 2020;222:415–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jamieson DJ, Steinberg JP, Martinello RA, Perl TM, Rasmussen SA. Obstetricians on the coronavirus disease 2019 (COVID-19) front lines and the confusing world of personal protective equipment. Obstet Gynecol 2020;135:1257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wu Y, Liu C, Dong L, Zhang C, Chen Y, Liu J, et al. Coronavirus disease 2019 among pregnant Chinese women: Case series data on the safety of vaginal birth and breastfeeding. BJOG 2020 May 5Doi: 10.1111/1471-0528.16276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Qi H, Luo X, Zheng Y, Zhang H, Li J, Zou L, et al. Safe delivery for pregnancies affected by COVID-19. BJOG 2020;127:927–9. [DOI] [PubMed] [Google Scholar]


