Abstract
The US FDA has approved pulsed electromagnetic fields (PEMFs) as a safe and effective treatment for nonunions of bone. Despite its clinical use, the mechanisms of action of electromagnetic stimulation of the skeleton have been elusive. Recently, cell membrane receptors have been identified as the site of action of PEMF and provide a mechanistic rationale for clinical use. This review highlights key processes in cell responses to PEMF as follows: (1) signal transduction through A2A and A3 adenosine cell membrane receptors and (2) dose-response effects on the synthesis of structural and signaling extracellular matrix (ECM) components. Through these actions, PEMF can increase the structural integrity of bone and cartilage ECM, enhancing repair, and alter the homeostatic balance of signaling cytokines, producing anti-inflammatory effects. PEMFs exert a proanabolic effect on the bone and cartilage matrix and a chondroprotective effect counteracting the catabolic effects of inflammation in the joint environment. Understanding of PEMF membrane targets, and of the specific intracellular pathways involved, culminating in the synthesis of ECM proteins and reduction in inflammatory cytokines, should enhance confidence in the clinical use of PEMF and the identification of clinical conditions likely to be affected by PEMF exposure.
The musculoskeletal system is highly responsive to its physicochemical environment. Bone and cartilage cells respond to changes in mechanical stress, fluid flow, pH, and pO2 by altering their phenotype and expressing a range of signaling and structural molecules that result, in particular, in an altered extracellular matrix (ECM) organization and associated biomechanical properties. Response to mechanical stress is perhaps the best recognized and intuitively obvious of skeletal environmental conditions, facilitating adaptation and modeling to changing biomechanical and environmental requirements perhaps through intermediary strain-associated signaling events. In addition to mechanical stress, skeletal tissues, both bone and cartilage, demonstrate an exquisite sensitivity to electrical and electromagnetic stimulation.
Responses of skeletal cells to pulsed electromagnetic field (PEMF) have been exploited therapeutically with devices that expose tissues to appropriately configured fields to stimulate ECM synthesis for bone and cartilage repair. This review highlights key processes in cell responses to PEMF as follows: (1) signal transduction through cell membrane adenosine receptors (ARs), (2) the activation of osteoinductive pathways, and (3) the synthesis of skeletal ECM including structural and signaling molecules. These actions are reflected physiologically in bone as the healing of fractures, osteotomies, and nonunions, and, in joints, as the modulation of cartilage damage and reduction in catabolic and inflammatory cytokines in arthritis. Understanding the cellular responses to PEMF will inform clinical studies, may point to key issues that need further investigation, and will be relevant in promoting bone and cartilage repair, tissue engineering and regeneration in a repair mode, and damping inflammation in arthritis. Understanding the pathways of the activity of PEMFs provides a solid mechanistic basis for their clinical use.
Mechanisms of Cellular Responses to Pulsed Electromagnetic Field Exposure
Signal Characterization and Dosimetry
In the past 20 years, the approach to the study of the biological effects of PEMF has changed notably and the investigation methodology of pharmacology has been adopted through (1) description of the relevant physical parameters: frequency, amplitude, pulse shape, and duration; (2) dose-response effects; and (3) investigation of the mechanisms at the molecular level. The relationships among PEMF signal characteristics, exposure conditions, dosimetry, and biological responses have been investigated. For proteoglycan synthesis in bovine cartilage explants, the largest effect has been observed with 1.5 mT magnetic field peak value and 4 hours of stimulation.1 Recently, Parate et al2 have extensively tested PEMF effects on the chondrogenic differentiation of human mesenchymal stem cells (MSCs) exposed to various magnetic field amplitudes and stimulation times. They reported a dose-dependent increase in MSC chondrogenesis up to an amplitude of 2 mT and a duration of 10 minutes of exposure, showing then decreased chondrogenic activity for higher doses or longer exposure times, indicating a dosing window of amplitude and exposure duration. In a study examining the effects of power frequency (60 Hz) fields on a model of endochondral ossification (EO), an amplitude dose effect was observed on chondrogenesis measured with biochemical, immunohistochemistry, and molecular end points with a clear maximum response at 0.1 mT.3 A maximum response in terms of daily exposure time was at 7 to 9 h/d with lesser response seen at shorter and longer exposure times.
Membrane Responses to Pulsed Electromagnetic Field Through Adenosine Membrane Receptors
There is strong evidence to support a role for adenosine and its receptors in bone homeostasis and in skeletal pathology, including osteoporosis and arthritis.4 Furthermore, adenosine, acting through the A2A receptor, inhibits osteoclast differentiation and increases the rate of new bone formation in bone defects.5 A2A signaling also promotes the Wnt/β-catenin pathway regulating bone formation.6
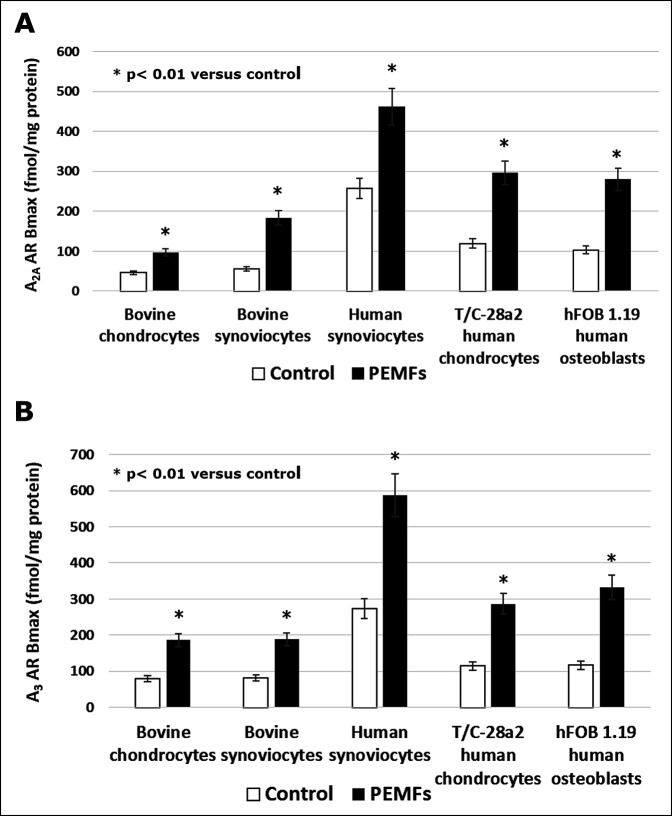
Although the transmembrane signal recognition processes of PEMF are incompletely understood, the specific mechanism of interaction between PEMF and the cell membrane was reported by Varani et al.7 They identified for the first time that ARs were the main target of PEMF stimulation in inflammatory cells; ARs play a pivotal role in the regulation of inflammatory processes, with both proinflammatory and anti-inflammatory effects.8 It has been demonstrated that PEMF exposure induces a notable increase in A2A and A3 AR density on the cell membrane of chondrocytes, synoviocytes, and osteoblasts8 (Figure 1). Notably, A1 and A2B receptors were not influenced by the same exposure conditions. Moreover, in the presence of the specific A2A receptor agonist, PEMF exposure was able to synergize with the agonist and induce a notable increase in intracellular cyclic adenosine monophosphate (cAMP) levels. On the contrary, the presence of the specific A2A receptor antagonist blocked the effects of both the agonist and PEMF stimulation, suggesting that PEMFs specifically act through the activation of A2A ARs with a pharmacologic-like mechanism. The agonist activity of PEMF for the A2A and the A3 ARs is particularly relevant because it inhibits the NF-kB pathway, which is a key regulator of the expression of matrix metalloproteinases and of several genes involved in responses to inflammation.9 Cohen et al10 showed in vivo that an experimental A2A agonist drug reduced cartilage damage in a rabbit model of septic arthritis of the knee. These observations formed the basis for the application of PEMF for chondroprotection of articular cartilage from the catabolic effects of joint inflammation, as discussed in more detail later.
Figure 1.
Bar graph showing A2A AR (A) and A3 AR (B) density in bovine chondrocytes and synoviocytes, human synoviocytes, T/C-28a2 human chondrocytes, and hFOB 1.19 human osteoblasts in the absence and in the presence of PEMFs. AR = adenosine receptor, PEMF = pulsed electromagnetic field. (Reproduced with permission from Varani K, Vincenzi F, Ravani A, et al: Adenosine receptors as a biological pathway for the anti-inflammatory and beneficial effects of low frequency low energy pulsed electromagnetic fields. Mediators Inflamm 2017;2017:2740963.)
Activation of Osteoinductive and Angiogenic Pathways by Pulsed Electromagnetic Field
The stimulation of growth factors and cytokines by PEMF as intermediary activation mechanisms has been summarized by Aaron et al.11 They presented the results of 9 studies showing activation by PEMF of the transforming growth factor (TGF)-β gene family, including bone morphogenetic proteins (BMPs) 2 and 4, and increased synthesis of corresponding proteins in a variety of skeletal models. In a detailed study of the effects of PEMF on TGF-β in a model of EO, it was shown that PEMF exposure increased chondrogenic differentiation and EO in association with a 343% increase in TGF-β immunopositive cells.12 PEMF exposure was observed to enhance, but not to disorganize, the developmental processes of chondrogenic differentiation and EO. Since those publications, several studies have appeared showing that PEMF exposure stimulates the Wnt/β-catenin signaling pathway resulting in osteogenesis. One study demonstrated improved trabecular microarchitecture by PEMF through activation of the Wnt/β-catenin pathway.13 Osteogenic differentiation of osteoblastic cells is increased by PEMF through the Wnt signaling pathway.14 PEMF has been shown to upregulate the canonical Wnt ligands, Wnt1,3a, and 10b, in association with increases in bone mass and strength.15 PEMF stimulation has also been shown to affect the Wnt signaling pathway in MC3T3-E1 osteoblast-like cells. Zhai et al16 demonstrated that PEMF exposure markedly enhanced the expression of components of the Wnt canonical signaling pathway, such as Wnt1, LRP6, and β-catenin. In ovariectomized rats, Jing et al17 showed that PEMF modulates bone microarchitecture and strength through the activation of the Wnt/LRP5/β-catenin signaling pathway. Recently, Wu et al18 proposed an interdependent Wnt/Ca2+ and Wnt/β-catenin signaling pathway for PEMF-induced osteoblastogenesis in C3H10T1/2 mesenchymal cells, suggesting that both Wnt canonical and noncanonical pathways are involved in osteogenic differentiation.
Angiogenesis is an important component of new bone formation including fracture healing and is deficient in nonunions. PEMF has been shown to increase angiogenesis and perfusion in a number of skeletal-related models.19-21 The mechanism of stimulation of angiogenesis by PEMF appears to be dependent on the stimulation of fibroblast growth factor 2, but not vascular endothelial growth factor. In femurs harvested from PEMF-treated mice, Goto et al22 reported increased expression levels of angiopoietin-2 and fibroblast growth factor 2 compared with control mice.
A variety of other pathways of intracellular and extracellular PEMF signaling have been described, perhaps dependent on the model system used. Petecchia et al23 showed that PEMFs enhance the early stages of osteogenesis in bone marrow stem cells (BMSCs) by increasing the expression of functional L-type voltage-gated calcium channels and the concentration of cytosolic-free calcium. Moreover, recent data from Bagheri et al24 showed that PEMF stimulates osteogenic differentiation of BMSCs and increases the expression of Notch4, Dll4, Hey1, Hes1, and Hes5 in osteogenic medium compared with controls, indicating that the activation of the Notch pathway is required for PEMF‐stimulated osteogenic differentiation. Poh et al25 demonstrated that PEMF induced the activation of protein kinase B (Akt) and the MAPK/ERK signaling cascade and notably upregulated collagen I, alkaline phosphatase, and osteocalcin.
Pulsed Electromagnetic Field Promotes the Synthesis of Extracellular Matrix in Skeletal Models
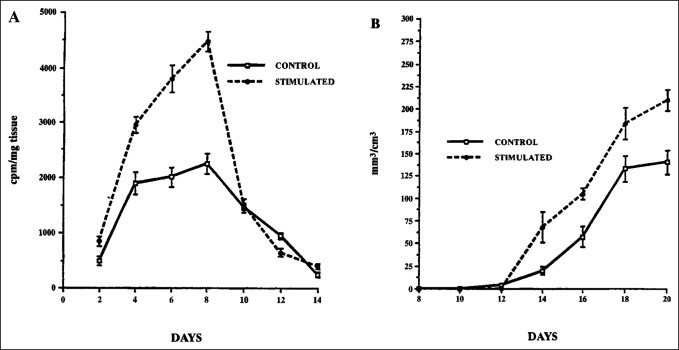
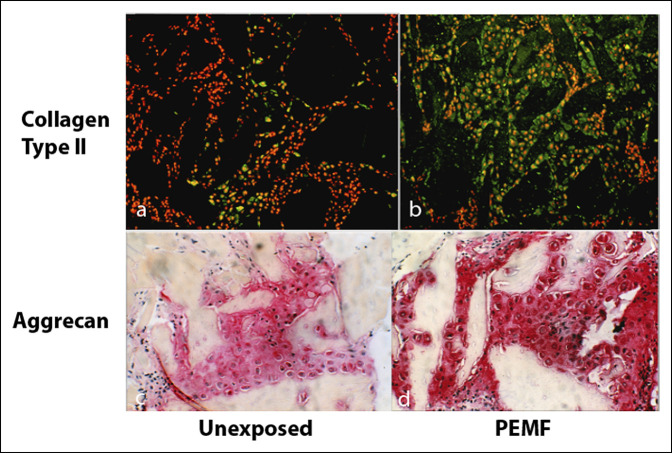
The physiologic mechanism of the response of skeletal cells to PEMF is the synthesis of ECM structural and signaling molecules in the context of repair. In a well-characterized in vivo model of EO, PEMF stimulation has been shown to increase the synthesis of cartilage and bone matrix growth factors and to enhance the proliferation and differentiation of osteoblast-like primary cells.11 The demineralized bone matrix–induced EO model developed by Hari Reddi has been extremely useful for examining the details of chondrogenesis and endochondral bone formation, including cell differentiation and ECM synthesis. It has been used in several studies to examine the response to PEMF of mesenchymal cells undergoing EO. In this model, demineralized bone matrix is prepared from rat long bones and is implanted subcutaneously along the thoracic musculature. It induces mesenchymal cell infiltration and differentiation resulting in mature bone, or ossicle, through the process of EO. Chondrogenesis peaks at day 8 of ossicle development, and then, the cartilage matrix is eroded by osteoclasts and replaced with bone that forms into mature trabeculae on days 18 to 20 of development. Exposure to PEMF of animals bearing developing ossicles resulted in an increase in chondrogenesis, on-schedule removal of cartilage, and accelerated bone formation (Figure 2).26 Immunohistochemistry demonstrates the spatial increase in proteoglycan induced by PEMF exposure (Figure 3). mRNA for aggrecan and type II collagen, aggrecan synthesis, glycosaminoglycan content, the spatial area of cartilage ECM, and the number of chondrocytes are all increased by PEMF exposure (Table 1). The ratio of chondrocytes to ECM is unchanged, indicating normal morphology with PEMF exposure. In a detailed study of chondrogenesis with this model, proteoglycan and glycosaminoglycan molecular sizes and chemical composition were normal with PEMF stimulation.27 The increased expression of cartilage macromolecules, greater immunoreactivity of 3B3 and 5D4 epitopes, and no change in DNA content or 3H-thymidine incorporation suggested increased cell differentiation as the most likely mechanism of PEMF activity. Studies with this model have shown an increase in TGF-β mRNA and protein levels in association with chondrogenic differentiation with rapid progression to EO and bone formation.12 The locus of the stimulation of endochondral bone formation by PEMF was shown to reside in the early mesenchymal stage before chondrogenesis.28
Figure 2.
Graph showing the incorporation of radiolabeled sulfate into proteoglycan and bone formation. A, Ossicles exposed to PEMF stimulation exhibit a notable increase in proteoglycan synthesis by day 4 of development, peaking during maximal chondrogenesis on day 8 (P = 0.001) and falling to control levels coincident with the onset of calcification. B, Trabecular bone formation is increased with PEMF stimulation. PEMF = pulsed electromagnetic field. (Adapted from Aaron RK, Ciombor DM: Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res 1996;14:582-589.)
Figure 3.
Immunohistochemistry of type II collagen and aggrecan in the DBM-EO model with and without exposure to PEMF. Dark areas in (A and B) and light areas in (C and D) are DBM particles. PEMF stimulation increases both ECM molecules. DBM = demineralized bone matrix, ECM = extracellular matrix, EO = endochondral ossification, PEMF = pulsed electromagnetic field
Table 1.
Increase in Indices of Chondrogenesis on Day 8 of Ossicle Development by Exposure to PEMF12
| Control | PEMF | Percent | P | |
| mRNA aggrecan | 6.1 | 22.5 | 269 | 0.02 |
| mRNA type II collagen | 11.8 | 21.9 | 86 | 0.05 |
| 35SO4 incorporation (cpm/mg) | 2166 ± 387 | 4448 ± 293 | 105 | 0.005 |
| GAG content (μg/mg) | 1.4 ± 0.2 | 2.5 ± 0.2 | 79 | 0.01 |
| Cartilage area (mm2) | 24 ± 2.1 | 148 ± 11.7 | 517 | 0.001 |
| Chondrocytes (n) | 701 ± 227 | 3582 ± 675 | 411 | 0.005 |
| Chondrocyte/cartilage | 29.2 | 24.2 | n.s. |
GAG = glycosaminoglycan, PEMF = pulsed electromagnetic field
Wang et al29 observed that PEMFs stimulate osteogenic differentiation and mineralization through the activation of the sAC-cAMP-PKA-CREB signaling pathway. Ongaro et al reported that PEMF stimulation increases alkaline phosphatase activity, osteocalcin, and matrix mineralization in MSCs isolated from both BMSCs and adipose tissue–derived MSCs.9 In particular, in BMSCs, PEMF showed a synergistic action with BMP-2, an essential growth factor for bone cells.30 Moreover, PEMFs have been shown to induce BMP-2 mRNA expression in human bone marrow stromal cells.31 Ehnert et al32 demonstrated increased proliferation and osteogenic differentiation with PEMF exposure in a coculture system of adipose tissue–derived MSCs and osteoblasts. The result of the signaling processes is to instruct skeletal cells to synthesize structural ECM and signaling molecules, enhance the ability of skeletal tissues to respond to changing physicochemical environments and biomechanical demands, and facilitate repair.
Other models have demonstrated PEMF effects on bone repair. Canè et al,33 in holes drilled into the metacarpal bone of male horses, showed that new trabecular growth was 3.4 µm/d in bone exposed to PEMF compared with 1.8 µm/d in controls. In a rat fibular osteotomy model, Midura et al34 observed, 9 days after surgery, a 2-fold faster rate of hard callus formation in PEMF-treated limbs, yielding a 2-fold increase in callus volume by 13 to 20 days after surgery. Fassina et al,35 using PEMF-stimulated SAOS-2 human osteoblasts, showed enhanced cellular proliferation and increased expression of decorin, fibronectin, osteocalcin, osteopontin, TGF-β1, type I collagen, and type III collagen. Together, the studies represent the rationale for the clinical use of PEMF in promoting bone healing.
Pulsed Electromagnetic Field Stimulation of Bone Fracture Healing
Fresh Fractures and Surgical Osteotomies
Clinical PEMF stimulation is widely applied in both the United States and Europe as a noninvasive and safe therapy to promote bone repair. Considerable level 1 evidence has accumulated, demonstrating clinical efficacy of PEMF exposure in accelerating healing of fresh fractures and osteotomies (Table 2). Borsalino, in patients undergoing femoral osteotomy, and Mammi, in patients undergoing tibial osteotomy, demonstrated the efficacy of PEMF in promoting bone healing.36,37 Fontanesi, in fresh tibial fractures, and Faldini, in femoral neck fractures, reported, respectively, a reduction in time to union and an increase in the percentage of fracture healing in PEMF-stimulated patients compared with controls.38,39
Table 2.
Level I Studies of Bone Healing With PEMF9
| Reference | Clinical application |
| Poli et al, 1985 | Congenital nonunion, intramedullary nail fixation |
| Borsalino et al36 | Femoral intertrochanteric osteotomies |
| Sharrard, 1990 | Tibial nonunion, cast |
| Mammi et al37 | Tibial osteotomies |
| Capanna et al, 1994 | Osteotomy, tumor resection, and bone graft |
| Simonis et al, 2003 | Tibial nonunion, osteotomy, and external fixator |
| Faldini et al39 | Femoral neck fractures treated with screws |
| Hannemann et al, 2012 | Acute scaphoid fractures treated with cast |
| Shi et al45 | Nonunion of long bone, nail, and plate |
PEMF = pulsed electromagnetic field
Fracture Nonunions
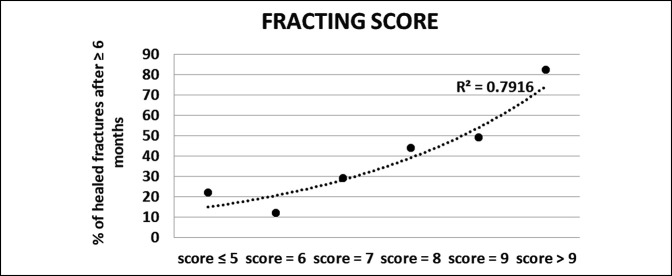
In the United States, PEMF exposure is FDA-approved for the treatment of fracture nonunions. Considerable clinical evidence supports the use of PEMF for nonunions with reported healing rates for nonunions after PEMF stimulation between 73% and 85%.40 Hinsenkamp et al41 reported a success rate above 70% in a European multicenter study including 308 patients. Traina et al42 achieved higher success rates (87.8% versus 69%) and shorter healing time with PEMF stimulation compared with surgery in nonunions of at least 9 months from trauma.42 Notably, in the PEMF group, the presence of infection did not negatively affect the effectiveness of the stimulation.42 In a Spanish retrospective cohort study of tibial nonunions, Cebriàn et al43 reported a healing rate of 91% in the PEMF-stimulated group compared with 83% of the control group. Recently, in a study of 1,382 patients with fracture nonunions, Murray and Pethica44 showed that patients who used PEMF stimulation for 9 hours or more per day healed 76 days earlier than patients who used PEMF stimulation for 3 hours or less per day. In a prospective randomized controlled study, Shi et al45 reported that early application of PEMF notably increased union rates in patients with long-bone delayed unions. Cost-benefit analysis appeared to justify early PEMF application for fractures that are likely to require a long time to heal. In this regard, it is worth highlighting the effort undertaken by the Italian group of orthopaedic surgeons, who developed the “FRACTING score” that can be applied to reliably estimate months needed for fracture healing and to accurately identify fractures at risk of nonunion46 (Figure 4).
Figure 4.
Graph showing that the FRACTING score reliably identifies fractures healing in greater than 6 months (delayed union). Multiple risk factors are combined to produce the FRACTING score.
Pulsed Electromagnetic Field Effects on Cartilage and Joint Biology
Articular Cartilage
Because cartilage is composed of a highly electrically charged ionic gel matrix, mechanical strain produces electrokinetic events, including streaming potentials, in addition to other physicochemical changes that may act as cell signaling events. Cartilage explants in vitro, and cartilage models in vivo, have been quite responsive to externally applied PEMFs with the response expressed as enhanced synthesis of ECM and supporting cytokines, particularly of the TGF-β/BMP family. Synthesis of cartilage ECM molecules with electromagnetic stimulation has been summarized in several reviews.47-49
The responsiveness of bovine cartilage explants to PEMF exposure has been studied in some detail. Using macromolecular radiolabeled sulfate incorporation into proteoglycan, notable increases in proteoglycan synthesis have been observed in PEMF-exposed, compared with unstimulated control, bovine cartilage explants (Figure 5).50 The proteoglycan molecules in PEMF-exposed explants were of equivalent size and degree of sulfation as were the controls, indicating the synthesis of normal proteoglycan molecules. An interesting observation was that exposure to PEMF increased proteoglycan synthesis in older cartilage to the level of that of cartilage from younger animals. PEMF stimulation of proteoglycan synthesis has been shown to be additive to that of IGF-1.51 PEMF + IGF-1 increased proteoglycan synthesis by 56% at maximum dosage compared with 25% by IGF-1 alone in bovine cartilage explants. Also, in bovine articular cartilage explants, PEMF stimulation has been shown to promote proteoglycan synthesis in the presence of IL-1β, thus antagonizing the catabolic activity of proinflammatory cytokines.52
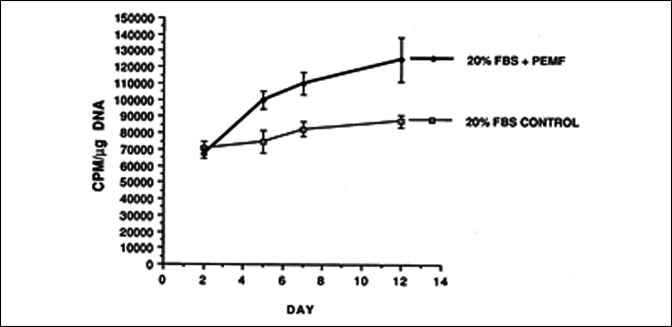
Figure 5.
Graph showing proteoglycan synthesis in adult bovine articular cartilage aged 4 to 6 years. Exposure to PEMF notably increases macromolecular radiolabeled sulfate into proteoglycans during 14 days of culture.50 FBS = fetal bovine serum, PEMF = pulsed electromagnetic field
In human osteoarthritic cartilage explants derived from knee arthroplasty, PEMF exposure stimulated proteoglycan synthesis in less pathologically involved cartilage, as shown by Mankin histological-histochemical scores.53 In cartilage with Mankin scores of eight or greater (of a possible score of 14), PEMF had no effect, suggesting that therapeutic effects of the fields were best expected in early osteoarthritis (OA), before major structural matrix damage. The increase in proteoglycan synthesis was paralleled by an increase in stiffness, or aggregate modulus, exhibiting a dose effect of exposure duration: 1-hr exposure was more effective than 4-hour exposure in increasing cartilage stiffness.
Articular Inflammation and Cytokines
The detrimental effect of inflammation on joint cartilage results in cartilage degradation and arthritis. An agonist activity for the A2A receptor can physiologically counteract inflammation and inhibit synthesis and release of proinflammatory cytokines. PEMF has a strong agonist activity for ARs. PEMF exposure induces a specific overexpression of A2A and A3 ARs in human chondrocytes. In particular, PEMFs have been shown to potentiate the responses of A2A and A3 AR agonists on cAMP production, suggesting a synergistic effect of biophysical stimulation and A2A and A3 AR activation. PEMFs also synergized with A2A AR agonists in potentiating the proliferative action on both human chondrocytes and osteoblasts.8 PEMF stimulation, through the activation of A2A and A3 ARs, has been shown to inhibit the NF-kB pathway, leading to decreased release of TNF-α and IL-1β by human synoviocytes and chondrocytes and reduced synthesis of prostaglandin E2 (PGE2) and COX-2 by bovine synoviocytes.9 Ongaro et al demonstrated in human synovial fibroblasts from patients with OA that PEMF stimulation reduces the synthesis of inflammatory mediators such as PGE2, IL-6, and IL-8, while stimulating the release of the anti-inflammatory interleukin-10.9 These observations suggest that PEMFs exert a proanabolic effect on the cartilage matrix and a chondroprotective effect counteracting the catabolic effects of inflammation in the joint environment. Together, these data suggest that PEMF may prevent or limit articular cartilage degradation, leading to joint preservation.
In Vivo Models of Osteoarthritis
The chondroprotective hypothesis has been tested in vivo in the Dunkin-Hartley guinea pig, an animal model that develops spontaneous OA at 9 to 12 months of age. The guinea pig exhibits the stereotypical loss of articular cartilage Safranin-O staining, surface fibrillation, and deep matrix clefts as observed in human OA. Preservation of ECM was observed in guinea pigs exposed to PEMF, and Mankin histological-histochemical scores demonstrated substantial differences in matrix and cell loss between the two groups (Figure 6).54,55 The pattern of articular cytokines was quite different between the PEMF-exposed and control groups. PEMF exposure resulted in an increase in TGF-β immunopositive cells and a corresponding decrease in IL-1 and matrix-degrading enzymes, suggesting that the cytokine environment of the arthritic joints was modified in this model (Table 3). Parallel studies with the same animal model concentrated on structural preservation of bone and cartilage by PEMF exposure.55 This study demonstrated both lower Mankin scores, indicating the preservation of cartilage ECM, and reductions in subchondral bone thickness in PEMF-treated animals compared with untreated controls, indicating reductions in pathological bone remodeling (Table 4).
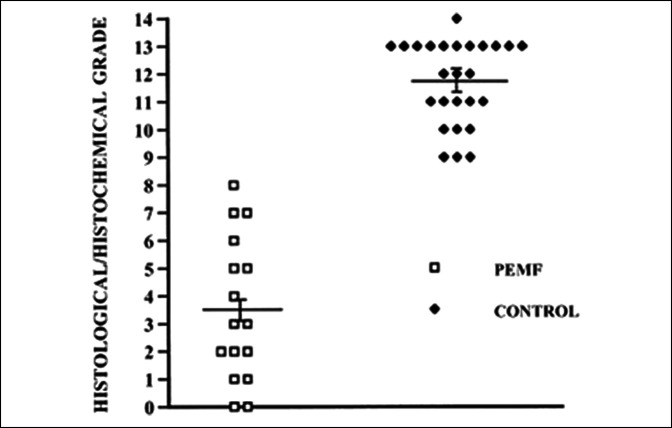
Figure 6.
Graph showing the histological/histochemical grade of tibial articular cartilage. Mean grade of control tibias was 11.7 ± 0.3 compared with 3.5 ± 0.7 of PEMF-treated cartilage (P = 0.0001), reflecting the preservation of cartilage morphology in the PEMF-treated group. PEMF = pulsed electromagnetic field. (Reprinted with permission from Ciombor DM, Aaron RK, Wang S, Simon B: Modification of osteoarthritis by pulsed electromagnetic field-a morphological study. Osteoarthritis Cartilage 2003; 11:455-462.)
Table 3.
Immunopositive Cells (Per Unit Area) With PEMF Exposure54
| Control | PEMF | % Change | P | |
| MMP-13 | 7.2 ± 0.8 | 0.0 ± 0.0 | — | 0.01 |
| MMP-3 | 13.8 ± 1.9 | 8.4 ± 1.0 | −39 | 0.02 |
| IL-1 | 13.9 ± 2.3 | 7.2 ± 0.6 | −48 | 0.01 |
| IRAP | 10.5 ± 1.0 | 17.3 ± 1.1 | +65 | 0.003 |
| TGF-β | 20.0 ± 3.5 | 34.4 ± 2.8 | +72 | 0.006 |
IRAP = Interleukin-1 receptor antagonist protein, MMP = matrix metalloproteinase, PEMF = pulsed electromagnetic field, TGF-β = transforming growth factor β
Table 4.
Cartilage Histological Score and Subchondral Bone Thickness Results for Sham-Treated and PEMF-Treated Animals
| Measurement Site | Cartilage Histological Score | Subchondral Bone Thickness (μ) | ||
| Sham Treated | PEMF Treated | Sham Treated | PEMF Treated | |
| Medial tibia plateau | 10.9 ± 0.9 | 3.9 ± 0.7a | 284.0 ± 38.0 | 242.2 ± 27.3a |
| Medial femoral condyle | 7.4 ± 0.8 | 2.5 ± 0.7a | 325.0 ± 32.8 | 271.0 ± 27.5a |
| Lateral tibia plateau | 6.4 ± 1.0 | 1.6 ± 0.4a | 304.3 ± 31.4 | 265.4 ± 29.2a |
| Lateral femoral condyle | 5.9 ± 0.9 | 1.8 ± 0.5a | 306.5 ± 34.4 | 261.7 ± 28.3a |
PEMF = pulsed electromagnetic field
Data adopted from Fini M, Giavaresi G, Torricelli P, Cavani F, Setti S, Cane V, Giardino R: Pulsed electromagnetic fields reduce knee osteoarthritic lesion progression in the aged Dunkin Hartley guinea pig. J Orthop Res 2005;23:899–908.
P < 0.05.
In agreement with these findings, Yang et al56 reported that preemptive and early PEMF treatment in low-dose monosodium iodoacetate–treated rats increased bone and cartilage synthesis, while decreasing bone and cartilage degradation. They concluded that the efficacy of PEMF exposure on OA is associated with an early application of treatment. Additional important PEMF effects on cytokine synthesis were observed in adult sheep treated with autologous osteochondral grafts. PEMF favored graft integration and prevented graft reabsorption. Notably, lower levels of inflammatory catabolic cytokines (IL-1β and TNF-α) and higher concentration of TGF-β were measured in the synovial fluid of PEMF-treated animals compared with controls. On the basis of these studies collectively, Fini et al55 suggested that PEMF stimulation could play a significant role in tissue engineering treatment protocols.
Clinical Observations of Pulsed Electromagnetic Field Effects After Joint Surgery
Joint surgery modifies the local cytokine environment resulting in increased levels of proinflammatory cytokines in synovial fluid that can have a catabolic effect on cartilage ECM, ultimately leading to the development of OA.57 Based on its expected chondroprotective and anti-inflammatory effects, PEMF treatment has been applied to the knee joint, after surgery, to preserve cartilage integrity and to promote functional recovery.
After arthroscopic treatment of cartilage lesions in the knee, Zorzi et al58 reported a notable reduction in the percentage of patients using NSAIDs in the PEMF-treated group compared with the controls. They also reported improvement in functional recovery in PEMF-treated patients 90 days after surgery and the maintenance of such advantage up to 3 years of follow-up. PEMF stimulation has also been shown to be effective in patients undergoing anterior cruciate ligament reconstruction. In a randomized double-blind study, Benazzo et al59 showed reduction in the recovery time and early return-to-sport activity. Similar results have been reported by Osti et al60 in patients undergoing microfracture for the treatment of OA of the knee. PEMF stimulation was able to markedly improve the American Orthopaedic Foot and Ankle Society score and to markedly reduce pain levels in patients with talar osteochondral lesions treated with collagen scaffolds seeded with bone marrow–derived cells at 6 and 12 months of follow-up.61 Similar results have been obtained with PEMF stimulation in association with matrix-assisted autologous chondrocyte implantation in the treatment of chondral lesions of the knee.62 Finally, two recent Italian studies on patients undergoing total knee arthroplasty reported notably reduced pain and knee swelling, together with improved functional scores in PEMF-exposed patients.63,64
As a conservative treatment, PEMF stimulation has been used to relieve pain in patients with early-stage OA. Thamsborg et al indicated that, compared with older subjects, younger patients (<65 years) had a better functional outcomes.65 Furthermore, in early-stage OA, Gobbi et al66 reported notable improvement in pain, knee function, and quality of life at 1-year follow-up in the PEMF-stimulated patients. Similar results have been described by Servodio Iammarrone et al67 in patients with patellofemoral pain; PEMF stimulation was able to improve joint function, favor pain resolution, and shorten the time to return-to-sport activity.
Summary
In 1979, the US FDA approved PEMF as a safe and effective treatment for nonunions of bone. Since then, the use of PEMF stimulation for bone repair has grown both in the United States and in Europe. In the United States, a survey showed that 72% of hospitals offer bone repair stimulation treatments for fractures that fail to heal. Analogous to pharmacodynamics as a key step in drug adoption, PEMF dose-response effects provide a solid conceptual basis for clinical use. The local field of biological activity, as opposed to systemic effects, represents a notable advantage of PEMF together with a lack of negative adverse effects in relation to its efficacy.
Despite its clinical use, the mechanisms of action of electromagnetic stimulation of the skeleton have been elusive, and PEMF has been viewed as a “black box.” In the past 25 years, research has been successful in identifying cell membrane receptors and osteoinductive pathways as sites of action of PEMF and provides a mechanistic rationale for clinical use. Understanding of PEMF membrane targets, and of the specific intracellular and extracellular pathways involved, culminating in the synthesis of ECM proteins and reduction in inflammatory cytokines, should enhance confidence in the clinical use of PEMF and the identification of clinical conditions likely to be affected by PEMF exposure.
The biological effects of PEMF treatment and favorable effects on the skeletal system are the result of notable research efforts conducted internationally by the orthopaedic community, and they have attracted much interest from other medical specialties such as wound and tendon healing, rheumatology, and neurology that may be able to take advantage of the experiences developed with bone and cartilage treatments.
Footnotes
Dr. Cadossi: president, IGEA. Dr. Aaron or an immediate family member serves as a paid consultant to Zimmer Biomet. Neither of the following authors nor any immediate family member has received anything of value from or has stock or stock options held in a commercial company or institution related directly or indirectly to the subject of this article: Dr. Massari and Ms. Racine-Avila.
References
- 1.De Mattei M, Fini M, Setti S, et al. : Proteoglycan synthesis in bovine articular cartilage explants exposed to different low-frequency low-energy pulsed electromagnetic fields. Osteoarthritis Cartilage 2007;15:163-168. [DOI] [PubMed] [Google Scholar]
- 2.Parate D, Franco-Obregon A, Frohlich J, et al. : Enhancement of mesenchymal stem cell chondrogenesis with short-term low intensity pulsed electromagnetic fields. Sci Rep 2017;7:9421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aaron RK, Ciombor DM, Keeping H, Wang S, Capuano A, Polk C: Power frequency fields promote cell differentiation coincident with an increase in transforming growth factor-beta(1) expression. Bioelectromagnetics 1999;20:453-458. [DOI] [PubMed] [Google Scholar]
- 4.Ham J, Evans BA: An emerging role for adenosine and its receptors in bone homeostasis. Front Endocrinol (Lausanne) 2012;3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mediero A, Wilder T, Cronstein B: Adenosine receptors stimulate bone regeneration, in Biology and Pathology of Bone and Joint: Osteoclasts, Osteoblasts and Bone Remodeling. Boston, MA, ACR/ARP Annual Meeting, 2014, Abstract 19. [Google Scholar]
- 6.Borhani S, Corciulo C, Larranaga Vera A, Cronstein B: Signaling at adenosine A2A receptor (A2aR) in osteoblasts; crosstalk with Wnt/β-catenin signaling pathway, in Osteoarthritis and Joint Biology—Basic Science Poster I. Chicago, IL, ACR/ARP Annual Meeting, 2018, Abstract 1047. [Google Scholar]
- 7.Varani K, Gessi S, Merighi S, et al. : Effect of low frequency electromagnetic fields on A2A adenosine receptors in human neutrophils. Br J Pharmacol 2002;136:57-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varani K, Vincenzi F, Ravani A, et al. : Adenosine receptors as a biological pathway for the anti-inflammatory and beneficial effects of low frequency low energy pulsed electromagnetic fields. Mediators Inflamm 2017;2017:2740963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massari L, Benazzo F, Falez F, et al. : Biophysical stimulation of bone and cartilage: State of the art and future perspectives. Int Orthop 2019;43:539-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SB, Gill SS, Baer GS, Leo BM, Scheld WM, Diduch DR: Reducing joint destruction due to septic arthrosis using an adenosine2A receptor agonist. J Orthop Res 2004;22:427-435. [DOI] [PubMed] [Google Scholar]
- 11.Aaron RK, Boyan B, Ciombor DM, Schwartz Z, Simon BJ: Stimulation of growth factor by electric and electromagnetic fields. Clin Orthop Rel Res 2004;419:30-37. [DOI] [PubMed] [Google Scholar]
- 12.Aaron RK, Wang S, Ciombor DM: Upregulation of basal TGFß1 levels by EMF coincident with chondrogenesis - implications of skeletal repair and tissue engineering. J Orthop Res 2002;20:233-240. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, He H, Yang L, et al. : Effects of pulsed electromagnetic fields on bone mass and Wnt/beta-catenin signaling pathway in ovariectomized rats. Arch Med Res 2012;43:274-282. [DOI] [PubMed] [Google Scholar]
- 14.Lin CC, Lin RW, Chang CW, Wang GJ, Lai KA: Single-pulsed electromagnetic field therapy increases osteogenic differentiation through Wnt signaling pathway and sclerostin downregulation. Bioelectromagnetics 2015;36:494-505. [DOI] [PubMed] [Google Scholar]
- 15.Cai J, Shao X, Yang Q, et al. : Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone 2020;133:115266. [DOI] [PubMed] [Google Scholar]
- 16.Zhai M, Jing D, Tong S, et al. : Pulsed electromagnetic fields promote in vitro osteoblastogenesis through a Wnt/beta-catenin signaling-associated mechanism. Bioelectromagnetics 2016;37:152-162. [DOI] [PubMed] [Google Scholar]
- 17.Jing D, Li F, Jiang M, et al. : Pulsed electromagnetic fields improve bone microstructure and strength in ovariectomized rats through a Wnt/Lrp5/beta-catenin signaling-associated mechanism. PLoS One 2013;8:e79377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu S, Yu Q, Lai A, Tian J: Pulsed electromagnetic field induces Ca(2+)-dependent osteoblastogenesis in C3H10T1/2 mesenchymal cells through the Wnt-Ca(2+)/Wnt-beta-catenin signaling pathway. Biochem Biophys Res Commun 2018;503:715-721. [DOI] [PubMed] [Google Scholar]
- 19.Pan Y, Dong Y, Hou W, et al. : Effects of PEMF on microcirculation and angiogenesis in a model of acute hindlimb ischemia in diabetic rats. Bioelectromagnetics 2013;34:180-188. [DOI] [PubMed] [Google Scholar]
- 20.Tepper OM, Callaghan MJ, Chang EI, et al. : Electromagnetic fields increase in vitro and in vivo angiogenesis through endothelial release of FGF-2. FASEB J 2004;18:1231-1233. [DOI] [PubMed] [Google Scholar]
- 21.Hopper RA, VerHalen JP, Tepper O, et al. : Osteoblasts stimulated with pulsed electromagnetic fields increase HUVEC proliferation via a VEGF-A independent mechanism. Bioelectromagnetics 2009;30:189-197. [DOI] [PubMed] [Google Scholar]
- 22.Goto T, Fujioka M, Ishida M, Kuribayashi M, Ueshima K, Kubo T: Noninvasive up-regulation of angiopoietin-2 and fibroblast growth factor-2 in bone marrow by pulsed electromagnetic field therapy. J Orthop Sci 2010;15:661-665. [DOI] [PubMed] [Google Scholar]
- 23.Petecchia L, Sbrana F, Utzeri R, et al. : Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca(2+)-related mechanisms. Sci Rep 2015;5:13856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagheri L, Pellati A, Rizzo P, et al. : Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J Tissue Eng Regen Med 2018;12:304-315. [DOI] [PubMed] [Google Scholar]
- 25.Poh PSP, Seeliger C, Unger M, Falldorf K, Balmayor ER, van Griensven M: Osteogenic effect and cell signaling activation of extremely low-frequency pulsed electromagnetic fields in adipose-derived mesenchymal stromal cells. Stem Cells Int 2018;2018:5402853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aaron RK, Ciombor DM, Jolly G: Stimulation of experimental endochondral ossification by low energy pulsing electromagnetic fields. J Bone Min Res 1989;4:227-233. [DOI] [PubMed] [Google Scholar]
- 27.Ciombor DM, Lester G, Aaron RK, Neame P, Caterson B: Low frequency EMF regulates chondrocyte differentiation and expression of matrix proteins. J Orthop Res 2002;20:40-50. [DOI] [PubMed] [Google Scholar]
- 28.Aaron RK, Ciombor DM: Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res 1996;14:582-589. [DOI] [PubMed] [Google Scholar]
- 29.Wang YY, Pu XY, Shi WG, et al. : Pulsed electromagnetic fields promote bone formation by activating the sAC-cAMP-PKA-CREB signaling pathway. J Cell Physiol 2019;234:2807-2821. [DOI] [PubMed] [Google Scholar]
- 30.Ongaro A, Pellati A, Bagheri L, Fortini C, Setti S, De Mattei M: Pulsed electromagnetic fields stimulate osteogenic differentiation in human bone marrow and adipose tissue derived mesenchymal stem cells. Bioelectromagnetics 2014;35:426-436. [DOI] [PubMed] [Google Scholar]
- 31.Jansen JH, van der Jagt OP, Punt BJ, et al. : Stimulation of osteogenic differentiation in human osteoprogenitor cells by pulsed electromagnetic fields: An in vitro study. BMC Musculoskelet Disord 2010;11:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ehnert S, van Griensven M, Unger M, et al. : Co-culture with human osteoblasts and exposure to extremely low frequency pulsed electromagnetic fields improve osteogenic differentiation of human adipose-derived mesenchymal stem cells. Int J Mol Sci 2018. Mar 27;19(4). pii: E994. doi: 10.3390/ijms19040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cane V, Botti P, Farneti D: Electromagnetic stimulation of bone repair: A histomorphometric study. J Orthop Res 1991;9:908-917. [DOI] [PubMed] [Google Scholar]
- 34.Midura RJ, Ibiwoye MO, Powell KA, et al. : Pulsed electromagnetic field treatments enhance the healing of fibular osteotomies. J Orthop Res 2005;23:1035-1046. [DOI] [PubMed] [Google Scholar]
- 35.Fassina L, Visai L, Benazzo F, et al. : Effects of electromagnetic stimulation on calcified matrix production by SAOS-2 cells over a polyurethane porous scaffold. Tissue Eng 2006;12:1985-1999. [DOI] [PubMed] [Google Scholar]
- 36.Borsalino G, Bagnacani M, Bettati E, et al. : Electrical stimulation of human femoral intertrochanteric osteotomies: Double-blind study. Clin Orthop Relat Res 1988;237:256-263. [PubMed] [Google Scholar]
- 37.Mammi GI, Rocchi R, Cadossi R, Massari L, Traina GC: The electrical stimulation of tibial osteotomies: Double-blind study. Clin Orthop Relat Res 1993;288:246-253. [PubMed] [Google Scholar]
- 38.Fontanesi G, Traina GC, Giancecchi F, et al. : Slow healing fractures: Can they be prevented? (Results of electrical stimulation in fibular osteotomies in rats and in diaphyseal fractures of the tibia in humans). Ital J Orthop Traumatol 1986;12:371-385. [PubMed] [Google Scholar]
- 39.Faldini C, Cadossi M, Luciani D, Betti E, Chiarello E, Giannini S: Electromagnetic bone growth stimulation in patients with femoral neck fractures treated with screws: Prospective randomized double-blind study. Curr Orthopaedic Pract 2010;21:282-287. [Google Scholar]
- 40.Assiotis A, Sachinis NP, Chalidis BE: Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. J Orthop Surg Res 2012;7:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hinsenkamp M, Tuerlinckx B, Rooze M: Effect of elf fields on bone growth and fracture repair, in Grandolfo M, Michaelson SM, Rindi A, eds: Biological Effects and Dosimetry of Static and ELF Electromagnetic Fields. Boston, MA, Springer, 1985, pp 441-476. [Google Scholar]
- 42.Traina GC, Fontanesi G, Costa P, et al. : Effect of electromagnetic stimulation on patients suffering from non-union. A retrospective study with a control group. J Bioelectricity 1991;10:101-117. [Google Scholar]
- 43.Cebrian JL, Gallego P, Frances A, et al. : Comparative study of the use of electromagnetic fields in patients with pseudoarthrosis of tibia treated by intramedullary nailing. Int Orthop 2010;34:437-440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murray HB, Pethica BA: A follow-up study of the in-practice results of pulsed electromagnetic field therapy in the management of nonunion fractures. Orthop Res Rev 2016;8:67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shi HF, Xiong J, Chen YX, et al. : Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: A prospective randomized controlled study. BMC Musculoskelet Disord 2013;14:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massari L, Benazzo F, Falez F, et al. : Can clinical and surgical parameters Be combined to predict how long it will take a tibia fracture to heal? A prospective multicentre observational study: The FRACTING study. Biomed Res Int 2018;2018:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fini M, Giavaresi G, Carpi A, Nicolini A, Setti S, Giardino R: Effects of pulsed electromagnetic fields on articular hyaline cartilage: Review of experimental and clinical studies. Biomed Pharmacother 2005;59:388-394. [DOI] [PubMed] [Google Scholar]
- 48.Aaron RK, Ciombor DM, Wang S, Simon B: Clinical biophysics: The promotion of skeletal repair by physical forces. Ann N Y Acad Sci 2006;1068:513-531. [DOI] [PubMed] [Google Scholar]
- 49.Vaca-Gonzalez JJ, Guevara JM, Moncayo MA, Castro-Abril H, Hata Y, Garzon-Alvarado DA: Biophysical stimuli: A review of electrical and mechanical stimulation in hyaline cartilage. Cartilage 2019;10:157-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ciombor D, Aaron R: Mechanomimetic effects of EMF on articular cartilage, in Orthopaedic Research Society, New Orleans, LA, 2003, vol 28, p 675. [Google Scholar]
- 51.De Mattei M, Pellati A, Pasello M, et al. : Effects of physical stimulation with electromagnetic field and insulin growth factor-I treatment on proteoglycan synthesis of bovine articular cartilage. Osteoarthritis Cartilage 2004;12:793-800. [DOI] [PubMed] [Google Scholar]
- 52.De Mattei M, Pasello M, Pellati A, et al. : Effects of electromagnetic fields on proteoglycan metabolism of bovine articular cartilage explants. Connect Tissue Res 2003;44:154-159. [PubMed] [Google Scholar]
- 53.Chan C, Ciombor D, Fleming B, Rotenberg J, Aaron R: Reconstitution of proteoglycans in human osteoarthritic knee cartilage and its biomechanical consequences, in Orthopaedic Research Society, Boston, MA, 2006, vol 31, p 1529. [Google Scholar]
- 54.Ciombor DM, Aaron RK, Wang S, Simon BJ: Modification of osteoarthritis by pulsed electromagnetic field - a morphological study. Osteoarthritis Cartilage 2003;11:455-462. [DOI] [PubMed] [Google Scholar]
- 55.Fini M, Pagani S, Giavaresi G, et al. : Functional tissue engineering in articular cartilage repair: Is there a role for electromagnetic biophysical stimulation? Tissue Eng Part B Rev 2013;19:353-367. [DOI] [PubMed] [Google Scholar]
- 56.Yang X, He H, Zhou Y, et al. : Pulsed electromagnetic field at different stages of knee osteoarthritis in rats induced by low-dose monosodium iodoacetate: Effect on subchondral trabecular bone microarchitecture and cartilage degradation. Bioelectromagnetics 2017;38:227-238. [DOI] [PubMed] [Google Scholar]
- 57.Bigoni M, Sacerdote P, Turati M, et al. : Acute and late changes in intraarticular cytokine levels following anterior cruciate ligament injury. J Orthop Res 2013;31:315-321. [DOI] [PubMed] [Google Scholar]
- 58.Zorzi C, Dall'Oca C, Cadossi R, Setti S: Effects of pulsed electromagnetic fields on patients' recovery after arthroscopic surgery: Prospective, randomized and double-blind study. Knee Surg Sports Traumatol Arthrosc 2007;15:830-834. [DOI] [PubMed] [Google Scholar]
- 59.Benazzo F, Zanon G, Pederzini L, et al. : Effects of biophysical stimulation in patients undergoing arthroscopic reconstruction of anterior cruciate ligament: Prospective, randomized and double blind study. Knee Surg Sports Traumatol Arthrosc 2008;16:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osti L, Del Buono A, Maffulli N: Application of pulsed electromagnetic fields after microfractures to the knee: A mid-term study. Int Orthop 2015;39:1289-1294. [DOI] [PubMed] [Google Scholar]
- 61.Cadossi M, Buda RE, Ramponi L, Sambri A, Natali S, Giannini S: Bone marrow-derived cells and biophysical stimulation for talar osteochondral lesions: A randomized controlled study. Foot Ankle Int 2014;35:981-987. [DOI] [PubMed] [Google Scholar]
- 62.Collarile M, Sambri A, Lullini G, Cadossi M, Zorzi C: Biophysical stimulation improves clinical results of matrix-assisted autologous chondrocyte implantation in the treatment of chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc 2018;26:1223-1229. [DOI] [PubMed] [Google Scholar]
- 63.Moretti B, Notarnicola A, Moretti L, et al. : I-ONE therapy in patients undergoing total knee arthroplasty: A prospective, randomized and controlled study. BMC Musculoskelet Disord 2012;13:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adravanti P, Nicoletti S, Setti S, Ampollini A, de Girolamo L: Effect of pulsed electromagnetic field therapy in patients undergoing total knee arthroplasty: A randomised controlled trial. Int Orthop 2014;38:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thamsborg G, Florescu A, Oturai P, Fallentin E, Tritsaris K, Dissing S: Treatment of knee osteoarthritis with pulsed electromagnetic fields: A randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage 2005;13:575-581. [DOI] [PubMed] [Google Scholar]
- 66.Gobbi A, Lad D, Petrera M, Karnatzikos G: Symptomatic early osteoarthritis of the knee treated with pulsed electromagnetic fields: Two-year follow-up. Cartilage 2014;5:78-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Servodio Iammarrone C, Cadossi M, Sambri A, Grosso E, Corrado B, Servodio Iammarrone F: Is there a role of pulsed electromagnetic fields in management of patellofemoral pain syndrome? Randomized controlled study at one year follow-up. Bioelectromagnetics 2016;37:81-88. [DOI] [PubMed] [Google Scholar]