Abstract
Pueraria lobata is one of the most important medicinal herbs used traditionally in China. According to Shanghan Lun (Treatise on Exogenous Febrile Disease), it has been used traditionally to relieve body heat, eye soring, dry mouth, headache associated with high blood pressure, and stiff neck problems. Modern studies in the 1970s revealed that isoflavonoids extracted from P. lobata were the bioactive components of an herbal remedy namely Yufeng Ningxin Tablets for the treatment of patients after stroke. This article reviews recent application of P. lobota in the treatment of diabetics and in reducing alcohol drinking. In view of its low toxicity profile, P. lobota stands an excellent chance to be developed as a phytomedicine for treating human diseases.
Keywords: diabetics, Pueraria lobata (Willd) Ohwi, reducing alcohol drinking
1. Historical use of Pueraria lobata
Pueraria lobata (Willd) Ohwi (Fig. 1), commonly named kudzu, is an ancient Chinese medicinal plant used for diabetes, alcohol intoxication, stroke, and cardiovascular disease. It is a long and intricate climbing vine that coils and trails. It is also known by other names like “mile-a-minute vine” and “foot-a-night vine” because of its ability to grow out of control in a very short time span.
Fig. 1.
P. lobata and its roots
The P. lobata root is just one of the parts of the P. lobata plant that is used for herbal remedies. It is a perennial leguminous vine and has been used also as food in Japan and China. The root of kudzu was first described in the Chinese literature (Shengnong Bencao Jing, 1278AD) as sweet and acrid in taste, cool in nature, and useful as an antipyretic, antidiarrhetic, diaphoretic, and anti-emetic agent (Keung and Vallee, 1998). Kudzu was also listed in the most comprehensive medical book of the time, Bencao Gangmu (Compendium of Materia Medica), compiled by Li Shi-zhen (Li, 1596), in which it was said to treat diabetes and reduce liver intoxication induced by alcohol. However, these claims of efficacy are difficult to assess because the evidence is anecdotal and there were many different preparations over the centuries.
2. General pharmacology of P. lobata
Modern clinical studies of kudzu and other herbal products tend to be flawed by large dropout rates, no measures of medication adherence, relatively few assessments, and no information on the source or dose of the preparation. We have analyzed a variety of commercial kudzu preparations and found them to vary widely in their isoflavone concentrations, even within the same manufacturer. These issues highlight the difficulties of evaluating the efficacy of dietary supplements. While randomized clinical trials are the best method for studying efficacy, such trials often fail because of methodological weaknesses (Linde, 2000). We found that commercial kudzu products do not contain enough of the active isoflavones to be effective, and so over the past 15 years we have developed a kudzu extract that has been standardized with 25% isoflavones, compared to the 0%-13% in the over-the-counter products that we have analyzed. Interestingly, the wild type of P. lobata contains much higher isoflavonoids (up to 12%) than cultivated and eatable species (1%-3%) (P. thomsonii Benth.).
Kudzu roots have low toxicity. In a study evaluating the acute toxic effect, a high dose of 5 g/kg/d (ethanolic extract), which corresponds to approximately 500 mg/kg puerarin, was administered to rats orally (po) for 21 d. No significant deviations from normal histology were found in liver, kidney, pancreas, and spleen. Indeed, there were no major abnormalities in a series of biochemical parameters such as creatinine, creatinine kinase, ALT, AST, and gamma-glutamyltransferase in serum compared with the control group (Bebrevskaet al, 2010). The LD50 intraperitoneally (ip) of crude isoflavone extract of kudzu roots was 5.97 g/kg in rats. The LD50 (iv) of puerarin was 700-800 mg/kg in mice (Liu, 2015). In a clinical trial, 500 mg of kudzu extract (19% puerarin, 4% daidzin, and 2% daidzein) po three times a day for 7 d produced no significant variation in vital signs, liver function, hematology, or urinalysis profile during the course and 4 weeks afterwards (Lukas et al, 2005; Lukas et al, 2013a). These data suggested that kudzu extract is safe at least sub-chronically. We believe that in any attempt to improve health and promote resilience with any kind of dietary supplement, safety is the utmost important factors to be considered.
3. HPLC fingerprint analysis of P. lobata extract
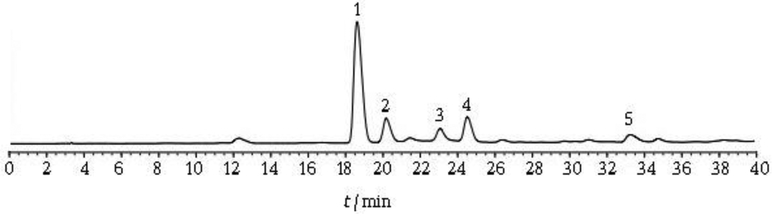
Analytical HPLC has been used routinely to establish quality assurance references prior to formulation. For instance, the crude kudzu extract received from China can be analyzed initially by a reverse phase (C-18) HPLC method that has been validated in Dr. Lee’s laboratory for the analysis of various isoflavones in P. lobata. It can be used as an internal standard for qualitative and quantitative quality assurance because puerarin is the major isoflavone component of the kudzu extract. Fig 2 showed the HPLC fingerprint of initial aqueous acetone extract of kudzu. The HPLC conditions are as follows: YMC C-18 analytical column (4.6 mm × 15 cm); 70% methanol: 30% water, at 1.0 mL/min; UV monitor at 254 nm. The major peaks are identified and assigned based on our studies. This standardized HPLC fingerprint serves as a reference standard for inter-batch quality control.
Fig. 2.
HPLC tracing of isoflavone of kudzu extract (1: puerarin; 2: 3'-methoxy puerarin; 3: 3'-methoxydaidzin; 4: daidzin; 5: daidzein).
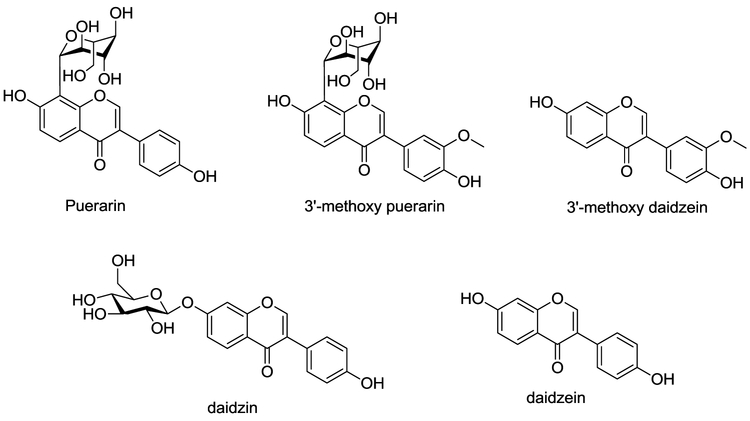
Kudzu extracts contain several compounds, with the highest concentration being isoflavones. The three major ones, puerarin, daidzin and daidzein have been the focus of attention for their likely contributions to the effects of the raw roots on drinking. While some investigators have focused on daidzin because of its weak antabuse-like effect (Arolfo et al, 2009), we believe that puerarin is responsible for bioactivities of kudzu. While it is less potent than the others, there is far more of it in the plant, and it turns out that puerarin has a unique pharmacology. Puerarin belonging to isoflavone compounds, is one of the major bioactive components of P. lobata. These isoflavones are common constituents of plants of the Leguminosae family and possess rearranged flavonoid skeletons (Fig. 3); i.e., the B ring is attached to the C-3-position instead of the C-2-position of the hetero ring. Interestingly, the glucose unit in puerarin is attached to the isoflavone through a unique carbon-carbon bond (C-glycoside) that is substantially more resistant to enzymatic nyarolysis and metabolic deactivation than the ordinary carbonoxygen linkage (O-glycoside).
Fig. 3.
Structures of isoflavone of kudzu extract.
4. Obesity and metabolic syndrome: major global health threats
Overweight and obesity have increased markedly in the last two decades in the United States. Among adults over age 20, 65.1% are overweight or obese, and more than 150 million adults have diabetes (Hedley et al, 2004). Even more alarming is the fact that about 25% of children in the US are overweight or obese, which could lead to the emergence of type 2 diabetes in this previously unaffected population. The numbers of overweight and obesity worldwide are expected to double by the year 2025, with especially severe health impact in less developed countries (Haslam, 2005; 2006). Obesity is associated with several risk factors and diseases, including metabolic syndrome, diabetes, cardiovascular disorders, and certain kinds of cancer. Obesity is a chronic disease and involves a complex interplay between genes and environment. Epidemiological studies suggest that environmental factors play a large role in the current prevalence. The main treatment options are dietary intervention, physical activity, behavior modification, pharmacotherapy, and surgery (AGA, 2002). Although surgery is the most effective, it is reserved for the severely obese. Caloric restriction, physical exercise, and behavior modification constitute the standard mode, but these methods alone or in combination generally have poor long-term outcomes (NTFPTB, 1996). Drugs have shown only limited success, and some, such as fenfluramine and dexfenfluramine, are unsatisfactory due to severe side effects and have been withdrawn from the market. None of the many dietary supplements has shown conclusive evidence of efficacy in the form of double-blind, placebo-controlled clinical trials, nor are they well-characterized, and safety is a major concern (Dwyer et al, 2005). Given the traditional use of kudzu extract in Asia, we believe that rigorous scientific investigation of it for preventing weight gain and metabolic syndrome has the potential to make a significant positive impact on public health.
5. Kudzu in preventing metabolic syndrome and treating diabetes
An estimated 371 million people worldwide have diabetes, and about 90% of cases are type 2 diabetes. The number has almost doubled since 1980. Based on the current trend, by 2030 there will be 550 million cases of type 2 diabetes. The increasing global prevalence is tied closely to the rising rate of obesity as a consequence of social trends toward higher energy intake and reduced energy expenditure. Obesity is a major risk factor for the development of diabetes and related metabolic disorders. Lifestyle changes, including diet, play a key role in the management of obesity and type 2 diabetes. There is increasing scientific evidence that certain natural product dietary supplements, including kudzu extract, provide additional benefits (Ricardi et al, 2005), but the specific bioactive components and their modes of action remain undefined. Adipose tissue plays an important role in the development of obesity and its co-morbidities. Two functionally different types of fat tissue are present in mammals: white adipose tissue (WAT) is the primary site of triglyceride storage, and brown adipose tissue (BAT) is for thermogenic energy expenditure. BAT is specialized to dissipate chemical energy in the form of heat and has recently been shown in humans. Thus, increasing the amount and function of brown fat has become an attractive approach for the prevention and treatment of obesity. Whether or not botanical dietary supplements directly affect adipocyte differentiation and function has not yet been clearly elucidated.
Previous studies have shown that kudzu extract and its bioactive isoflavones such as puerarin (Fig. 1) could be useful clinically for treatment of diabetes (Hsu et al, 2003; Yang et al, 2016; Liu et al, 2018) and cardiovascular diseases such as hypertension (Fen et al, 2015), atherosclerosis (Xiao et al, 2004; Bao et al, 2015), dyslipidemia (Duan et al, 2000; Liu et al, 2011), and myocardial infarction, as well as metabolic syndrome (Caballero, 2003; Yudkin, 2003; Wenjun et al, 2015). Although the common pathogenesis of metabolic syndrome is still not fully understood, recent studies suggest that kudzu root extract and puerarin could reverse the pathological abnormalities induced by high glucose levels in vitro and In vivo. In high glucose-treated preadipocytes, co-incubation of puerarin considerably potentiated glucose uptake dose-dependently (Xu et al, 2005). A similar effect was observed in isolated rat soleus muscle (Hsu et al, 2003). Puerarin promoted insulin-induced preadipocyte differentiation and upregulated mRNA expression of PPAR (Xu et al, 2005). PPAR is a nuclear receptor that plays a regulatory role in the expression of genes related to preadipocyte differentiation, insulin sensitivity, and inflammation (Xu et al, 2005; Semple et al, 2005). Activation of PPAR enhanced insulin sensitivity and triggered adipogenesis, which is responsible for the reduction of adiposity and release of detrimental adipokines (Chiarelli and Di Marzio, 2008).
Another mechanism that may contribute to the glucose lowering activity of puerarin is alteration in the expression of the glucose transporter (GLUT). Diabetes was induced by streptozotocin (STZ) 60 mg/kg iv Puerarin 15 mg/kg iv noticeably diminished blood glucose level and upregulated the mRNA and protein expression of the GLUT subtype 4 (GLUT-4) in soleus muscle cells (Hsu et al, 2003). Puerarin 100 mg/kg/day ip decreased the levels of blood glucose and insulin and the protein expression of GLUT-4 at the plasma membrane of skeletal muscle (Song and Bi, 2004). In a separate study, puerarin 15 mg/kg iv reduced blood glucose level and increased endorphin content in the absence of insulin stimulation in STZ-diabetic rats (Hsu et al, 2004). Interestingly, supplementation of 0.2% kudzu root extract in normal diet for 2 months reduced arterial pressure, body weight, fasting blood glucose, plasma total cholesterol, and insulin levels in both ovariectomized and non-ovariectomized SHR rats (Peng et al, 2009). Excessive intrahepatic triglyceride content in obese persons is a robust marker of metabolic abnormalities. Recent results showed that puerarin significantly decreased the triglyceride and total cholesterol content in liver of non-alcoholic fatty liver disease.
6. Kudzu in reducing alcohol drinking
Ethyl alcohol is the most widely used psychoactive drug in the world and was recently rated as “the most harmful” of all drugs to self plus others, exceeding that of heroin, crack cocaine, methamphetamine, and tobacco (Nutt et al, 2010). Because it disrupts psychomotor performance while altering mood, it contributes to about 100 000 deaths annually in the U.S. alone. In its most recent publication, The National Survey on Drug Use and Health (SAMSHA, 2011) reported that rates of current alcohol use were 51.8% among persons aged 12 or older or about 131.3 million people. These estimates were similar to the rates reported in 2009 so there is no change in drinking among our young adults. However, 23% (58.6 million) of persons 12 or older engaged in binge drinking in the 30 d prior to the survey and 6.7% of those surveyed reported heavy drinking.
There are no uniformly effective pharmacotherapies for treating alcohol use disorders (AUD). Although there are some promising medications, many gaps remain (Kranzler, 2000). Litten et al (2005) and Johnson et al (2010) reviewed and identified the various agents used to treat alcoholism and noted that none of these medications are universally effective in reducing drinking and have modest effects; Some are not yet FDA approved; All are available only by prescription and most have side effects that limit their use. The efficacy of the four FDA-approved medications is modest and all have significant side effect profiles. Garbutt (2009) reviewed the current status of medications and noted that disulfiram is somewhat effective when taken, but prescription rates were the lowest of all (Mark et al, 2003) and have plummeted in recent years (Mark et al, 2009). The toxic reaction with alcohol can be frightening and as such it may be useful only in short-term treatment with careful monitoring (Skinner et al, 2014) as 87% of treatments were stopped at (or before) the expiration of the first prescription (Pedersen et al, 2011). Acamprosate was initially promising in European trials, but the results of Project COMBINE (Anton et al, 2006) and another large U.S. based trial (Mason et al, 2006) found that it is no different from placebo. It was demonstrated that oral naltrexone (NTX) was effective, but it had little therapeutic effect (Krystal et al, 2001) and nearly 86% are never refilled of all prescriptions written for NTX(Kranzler et al, 2008). The Bouza et al, (2004) meta-analysis of NTX trials revealed: (a) it reduces the likelihood of relapse to heavy drinking; (b) there is some evidence that it enhances abstinence rate; (c) efficacy is modest; (d) the overall tolerability is acceptable. The COMBINE Study (Anton et al, 2006) supported these findings and reported that patients who received oral NTX were significantly more likely to experience vomiting, diarrhea, somnolence and AST or ALT levels more than five times the upper normal limits. Clinician concerns about efficacy contribute to limited use of NTX (Mark et al, 2003) and parallels the low efficacy literature. It is not surprising that some clinicians fail to see a robust clinical response if they prescribe oral NTX to a limited number of patients. No significant effect of extended release NTX was found for number of heavy drinking days, but it delayed the time to first drink. Injection site reactions were more common in the NTX group (Kranzler et al, 2004) and nausea, decreased appetite, dizziness, and injection-site pain were significantly more common among patients receiving 380 mg extended-release NTX (Garbutt et al, 2005). In conclusion, many patients cannot tolerate the side effects of these prescription medications and are unwilling to take them. Thus, the importance of offering patients viable options for treatment of such a widely prevalent disorder such as herbal therapy is extremely important.
The use of complementary and alternative therapies has increased at an explosive pace (Barnes et al, 2008). The majority of the increase in use of alternative therapies was due to herbal medicines as they were used by 2.5% of the population in 1990 by 12.1% in 19 97, and in 2007, 38.3% of adults used some form of CAM. The rates of use, as determined by this national survey, ranged from 32% to 54% of the population and spanned all socio-demographic groups. In general, use was the highest among women (48.9% vs 37.8%), those with some college education versus no college (50.6% versus 36.4%) and in 35-49 year olds (50.1%). Also, 58.3% of the respondents paid for their alternative therapy out-of-pocket, which equated to $33.9 billion in 2009. These data indicate that the U.S. public makes millions of visits to CAM providers each year and spends billions of dollars for these services. Wolsko et al. (2000) concluded that the CAM use is wider than generally appreciated and includes individuals from a wide spectrum of socioeconomic status. Thus, a CAM product that reduces alcohol consumption would be well received by the public.
In fact, the use of medicinal plants to treat alcohol-related diseases dates to 600 A.D. One such Chinese herbal medicine, Xian Jiu Ling (NPI-028), has long been used to reduce the inebriation that results from alcohol consumption. NPI-028 contains the extracts of several plants including P. lobata and Citrus reticulata Blanco, which were recorded in an ancient Chinese Materia Medica entitled Bencao Gangmu (Li, 2006) and have long been used to lower intoxication (Sun, 600). Until recent publication by Lukas et al in 2005, there had been only one clinical trial of kudzu as a treatment for alcohol abuse. Shebek and Rindone (2000) found no effect of kudzu in promoting sobriety or reducing alcohol craving among veterans. However, their study has a number of weaknesses (many of which the authors noted). These included a large dropout rate, no measure of compliance, only one assessment per month, and no information on the source or dose of kudzu was provided. A variety of OTC preparations were analyzed and found them to vary widely in their isoflavone concentration. A lack of standardization highlights the difficulties of evaluating the efficacy of complementary and alternative therapies. While randomized clinical trials are the best method for studying efficacy, such trials often fail because of significant methodological weaknesses (Linde, 2000). Keung and Vallee (1993b) performed the first study demonstrating that kudzu reduced alcohol intake in an animal model—after a single injection! However, the hamsters received a concentrated injection of the kudzu root—a dose that is difficult to achieve using the supplements available in health food stores. The original Chinese method of preparing kudzu root involves concentrating it in a tea for drinking; the commercial products just do not contain enough of the active isoflavones to be effective. Lukas et al, developed a kudzu extract with a highly standardized total isoflavone content of 25% including the individual isoflavones puerarin, daidzin and daidzein in a ratio of 19:4:2 (Fig 2).
6.1. Animal Study
According to several preclinical studies, these isoflavones suppressed alcohol intake in Syrian Golden hamsters (Keung and Valle, 1993a, b; Guerra et al, 2000), in alcohol preferring P rats (Benlhabib et al, 2004) and in HAD, FH rats and monkeys (Overstreet et al, 1996, 1997, 2011; Penetar et al, 2015).
Interestingly, there is a great deal of preclinical evidence that isoflavones/phytoestrogens contained in kudzu are “protective” of liver function. Based on our animal drinking study, daidzin is the most active isoflavone isolated from kudzu and is three times more potent than puerarin. Lin et al, (1996) showed that 100 mg/kg/d (orally in food) of daidzein, daidzin, and puerarin in female P rats decreased alcohol consumption by 75%, 50%, and 42%, respectively. The effectiveness of was increased to 65% reduction when the puerarin dose was increased to 300 mg/kg/d. However, daidzin is an O-glycoside, and its sugar unit can be easily hydrolyzed under physiological conditions to give the aglycone (daidzein), which is a less hydrophilic metabolite. The glucose unit not only enhances water solubility but also facilitates the molecule passing the blood-brain barrier because puerarin has a non-cleavable glucose moiety. Interestingly, flavonoid compounds such as 7,8-dihydroxyflavone and chrysin (5,7-dihydroxyflavone) were totally inactive in suppression of alcohol intake (Keung and Vallee, 1993a). These preliminary findings demonstrated that a minor structural variation could result in a significant difference in effect on ethanol intake. Based on our animal drinking studies, we found that the 3’-methoxy analogs of puerarin is three times more potent than puerarin, and the 7-hydroxyl group is not necessary for the observed activity. With this limited SAR information at two separate regions (A and C), it is conceivable that the activity of the extract of kudzu is attributed to a mixture of three major isoflavones (Fig 3) as a whole, and our formulation contains all three (as well as the active 3’methoxy compounds) in the same proportion that is found in nature.
6.2. Mechanism of action of puerarin in reducing alcohol consumption and secondary benefits
The actual mechanisms of how kudzu-related compounds reduce alcohol consumption are still not clear. Some postulated options include enhancing hepatic anti-oxidant defense systems by increasing alcohol and aldehyde dehydrogenase activity (Penetar et al, 2015), and increased superoxide dismutase and catalase (Pandey et al, 2013), all of which can help clear alcohol from the system and protect the liver from damage that can ensue from chronic alcohol. An added benefit of this approach will be a reduction in liver morbidity. Puerarin appears to increase cerebral perfusion and reduces vascular resistance. Intravenous puerarin (NPI-031G) improved heart function in patients with chronic heart failure (Duan et al, 2000). Similar iv puerarin administration in rats observed increased cerebral blood flow in the pia mater in SHRs (Wu et al, 2014). Puerarin is injected iv in patients to treat coronary heart disease (Gao et al, 2015). Finally, we have just completed a study revealing that puerarin upregulates VMAT2 (dopamine transporter) gene expression, which has been implicated in the etiology of alcoholism (Xiong et al, 2016).
Alkontrol-herbal may have an additional positive effect in alcoholic brain if it improves cerebral perfusion. This would be important since alcoholism is associated with reduced cerebral perfusion (Christie et al, 2008; Tolentino et al, 2011). The frontal lobes appear especially vulnerable to chronic alcohol-induced cerebral perfusion changes (Gundersen et al, 2013; Day et al, 2015). As the frontal lobes play pivotal roles in mediating executive function, a cognitive domain critical for appropriate behavioral responses (e.g., treatment compliance) and one that is impaired in alcoholics (Nixon et al, 2013; Day et al, 2015), frontal impairments could negatively impact on treatment outcome (Loeber et al, 2010; Bernardin et al, 2014). While executive function appears to recover in abstinence (Fein et al, 2006), the recovery rate is slow (e.g., versus the rate of perfusion improvements), at least in older alcoholics (Caputo et al, 2012). Thus, treatments that enhance frontal blood flow may also accelerate functional recovery, which would provide additional benefits from the treatment with this product.
6.3. Clinical Studies
6.3.1. Kudzu extract reduces alcohol consumption in outpatient setting
Seventeen non-treatment seeking alcohol-dependent 23-24 years old males who had been drinking regularly since age 17-18 participated. Although the sample size was relatively small (n = 10 and 7 completers of 3 weeks of kudzu and placebo, respectfully), there was an overall main effect for time on number of drinks per week [F (5,75) = 9.49, P < 0.001, η2Partial = 0.388] and there was an interaction for all kudzu extract treatment weeks [F (5,75) = 2.56, P = 0.034, η2Partial = 0.146] (Fig 4). Both kudzu extract and placebo treated individuals reduced their drinking during the first treatment week (Tx1), but they were significant only for the kudzu treated group (Lukas et al, 2013a). As shown in Fig 4, a significant reduction from baseline drinking continued for the kudzu treated group for treatment weeks 2, 3, and 4.
Fig. 4.
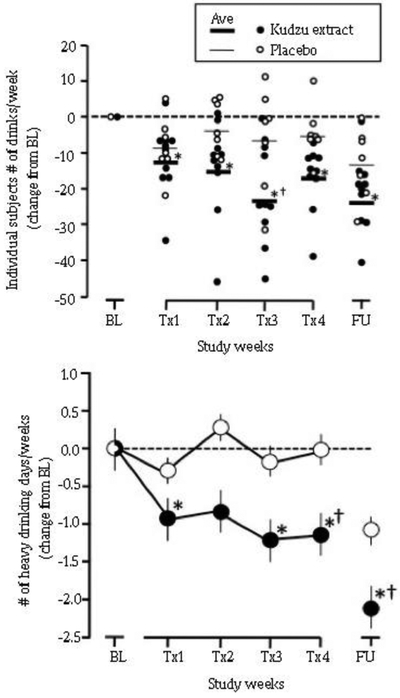
Panel-Kudzu extract and Placebo effects on weekly alcohol consumption among heavy drinkers in an outpatient setting (A). Subjects reported drinking via ActiWatchScore wrist actigraphy and daily diaries. Individual data are plotted as symbols and the average value as a thick (kudzu extract) or thin (placebo) line. Panel-Kudzu extract and Placebo effects on number of heavy drinking days per week (B). BL-Baseline; Tx1-Treatment Week 1; Tx2-Treatment Week 2; Tx3-Treatment Week 3; Tx4-Treatment Week 4; FU-Follow-up. *Indicates significantly different from baseline; †indicates significantly different from placebo (Lukas et al, 2013a)
There was a trend towards a significant difference between placebo and kudzu extract at Tx3 (P = 0.055). Fig 4B shows the effects of treatment on the number of heavy drinking days per week. An overall main effect was observed for both time ([F (5, 75) = 3.684, P < 0.005, η2Partial = 0.197] and for drug [F (1,15) = 9.428, P = 0.008, η2Partial = 0.386]. There were no significant changes in the placebo-treated group, while significant reductions were observed in treatment weeks 1, 3, and 4 for the kudzu-treated group. Additionally, there was a significant difference between the two drug treatments during treatment week 4. Many subjects, regardless of treatment, reduced consumption during the follow up, which included additional briefings about the values of lower alcohol intake.
Other metrics of alcohol consumption are percent days abstinent and number of consecutive days of abstinence. Kudzu extract also caused significant changes in these as compared to placebo treated individuals (Fig 5). It is noteworthy that these participants were daily drinkers and only one of the placebo-treated participants and two of the kudzu-treated participants achieved 3 or more consecutive days of abstinence during baseline phase (4 and 5 d, respectively). However, during the treatment phase, 7 out of 9 of the kudzu-treated and only 1 out of 5 of the placebo-treated participants achieved 3 or more consecutive days of abstinence. This resulted in an odds ratio of 14.0 (Exact Test, StatXact v6.3, Cytel Softward Corp, Cambridge, MA) to achieve 3 or more consecutive days of abstinence.
Fig. 5.
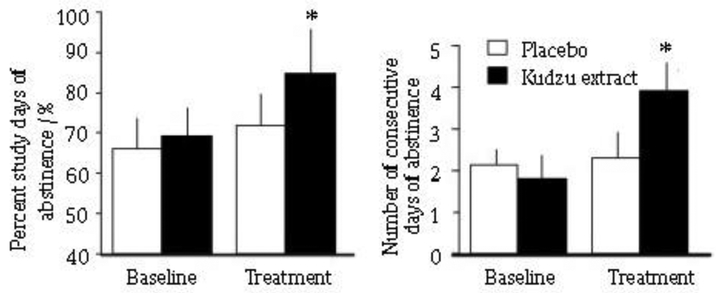
Effects of kudzu extract and placebo on percent study days in which participants were abstinent (A) and number of consecutive days of abstinence (B) from drinking during weeks 3-6 of the study. *Indicates significantly different from placebo treatment using baseline drinking as a covariate at P < 0.05.
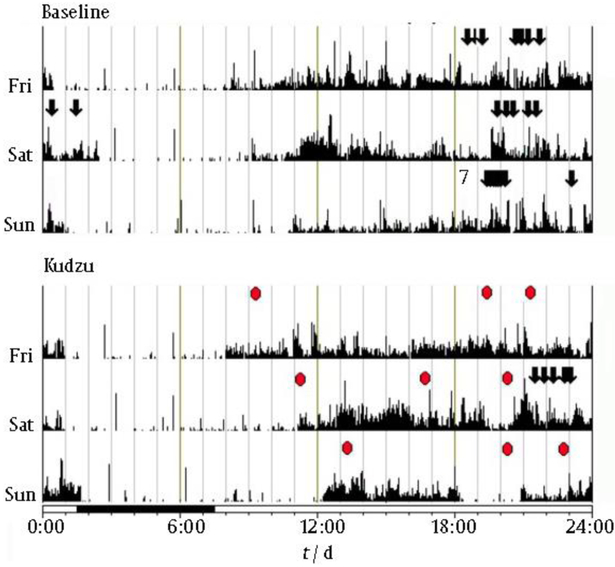
We have made extensive use of wrist actigraphy as a measure of sleep/wake activity and as a method of tracking alcohol drinking behavior. The patterns of drinking during baseline and after kudzu treatment are shown for one representative subject in Fig 6. Note that binge drinking (arrows) occurred on all three nights during baseline, but drinking occurred on only one night while this subject was taking kudzu extract. Note also the level of medication compliance (red circles). The vertical lines represent locomotor activity.
Fig. 6.
Three-day samples of wrist actigraphy data from one subject during baseline (top) and after 10 d of kudzu extract treatment (bottom); arrows depict alcoholic drinks. Vertical lines depict locomotor activity. Kudzu administration is depicted by red ovals.
6.3.2. Puerarin is an active isoflavone in kudzu extract
Natural Pharmacia International supplied us with puerarin (NPI-031G) so that we could study the effects of the major isoflavone in kudzu extract on alcohol consumption in the same naturalistic setting that we used to study kudzu extract (Lukas et al, 2005). Puerarin (600 mg, b.i.d.) was administered in a double-blind, placebo-controlled, crossover design for one week prior to an afternoon 1.5 h drinking session. During the drinking session, participants had access to up to six bottles of their preferred brand of beer (in addition to juice and water). Drinking behavior was recorded by a custom-built end table that housed a concealed electronic scale that was connected to a computer in the control room.
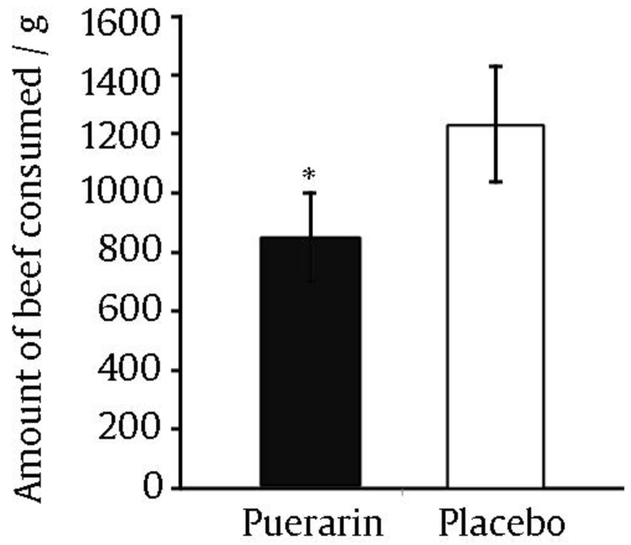
A time course of drinking and sip volumes were obtained by recording the weight of the mug and beer between sips. Over the 90-minute session, participants consumed an average (3.29 ± 0.7) beers when treated with placebo and (2.28 ± 0.6) beers when treated with puerarin. Participants delayed opening beers by an average of 10 additional minutes (23%) and took 4 min longer to consume beers (18%). Fig. 7 depicts the effects of puerarin on the amount of beer consumed (Penetar et al, 2012). We conclude that puerarin is an active isoflavone that has effects that parallel kudzu extract (Lukas et al, 2005). However, the combination of the other two major isoflavones (daidzin and daidzein) in the Alkontrol-herbal preparation enhances the total efficacy in reducing alcohol drinking, and therefore is a superior strategy than focusing in a single entity.
Fig. 7.
Amount of beer consumed during drinking session after either placebo or puerarin treatment for 7 d.
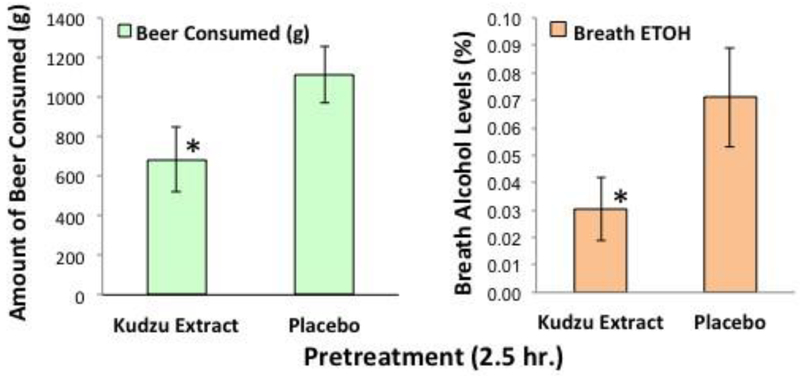
6.3.3. Effects of a single dose of kudzu extract on drinking behavior
In a recent completed study, 20 alcohol drinkers participated in a baseline drinking session and then a second session during wnich they consumed either Kudzu extract (2g) or matched placebo, 2.5 h before a 90-minute drinking session began (Lukas et al, 2005). Consumption of their preferred brand of beer was recorded using a custom end table that contains a digital scale and records the sip-by-sip drinking activity (Fig. 8). An acute dose of kudzu extract significantly (P = 0.014) reduces alcohol intake after a single dose (Fig. 8A) and a reduction in breath alcohol level at the end of the drinking session (Fig. 8B) (P = 0.026). The effects of a 2.5 h pretreatment are strikingly similar to our 7-day pretreatment study (Lukas et al, 2005). Collectively, our progress to date reveals that kudzu extract represents a safe and modestly effective treatment for alcohol abuse/dependence
Fig. 8.
Effects of a single dose of kudzu extract or matched placebo on intake of preferred brand of beer (A). Right-Breath alcohol levels at end of drinking sessions (B). (mean ± SEM, n = 10)
6.4. Objective measures of drinking
A number of methods have been used to monitor alcohol intake during an extended period of time. The values obtained with a swab test for salivary ethanol levels (QED®, STC Diagnostics, Bethlehem, PA) correspond well with breath estimates and blood levels (Drummer, 2008). We will continue to monitor all drinking activity via four methods: 1) daily diaries, 2) Actiwatch Score® entries (Fig. 9), 3) frequent breathalyzer samples, and 4) the QED® units. In addition, because of the added value that biomarkers offer, we will collect blood samples for measuring liver function and CDT (Marques et al, 2010). Both liver function tests and carbohydrate deficient transferrin (CDT) were used in combination to identify different patterns of drinking (Tavakoli et al, 2011).
Fig. 9.
ActiWatch Score wrist actigraphy device. Note large response button that advances the two-digit display (currently showing a "4").
7. Conclusion
In summary, P. lobata has been used traditionally in China for treating various diseases including heart, stroke, high blood pressure, etc. This review summarized its recent applications in obesity, diabetic and alcohol drinking. Further investigation by using a combination of cellular, molecular, genomics, and proteomics approaches to understand the mechanisms of P. lobata will add valuable scientific information and provide a conduit for speeding up the development of P. lobata as an evidence-based phytomedicine in managing obesity, diabetes, and metabolic syndrome.
Acknowledgments
This work was supported by grants from NIH (1R42AA022577).
Footnotes
Conflict of interest
The authors declare no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai F, Chen M, Yu B, Yang Y, Xu G, Gui F, Liu Z, Bai X, Chen Z (2015). Puerarin accelerate scardiac angiogenesis and improves cardiac function of myocardial infarction by upregulating VEGFA, Ang-1 and Ang-2 in rats. Int J Clin Exp Med, 8, 20821–20828. [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM (2006). Combined pharmacotherapies and behavioral interventions for alcohol dependence: The COMBINE Study: A randomized controlled trial. JAMA, 295, 2003–2017. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Overstreet DH, Yao L, Fan P, Lawrence AJ, Tao G, Keung WM, Vallee BL., Olive MF, Gass JT, Rubin E, Anni H, Hodge CW, Besheer J, Zablocki J, Leung K, Blackburn BK, Lange LG, Diamond I (2009). Suppression of heavy drinking and alcohol seeking by a selective ALDH-2 inhibitor. Alcohol Clin Exp Res, 33, 1935–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benlhabib E, Baker JI, Keyler DE, Singh AK 2004. Kudzu root extract suppresses voluntary alcohol intake and alcohol withdrawal symptoms in P rats receiving free access to water and alcohol. J Med Food, 7, 168–79. [DOI] [PubMed] [Google Scholar]
- Bao L, Zhang Y, Wei G, Wang Y, Ma R, Cheng R, Ren X, Agula B 2015. The anti-atherosclerotic effects of puerarin on induced-atherosclerosis in rabbits. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub, 159, 53–59. [DOI] [PubMed] [Google Scholar]
- Barnes PM, Bloom B, Nahin R (2008). CDC National Health Statistics Report #12. Complementary and Alternative Medicine Use Among Adults and Children: United States, 2007. December 2008. [PubMed] [Google Scholar]
- Bebrevska L, Foubert K, Hermans N, Chatterjee S, Van ME, De MG, Vlietinck A, Pieters L, Apers S (2010). In vivo antioxidative activity of a quantified Pueraria lobata root extract. J Ethnopharmacol, 127, 112–117. [DOI] [PubMed] [Google Scholar]
- Bernardin F, Maheut-Bosser A, Paille F (2014). Cognitive impairments in alcohol-dependent subjects. Front Psychiatry, 5, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouza C, Angeles M, Muñoz A, Amate JM (2004). Efficacy and safety of naltrexone and Acamprosate in the treatment of alcohol dependence: A systematic review. Addiction, 99, 811–828. [DOI] [PubMed] [Google Scholar]
- Caballero E (2003). Obesity, diabetes, and the metabolic syndrome: new challenges in antipsychotic drug therapy. CNS Spectr, 8, 19–22. [DOI] [PubMed] [Google Scholar]
- Caputo F, Vignoli T, Leggio L, Addolorato G, Zoli G, Bernardi M (2012). Alcohol use disorders in the elderly: A brief overview from epidemiology to treatment options. Exp Gerontol, 47, 411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli F, Marzio D (2008). Peroxisome proliferator-activated receptor-gamma agonists and diabetes: Current evidence and future perspectives. Vasc Health Risk Manag, 4, 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie IC, Price J, Edwards L, Muldoon M, Meltzer CC, Jennings JR (2008). Alcohol consumption and cerebral blood flow among older adults. Alcohol, 42, 269–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day AM, Kahler CW, Ahern DC, Clark US (2015). Executive Functioning in Alcohol Use Studies: A Brief Review of Findings and Challenges in Assessment. Curr Drug Abuse Rev, 8, 26–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummer OH (2008). Introduction and review of collection techniques and applications of drug testing of oral fluid. Ther Drug Monit, 30, 203–206. [DOI] [PubMed] [Google Scholar]
- Duan S, Li YF, Luo XL (2000). Effect of puerarin on heart function and serum oxidized-LDL in the patients with chronic cardiac failure. Hunan Yi Ke Da Xue Xue Bao, 25, 176–178. [PubMed] [Google Scholar]
- Dwyer JT, Allison DB, Coates PM (2005). Dietary supplements in weight reduction. J Am Diet Assoc, 105, S80–86. [DOI] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di, Sclafani V (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res, 30, 1538–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Wei B, Qian C (2015). Puerarin injection for treatment of unstable angina pectoris: a meta-analysis and systematic review. Int J Clin Exp Med, 8, 14577–14594. [PMC free article] [PubMed] [Google Scholar]
- Garbutt JC (2009). The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat, 36, S15–23. [PubMed] [Google Scholar]
- Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL (2005). Efficacy and tolerability of long acting injectable naltrexone for alcohol dependence: A randomized controlled trial. JAMA, 293, 1617–1625. [DOI] [PubMed] [Google Scholar]
- Guerra MC, Speroni E, Broccoli M, Cangini M, Pasini P, Minghett A, Crespi-Perellino N, Mirasoli M, Cantelli-Forti G, Paolini M (2000). Comparison between chinese medical herb Pueraria lobata crude extract and its main isoflavone puerarin antioxidant properties and effects on rat liver CYP-catalysed drug metabolism. Life Sci, 67, 2997–3006. [DOI] [PubMed] [Google Scholar]
- Gundersen H, van Wageningen H, Grüner R (2013). Alcohol-induced changes in cerebral blood flow and cerebral blood volume in social drinkers. Alcohol Alcohol, 48, 160–165. [DOI] [PubMed] [Google Scholar]
- Haslam DW, James WP (2005). Obesity. Lancet, 366, 1197–209. [DOI] [PubMed] [Google Scholar]
- Haslam DW (2006). Obesity-time to wake up. BMJ, 333, 640–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM (2004). Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA, 291, 2847–2850. [DOI] [PubMed] [Google Scholar]
- Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC, Cheng JT (2003). Antihyperglycemic effect of puerarin in streptozotocin-induced diabetic rats. J Nat Prod, 66, 788–792. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Wu YC, Liou SS, Liu IM, Huang LW, Cheng JT (2004). Mediation of Endogenous beta-endorphin by Tetrandrine to Lower Plasma Glucose in Streptozotocin-induced Diabetic Rats. Evid Based Complement Alternat Med, 1, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA (2010). Medication treatment of different types of alcoholism. Am J Psychiatry, 167, 630–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL (1993a). Daidzin, a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc Natl Acad Sci USA, 90, 1247–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL (1993b). Daidzin and daidzen suppress free-choice ethanol intake by Syrian Golden hamsters Proc Natl Acad Sci USA, 90, 10008–10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keung WM, Vallee BL (1998). Kudzu root, an ancient Chinese source of modern antidipsotropic agents. Phytochemistry, 47, 499–506. [DOI] [PubMed] [Google Scholar]
- Kranzler HR (2000). Pharmacotherapy of alcoholism: gaps in knowledge and opportunities for research. Alcohol Alcohol, 35, 537–547. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Stephenson JJ, Montejano L, Wang S, Gastfriend DR (2008). Persistence with oral naltrexone for alcohol treatment: implications for health-care utilization. Addiction, 103, 1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L (2004). Naltrexone depot for treatment of alcohol dependence: A multicenter randomized, placebo controlled clinical trial. Alcohol Clin Exp Res 28, 1051–1059. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA (2001). For the Veterans Affairs Naltrexone Cooperative Study 425 Group. Naltrexone in the treatment of alco- hol dependence. N Engl J Med, 345, 1734–1739. [DOI] [PubMed] [Google Scholar]
- Li SZ (2006). Compendium of Materia Medica (Bencao Gangmu) 6 vols. Foreign Language Press, Beijing [Google Scholar]
- Linde K (2000). How to evaluate the effectiveness of complementary therapies. J Altern Complement Med, 6, 253–256. [DOI] [PubMed] [Google Scholar]
- Litten RZ, Fertig J, Mattson M, Egli M (2005). Development of medications for alcohol use disorders, recent advances and ongoing challenges. Expert Opin Emerg Drugs, 10, 323–343. [DOI] [PubMed] [Google Scholar]
- Liu CM, Ma JQ, Sun YZ (2011). Protective role of puerarin on lead-induced alterations of the hepatic glutathione antioxidant system and hyperlipidemia in rats. Food Chem Toxicol, 49, 3119–3127. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li W (2018) Research progress on puerarin in treatment of diabetic nephropathy. Chinese Traditional and Herbal Drugs, 49 (4), 981–986. [Google Scholar]
- Liu YZ (2015). Dietary Chinese Herbs: Chemistry, Pharmacology and Clinical Evidence. Springer-Verlag; Wien. [Google Scholar]
- Loeber S, Duka T, Welzel, Márquez H, Nakovics H, Heinz A, Mann K, Flor H (2010). Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol, 45, 541–547. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Berko J, Vicens L, Palmer C, Mallya G, Macklin EA, Lee DY (2005). An extract of the Chinese herbal root kudzu reduces alcohol drinking by heavy drinkers in a naturalistic setting. Alcohol Clin Exp Res, 29, 756–62. [DOI] [PubMed] [Google Scholar]
- Lukas SE, Penetar D, Su Z, Geaghan T, Maywalt M, Tracy M, Rodolico J, Palmer C, Lee DYW (2013a). A standardized kudzu extract (NPI-031) reduces alcohol consumption in nontreatment-seeking male heavy drinkers. Psychopharmacology (Berl), 226, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas SE, Lowen SB, Lindsey KP, Conn N, Tartarini W, Rodolico J, Mallya G, Palmer C, Penetar DM (2013b). Extended-release naltrexone (XR-NTX) attenuates brain responses to alcohol cues in alcohol-dependent volunteers, a bold FMRI study. Neuroimage, 78, 176–185. [DOI] [PubMed] [Google Scholar]
- Mark TL, Kassed CA, Vandivort-Warren R, Levit KR, Kranzler HR (2009). Alcohol and Opioid Dependence Medications: Prescription Trends, Overall and by Physician Specialty. Drug Alcohol Depend, 99, 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X, Bransberger P, Poole VH, Crosse S (2003). Physicians' opinions about medications to treat alcoholism. Addiction, 98, 617–626. [DOI] [PubMed] [Google Scholar]
- Marques P, Tippetts S, Allen J, Javors M, Ailing C, Yegles M, Pragst F, Wurst F (2010). Estimating driver risk using alcohol biomarkers, interlock blood alcohol concentration tests and psychometric assessments: initial descriptives. Addiction, 105, 226–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Goodman AM, Chabac S, Lehert P (2006). Effect of oral acamprosate on abstinence in patients with alcohol dependence in a double-blind, placebo-controlled trial: The role of patient motivation. J Psychiatr Res, 40, 383–393. [DOI] [PubMed] [Google Scholar]
- Nixon SJ (2013). Executive functioning among young people in relation to alcohol use. Curr Opin Psychiatry, 26, 305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTPPTB. (1996). Long-term pharmacotherapy in the management of obesity. National Task Force on the Prevention and Treatment of Obesity. JAMA, 276, 1907–1915 [DOI] [PubMed] [Google Scholar]
- Nutt DJ, King LA, Philips LD (2010). Drug harms in the UK: A multicriteria decision analysis. Lancet, 376, 1558–1565. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, McArthur RA, Rezvani AH, Post C (1997). Selective inhibition of alcohol intake in diverse alcohol-preferring rat strains by the 5-HT2A antagonists amperozide and FG 5974. Alcohol Clin Exp Res, 21, 1448–1454. [PubMed] [Google Scholar]
- Overstreet DH, Lee YW, Rezvani AH, Pei YH, Criswell ME, Janowsky DS (1996). Suppression of alcohol intake after administration of the Chinese herbal medicine, NPI-028, and its derivatives. Alcohol Clin Exp Res, 20, 221–227. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Lee YW Amir HR (2011). The Chinese herbal medicine NPI028 suppresses alcohol intake in alcohol-preferring rats and monkeys without inducing taste aversion. Article in Focus on Alternative and Complementary Therapies, 2, 194–194. [Google Scholar]
- Pandey N, Yadav D, Pandey V, Tripathi YB (2013). Anti-inflammatory effect of Pueraria tuberosa extracts through improvement in activity of red blood cell anti-oxidant enzymes. Ayu, 34, 297–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen B, Alva-Jørgensen P, Raffing R, Tønnesen H (2011). Fractures and alcohol abuse - patient opinion of alcohol intervention. Open Orthop J, 7, 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar D, Toto L, Farmer S, Lukas SE (2012). The isoflavone puerarin reduces alcohol drinking by heavy drinkers in a naturalistic setting. Drug Alcohol Dependence, 126, 251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penetar D, Toto L, Lee DY, Lukas SE (2015). A single dose of kudzu extract reduces alcohol consumption in a binge drinking paradigm. Drug Alcohol Depend, 153, 194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng N, Prasain JK, Dai Y, Moore R, Arabshahi A, Barnes S, Carlson S, Wyss JM (2009). Chronic dietary kudzu isoflavones improve components of metabolic syndrome in stroke-prone spontaneously hypertensive rats. J Agric Food Chem, 57, 7268–7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi G, Capaldo B, Vaccaro O (2005). Functional foods in the management of obesity and type 2 diabetes. Curr Opin Clin Nutr Metab Care, 8, 630–635. [DOI] [PubMed] [Google Scholar]
- SAMSHA. (2011). Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-41, HHS Publication No. (SMA) 11–4658. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Semple RK, Meirhaeghe A, Vidal-Puig AJ, Schwabe JW, Wiggins D, Gibbons GF, Gurnell M, Chatterjee VK, O'Rahilly S (2005). A dominant negative human peroxisome proliferator-activated receptor (PPAR) {alpha} is a constitutive transcriptional corepressor and inhibits signaling through all PPAR isoforms. Endocrinology, 146, 1871–1882. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Lahmek P, Pham H, Aubin HJ 2014. Disulfiram efficacy in the treatment of alcohol dependence: a meta-analysis. PLoS One, 9, e87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shebek J, Rindone JP (2000). A pilot study exploring the effect of kudzu root on the drinking habits of patients with chronic alcoholism. J Altern Complemen Med, 6, 45–48. [DOI] [PubMed] [Google Scholar]
- Song CY, Bi MM (2004). Effects of puerarin on plasma membrane GLUT4 content in skeletal muscle from insulin-resistant Sprague-Dawley rats under insulin stimulation. Zhongguo Zhongyao Zazhi, 29, 172–175. [PubMed] [Google Scholar]
- Sun SM (circa 600 AD) Beiji-Quianjin-Yaofang. [Google Scholar]
- Tavakoli HR, Hull M, Michael., Okasinski L (2010). Review of current clinical biomarkers for the detection of alcohol dependence. Innov Clin Neurosci, 8, 26–33. [PMC free article] [PubMed] [Google Scholar]
- Tolentino NJ, Wierenga CE, Hall S, Tapert SF, Paulus MP, Liu TT, Smith TL, Schuchit MA (2011). Alcohol effects on cerebral blood flow in subjects with low and high responses to alcohol. Alcohol Clin Exp Res, 35, 1034–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright C, Moore RD (1990). Disulfiram treatment of alcoholism. Am J Med, 88, 647–655. [DOI] [PubMed] [Google Scholar]
- Xiao LZ, Huang Z, Ma SC, Zen Z, Luo B, Lin X, Xu X (2004). Study on the effect and mechanism of puerarin on the size of infarction in patients with acute myocardial infarction. Zhongguo Zhongxiyi Jiehe Zachi, 24, 790–792. [PubMed] [Google Scholar]
- Xiong N, Li N, Martin E, Yu J, Li J, Liu J, Lee YWD, Isacson O, Vance J, Qing H, Wang T, Lin Z (2016). hVMAT2, A Target of Individualised Medication for Parkinson's Disease. Neurotherapeutics, 13, 623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenjun H, Jing W, Tao L, Ling M, Van Y, Xiaorong Z, Rui Z (2015). The Protective Effect of Puerarin on Myocardial Infarction Reperfusion Injury (MIRI): A Meta-Analysis of Randomised Studies in Rat Models. Med Sci Monit, 21, 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolsko P, Ware L, Kutner J, Lin CT, Albertson G, Cyran L, Schilling L, Anderson RJ (2000). Alternative/Complementary Medicine, Wider usage than generally appreciated. J Altern Complement Med, 6, 321–326. [DOI] [PubMed] [Google Scholar]
- Wu XD, Wang C, Zhang ZY, Lu Y, Liu FY, Liu XH (2014). Puerarin attenuates cerebral damage by improving cerebral microcirculation in spontaneously hypertensive rats. Evid Based Complement Alternat Med, 2014, 408501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ME, Xiao SZ, Sun YH, Zheng XX, Qu-Yang Y, Guan C (2005). The study of anti-metabolic syndrome effect of puerarin in vitro. Life Sci, 77, 3183–3196. [DOI] [PubMed] [Google Scholar]
- Yang L, Yao D, Vang H, Wei Y, Peng Y, Ding Y, Shu L (2016). Puerarin Protects Pancreatic β-Cells in Obese Edabetic Mice via Activation of GLP-1R Signaling. Mol Endocrinol, 30, 361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudkin JS (2003). Adipose tissue, insulin action and vascular disease: inflammatory signals. Int J Obes Relat Metab Disord. Suppl, 3, 325–28. [DOI] [PubMed] [Google Scholar]