Abstract
Purpose
Evidence-based guidelines inform treatment decisions for patients for whom germline genetic information is available. Our real-time tumor sequencing program, which makes precision treatment decisions for patients with cancer, produces matched germline information, providing a unique opportunity to efficiently implement pharmacogenetics and benefit patients.
Methods
The germline genetic database from the Michigan Oncology Sequencing (MI-Oncoseq) program was searched for 21 clinically actionable polymorphisms in five cancer-relevant genes: TPMT, DPYD, CYP2C19, CYP3A5, and UGT1A1. Residual germline DNA was sent to an external Clinical Laboratory Improvement Amendments–approved laboratory for confirmatory genotyping. The medical records of MI-Oncoseq patients with actionable phenotypes were searched for receipt of relevant drugs and to determine whether having genetic information at the time of treatment would have led to a treatment recommendation.
Results
All nine variants in TPMT, DPYD, and CYP2C19 that were detected in MI-Oncoseq were confirmed by external genotyping. Genotype determinations could not be made for CYP3A5*3, UGT1A1*28, or UGT1A1*80. On the basis of retrospective assessment of 115 adult and pediatric patient records, 4.3% (n = 5) had a potentially clinically actionable phenotype for TPMT, DPYD, or CYP2C19 and received a relevant medication. After accounting for differences in adult and pediatric recommendations, three of these patients could have received a treatment recommendation at the time of prescribing.
Conclusion
Germline genotype determinations for TPMT, DPYD, and CYP2C19 can be used to make evidence-based treatment recommendations in MI-Oncoseq patients. Although the proportion of patients for whom recommendations can be made is small, this added value to MI-Oncoseq and patient care comes at no additional genotyping cost. Pharmacogenetic assessment should be integrated into tumor sequencing programs that genotype matched germline DNA; however, the complexity and additional cost of implementing pharmacogenetics remain challenging.
INTRODUCTION
The genomes of patients (germline) and their tumors (somatic) each provide useful information to guide precision medicine in oncology. Validated pharmacogenetic associations exist for several germline polymorphisms with commonly used cancer treatments, including thiopurines (TPMT),1 fluorouracil (FU)/capecitabine (DPYD),2 irinotecan (UGT1A1),3 and tacrolimus (CYP3A5).4 Additionally, there are several known associations with supportive care agents commonly used in patients during treatment,such as fungal prophylaxis with voriconazole (CYP2C19)5 and antiemetic treatment with ondansetron (CYP2D6).6
Despite their established clinical validity, few of these associations have been implemented into clinical practice. St Jude Children’s Research Hospital has led the way, implementing preemptive pharmacogenetic testing to guide personalized treatment of several gene-drug pairs.7 The experiences of early adopters has identified formidable challenges to implementing pharmacogenetics into clinical practice,8,9 which require substantial investment and expertise.10,11 The primary bottleneck in the implementation of pharmacogenetics is a lack of evidence that pharmacogenetic testing meaningfully improves patient outcomes. Although there is debate about the necessity and feasibility of demonstrating clinical utility for pharmacogenetic implementation,12,13 the lack of uptake indicates that health systems are not willing to incur the costs of pharmacogenetic implementation for unproven clinical benefit. However, available germline genetic information should be considered when making treatment decisions.14 In anticipation of a time when genetic information is available for many patients, perhaps as a result of the proliferation of direct-to-consumer genotyping,15 the pharmacogenetic community has developed evidence-based pharmacogenetic treatment guidelines.3,16
In oncology, there has been a tremendous expansion in the availability of genetic information as a result of the proliferation of tumor sequencing programs that use somatic genetic information to personalize selection of targeted cancer treatments.17 Although some programs analyze only the somatic genome, others have found that matched germline analysis improves quality control18 and enables simultaneous assessment of familial predisposition to cancer.19 This creates a unique situation in which germline genetic information for clinically relevant pharmacogenes is freely available.20 However, the opportunity to use these data in clinical practice has not yet been capitalized upon.
The Michigan Oncology Sequencing (MI-Oncoseq) program at The University of Michigan Comprehensive Cancer Center (UMCCC) performs targeted sequencing of somatic and matched germline DNA, in addition to somatic whole-transcriptome analysis.21 The targeted sequencing panel includes approximately 100 pharmacogenes that could be used to provide evidence-based pharmacogenetic treatment recommendations, if the accuracy of the germline genetic determinations are verified. Our primary objective was to confirm the reliability of germline genotype determinations made by in-house MI-Oncoseq sequencing for polymorphisms in cancer-relevant pharmacogenes. After retrospectively assessing the proportion of MI-Oncoseq patients who carried actionable phenotypes and received a drug relevant to their genotype status, we then reviewed treatment outcomes in these patients to assess the clinical usefulness of integrating germline pharmacogenetics into MI-Oncoseq.
METHODS
MI-Oncoseq Patients
The primary objective of Mi-Oncoseq is to provide precision cancer treatment recommendations based on profiling of the tumor and the patient. Detailed study information including inclusion and exclusion criteria, data analysis and processing, and return of results has been previously published.21-23 Briefly, patients treated at UMCCC with refractory tumors are invited to participate in a research protocol in which they provide tumor and blood samples for matched genetic sequencing, among other somatic analyses. Patients sign informed consent, including an opt-out option for germline information. Since the initiation of MI-Oncoseq, the DNA sequencing platform has transitioned from whole-exome sequencing to a targeted exon sequencing panel, and sequencing methods have been previously described in detail.21,23 This targeted panel sequences primarily exonic regions of approximately 1,700 genes, including approximately 100 pharmacogenes selected based on curation within PharmGKB (Appendix Table A1). The Onco1700 has gone through several updates as sequencing issues have been identified and corrected. Patients for whom germline genetic information was available from the fourth version of Onco1700 (Onco1700_V4; Onco1500 v4_160111_HG19_OncoPanelV4_XC_EZ_HX1) as of December 28, 2016, were included in the retrospective analysis of MI-Oncoseq germline pharmacogenetics.
Onco1700 Genotype Determinations
The genetic data for 21 single-nucleotide polymorphisms (SNPs) in five cancer-related pharmacogenes were collected: TPMT: *2 (rs1800462), *3B (rs1800460), *3C (rs1142345), and *4 (rs1800584); DPYD: *2 (rs3918290), *13 (rs55886062), and rs67376798 (no * designation); CYP2C19: *2 (rs4244285), *3 (rs4986893), *4 (rs28399504), *5 (rs56337013), *6 (rs72552267), *7 (rs72558186), *8 (rs41291556), and *17 (rs12248560); CYP3A5: *3 (rs776746), *6 (rs10264272), and *7 (rs41303343); and UGT1A1: *6 (rs4148323), *28 (rs8175347), and *80 (rs887829). These five genes were selected based on their relevance to cancer treatment or supportive care, likelihood of clinician interest in prospective implementation of the gene-drug pairs, and existence of evidence-based treatment guidelines from the Clinical Pharmacogenetics Implementation Consortium (CPIC)16 or the Dutch Pharmacogenetics Working Group (DPWG).3 The polymorphisms in each gene were selected based on the validated variant lists within each guideline. Note that UGT1A1*28 (rs8175347) and *80 (rs887829) were both included to represent the UGT1A1*28 genotype, because they are highly linked, and *80 is often substituted for *28 as a result of its relative ease of genotyping.24 The MI-Oncoseq germline genetic database was screened to identify all variant calls at any of these polymorphisms for all patients sequenced on Onco1700_V4, and variant calls were compiled in a single data set for further analysis.
Confirmatory Genotyping
Genotype determinations from Onco1700 were manually screened for potentially unreliable calls by assessing standard sequencing quality control parameters, including read depth. Reliable genotype determinations were then screened to identify patients to send for Clinical Laboratory Improvement Amendments–approved confirmatory genotyping. Up to five samples that were heterozygous and five that were homozygous variant for each polymorphism were selected, while attempting to minimize the total number of samples regenotyped. An iterative manual process was undertaken in which every sample was selected for all polymorphisms with ≤ five occurrences. In the second stage, polymorphisms with > five but < 30 occurrences were selected, by prioritizing patients who also carried a variant at one of the common SNP positions. Finally, as many samples as necessary were selected that carried these common variants so that each variant was represented in five samples.
Selected samples were sent for College of American Pathologists–accredited, Clinical Laboratory Improvement Amendments–approved genotyping at Genelex Laboratories (Seattle, WA). Genotypes were obtained using a laboratory-developed, multiplex polymerase chain reaction–based test followed by single base primer extension for variant detection by mass spectrometry (MassArray Analyzer 4 System; Agena Bioscience, San Diego, CA). Analytic sensitivity and specificity was > 99%.25 All samples were genotyped for all polymorphisms of interest, and this was conducted blinded to the Onco1700 genotype determinations. The Onco1700 genotype determinations were compared with the Genelex confirmatory genotype determinations to assess concordance. Percent concordance for each SNP was calculated as the number of concordant genotypes divided by the total number of samples compared.
Retrospective Analysis of Clinically Actionable Phenotypes
Patient genotypes were translated to activity phenotypes based on the appropriate CPIC26 or DPWG3 guidelines (Appendix Table A2). Clinically actionable phenotypes were defined on the basis of guideline recommendations to adjust dose or select an alternative drug. Genotype data for UGT1A1*28 (*80) and CYP3A5*3 was only available for the 25 samples sent for confirmatory genotyping; therefore, these patients and genotypes were included in assessments of clinical usefulness. The electronic medical records for all patients with actionable phenotypes were screened to determine whether they received the relevant drugs. Medical record screening was performed using an automated screening tool (EMERSE27) that searches the text of notes in MiChart, the version of EPIC used at Michigan Medicine. Text used to screen the medical records included all generic and brand drug names and commonly used acronyms (eg, FU for fluourouracil).
A pharmacy student, in consultation with a pharmacist with oncology pharmacogenomic expertise, manually reviewed the electronic medical record for each patient who had an actionable phenotype and received the relevant drug to determine whether a treatment modification would have been recommended had this genetic information been available to the clinician before treatment initiation. Treatment outcomes relevant to the identified gene-drug interactions were also manually evaluated. Descriptive statistical results for this analysis include the percentages of patients with actionable phenotypes, those who received the relevant drug, and those who would have been eligible for a pharmacogenetic treatment recommendation based on evidence-based guidelines.
RESULTS
MI-Oncoseq Patients and Confirmatory Genotyping
A search of all patients with germline sequence data available from Onco1700_V4 identified 115 patients. These patients represent the diversity of the MI-Oncoseq cohort, including adult (n = 82; 71%) and pediatric patients (n = 33; 29%) who were evenly divided between male (n = 58; 50%) and female sex (n = 57; 50%), were primarily white (n = 97; 84%), and had a variety of solid (n = 80; 70%) and liquid tumor types (n = 35; 30%).
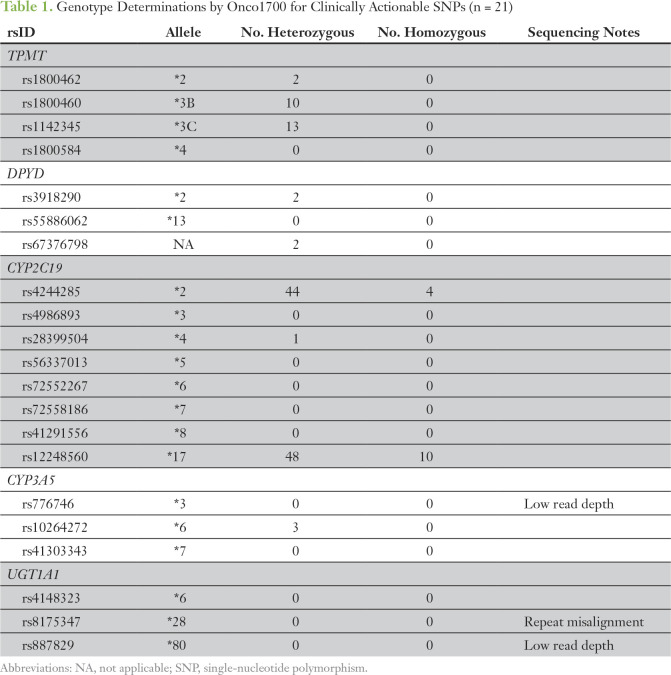
No genotype determinations for CYP3A5*3, UGT1A1*28, or UGT1A1*80 were made by Onco1700 because of low read depth (CYP3A5*3 and UGT1A1*80) or sequence repeat misalignment (UGT1A1*28); therefore, these polymorphisms were excluded from genotype concordance analyses. Across the 18 remaining SNPs, a total of 139 variant calls were made in these 115 samples, and nine unique SNPs were detected in at least one patient (Table 1). No variants were detected for the remaining nine SNPs.
Table 1.
Genotype Determinations by Onco1700 for Clinically Actionable SNPs (n = 21)
Using the previously described selection process, residual germline DNA samples from 25 patients were sent for confirmatory genotyping. All variant determinations made by Onco1700 were confirmed, and no variant genotypes were identified by confirmatory genotyping that were not detected by Onco1700. Therefore, confirmatory genotyping was 100% concordant with Onco1700 genotype determinations.
Retrospective Analysis of Clinically Actionable Phenotypes
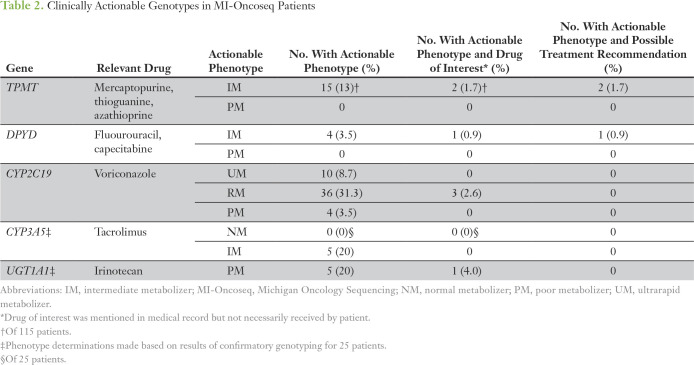
A determination of clinically actionable phenotype, based on CPIC criteria, was made for each patient for each gene for which he or she had usable genotype data (ie, n = 25 for CYP3A5 and UGT1A1). The number and percentage of patients carrying actionable phenotypes for each gene are listed in Table 2. The frequency of actionable phenotypes was highest for CYP2C19 (43.5%), followed by CYP3A5 (20%), UGT1A1 (20%), and TPMT (13%), and lowest for DPYD (3.5%), as expected.
Table 2.
Clinically Actionable Genotypes in MI-Oncoseq Patients
The electronic medical record for each patient carrying an actionable phenotype was screened for relevant drugs. Initial screening identified seven patients with actionable phenotypes whose medical records contained mention of a relevant drug. For four of these seven patients, having genetic information would not have led to a treatment recommendation. One patient with CYP2C19 rapid metabolizer phenotype never received voriconazole; two patients with CYP2C19 rapid metabolizer phenotype received voriconazole treatment, but they were pediatric patients, so no dose adjustment is recommended per CPIC guidelines5; and one patient with UGT1A1 poor metabolizer phenotype treated with irinotecan received a standard pediatric dose (49 mg/m2), which is below the recommended threshold for dose adjustment based on DPWG guidelines.3
Three patients with actionable phenotypes who received the relevant drug, in whom a treatment recommendation could have been made, were identified. Two pediatric patients diagnosed with lymphoblastic leukemia had TPMT genotype ordered from an outside laboratory and were determined to be intermediate metabolizers. Per Children’s Oncology Group protocol, both patients started mercaptopurine at standard oral dose (75 mg/m2 per day). One patient’s dose was held after 5 days because of neutropenia (absolute neutrophil count [ANC], 200), and the second patient’s dose was reduced to 70% of the standard dose after 7 days because of neutropenia (ANC, 500). The third patient was diagnosed with pancreatic adenocarcinoma. The patient’s DPYD genotype was available within MI-Oncoseq, but the intermediate metabolizer phenotype was not known by the clinical team. The patient received standard-dose FOLFIRI (FU, leucovorin, and irinotecan), including FU continuous infusion at 2,400 mg/m2, which caused moderate neutropenia (ANC, 900) but otherwise no notable toxicity.
DISCUSSION
Despite the discovery and validation of germline genotypes that predict treatment outcomes, pharmacogenetics has been slow to be implemented into clinical practice.28-30 There are many challenges to pharmacogenetic implementation,10 but perhaps the primary challenge is the current lack of evidence of clinical utility to justify the upfront cost of establishing a pharmacogenetic service.31,32 In anticipation of a future in which genomic information is more readily available, CPIC and other groups have published evidence-based treatment recommendations for patients with known genotypes.3,16 There is a unique opportunity to integrate evidence-based pharmacogenetic treatment into tumor sequencing programs that analyze germline genetic information,20 such as the MI-Oncoseq program at UMCCC.21 The objective of this analysis was to confirm the accuracy of germline genotype determinations produced during MI-Oncoseq sequencing and then to retrospectively assess the clinical usefulness of integrating pharmacogenetics into MI-Oncoseq.
Attempted confirmatory genotyping of 21 clinically actionable SNPs in five cancer-relevant pharmacogenes confirmed genotyping accuracy for common and uncommon variants in three genes (TPMT, DPYD, and CYP2C19) but revealed an inability to genotype common variants in CYP3A5 and UGT1A1. This finding is easily explained by the targeted exonic coverage of Onco1700 and the location of these polymorphisms at a splice site (CYYP3A5*3, rs776746) and in the promoter region (UGT1A1*28, rs8175347). Other variants that were not detected are extremely rare, and several have not been found in white patients.
Prior studies have estimated that > 90% of the population has an actionable phenotype of at least one candidate gene33,34; however, this is only relevant if the patient is treated with the drug of interest and the guidelines apply to the patient. In our cohort, 4% (five of 115) of patients had a potentially actionable phenotype in CYP2C19, TPMT, or DPYD and received the relevant drug, and in 2.6% (three of 115) of patients, a guideline-based treatment recommendation could have been made.
Manual review of these three patients identified several interesting findings. Despite TPMT genotype information available at the time of treatment, two patients with heterozygous genotypes initiated treatment at standard mercaptopurine doses, per the Children’s Oncology Group protocols on which they were enrolled. CPIC recommends a preemptive 30% to 70% dose decrease with enhanced monitoring and titration based on tolerability.1 Although germline TPMT determination from MI-Oncoseq would not have changed these patients’ treatment in any way, it would have prevented external genetic testing, resulting in cost savings to the health system. The third patient carried a DPYD genotype that confers risk of severe toxicity with FU, which was not known at the time of treatment. CPIC guidelines recommend a preemptive dose reduction of 50% with monitoring and titration.2 Although it is impossible to attribute toxicity to any single factor, it is interesting that two patients experienced toxicity requiring a reactive dose reduction, which may have been prevented if care had been based on CPIC guidelines.
The 2% to 4% absolute increase in the proportion of patients with clinically actionable findings from germline pharmacogenetics represents a minimal estimate, because the EMERSE screening tool does not automatically screen prescribing data, and there is some chance that a patient with an actionable phenotype received a relevant medication that was never mentioned in a clinical note. Regardless, this represents a meaningful increase in the clinically actionable findings from our tumor sequencing program.35 In addition to its usefulness for quality control,18,36 matched germline analysis identifies validated cancer predisposition variants in an estimated 15% of patients.19 Several tumor sequencing programs, including MI-Oncoseq,21 use their matched germline DNA for this purpose.37,38 Our results represent a critical first step toward integration of germline pharmacogenetics into MI-Oncoseq.
Although several programs have reported that pharmacogenetics is considered in their decision making,39,40 we are not aware of any detailed reports of the integration of pharmacogenetics into these programs. A summary of the experience of the Precision in Pediatric Sequencing Program at Columbia University Medical Center mentioned that pharmacogenetic investigation detected clinically meaningful variants in UGT1A1.41 Although the UGT1A1*28 variant was reported in two patients, according to guideline recommendations, only the patient who was homozygous for UGT1A1*28 had a clinically actionable phenotype.3 The implication of drug sensitivity was noted; however, no information was provided on how this information would be used to inform irinotecan treatment decisions3 or how the incidental finding of Gilbert’s syndrome would be conveyed to the patient.42,43
Generalization of our estimate of the proportion of patients who could benefit from pharmacogenetic implementation in other tumor sequencing programs is challenging for several reasons. First, there are differences in the frequencies of clinically actionable alleles or phenotypes among racial cohorts.44 Additionally, institutional differences in the distribution of tumors that are treated and sent for sequencing could dramatically affect this estimate. Furthermore, the potential utility of this approach is highly dependent on when the sequencing occurs. Many programs sequence primarily refractory tumors, at which time most standard treatment has already been exhausted. Pharmacogenetic integration would be most beneficial in programs that sequence tumors early in treatment, particularly at institutions that treat many pediatric patients with ALL.
The proportion of patients who would benefit from pharmacogenetic implementation could be increased in several ways. The next update of Onco1700 will include targeted sequencing coverage of CYP3A5*3, UGT1A1*28, and UGT1A1*80. Another gene that will be included in the next update is NUDT15, which contributes to mercaptopurine toxicity45,46 and is being added to the CPIC thiopurine guidelines. Another high-priority target is CYP2D6, which has CPIC guidelines for several drugs used commonly in cancer supportive care, including narcotic analgesics,47 5-hydroxytryptamine-3 antiemetics,6 and antidepressants.48 Unfortunately, CYP2D6 had to be excluded from this initial analysis because of the complexity of assigning genotype determinations from sequencing data49 and making genotype-based clinical recommendations.50
In addition to genotyping cost, prospective implementation of pharmacogenetics requires substantial upfront investment to build and maintain clinical decision support within the electronic health record, hire or train individuals with pharmacogenetic expertise, and provide clinician and patient education. Pharmacogenetic implementation within tumor sequencing programs that analyze matched germline DNA is particularly efficient because there is no genotyping cost and the bioinformatic workflow to detect actionable germline phenotypes can be integrated into the existing infrastructure. However, substantial investment is still required to integrate pharmacy and pharmacogenomic expertise51 into the multidisciplinary precision medicine tumor board that oversees genetically informed treatment decision making. In the MI-Oncoseq model, pharmacists will work alongside medical oncologists and genetic counselors,52 providing pharmacogenetic expertise and education. Additional work is necessary to develop infrastructure to embed active clinical decision support into the electronic health record so that actionable phenotypes can be stored and used indefinitely.7,11
In conclusion, Onco1700 produces reliable germline pharmacogenetic information for three clinically relevant pharmacogenes (TPMT, DPYD, and CYP2C19). Prospective implementation of pharmacogenetics within MI-Oncoseq will enable evidence-based treatment recommendations in 2% to 4% of MI-Oncoseq patients. Integration of germline pharmacogenetics into a tumor sequencing program is a uniquely efficient opportunity to maximize the clinical benefit of genomic testing, taking another step toward precision medicine for patients with cancer.
ACKNOWLEDGMENT
We thank the patients with cancer and their families for their kindness and generosity in participating in this study.
Appendix
Table A1.
Pharmacogenes Sequenced on Onco1700
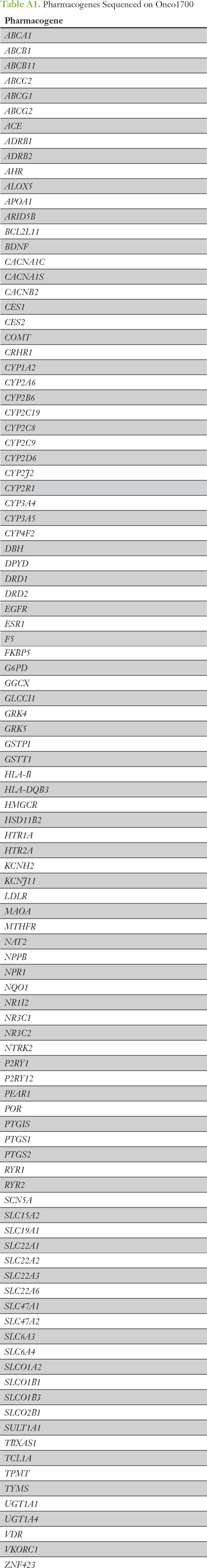
Table A2.
Results of Confirmatory Genotyping
Footnotes
Supported by National Institutes of Health (NIH) Clinical Sequencing Exploratory Research Award No. 1UM1HG006508, NIH National Cancer Institute Awards No. U24CA204863 and P30CA046592, NIH National Center for Advancing Translational Sciences Awards No. UL1TR000433 and UL1TR002240, the Prostate Cancer Foundation, Stand Up 2 Cancer Prostate Cancer Foundation Prostate Dream Team Grant No. SU2C-AACR-DT0712, Early Detection Research Network Grant No. U01 CA214170, and Prostate Specialized Programs of Research Excellence Grant No. P50 CA186786; A.C. is a Howard Hughes Medical Institute Investigator, A. Alfred Taubman Scholar, and American Cancer Society Professor.
Presented in preliminary form at the 2016 American Society of Clinical Oncology Annual Meeting, Chicago, IL, June 2-7, 2016.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel L. Hertz, Erika Mora, Kevin Frank, Rajen J. Mody, Arul Chinnaiyan
Financial support: Rajen J. Mody, Arul Chinnaiyan
Administrative support: Bailey Anderson, Rajen J. Mody
Provision of study material or patients: Yi-Mi Wu, Rajen J. Mody, Arul Chinnaiyan
Collection and assembly of data: Andrew Glatz, Pankaj Vats, Yi-Mi Wu, Bailey Anderson, Erica Rabban, Kevin Frank, Daniel R. Robinson, Rajen J. Mody
Data analysis and interpretation: Daniel L. Hertz, Andrew Glatz, Amy L. Pasternak, Robert J. Lonigro, Pankaj Vats, Yi-Mi Wu, Kevin Frank, Daniel R. Robinson, Rajen J. Mody, Arul Chinnaiyan
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po-author-center.
Daniel L. Hertz
No relationship to disclose
Andrew Glatz
No relationship to disclose
Amy L. Pasternak
No relationship to disclose
Robert J. Lonigro
No relationship to disclose
Pankaj Vats
No relationship to disclose
Yi-Mi Wu
No relationship to disclose
Bailey Anderson
No relationship to disclose
Erica Rabban
No relationship to disclose
Erika Mora
Employment: Amgen
Kevin Frank
No relationship to disclose
Daniel R. Robinson
No relationship to disclose
Rajen J. Mody
No relationship to disclose
Arul Chinnaiyan
Stock and Other Ownership Interests: Oncopia, Tempus, Esanik, OncoFusion Therapeutics, Medsyn
Consulting or Advisory Role: Tempus
Patents, Royalties, Other Intellectual Property: University of Michigan royalties
REFERENCES
- 1.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caudle KE, Thorn CF, Klein TE, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther. 2013;94:640–645. doi: 10.1038/clpt.2013.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swen JJ, Nijenhuis M, de Boer A, et al. Pharmacogenetics: From bench to byte—An update of guidelines. Clin Pharmacol Ther. 2011;89:662–673. doi: 10.1038/clpt.2011.34. [DOI] [PubMed] [Google Scholar]
- 4.Birdwell KA, Decker B, Barbarino JM, et al. Clinical pharmacogenetics implementation consortium (CPIC) guidelines for CYP3A5 genotype and tacrolimus dosing. Clin Pharmacol Ther. 2015;98:19–24. doi: 10.1002/cpt.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moriyama B, Obeng AO, Barbarino J, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for CYP2C19 and voriconazole therapy. Clin Pharmacol Ther. doi: 10.1002/cpt.583. epub ahead of print on December 16, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell GC, Caudle KE, Whirl-Carrillo M, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 genotype and use of ondansetron and tropisetron. Clin Pharmacol Ther. 2017;102:213–218. doi: 10.1002/cpt.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman JM, Haidar CE, Wilkinson MR, et al. PG4KDS: A model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet. 2014;166C:45–55. doi: 10.1002/ajmg.c.31391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel JN. Cancer pharmacogenomics, challenges in implementation, and patient-focused perspectives. Pharm Genomics Pers Med. 2016;9:65–77. doi: 10.2147/PGPM.S62918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volpi S, Bult CJ, Chisholm RL, et al. Research directions in the clinical implementation of pharmacogenomics: An overview of US programs and projects. Clin Pharmacol Ther. 2018;103:778–786. doi: 10.1002/cpt.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shuldiner AR, Relling MV, Peterson JF, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Overcoming challenges of real-world implementation. Clin Pharmacol Ther. 2013;94:207–210. doi: 10.1038/clpt.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunnenberger HM, Crews KR, Hoffman JM, et al. Preemptive clinical pharmacogenetics implementation: Current programs in five US medical centers. Annu Rev Pharmacol Toxicol. 2015;55:89–106. doi: 10.1146/annurev-pharmtox-010814-124835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudle KE, Gammal RS, Whirl-Carrillo M, et al. Evidence and resources to implement pharmacogenetic knowledge for precision medicine. Am J Health Syst Pharm. 2016;73:1977–1985. doi: 10.2146/ajhp150977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillis NK, Innocenti F. Evidence required to demonstrate clinical utility of pharmacogenetic testing: The debate continues. Clin Pharmacol Ther. 2014;96:655–657. doi: 10.1038/clpt.2014.185. [DOI] [PubMed] [Google Scholar]
- 14.Altman RB. Pharmacogenomics: “noninferiority” is sufficient for initial implementation. Clin Pharmacol Ther. 2011;89:348–350. doi: 10.1038/clpt.2010.310. [DOI] [PubMed] [Google Scholar]
- 15.Filipski KK, Murphy JD, Helzlsouer KJ. Updating the landscape of direct-to-consumer pharmacogenomic testing. Pharm Genomics Pers Med. 2017;10:229–232. doi: 10.2147/PGPM.S140461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Relling MV, Klein TE. CPIC: Clinical Pharmacogenetics Implementation Consortium of the Pharmacogenomics Research Network. Clin Pharmacol Ther. 2011;89:464–467. doi: 10.1038/clpt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heuckmann JM, Thomas RK. A new generation of cancer genome diagnostics for routine clinical use: Overcoming the roadblocks to personalized cancer medicine. Ann Oncol. 2015;26:1830–1837. doi: 10.1093/annonc/mdv184. [DOI] [PubMed] [Google Scholar]
- 18.Jones S, Anagnostou V, Lytle K, et al. Personalized genomic analyses for cancer mutation discovery and interpretation. Sci Transl Med. 2015;7:283ra53. doi: 10.1126/scitranslmed.aaa7161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schrader KA, Cheng DT, Joseph V, et al. Germline variants in targeted tumor sequencing using matched normal DNA. JAMA Oncol. 2016;2:104–111. doi: 10.1001/jamaoncol.2015.5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hertz DL, McLeod HL. Integrated patient and tumor genetic testing for individualized cancer therapy. Clin Pharmacol Ther. 2016;99:143–146. doi: 10.1002/cpt.294. [DOI] [PubMed] [Google Scholar]
- 21.Roychowdhury S, Iyer MK, Robinson DR, et al. Personalized oncology through integrative high-throughput sequencing: A pilot study. Sci Transl Med. 2011;3:111ra121. doi: 10.1126/scitranslmed.3003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mody RJ, Wu YM, Lonigro RJ, et al. Integrative clinical sequencing in the management of refractory or relapsed cancer in youth. JAMA. 2015;314:913–925. doi: 10.1001/jama.2015.10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson DR, Wu YM, Lonigro RJ, et al. Integrative clinical genomics of metastatic cancer. Nature. 2017;548:297–303. doi: 10.1038/nature23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gammal RS, Court MH, Haidar CE, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for UGT1A1 and atazanavir prescribing. Clin Pharmacol Ther. 2016;99:363–369. doi: 10.1002/cpt.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott LS, Henderson JC, Neradilek MB, et al. Clinical impact of pharmacogenetic profiling with a clinical decision support tool in polypharmacy home health patients: A prospective pilot randomized controlled trial. PLoS One. 2017;12:e0170905. doi: 10.1371/journal.pone.0170905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caudle KE, Dunnenberger HM, Freimuth RR, et al. Standardizing terms for clinical pharmacogenetic test results: Consensus terms from the Clinical Pharmacogenetics Implementation Consortium (CPIC) Genet Med. 2017;19:215–223. doi: 10.1038/gim.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: A report of University of Michigan’s nine-year experience in developing and using the Electronic Medical Record Search Engine (EMERSE) J Biomed Inform. 2015;55:290–300. doi: 10.1016/j.jbi.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Relling MV, Evans WE. Pharmacogenomics in the clinic. Nature. 2015;526:343–350. doi: 10.1038/nature15817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weitzel KW, Alexander M, Bernhardt BA, et al. The IGNITE network: A model for genomic medicine implementation and research. BMC Med Genomics. 2016;9:1. doi: 10.1186/s12920-015-0162-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luzum JA, Pakyz RE, Elsey AR, et al. The Pharmacogenomics Research Network Translational Pharmacogenetics Program: Outcomes and metrics of pharmacogenetic implementations across diverse healthcare systems. Clin Pharmacol Ther. 2017;102:502–510. doi: 10.1002/cpt.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirmohamed M. Acceptance of biomarker-based tests for application in clinical practice: Criteria and obstacles. Clin Pharmacol Ther. 2010;88:862–866. doi: 10.1038/clpt.2010.245. [DOI] [PubMed] [Google Scholar]
- 32.Khoury MJ. Dealing with the evidence dilemma in genomics and personalized medicine. Clin Pharmacol Ther. 2010;87:635–638. doi: 10.1038/clpt.2010.4. [DOI] [PubMed] [Google Scholar]
- 33.Van Driest SL, Shi Y, Bowton EA, et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther. 2014;95:423–431. doi: 10.1038/clpt.2013.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji Y, Skierka JM, Blommel JH, et al. Preemptive pharmacogenomic testing for precision medicine: A comprehensive analysis of five actionable pharmacogenomic genes using next-generation DNA sequencing and a customized CYP2D6 genotyping cascade. J Mol Diagn. 2016;18:438–445. doi: 10.1016/j.jmoldx.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gornick MC, Cobain E, Le LQ, et al. Oncologists’ use of genomic sequencing data to inform clinical management. JCO Precis Oncol. doi: 10.1200/PO.17.00122. doi:10.1200/PO.17.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cabanillas R, Diñeiro M, Castillo D, et al. A novel molecular diagnostics platform for somatic and germline precision oncology. Mol Genet Genomic Med. 2017;5:336–359. doi: 10.1002/mgg3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stockley TL, Oza AM, Berman HK, et al. Molecular profiling of advanced solid tumors and patient outcomes with genotype-matched clinical trials: The Princess Margaret IMPACT/COMPACT trial. Genome Med. 2016;8:109. doi: 10.1186/s13073-016-0364-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uzilov AV, Ding W, Fink MY, et al. Development and clinical application of an integrative genomic approach to personalized cancer therapy. Genome Med. 2016;8:62. doi: 10.1186/s13073-016-0313-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radovich M, Kiel PJ, Nance SM, et al. Clinical benefit of a precision medicine based approach for guiding treatment of refractory cancers. Oncotarget. 2016;7:56491–56500. doi: 10.18632/oncotarget.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oberg JA, Glade Bender JL, Sulis ML, et al. Implementation of next generation sequencing into pediatric hematology-oncology practice: Moving beyond actionable alterations. Genome Med. 2016;8:133. doi: 10.1186/s13073-016-0389-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bosma PJ, Chowdhury JR, Bakker C, et al. The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med. 1995;333:1171–1175. doi: 10.1056/NEJM199511023331802. [DOI] [PubMed] [Google Scholar]
- 43.Pasternak AL, Ward KM, Luzum JA, et al. Germline genetic variants with implications for disease risk and therapeutic outcomes. Physiol Genomics. 2017;49:567–581. doi: 10.1152/physiolgenomics.00035.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel JN. Cancer pharmacogenomics: Implications on ethnic diversity and drug response. Pharmacogenet Genomics. 2015;25:223–230. doi: 10.1097/FPC.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 45.Moriyama T, Yang YL, Nishii R, et al. Novel variants in NUDT15 and thiopurine intolerance in children with acute lymphoblastic leukemia from diverse ancestry. Blood. 2017;130:1209–1212. doi: 10.1182/blood-2017-05-782383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moriyama T, Nishii R, Perez-Andreu V, et al. NUDT15 polymorphisms alter thiopurine metabolism and hematopoietic toxicity. Nat Genet. 2016;48:367–373. doi: 10.1038/ng.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crews KR, Gaedigk A, Dunnenberger HM, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guidelines for codeine therapy in the context of cytochrome P450 2D6 (CYP2D6) genotype. Clin Pharmacol Ther. 2012;91:321–326. doi: 10.1038/clpt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hicks JK, Bishop JR, Sangkuhl K, et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline for CYP2D6 and CYP2C19 genotypes and dosing of selective serotonin reuptake inhibitors. Clin Pharmacol Ther. 2015;98:127–134. doi: 10.1002/cpt.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Numanagić I, Malikić S, Pratt VM, et al. Cypiripi: Exact genotyping of CYP2D6 using high-throughput sequencing data. Bioinformatics. 2015;31:i27–i34. doi: 10.1093/bioinformatics/btv232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks JK, Swen JJ, Gaedigk A. Challenges in CYP2D6 phenotype assignment from genotype data: A critical assessment and call for standardization. Curr Drug Metab. 2014;15:218–232. doi: 10.2174/1389200215666140202215316. [DOI] [PubMed] [Google Scholar]
- 51.Walko C, Kiel PJ, Kolesar J. Precision medicine in oncology: New practice models and roles for oncology pharmacists. Am J Health Syst Pharm. 2016;73:1935–1942. doi: 10.2146/ajhp160211. [DOI] [PubMed] [Google Scholar]
- 52.Mills R, Haga SB. Clinical delivery of pharmacogenetic testing services: A proposed partnership between genetic counselors and pharmacists. Pharmacogenomics. 2013;14:957–968. doi: 10.2217/pgs.13.76. [DOI] [PMC free article] [PubMed] [Google Scholar]