Abstract
Purpose:
The need for longitudinal studies on prenatal substance exposure (PSE) extending into adulthood is widely recognised. In particular, studies on the dual effect of exposure to substances and adverse childhood experiences are needed. This register-based matched cohort study investigates the effect of this dual exposure on the health and development of youth with PSE. The follow-up is from birth to young adulthood.
Participants:
The exposed youth were born in 1992–2001 to mothers with a significant substance misuse problem during pregnancy. The mothers were identified in primary care maternity clinics in the Helsinki metropolitan area and referred for intensified pregnancy follow-up in a tertiary care setting (HAL-clinics). Data from hospital medical records were collected for the mothers during the pregnancy follow-up and linked with register data from multiple national health and social welfare registers obtained for each mother–child dyad from birth until the end of 2015–2018. Similar register data were gathered for three matched mother–child dyads without any evidence of the mother’s substance misuse in national health and social welfare registers. The study consists of 615 exposed and 1787 unexposed youth aged 15–24 years.
Findings to date:
A majority of the exposed youth (64%) had been in out-of-home care at least once compared with 8% among the unexposed. Outpatient and inpatient hospital care due to mental or behavioural disorders were two to three times more common among the exposed than among the unexposed. The exposed had less often completed secondary school education and had more often needed social assistance.
Future plans:
The data comprise a wide range of information on infant health, youth’s mental and somatic health and development, out-of-home care history, and mother’s life situation at the delivery and later health. Risk and protective factors for different long-term developmental outcomes in adolescence or in young adulthood will be studied.
Keywords: adolescents, alcohol, FASD, illicit drugs, prenatal substance exposure, young adults
Introduction
Alcohol crosses the placenta easily and damages the development of the brain and other organs of the foetus (Popova, Lange, Probst, Gmel, & Rehm, 2017). Detrimental effects of prenatal alcohol exposure (PAE) manifest as cognitive dysfunction (Riley, Infante, & Warren, 2011), congenital anomalies and morbidity (Popova et al., 2016) and mental and behavioural disorders later in life (Khoury, Jamieson, & Milligan, 2018; Weyrauch, Schwartz, Hart, Klug, & Burd, 2017). Other substances also cross the placenta rapidly and can alter brain neurotransmitters and brain biochemistry (Behnke, Smith, & Committee on Substance Abuse, 2013) but less is known about the long-term effects of exposure to substances other than alcohol (Irner, 2012). However, problems in cognitive development (Bunikowski et al., 1998; Eriksson, Jonsson, Steneroth, & Zetterström, 2000), attention, language development and behaviour (Behnke et al., 2013; Irner, 2012) have also been found in adolescents with prenatal exposure to illegal drugs. No excess risk for congenital anomalies has been reported (Behnke et al., 2013; Minnes, Lang, & Singer, 2011).
Because women with substance misuse problems typically are polydrug users (Pajulo, Savonlahti, Sourander, Helenius, & Piha, 2001; Sarkola, Kahila, Gissler, & Halmesmäki, 2007) it is difficult to differentiate the effect of an individual drug on the development of the exposed child. Moreover, childhood maltreatment can cause similar structural damage to the brain as PAE, which manifests as cognitive and behavioural dysfunction (Glaser, 2000; Hart & Rubia, 2012). However, the interaction between PAE and adverse experiences has not been well studied (Koponen, 2006; Koponen, Kalland, & Autti-Rämö, 2009; Koponen, Kalland, Autti-Rämö, Laamanen, & Suominen, 2013; Price, Cook, Norgate, & Mukherjee, 2017). Studies on prenatal drugs exposure (PDE) have more systematically investigated the effects of environmental risk factors. These studies share the view that adverse childhood experiences (ACEs) have a strong negative effect on the exposed children’s and adolescents’ cognitive and emotional development (Ackerman, Riggins, & Black, 2010; Buckingham-Howes, Berger, Scaletti, & Black, 2013; Lambert & Bauer, 2012; Minnes et al., 2011). Still, more longitudinal studies extending into adolescence and adulthood are needed (Irner, 2012). Previous studies on PAE and PDE have also been criticised for moderate sample sizes and insufficient numbers of variables describing adversity (Irner, 2012; Minnes et al., 2011; Price et al., 2017). In the ADEF Helsinki research project (Alcohol/Drugs Exposure during Foetal life), we are able to avoid many of these problems.
ADEF Helsinki is a longitudinal register-based matched (exposed/unexposed youth) cohort study. The study investigates (1) the overall developmental outcomes (congenital anomalies, somatic health, mental and behavioural disorders, attention deficit hyperactivity disorder (ADHD) and learning problems, medication, education, need for income support, problems with the law, rehabilitation, abortions, parity, pregnancies, and health of the next generation) of adolescents and young adults with prenatal substance exposure (PSE), and (2) the effect of the dual exposure to PSE and ACES on the developmental outcomes of these young people. The variables measuring ACEs are: mother’s sociodemographic characteristics (age, marital status, and socioeconomic status), mother’s health and well-being (mental health problems, continued substance misuse, criminal record, income support, and death), and youth’s out-of-home care history (age at the first placement, number of placements, proportion of time living in out-of-home care, and family/residential care). Developmental outcomes of exposed and unexposed youth are compared, as well as the effects of ACEs in both groups.
We hypothesise that developmental problems are more common among the exposed than among the unexposed, and that ACEs increase all developmental problems in both groups and decrease the differences between them. In the present cohort profile, we describe the data and report some first results in the long-term developmental outcomes of exposed and unexposed adolescents and young adults.
Cohort description
This is a longitudinal population- and register-based cohort study comparing adolescents and young adults with and without PSE. Mothers of the exposed youth were first identified by public health nurses in the maternity clinics of primary healthcare centres in the Helsinki metropolitan area. The nurses were educated and advised to refer pregnant women with a significant substance misuse problems (The Alcohol Use Disorders Identification Test ≥ 8 or illegal drug misuse or nonmedical use of central nervous system medication or opioid maintenance treatment) to special antenatal HAL-clinics (illicit drugs, alcohol, medicines) at HUS (The Hospital District of Helsinki and Uusimaa) for counselling and intensified follow-up. Pregnancies were followed up in a multidisciplinary service setting consisting of outpatient antenatal treatment and counselling provided by an obstetrician, a nurse, a midwife, a psychiatric nurse, and a social worker experienced in addiction treatment teamwork. Follow-up visits were individualised from daily visits to visits every four weeks, and women were offered intensified support and easy access to addiction treatment and/or psychiatric care (Kahila, Gissler, Sarkola, Autti-Rämö, & Halmesmäki, 2010; Kahila, Saisto, Kivitie-Kallio, Haukkamaa, & Halmesmäki, 2007).
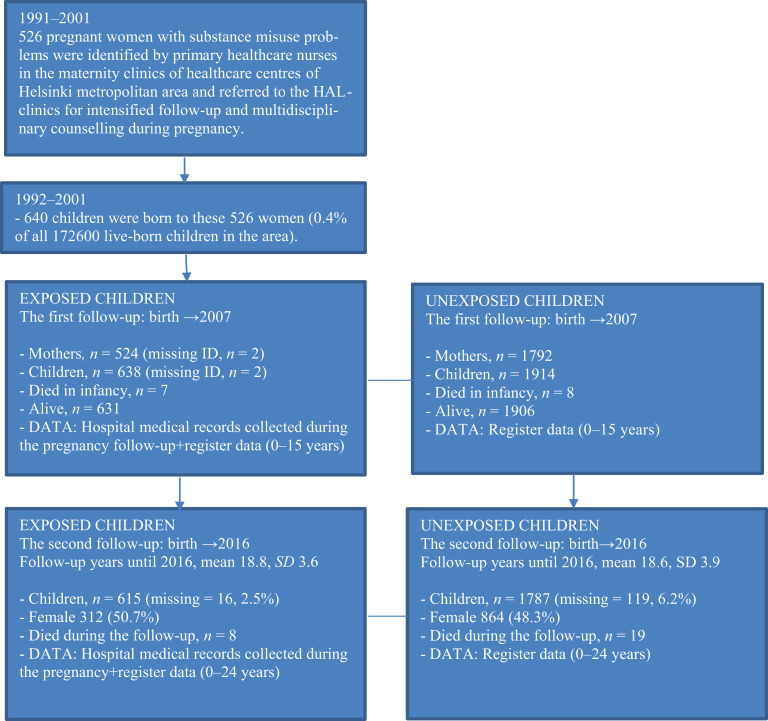
In 1992–2001, 526 pregnant women with identified substance misuse problem (substance misusing mothers), were followed at the HAL-clinics giving birth to 640 infants (0.4% of all children born in the region). Two of the infants could not be linked due to an incomplete maternal identification number. Thus, the original exposed cohort in the first follow-up study consisted of 638 exposed children and 524 substance misusing mothers (Figure 1).
Figure 1.
The data collection process.
The matched control cohort was obtained from the national Medical Birth Register and consisted of 1914 unexposed children born in 1992–2001 to women with no evidence of alcohol and/or substance misuse (n = 1792) in any of the national health and social welfare registers. Three mother–child unexposed dyads were matched with each exposed mother–child dyad for maternal age, parity, number of foetuses, the month of delivery, and delivery hospital of the index child. Because matching was carried out for maternal characteristics, children were not matched by sex. The proportion of boys among the born children in Finland is 51.1% (Statistics Finland). In our data, the proportion of boys among exposed is 49.3%, and among unexposed 51.7% (Figure 1).
Hospital medical records were reviewed for substance misusing mothers during the antenatal and perinatal follow-up as well as for newborns until discharge from the maternity hospital. Register data were obtained from the multiple mandatory national health and social welfare registers for each exposed and unexposed dyad (Table 1). Data linkage of all registers was made by using the unique identification number assigned to each Finnish citizen at birth or immigration (Kahila et al., 2007). Mothers’ and children’s identification numbers were concealed and replaced with study numbers after the data linkages.
Table 1.
Summary of the registers and data collected of exposed and unexposed children and their mothers.
| Register | Data | Children | Period covered | Mothers | Period covered |
|---|---|---|---|---|---|
| Hospital medical records (HAL-clinics) | Pregnancy follow-up, prenatal substance exposure and infant health (exposed only) | x | 1991–2001 | x | 1991–2001 |
| Medical Birth Register (THL) | Pregnancy and birth outcomes, smoking during pregnancy, socioeconomic status, marital status at birth | x | 1992–2001 | x | 1992–2001 |
| Cause-of-Death Register (Statistics Finland) | Dates and causes of death | x | 1992–2015 | x | 1992–2015 |
| Register on Congenital Anomalies (THL) | FASD-diagnoses, congenital anomalies, ICD-9 | x | 1992–2016 | ||
| Central Population register (VRK) | Sex, birth year, marital status, born children, follow-up time, municipality, language | x | 1992–2016 | ||
| Population census data (Statistics Finland), collected every year | Completed secondary school education, socioeconomic status | x | 2010–2015 | ||
| Finnish Employment Register and Pensions Register (ETK) | Earning periods, disability pensions, diagnoses that entitle disability pensions | x | 2008–2015 | ||
| Register on Pensions (KELA) | Pension type, diagnosis | x | 2010–2015 | ||
| Hospital Discharge Register (THL) | Inpatient visits (ICD-9) Inpatient visits (ICD-10) Outpatient visits (ICD-10) Diagnoses, number of out- and inpatient visits, and duration of inpatient visits. |
x x x |
1992–1995 1996–2016 1998–2016 |
x x x |
1992–1995 1996–2016 1998–2016 |
| Register on Primary Healthcare Visits (THL) | Use of primary healthcare (ICD-10) | x | 2011–2016 | ||
| Drug Prescription Register (KELA) | ATC-code and reimbursed prescribed medicines | x | 2008–2016 | ||
| Register on Induced Abortions (THL) | Pregnancies and abortions | x | 1992–2016 | ||
| Register on Child Welfare (THL) | Out-of-home care history | x | 1992–2016 | ||
| Criminal records (LRC) | Criminal records |
x | 2012–2017 |
x | 1985–2018 |
| Register on Social Assistance (THL) | Received social assistance, months, years, euros | x | 2002–2016 | x | 2002–2016 |
| The Register of Child Sickness Benefits (KELA) | Allowances granted due to need of prolonged care of the child caused by sickness. Code, the first and last date, diagnosis. | x | 1998–2016 | x | 1998–2016 |
| The Register on Rehabilitation and Disability Benefits (KELA) | The legal basis of rehabilitation, type of rehabilitation, ICD-10 code, time and duration of rehabilitation. | x | 1996–2016 | x | 1996–2016 |
| The Register on Institutional Care and Housing Services in Social Welfare (THL) | Institutional care in social welfare institutions | x | 1996–2016 | x | 1996–2016 |
HAL-clinics = intensified pregnancy follow-up in a tertiary care setting; THL = Finnish National Institute for Health and Welfare; FASD = foetal alcohol spectrum disorders; ICD = International Statistical Classification of Diseases and Related Health Problems by World Health Organization, WHO; VRK = Central Population Register; ETK = Finnish Centre for Pensions; KELA = Social Insurance Institution; ATC = Anatomical Therapeutic Chemical Classification System by WHO; LRC = Legal Register Centre.
Follow-ups
The first follow-up for exposed dyads (N = 638) and matched unexposed dyads (N = 1914) extending from birth until the end of 2007 (or emigration or death) has been completed (median follow-up = 9 years, range 5–15 years). In the second (ongoing) follow-up, data collection extended from birth until the end of 2015–2018 depending on the availability of register data (Table 1). Most of the register data were available until the end of 2015 or 2016. In 2016 the median age in the youth cohort was 18 years (range 15–24 years). Youth’s criminal records were received until the end of 2017, and mothers’ criminal records until the end of 2018.
The second follow-up consists of 615 exposed and 1787 unexposed dyads (Figure 1). Seven of the 638 exposed and eight of the 1914 unexposed children were stillborn. Of the 631 exposed and 1906 unexposed children born alive, one exposed and 74 unexposed had missing/secured ID information, which resulted in 630 exposed and 1832 unexposed children. Due to the inclusion criteria of being born between 1992 and 2001, in total 15 exposed and 42 unexposed children were excluded due to incorrect birth year (either 1991 or 2002–2003). Further, one unexposed with FAE-diagnosis (foetal alcohol effects) and two others with NAS-diagnosis (neonatal abstinence syndrome) were excluded. The final study population consists of 615 exposed and 1787 unexposed youths born between 1992 and 2001. A total of 15 (2.4%) exposed and 27 (1.4%) unexposed children had died either in infancy or later.
Measures
In the ongoing second follow-up, we use the hospital medical records from the HAL-clinics and register data that were collected from the national health and social welfare registers for the first follow-up, and we have extended the register data collection from 2007 to 2015–2018. Additional register data (Criminal Records, Register of Primary Healthcare Visits, and Register of Induced Abortions) have been added to the database at this stage to complete the long-term evaluation.
Maternal characteristics, prenatal substance exposure, and infant health at birth
Data on prenatal substance exposure (alcohol, illegal/legal drugs) were received from the medical follow-up records of HAL-clinics (based on observations of healthcare personnel, mothers’ own reporting and voluntary urine toxicology screenings if consent was given). Diagnoses on FASD (foetal alcohol spectrum disorders) and congenital anomalies (ICD-9 diagnoses) International Statistical Classification of Diseases and Related Health Problems were received from the Register on Congenital Anomalies provided by the Finnish Institute for Health and Welfare (THL). The Medical Birth Register (THL) was the data source for the health of the neonates (exposure to smoking, gestational age, birth weight and height, Apgar scores, need of intensive care and birth defects) and for mother’s life situation at the time of delivery (age, marital status, cohabitation, socioeconomic status, pregnancy complications, and numbers of previous births, pregnancies, miscarriages, and abortions) (Table 1).
Follow-up data on youth
Youth follow-up data include education, socioeconomic status (Statistics Finland), marital status and live-born children (Population Register Centre), earning periods and disability pensions (Finnish Centre for Pensions, ETK; Social Insurance Institution, KELA), criminal records (Legal Register Centre, LRC), social assistance (THL), and special care allowances (KELA). Health data consist of primary diagnoses (ICD-9, ICD-10) from inpatient and/or outpatient hospital care (THL), primary diagnoses (ICD-10) from primary care health centres, induced abortions, deliveries, and congenital anomalies among the offspring (THL), reimbursed prescribed medicines (KELA), dates and causes of deaths (Statistics Finland), and rehabilitation (KELA). Data on out-of-home care including age at first placement, number of placements, years and type (foster home, institution, kinship care) were provided by THL.
Follow-up data on mothers
Maternal follow-up data, obtained from the same registers as data on youth, included primary diagnoses (ICD-9, ICD-10) based on inpatient and/or outpatient visits in hospitals (THL). When the mother’s substance misuse was defined, secondary diagnoses and external causes were also used. Additional follow-up data consisted of dates and causes of deaths (Statistics Finland), criminal records (LRC), social assistance (THL), rehabilitation (KELA), special care allowances (KELA), and institutional care in social welfare institutions, including care for mental health disorders and care for alcohol- and drug-related problems (THL). Table 1 shows the summary of the registers and data.
Results
Findings and publications of the first follow-up
Results of the first follow-up from birth until the end of 2007 have been reported in articles by Kahila et al. (2007, 2010), Sarkola, Gissler, Kahila, Autti-Rämö, and Halmesmäki (2011, 2012) and Sarkola et al. (2007). The results showed that the mean age of the substance misusing and non-misusing mothers was 27 years (SD = 6 years), and about half the mothers in both groups were expecting their first child. Substance misusing mothers displayed significant long-term morbidity, mortality and loss of productivity after delivery (Kahila et al., 2007). Seven (1.1%) of the exposed children and 8 (0.4%) of the unexposed children were born dead or died during infancy. During their first decade of life, the exposed children frequently used healthcare services for mental and behavioural disorders, especially those who were placed in out-of-home care (Sarkola et al., 2011). Postnatal maternal substance misuse-related morbidity was associated with significant early child morbidity, use of medication and timing of out-of-home care (Sarkola et al., 2012).
First results of the second follow-up
Of the 615 exposed adolescents and adults in the ongoing second follow-up, 175 (28.5%) were exposed to alcohol only, 63 (10.2%) to illegal drugs only, 63 (10.2%) to both alcohol and illegal drugs, and 314 (51.1%) had an unspecified substance exposure (multiple substance use, not possible to classify to the mentioned ‘pure’ categories) history during pregnancy. These figures were based on hospital medical records. NAS was reported in 50 (8.1%), and FASD (diagnosed FAS (foetal alcohol syndrome), FAE/suspected FAS, and ARBD (alcohol-related birth defect)) later diagnosed in 46 (7.5%) among the exposed. Ongoing maternal smoking during pregnancy was prevalent (75.3%) among the exposed. The corresponding proportion of daily maternal smoking among the unexposed was 18.9%. The exposed children had lower birth weight and were more often followed in neonatal intensive care units. Sixteen per cent of them had at least one congenital anomaly (unexposed 9.2%), and 2.1% were intellectually disabled (unexposed 0.8%) (Table 2).
Table 2.
Follow-up data of the 1992–2001 cohort (second follow-up, exposed n = 615, unexposed n = 1787).
| Exposed n (%) | Unexposed n (%) | p-value | |
|---|---|---|---|
| Infant health | |||
| Daily maternal smoking during pregnancy | 463 (75.3) | 337 (18.9) | < 0.001 |
| Birth weight < 2500 g | 77 (12.5) | 120 (6.7) | < 0.001 |
| Apgar score at one minute 0–6 | 25 (4.1) | 60 (3.4) | 0.409 |
| Neonatal intensive care during the first 7 days of life | 127 (20.7) | 176 (9.8) | < 0.001 |
| Any congenital anomaly | 96 (15.6) | 164 (9.2) | < 0.001 |
| Major congenital anomaly | 68 (11.1) | 80 (4.5) | < 0.001 |
| Mother’s life situation at delivery | |||
| Mother’s age less than 20 years | 71 (11.5) | 195 (10.9) | 0.967 |
| Married | 125 (20.5) | 1059 (60.1) | < 0.001 |
| Higher socioeconomic status (mother self-employed or upper- or lower-level employee at the time of the child’s birth) | 190 (34.3) | 966 (57.3) | < 0.001 |
| Life situation in adolescence or young adulthood | |||
| Married | 10 (1.6) | 28 (1.6) | 0.909 |
| Completed secondary school education | 110 (17.9) | 419 (23.4) | 0.004 |
| Social assistance starting at age 18 years or later | 214 (34.8) | 248 (13.9) | < 0.001 |
| At least one episode of out-of-home care | 393 (63.9) | 147 (8.2) | < 0.001 |
| Criminal record | 32 (5.2) | 16 (0.9) | < 0.001 |
| Maternal death during the follow-up | 71 (11.5) | 13 (0.7) | < 0.001 |
| Health in adolescence or young adulthood | |||
| At least one outpatient visit, all diagnoses | 592 (96.3) | 1594 (89.2) | < 0.001 |
| At least one inpatient visit, all diagnoses | 420 (68.3) | 917 (51.3) | < 0.001 |
| Child death during the follow-up | 8 (1.3) | 19 (1.1) | 0.630 |
| Mental and behavioural disorders in adolescence and young adulthood | |||
| Outpatient visit with F-diagnosis | 333 (54.1) | 466 (26.1) | < 0.001 |
| Inpatient visit with F-diagnosis | 129 (21.0) | 121 (6.8) | < 0.001 |
| Outpatient visit due to ADHD ICD-10: F90 | 76 (12.4) | 64 (3.6) | < 0.001 |
| Inpatient visit due to ADHD ICD-10: F90 | 16 (2.6) | 6 (0.3) | < 0.001 |
| Intellectual disability ICD-10: F70-79 | 13 (2.1) | 15 (0.8) | < 0.001 |
F-diagnosis = Mental and behavioral disorders; ADHD = attention deficit hyperactivity disorder; ICD = International Statistical Classification of Diseases and Related Health Problems by WHO.
Compared with non-misusing mothers, substance misusing mothers had lower socioeconomic status and were less often married at the time of the child’s birth, and many died during the follow-up. Of the exposed youth, 11.5% had lost their mother compared with 0.7% among unexposed (Table 2).
Approaching adulthood, exposed adolescents and adults faced more difficulties than their unexposed age-mates. Exposed youths had less often completed secondary school education, more often received social assistance and had more outpatient and inpatient hospital visits due to any diagnoses and due to mental and behavioural disorder diagnoses, including ADHD and intellectual disability. A majority of exposed youths (63.9%) had been placed outside the biological parent’s home at least once during the follow-up (unexposed 8.2%), and 5.2% had at least one criminal record (unexposed 0.9%).
Discussion
The data of the present study consist of a unique combination of medical records and register data with large sample size, a large number of variables describing adverse childhood experiences and other background factors extending from birth to young adulthood. We are able to provide a broad and reliable overview of the nature and extent of the potential problems that adolescents and young adults with a history of prenatal substance exposure face in their life and to identify factors associated with these problems. This research will be of interest both internationally and nationally.
The reporting to the national health and social welfare registers used in this study is obligatory, and their good coverage and quality have been ascertained (Aro, Koskinen, & Keskimäki, 1990; Gissler & Haukka, 2004). Medical data are based on primary diagnoses and statements made by medical professionals and registered in national databases. The Finnish Hospital Discharge Register has been found to be a valid and reliable tool for epidemiological research (Sund, 2012). A register-based study design avoids problems related to the participation, response, and recall bias. No participants are contacted and personal identification is impossible after the data linkages. All data are confidential, and no harm is caused to the participants.
In Finland, 99.7% of all pregnant women attend antenatal services provided by maternity clinics at healthcare centres (Kahila et al., 2010), which made it possible to identify pregnant women with a substance misuse problem. Prenatal substance exposure was confirmed and was considered significant during the pregnancy follow-up. Identification of mothers’ substance use was based on mothers’ own reporting, voluntary urine toxicology screenings, and observations and other information received by healthcare personnel in the maternity clinics and during the pregnancy follow-up. The substance-exposed children in the present study represent 0.4% of all children born in the Helsinki metropolitan area in 1992–2001 (Kahila et al., 2007). International (Popova et al., 2017) and national (Pajulo et al., 2001) prevalence estimations of the substance misuse problem during pregnancy are much higher, which indicates that only the most severe cases were identified and included in the study. Only a few exposed children were diagnosed, which shows that FASD is an underdiagnosed disorder.
The study has also limitations. As in all studies on prenatal substance exposure (Behnke et al., 2013), detailed information on the type, timing and amount of exposure is limited, and all pregnant women with substance misuse problems in the catchment area may not have been identified. Therefore, the data are likely to be biased to those with the heaviest substance use, and they do not reveal the effects of slight and/or occasional substance use. Another limitation is that we cannot be sure that women in the non-misuse group had not used any substances. However, all registers were carefully checked in order to rule out this possibility, and if any sign was noticed, these women and their children were removed from the data.
The proportion of missing cases lost from the first follow-up to the second was somewhat larger among the unexposed children. It is unlikely that this has any effect on the results as three unexposed children were matched with each exposed child.
Child abuse and neglect have a strong negative effect on child development (Hart, & Rubia, 2012) but this information could not be obtained from the registers. However, a majority of exposed adolescents and young adults had been placed in out-of-home care at least once which indicates that many of them may have faced these adversities as previously reported by Kalland and Sinkkonen (2001). Finally, one limitation of register studies is that it is impossible to collect additional primary data if needed.
The first results of this second follow-up of the data showed that a very high percentage of the exposed youth had been in out-of-home care, their mental health problems continued to youth age, and their educational level was lower compared with the unexposed. The results are in line with earlier studies on individuals with PSE (Irner, 2012; Popova et al., 2016; Streissguth, Barr, Kogan, & Bookstein, 1996; Streissguth et al., 2004). In upcoming research articles, we will focus on analysing interactions between PAE, PDE, ACEs, and developmental outcomes among youth with and without PSE.
Footnotes
Research ethics: The study has been approved by the local ethical committee of The Hospital District of Helsinki and Uusimaa (Dnro 333/E8/02). All register organisations have approved the use of their register data.
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: Samfundet Folkhälsan i svenska Finland, Juho Vainio Foundation, Signe and Ane Gyllenberg Foundation, Medicinka Understödsföreningen Liv och Hälsa, and the Finnish Foundation for Alcohol Studies.
ORCID iD: Anne M. Koponen  https://orcid.org/0000-0003-2644-9095
https://orcid.org/0000-0003-2644-9095
Contributor Information
Anne M Koponen, Folkhälsan Research Center, and University of Helsinki, Finland.
Niina-Maria Nissinen, Folkhälsan Research Center, and University of Tampere, Finland.
Mika Gissler, National Institute for Health and Welfare, Finland.
Taisto Sarkola, Children’s Hospital, University of Helsinki, and Helsinki University Hospital, Finland.
Ilona Autti-Rämö, Ministry of Social Affairs and Health, Helsinki, Finland.
Hanna Kahila, Helsinki University Central Hospital, Helsinki, Finland.
References
- Ackerman J. P., Riggins T., Black M. M. (2010). A review of the effects of prenatal cocaine exposure among school-aged children. Pediatrics, 125(3), 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro S., Koskinen R., Keskimäki I. (1990). Reliability of hospital discharge data concerning diagnosis, treatments and accidents. Duodecim: Lääketieteellinen Aikakauskirja, 106(21), 1443–1450. [PubMed] [Google Scholar]
- Behnke M., Smith V. C., & Committee on Substance Abuse. (2013). Prenatal substance abuse: Short-and long-term effects on the exposed fetus. Pediatrics, 131(3), 1009–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham-Howes S., Berger S. S., Scaletti L. A., Black M. M. (2013). Systematic review of prenatal cocaine exposure and adolescent development. Pediatrics, 131(6), e1917–e1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunikowski R., Grimmer I., Heiser A., Metze B., Schäfer A., Obladen M. (1998). Neurodevelopmental outcome after prenatal exposure to opiates. European Journal of Pediatrics, 157(9), 724–730. [DOI] [PubMed] [Google Scholar]
- Eriksson M., Jonsson B., Steneroth G., Zetterström R. (2000). Amphetamine abuse during pregnancy: Environmental factors and outcome after 14–15 years. Scandinavian Journal of Public Health, 28(2), 154–157. [DOI] [PubMed] [Google Scholar]
- Gissler M., Haukka J. (2004). Finnish health and social welfare registers in epidemiological research. Norsk Epidemiologi, 14, 113–120. [Google Scholar]
- Glaser D. (2000). Child abuse and neglect and the brain: A review. The Journal of Child Psychology and Psychiatry and Allied Disciplines, 41(1), 97–116. [PubMed] [Google Scholar]
- Hart H., Rubia K. (2012). Neuroimaging of child abuse: A critical review. Frontiers in Human Neuroscience, 6, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irner T. B. (2012). Substance exposure in utero and developmental consequences in adolescence: A systematic review. Child Neuropsychology, 18(6), 521–549. [DOI] [PubMed] [Google Scholar]
- Kahila H., Gissler M., Sarkola T., Autti-Rämö I., Halmesmäki E. (2010). Maternal welfare, morbidity and mortality 6–15 years after a pregnancy complicated by alcohol and substance abuse: A register-based case-control follow-up study of 524 women. Drug and Alcohol Dependence, 111(3), 215–221. [DOI] [PubMed] [Google Scholar]
- Kahila H., Saisto T., Kivitie-Kallio S., Haukkamaa M., Halmesmäki E. (2007). A prospective study on buprenorphine use during pregnancy: Effects on maternal and neonatal outcome. Acta Obstetricia et Gynecologica Scandinavica, 86(2), 185–190. [DOI] [PubMed] [Google Scholar]
- Kalland M., Sinkkonen J. (2001). Finnish children in foster care: Evaluating the breakdown of long-term placements. Child Welfare, 80(5), 513–527. [PubMed] [Google Scholar]
- Khoury J. E., Jamieson B., Milligan K. (2018). Risk for childhood internalizing and externalizing behavior problems in the context of prenatal alcohol exposure: A meta-analysis and comprehensive examination of moderators. Alcoholism: Clinical and Experimental Research, 42(8), 1358–1377. [DOI] [PubMed] [Google Scholar]
- Koponen A. (2006). Sikiöaikana päihteille altistuneiden lasten kasvuympäristö ja kehitys [Life of children exposed to alcohol or drugs in utero]. The Kotu Research Publications 5. Helsinki: Finnish Association on Intellectual and Developmental Disabilities. (Doctoral dissertation, Faculty of Social Sciences, Department of Social Research, University of Helsinki, Finland). Retrieved from http://ethesis.helsinki.fi/julkaisut/val/sosps/vk/koponen/ [Google Scholar]
- Koponen A. M., Kalland M., Autti-Rämö I. (2009). Caregiving environment and socio-emotional development of foster-placed FASD-children. Children and Youth Services Review, 31(9), 1049–1056. [Google Scholar]
- Koponen A. M., Kalland M., Autti-Rämö I., Laamanen R., Suominen S. (2013). Socio-emotional development of children with foetal alcohol spectrum disorders in long-term foster family care: A qualitative study. Nordic Social Work Research, 3(1), 38–58. [Google Scholar]
- Lambert B. L., Bauer C. R. (2012). Developmental and behavioral consequences of prenatal cocaine exposure: A review. Journal of Perinatology, 32(11), 819–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minnes S., Lang A., Singer L. (2011). Prenatal tobacco, marijuana, stimulant, and opiate exposure: Outcomes and practice implications. Addiction Science & Clinical Practice, 6(1), 57–70. [PMC free article] [PubMed] [Google Scholar]
- Pajulo M., Savonlahti E., Sourander A., Helenius H., Piha J. (2001). Antenatal depression, substance dependency and social support. Journal of Affective Disorders, 65(1), 9–17. [DOI] [PubMed] [Google Scholar]
- Popova S., Lange S., Probst C., Gmel G., Rehm J. (2017). Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. The Lancet Global Health, 5(3), e290–e299. [DOI] [PubMed] [Google Scholar]
- Popova S., Lange S., Shield K., Mihic A., Chudley A. E., Mukherjee R. A.…Rehm J. (2016). Comorbidity of fetal alcohol spectrum disorder: A systematic review and meta-analysis. The Lancet, 387(10022), 978–987. [DOI] [PubMed] [Google Scholar]
- Price A., Cook P. A., Norgate S., Mukherjee R. (2017). Prenatal alcohol exposure and traumatic childhood experiences: A systematic review. Neuroscience & Biobehavioral Reviews, 80, 89–98. [DOI] [PubMed] [Google Scholar]
- Riley E. P., Infante M. A., Warren K. R. (2011). Fetal alcohol spectrum disorders: An overview. Neuropsychology Review, 21(2), 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkola T., Gissler M., Kahila H., Autti-Rämö I., Halmesmäki E. (2011). Early healthcare utilization and welfare interventions among children of mothers with alcohol and substance abuse: A retrospective cohort study. Acta Paediatrica, 100(10), 1379–1385. [DOI] [PubMed] [Google Scholar]
- Sarkola T., Gissler M., Kahila H., Autti-Rämö I., Halmesmäki E. (2012). Alcohol and substance abuse identified during pregnancy: Maternal morbidity, child morbidity and welfare interventions. Acta Paediatrica, 101(7), 784–790. [DOI] [PubMed] [Google Scholar]
- Sarkola T., Kahila H., Gissler M., Halmesmäki E. (2007). Risk factors for out-of-home custody child care among families with alcohol and substance abuse problems. Acta Paediatrica, 96(11), 1571–1576. [DOI] [PubMed] [Google Scholar]
- Statistics Finland. (2019). Väestötieteen perusteet [Introduction to population demographics]. Retrieved from https://tilastokoulu.stat.fi/verkkokoulu_v2.xql?course_id=tkoulu_vaesto&lesson_id=5&subject_id=3&page_type=sisalto
- Streissguth A. P., Barr H. M., Kogan J., Bookstein F. L. (1996). Understanding the occurrence of secondary disabilities in clients with Fetal Alcohol Syndrome (FAS) and Fetal Alcohol Effects (FAE). Final Report. Seattle, WA: University of Washington School of Medicine. [Google Scholar]
- Streissguth A. P., Bookstein F. L., Barr H. M., Sampson P. D., O’Malley K., Young J. K. (2004). Risk factors for adverse life outcomes in fetal alcohol syndrome and fetal alcohol effects. Journal of Developmental and Behavioral Pediatrics, 25(4), 228–238. [DOI] [PubMed] [Google Scholar]
- Sund R. (2012). Quality of the Finnish Hospital Discharge Register: A systematic review. Scandinavian Journal of Public Health, 40(6), 505–515. [DOI] [PubMed] [Google Scholar]
- Weyrauch D., Schwartz M., Hart B., Klug M. G., Burd L. (2017). Comorbid mental disorders in fetal alcohol spectrum disorders: A systematic review. Journal of Developmental & Behavioral Pediatrics, 38(4), 283–291. [DOI] [PubMed] [Google Scholar]