Abstract
SARS-Cov-2 has erupted across the globe, and confirmed cases of COVID-19 pose a high infection risk. Infected patients typically receive their treatment in specific isolation wards, where they are confined for at least 14 days. The virus may contaminate any surface of the room, especially frequently touched surfaces. Therefore, surface contamination in wards should be monitored for disease control and hygiene purposes. Herein, surface contamination in the ward was detected on-site using an RNA extraction-free rapid method. The whole detection process, from surface sample collection to readout of the detection results, was finished within 45 min. The nucleic acid extraction-free method requires minimal labor. More importantly, the tests were performed on-site and the results were obtained almost in real-time. The test confirmed that 31 patients contaminated seven individual sites. Among the sampled surfaces, the electrocardiogram fingertip presented a 72.7% positive rate, indicating that this surface is an important hygiene site. Meanwhile, the bedrails showed the highest correlation with other surfaces, so should be detected daily. Another surface with high contamination risk was the door handle in the bathroom. To our knowledge, we present the first on-site analysis of COVID-19 surface contamination in wards. The results and applied technique provide a potential further reference for disease control and hygiene suggestions.
Keywords: Surface contamination, Wards, COVID-19, Isothermal amplification
Graphical abstract
1. Introduction
In early 2020, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-Cov-2) spread across the globe, causing more than 20 million infections and 700,000 deaths as of Aug 13, 2020 (Dong et al., 2020) Coronavirus can spread through the air and survives on various surfaces for considerable periods. On June 18, Beijing reported new incidents related to the Xinfadi Market cluster (Owen, 2020) COVID-19 was discovered on the surface of chopping boards used for imported salmon at the Xinfadi food market, providing that COVID-19 could survive on material surfaces.
Previous researches reported that the COVID-19 virus can inhabit the surface of materials in wards (Guo et al., 2020; H. Wang et al., 2020; J. Wang et al., 2020). These researchers confirmed the virus by real time reverse transcription polymerase chain reaction (RT-PCR), which typically performs deactivation, nucleic acid extraction, and RT-PCR amplification of the collected samples. However, nucleic acid extraction risks nucleic acid losses and places high demands on the detection limit. Furthermore, the whole nucleic-acid extraction and amplification process requires approximately 2.5–4 h for one batch of detection. Therefore, a rapid detection method should be applied for on-site COVID-19 identification in the environment. Loop-mediated isothermal amplification (LAMP) has achieved brilliant performance in pathogenic virus detection, accomplishing amplification within 45 min (Liu et al., 2018). LAMP assay also performs nucleic acid amplification without requiring nucleic acid extraction (Lalli et al., 2020), thus preventing RNA damage through a tedious process. For these reasons, we applied LAMP in our present report on surface-contamination detection.
Patients with confirmed COVID-19 are retained for over two weeks in rooms with many living and medical apparatuses. Nosocomial transmission plays a major role in viral spread and infection, especially in wards. Confirmed patients living in the ward can spread viruses through coughing or even shortness of breath (Ghinai et al., 2020). Exhaled virus particles can sediment on the surface of materials in the wards. The transmission and distribution of coronavirus are still unclear and remain under investigation. By systematically learning how viruses contaminate surfaces, we could better control the spread of the virus and avoid cross-infection among patients or between patients and medical care workers.
Applying the extraction-free LAMP-based detection method, we performed an on-site surface contamination analysis in wards. Multiple surface contamination samples were collected from different wards before the daily cleaning and disinfection processes. After assessing the risk of contamination on different surfaces, we provide recommendations for cleaning.
2. Material and methods
2.1. Study design and surface contamination collection
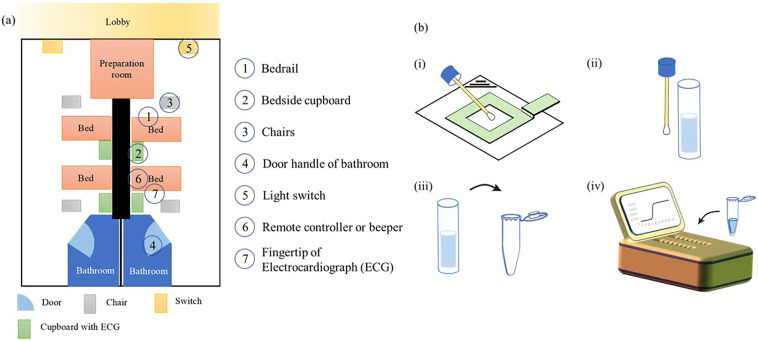
This experiment aimed to determine the concentration of surface contaminants in wards of the Chengdu Center of Disease Control (Chengdu CDC), which has been designated for the treatment of COVID-19 patients during the disease outbreak. Samples were collected from seven sites: 1) bedrail; 2) bedside cupboard; 3) chairs; 4) door handles of the bathroom; 5) light switches; 6) remote controller or beeper; 7) fingertip of electrocardiograph (ECG) monitoring. The samples were collected on 18th, 19th and 24th Mar, 27th and 28th Apr of 2020. The sampling site was illustrated in Fig. 1 (a). The correspondences between patient clinical information and collected samples were listed in Table S.2.
Fig. 1.
Illustration of the sampling sites and detection process. (a) Layout of the sampling ward. Each room contains 2 beds with chairs and a bedside cupboard. The sampling sites are labeled 1–7. (b) Detection process of the surface contaminants. The test surface was wiped with a wetted cotton swab. The swab was then immersed in 3 mL sodium chloride solution. From this solution, a 5 μL sample was pipetted into the reaction solution.
The surface contamination samples were collected as described Index A of the Hygienic Standard for Disinfection in Hospital (Chinese National Standard, GB 15892-2012). Briefly, a 5 cm × 5 cm standard scale board was placed on the surface of sampling material, and the surface was evenly rubbed within the 25-cm2 area with a cotton swab wetted with 0.9% sodium chloride. After swiping, the cotton swab was immersed in 1 mL 0.9% sodium chloride solution prepared for nucleic-acid amplification detection.
2.2. Analytical method
The collected surface-contamination samples were immediately transferred to the BSL-2 laboratory next to the Chengdu CDC wards for analysis. Viral contamination was detected by a nucleic acid extraction-free isothermal detection kit, specifically, a novel coronavirus real-time isothermal amplification kit (Cat. No. PCSYHE), acquired from Shanghai Fosun Long March Medical Science Co., Ltd. and certificated by CE-IVD (Ref No. 8821-2020). Details of the detection kit are available at http://en.lm-diagnostics.com.cn/prod_view.aspx?nid=3&typeid=89&id=573. The kit applies the LAMP technique and targets the N and S genes of the SARS-Cov-2 genome for clinical diagnostic use. Here, we detected contamination by the presence of the N gene, whose sequence is shown in Table S1.
The detection process was performed following manufacturer's instructions. For each batch, we prepared sufficient reaction reagent for n tested samples plus two control samples. That is, n × 1 μL of Bst enzyme, n × 1 μL of RT II enzyme, and n × 18 μL of COVID-19 gene reaction reagent were added to a centrifuge tube, mixed by shaking, and centrifuged at low speed for a few seconds. After separation, 20 μL aliquots were pipetted into PCR reaction tubes. The reaction tubes could be placed at 2–8 °C for 3 h at most after separation. Next, 5 μL of the samples were added to different PCR reaction tubes. A 5-μL Negative Control and 5 μL-Positive Control were added to the control wells.
The reaction mixture was mixed by shaking and centrifuging at low speed for a few seconds. The amplification process was performed in the authorized real-time nucleic acid amplification instrument (Cat. No. PCIPAA, Shanghai Fosun Long March Medical Science Co., Ltd., Fig. S1). The device is powered by chargeable batteries and is entirely portable. Nucleic acid amplification was implemented at 63 °C for 30 min. The signal of the SYBR or FAM channel was collected every 30 s.
The times at which the fluorescent intensity exceeded the default detection threshold of the device were recorded as the threshold times T t. The T t values provided a reference for the results. The amplification curve of a positive result should follow a typical logistic curve and the Tt should be less than 30.
2.3. Data analysis
The T t of each sample taken from each surface was recorded. The T t reflects the relative concentration of nucleic acid on the surfaces; specifically, it decreases with increasing concentration of nucleic acid.
The relationships between two different sampling sites were analyzed by the Kendall algorithm. Positive and negative results were labeled as 1 and 0, respectively. The correlation coefficient and p-value were calculated for each relationship.
3. Results and discussion
The established method detects surface contaminants on-site, and is labor-saving because it requires no RNA extraction step. Following the protocol, the whole detection process was completed on-site within 45 min. The labor-intensive RNA extraction step is replaced by a simple reaction MIX preparation, and addition of the sample elution into the reaction MIX. The detection kit integrates the nucleic-acid releasing reagents with the reaction MIX, achieving fast amplification. Detection can be performed by a standard isothermal amplification instrument with a FAM channel, or a standard real-time PCR instrument. Furthermore, as described in the kit instruction, the detection sensitivity reached 20 copies/reaction, satisfying the demands of surface- contamination sample detection.
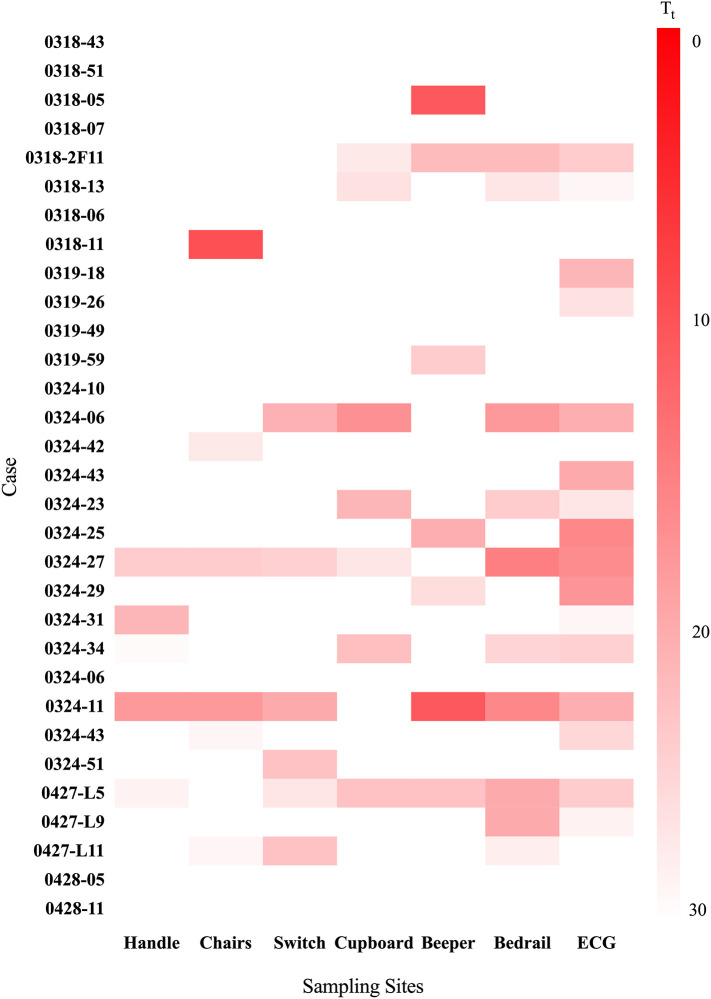
The detection results of the different cases and sampling sites are summarized in Fig. 2 and Table S.1. The color intensities of the blocks represent the T t values of the real-time isothermal amplification process (ranging from red at T t ~0, pink at intermediate T t, to white at high T t). Intense red indicates a high concentration of the virus.
Fig. 2.
Amplification results in all cases. Tt values of 31 cases paired with different contaminated surfaces. Scale bar ranges from intense red (Tt ~0) to white (high Tt). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Thirty-one surface-contamination samples were collected from 18 Mar to 28 April of 2020. Among the samples, nine confirmed cases reported no surface contamination sites (no positive amplification results), five cases reported two contamination sites, three cases reported three contamination sites, three cases reported four contamination sites, and three cases reported six contamination sites. Generally, the live COVID-19 positive rate of surface contamination by confirmed SARS-Cov-2 cases exceeded 70%, indicating a high risk of cross-infection from various surfaces in isolation wards.
Sixteen of the positive results (72.7% of all positive cases) were reported from the ECG fingertip, where the virus was also more concentrated than on the other surfaces. We believe that ECG fingertips are high-risk surfaces for spreading the virus, and should be targeted for extra cleaning. The second risk to virus control in patients' rooms was the bedrail. Ten samples from the bedrail yielded a positive amplification result, most likely transferred by the patients themselves. Patients prefer to grab the bedrail when rising or lying down in wards. The above were defined as ultrahigh-contamination risk surfaces. Seven samples from the beeper (remote controller) and seven cases from the cupboard also reported positive results.
Second, the correlations between the sampling sites were analyzed and the results are presented in Fig. 3 . The highest correlation (0.78, p-value 1.81 × 10-5) was found between the bedrail and cupboard, meaning that the positive results from bedrail surfaces were highly relevant to the detection results from cupboards. Meanwhile, bedrail contamination was positively correlated with three other surface contaminations, more than any other surface. In future surface-contamination detection works, the bedrail should be listed as the most frequent detection site. The results from the bedrail might reflect the contamination degree, providing guidance for medical care advice.
Fig. 3.
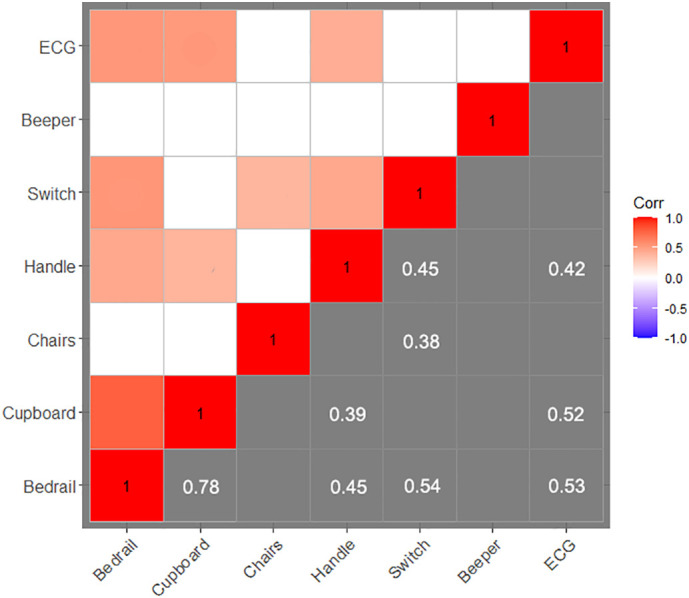
Relationship between detection results in different sites. Correlations between two sampling sites. The numbers in the blocks are the correlations between the paired surfaces (p-value <0.05). Blank blocks denote no significant correlation between the contamination results of the paired surfaces.
The five positive samples from the handles were 100% associated with positive results from the ECG fingertips positive results. Four positive cases from the handles were also associated with positive results from bedrails, and three cases were associated with positive results from cupboards and light switches. Therefore, a surface contamination sample from the door handle can be interpreted as an ultra-high risk label. In daily monitoring, the bathroom door handles should be swabbed and analyzed. When the samples from a door handle report positive results, the corresponding ward poses an enhanced hygienic challenge.
Three of the collected samples (samples #0324-27, #0324-11, and #0427-L5) presented on six contaminated surfaces. All of these confirmed cases came from outside mainland China and presented positive symptoms. On a 1–4 scale of clinical severity, where type 1 denotes mild cases and type 4 represents the severest cases, sample #0324-27 was classified as type 2, and samples #0324-11 and #0427-L5 were classified as type 3. The clinical symptoms might influence the contamination degree and infection risk. Further research on these relationships is ongoing.
4. Conclusion
We successfully applied an extraction-free SARS-Cov-2 isothermal amplification detection method to on-site analysis of surface contamination by COVID-19 patients in wards. For each confirmed case, seven sites in the ward were collected and analyzed. The detection process is efficient and labor-saving, as desired for on-site COVID-19 contamination detection. Among 31 cases collected from 18 March to 27 April of 2020, 72.7% reported positive amplifications on the ECG fingertip, indicating that this surface is an important hygiene site.
The correlation results also confirmed that bedrails should be regularly monitored, as contamination on bedrail surfaces is relevant to many other contaminated surfaces. Meanwhile, bathroom door handles were recognized as alert factors of high contamination risk. These results and the applied techniques provide a further reference for disease control and hygiene suggestions. Virus monitoring should be added as a routine procedure in ward management. The door handles and fingertips of ECG monitors should be sampled daily for contamination analysis. If the sample from a door handle obtains a positive result, a more precise and thorough cleaning should be performed. As surface contamination may cause nosocomial viral infection, general cleaning is mandatory in wards.
Although the analysis can be performed by standard real-time PCR instruments, simpler isothermal amplification fluorescent instruments are suggested for on-site analysis, as they are less expensive, smaller in size, and more easily transported than standard PCR instruments. In future works, we will analyze the relationship between different clinical symptoms and surface contamination, which may reveal the transmission mode of COVID-19 in wards.
CRediT authorship contribution statement
Bin Wan, Xia Zhao, Limei Lei, and Chunmei Liu collected samples from wards. Xinlian Zhang, Dongxia Luo, and Xi Chen compiled the data and draw up the draft. Tong Zhang, Yuhan Yao, Wang Zhao, and Lin Zhou developed the method and validated the method. Yuqing Ge, Hongju Mao, Sixiu Liu, Jianmin Chen, Xunjia Cheng, Jianlong Zhao, and Guodong Sui conceived the idea, coordinated the project, and wrote the manuscript.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgment
The Special Fund of Chengdu Science and Technology COVID-19 Prevention and Control (2020-YF05-00033-SN); the Major Special Project of “Prevention and Control of Major Infectious Diseases such as AIDS and Viral Hepatitis” (2018ZX10732401-003-016); the Science and Technology Commission of Shanghai Municipality (Nos. 17JC1401000, 19441903700, 18DZ1113000 and 16441902100), the National Natural Science Foundation of China (Nos. 61971410, 61801464, 21577019, 21527814).
Editor: Jay Gan
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2020.141758.
Appendix A. Supplementary data
Supplementary material
References
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Wang Z., Zhang S., Li X., Li L., Li C., Cui Y., Fu R., Dong Y., Chi X., Zhang M., Liu K., Cao C., Liu B., Zhang K., Gao Y., Lu B., Chen W. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg. Infect. Dis. 2020;26 doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalli M.A., Chen X., Langmade S.J., Fronick C.C., Sawyer C.S., Burcea L.C., Fulton R.S., Heinz M., Buchser W.J., Head R.D., Mitra R.D., Milbrandt J. Rapid and extraction-free detection of SARS-CoV-2 from saliva with colorimetric LAMP. Serv. Heal. Sci. 2020 doi: 10.1101/2020.05.07.20093542. medRxiv Prepr. 2020.05.07.20093542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Chen L., Yao Y., Ke S., Zhao W., Yang Z., Sui G. A sample-to-answer labdisc platform integrated novel membrane-resistance valves for detection of highly pathogenic avian influenza viruses. Sensors Actuators B Chem. 2018;270:371–381. doi: 10.1016/j.snb.2018.05.044. [DOI] [Google Scholar]
- Owen J. Covid-19: WHO raises concerns about new cases in Beijing. BMJ. 2020;369 doi: 10.1136/bmj.m2415. [DOI] [PubMed] [Google Scholar]
- Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y., Zhuo L., Zhong Z., Qu T. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int. J. Infect. Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Mo P., Li G., Chen P., Liu J., Wang F., Zhang Y., Zhao Q. Environmental virus surveillance in the isolation ward of COVID-19. J. Hosp. Infect. 2020;105:373–374. doi: 10.1016/j.jhin.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material