Abstract
Objectives
In Germany the coronavirus disease 2019 (COVID-19) pandemic situation is unique among large European countries in that incidence and case fatality rate are distinctly lower. We describe the clinical course and examine factors associated with outcomes among patients hospitalized with COVID-19 in Germany.
Methods
In this retrospective cohort study we included patients with COVID-19 admitted to a national network of German hospitals between February 12 and June 12, 2020. We examined demographic characteristics, comorbidities and clinical outcomes.
Results
We included 1904 patients with a median age of 73 years, 48.5% (924/1904) of whom were female. The mortality rate was 17% (317/1835; 95% confidence interval (95%CI) 16–19), the rate of admission to the intensive care unit (ICU) was 21% (399/1860; 95%CI 20–23), and the rate of invasive mechanical ventilation was 14% (250/1850: 95%CI 12–15). The most prominent risk factors for death were male sex (hazard ratio (HR) 1.45; 95%CI 1.15–1.83), pre-existing lung disease (HR 1.61; 95%CI 1.20–2.16), and increased patient age (HR 4.11 (95%CI 2.57–6.58) for age >79 years versus <60 years). Among patients admitted to the ICU, the mortality rate was 29% (109/374; 95%CI 25–34) and higher in ventilated (33% [77/235; 95%CI 27–39]) than in non-ventilated ICU patients (23%, 32/139; 95%CI 16–30; p < 0.05).
Conclusions
In this nationwide series of patients hospitalized with COVID-19 in Germany, in-hospital and ICU mortality rates were substantial. The most prominent risk factors for death were male sex, pre-existing lung disease, and greater patient age.
Keywords: Coronavirus, COVID-19, Germany, Observational cohort study, Pandemic
Introduction
The outbreak of coronavirus disease 2019 (COVID-19) in Germany differs from that in other countries in certain aspects. First, the estimated incidence of COVID-19 in Germany (0.85%) is distinctly lower than in other large European countries with a similar demographic and economic structure, such as Spain (5.5%), Italy (4.6%), the UK (5.1%), France (3.4%), or Belgium (8.0%) [1]. Second, in Germany the pandemic has been associated with the substantially lower case fatality rate (CFR) of 4.6% compared with Spain (11.3%), Italy (14.4%), the UK (15.5%), and even neighbouring countries such as France (14.6%) and Belgium (15.8%) [2].
These comparatively low figures may be due to non-pharmaceutical interventions against COVID-19 being more effective in Germany than in other European countries [1]. However, Germany's lower incidence of COVID-19 and the resulting lack of population immunity may render the German population more vulnerable to a potential second wave of the pandemic. It is unclear whether there is an association between a lower incidence of COVID-19 and a low CFR. In examining this issue, one of the major limitations is that, to date, no comprehensive nationwide clinical data on COVID-19 have emerged from Germany. Such evidence may be important in understanding whether the course of COVID-19 in a country with a lower disease burden differs from that observed in other countries, and may shed light on methods to prevent a second wave of infection or limit its impact on the population. We therefore describe clinical characteristics of all patients with COVID-19 admitted to a nationwide German hospital network and report risk factors associated with patient outcomes.
Methods
Study design and endpoints
This retrospective multicentre observational clinical study consecutively enrolled all patients admitted with laboratory-confirmed COVID-19 to any of the 86 hospitals of the Helios network. As the largest private healthcare provider in Germany, the Helios network accounts for 6.5% of patient hospitalizations nationwide; it represents small and large as well as general and academic hospitals in rural and urban areas in 13 of the 16 federal states of Germany (Supplementary Material Fig. S1) [3]. During this study, patients with COVID-19 were admitted to 75 network hospitals; the remaining 11 centres did not see any COVID-19 patients. The study was approved by the internal review board of the Brandenburg Medical School (Neuruppin, Germany) on March 24, 2020 (E01-20200319) and registered with the German Clinical Trials Register (DRKS00021161). Individual informed consent was waived because of the retrospective nature of this study. The inclusion criteria were laboratory-confirmed COVID-19 and admission to a hospital within the Helios network. The only exclusion criterion was a lack of laboratory confirmation of COVID-19. The study endpoints were process variables such as admission to the ICU and use of invasive mechanical ventilation, and outcome variables such as length of stay and death from any cause.
Data sources
Eligible patients were admitted between February 12, 2020 and June 12, 2020, and had laboratory-confirmed COVID-19 according to the WHO interim guidance [4]. The diagnosis was based on real-time reverse transcription polymerase chain reaction (rtPCR) on nasal and pharyngeal swab specimens. Of the 1933 eligible patients, 29 were excluded since they had been transferred from other hospitals and data on the preceding hospitalization were not available. For three patients we used the date of the positive COVID-19 test as time of admission since they had been hospitalized for other reasons prior to the study period. For 25 patients with hospital stay in two different time periods we used only information on the first one. Demographic, clinical, laboratory, management and outcome data were collected from the paper medical records by trained hospital staff and entered into a separate registry, which serves as an addition to our hospitals' routine infection control system. Registry data were consecutive and compared with routine administrative healthcare data and inconsistencies were resolved by individual review of medical records. More details are provided in the online Supplementary Material.
Statistical analysis
Continuous variables were summarized using medians and interquartile ranges (IQRs); categorical variables were summarized with counts and percentages. Mortality rate was the percentage of patients who died while in hospital relative to all patients discharged (alive or dead). Mortality rate on ICU was the percentage of patients who died in the ICU relative to all patients ever admitted to the ICU who had been discharged (alive or dead). We also calculated the percentage of patients who were admitted to the ICU relative to all patients currently in the ICU or discharged (alive or dead), and the percentage of patients who were invasively mechanically ventilated relative to all patients currently being invasively mechanically ventilated or discharged (alive or dead). Confidence intervals for percentages were based on the exact binomial distribution. Cumulative incidences and hazard ratios (HRs) for time to the following endpoints were calculated: admission to the ICU, invasive mechanical ventilation, and death (among all patients and among those admitted to the ICU). Time at risk started on the date of hospitalization and ended on the date of ICU admission, the start of invasive mechanical ventilation, and the date of death, depending on the outcome studied. For the analyses of mortality among patients admitted to the ICU, time at risk started on the date of ICU admission. Patients were censored on the date of the last updated information. All survival analyses were conducted using competing risk models that considered hospital discharge a competing event. For endpoints other than mortality, death was considered a competing event. Multivariable proportional hazards models were used to assess associations between clinical characteristics and cause-specific incidences [5], including age, sex, symptoms on admission and a set of prespecified comorbidities. Invasive mechanical ventilation was used as a time-dependent variable in analyses of mortality among patients admitted to the ICU. Due to the small number of events, these analyses were adjusted only for gender and continuous age. Visual inspection of the Schoenfeld residuals revealed that hazards were roughly proportional during the first 20 days of follow-up, which covered at least 90% of all events for all analyses. P-values ≤0.05 were considered statistically significant. STATA statistical software (version SE16; STATA, College Station, TX) was used for analysis.
Results
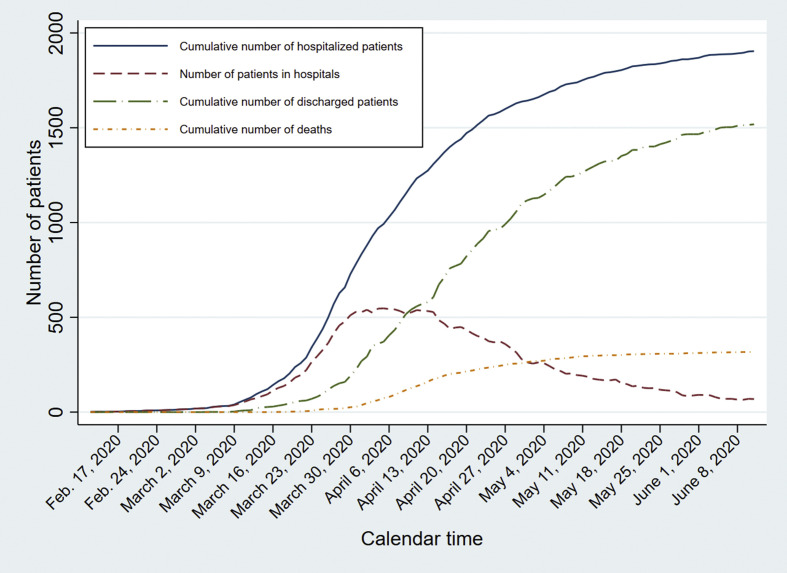
In total, 1904 patients with COVID-19 were admitted to 75 hospitals between February 12, 2020 and June 12, 2020. The cumulative number of hospitalized patients increased steeply between March 16 and early April (Fig. 1 ). The absolute number of patients in hospitals initially increased as well, reaching a maximum at the end of March and subsequently decreasing.
Fig. 1.
Number of patients over time by hospitalization, discharge and death.
The median age of the patients was 73 years (IQR 57–82); 34.2% of them (652/1904) were older than 79 years, and 48.5% (924/1904) were female (Table 1 ). Information on symptoms and comorbidities was available for 90% of all patients (1709/1904). The most frequent symptoms on admission were fever (42.8%; 731/1709) and cough (37.6%; 642/1709). Muscle or body aches (7.4%; 127/1709) and rhinorrhoea (3.2%; 55/1709) were rarely documented. At least one of the examined comorbidities was present in 46.6% of patients (797/1709), with cardiovascular disease being the most frequent (36.1%; 617/1709).
Table 1.
Demographic and clinical characteristics of the entire patient cohort
| Characteristic | |
| Total n | 1904 |
| Male sex | 980 (51.5) |
| Female sex | 924 (48.5) |
| Age (years) | 73 (57–82) |
| Age groups: | |
| Age <50 years | 279 (14.7) |
| Age 50–59 years | 271 (14.2) |
| Age 60–69 years | 290 (15.2) |
| Age 70–79 years | 412 (21.6) |
| Age >79 years | 652 (34.2) |
| Symptoms on admissiona | |
| Fever | 731 (42.8) |
| Cough | 642 (37.6) |
| Diarrhoea | 169 (9.9) |
| Muscle or body ache | 127 (7.4) |
| Rhinorrhoea | 55 (3.2) |
| Comorbidities | |
| At least one comorbiditya | 797 (46.6)a |
| Cardiovascular diseaseb | 617 (36.1) |
| Diabetesc | 260 (15.2) |
| Lung diseased | 201 (11.8) |
| Malignancye | 92 (5.4) |
| Currently in hospital | 69 (3.6) |
| No longer in hospital | 1835 (96.4) |
| Discharged alive | 1518 (79.7) |
| Ever intensive care | 265/1518 (17.5) |
| Ever invasive mechanical ventilation | 158/1518 (10.4) |
| Death | 317 (16.6) |
| Ever intensive care | 109/317 (34.4) |
| Ever invasive mechanical ventilation | 77/317 (24.3) |
| Hospital stay, total patient cohort (days) | 9 (4–17) |
| Hospital stay, ICU patients (days) | 15 (7–26) |
Data are n (%) or median interquartile range (IQR).
Information on symptoms and comorbidities was available for 1709 patients.
Cardiovascular disease is defined as any cardiac injury, such as myocardial infarction, angina pectoris, hypertensive heart disease, cardiomyopathy, aortic aneurysms, congenital heart disease, or peripheral heart disease, among others.
Diabetes mellitus is defined as prolonged high blood sugar levels due to metabolic disorders, such as type-1 diabetes and type-2 diabetes.
Lung disease is defined as any respiratory tract disease such as lung injury, chronic obstructive lung disease, asthma, bronchiectasis, chronic bronchitis, or pulmonary tuberculosis, among others.
Malignancy is defined as any cancer (malign tumour) with potential invasion of or spreading to other parts of the body, such as glioblastoma, stomach cancer, colorectal cancer, melanoma, renal cancer, breast cancer or prostate cancer, among others.
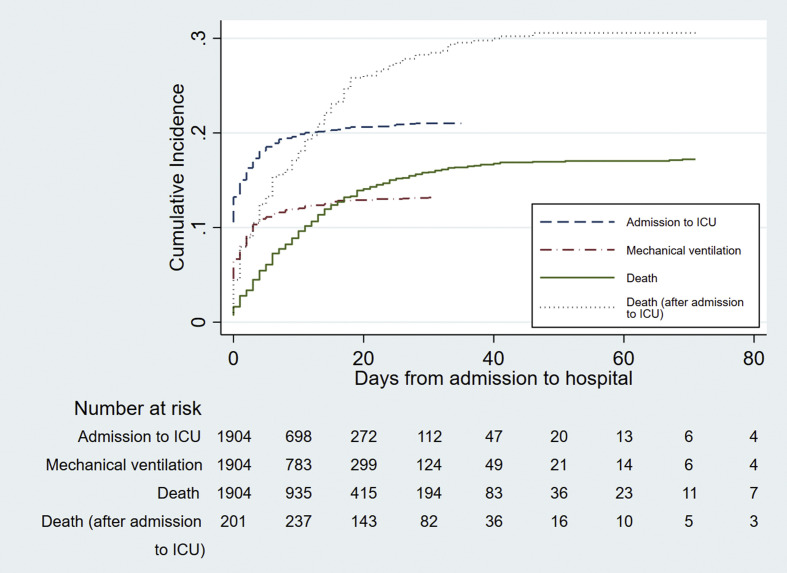
At the time of data analysis, 1518/1904 patients (79.7%) had been discharged alive, 317/1904 (16.6%) had died before discharge, and 69/1904 (3.6%) were still hospitalized. Cumulative incidence of admission to the ICU and invasive mechanical ventilation increased sharply during the first week and then flattened out (Fig. 2 ). The incidence of death increased in a more linear fashion especially during the first 3 weeks, both in the hospital and in the ICU cohort.
Fig. 2.
Cumulative incidence of admission to intensive care unit (ICU), invasive mechanical ventilation and death.
The rate of admission to the ICU was 21% (399/1860; 95%CI 20 –23) (Table 2 ). It was higher in male (27%, 256/954; 95%CI 24–30) than in female patients (16%, 143/906; 95%CI 13–18; HR 1.5, 95%CI 1.2–1.9). Men were also at higher risk of requiring invasive mechanical ventilation than women (HR 1.5, 95%CI 1.1–2.0).
Table 2.
Rate of admission to the intensive care unit (ICU) and of receiving invasive mechanical ventilation (IMV)
| ICU admission |
IMV |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients in analysis | ICU admissions | Rate (95%CI) |
Mean FU duration (d)a | Hazard ratio (95%CI)b |
Patients in analysis | Patients with IMV | Rate (95%CI) |
Mean FU duration (d)a | Hazard ratio (95%CI)b |
|
| Total no. | 1860 | 399 | 0.21 (0.20–0.23) | 9.74 | — | 1850 | 250 | 0.14 (0.12–0.15) | 10.67 | — |
| Female sex | 906 | 143 | 0.16 (0.13–0.18) | 10.55 | 1.00 (ref) | 903 | 85 | 0.09 (0.08–0.11) | 11.40 | 1.00 (ref) |
| Male sex | 954 | 256 | 0.27 (0.24–0.30) | 8.97 | 1.53 (1.24–1.90)c | 947 | 165 | 0.17 (0.15–0.20) | 9.99 | 1.52 (1.14–2.02)d |
| Age groups | ||||||||||
| Age <60 years | 532 | 81 | 0.15 (0.12–0.18) | 6.38 | 0.50 (0.37–0.69)c | 530 | 50 | 0.09 (0.07–0.12) | 7.11 | 0.48 (0.32–0.72)c |
| Age 60–69 years | 277 | 87 | 0.31 (0.26–0.37) | 8.03 | 1.00 (ref) | 275 | 62 | 0.23 (0.18–0.27) | 9.29 | 1.00 (ref) |
| Age 70–79 years | 405 | 116 | 0.29 (0.24–0.33) | 10.12 | 0.98 (0.73–1.31) | 402 | 80 | 0.20 (0.16–0.24) | 11.11 | 0.96 (0.67–1.37) |
| Age >79 years | 646 | 115 | 0.18 (0.15–0.21) | 13.08 | 0.59 (0.43–0.80)c | 643 | 58 | 0.09 (0.07–0.11) | 14.02 | 0.41 (0.27–0.62)c |
| Symptoms on admission | ||||||||||
| Fever | 728 | 228 | 0.31 (0.28–0.35) | 7.95 | 2.35 (1.85–3.00)c | 728 | 155 | 0.21 (0.18–0.24) | 9.03 | 2.80 (2.03–3.87)c |
| Diarrhoea | 168 | 44 | 0.26 (0.20–0.33) | 8.86 | 1.03 (0.75–1.41) | 168 | 26 | 0.15 (0.10–0.21) | 10.09 | 0.90 (0.60–1.35) |
| Muscle or body ache | 127 | 26 | 0.20 (0.13–0.27) | 7.06 | 0.69 (0.47–1.04) | 127 | 15 | 0.12 (0.06–0.17) | 8.34 | 0.59 (0.34–0.99)e |
| Cough | 639 | 169 | 0.26 (0.23–0.30) | 7.97 | 1.08 (0.85–1.36) | 639 | 110 | 0.17 (0.14–0.20) | 8.97 | 1.09 (0.81–1.47) |
| Rhinorrhoea | 55 | 17 | 0.31 (0.19–0.43) | 6.73 | 1.55 (0.96–2.48) | 55 | 10 | 0.18 (0.08–0.28) | 7.93 | 1.43 (0.75–2.74) |
| Comorbidities: | ||||||||||
| Diabetes | 260 | 86 | 0.33 (0.27–0.39) | 9.74 | 1.46 (1.13–1.88)d | 260 | 54 | 0.21 (0.16–0.26) | 11.15 | 1.35 (0.97–1.88) |
| Malignancy | 91 | 23 | 0.25 (0.16–0.34) | 14.13 | 0.88 (0.56–1.36) | 91 | 17 | 0.19 (0.11–0.27) | 14.97 | 1.01 (0.59–1.72) |
| Cardiovascular disease | 616 | 169 | 0.27 (0.24–0.31) | 10.54 | 1.26 (1.00–1.58) | 616 | 113 | 0.18 (0.15–0.21) | 11.62 | 1.47 (1.10–1.98)e |
| Lung disease | 200 | 62 | 0.31 (0.25–0.37) | 10.79 | 1.26 (0.95–1.67) | 200 | 40 | 0.20 (0.14–0.26) | 12.35 | 1.18 (0.82–1.69) |
FU, follow-up; CI, confidence interval; ref, reference.
Mean follow-up for all patients, gender and age based on 1904 patients and for symptoms and comorbidities based on 1709 patients.
Hazard ratios adjusted for all variables listed in the table and calculated with data of 1709 patients.
p < 0.001 according to the Wald test.
p < 0.05 according to the Wald test.
p < 0.01 according to the Wald test.
The highest rate of admission to the ICU was observed among patients between 60 and 69 years of age (31%, 87/277; 95%CI 26–37) and among patients between 70 and 79 years (29%, 116/405; 95%CI 24–33). The same age groups also displayed the highest rates of invasive mechanical ventilation. Patients younger than 60 years (HR 0.5, 95%CI 0.4–0.7) and in those older than 79 years (HR 0.6, 95%CI 0.4–0.8) were less likely to be admitted to the ICU compared with those 60–69 years. In a sample of 19% (28/147) of all patients older than 79 years who died without admission to the ICU, all had do-not-intubate (DNI) orders.
Patients displaying fever on admission were at increased risk for admission to the ICU (HR 2.4, 95%CI 1.9–3.0) and for invasive mechanical ventilation (HR 2.8, 95%CI 2.0–3.9) than those without fever. Of the examined comorbidities, diabetes (HR 1.5, 95%CI 1.1–1.9) was associated with increased risk of admission to the ICU, and cardiovascular disease was associated with a higher risk of invasive mechanical ventilation (HR 1.5, 95%CI 1.1–2.0).
Men were at higher risk of death (HR 1.5, 95%CI 1.2–1.8) than female patients (Table 3 ). Once admitted to the ICU, HRs for death were similar between men and women. Among different age groups, the highest mortality rate was observed in patients older than 79 years, both in the entire cohort (30%, 191/634; 95%CI 27–34) and in the ICU group (43%, 44/103; 95%CI 33–52). Of the 279 patients younger than 50 years, 27/279 (10%) were admitted to the ICU, 15/279 (5%) required invasive mechanical ventilation, and 2/279 (1%) died. Among all patients, death was more likely in patients presenting with fever (HR 1.3, 95%CI 1.0–1.7), cardiovascular comorbidity (HR 1.3, 95%CI 1.0–1.7), or pre-existing lung disease (HR 1.6, 95%CI 1.2–2.2). Among patients admitted to the ICU, death was associated with pre-existing lung disease (HR 1.7, 95%CI 1.0–2.7) but not with any of the other examined comorbidities or symptoms on admission.
Table 3.
Mortality rate among all hospitalized patients and among patients admitted to the intensive care unit (ICU)
| All hospitalized patients |
Patients admitted to the ICU |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of patients | Number of deaths | Mortality rate (95%CI) |
Mean FU duration (d)a | Hazard ratio (95%CI)b |
Number of patients | Number of deaths | Mortality rate (95%CI) |
Mean FU duration (d)c | Hazard ratio (95%CI)b |
|
| Total No. | 1835 | 317 | 0.17 (0.16–0.19) | 13.07 | — | 374 | 109 | 0.29 (0.25–0.34) | 15.92 | — |
| Female sex | 898 | 146 | 0.16 (0.14–0.19) | 12.99 | 1.00 (ref) | 135 | 42 | 0.31 (0.23–0.39) | 15.76 | 1.00 (ref) |
| Male sex | 937 | 171 | 0.18 (0.16–0.21) | 13.15 | 1.45 (1.15–1.83)d | 239 | 67 | 0.28 (0.22–0.34) | 16.01 | 1.10 (0.72–1.68) |
| Age groups: | ||||||||||
| Age <60 years | 527 | 13 | 0.02 (0.01–0.04) | 8.92 | 0.33 (0.16–0.68)d | 76 | 7 | 0.09 (0.03–0.16) | 17.21 | 0.62 (0.23–1.67) |
| Age 60–69 years | 274 | 24 | 0.09 (0.05–0.12) | 12.91 | 1.00 (ref) | 84 | 12 | 0.14 (0.07–0.22) | 16.26 | 1.00 (ref) |
| Age 70–79 years | 400 | 89 | 0.22 (0.18–0.26) | 14.52 | 2.75 (1.69–4.47)e | 111 | 46 | 0.41 (0.32–0.51) | 15.64 | 3.52 (1.77–7.01)e |
| Age >79 years | 634 | 191 | 0.30 (0.27–0.34) | 15.73 | 4.11 (2.57–6.58)e | 103 | 44 | 0.43 (0.33–0.52) | 15.03 | 3.68 (1.82–7.42)e |
| Symptoms on admission: | ||||||||||
| Fever | 725 | 130 | 0.18 (0.15–0.21) | 12.79 | 1.33 (1.04–1.70)f | 225 | 64 | 0.28 (0.23–0.34) | 15.51 | 1.08 (0.71–1.64) |
| Diarrhoea | 168 | 25 | 0.15 (0.09–0.20) | 12.95 | 0.87 (0.58–1.32) | 44 | 9 | 0.20 (0.09–0.32) | 15.70 | 0.59 (0.28–1.25) |
| Muscle or body ache | 126 | 9 | 0.07 (0.03–0.12) | 10.96 | 0.66 (0.33–1.32) | 25 | 5 | 0.20 (0.04–0.36) | 19.04 | 0.67 (0.26–1.73) |
| Cough | 636 | 92 | 0.14 (0.12–0.17) | 12.32 | 0.76 (0.58–0.99)f | 166 | 42 | 0.25 (0.19–0.32) | 16.51 | 0.88 (0.58–1.35) |
| Rhinorrhoea | 55 | 8 | 0.15 (0.05–0.24) | 10.65 | 1.11 (0.54–2.32) | 17 | 4 | 0.24 (0.03–0.44) | 12.71 | 1.00 (0.34–2.93) |
| Comorbidities: | ||||||||||
| Diabetes | 258 | 57 | 0.22 (0.17–0.27) | 15.67 | 1.02 (0.75–1.38) | 84 | 25 | 0.30 (0.20–0.40) | 17.92 | 0.94 (0.57–1.55) |
| Malignancy | 91 | 29 | 0.32 (0.22–0.41) | 18.61 | 1.38 (0.94–2.05) | 23 | 10 | 0.43 (0.23–0.64) | 17.91 | 1.81 (0.85–3.82) |
| Cardiovascular diseases | 613 | 156 | 0.25 (0.22–0.29) | 14.70 | 1.31 (1.02–1.70)f | 166 | 57 | 0.34 (0.27–0.42) | 15.20 | 1.02 (0.67–1.56) |
| Lung disease | 199 | 57 | 0.29 (0.22–0.35) | 15.99 | 1.61 (1.20–2.16)d | 61 | 27 | 0.44 (0.32–0.57) | 16.87 | 1.68 (1.04–2.72)f |
FU, follow up; CI, confidence interval; ref, reference; HR, hazard ratio.
Mean follow-up for all patients, gender and age based on 1904 and 399 patients and for symptoms and comorbidities based on 1709 and 354 patients for death among all patients hospitalized and admitted to the ICU, respectively.
Hazard ratios adjusted for all variables listed in the table and calculated with data of 1709 patients and 354 for death among all patients hospitalized and admitted to the ICU, respectively.
Mean follow-up for all patients, gender and age based on 399 patients and for symptoms and comorbidities based on 354 patients.
p < 0.01 according to the Wald test.
p < 0.001 according to the Wald test.
p < 0.05 according to the Wald test.
During their stay on the ICU, 250/399 patients (63%) received invasive mechanical ventilation and 149/399 (37%) did not. Among discharged ICU patients requiring invasive mechanical ventilation the mortality rate was 33% (77/235) compared to 23% (32/139) for discharged non-ventilated ICU patients (HR 1.8, 95%CI 1.2–2.7; p < 0.01).
Discussion
We provide analyses of 1904 consecutive patients with laboratory-confirmed COVID-19 admitted to 75 hospitals in Germany. The in-hospital mortality rate was 17% and the risk of death was higher for older age, male sex and pre-existing cardiovascular or lung disease. Men were also more likely to be admitted to the ICU and to receive invasive mechanical ventilation. The rate of ICU admission was 21% and that of invasive mechanical ventilation 14%. Among patients admitted to the ICU, we observed a mortality rate of 29% and a higher risk of death among patients receiving invasive mechanical ventilation.
Up until the time of analysis, 28 260 patients were hospitalized with COVID-19 in Germany [6]. This means that our study represents 7% of the entire hospitalized COVID-19 patient population in Germany. According to government data, 26% of all COVID-19 patients admitted to the ICU in Germany died [6]. This figure is in line with the mortality rate observed among ICU patients in our analysis, which suggests that our cohort may offer a realistic representation of the clinical course of the pandemic in Germany. The only non-government data on patients hospitalized with COVID-19 in Germany stem from a study that selectively examined health insurance claims data of one specific insurance fund (AOK) [7]. While this study included more than 10 000 patients, its generalizability to the German population may be limited, since AOK members are known to have a higher prevalence of chronic diseases such as hypertension, diabetes and coronary artery disease [8]. Indeed, in our study, which consecutively included all patients irrespective of insurance fund, the prevalence of diabetes was 15% compared to 28% in the AOK study. This may contribute to a higher mortality rate in the AOK study (22%) compared to our study (17%).
The incidence of COVID-19 in Germany is estimated to be substantially lower than in other large European countries [1], as is the overall CFR [2]. Furthermore, Germany's health infrastructure may be more resistant to overburdening due to comparably ample hospital bed and ICU bed capacity [9,10]. For example, Germany's ICU bed capacity is 29 per 100 000 population, which is substantially higher than in most European countries such as Belgium (16), France (12), the UK (7), Italy (13), and Spain (10) [10]. Even though the benefit of a high ICU bed capacity is uncertain in this current global pandemic [11], healthcare professionals in China and Italy have suggested that avoiding strain on ICU bed capacity may directly impact disease outcomes [12,13].
Descriptions of the clinical course of COVID-19 on a national scale are scarce. A recent nationwide series from the UK examined 20 133 patients hospitalized with COVID-19 [14]. The authors describe mortality rates of 26% among all patients and 32% among patients on ICU or high dependency units. These numbers are slightly higher than those observed in our study, which may be explained by differences in age distributions among patients receiving invasive mechanical ventilation.
Another nationwide study from China describes 1099 patients hospitalized with COVID-19 [15]. The ICU admission rate was 5.0%, 2.3% received mechanical ventilation, and 1.4% died. These figures are up to ten-fold lower than those observed in the UK and in our cohort, which may be explained by the Chinese study's strikingly lower median age of 47 years. Regionally limited case series from China describe older patient cohorts with poorer outcomes compared to the national study [[16], [17], [18]].
Other large clinical series on COVID-19 have emerged from narrow geographic locations with high infection rates. In the Lombardy region [13], among 1591 consecutive patients admitted to the ICU the total mortality rate was 26%, which is comparable to the mortality rate among ICU patients in our study. Another large clinical case series on COVID-19 originates from the New York City metropolitan area [19] and presents outcomes for 2634 patients, of which 14% were admitted to the ICU, which is comparable to the 21% observed in our study.
The fact that hospitalized patients in our study were older than those in cluster regions with massive outbreaks may suggest that, in Germany, a higher proportion of older patients with COVID-19 was admitted to hospitals. This may have been facilitated by a large pool of vacant hospital beds. In addition, more widespread COVID-19 testing ability in Germany than in other countries [20] may have led to improved identification of older patients with flu-like symptoms, who may have otherwise gone unnoticed and remained in their usual environment as potential virus spreaders. Considering that in other countries large sources of transmission were long-term and elderly care facilities [21,22], Germany may have avoided such outbreaks by being able to isolate even older symptomatic patients by hospitalizing them. The fact that in our study patients older than 79 years were at highest risk of death, but at decreased risk of admission to the ICU or initiation of invasive mechanical ventilation, suggests strict implementation of DNI orders, which appear to have been common in this age group in our study.
Our study has several limitations. First, it focuses exclusively on hospitalized patients, and our findings therefore cannot be generalized to patients displaying either no or only mild symptoms of COVID-19. Second, we focus on patients with laboratory-confirmed COVID-19 and do not include cases with typical clinical symptoms but negative test results. Third, information on comorbidities was limited due to the nature of the hospital infection control registry and in keeping with national data protection laws; however, in a review of the literature we are unlikely to have missed key predictors for our endpoints. Finally, we used a limited control sample to estimate that patients older than 79 years were less likely to be admitted to the ICU than their younger peers due to DNI orders.
In conclusion, in-hospital and ICU mortality rates among patients with COVID-19 were substantial in this nationwide series. The most prominent risk factors for death were male sex, pre-existing lung disease, and increased patient age.
Author contributions
IN, JD, PL, PT, JB, TB, JT, and KS collected the epidemiological and clinical data. MH and KJ performed all statistical analyses of the data. JD, IN, and MH drafted the manuscript. JD, IN, PT, PL, KJ, AMH, RK, JB, TB, JT, and KS revised the final manuscript.
Transparency declaration
IN, PL, PT, AMH, RH, JB, TB, JT, KS and JD are employees of Helios. RK holds shares in Helios AG. This project is funded by the Helios Center for Research and Innovation via a grant (HCRI ID 2020-0078) to IN and JD. The authors declare that they have no conflicts of interest.
Acknowledgements
We would like to thank the members of the nursing and medical staff at all HELIOS hospitals for their efforts in caring for patients in these difficult times. We also thank all hygiene specialists involved in this study.
Editor: L. Leibovici
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2020.08.011.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Flaxman S., Mishra S., Gandy A., Unwin H.J.T., Mellan T.A., Coupland H. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020 doi: 10.1038/s41586-020-2405-7. published online Jun 8. [DOI] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center Mortality analyses. https://coronavirus.jhu.edu/data/mortality
- 3.German Federal Statistical Office https://www.destatis.de/DE/Themen/Gesellschaft-Umwelt/Gesundheit/Krankenhaeuser/Tabellen/gd-krankenhaeuser-jahre.html;jsessionid=00007A94ACC46038CE3DBF921701BBE1.internet8732
- 4.WHO Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf Jan 28.
- 5.Fine J., Gray R.J. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 6.Coronavirus disease 2019 (COVID-19) daily situation report of the Robert Koch institute. https://www.rki.de/DE/Content/InfAZ/N/Neuartiges_Coronavirus/Situationsberichte/Archiv_Juni.html
- 7.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30316-7. published online Jul 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann F., Icks A. Structural differences between health insurance funds and their impact on health services research: results from the Bertelsmann Health-Care Monitor. Gesundheitswesen. 2012;74:291–297. doi: 10.1055/s-0031-1275711. [DOI] [PubMed] [Google Scholar]
- 9.OECD . 2020. Hospital beds (indicator)https://data.oecd.org/healtheqt/hospital-beds.htm [Google Scholar]
- 10.Rhodes A., Ferdinande P., Flaatten H., Guidet B., Metnitz P.G., Moreno R.P. The variability of critical care bed numbers in Europe. Intensive Care Med. 2012;38:1647–1653. doi: 10.1007/s00134-012-2627-8. [DOI] [PubMed] [Google Scholar]
- 11.Phua J., Hashmi M., Haniffa R. ICU beds: less is more? Not sure. Intensive Care Med. 2020 doi: 10.1007/s00134-020-06162-8. published online Jun 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan A., Liu L., Wang C., Guo H., Hao X., Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.6130. published online Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020 doi: 10.1001/jama.2020.5394. published online Apr 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020:369. doi: 10.1136/bmj.m1985. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease in China 2019. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. published online Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. published online Feb 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. published online Feb 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020 doi: 10.1001/jama.2020.6775. published online Apr 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foundation for innovative new diagnostics. SARS- COV-2 TEST TRACKER; 2020. https://www.finddx.org/COVID-19/test-tracker/ [Google Scholar]
- 21.McMichael T.M., Currie D.W., Clark S., Pogosjans S., Kay M., Schwartz N.G. Epidemiology of covid-19 in a long-term care facility in king county, Washington. N Engl J Med. 2020;382:2005–2011. doi: 10.1056/NEJMoa2005412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Euronews The deadly impact of COVID-19 on Europe's care homes. 2020. https://www.euronews.com/2020/05/08/the-deadly-impact-of-covid-19-on-europe-s-care-home May 14.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.