Abstract
Introduction
This study examines the frequency, associated factors, and characteristics of healthcare personnel coronavirus disease 2019 cases in a healthcare department that comprises a tertiary hospital and its associated 12 primary healthcare centers.
Methods
This study included healthcare personnel that showed symptoms or were in contact with a coronavirus disease 2019 case patient from March 2, 2020 to April 19, 2020. Their evolution and characteristics (age, sex, professional category, type of contact) were recorded. Correlations between the different characteristics and risk of developing coronavirus disease 2019 and severe coronavirus disease 2019 were analyzed using chi-square tests. Their magnitudes were quantified with ORs, AORs, and their 95% CIs using a logistic regression model.
Results
Of the 3,900 healthcare professionals in the department, 1,791 (45.9%) showed symptoms or were part of a contact tracing study. The prevalence of those with symptoms was 20.1% (784/3,900; 95% CI=18.8, 21.4), with coronavirus disease 2019 was 4.0% (156/3,900; 95% CI=3.4, 4.6), and with severe coronavirus disease 2019 was 0.5% (18/3,900; 95% CI=0.2, 0.7). The frequency of coronavirus disease 2019 in symptomatic healthcare personnel with a nonprotected exposure was 22.8% (112/491) and 13.7% (40/293) in those with a protected exposure (AOR=2.2, 95% CI=1.2, 3.9). The service in which the healthcare personnel performed their activity was not significantly associated with being diagnosed with coronavirus disease 2019. A total of 26.3% (10/38) of male healthcare personnel with coronavirus disease 2019 required hospitalization, compared with 6.8% (8/118) among female healthcare personnel (OR=4.9, 95% CI=1.8, 13.6).
Conclusions
A surveillance and monitoring program centred on healthcare personnel enables an understanding of the risk factors that lead to coronavirus disease 2019 among this population. This knowledge allows the refinement of the strategies for disease control and prevention in healthcare personnel during the coronavirus disease 2019 pandemic.
INTRODUCTION
In December 2019 in Wuhan (Hubei, China), a new coronavirus that had not been previously identified in humans was associated with a cluster of pneumonia cases.1 It rapidly propagated around various countries and on January 30, 2020, the WHO classified the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus disease 2019 (COVID-19), as a Public Health Emergency of International Concern.2 On March 11, 2020, the WHO declared the state of pandemic.3 To date, the vast majority of countries worldwide have reported COVID-19 cases.4
Healthcare personnel (HCP) are at the forefront of the response to the COVID-19 pandemic, and as such, they are exposed to a higher infection risk.5 Up to 10% of the cases reported in China and Italy were among HCP.6 , 7 In Spain, 20.4% of the confirmed cases were among HCP.8
The protection of HCP is recognized as a critical priority to guarantee their health and the continuity of healthcare services.9 The occupational exposure of this population must be addressed widely. One of the strategies for disease control and prevention in healthcare institutions is the surveillance and management of exposed personnel.10 , 11 A total of 2 fundamental approaches form part of this strategy: (1) continuous monitoring and individual risk assessment of all HCP exposed to COVID-19 and (2) identification of HCP that develop symptoms consistent with the disease. These strategies enable the establishment of appropriate diagnostic testing and isolation measures in the workplace to stop the spread of the disease.
There is limited information on the risk of infection by SARS-CoV-2 among HCP, and the available data come from limited case series that target specific aspects of this population (Folgueira et al., unpublished data, April 2020).12, 13, 14, 15, 16, 17 No studies have been reported that analyze the risk of HCP in diverse environments (healthcare assistance and social interactions). Improving the surveillance and understanding of how HCP exposure to SARS-CoV-2 translates to a risk of infection is essential to establish the most effective disease control and prevention recommendations among HCP.18
The aim of this study is to uncover the frequency, associated factors, and characteristics of COVID-19 and severe COVID-19 in HCP at Alicante's Healthcare Department (HCD) in Spain.
METHODS
Study Sample
An observational study was carried out in an HCD comprised by a tertiary hospital, 12 primary healthcare centers, and a total of 3,900 HCP. It spanned 7 epidemiologic weeks, from Week 10 (starting March 2, 2020) up until Week 16 (ending April 19, 2020). During this period, it was compulsory for all HCP to inform the Preventive Medicine Service if they showed any compatible COVID-19 symptoms or if they had had contact with a patient with confirmed or suspected COVID-19 case.
Measures
The HCP were evaluated under the 2 assistance programs deployed by the Preventive Medicine Service. The first was the Biological Risk HCP Evaluation Program, in which HCP were tested when they showed any severity of compatible SARS-CoV-2 infection symptoms: mild fever (≥37°C) or fever (≥38°C), cough, nasal congestion, anosmia, rhinorrhea, sore throat, dysgeusia, headache, asthenia, or dyspnea. The second was the HCP Contact Tracing Program, through which all HCP who were exposed to a suspected or confirmed patient with COVID-19 case in any environment (workplace, family, or social) were evaluated. As part of this second program, they were subjected to a contact tracing study during the 14 days following the exposure to identify all the potential contacts that had shown symptoms. Both programs were stablished before the beginning of the pandemic, and the HCD personnel were already familiar with the procedures.
A contact of an HCP with a confirmed patient with COVID-19 case was defined as someone being in the same place in which a patient with confirmed case was showing symptoms. All HCP with symptoms compatible with COVID-19 had a SARS-CoV-2 respiratory tract sample test via polymerase chain reaction (PCR). HCP were considered as a patient with confirmed COVID-19 case when they showed symptoms and had 2 positive microbiological PCR tests for SARS-CoV-2 (a first PCR screening test that when rendered positive was confirmed with a second PCR test).19 HCP were considered to have severe COVID-19 when they required hospitalization.
Among all evaluated HCP, the following data were collected: age, sex, professional category (physician, nurse, nursing assistant or others not performing strict clinical care like hospital wardens, administrative assistants, or physiotherapists), and the service in which they were working. Regarding the exposure risk, the reason, type, and characteristics of the contact were registered. The contact reason was classified based on the exposure 14 days before initiation of symptoms: (1) healthcare-associated, when the contact occurred when providing clinical care to a patient with confirmed COVID-19; (2) workplace social interactions among HCP, when the contact occurred with another HCP with confirmed COVID-19 via interactions, work meetings, or breaks; and (3) other social interactions, when the HCP had contact with a patient with positive COVID-19 case in the community outside the work environment. The type of contact was classified as: (1) close, when the evaluated HCP was at a distance of <2 meters for ≥15 minutes with a case patient who showed symptoms; (2) occasional, when the HCP was in the same place and the conditions did not comply with the above; and (3) unknown, when the potential infection source was unidentified. For healthcare-associated contacts, the protection level was classified as: (1) protected, when the appropriate extended droplet precautions were followed (use of a surgical mask or an N95, FFP2, or FFP3 respirator) and additional eye protection was used depending on the risk of the maneuver (aerosol-generating procedures were considered high risk),10 and (2) not protected, when the extended droplet precautions were not followed. For those contacts related to social environments (workplace and community), the protection level was classified as: (1) protected, when the HCP used a surgical mask during the contact, and (2) not protected, when a mask was not used during the contact. When more than 1 type of contact occurred, it was assigned to the least protected exposure. When a social contact could not be clearly established, the type of contact was considered healthcare-associated.
The HCP with confirmed COVID-19 were interviewed using the epidemiologic survey of the Regional Public Health Department Compulsory Disease Declaration. In this survey, the following data were included: presence of symptoms, clinical comorbidities, influenza vaccination status, and hospital admission requirement or intensive care unit (ICU) requirement, among others.
Statistical Analysis
The evolution over time and the characteristics of all evaluated HCP were described, both symptomatic with confirmed COVID-19 and those that required hospitalization (severe COVID-19 cases). The absolute and relative frequencies shown as percentages were calculated for each variable. Prevalence and 95% CIs were calculated for the following groups: evaluated HCP, HCP with symptoms, HCP with COVID-19, HCP with severe COVID-19, and HCP who required admission to the ICU. To evaluate the association between the different variables and the main outcome (confirmed COVID-19), chi-square tests were performed. To quantify the effect of the association, ORs and 95% CIs were calculated. AORs and 95% CIs were calculated using a logistic regression model. This model was based on the variables that showed an association in the bivariate analysis. Statistical significance was set at p<0.05 for all hypothesis determinations. Analysis was conducted using SPSS, version 25.0.
RESULTS
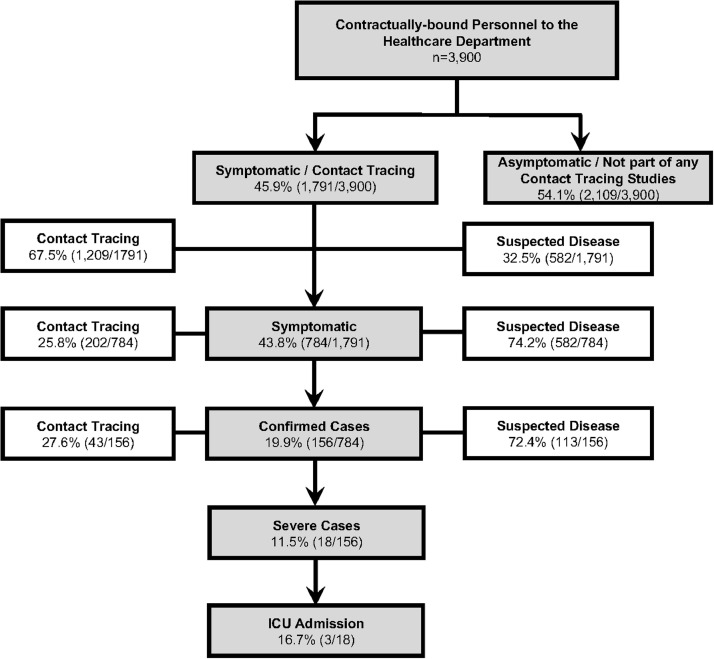
Between March 2, 2020 and April 19, 2020, a total 45.9% (1,791/3,900) of the HCP in the HCD were evaluated either because they showed symptoms or because they were part of a contact tracing study. The remaining 54.1% (2,109/3,900) did not show any symptoms and were not part of any contact tracing studies (Figure 1 ). Of the 1,791 evaluated HCP, 32.5% (582/1,791) presented with symptoms, and 67.5% (1,209/1,791) were identified through a contact tracing investigation. Of the latter, 16.7% (202/1,209) showed symptoms within the 14 days after they were exposed to a patient with suspected or confirmed COVID-19 case. All the HCP personnel who showed symptoms at any point in time (n=784) had a PCR test performed. Of these, 19.9% (156/784) were confirmed to have COVID-19. Of these HCP with confirmed COVID-19, 11.5% (18/156) required hospital admission. Of these, 16.7% (3/18) required admission to the ICU.
Figure 1.
HCP characteristics and type of study in which it was evaluated.
HCP, healthcare personnel; ICU, intensive care unit.
The daily distribution of new cases in evaluated HCP, both symptomatic and those that required hospital admission, is shown in Appendix Figure 1 (available online). A significant increase in the number of evaluated HCP from both populations was observed between March 11, 2020 and March 12, 2020. This was followed by a plateau and a steady decrease of new cases from March 25, 2020. Characteristics of HCP are shown in Table 1 .
Table 1.
Distribution of the HCP Evaluated
| Characteristic | Evaluated (n=1,791), | Symptomatic (n=784), | Confirmed (n=156), | Severe confirmed (n=18), |
|---|---|---|---|---|
| % (n) | % (n) | % (n) | % (n) | |
| Age, years | ||||
| <60 | 85.1 (1,525) | 88.1 (691) | 86.5 (135) | 72.2 (13) |
| ≥60 | 14.9 (266) | 11.9 (93) | 13.5 (21) | 27.8 (5) |
| Sex | ||||
| Male | 24.8 (444) | 21.6 (169) | 24.4 (38) | 55.6 (10) |
| Female | 75.2 (1,347) | 78.4 (615) | 75.6 (118) | 44.4 (8) |
| Professional category | ||||
| Physician | 22.7 (407) | 27.2 (213) | 38.5 (60) | 50.0 (9) |
| Nurse | 36.6 (655) | 34.4 (270) | 31.4 (49) | 16.7 (3) |
| Nursing assistant/technicians | 20.3 (363) | 23.6 (185) | 17.3 (27) | 16.7 (3) |
| Othera | 20.4 (366) | 14.8 (116) | 12.8 (20) | 16.7 (3) |
| Contact reason | ||||
| Healthcare-associated | 29.6 (531) | 26.7 (209) | 17.9 (28) | 27.8 (5) |
| Workplace social interactions | 60.6 (1,085) | 53.3 (418) | 53.8 (84) | 50.0 (9) |
| Other social interactions | 9.8 (175) | 20.0 (157) | 28.2 (44) | 22.2 (4) |
| Contact type | ||||
| Close | 47.7 (854) | 66.7 (523) | 76.3 (119) | 72.2 (13) |
| Occasional | 42.9 (769) | 23.2 (182) | 17.3 (27) | 22.2 (4) |
| Unknown | 9.4 (168) | 10.1 (79) | 6.4 (10) | 5.6 (1) |
| Department | ||||
| Emergency service | 5.1 (91) | 4.7 (37) | 3.8 (6) | 11.1 (2) |
| Pediatric areasb | 6.5 (45) | 6.1 (48) | 2.6 (4) | 0.0 (0) |
| Critical care unitsc | 15.2 (24) | 15.3 (120) | 19.2 (30) | 22.2 (4) |
| Medical wards—adults | 35.5 (635) | 35.8 (281) | 26.9 (42) | 16.7 (3) |
| Primary health care | 12.6 (226) | 12.4 (97) | 14.7 (23) | 16.7 (3) |
| Non–COVID-19 wardsd | 25.1 (450) | 25.6 (201) | 32.7 (51) | 33.3 (6) |
Hospital wardens, administrative assistants, physiotherapist, etc.
Pediatric emergency service, surgical pediatric wards, medical pediatric wards, and pediatric ICU.
Medical ICU, surgical ICU, and operating theatre.
Non–COVID-19 wards: surgical wards adults; administrative areas; central services: laboratory, microbiology, pharmacy, radiology, blood bank, etc.
COVID-19, coronavirus disease 2019; HCP, healthcare personnel; ICU, intensive care unit.
The prevalence of symptomatic HCP was 20.1% (95% CI=18.8, 21.4). The prevalence of symptomatic confirmed COVID-19 was 4.0% (95% CI=3.4, 4.6). Severe COVID-19 cases accounted for 0.5% (95% CI=0.2, 0.7) and 0.1% (95% CI=0.0, 0.2) ultimately requiring admission to the ICU (Appendix Table 1, available online).
Among symptomatic HCP, the only significant and independent variable associated with the development of confirmed COVID-19 was the type of contact (protected or not protected). The frequency of patients with confirmed COVID-19 HCP case with symptoms who had a nonprotected exposure was 22.8% (112/491) and 13.7% (40/293) when its exposure was protected (AOR=2.2, 95% CI=1.2, 3.9) (Table 2 ).
Table 2.
Risk Factors for Disease Development Among HCP With Symptoms That Were Subject to a PCR Test (n=784)
| Characteristic | COVID-19 case frequency, % (n) | OR (95% CI) | p-value | AOR (95% CI) | p-value |
|---|---|---|---|---|---|
| Age, years | |||||
| <60 | 19.5 (135/691) | 1 | — | — | — |
| ≥60 | 22.6 (21/93) | 1.2 (0.7, 2.0) | 0.490 | — | — |
| Sex | |||||
| Male | 22.5 (38/169) | 1.2 (0.8, 1.8) | 0.341 | — | — |
| Female | 19.2 (118/615) | 1 | — | — | — |
| Professional category | |||||
| Physician | 28.2 (60/213) | 1.9 (1.1, 3.3) | 0.029 | 1.5 (0.7, 3.1) | 0.262 |
| Nurse | 18.1 (49/270) | 1.1 (0.6, 1.9) | 0.831 | 1.3 (0.6, 2.7) | 0.459 |
| Nurse assistant/technician | 14.6 (27/185) | 0.8 (0.4, 1.5) | 0.539 | 0.6 (0.3, 1.2) | 0.154 |
| Othera | 17.2 (20/116) | 1 | — | 1 | — |
| Contact reason | |||||
| Healthcare-associated | 13.4 (28/209) | 1 | — | 1 | — |
| Workplace social interactions | 20.1 (84/418) | 1.6 (1.0, 2.6) | 0.040 | 0.6 (0.3, 1.1) | 0.125 |
| Other social interactions | 28.0 (44/157) | 2.5 (1.5, 4.3) | 0.001 | 1.5 (0.8, 3.0) | 0.241 |
| Contact type | |||||
| Close | 22.8 (119/523) | 2.0 (1.0, 4.1) | 0.045 | 2.1 (0.8, 5.1) | 0.105 |
| Occasional | 14.8 (27/182) | 1.2 (0.6, 2.6) | 0.644 | 1.4 (0.5, 3.9) | 0.479 |
| Unknown | 12.7 (10/79) | 1 | — | 1 | — |
| Contact characteristics | |||||
| Not protected | 22.8 (112/491) | 1.9 (1.3, 2.7) | 0.001 | 2.2 (1.2, 3.9) | 0.009 |
| Protected | 13.7 (40/293) | 1 | — | 1 | — |
| Department | |||||
| Emergency service | 16.2 (6/37) | 0.6 (0.2, 1.4) | 0.235 | 0.7 (0.2, 1.9) | 0.442 |
| Pediatric areasb | 8.3 (4/48) | 0.3 (0.1, 0.8) | 0.016 | 0.4 (0.1, 2.2) | 0.112 |
| Critical care unitsc | 25.0 (30/120) | 1.0 (0.6, 1.7) | 0.941 | 0.8 (0.3, 1.7) | 0.296 |
| Adults medical wards | 14.9 (42/281) | 0.5 (0.3, 0.8) | 0.005 | 0.7 (0.4, 1.4) | 0.370 |
| Primary health care | 23.7 (23/97) | 0.9 (0.5, 1.6) | 0.756 | 1.1 (0.5, 2.2) | 0.888 |
| Non–COVID-19 wardsd | 25.4 (51/201) | 1 | — | 1 | — |
Note: AOR based on professional category, reason for contact, type of contact, contact characteristics, and department.
Hospital wardens, administrative assistants, physiotherapist, etc.
Pediatric emergency service, surgical pediatric wards, medical pediatric wards and pediatric ICU.
Medical ICU, surgical ICU, and operating theatre.
Non–COVID-19 wards: adults surgical wards; administrative areas; central services: laboratory, microbiology, pharmacy, radiology, blood bank, etc.
COVID-19, coronavirus disease 2019; HCP, healthcare personnel; ICU, intensive care unit; PCR, polymerase chain reaction.
Among the HCP who required hospital admission (severe COVID-19 cases), the variables that were significantly associated with this outcome were sex and the presence of pneumonia. Hospital admission was required for 26.3% (10/38) of male HCP with COVID-19 compared with 6.8% (8/118) of female HCP (OR=4.9, 95% CI=1.8, 13.6). Hospital admission was required for 64.3% (9/14) of HCP with COVID-19 who were diagnosed with pneumonia, compared with 6.3% (9/142) of HCP with COVID-19 who were not diagnosed with pneumonia (OR=26.6, 95% CI=7.4, 96.1) (Table 3 ).
Table 3.
Prognostic Factors for the Development of Severe COVID-19 Among HCP (n=156)
| Characteristic | Severe COVID-19 case frequency, % (n) | OR (95%CI) | p-value |
|---|---|---|---|
| Age, years | |||
| <60 | 9.6 (13/135) | 1 | — |
| ≥60 | 23.8 (5/21) | 2.9 (0.9, 9.3) | 0.071 |
| Sex | |||
| Male | 26.3 (10/38) | 4.9 (1.8, 13.6) | 0.002 |
| Female | 6.8 (8/118) | 1 | — |
| Pre-existing conditions | |||
| Shows a pre-existing condition (yes) | 12.5 (6/48) | 1.1 (0.4, 3.2) | 0.802 |
| Diabetes (yes) | 0.0 (0/2) | N/A | — |
| Chronic hepatic disease (yes) | 0.0 (0/1) | N/A | — |
| Chronic kidney disease (yes) | 100.0 (2/2) | N/A | — |
| Immunodeficiency (yes) | 100.0 (1/1) | N/A | — |
| Cardiovascular disease (yes) | 22.2 (2/9) | 2.3 (0.4, 12.1) | 0.278 |
| Chronic lung disease (yes) | 0.0 (0/7) | N/A | — |
| Chronic neurologic disease (yes) | 0.0 (0/1) | N/A | — |
| Pregnancy (yes) | 0.0 (0/1) | N/A | — |
| Cancer (yes) | 0.0 (0/2) | N/A | — |
| Arterial hypertension (yes) | 27.3 (3/111) | 3.2 (0.7, 13.5) | 0.118 |
| Other pre-existing conditions (yes) | 6.3 (2/32) | 0.4 (0.1, 2.0) | 0.370 |
| Influenza vaccination | |||
| Yes | 11.9 (8/67) | 1.1 (0.4, 2.9) | 0.892 |
| No | 11.2 (10/89) | 1 | — |
| Clinical characteristics | |||
| Fever (yes) | 13.2 (14/106) | 1.8 (0.6, 5.6) | 0.428 |
| Cough (yes) | 10.7 (13/121) | 0.7 (0.2, 2.2) | 0.564 |
| Pneumonia (yes) | 64.3 (9/14) | 26.6 (7.4, 96.1) | <0.001 |
| Headache (yes) | 9.7 (6/62) | 0.7 (0.3, 2.1) | 0.555 |
| Shivers (yes) | 17.0 (9/53) | 2.1 (0.8, 5.8) | 0.127 |
| Coryza (yes) | 14.3 (3/21) | 1.3 (0.4, 5.1) | 0.713 |
| Myalgia (yes) | 12.5 (11/88) | 1.2 (0.5, 3.4) | 0.669 |
| Vomits (yes) | 30.0 (3/10) | 3.7 (0.9, 16.0) | 0.093 |
| Diarrhea (yes) | 15.4 (6/39) | 1.6 (0.6, 4.6) | 0.393 |
| ARDS (yes) | 100.0 (2/2) | N/A | — |
| Acute kidney failure (yes) | 100.0 (1/1) | 9.1 (5.8, 14.3) | 0.115 |
| Ageusia (yes) | 14.3 (5/35) | 1.4 (0.4, 4.2) | 0.556 |
| Anosmia (yes) | 13.5 (5/37) | 1.3 (0.4, 3.8) | 0.768 |
| Ocular symptoms (yes) | 20.0 (1/5) | 2.0 (0.2, 18.7) | 0.463 |
| Migraine (yes) | 8.0 (6/75) | 0.5 (0.2, 1.4) | 0.183 |
| Other (yes) | 8.7 (10/115) | 0.4 (0.2, 1.1) | 0.086 |
Note: Boldface indicates statistical significance (p<0.05).
ARDS, acute respiratory distress syndrome; COVID-19, coronavirus disease 2019; HCP, healthcare personnel; N/A, not applicable.
DISCUSSION
The data presented here do not only show the frequency of HCP with COVID-19, but also the risk factors, prognosis, the evolution of HCP COVID-19 cases over time. This work represents a comprehensive study on almost half of the HCP of an HCD constituted by a tertiary general hospital and its 12 primary care centers.
In this study, the prevalence of COVID-19 among HCP was 4.0% (95% CI=3.4, 4.6). The first sets of international data showed that up to 10% of the COVID-19 cases in China and Italy were among HCP.6 , 7 More recent data from the U.S. between February 12, 2020 and April 9, 2020 showed that only in 16% of the reported cases a variable was available to record if the patient was an HCP. Of these, 19% were identified as HCP.12 In England, between March 10, 2020 and 31, 2020, a total of 1,666 SARS-CoV-2 tests were performed among 1,654 HCP.20 SARS-CoV-2 was detected in 240 (14%) of these tests. In a Spain-wide study, 20.4% of the cases through April 23, 2020 were reported as HCP.8 According to preliminary results from a hospital in Madrid, through March 3, 2020, HCP comprised 11.6% of their cases (Folgueira et al., unpublished data, April 2020).
Considering these data on the prevalence of COVID-19 among HCP, the rate of COVID-19 cases in this department is considered lower than what has been published to date. This statement requires further clarification as the different studies cannot be directly compared owing to the discordant methodologies used. This study did not test asymptomatic HCP, even though the existence of asymptomatic COVID-19 cases has been decribed.21 According to the published data, the proportion of asymptomatic cases would be in the range of 17.9%22–32%.23 Taking this into account, the prevalence in this department would increase to 4.7%–5.3%.
A critical point would be to determine if the prevalence of COVID-19 among HCP is higher than that of the general population. This would assess the potential additional risk to which HCP are exposed and would allow understanding of whether the measures of the programs for disease control and prevention are effective. Unfortunately, this direct comparison is not possible as the true percentage of the population affected by COVID-19 in Spain is not known. Following a model developed by Imperial College London, the proportion of the infected population in Spain as of March 28, 2020 was 15.0% (95% CI=3.7, 41.0).24 Although these estimations can aid understanding of the impact of COVID-19 in the general population, to establish comparisons with HCP, it would be necessary to obtain prevalence data of COVID-19 in the population based on determination of immunoglobulin G after the epidemic stage of the disease.
In this study, the only variable that was significantly and independently associated with the development of COVID-19 was having had a nonprotected contact (AOR=2.2, 95% CI=1.2, 3.9). Some of these nonprotected contacts took place while providing clinical care to patients who had not originally been suspected to have COVID-19; in these cases, appropriate precautionary measures were not taken. Nonprotected contacts also took place during social interactions in the workplace or in the community where a surgical mask was not used. The age, sex, professional category, contact reason, and the contact type were not independently associated with the development of COVID-19. The previous statement also applies to the different services in which the HCP could potentially be more exposed, like the emergency service or critical care units, in line with what was determined in other case series (Folgueira et al., unpublished data, April 2020). In fact, the services with the highest proportion of symptomatic HCP with confirmed COVID-19 were the non–COVID-19 wards. This is in line with the HCP screening study carried out in England,20 which is in favor of the effectiveness of the isolation protocols and personal protective equipment (PPE). As long as the exposure is protected, independently of the contact carried out, the infection risk diminishes. This statement is supported by a study in California in which among the 121 HCP with a nonprotected exposure to a not-yet-diagnosed COVID-19 patient, 3 resulted positive for SARS-CoV-2, in comparison with the 146 HCP who had a protected exposure using the appropriate PPE of whom none tested positive for SARS-CoV-2.17 In addition, a Chinese study reported a high COVID-19 transmission rate among HCP, but when the appropriate PPE measures were implemented, the transmission among HCP drastically reduced.6 Hence, the use of the appropriate PPE by all HCP is the key to their protection, and as such it should be taken into account when directing resources to guarantee their availability. However, it is thought provoking that in this series, 82.1% (128/156) of the HCP COVID-19 cases were associated with social interactions in the work environment with other colleagues or outside of work with friends or family, in contrast to the 17.9% (28/156) of cases that were strictly associated to direct clinical care.
If the goal is to reduce the infection risk among HCP, the disease control and prevention strategies should not just be targeted to direct clinical care but also the rest of the situations occurring in the healthcare and community settings. This is the reason behind the decision to require the universal use of a surgical mask among HCP. This applied when they were within 1 m of other colleagues or patients (in the work environment), but also of other friends and relatives (in the social and family environments). The evaluation of the effectivity of this strategy was evaluated in a separate study.25
Regarding the HCP characteristics, the number of evaluated female HCP was clearly higher than the number of male HCP, in line with the sex distribution among the department HCP, were the proportion of female HCP is also higher. The prevalence of COVID-19 among male and female HCP was not different, but being male was associated with the development of a more severe form of disease (OR=4.9, 95% CI=1.8, 13.6). This was also shown in a preliminary study on the influence of sex in the morbidity and mortality of COVID-19, which determined that male case patients tended to be more severe than those in female patients (p=0.035); the mortality (not studied in this manuscript) was also higher in male than female case patients (70.3% vs 29.7%, p=0.016).26 The second factor associated with admission was the presence of pneumonia. These data are relevant as an early identification of the risk factors associated with the severity of the disease can facilitate a prompt therapeutic response for the patient in case it is necessary. However, as the date of onset of symptoms was not available, it was not possible to identify which early symptoms help predict severe disease.
Because data collection was not focused on studying the severity of the disease, to perform a more in-depth analysis of the associated COVID-19 prognostic factors among HCP, it would be necessary to gather more refined clinical data.
Limitations
There are various limitations to this study. First, there was potential selection bias owing to the HCP who were not evaluated (54.1%) either because they did not present with symptoms or because when they were contacted as part of a contact tracing studies, they were not symptomatic (a PCR test was not indicated). This could potentially have led to underdiagnosis of COVID-19 among HCP. Second, this study was observational in nature. Third, classification bias that may have arisen when an infection in an HCP was attributed to a specific contact when multiple contacts had taken place. Even when there is a clear hypothesis for the infection source, this cannot always be proven. Fourth, the presence of comorbidities (pre-existing conditions) was obtained through telephone interviews as part of epidemiologic surveys.
CONCLUSIONS
A surveillance and monitoring program centred on HCP allows for ascertainment of the COVID-19 risk factors in this population, and can thus reshape the disease prevention and control strategies to improve HCP protection.
ACKNOWLEDGMENTS
The authors acknowledge Álvaro Sánchez Vela for contributing to the editing and critical review of the manuscript.
No financial disclosures were reported by the authors of this paper.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2020.07.014.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm, Sweden: 2020. Threat assessment brief: Pneumonia cases possibly associated with a novel coronavirus in Wuhan, China.https://www.ecdc.europa.eu/sites/default/files/documents/Threat-assessment-Pneumonia-cases-possibly-associated-to-a-novel-coronavirus-in-Wuhan-China.pdf Published January 9. [Google Scholar]
- 2.WHO . WHO; Geneva, Switzerland: 2020. Statement on the second meeting of the international health regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV)https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-%282005%29-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-%282019-ncov%29 Published January 30. [Google Scholar]
- 3.WHO . WHO; Geneva, Switzerland: 2020. WHO Director-general's opening remarks at the media briefing on COVID-19.https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020 Published March 11. [Google Scholar]
- 4.WHO . WHO; Geneva, Switzerland: 2020. Coronavirus disease 2019 (COVID-19) Situation report–95.https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200424-sitrep-95-covid-19.pdf?sfvrsn=e8065831_4 Published April 24. [Google Scholar]
- 5.WHO . WHO; Geneva, Switzerland: 2020. Coronavirus disease (COVID-19) outbreak: rights, roles and responsibilities of health workers, including key considerations for occupational safety and health.https://www.who.int/publications/i/item/coronavirus-disease-(covid-19)-outbreak-rights-roles-and-responsibilities-of-health-workers-including-key-considerations-for-occupational-safety-and-health Published March 18. [Google Scholar]
- 6.WHO . WHO; Geneva, Switzerland: 2020. Report of the WHO-China joint mission on coronavirus Disease 2019 (COVID-19)https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf Published February 16–24. [Google Scholar]
- 7.Istituto Superiore di Sanità; Rome, Italy: 2020. The COVID-19 Task Force of the Department of Infectious Diseases and the IT Service Istituto Superiore di Sanità. Integrated surveillance of COVID-19 in Italy.https://www.epicentro.iss.it/coronavirus/bollettino/Infografica_2aprile%20ENG.pdf Published February 27. [Google Scholar]
- 8.Red Nacional R de Vigilancia Epidemiológica . Red Nacional R de Vigilancia Epidemiológica; Madrid, Spain: 2020. Informe sobre la situación de COVID-19 en España: Informe COVID-19 n° 25.https://www.isciii.es/QueHacemos/Servicios/VigilanciaSaludPublicaRENAVE/EnfermedadesTransmisibles/Documents/INFORMES/Informes%20COVID-19/Informe%20n%C2%BA%2025.%20Situaci%C3%B3n%20de%20COVID-19%20en%20Espa%C3%B1a%20a%2023%20de%20abril%20de%202020.pdf Published April 23. [Google Scholar]
- 9.WHO . WHO; Geneva, Switzerland: 2018. Occupational safety and health in public health emergencies: a manual for protecting health workers and responders.https://www.who.int/publications-detail/occupational-safety-and-health-in-public-health-emergencies-a-manual-for-protecting-health-workers-and-responders Published May 23. [Google Scholar]
- 10.European Centre for Disease Prevention and Control. Infection prevention and control and preparedness for COVID-19 in healthcare settings – fourth update. Stockholm, Sweden: European Centre for Disease Prevention and Control.https://www.ecdc.europa.eu/en/publications-data/infection-prevention-and-control-and-preparedness-covid-19-healthcare-settings. Accessed July 23, 2020.
- 11.Interim Operational Considerations for Public Health Management of Healthcare Workers Exposed to or Infected with COVID-19: non-U.S. Healthcare Settings. Centers for Disease Control and Prevention.https://www.cdc.gov/coronavirus/2019-ncov/hcp/non-us-settings/public-health-management-hcw-exposed.html. Updated April 22, 2020. Accessed April 25, 2020.
- 12.CDC COVID-19 Response Team Characteristics of health care personnel with COVID-19 - United States. MMWR Morb Mortal Wkly Rep. 2020;69(15):477–481. doi: 10.15585/mmwr.mm6915e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y, Mo P, Xiao Y, Zhao O, Zhang Y, Wang F. Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China, January to February 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng K, Poon BH, Kiat Puar TH. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020;172(11):766–767. doi: 10.7326/L20-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tostmann A, Bradley J, Bousema T. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill. 2020;25(16) doi: 10.2807/1560-7917.ES.2020.25.16.2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keeley AJ, Evans C, Colton H. Roll-out of SARS-CoV-2 testing for healthcare workers at a large NHS Foundation Trust in the United Kingdom, March 2020. Euro Surveill. 2020;25(14) doi: 10.2807/1560-7917.ES.2020.25.14.2000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heinzerling A, Stuckey MJ, Scheuer T. Transmission of COVID-19 to health care personnel during exposures to a hospitalized patient - Solano County, California, February 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):472–476. doi: 10.15585/mmwr.mm6915e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO . WHO; Geneva, Switzerland: 2020. Risk assessment and management of exposure of health care workers in the context of COVID-19.https://apps.who.int/iris/bitstream/handle/10665/331496/WHO-2019-nCov-HCW_risk_assessment-2020.2-eng.pdf Published March 19. [Google Scholar]
- 19.Instituto de Salud Carlos III. Estrategia de detección precoz, vigilancia y control de COVID-19. Madrid, Spain: Instituto de Salud Carlos III.https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/COVID19_Estrategia_vigilancia_y_control_e_indicadores.pdf. Accessed July 9, 2020.
- 20.Hunter E, Price DA, Murphy E. First experience of COVID-19 screening of health-care workers in England. Lancet. 2020;395(10234):e77–e78. doi: 10.1016/S0140-6736(20)30970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Euro Surveill. 2020;25(10) doi: 10.2807/1560-7917.ES.2020.25.10.2000180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabata S, Imai K, Kawano S. Clinical characteristics of COVID-19 in 104 people with SARS-CoV-2 infection on the Diamond Princess cruise ship: a retrospective analysis. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30482-5. In press. Online June 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaxman S, Mishra S, Gandy A. Imperial College; London, United Kingdom: 2020. Estimating the number of infections and the impact of non-pharmaceutical interventions on COVID-19 in 11 European countries.https://spiral.imperial.ac.uk:8443/bitstream/10044/1/77731/10/2020-03-30-COVID19-Report-13.pdf [Google Scholar]
- 25.Gras-Valentí P, Mora-Muriel JG, Chico-Sánchez P. Bulletin of the World Health Organization; Geneva, Switzerland: 2020. Effectivity of a programme for the control & prevention of COVID-19 healthcare-associated infections in a Spanish Academic Hospital.https://www.who.int/bulletin/online_first/20-263384.pdf [Google Scholar]
- 26.Jin JM, Bai P, He W. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.