Abstract
Background
Neither pre-exposure nor post-exposure chemo-prophylaxis agents are currently available to prevent COVID-19. On the other hand, high loads of SARS-CoV-2 are shed from the nasal cavity before and after symptoms onset.
Objective
To conduct a scoping review on the available evidence on tolerable nasal disinfectants with encouraging health outcomes against SARS-CoV-2, i.e., agents effective against at least two different viruses beyond SARS-CoV-2.
Methods
Online databases were searched to identify papers published during 2010–2020. Publications were selected if they were relevant to the scoping review. The review was narrative, describing for each treatment the mechanism(s) of action, tolerability, in vitro and in vivo evidence of the effects against SARS-CoV-2 and whether the product had been marketed.
Results
Eight treatments were scrutinized: hypothiocyanite, lactoferrin, N-chlorotaurine, interferon-alpha, povidone-iodine, quaternary ammonium compounds, alcohol-based nasal antiseptics and hydroxychloroquine. In vitro viricidal effect against SARS-CoV-2 was reported for ethanol, alcohol-based hand sanitizers and povidone-iodine. Inhibition of other coronaviruses was described for lactoferrin, ethanol, hydroxychloroquine and quaternary ammonium compound. No treatment has been tested against SARS-CoV-2 in randomized controlled clinical trials thus far. However, interferon-alpha, lactoferrin and hydroxychloroquine were tested in one-arm open label uncontrolled clinical trial. Oxidant activity (hypothiocyanite, N-chlorotaurine and povidone-iodine), enhancement of endocytic and lysosomal pH (quaternary ammonium compounds and hydroxychloroquine) and destruction of the viral capsid (quaternary ammonium compounds, alcohol-based nasal antiseptics) were the main mechanisms of action. Lactoferrin and interferon-alpha have subtle biological mechanisms. With the exception of N-chlorotaurine, all other products available on the market.
Conclusions
Effective and safe chemo-prophylactic drugs against SARS-CoV-2 do not exist yet but most eligible candidates are already in the market. Whilst the human nasal cavity is the port of entry for SARS-CoV-2, the mouth is involved as exit site through emission of respiratory droplets. The well-known hand-to-nose-to-hand cycle of contamination requires appropriate additional strategies for infection control. To narrow down the subsequent laboratory and clinical investigations, a case-control approach could be employed to compare the use of candidate drugs among individuals testing positive and negative to COVID-19 swabs.
Keywords: COVID-19, SARS-CoV-2, Nasal disinfection, Hypothiocyanite, Lactoperoxidase, Lactoferrin
1. Background
1.1. Rationale
The current coronavirus disease 2019 (COVID-19), caused by the novel beta coronavirus SARS-CoV-2, has been declared a pandemic affecting 213 countries as of August 26, 2020 (Worldometers, 2020).
Unlike SARS-CoV-1, SARS-CoV-2 seems to replicate efficiently in the upper airways during the incubation period, which is estimated to last up to 14 days (Heymann and Shindo, 2020; World Health Organization, 2020a). During this prodromal stage, asymptomatic and pre-symptomatic individuals release large amounts of viruses from infected cells (World Health Organization, 2020a). As a result, viral transmission is more effective with SARS-CoV-2 than with SARS-CoV-1, which conversely was contagious only during the active/critical phase of the disease (World Health Organization, 2020a). Furthermore, human coronaviruses which may cause common cold are known to cause respiratory re-infections regardless of pre-existing humoral immunity (Gorse et al., 2020; Cegolon et al., 2020). Therefore, there are two issues with COVID-19: the high transmissibility of the virus and a variable clinical pattern of the disease, which can range from pre-symptomatic/asymptomatic condition to life threatening pneumonia featured by severe acute respiratory syndrome (SARS) and hypercoagulable state (World Health Organization, 2020a, Cegolon et al, 2020).
The high risk of contagion from SARS-CoV-2 has been tackled so far by infection prevention and control (IPC) measures such as self-isolation, social distancing, quarantine of contacts, use of personal protective equipment (PPE), travel restrictions and other limitations to the freedom of movement of individuals. However, the effect of each intervention in containing the spread of SARS-CoV-2 has not been evaluated yet (Kucharski and Eggo, 2020).
Currently, the World Health Organization and the European Center for Disease Prevention and Control guidelines recommend testing only symptomatic individuals and close contacts of a confirmed COVID-19 case (European Centre for Disease Prevention and Control, 2020;; World Health Organization, 2020b). Whilst in the initial stage of the epidemic a symptom-based screening strategy was useful with the aim to treat and isolate infected cases, COVID-19 outbreaks in care homes and hospitals suggest that the current IPC measures are inadequate and have failed in several countries (Gandhi et al., 2020; Arons et al., 2020). Furthermore, the above IPC measures are not sustainable in the long run, as they would severely ruin the economies of countries heavily affected by COVID-19. The clear need to relax/avoid the lock down measures enforced in many countries prompts for a revised approach to reduce the transmission of SARS-CoV-2 from asymptomatic/pre-symptomatic individuals, until a pharmacological intervention (ideally a vaccine or a chemoprophylaxis) will hopefully become available (Gandhi et al., 2020). As explained above, the problem with COVID-19 is the transmission from asymptomatic/pre-symptomatic individuals, a phenomenon particularly critical in care homes and hospitals, where more than half residents testing positive might not develop symptoms (Heymann and Shindo, 2020; Gandhi et al., 2020). In Los Angeles families have been advised by the Public Health Department to remove their relatives from care homes to reduce health risk of community outbreaks of COVID-19 (Dolan and Hamilton, 2020).
Mass testing has been considered in various countries as a possible strategy to reduce the transmission of SARS-CoV-2 from asymptomatic/pre-symptomatic individuals (Gandhi et al., 2020). Nonetheless, mass testing on defined and contained outbreak clusters (as done in South Korea) may be sensible, not in the current global scenario though, with an ongoing pandemic (Balilla, 2020). Not to mention that mass testing is probably far beyond the capacity of microbiology laboratories even in high-income countries. However, restricting mass testing to high risk congregate settings, such as care homes, hospitals, mental health facilities, prisons and schools may be appropriate (Gandhi et al., 2020). In an outbreak reported from a care home in Washington State, hosting a total of 76 residents, 27 of the 48 residents testing positive for COVID-19 at real time PCR were asymptomatic during the 14 days preceding the test (Arons et al., 2020).
High loads of SARS-CoV-2 are shed from the nasal cavity into the environment also before symptoms onset (Heymann and Shindo, 2020; Gandhi et al., 2020) and transmission of SARS-CoV-2 from asymptomatic individuals has been described (Rothe et al., 2020; Yu et al., 2020; Bai et al., 2020). Unnoticed, asymptomatic cases of COVID-19 might therefore constitute an important source of contagion, as endorsed by recent evidence from the China's National Health Commission, reporting that the vast majority of infections (four out of five) were totally asymptomatic. In particular, 130 out of the total 166 new infections identified in China on April 1, 2020 had no symptoms whatsoever (Day, 2020).
Although asymptomatic individuals testing positive for SARS-CoV-2 are enforced to quarantine for 14 days, mass testing would yet not resolve the endemic presence of the virus in the general population, a condition posing the risk of repeated resurgences of outbreaks, especially with cold weather conditions (Cegolon et al, 2020; Cegolon and Mastrangelo, 2020). There is in fact evidence that the transmissibility and viability of SARS-CoV-2 reduces importantly with hot and humid climate (Cegolon et al., 2020; Matson et al., 2020; Wang et al., 2020), as confirmed by the diminished spread of the novel coronavirus with increasing relative humidity from 23.33% to 82.67% (p-value = 0.002) and with increased weather temperature from −13.17 °C to 19 °C (p-value = 0.003) observed in Chinese cities during January–March 2020 (Yao et al., 2020).
The protective effect of humoral immunity against human coronaviruses is still debated, and some evidence even points out a potential Antibody Dependent Enhancement (ADE) triggered by secondary infections featuring the severe/critical forms of COVID-19, as with other viral diseases such as Dengue, SARS-CoV-1, MERS-CoV and the West Nile Virus. (Cegolon et al. 2020). Given the potential of reversed and untoward consequences following relaxation of the above IPC measures, especially during cold months, and in absence of a specific registered treatment or vaccine against SARS-CoV-2, there is a clear and urgent need to find alternative solutions to prevent and control the replication of the virus and the spread of COVID-19 among humans (Yu et al., 2020).
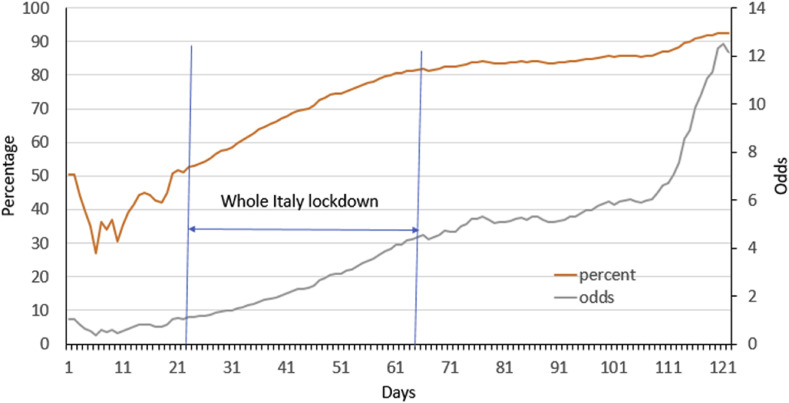
COVID-19 is a rapidly evolving pandemic. Asymptomatic individuals testing positive for SARS-CoV-2 and enforced to self-isolate at home were in Italy about 40% of all positive individuals at the beginning of the epidemic, then becoming >80% following the end of full lockdown (GEDI visual, 2020). Fig. 1 reports the corresponding changes as percentage or odds; the latter detects the improvement of the index score better than the former because it is able to overcome the ceiling effects.
Fig. 1.
Daily trend of asymptomatic persons who tested positive for SARS-Cov-2 and were enforced to home self-isolation in Italy, from March 1 to June 30, 2020. Prevalence is expressed as percentage (orange line and left vertical axis) or odds (grey line ad right vertical axis). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Therefore, in addition to an effective treatment for symptomatic patients, there is an urgent need to abate the carriage of SARS-CoV-2 in the human nasal cavity of asymptomatic/pre-symptomatic individuals, in order to contain the transmission of the novel coronavirus within the community.
1.2. Objectives
To find tolerable topical nasal disinfectants featured by encouraging efficacy against SARS-CoV-2, e.g. drugs effective against at least two different viruses beyond SARS-CoV-2, with similar viral/molecular structure.
2. Methods
PubMed was investigated using “Anti-Infective Agents, Local” and “Anti-Infective Agents, Nasal” as key words and applying the filters “Age: 19+ years”, “Humans” and “English” to identify papers published during 2010–2020. The 255 publications returned by the system were scrutinized by inspection of title and abstract. The majority of papers dealt with drugs against methicillin resistant Staphylococcus aureus (generally, mupirocin in nose associated with either chlorhexidine or hexachlorophene body wash). The pharmacological agents relevant for the present scoping review were: povidone-iodine solution (reported by 7 papers), alcohol-based nasal antiseptics (2 papers), quaternary ammonium compounds (1 paper) and N-chlorotaurine (1 paper). Furthermore, “nasal disinfection” was used as search term in three online repositories of preliminary not peer-reviewed reports. The treatments found were: povidone-iodine solution and interferon-alpha (2 papers from MedRxiv), hypothiocyanite (2 papers, of which 1 from SSRN) and alcohol-based nasal antiseptics (1 paper from arXiv).
Every cited treatment was used as key term to find additional information from different electronic databases. The abstracts of the original articles were explored for the following terms: mechanism(s) of action, tolerability and any evidence of toxic effects or selection of resistant strains, whether the treatment was tested in vitro (in particular against SARS-CoV-2), or reached the clinical trials stage, or is currently marketed/promoted/sold.
3. Results
8 treatments were scrutinized: hypothiocyanite (OSCN−), lactoferrin (LF), N-chlorotaurine (NCT), interferon-alpha (IFN-α), polyvinylpyrrolidone-iodine (PVP–I), hydroxychloroquine (HCQ), quaternary ammonium compounds and alcohol-based nasal antiseptics.
3.1. Hypothiocyanite
The lactoperoxidase (LPO) is a heme peroxidase present in several exocrine secretions, including the interface of human airways epithelium, which produces different anti-infectious agents in presence of hydrogen peroxide (H2O2), principally from halide (the iodide anion I−) and pseudo-halide (thiocyanate, also known as anion SCN−) substrates (Bafort et al., 2014). Both SCN− and I− act as one-electron donors, H2O2 works as a relatively specific electron acceptor, whereas LPO is the catalyzing enzyme. By undergoing two oxidations, the native LPO donates two electrons to H2O2, which is reduced to water. At that point, LPO is reduced back to its native state. Whether the required biochemical/environmental conditions are satisfied, LPO produces the active antimicrobial molecules OSCN− (hypothiocyanite) or OI− (hypoiodite) in presence of SCN− or I− respectively (Bafort et al., 2014).
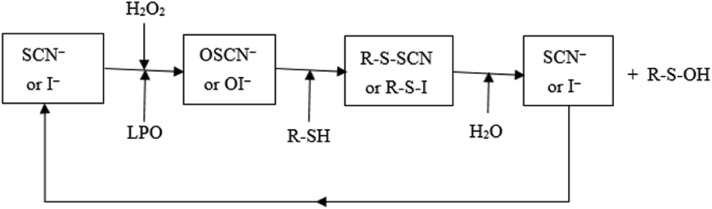
In Fig. 2 , R–SH is a peptide or protein with a thiol moiety essential for the activity of numerous enzymes and proteins. Sulphydryl oxidation of R–SH by OSCN− or OI− generates sulfenyl thiocyanate (R-S-SCN) or sulfenyl iodide (R-S-I), determining inhibition of bacterial glycolysis, respiration and glucose transport. Inhibition of the pentose phosphate pathway was also observed only for OI−, with sulfenic acid (R-S-OH) as well as SCN− or I− formed at the end of the cycle. Either SCN− or I− react with the native LPO and the cycle restarts. The anti-microbial activity of the entire system (enzyme plus substrates) is known to be more effective than hypothiocyanite or hypoiodite alone and has been explained by the production of short-lived, highly reactive intermediates (Bafort et al., 2014). Furthermore, the activity of the I− peroxidase system is more effective against E. coli than the SCN− system, in that lower I− concentrations are necessary (Bafort et al, 2014).. The I− peroxidase system deserves to be tested in vitro against the new coronavirus.
Fig. 2.
Lactoperoxidase cycle [from Bafort et al., 2014), modified]: SCN−, thiocyanate; I−, iodide; H2O2, hydrogen peroxide; LPO, lactoperoxidase; OSCN−, hypothiocyanite; OI− hypoiodite; R–SH, peptide or protein with a thiol moiety; R-S-SCN or R-S-I, sulfenyl thiocyanate or sulfenyl iodide; and R–SOH, sulfenic acid.
Additionally, OSCN− seems able to alter the surface proteins of different respiratory viruses, probably by oxidizing free thiol radicals and creating disulfide bonds (Alaxia, 2020), thus contrasting the binding of viruses with the human airways mucosae. OSCN− may also arguably hamper the synthesis and assemblance of viral proteins and nucleic acids, thus interfering with the release of viruses from infected cells (Day, 2020; Cegolon et al., 2014; Cegolon, 2020).
The products of the LPO extracellular oxidative complex is part of the human natural protective system of central airways against pathogen threats (Day, 2020; Alaxia, 2020; Cegolon et al., 2014; Cegolon, 2020). Due to the need of maximizing gas exchange, alveolar epithelial cells cannot contain strong protective structures. The latter cells are fragile and vulnerable to infectious agents, as shown by the diffuse alveolar damage discovered in two patients undergoing surgical resections for lung adenocarcinoma, later discovered to be affected also by COVID-19 pneumonia (Tian et al., 2020). Bronchi were not reported to be as vulnerable as alveoli in the same study (Tian et al., 2020).
The lack of LPO/H2O2/SCN− system in nasal and eye secretions of humans may explain the survival and proliferation of bacteria and respiratory viruses in the mucosae of conjunctiva and nose and their subsequent shedding in the environment (Tenovuo et al., 1986; Marcozzi et al., 2003; Mastrangelo et al., 2005, 2009; Schaffer et al., 1976; Couch, 1995). At micromolar concentration, the reactive mixture LPO/H2O2/OSCN− proved cidal activity against a range of bacteria (Gram positive and negative), fungi (Candida albicans and Candida krusei) and viruses as HIV, herpes-simplex virus (HSV-1), adenovirus, echovirus, respiratory syncytial virus (RSV) and influenza virus (Moskwa et al., 2007; Conner et al., 2007; Carlsson et al., 1984; Thomas and Aune, 1978; Ihalin et al., 1998; Reiter et al., 1976; Patel et al., 2018; Lenander-Lumikari, 1992; Mikola et al., 1995; Pourtois et al., 1990; Gingerich et al., 2016).
In a recent experiment in-vitro, OSCN− produced by the oxidase/LPO/H2O2/thiocyanate system rapidly and effectively inactivated A/swine/Illinois/02860/09 (swH1N2) influenza A virions, successfully preventing the infection of both primary human and male Sprague-Dawley rat trachea-bronchial epithelial cells (Gingerich et al., 2016).
In a more recent in-vitro cell-free experiment, all 12 different strains of influenza A and B viruses (the major circulating serotypes and species causing epidemics) were effectively inactivated by the LPO/H2O2/(SCN−/I−) system (Patel et al., 2018). Considering the strain-independent effect, the authors of the latter study encouraged a pharmaceutical application of the LPO/H2O2/(SCN−/I−) system in vivo to contribute to the clearance of influenza virus (Patel et al., 2018).
Another laboratory experiment challenged enzyme free OSCN− against A/H1N1 2009 pandemic influenza virus in-vitro, showing an evident dose-dependent viricidal activity, without cell toxicity (Cegolon et al., 2014). Considering a demonstrated wide spectrum cidal effect (Cegolon and Mastrangelo, 2020; Patel et al., 2018), not targeting specific proteins, it could be reasonably argued that OSCN− may be effective also against SARS-CoV-2 and would therefore deserved to be tested in vitro (Cegolon et al., 2014; Cegolon, 2020). Since it is already naturally present in human airways secretion and considering its large spectrum of action, a low level of resistance due to viral mutations and limited adverse reactions can be predicted with OSCN− (Cegolon and Mastrangelo, 2020; Cegolon et al., 2014; Cegolon, 2020). If effective in vitro against SARS-CoV-2, in vivo aerosol trials with enzyme free OSCN− could be subsequently undertaken with the aim of providing a short term-prophylaxis for the transmissibility of SARS-CoV-2, by clearing any individual COVID-19 positive at real time PCR.
OSCN−, alone or in combination with lactoferrin (LF), is already being tested in a phase 1 clinical trial (RCT02598999) on healthy volunteers and patients affected by cystic fibrosis and bronchiectasis (Alaxia, 2020).
The LPO/H2O2/SCN− system is also a commercially available non-prescription product (Zendium) used as toothpaste and mouthwash.
3.2. Lactoferrin
Lactoferrin (LF) is a natural multifunctional protein belonging to the family of transferrin. It is present in the human milk and can be found in external secretions such as saliva, tears, milk, nasal and bronchial secretions, gastrointestinal fluids and urine mucosal secretions. LF is an important constituent of the neutrophilic granules of leukocytes (Farnaud and Evans, 2003).
Due to its similarities to transferrin, LF has iron binding capabilities; its iron is not released even at pH 3.5. This property ensures iron sequestration in infected tissues, where the pH is typically acidic, preventing the utilization of iron by pathogenic bacteria (Kell et al., 2020).
LF is a constituent of the human innate immune response and during viral infections the expression of genes encoding LF was found to be elevated by approximately 150 fold in SARS-CoV-1 patients compared with healthy controls (Reghunathan et al., 2005). LF possesses strong antiviral activity against a broad spectrum of RNA and DNA viruses, such as HIV, Zika virus, Chikungunya, hepatitis C virus, cytomegalovirus, rotavirus, among others (Martorell et al., 2016; Serrano et al., 2020; Carvalho et al., 2016; Berlutti et al., 2011; Wakabayashi et al., 2004; Wang et al., 2018; Wrapp et al., 2020; Hofmann and Pohlmann, 2004).
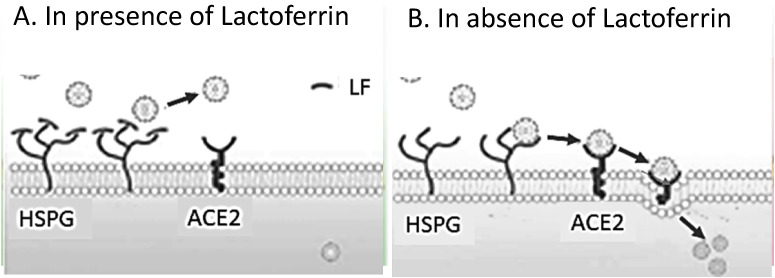
The antiviral activity of LF takes place particularly when the virus attacks the host cell, since LF prevents the virus from anchoring and then entering the host cell (Lang et al., 2011). It has been reported that LF binds to heparan sulfate proteoglycans (HSPGs), which are cell-surface and extracellular matrix macromolecules that are composed of a core protein decorated with covalently linked glycosaminoglycan chains (Kell et al., 2020). HSPGs could serve as preliminary docking sites on the host cell membrane and play an important role in the process of entry of SARS-CoV-1 into the cell (Lang et al., 2011). As shown in Fig. 3 , LF blocks the infection of SARS-CoV-1 by competing with the virus for HSPGs (Lang et al., 2011). This mechanism of action could prevent the viral concentration on the cell surface, as well as to the specific entry receptors (ACE2). It is still not presently known whether LF binds to ACE2 though (Lang et al., 2011). LF may likely inhibit SARS-CoV-2 invasion at micromolar concentrations and in a dose dependent fashion, just as for SARS-CoV-1 (Lang et al., 2011). However, there is no current confirmed evidence that SARS-CoV-2 binds to HSPGs, and whether SARS-CoV-2 also enters host cells via HPSGs in the same way as SARS-CoV-1 clearly warrants further investigation.
Fig. 3.
Protective role of Lactoferrin (LF) in SARS-CoV infection. (A) LF blocks the infection of SARS-CoV by binding to cell surface heparan sulfate proteoglycans (HSPGs). (B) In absence of LF, the anchoring sites provided by HSPGs allow the initial contact between SARS-CoV and host cells. SARS-CoV scans for specific entry receptors, which leads to subsequent cell entry. [From Lang et al., 2011), modified].
LF is available as an oral supplement and is widely used as a nutritional additive for infants at doses ranging from 100 mg to 4.5g a day for various indications without apparent toxicities (Peroni, 2020). There is a list of 24 commercial products containing LF and available by the American corporation Amazon.
A randomized, open-label, parallel-group clinical trial was conducted to examine the effectiveness of sucking tablets containing LF and LPO (LF + LPO) in alleviating symptoms of common cold and/or influenza infection. Treatment and non-treatment groups (overall 407 subjects) were further classified into subgroups habitually wearing a face mask, washing their hands, or gargling (Shin et al., 2018). The incidence and duration of common cold, influenza infection and gastrointestinal symptoms was not statistically different between treatment and non-treatment groups. (LF + LPO) tablets were moderately effective in significantly reducing the duration of fever higher than 38 °C in the subgroup that did not wear a protective face mask (Shin et al., 2018).
LF in its free form is degraded within the stomach by the action of hydrochloric acid and hydrolytic enzymes. Newer formulations of LF including encapsulation and liposomalization have been explored. Another interesting observation is that zinc saturated lactoferrin can apparently exert a more potent antiviral effect. This is of particular relevance in COVID-19 as zinc supplementation has been proposed as a possible therapeutic intervention for the disease (Zhang and Liu, 2020). An oral supplement combining a liposomal bovine lactoferrin (LLF) syrup (32 mg of LF/10 ml plus 12 mg of ascorbic acid) and a Zinc solution 10 mg/10 ml 2–3 times a day and 4–6 times/day was administered for 10 days to 75 symptomatic COVID-19 patients tested positive for IgM/IgG rapid test and self-isolated at home. A control group was treated only with LLF. All patients, who were followed up for one month, resolved their symptoms within 4-5- days since treatment inception and a half dose of LLF on 256 household contacts was effective to prevent SARS-CoV-2 infection (Serrano et al., 2020). Last but not least, out of seven authors, six worked for Sesderma Laboratories, the producer of all treatments used in the trial (Serrano et al., 2020).
As mentioned above, LF alone or in combination with OSCN− is already being tested in a phase 1 clinical trial (RCT02598999) on healthy volunteers and patients affected by cystic fibrosis (Alaxia, 2020; Cegolon, 2020).
3.3. N-chlorotaurine
N-chlorotaurine (NCT) is a natural oxidant part of the human defense system which is produced in the body (HOCl + taurine → NCT + H2O) by the strongly toxic hypochlorous acid and the amino-acid taurine (Gottardi and Nagl, 2010). Recently NCT was obtained by synthesis and large quantities were available. This compound revealed an optimal compromise between sufficient disinfecting power and good tissue tolerability. NCT can be stored long-term at low temperatures, and has killing activity against bacteria, fungi, parasites and viruses (Herpes simplex virus type 1; Herpes simplex virus type 2; Adenovirus; Influenza virus A and HIV-1). NCT can be applied to sensitive body regions as an endogenous antiseptic. Tolerability was very good in the eye, skin, mucous membranes and paranasal sinuses. The ciliary beat frequency of epithelial cells of the nasal mucosa, a very sensitive parameter for toxicity, was decreased only moderately and reversibly by 1% NCT (Nagl et al., 2018). A special field of application of NCT is inhalation for treatment of various infections. A phase I double-blind randomized clinical study with test group (inhalation of 1% NCT) and a parallel control group (0.9% NaCl as placebo) was carried out in two Austrian centers. Among treated subjects the forced expiratory volume in the first second (FEV1) was not reduced and blood analyses showed no abnormalities compared to the baseline values and to the control arm (Nagl et al., 2018). Overall, there is no evidence of efficacy of NCT to clear the nasopharynx from SARS-CoV-2.
3.4. Interferon-alpha
The interferon, discovered by virologists in 1957, is part of the human anti-viral innate defenses and plays a critical role in anti-viral immunity, interfering with the viral replication and spread with different mechanisms, including inhibition of the cell metabolism and downregulating the secretion of cytokines which stimulate the adaptive immunity (Jakimovski et al., 2018). An in vitro investigation confirmed the efficacy of IFN-α against SARS-like coronavirus infections (Zorzitto et al., 2006). Animal tests have confirmed that IFN-α nasal spray can effectively block or reduce SARS-CoV-1 infection-related damage in monkeys (Enserink, 2004; Gao et al., 2005).
SARS-CoV-2 can inhibit the endogenous secretion of IFN by host cells, thus in turn reducing their ability to suppress viral infections (Cegolon et al, 2020). Therefore, the use of exogenous IFN is critical with the severe form of COVID-19 (Zhou et al., 2020). A recent open-label prospective study tested nasal drops of recombinant human interferon alpha (rhIFN-α) on 2944 health care workers in China during SARS-CoV-2 epidemics as follows (Meng et al., 2020):
-
•
2415 subjects (997 doctors and 1418 nurses with average ages of 37.38 and 33.56 years respectively) included in a “low-risk group” were administered 2–3 drops/nostril/time of rhIFN-α, 4 times/day for 28 days.
-
•
529 subjects (122 doctors and 407 nurses with average ages of 35.24 and 32.16 years respectively) were included in a “high-risk group” and administered 2–3 drops/nostril/time of rhIFN-α, 4 times/day for 28 days, in addition to thymosin-α 11.6 mg hypodermic injection once a week.
At day 28 no study subject was positive for COVID-19 and nobody developed respiratory symptoms (Meng et al., 2020). The latter investigation was not a clustered, randomized study though. The control group used was composed by medical staff affected by COVID-19 pneumonia and recruited from the epidemic area during the same period reported in the literature, rather than a strictly parallel, placebo-controlled group. In addition, the paper in its current form is a preliminary report which has not been certified by peer review.
Although IFN is seemingly effective to disinfect the upper airways from COVID-19, its use may be more appropriate for front-line individuals such as health care staff, especially considering its likely high cost. Since the protocol entails also the injection of thymosin-α 11.6 mg once a week, IFN does not seem of practical use in the general population.
3.5. Polyvinylpyrrolidone-iodine
Polyvinylpyrrolidone polymer with iodine (PVP–I), also known as povidone iodine, was discovered and marketed as disinfectant since 1955.
The air-liquid interface of human nasal epithelial cells cultures collected from patients affected by chronic rhinosinusitis were recently exposed in vitro to a 0.5% solution of PVP-I (Nasodine® licensed by Firebrick Pharma) (Ramezanpour et al., 2020). No cell toxicity was observed on paracellular permeability or cilia beat frequency. The trans epithelial electrical resistance of cultured cells was significantly reduced only after 30 minutes exposure to Nasodine® (Meng et al., 2020). Consequently, PVP-I could be considered as a safe therapy when used as a mouthwash or taken nasally or used during ophthalmic surgeries.
The viricidal activity in-vitro of topical and oral PVP‐I products against SARS‐CoV‐2 was recently reported (Bidra et al., 2020). All four products (antiseptic solution, PVP-I at 10%; skin cleanser, PVP-I at 7.5%; gargle and mouth wash, PVP-I at 1%; and throat spray, PVP-I at 0.45%) achieved ≥ 99.99% viricidal activity against SARS-CoV-2 within 30 seconds of being in contact with the virus (Bidra et al., 2020). The use of 0.5% PVP-I was proposed among healthcare workers and their patients to minimize the risk of the spread of COVID-19 in addition to the recommended IPC measures and PPE. A mouthwash or nasal spray containing PVP-I may be an effective strategy both to combat the virus at its point of entry and to reduce SARS-CoV-2 transmission through its droplet emission from the mouth
A phase III clinical trial (ACTRN12619000764134) is ongoing to assess the safety and efficacy of povidone-iodine nasal spray (Nasodine®) in the treatment of subjects affected by common cold, potentially caused by human coronaviruses (Firebrick Pharma Pty Ltd., 1261). PVP-I awaits clinical trial data confirming an effective activity of drug against SARS-CoV-2.
Although it is an effective antiseptic, PVP-I may not be optimal for pregnant women and children though. Hypothyroidism has in fact been reported with povidone-iodine antiseptics in neonates. Furthermore, transmission of SARS-COV-2 to breastfeeding infants through close contact with an asymtpomatic mother has been reported (Jafari et al., 2020). Transient hyper-thyrotropinemia can occur in neonates whose mothers had been exposed to povidone-iodine as a skin disinfectant during and after labour (Casteels et al., 2000).
3.6. Hydroxychloroquine
Hydroxychloroquine (HCQ), a less toxic derivative of chloroquine (CQ), at micromolar concentration proved anti-viral activity against SARS-CoV-2 in vitro (Liu et al., 2020), and its mechanism of action entails the increase of pH of intracellular organelles, such as endosomes/lysosomes, essential for membrane fusion (Ashfaq et al., 2011). In addition, CQ could inhibit SARS-CoV-1 entry through changing the glycosylation of ACE2 receptor and the spike protein (Savarino et al., 2006). Along with the derivative HCQ, CQ has even entered multiple clinical trials. The evidence on the efficacy of HCQ to clear the nasopharynx from SARS-CoV-2 is relatively low, being founded only on one small open-label non-randomized clinical trial, which did not confirm clinical improvement of COVID-19 patients though (Gautret et al., 2020). Furthermore, HCQ is featured by risk of side effects in terms of QTc prolongation and haemolysis associated with deficiency of glucose-6-phosphate dehydrogenase (Tilangi et al., 2020). On this note, the Mayo clinic recommended baseline electrocardiogram monitoring before commencing treatment of COVID-19 patients with HCQ (Giudicessi et al., 2020). The efficacy of HCQ, also in combination with azithromycin, has been questioned too, since it was not found to be associated with a significantly reduced mortality among 1428 randomly sampled COVID-9 patients admitted to 25 hospitals of the New York City Metropolitan region (Rosenberg et al., 2020).
Lastly, nasal antimicrobials such as HCQ are typically not used on a regular basis to reduce the subclinical colonization of micro-organisms in humans because this strategy might lead to increased development of resistant strains of bacteria/viruses.
3.7. Alcohol-based nasal antiseptics
SARS-CoV-2, SARS-CoV-1 and MERS-CoV are lipophilic enveloped viruses relatively easy to inactivate by exposure to alcohols. Ethanol 62–71% is among the reagents already proven effective to disinfect fomites from SARS-CoV-1 and MERS-CoV within 1 minute (Kampf et al., 2020). Sanitizers with at least 60% ethanol are recommended by the USA Centre for Disease Control and Prevention (CDC) for hand hygiene of health care staff against SARS-CoV-2 and (if foam/water are not available) the general public too (CDC, 2020). In a recent study ethanol at concentration >30% efficiently inactivated SARS-CoV-2 in suspension in 30 seconds (Kratzel et al, 2020). A further study confirmed that two commercial formulated alcohol-based hand sanitizers (a gel and a foam both with a 70% concentration of ethanol) marketed in the USA achieved the abatement of SARS-CoV-2 in suspension below detectable thresholds (>3 log10 reduction) (Leslie et al, 2020). Saline nasal irrigations and ethanol oral rinses have been suggested as potential topical measures for the prevention and control of COVID-19 (Casale et al., 2020).
Two studies tested the effectiveness of alcohol-based antiseptics in reducing nasal bacterial carriage in health care professionals at an urban hospital center (Steed et al., 2014) or among colonized patients (Kanwar et al., 2019), providing conflicting results though. A commercially available, non-prescription product, Nozin Nasal Sanitizer antiseptic was used as test agent in both latter studies. The safety-tested formulation was composed of ethanol active combined with a mixture of natural oil emollients and the preservative benzalkonium chloride. Sterile phosphate-buffered saline with 0.017% peppermint oil as a masking agent was used as placebo treatment control. Whilst 3 nasal applications of the latter alcohol based antiseptic over 8 hours significantly reduced the nasal carriage of S. aureus in health care staff (Steed et al., 2014), the respective reduction among colonized patients was only transient, becoming non significant at 8 hours (Kanwar et al., 2019). Shintake recently proposed a controlled inhalation of ethanol vapor obtained from readily available alcoholic beverages (whisky or Japanese sake), to disinfect the human airways from SARS-CoV-2 (Shintake, 2020). Clinical research is recommended to investigate the potential effect of alcohol-based nasal antiseptics against SARS-CoV-2, evaluating also their tolerability, especially among vulnerable groups as pregnancy women and children.
3.8. Quaternary ammonium compounds
Quaternary ammonium compounds (QACs) are active ingredients in over 200 disinfectants currently recommended by the USA Environmental Protection Agency (EPA) to inactivate SARS-CoV-2. The amounts of QACs used in household settings (including detergents for personal care, soaps and liquid hand washes), workplace and industry settings has likely increased a lot, and their usage will continue to be elevated considering the trend of the COVID-19 pandemic (Hora et al., 2020).
Inactivation of SARS-CoV-2 by formulated detergents is believed to occur as a result of disruption of the virally modified, host-cell-derived, phospholipid bilayer glycoproteinaceous envelope, and the associated spike glycoproteins interacting with the ACE2 receptor in the infection of host cells. Another mechanism of action entails raising the endocytic and lysosomal pH (Hora et al., 2020).
Using a text mining approach, Baker (Baker et al., 2020) identified the following three classes of QACs having a possible antiviral activity against coronaviruses.
-
•
Ammonium chloride. Various uses, including metabolic acidosis. Viral activity against murine coronavirus and hepatitis C.
-
•
Cetylpyridinium chloride. Used as antiseptic, mouthwash, personal care products, cleaning agents etc. This product, also being used as an antimicrobial agent for meat and poultry products was tested in a clinical trial as a treatment against respiratory infections (Mukherjee et al., 2017). Cetylpyridinium chloride has anti-viral activity against Influenza, hepatitis B, poliovirus 1.
-
•
Miramistin. Antiseptic with antiviral activity against HIV, influenza, herpes simplex virus and SARS-CoV-1.
QACs such as cetylpyridinium chloride and miramistin have not been tested yet against SARS-CoV-2 in vitro or in clinical trials.
Although the clear and severe threat posed by COVID-19 prompts a massive use of QACs as a reasonable strategy to mitigate and contain the spread of the infection, the potential environmental impact of QAC, which may include disruption of wastewater treatment unit operations, proliferation of antibiotic resistance, formation of nitrosamine disinfection byproducts, and negative effects on biota of surface waters, should also be considered. Exploration of potential technologies to minimize the environmental releases of QACs is highly warranted (Hora et al., 2020).
4. Conclusions
The in vitro viricidal effect against SARS-CoV-2 has been described for ethanol, alcohol-based hand sanitizers and povidone-iodine only so far. In vitro inhibition of other coronaviruses was reported for LF, HCQ, ethanol and AQCs. No treatment was tested against SARS-CoV-2 in randomized controlled clinical trials. The lack of effective and safe drugs against SARS-CoV-2 implies primarily that the relevant literature on this topic does not exist.
Most drug candidates are already in the market. As pointed out by Baker et al. “As simple as it sounds, it is entirely possible that we should be looking in our bathroom cupboards for potential remedies against COVID-19” (Baker et al., 2020).
Whilst the nasal cavity is the main port of entry for SARS-CoV-2, the mouth is also relevant as exit site of the virus through emission of saliva droplets. The well-known hand-to-nose-to-hand cycle of contamination requires appropriate additional methods for infection control.
The present scoping review identified knowledge gaps more than available evidence on the topic of chemoprevention of COVID-19. Since speed is essential in responding to the COVID-19 pandemic (Hurley, 2020), undertaking clinical trials on all the candidate agents identified could be too demanding and would require too much time.
Taking everything into account, these data suggest that the search for convenient non-antibiotic drug(s) could be pursued with a case-control study, comparing the use of candidate drugs among individuals positive and negatives to coronavirus swab tests. The results of such study could help in narrowing down the subsequent laboratory and clinical investigations.
Funding
None.
Author's contributions
LC and GM conceived the idea, participated in the review process and drafted the paper; MJ contributed to draft the paper.
Declaration of competing interest
None to declare.
References
- ECDC Guidance for health system contingency planning during widespread transmission of SARS-CoV-2 with high impact on healthcare services. 2020. https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-guidance-health-systems-contingency-planning.pdf Available from:
- Alaxia ALX – 009. https://www.alaxia-pharma.eu/alx-009/ Available from:
- Arons M.M., Hatfield K.M., Reddy S.C. Pre-symptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N. Engl. J. Med. 2020 May 28;382(22):2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashfaq U.A., Javed T., Rehman S., Nawaz Z., Riazuddin S. Lysosomotropic agents as HCV entry inhibitors. Virol. J. 2011;8:163. doi: 10.1186/1743-422X-8-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bafort F., Parisi O., Perraudin J.P., Jijakli M.H. Mode of action of lactoperoxidase as related to its antimicrobial activity: a review. Enzym. Res. 2014;2014:517164. doi: 10.1155/2014/517164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. https://www.ncbi.nlm.nih.gov/pubmed/32083643 Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker N., Williams A.J., Tropsha A., Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm. Res. (N. Y.) 2020;37:104. doi: 10.1007/s11095-020-02842-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balilla J. SSRN; 2020. Assessment of COVID-19 Mass Testing: the Case of South Korea. [Google Scholar]
- Berlutti F., Pantanella F., Natalizi T., Frioni A., Paesano R., Polimeni A. Antiviral properties of lactoferrin-A natural immunity molecule. Molecules. 2011;16:6992–7018. doi: 10.3390/molecules16086992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidra A.S., Pelletier J.S., Westover J.B., Frank S., Brown S.M., Tessema B. Rapid in‐vitro inactivation of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) using povidone‐iodine oral antiseptic rinse. J. Prosthodont. 2020;29(6):529–553. doi: 10.1111/jopr.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Edlund M.B.K., Hanstrom L. Bactericidal and cytotoxic effects of hypothiocyanite-hydrogen peroxide mixtures. Infect. Immun. 1984;44:581–586. doi: 10.1128/iai.44.3.581-586.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A.M., Casseb S., Gonçalves R.B. Bovine lactoferrin activity against Chikungunya and Zika viruses. J. Gen. Virol. 2016;98(7):1749–1775. doi: 10.1099/jgv.0.000849. 20. [DOI] [PubMed] [Google Scholar]
- Casale M., Rinaldi V., Sabatino L., Moffa A., Ciccozzi M. Could nasal irrigation and oral rinse reduce the risk for COVID-19 infection? Int. J. Immunopathol. Pharmacol. 2020 Jan–Dec;34 doi: 10.1177/2058738420941757. 2058738420941757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels K., Pünt S., Brämswig J. Transient neonatal hypothyroidism during breastfeeding after post-natal maternal topical iodine treatment. Eur. J. Pediatr. 2000;159(9):716. doi: 10.1007/s004310000496. [DOI] [PubMed] [Google Scholar]
- CDC Coronavirus Disease 2019 (COVID-19):How to Protect Yourself & Others. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19) 2020 https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/prevention.html [Google Scholar]
- Cegolon L. Investigating hypothiocyanite against SARS-CoV-2. Int. J. Hyg Environ. Health. 2020;227:113520. doi: 10.1016/j.ijheh.2020.113520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegolon L., Mastrangelo G. April 18, 2020. Hypothiocyanite for the Prevention and Control of COVID-19.https://ssrn.com/abstract=3579762 Available at: SSRN: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegolon L., Salata C., Piccoli E., Juarez V., Palu G., Mastrangelo G. In vitro antiviral activity of hypothiocyanite against A/H1N1/2009 pandemic influenza virus. Int. J. Hyg Environ. Health. 2014;217(1):17–22. doi: 10.1016/j.ijheh.2013.03.001. [DOI] [PubMed] [Google Scholar]
- Cegolon L., Pichierri J., Mastrangelo G., Cinquetti S., Sotgiu G., Bellizzi S., Pichierri G. Hypothesis to explain the severe form of the disease. Glob. Health. 2020;5(6) doi: 10.1136/bmjgh-2020-002564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner G.E., Wijkstrom-Frei C., Randell S.H., Fernandez V.E., Salathe M. The lactoperoxidase system links anion transport to host defense in cystic fibrosis. FEBS Lett. 2007;581:271–278. doi: 10.1016/j.febslet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couch R.B. University of Texas Medical Branch; Galveston: 1995. Medical Microbiology; pp. 1–22. [Google Scholar]
- Day M. Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ. 2020 Apr 2;369:m1375. doi: 10.1136/bmj.m1375. [DOI] [PubMed] [Google Scholar]
- Dolan J., Hamilton M. Los Angeles Times; April 7, 2020. Consider Pulling Residents from Nursing Homes over Coronavirus, Says County Health Director.https://www.latimes.com/california/story/2020-04-07/coronavirus-nursing-homes-residents-remove-la-county [Google Scholar]
- Enserink M. SARS treatment: interferon shows promise in monkeys. Science. 2004;303(5662):1273a–1275. doi: 10.1126/science.303.5662.1273a. [DOI] [PubMed] [Google Scholar]
- Farnaud S., Evans R.W. Lactoferrin—a multifunctional protein with antimicrobial properties. Mol. Immunol. 2003;40:395–405. doi: 10.1016/s0161-5890(03)00152-4. [DOI] [PubMed] [Google Scholar]
- Firebrick Pharma Pty Ltd Trial Registration ACTRN12619000764134. Australian-New Zealand Clinical Trial Registry. https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=377353 Available from:
- Gandhi M., M D., Yokoe D.S., Havlir D.V. Asymptomatic transmission, the achilles’ heel of current strategies to control covid-19. NEJM. Engl J Med. 2020 Apr 24;382(22):2158–2160. doi: 10.1056/NEJMe2009758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Zhang L.L., Wei Q. Preventive and therapeutic effects of recombinant IFN-alpha2b nasal spray for SARS-CoV infection in Macaca mulata. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 2005;19(3):207–210. [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open- label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;2020 doi: 10.1016/j.ijantimicag.2020.105949. published online March 20. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gedi visual Coronavirus - the Situation in Italy. https://lab.gedidigital.it/gedi-visual/2020/coronavirus-i-contagi-in-italia/ Available from:
- Gingerich A., Pang L., Hanson J., Dlugolenski D., Streich R., Lafontaine E.R., Nagy T., Tripp R.A., Rada B. Hypothiocyanite produced by human and rat respiratory epithelial cells inactivates extracellular H1N2 influenza A virus. Inflamm. Res. 2016;65:71–80. doi: 10.1007/s00011-015-0892-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin. Proc. 2020 doi: 10.1016/j.mayocp.2020.03.024. published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorse G.J., Donovan M.M., Patel G.B. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. J. Med. Virol. 2020:1–6. doi: 10.1002/jmv.25715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi W., Nagl M. N-chlorotaurine, a natural antiseptic with outstanding tolerability. J. Antimicrob. Chemother. 2010 Mar;65(3):399–409. doi: 10.1093/jac/dkp466. [DOI] [PubMed] [Google Scholar]
- Heymann D.L., Shindo N. COVID-19: what is next for public health? Lancet. 2020;395(10224):542–545. doi: 10.1016/S0140-6736(20)30374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Pohlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hora P., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020 doi: 10.1021/acs.estlett.0c00437. https://pubs.acs.org/doi/abs/10.1021/acs.estlett.0c00437 Available from: (last accessed on 24th August 2020) [DOI] [PubMed] [Google Scholar]
- Hurley R. Moving quickly in pandemics. BMJ. 2020;370:m2730. [Google Scholar]
- Ihalin R., Loimaranta V., Lenander-Lumikari M., Tenovuo J. The effects of different (pseudo) halide substrates on peroxidase-mediated killing of Actinobacillus actinomycetemcomitans. J. Periodontal. Res. 1998;33:421–427. doi: 10.1111/j.1600-0765.1998.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Jafari R, Cegolon L, Torkaman M, Kashaki M, Dehghanpoor F, Cheraghalipoor F, Javanbakht M. A 6 months old infant with fever, dyspnea and poor feeding, diagnosed with COVID-19. Travel Medicine and Infectious Disease. 2020;36(101789) doi: 10.1016/j.tmaid.2020.101789. In this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakimovski D., Kolb C., Ramanathan M., Zivadinov R., Weinstock-Guttman B. Interferon β for multiple sclerosis. Cold Spring Harb. Perspect. Med. 2018;8:1–20. doi: 10.1101/cshperspect.a032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. Persistence of coronaviruses on inanimate surfaces and their inactivation with biocidal agents. J. Hosp. Infect. 2020;104(3):246–251. doi: 10.1016/j.jhin.2020.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar A., Kumar J.A., Ng-Wong Y.K., Thakur M., Cadnum J.L., Alhmidi H., Mana T.S.C., Jencson A., a2), Donskey C.J. Evaluation of an alcohol-based antiseptic for nasal decolonization of methicillin-resistant Staphylococcus aureus in colonized patients. Infect. Control Hosp. Epidemiol. 2019 Dec;40(12):1436–1437. doi: 10.1017/ice.2019.266. Epub 2019 Sep 30. PMID: 31566147. [DOI] [PubMed] [Google Scholar]
- Kell D.B., Heyden E.L., Pretorius E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020;11:1221. doi: 10.3389/fimmu.2020.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzel A, Todt D, V’kovski P, Steiner S, Gultrom M. Inactivation of severe acute respiratory syndrome coronavirus 2 by WHO-Recommended Hand Rub Formulations and Alcohols. Emerg Infect Dis. 2020;26(7):1592–1595. doi: 10.3201/eid2607.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Eggo R.M. Invisible spread of SARS-CoV-2. Lancet. 2020;S1473 3099(20):30275–30279. doi: 10.1016/S1473-3099(20)30275-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang J., Yang N., Deng J., Liu K., Yang P. Inhibition of SARS pseudovirus cell entry by lactoferrin binding to heparan sulfate proteoglycans. PloS One. 2011;6(8) doi: 10.1371/journal.pone.0023710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenander-Lumikari M. Inhibition of Candida albicans by the peroxidase/SCN/H2O2 system. Oral Microbiol. Immunol. 1992;7:315–320. doi: 10.1111/j.1399-302x.1992.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Leslie Rachel, Zhou Steve, Macinga David. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Amerian Journal of Infection Control. 2020 doi: 10.1016/j.ajic.2020.08.020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcozzi G., Liberati V., Madia F., Pizzinga A., de Feo G. Effect of hormone replacement therapy on lacrimal fluid peroxidase activity in woman. Maturitas. 2003;45(3):225–229. doi: 10.1016/s0378-5122(03)00146-4. [DOI] [PubMed] [Google Scholar]
- Martorell P., Llopis S., Gonzalez N., Ramón D., Serrano G., Torrens A., Serrano J.M., Navarro M., Genovés S. A nutritional supplement containing lactoferrin stimulates the immune system, extends lifespan, and reduces amyloid β peptide toxicity in Caenorhabditis elegans. Sci Nutr. 2016 Jul 28;5(2):255–265. doi: 10.1002/fsn3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastrangelo G., Zanibellato R., Fedeli U., Fadda E., Lange J.H. Exposure to hydrogen peroxide at TLV level does not induce lung function changes: a longitudinal study. Int. J. Environ. Health Res. 2005;15:313–317. doi: 10.1080/09603120500156003. [DOI] [PubMed] [Google Scholar]
- Mastrangelo G., Zanibellato R., Fadda E., Lange J.H., Scoizzato L., Rylander R. Exposure to hydrogen peroxide and eye and nose symptoms among workers in a beverage processing plant. Ann. Occup. Hyg. 2009;53:161–165. doi: 10.1093/annhyg/men077. [DOI] [PubMed] [Google Scholar]
- Matson N.J., Kwe Yinda C., Seifert S.N., Bushmaker T., Fischer R.J., van Doremalen N., Lloyd-Smith J.O., Munster V.J. Effect of environmental conditions on SARS-CoV-2 stability in human nasal mucus and sputum. Emerg. Infect. Dis. 2020 Sep;26(9):2276–2278. doi: 10.3201/eid2609.202267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z., Wang T., Li C., Chen X., Li L., Qin X., Li H., Luo J. An experimental trial of recombinantX human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. MedRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.04.11.20061473v2 Available from: (last accessed on 24th August 2020) [Google Scholar]
- Mikola H., Waris M., Tenovuo J. Inhibition of herpes simplex virus type 1, respiratory syncytial virus and echovirus type 11 by peroxidase-generated hypothiocyanite. Antivir. Res. 1995;26:161–171. doi: 10.1016/0166-3542(94)00073-h. [DOI] [PubMed] [Google Scholar]
- Moskwa P., Lorentzen D., Excoffon K.J., Zabner J., McCray P.B., Jr., Nauseef W.M., Dupuy C., Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee P.K., Esper F., Buchheit K., Arters K., Adkins I., Ghannoum M.A. Randomized, double-blind, placebo controlled clinical trial to assess the safety and effectiveness of a nove dual-action oral topical formulation against upper respiratory infections. BMC Infect. Dis. 2017;17(1):74. doi: 10.1186/s12879-016-2177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagl M., Arnitz R., Lackner M. N-chlorotaurine, a promising future candidate for topical therapy of fungal infections. Mycopathologia. 2018;183(1):161–170. doi: 10.1007/s11046-017-0175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel U., Gingerich A., Widman L., Sarr D., Tripp R.A., Rada B. Susceptibility of influenza viruses to hypothiocyanite and hypoiodite produced by lactoperoxidase in a cell-free system. PloS One. 2018;13(7) doi: 10.1371/journal.pone.0199167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peroni D.G. Viral infections: lactoferrin, a further arrow in the quiver of prevention. Journal of Pediatric and Neonatal Individualized Medicine. 2020;9(1) [Google Scholar]
- Pourtois M., Binet C., Van Tieghem N., Courtois P., Vandenabbeele A., Thiry L. Inhibition of HIV infectivity by lactoperoxidase-produced hypothiocyanite. J. Biol. Buccale. 1990;18:251–253. [PubMed] [Google Scholar]
- Ramezanpour M., Smith J.L.P., Psaltis A.J., Wormald P.J., Vreugde S. In vitro safety evaluation of a povidone-iodine solution applied to human nasal epithelial cells. Int Forum Allergy Rhinol. 2020 Apr 6;2020 doi: 10.1002/alr.22575. ([Online ahead of print]) [DOI] [PubMed] [Google Scholar]
- Reghunathan R., Jayapal M., Hsu L., Chng H., Tai D. Expression profile of immune response genes in patients with Severe Acute Respiratory Syndrome. BMC Immunol. 2005;6:2. doi: 10.1186/1471-2172-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter B., Marshall V.M.E., Bjorck L., Rosen C.-G. Nonspecific bactericidal activity of the lactoperoxidase-thiocyanate hydrogen peroxide system of milk against Escherichia coli and some gram-negative pathogens. Infect. Immun. 1976;13:800–807. doi: 10.1128/iai.13.3.800-807.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., Udo T. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. J. Am. Med. Assoc. 2020;323(24):2493–2502. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Di Trani L., Donatelli I., Cauda R., Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect. Dis. 2006;6(2):67–69. doi: 10.1016/S1473-3099(06)70361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffer F.L., Soergel M.E., Straure D.C. Survival of airborne influenza virus: effects of propagating host, relative humidity, and composition of spray fluids. Arch. Virol. 1976;51:263–273. doi: 10.1007/BF01317930. [DOI] [PubMed] [Google Scholar]
- Serrano G., Kochergina J., Albors A., Diaz E., Oroval M., Hueso G., Serran J.M. Liposomial lactoferrin as potential preventive and cure for COVOD-19 - Int. J of Reasearch in Health Science. 2020;8(1):8–15. [Google Scholar]
- Shin K., Wakabayashi H., Sugita C., Yoshida H., Sato K., Sonoda T., Yamauchi K., Abe F., Kurokawa M. Effects of orally administered lactoferrin and lactoperoxidase on symptoms of the common cold. Int. J. Health Sci. 2018;12(5):44–50. [PMC free article] [PubMed] [Google Scholar]
- Shintake T. Possibility of Disinfection of SARS-CoV-2 (COVID-19) in Human Respiratory Tract by Controlled Ethanol Vapor Inhalation. ArXiv - Cornell University. https://arxiv.org/abs/2003.12444 Available from:
- Steed L.L., Costello J., Lohia S., Jones T., Spannhake E.W., Nguyen S. Reduction of nasal Staphylococcus aureus carriage in health care professionals by treatment with a nonantibiotic, alcohol-based nasal antiseptic. Am. J. Infect. Contr. 2014 Aug;42(8):841–846. doi: 10.1016/j.ajic.2014.04.008. [DOI] [PubMed] [Google Scholar]
- Tenovuo J., Lehtonen O.P., Aaltonen A.S., Vilja P., Tuohimaa P. Antimicrobial factors in whole saliva of human infants. Infect. Immun. 1986;51:49–53. doi: 10.1128/iai.51.1.49-53.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E.L., Aune T.M. Lactoperoxidase, peroxide, thiocyanate antimicrobial system: correlation of sulfhydryl oxidation with antimicrobial action. Infect. Immun. 1978;20:456–463. doi: 10.1128/iai.20.2.456-463.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilangi P., Desai D., Khan A., Soneja M. Hydroxychloroquine prophylaxis for high-risk COVID-19 contacts in India: a prudent approach. Lancet. 2020;S1473–3099(20):30430–30438. doi: 10.1016/S1473-3099(20)30430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi H., Kurokawa M., Shin K., Teraguchi S., Tamura Y., Shiraki K. Oral lactoferrin prevents body weight loss and increases cytokine responses during herpes simplex virus type 1 infection of mice. Biosci. Biotechnol. Biochem. 2004;68:537–544. doi: 10.1271/bbb.68.537. [DOI] [PubMed] [Google Scholar]
- Wang A., Duncan S.E., Lesser G.L. Effect of lactoferrin on taste and smell abnormalities induced by chemotherapy: a proteome analysis. Food Funct. 2018;9:4948–4958. doi: 10.1039/c8fo00813b. [DOI] [PubMed] [Google Scholar]
- Wang J., Tang K., Feng K. SSRN; 2020. High Temperature and High Humidity Reduce the Transmission of COVID-19. [Google Scholar]
- World Health Organization Laboratory testing for coronavirus disease (COVID-19) in suspected human cases- interim guidance. https://apps.who.int/iris/handle/10665/331501 Available from:
- World Health Organization Coronavirus Disease 2019 (CVOID-19) https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200219-sitrep-30-covid-19.pdf?sfvrsn=3346b04f_2 Situation reports - 30. Available from:
- Worldometers Coronavirus. https://www.worldometers.info/coronavirus/ Available from:
- Wrapp D., Wang N., Corbett K., Goldsmith J.A., Hsieh C.L., Abiona O. Cryo- EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao M., Zhang L., Ma J., Zhou L. On airborne transmission and control of SARS-Cov-2. Sci. Total Environ. 2020;731:139178. doi: 10.1016/j.scitotenv.2020.139178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P., Zhu J., Zhang Z., Han Y., Huang L. A familial cluster of infection associated with the 2019 novel coronavirus indicating potential person-to-person transmission during the incubation period. J. Infect. Dis. 2020;221(11):1757–1761. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systematic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q., Chen V., Shannon C.P., Wei X.S., Xiang X., Wang X., Wang Z.H., Tebbutt S.J., Kollmann T.R., Fish E.N. Interferon-α2b treatment for COVID-19. Front. Immunol. 2020 May 15;11:1061. doi: 10.3389/fimmu.2020.01061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzitto J., Galligan C.L., Ueng J.J., Fish E.N. Characterization of the antiviral effects of interferon-alpha against a SARS-like coronavirus infection in vitro. Cell Res. 2006;16(2):220–229. doi: 10.1038/sj.cr.7310030. [DOI] [PMC free article] [PubMed] [Google Scholar]