Abstract
Objective
Ovarian cancer (OC) is the leading cause of death among gynecological tumors; however, no effective treatment is currently available. Fucoidan, which is extracted from marine algae, has significant anti-cancer effects. The aim of this study was to determine the effects of fucoidan on the proliferation and apoptosis of OC cells through inhibition of the PI3K/Akt signaling pathway.
Methods
Human ovarian normal epithelial cells (IOSE80) and human OC cells (SKOV-3, A2780, OVCAR-3, TOV-112D, and Caov-3) were selected to verify the safety of fucoidan at various doses in SKOV-3 and Caov-3 cells as well as a xenograft mouse model using various molecular biology techniques.
Results
Fucoidan had no significant effect on normal ovarian epithelial cells, but had significantly inhibited the proliferation of OC cells, induced cell cycle arrest at the G0/G1 phase, increased the proportion of apoptotic cells and expression of pro-apoptotic proteins, and inhibited the expression of PI3K and phosphorylation of Akt, which could be partly rescued by IGF-1.
Conclusion
Fucoidan had anti-tumor effects both in vivo and in vitro via a mechanism that is related to the inhibition of the PI3K/Akt signaling pathway.
Keywords: ovarian cancer, fucoidan, cell cycle, apoptosis, PI3K/Akt
Introduction
Ovarian cancer (OC), endometrial cancer, and cervical cancer are the most common malignant tumors that affect the female genital organs. Although the incidence of OC is lower than that of the other two types, early clinical diagnosis remains difficult.1 In addition, about 70% of OC patients display peripheral tissue invasion at the time of diagnosis, which greatly increases the risk of mortality, Thus, OC ranks first in mortality among all gynecological tumors. At present, the main primary treatment for OC consist of surgery, radiotherapy, and chemotherapy.2 However, radical surgery deprives some women of their childbearing ability and seriously affects quality of life. At the same time, surgical resection while retaining one side of the appendix provides additional opportunities and risks for the further deterioration of tumors. Although some chemotherapy regimens can effectively improve the median survival of patients, the recurrence rate of OC remains high and multiple rounds of chemotherapy can also increase the pain and economic burden.3–5 Accordingly, the five-year survival rate of OC is only 46%.6 Therefore, the development of new therapeutic approaches as well as safe, effective, and economical drugs are urgently required.
Fucoidan is abundantly produced by marine algae and has many biological functions, including anti-tumor, anti-inflammatory, anti-oxidation effects, and the regulation of glucose metabolism.7–11 In animal experiments, Beppu and Peng confirmed that the repeated oral administration of high purity fucoidan at a maximum dose of 1000 mg/kg within 30 days caused no abnormal changes to the liver, kidney, spleen, or gonad tissues.12,13 At the same time, fucoidan was not associated with any genotoxic effects in mouse bone marrow cells.14,15 Hence, fucoidan is considered a safe, non-toxic, and natural product in mice.16 Studies have shown that the specific anti-cancer mechanisms of fucoidan include the induction of apoptosis, inhibition of proliferation, and enhancement of both the immune response and the sensitivity of tumors to chemotherapeutic drugs.7,10,17,18 Moreover, He and colleagues demonstrated that fucoidan could inhibit the invasion and migration, and promote the apoptosis of breast cancer MCF-7 cells.19 In addition, HT-29 colon cancer cell viability was inhibited by fucoidan, possibly due to the downregulation IGF-IR signaling through the IRS-1/PI3K/AKT pathway.20 As a significant clinical application, fucoidan has been found to improve the sensitivity of lung and breast cancer to chemotherapeutic drugs, which not only reduced the severity of related side effects, but also reduced the economic burden to patients.10,11 In addition, many studies have reported that fucoidan also plays an important anti-cancer role in other tumors, including prostate cancer, gastric cancer, liver cancer, and large B cell lymphoma.18,21–25 However, the effects of fucoidan have not been verified in OC.
Although the etiology remains unclear, heredity, environment, obesity, and endocrine factors are believed to promote the onset and progression of OC. The mechanism underlying the occurrence, development, and metastasis of OC cannot be fully explained by a single factor or gene. Moreover, the role of signal transduction pathways in the oncogenesis of OC has been an area of increased interest and challenges in clinical research. In fact, the study of epidermal growth factor receptor (EGFR) and its downstream PI3K/Akt signaling pathway has gradually become the focus of continuing research. Oh et al selected sic EGFR-amplified cancer cell lines to prove that fucoidan has the potential to increase or decrease the effects of certain anticancer drugs.26 And, studies have shown that EGFR expression is relatively high in 70–100% of OC tissues, thus, the EGFR can be used as an indicator of the degree of OC malignancy.27 Highly expressed EGFR activates the downstream PI3K/Akt signaling pathway, inhibits cellular apoptosis induced by multiple stimuli, promotes cell survival and proliferation, participates in angiogenesis, transmits integrin-mediated invasion signals, and plays an important role in the formation, proliferation, and metastasis of tumors.28–30 In related studies, fucoidan has been shown to play a biological role in different types of tumors through the inhibition of the PI3K/Akt signaling pathway.7,8,30–32 In addition, fucoidan had been proved to induce cell death in female cancer cell lines including OC cells.17,33 Therefore, we inferred that fucoidan may play an inhibitory role in the physiological processes of proliferation and apoptosis mediated by the PI3K/Akt signaling pathway in OC cells.
Therefore, the aim of the present study was to evaluate the effects of fucoidan on the proliferation and apoptosis of OC cells through inhibition of the PI3K/Akt signaling pathway using a series of molecular biological methods. It is expected that this study will provide effective targets and strong evidence for the screening of clinical therapeutic drugs for OC.
Materials and Methods
Chemicals and Regents
Fucoidan (Cat. No. F8190) from Fucus vesiculosus and insulin-like growth factor 1 (IGF-1, Cat. No. GF306) were purchased from Sigma-Aldrich Corporation (St. Louis, MO, USA). Fucoidan was dissolved in saline to a concentration of 1 mg/mL and IGF-1 was dissolved in dimethyl sulfoxide (Sigma-Aldrich Corporation) to yield a 20 mM stock solution, which was stored at −20°C for future use. The required concentration was prepared using basic culture medium without the addition of serum or antibiotics. A cell counting kit (CCK8) was purchased from Dojindo Laboratories (Cat. No. CK04, Tokyo, Japan). Primary antibodies against Bcl-2 (Cat. No. 3498), Bax (Cat. No. 2772), caspase-3 (Cat. No. 9662), caspase-9 (Cat. No. 9508), PI3K (Cat. No. 4249), Akt (Cat. No. 2920), p-Akt (Ser473) (Cat. No. 4060), cyclin D1 (Cat. No. 55,506), cyclin-dependent kinase (CDK)-4 (Cat. No. 12,790), CDK-6 (Cat. No. 13,331), cyclin-E1 (Cat. No. 20,808), P21 (Cat. No. 2947), P27 (Cat. No. 3888), E2F transcription factor 1 (Cat. No. 3742), retinoblastoma (Rb) (Cat. No. 9309), phospho-retinoblastoma (Rb) (Ser780) (Cat. No. 3590), cytochrome c (Cat. No. 4280), and GAPDH (Cat. No. 5174) were all obtained from Cell Signaling Technology (Danvers, MA, USA). The pan-caspase inhibitor Z-VAD-FMK was obtained from Selleck Chemicals (Cat. No. S7023, Houston, TX, USA). A ribonucleic acid (RNA) polymerase chain reaction (PCR) kit was purchased from TaKaRa Biotechnology (Cat. No. RR420A, Dalian) Co., Ltd. (Dalian, China) and an Annexin V/PI apoptosis detection kit were purchased from BD Biosciences (Cat. No. 556,547, San Jose, CA, USA). A Cell Mitochondrial Isolation Kit was obtained from Beyotime Institute of Biotechnology (Cat. No. C3606, Haimen, China).
Cell Lines and Culture
Human ovarian normal epithelial cells (IOSE80) and human OC cells (SKOV-3, A2780, OVCAR-3, TOV-112D, and Caov-3) were purchased from the Academy of Sciences Committee Type Culture Collection (Shanghai, China). All cells were inoculated in Roswell Park Memorial Institute 1640 medium (containing 10% fetal bovine serum, 100 U/mL of streptomycin and 100 U/mL penicillin) and incubated at 37°C under an atmosphere of 95% humidity and 5% CO2. The cells were sub-cultured every two to three days. Cells in the logarithmic growth phase were used in the following experiments.
CCK-8 Assay
The cells were digested and diluted to a concentration of 1×105/mL, and then 100-μL aliquots of the cell suspension were added to the wells of 96-well plates. Different concentrations of drugs were prepared for as a treatment for the cells. Five additional wells were allocated for each drug concentration in triplicate. The experiment was terminated at the corresponding time points after the adding the drug. Next, 10 μL the of CCK-8 reagent was directly added to each well and the reaction was allowed to proceed for approximately 2 h. Afterward, the absorbance at 450 nm was measured using a Synergy H4 Hybrid Multi-Mode Microplate Reader (BioTek Instruments, Inc., Winooski, VT, USA). The cell inhibition rate was calculated as 1 - (average value of the experimental group - average value of the blank group)/(average value of the control group - average value of the blank group) × 100%. The appropriate drug concentration was screened for subsequent experiments. SKOV-3 and Caov-3 cells were the most sensitive and selected for further experiments.
Flow Cytometry
The cells were digested and collected according to the experimental scheme. After washing twice with high-pressure sterilized phosphate-buffered saline (PBS), the cells were centrifuged at 1000 rpm for 5 min. After discarding the supernatant, the cells were suspended in 500 μL of 1× buffer solution and mixed with 5 μL of Annexin V- fluorescein isothiocyanate (FITC) solution at room temperature for 20 min. Afterward, the cells were incubated in 5 μL of propidium iodide (PI) for 5 min. For flow cytometry, the excitation wavelength was set at 488 nm, FITC fluorescence was detected at a wavelength of 515 nm, and PI was detected at 560 nm (Cytomics FC500; Beckman Coulter, Fullerton, CA, USA). In the flow chart, early apoptotic cells are in the lower right and late apoptotic cells in the upper right. The ratio of apoptotic cells was determined as the sum of the two.
Hoechst 33,342 Staining
Cells in the logarithmic phase were inoculated into the wells of a 12-well plate. After 24 h, fucoidan was added to the wells when the cells had adhered to the wall and the fusion rate reached 50%. The supernatant culture medium was removed and the cells were fixed for 10 min with 4% formaldehyde before washing twice with PBS for 5 min each time. A Hoechst 33,342 (10 μg/mL) dye solution for 5 min in the dark and the cells were washed twice. The 12-well plate was placed under a fluorescence microscope and blue fluorescence was observed under ultraviolet light (LI-COR Biosciences, Lincoln, NE, USA).
Mitochondrial Protein Extraction
The treated cells were lightly suspended in pre-cooled PBS and counted, so that the concentration of cells was 20–50 × 106. The cells were gently suspended in 2 mL of mitochondrial isolation reagent (mixed with 10 μL of phenylmethylsulfonyl fluoride before use) on ice. After 10–15 min, the cell suspension was transferred to a glass homogenizer about 20 times until the proportion of positive blue cells was > 50%, as determined by Trypan blue staining. The cell homogenate was centrifuged at 800 rpm for 10 min, followed by 1500 rpm for 10 min. The mitochondria were separated and the supernatant containing the cytoplasmic proteins was collected. The mitochondrial samples were suspended in 150 mL of mitochondrial lysate and centrifuged to collect the mitochondrial proteins. All proteins were stored at −20°C until used for further analysis.
RNA Extraction and Quantitative Real-Time (qRT-PCR)
The cells were lysed with 1 mL of TRIzol reagent on ice for 5 min, then centrifuged at 12,000 rpm for 5 min. The supernatant was transferred to another centrifugal tube and mixed with 200 μL chloroform. After vigorous mixing on an oscillator for 20 s, the supernatant was placed on ice for 10 min and then centrifuged at 12,000 rpm for 15 min at low temperature. Afterward, the supernatant was carefully transferred to a new tube and mixed with an equal volume of isopropanol for 10 min at room temperature to precipitate the RNA. The ethanol was removed as much as possible with a micro-pipette, and the sample was air-dried for 10 min at room temperature. The RNA was dissolved in 20 μL of diethyl pyrocarbonate-treated water. After verifying the purity and concentration, the RNA was reverse transcribed into cDNA, which was then stored at −20°C. A 7900HT fast real-time PCR system (Applied Biosystems, Foster City, CA, USA) was used to determine the level of target gene expression. The primers used for qRT-PCR are shown in Table 1.
Table 1.
Nucleotide Sequences of Primers Used for qRT-PCR
| Gene | Primer Sequences (5′—3′) | |
|---|---|---|
| GAPDH | Forward | TGTGGGCATCAATGGATTTGG |
| Reverse | ACACCATGTATTCCGGGTCAAT | |
| PCNA | Forward | GCTGACATCGGACACTTA |
| Reverse | CTCAGGTACAAACTTGGTG |
Western Blot Analysis
The required cells were washed twice with pre-cooled PBS and centrifuged at 3000 rpm for 3 min. The bottom sediment was collected and 20 μL of cell hematocrit was added with 100 μL precooled radioimmunoprecipitation assay buffer containing 1% phosphatase inhibitor and 1% phenylmethylsulfonyl fluoride. The cell suspensions were repeatedly vortexed and incubated on ice for 40 min. After centrifugation at 12,000 rpm for 10 min, the supernatant was collected to obtain the total protein samples, which were quantified using a bicinchoninic acid protein assay (Thermo Fisher Scientific, Waltham, MA, USA). The remaining suspension was boiled for 10 min in 5 × sodium dodecyl sulfate sample buffer and stored at −20°C as a reserve. The same amount of protein was added to prepared gels (12.5% for 10–40kDa, 10% for 40–70kDa, 7.5% for 70–150kDa) and separated by electrophoresis at 80 V. When bromophenol blue appeared in the separation gel, the voltage was adjusted to 120 V. Electrophoresis was continued until the bromophenol blue dye reached the end of the gel. After steady-flow membrane transfer at 200 mA, non-specific loci were blocked by incubating the membrane in a pre-prepared 5% skim milk (phosphorylated protein using 5% bovine serum albumin blocking solution). After incubation with primary and secondary antibodies and washing, the membranes were scanned using an Odyssey dual-color infrared laser imaging system (LI-COR Biosciences). The band densities of the target proteins were assessed using GAPDH as an internal reference.
Animal Experiments
A total of six BALB/C nu/nu male nude mice (aged 6–8 weeks) were purchased from Shanghai Shrek Biotechnology Co., Ltd. (Shanghai, China) and housed at a constant temperature of 22°C and 55% humidity under a 12-h light/dark cycle. All animal experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by Animal Care and Use Committee of Qingdao University (Qingdao, China, No. SYXK2015-0003). Animals were observed daily after dosing, weighed once a day, and calculated the growth rate of animals. The mice were anesthetized by an intraperitoneal standard dosage (30mg/kg) of sodium pentobarbital (Nembutal, St. Louis, MO, USA). At the end of the observation period, all mice were euthanized by carbon dioxide induced apnea (20% of chamber volume that the flow rate of CO2 displaced per minute). After staying for 5min, take it out and expose it to air for 5min to confirm its death. No animals died or became severely ill prior to the experimental endpoint. Throughout the course of this study, all efforts were made to minimize animal suffering. SKOV-3 cells in the logarithmic growth phase (cell concentration > 5×106/mL) were subcutaneously injected into the upper flank region of all mice. After 5 to 7 days, white spherical protuberances with diameters of approximately 5 mm were observed at the injection sites. All mice were randomly divided to two groups. The mice in one group (n = 3) were administered fucoidan at 10 mg/kg by gavage and those in the other group were administered the same volume of saline. The mice were weighed and the lengths and diameters of the tumors were measured every three days (The maximum tumor volume is 4000 mm3). After three weeks, the mice were sacrificed under anesthesia.
Immunohistochemistry
Prepared paraffin sections were dewaxed and rehydrated by the xylene and different concentrations of alcohol. Antigens immersed in the citrate buffer were recovered by microwave in the water-bath of 95 °C for 10 min called heat-induced antigen retrieval technique, and then cooling at room temperature. The 3% hydrogen peroxide solution was added to the specimen that was stored for 20 minutes at 37 °C to block endogenous peroxidase activity. After that, the section were washed by phosphate buffer solution (PBS) for three times and blocked with 5% bovine serum albumin (BSA) at 37 °C for 20 minutes, followed by a 10-minute incubation at room temperature. The slices were then incubated with antibodies directed against Ki-67 (1:100), Bax (1:500) and Bcl-2 (1:100) for 24 h at 4 °C and with a secondary antibody (1:50) for 1 h at 37 °C after washing. A diaminobenzidine (DAB) kit was used to show granular brown substances which were observed and captured by a microscope with a digital camera (Leica Wetzlar, Germany). The integrated optical densities (IODs) of different indicators were calculated using Image-Pro Plus software 6.0 (Media Cybernetics, Silver Spring, MD, USA).
Statistical Analyses
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 20.0 (IBM Corporation, Armonk, NY, USA). Quantitative data are expressed as the mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used to detect differences among normally distributed data when F was significant. A least significant difference test was applied for non-normally distributed data, as determined using the K-test. In all cases, a probability (p) value of < 0.05 was considered statistically significant. Quantitative results of Western blotting/flow cytometry are performed by Image Pro 6.0.
Results
Fucoidan Inhibited OC Cell Proliferation
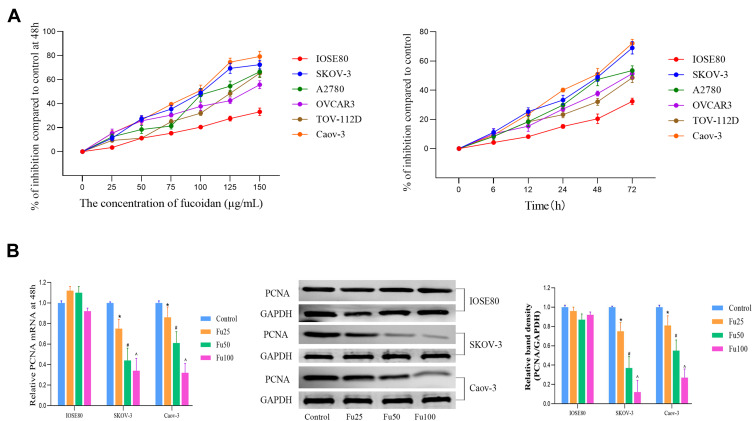
According to American type culture collection (ATCC), the OC cells (SKOV-3, A2780, OVCAR-3, TOV-112D, and Caov-3) are all from human ovarian adenocarcinoma. SKOV-3, OVCAR-3 and Caov-3 are resistant to several cytotoxic drugs, such as diphtheria toxin, cisplatin and adriamycin. The dose range of fucoidan was based on a literature review.9,21,32 First, different concentrations of fucoidan (0–150 μg/mL) were used to treat normal ovarian epithelial cells (IOSE80) and OC cells (SKOV-3, A2780, OVCAR-3, TOV-112D, and Caov-3). Cell proliferation was measured at different time points (0–72 h) and a cell growth curve was constructed (Figure 1A). The results showed that fucoidan could effectively inhibit the proliferation of OC cells in a concentration- and time-dependent manner, but had little effect on normal epithelial cells. Further, the half maximal inhibitory concentration of all cells (IOSE80, SKOV-3, A2780, OVCAR-3, TOV-112D, and Caov-3) was determined at 48 h. The half maximal inhibitory concentration (IC50) of cells is as follows: IOSE80 393.46 μg/mL, SKOV-3 91.49 μg/mL, A2780 116.269 μg/mL, OVCAR3 155.02 μg/mL, TOV-112D 113.021 μg/mL and Caov-3 82.32 μg/mL. Based on the IC50 value, dosages of 1/4, 1/2, and 3/2 were chosen for subsequent experiments. Two cell lines (SKOV-3 and Caov-3) and three doses (25, 50, and 100 μg/mL) were screened to optimize the experimental time and cost. Proliferating cell nuclear antigen (PCNA) is an important marker of cell proliferation. After treatment with different concentrations of fucoidan, the level of PCNA gene and protein expression in cancer cell lines had decreased, with significant differences among groups, while IOSE80 had no obvious changes (Figure 1B).
Figure 1.
The effects of fucoidan on OC cell proliferation. (A) Normal ovarian epithelial cells (IOSE80) and OC cells (SKOV-3, A2780, OVCAR-3, TOV-112D and Caov-3) were treated with fucoidan (0–150 μg/mL) for 0–72 h. A CCK8 kit was used to monitor cell proliferation (n = 5). (B) The mRNA and protein levels of PCNA were detected by PCR and Western blot analyses. The data are expressed as the mean ± SD (n=3, *p < 0.05 for Fu25 vs Control; #p < 0.05 for Fu50 vs Fu25; and ^p < 0.05 for Fu100 vs Fu50).
Fucoidan Provoked Cell Cycle Arrest in OC Cells
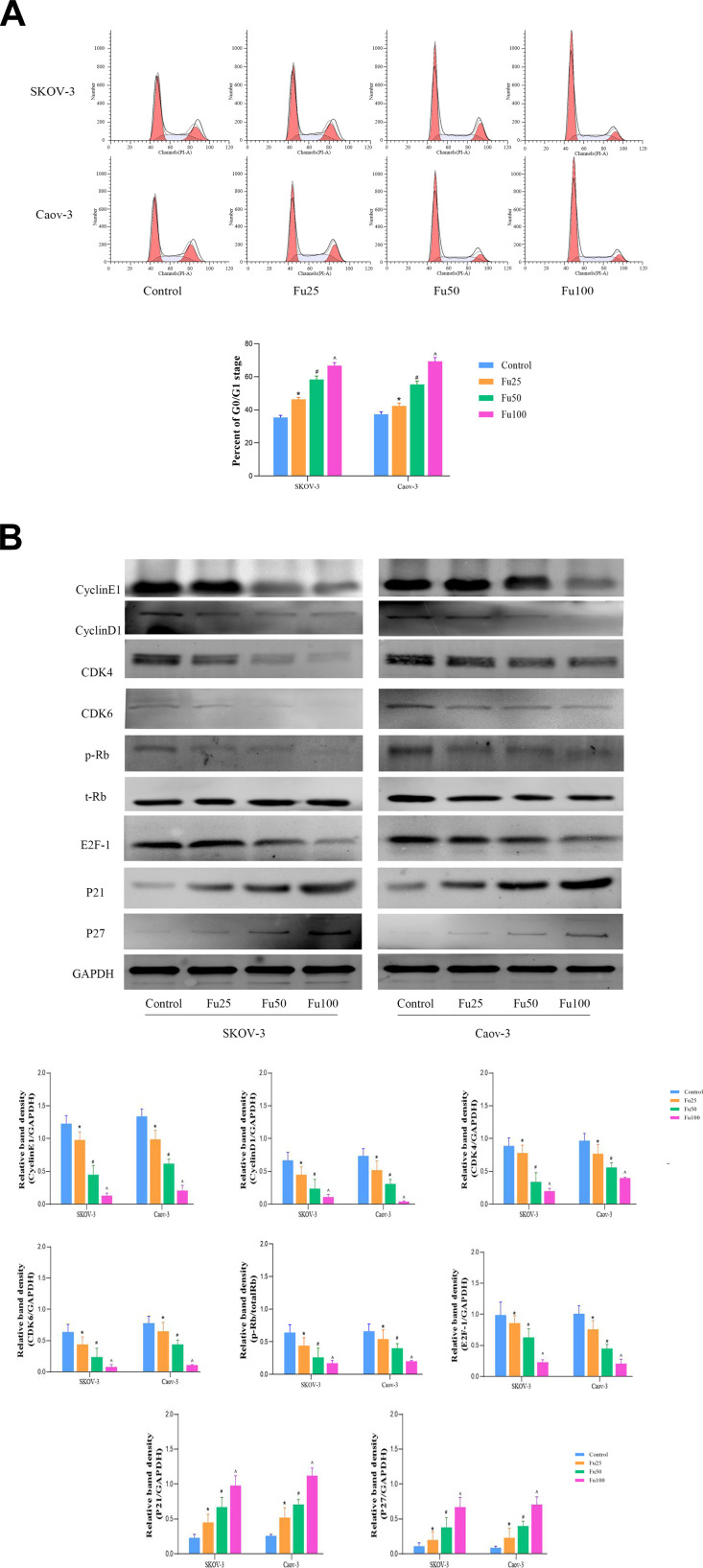
Dysregulation of the cell cycle can lead to disordered cell division, proliferation, and differentiation, and as well as subsequent aging.34 Many growth factors, cytokines, hormones, and oncogene products regulate DNA metabolism through the cell cycle.35,36 Studies of the cell cycle have deepened the understanding of the mechanism of tumorigenesis. As an antineoplastic drug, fucoidan has been found to induce G0/G1 phase arrest in SKOV-3 and Caov-3 cells (Figure 2A). Therefore, we further detected the proteins associated with the G0/G1 phase. Western blot analysis showed that fucoidan significantly decreased the protein expression levels of CDK-4, CDK-6, cyclin-E, cyclin-D1, E2F-1, and pRb, and increased that of the cyclin-dependent kinase inhibitor p21 and p27 (Figure 2B). So, fucoidan has a significant role in inducing cell cycle arrest, mainly at the G0/G1 phase.
Figure 2.
The effects of fucoidan on OC cell cycle. (A) Cell cycle progression in SKOV-3 and Caov-3 cells was determined by flow cytometry. (The two peaks represent G0/G1 and G2/M phases respectively, and the light blue area in the middle represents S phase). (B) Western blot analysis was performed to determine the levels of proteins related to the cell cycle. The data are expressed as the mean ± SD (n=3, *p < 0.05 for Fu25 vs Control; #p < 0.05 for Fu50 vs Fu25; and ^p < 0.05 for Fu100 vs Fu50).
Fucoidan Induced Caspase-Dependent Apoptosis of OC Cells
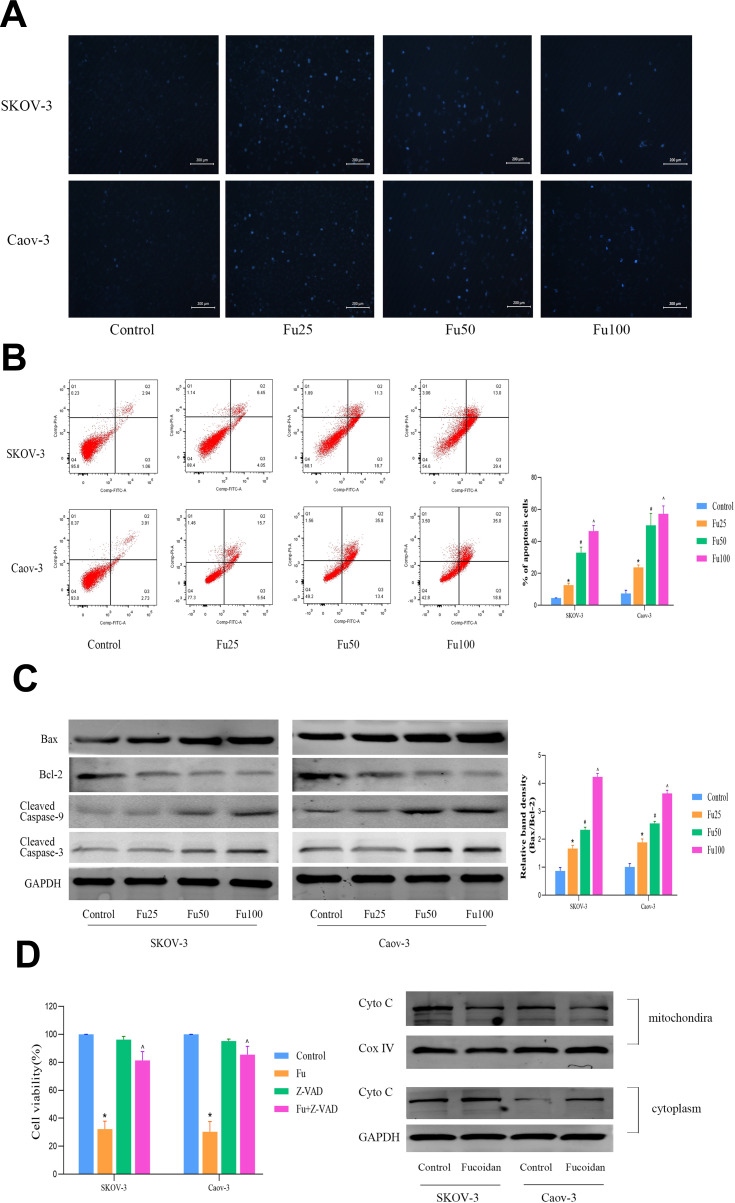
Two OC cell lines, SKOV-3 and Caov-3, were treated with different concentrations of fucoidan for 48 h. Hoechst 33,342 staining and flow cytometry were performed. The Hoechst staining results showed that the cells in the control group were dense and light blue, whereas the blue fluorescence intensity in the fucoidan-treated groups was bright blue as the dosage increase (Figure 3A). Similarly, the flow cytometry results (Annexin V-FITC/PI) confirmed these findings (Figure 3B). These results revealed that the proportion of apoptotic cells in the fucoidan-treatment group increased significantly in a dose-dependent manner. Since apoptosis is closely related to mitochondrial energy metabolism, we further detected the expression of Bcl-2, Bax, caspase-3, and caspase-9, as well as other proteins related to mitochondrial apoptosis under the same treatment conditions (Figure 3C). The results showed that the expression levels of activated caspase enzyme expression after fucoidan treatment were significantly higher than that of the control group, whereas the level of expression of anti-apoptotic protein, Bcl-2, were decreased. However, the number of apoptotic cells had significantly decreased when 50 μM Z-VAD-FMK (a permeable and irreversible pan-Caspases inhibitor) was added at 1 h before fucoidan (100 μg/mL) administration (Figure 3D). These results showed that fucoidan can effectively promote mitochondrial apoptosis in OC cells through a caspase-dependent pathway.
Figure 3.
The effects of fucoidan on OC cells apoptosis. (A) Nuclear fragmentation of SKOV-3 and Caov-3 cells was observed by fluorescence microscopy and staining with Hoechst 33,342 dye (magnification, 200 ×). (B) Apoptosis of SKOV-3 and Caov-3 cells was determined by flow cytometry. (C) Western blot analysis was performed to determine the levels of proteins related to cell apoptosis. The data are expressed as the mean ± SD (n=3, *p < 0.05 for Fu25 vs Control; #p < 0.05 for Fu50 vs Fu25; and ^p < 0.05 for Fu100 vs Fu50). (D) SKOV-3 and Caov-3 cells were pretreated with or without 50 μM of the pan-caspase inhibitor Z-VAD-FMK for 1 h. Western blot analysis was performed to determine the levels of Cyto C of mitochondria and cytoplasm. The data are expressed as the mean ± SD (n=3, *p < 0.05 for Fu100 vs Fu100+Z-VAD-FMK, ^p < 0.05 for Z-VAD-FMK vs Fu100+Z-VAD-FMK).
Fucoidan Functions by Downregulating the PI3K/Akt Signaling Pathway
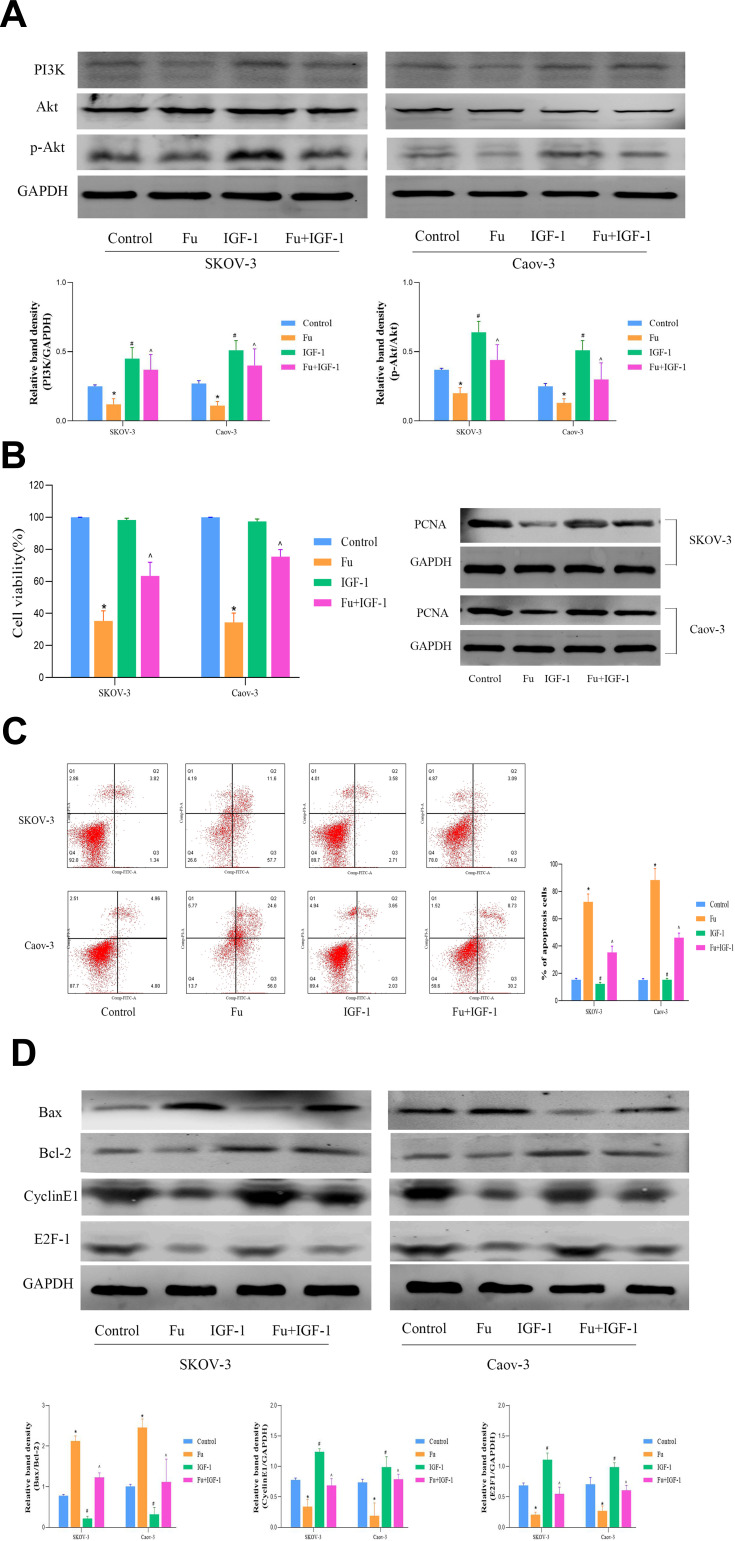
PI3K promotes Akt phosphorylation and activation, which regulates cell function by phosphorylating downstream factors, including enzymes, kinases, and transcription factors. Based on the findings of previous studies, we examined changes in PI3K/Akt-related pathways in fucoidan-treated cells. The results showed that fucoidan (100 μg/mL) administration significantly reduced the expression of PI3K and phosphorylated Akt (Figure 4A). However, PI3K activity was restored by the addition of the PI3K activator IGF-1 (5 μM). We further examined the expression levels of proteins related to cell proliferation, the cell cycle, and apoptosis after PI3K activation (Figure 4B–D). The results showed that the proliferation rate of cells treated with IGF-1 was higher than that of those treated with fucoidan alone, however, the apoptotic rate was lower, which indicated that the inhibitory effect of fucoidan was reversed by IGF-1-induced PI3K. Therefore, fucoidan can convey anti-tumor effects through the PI3K/Akt pathway in vitro.
Figure 4.
The effects of fucoidan on OC cells apoptosis. (A) The levels of PI3K, Akt, and p-Akt protein expression was determined by Western blot analysis. (B) A CCK8 kit was used to monitor cell proliferation (n = 5) and the level of PCNA protein expression was detected by Western blot. (C) Apoptosis of SKOV-3 and Caov-3 cells was determined by flow cytometry. (D) Western blot analysis was performed to determine the levels of proteins related to cell apoptosis and the cell cycle. The data are expressed as the mean ± SD (*p < 0.05). (n=3, *p < 0.05 for Fu vs Control; and ^p < 0.05 for Fu+IGF-1 vs Fu).
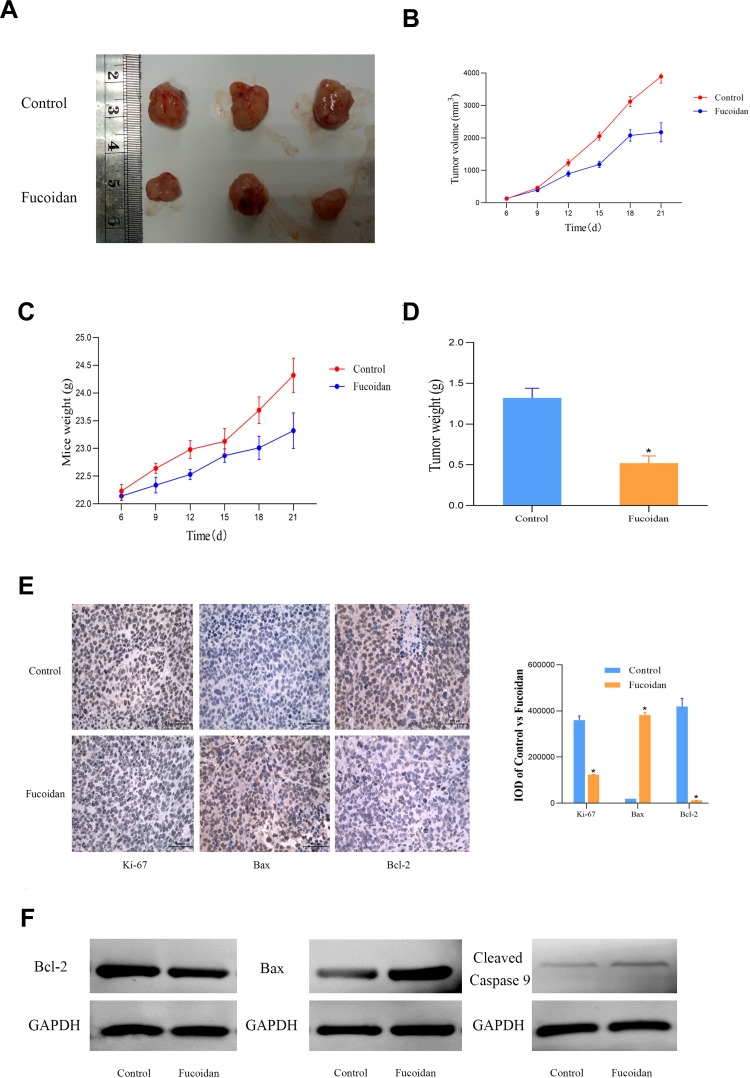
Fucoidan-Mediated Inhibition of Tumor Growth in vivo
These above experiments demonstrated that fucoidan treatment inhibited the proliferation and promoted apoptosis of cancer cells in vitro. Based on these findings, we studied the effect of fucoidan on tumor growth in mice. After three weeks of treatment, the sizes of the tumors had changed significantly (Figure 5A). Fucoidan (10mg/kg) administration had significantly reduced the tumor weight and slowed down the growth rate and weight of the mice, as well as the tumor volume (Figure 5B–D). The immunohistochemical staining and protein expression of Ki-67, Bax and Bcl-2 were performed to evaluate the proliferation and apoptosis changes in tumor tissues (Figure 5E and F). The differences among the groups were statistically significant, which suggested that fucoidan had anti-tumor effects in vivo.
Figure 5.
The anti-tumor effects of fucoidan in vivo. (A). Gross observation of HCC-SKOV-3 cell xenograft tumors in nude mice. (B and C) Changes in the tumor volume and mouse body weight. (D) Changes in the tumor weights of the experimental mice. The data are expressed as the mean ± SD. (E) Immunohistochemical staining (magnification ×400) of tumors show the levels of Ki-67, Bax and Bcl-2. (F) The levels of proteins related to the apoptosis was detected by Western blot. (n=3, *p < 0.05 Fucoidan-10mg/kg vs Control).
Discussion
Compared with the rapid development of therapies and the emergence of new drugs in most types of cancer, little progress has been made regarding the treatment of OC. Due to the lack of effective treatments and intolerance to chemotherapy, approximately 70% of patients with advanced OC eventually either actively or passively stop undergoing treatment. As a resource-rich seaweed extract, fucoidan has attracted the increased attention of researchers.9 In the present study, fucoidan was found to inhibit cell proliferation and induce cell cycle arrest and apoptosis. Furthermore, these effects were identified to be mediated via the inhibition of the PI3K/Akt signaling pathway.
Infinite replication and the ability to resist death are important characteristics of cancer cells.37 Therefore, we first studied the ability of fucoidan to inhibit cell growth and induce apoptosis. As a preliminary examination, a CCK-8 assay was used to screen normal ovarian epithelial cells and five OC cell lines to determine the appropriate administration times and dosages. The results showed that fucoidan had a time-and dose-dependent effect on the growth of cancer cells. Moreover, SKOV-3 and Caov-3 cells were the most sensitive, and within the range of action, fucoidan had little effect on normal cells. Afterward, the level of PCNA gene and protein expression was determined. The proliferation ability of tumor cells was obviously weakened by fucoidan treatment with a dose-dependent effect.
The abnormality of cell cycle litter-controlling factors is the main reason for the uncontrolled proliferation of cancer cells and the resistance of apoptotic cells can easily lead to the drug resistance of cancer cells.34,36 Our data show that fucoidan induced the accumulation of SKOV-3 and Caov-3 cells in the G0/G1 phase, which was accompanied by a decreased numbers of cells in the S and G2/M phases. At the same time, fucoidan increased the level of cyclin-dependent kinase inhibitor 1A (P21) and decreased the Rb phosphorylation at Ser795. In conjunction with the above changes, the marker proteins cyclin D1, CDK4, CDK6, and E2F-1 in the G0/G1 phase were significantly inhibited by fucoidan, indicating that fucoidan induced the cycle arrest of OC cells. However, there was no positive control included in this experiment. At present, the treatment of ovarian cancer is mainly surgical treatment and adjuvant chemotherapy. Major chemotherapy drugs are paclitaxel combined with platinum, which are non-specific drugs, difficult to be a meaningful positive control. This finding is consistent with those of previous studies of colorectal and breast cancer cells.38,39
On the other hand, flow cytometry and Hoechst staining showed that the number of apoptotic cells had increased significantly following fucoidan treatment with increased expression of the pro-apoptotic protein Bax and decreased expression of the anti-apoptotic protein Bcl-2, suggesting that fucoidan-induced apoptosis may be related to the mitochondria. Therefore, increased expression levels of cleaved caspase-3 and caspase-9 expression, as determined by Western blot analysis were consistent with our expectations. Then, the isolation of mitochondrial proteins showed that cytochrome C acts through displacement to ensure its release from the mitochondria to the cytoplasm.40 In addition, apoptosis can be either caspase-dependent or in-dependent. Thus, the pan-caspase inhibitor, Z-VAD-FMK, was used to inhibit the activity of caspases and assess the effect of fucoidan treatment. The results showed that the apoptotic effect of fucoidan was inhibited, suggesting that the pro-apoptotic effect of fucoidan was dependent on caspase activation, which was consistent with the findings of previous studies.10,19,21 This evidence suggests that different concentrations of fucoidan results in decreased proliferation and increased apoptosis of OC cells. Based on these findings, the mechanism underlying fucoidan activity was investigated.
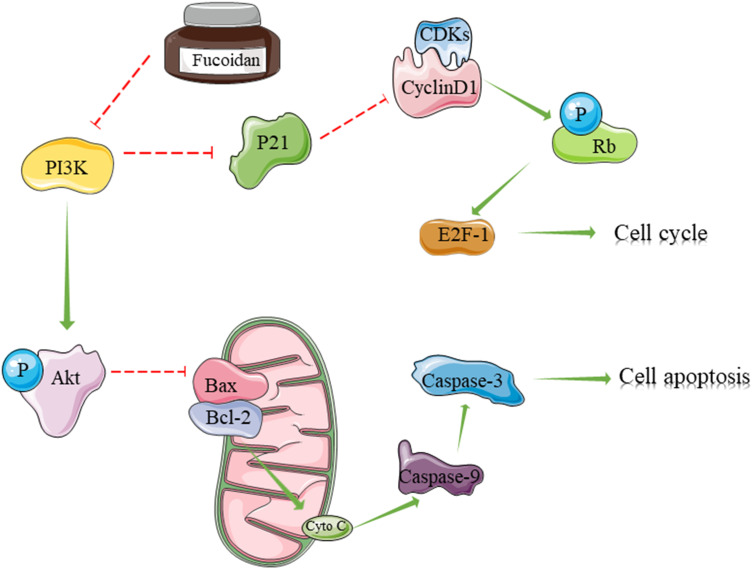
Extracellular stimulus signals are dependent on inositol lipid signaling molecules, which are transported to the nucleus through cell membrane transformation, whereas PI3K is an important cellular signal transduction molecule.41 Previous cancer studies have found that fucoidan can inhibit the PI3K/Akt signaling pathway, which has been further confirmed in OC cells.7,31,32 The results of the present study showed that PI3K gene transcription and protein expression were inhibited by fucoidan via Akt phosphorylation. However, the effect of fucoidan on SKOV-3 and Caov-3 cells was weakened by IGF-1, a PI3K activator, suggesting that fucoidan may play an anti-cancer role through the PI3K/Akt signaling pathway. Therefore, how does this crucial pathway affect the cell cycle and apoptosis? In cancer cells, activated PI3K produces PIP3 on the plasma membrane, as a second messenger, which binds to the signaling proteins, Akt and PDK1, containing a PH domain in the cells, and promotes the phosphorylation and activation of Akt at Ser308 by PDK1.42 On one hand, phosphorylated Akt inhibits the expression of the CDK inhibitor P21, so that CDK4/6 and cyclin D1 can form a complex to promote the proliferation and growth of cancer cells.43,44 However, fucoidan caused dissociation of the complexes, which prevented Rb phosphorylation and the subsequent release of the transcription factor E2F-1.45 This effect eventually leads to cell arrest from the G1 phase to the S phase. On the other hand, Bax expression was down-regulated by activated Akt, so that Bax could not bind with Bcl-2 to form a dimer.46–48 Fucoidan inhibited this process and increased the Bax/Bcl-2 ratio, thereby promoting the mitochondrial-mediated apoptosis of cancer cells. At the same time, cytochrome C was released from the mitochondria into the cytoplasm to induce the cleavage of caspases 9 and 3, which eventually induced apoptosis.49,50 However, Dawaguchi and his colleagues demonstrated that fucoidan inhibited proliferation through AMPK-associated suppression of fatty acid synthesis and G1/S transition in HLF cells, so AMPK signaling activation could be as another possible mechanism for the anti-cancer effects of fucoidan.18 Finally, we demonstrated that fucoidan could inhibit tumor growth in vivo (Figure 6).
Figure 6.
The mechanism of fucoidan action. Fucoidan inhibited PI3K leading to the reduce of phosphorylated Akt. On one hand, phosphorylated Akt inhibits the expression of the CDK inhibitor P21 to cause cell cycle arrest. On the other hand, the expression of Bax was up-regulated by fucoidan to promote the mitochondrial-mediated apoptosis of cancer cells.
In conclusion, the results of the present study showed that fucoidan can induce cell cycle arrest at the G0/G1 phase and apoptosis via a mechanism related to the inhibition of the PI3K/Akt signaling pathway. In addition, the oral administration of fucoidan can also inhibit the growth of tumor cells in xenotransplanted mice. However, because of the limited conditions at that time and the significant differences in the results, the sample size of animal was not expanded and normal cells (IOSE80) were not as comparison in further experiments of mechanism exploration. Future work needs to be planned to use a larger number of samples to further study the mechanism of drug action in vivo and in vitro. Another, although activation of PI3K by IGF-1 expression was further verification of our conclusion, inhibition of PI3K was a good negative control which need further exploration. In light of the above safe and effective anti-tumor effects, fucoidan may represent a potential drug for the treatment of OC patients.
Acknowledgments
This work was Funded by Shandong Provincial Natural Science Foundation, China, grant numbers ZR201808220011 and ZR201808120020.
Disclosure
The authors report no conflicts of interest in the present study.
References
- 1.Momenimovahed Z, Tiznobaik A, Taheri S, et al. Ovarian cancer in the world: epidemiology and risk factors. Int J Women's Health. 2019;11:287–299. doi: 10.2147/IJWH.S197604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flaum N, Crosbie EJ, Edmondson RJ, et al. Epithelial ovarian cancer risk: a review of the current genetic landscape. Clin Genet. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019. doi: 10.3322/caac.21559 [DOI] [PubMed] [Google Scholar]
- 4.O’Malley DM. New therapies for ovarian cancer. J Natl Compr Canc Netw. 2019;17(5.5):619–621. [DOI] [PubMed] [Google Scholar]
- 5.Yan ZZ, Huang YP, Wang X, et al. Integrated omics reveals tollip as an regulator and therapeutic target for hepatic ischemia-reperfusion injury in mice. Hepatology. 2019;70(5):1750–1769. doi: 10.1002/hep.30705 [DOI] [PubMed] [Google Scholar]
- 6.Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253. doi: 10.1016/S0140-6736(18)32552-2 [DOI] [PubMed] [Google Scholar]
- 7.Choo GS, Lee HN, Shin SA, et al. Anticancer effect of fucoidan on DU-145 prostate cancer cells through Inhibition of PI3K/Akt and MAPK pathway expression. Mar Drugs. 2016;14(7). doi: 10.3390/md14070126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han MH, Lee DS, Jeong JW, et al. Fucoidan induces ROS-dependent apoptosis in 5637 human bladder cancer cells by downregulating telomerase activity via inactivation of the PI3K/Akt signaling pathway. Drug Dev Res. 2017;Vol.78(1):37–48. [DOI] [PubMed] [Google Scholar]
- 9.van Weelden G, Bobinski M, Okla K, et al. Fucoidan structure and activity in relation to anti-cancer mechanisms. Mar Drugs. 2019;17(1):32. doi: 10.3390/md17010032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu HY, Lin TY, Hu CH, et al. Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP activation to enhance cisplatin-induced cytotoxicity in human lung cancer cells. Cancer Lett. 2018;432:112–120. doi: 10.1016/j.canlet.2018.05.006 [DOI] [PubMed] [Google Scholar]
- 11.Pawar VK, Singh Y, Sharma K, et al. Improved chemotherapy against breast cancer through immunotherapeutic activity of fucoidan decorated electrostatically assembled nanoparticles bearing doxorubicin. Int J Biol Macromol. 2019;122:1100–1114. doi: 10.1016/j.ijbiomac.2018.09.059 [DOI] [PubMed] [Google Scholar]
- 12.Peng J, Yuan JP, Wu CF, et al. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: metabolism and bioactivities relevant to human health. Mar Drugs. 2011;9(10):1806–1828. doi: 10.3390/md9101806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beppu F, Niwano Y, Tsukui T, et al. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J Toxicol Sci. 2009;34(5):501–510. doi: 10.2131/jts.34.501 [DOI] [PubMed] [Google Scholar]
- 14.Iio K, Okada Y, Ishikura M. [Bacterial reverse mutation test and micronucleus test of fucoxanthin oil from microalgae]. Shokuhin Eiseigaku Zasshi. 2011;52(3):190–193. Japanese. [DOI] [PubMed] [Google Scholar]
- 15.Iio K, Okada Y, Ishikura M. [Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats]. Shokuhin Eiseigaku Zasshi. 2011;52(3):183–189. doi: 10.3358/shokueishi.52.183. Japanese. [DOI] [PubMed] [Google Scholar]
- 16.Mathew L, Burney M, Gaikwad A, et al. Preclinical evaluation of safety of fucoidan extracts from undaria pinnatifida and fucus vesiculosus for use in cancer treatment. Integr Cancer Ther. 2017;Vol.16(4):572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Riby JE, Conde L, et al. A fucus vesiculosus extract inhibits estrogen receptor activation and induces cell death in female cancer cell lines. BMC Complement Altern Med. 2016;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Hayakawa M, Koga H, et al. Effects of fucoidan on proliferation, AMP-activated protein kinase, and downstream metabolism- and cell cycle-associated molecules in poorly differentiated human hepatoma HLF cells. Int J Oncol. 2015;46(5):2216–2222. doi: 10.3892/ijo.2015.2928 [DOI] [PubMed] [Google Scholar]
- 19.He X, Xue M, Jiang S, et al. Fucoidan promotes apoptosis and inhibits EMT of breast cancer cells. Biol Pharm Bull. 2019;42(3):442–447. doi: 10.1248/bpb.b18-00777 [DOI] [PubMed] [Google Scholar]
- 20.Kim IH, Nam TJ. Fucoidan downregulates insulin-like growth factor-I receptor levels in HT-29 human colon cancer cells. Oncol Rep. 2018;Vol.39(3):1516–1522. [DOI] [PubMed] [Google Scholar]
- 21.Yang G, Zhang Q, Kong Y, et al. Antitumor activity of fucoidan against diffuse large B cell lymphoma in vitro and in vivo. Acta Biochim Biophys Sin. 2015;47(11):925–931. doi: 10.1093/abbs/gmv094 [DOI] [PubMed] [Google Scholar]
- 22.Boo HJ, Hong JY, Kim SC, et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar Drugs. 2013;11(8):2982–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto M, Higaki K, Nanba E, et al. Anti-proliferation activity of fucoidan in MKN45 gastric cancer cells and downregulation of phosphorylated ASK1, a cell cycle-regulated kinase. Yonago Acta Med. 2015;58(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 24.Burney M, Mathew L, Gaikwad A, et al. Evaluation fucoidan extracts from undaria pinnatifida and fucus vesiculosus in combination with anticancer drugs in human cancer orthotopic mouse models. Integr Cancer Ther. 2018;17(3):755–761. doi: 10.1177/1534735417740631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu HY, Hwang PA. Clinical applications of fucoidan in translational medicine for adjuvant cancer therapy. Clin Transl Med. 2019;8(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh B, Kim J, Lu W, et al. Anticancer effect of fucoidan in combination with tyrosine kinase inhibitor lapatinib. Evid Based Complement Alternat Med. 2014;2014:865375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alper O, Bergmann-Leitner ES, Bennett TA, et al. Epidermal growth factor receptor signaling and the invasive phenotype of ovarian carcinoma cells. J Natl Cancer Inst. 2001;93(18):1375–1384. doi: 10.1093/jnci/93.18.1375 [DOI] [PubMed] [Google Scholar]
- 28.Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2(7):489–501. doi: 10.1038/nrc839 [DOI] [PubMed] [Google Scholar]
- 29.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275(5300):661–665. doi: 10.1126/science.275.5300.661 [DOI] [PubMed] [Google Scholar]
- 30.Han YS, Lee JH, Lee SH. Fucoidan inhibits the migration and proliferation of HT-29 human colon cancer cells via the phosphoinositide-3 kinase/Akt/mechanistic target of rapamycin pathways. Mol Med Rep. 2015;12(3):3446–3452. doi: 10.3892/mmr.2015.3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xue M, Ji X, Xue C, et al. Caspase-dependent and caspase-independent induction of apoptosis in breast cancer by fucoidan via the PI3K/AKT/GSK3beta pathway in vivo and in vitro. Biomed Pharmacother. 2017;94:898–908. doi: 10.1016/j.biopha.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Kim JS, Kim E. Fucoidan from seaweed fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One. 2012;7(11):e50624. doi: 10.1371/journal.pone.0050624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukahori S, Yano H, Akiba J, et al. Fucoidan, a major component of brown seaweed, prohibits the growth of human cancer cell lines in vitro. Mol Med Rep. 2008;1(4):537–542. [PubMed] [Google Scholar]
- 34.Maddox AS, Skotheim JM. Cell cycle, cell division, cell death. Mol Biol Cell. 2019;30(6):732. doi: 10.1091/mbc.E18-12-0819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SK, Torii KU. Linking cell cycle to stomatal differentiation. Curr Opin Plant Biol. 2019;51:66–73. [DOI] [PubMed] [Google Scholar]
- 36.Perez-Pena J, Diaz-Rodriguez E, Sanz E, et al. Central role of cell cycle regulation in the antitumoral action of ocoxin. Nutrients. 2019;11(5). doi: 10.3390/nu11051068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 38.Park HY, Park SH, Jeong JW, et al. Induction of p53-independent apoptosis and g1 cell cycle arrest by fucoidan in HCT116 human colorectal carcinoma cells. Mar Drugs. 2017;15(6):154. doi: 10.3390/md15060154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banafa AM, Roshan S, Liu YY, et al. Fucoidan induces G1 phase arrest and apoptosis through caspases-dependent pathway and ROS induction in human breast cancer MCF-7 cells. J Huazhong Univ Sci Technolog Med Sci. 2013;33(5):717–724. doi: 10.1007/s11596-013-1186-8 [DOI] [PubMed] [Google Scholar]
- 40.Xiao D, He H, Huang W, et al. Analysis of mitochondrial markers of programmed cell death. Methods Mol Biol. 2018;1743:65–71. [DOI] [PubMed] [Google Scholar]
- 41.Yang Q, Jiang W, Hou P. Emerging role of PI3K/AKT in tumor-related epigenetic regulation. Semin Cancer Biol. 2019;59:112–124. doi: 10.1016/j.semcancer.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 42.Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/AKT signalling pathway and cancer. Gene. 2019;698:120–128. doi: 10.1016/j.gene.2019.02.076 [DOI] [PubMed] [Google Scholar]
- 43.Kim HD, Jang CY, Choe JM, et al. Phenylbutyric acid induces the cellular senescence through an Akt/p21(WAF1) signaling pathway. Biochem Biophys Res Commun. 2012;422(2):213–218. [DOI] [PubMed] [Google Scholar]
- 44.Guo Q, Xiong Y, Song Y, et al. ARHGAP17 suppresses tumor progression and up-regulates P21 and P27 expression via inhibiting PI3K/AKT signaling pathway in cervical cancer. Gene. 2019;692:9–16. doi: 10.1016/j.gene.2019.01.004 [DOI] [PubMed] [Google Scholar]
- 45.Hengst L, Gopfert U, Lashuel HA, et al. Complete inhibition of Cdk/cyclin by one molecule of p21(Cip1). Genes Dev. 1998;12(24):3882–3888. doi: 10.1101/gad.12.24.3882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadidi M, Lentz SI, Feldman EL. Hydrogen peroxide-induced Akt phosphorylation regulates Bax activation. Biochimie. 2009;91(5):577–585. doi: 10.1016/j.biochi.2009.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonyan L, Renault TT, Novais MJ, et al. Regulation of Bax/mitochondria interaction by AKT. FEBS Lett. 2016;590(1):13–21. [DOI] [PubMed] [Google Scholar]
- 48.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pistritto G, Trisciuoglio D, Ceci C, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8(4):603–619. doi: 10.18632/aging.100934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mishra AP, Salehi B, Sharifi-Rad M, et al. Programmed cell death, from a cancer perspective: an overview. Mol Diagn Ther. 2018;22(3):281–295. doi: 10.1007/s40291-018-0329-9 [DOI] [PubMed] [Google Scholar]