Abstract
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also referred to as COVID-19, was declared a pandemic by the World Health Organization in March 2020. The manifestations of COVID-19 are widely variable and range from asymptomatic infection to multi-organ failure and death. Like other viral illnesses, acute myocarditis has been reported to be associated with COVID-19 infection. However, guidelines for the diagnosis of COVID-19 myocarditis have not been established.
Methods
Using a combination of search terms in the PubMed/Medline, Ovid Medline and the Cochrane Library databases and manual searches on Google Scholar and the bibliographies of articles identified, we reviewed all cases reported in the English language citing myocarditis associated with COVID-19 infection.
Results
Fourteen records comprising a total of fourteen cases that report myocarditis/myopericarditis secondary to COVID-19 infection were identified. There was a male predominance (58%), with the median age of the cases described being 50.4 years. The majority of patients did not have a previously identified comorbid condition (50%), but of those with a past medical history, hypertension was most prevalent (33%). Electrocardiogram findings were variable, and troponin was elevated in 91% of cases. Echocardiography was performed in 83% of cases reduced function was identified in 60%. Endotracheal intubation was performed in the majority of cases. Glucocorticoids were most commonly used in treatment of myocarditis (58%). Majority of patients survived to discharge (81%) and 85% of those that received steroids survived to discharge.
Conclusion
Guidelines for diagnosis and management of COVID-19 myocarditis have not been established and our knowledge on management is rapidly changing. The use of glucocorticoids and other agents including IL-6 inhibitors, IVIG and colchicine in COVID-19 myocarditis is debatable. In our review, there appears to be favorable outcomes related to myocarditis treated with steroid therapy. However, until larger scale studies are conducted, treatment approaches have to be made on an individualized case-by-case basis.
Keywords: Myocarditis, COVID-19, Glucocorticoids, Tocilizumab, Pandemic
1. Introduction
Infection with the novel pathogen severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), also referred to as COVID-19, was first reported in Wuhan, China in December 2019 and declared a pandemic by the World Health Organization (WHO) in March 2020 [1]. SARS-CoV-2 is one of the zoonotic coronaviruses similar to severe acute respiratory syndrome coronavirus (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) and believed to have resulted from a zoonotic transmission to humans from bats [2]. The manifestations of COVID-19 are widely variable and range from asymptomatic infection to multi-organ failure and death. Pulmonary involvement is the most dominant clinical manifestation of COVID-19 including acute respiratory distress syndrome (ARDS) which is associated with higher mortality, up to 52.4% in one series [3]. With rapidly evolving research on COVID-19, cardiovascular manifestations were found to occur in 20–30% of hospitalized patients and associated with worse outcomes [4,5]. COVID-19 related viral myocarditis has been reported in multiple case reports and review articles. The mechanism of cardiac injury remains poorly understood which makes management challenging. Multiple institutions have established guidelines for the management of COVID-19 however focus on respiratory distress and ARDS management. No guidelines for the management of myocarditis currently exist. Current practice is limited to case reports and our understanding of the pathophysiology of the disease is still to be determined.
Several reviews on cardiovascular complications have been done recently, yet the management and outcomes of myocarditis was not discussed in details. In this paper, we present an extensive systematic review of the reported cases of COVID-19 related myocarditis. We aim to describe the clinical characteristics and management of currently published COVID-19 myocarditis patients. We also aim to investigate the most common presenting features, workup and outcomes in the reported cases to identify a common pattern to aid in the diagnosis and management.
2. Methods
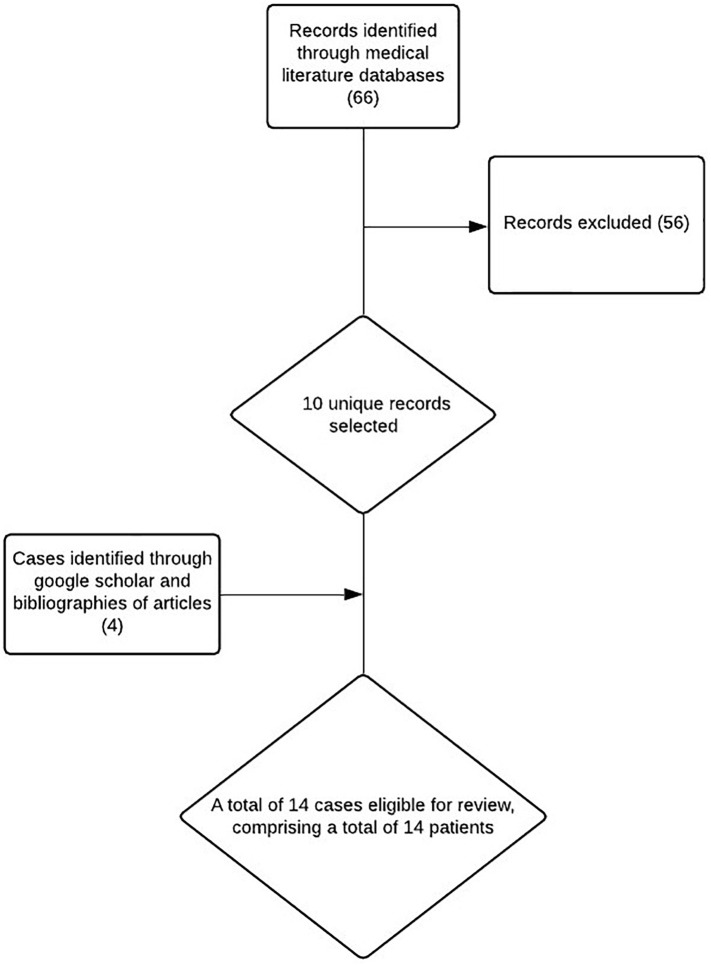
We conducted a systematic search of the medical literature of online databases including PubMed/Medline, Ovid Medline and the Cochrane Library from December 1st 2019 to June 30th 2020. We searched for the following medical subjects heading (MeSH) terms: (((COVID-19 OR coronavirus OR novel coronavirus OR SARS-CoV-2 OR SARS CoV 2))) in combination with terms “Myocarditis”, “Pericarditis”, and “myopericarditis”. We also screened all primary articles bibliography for addition cases. We limited our search to articles written in the English language. We limited our search to case reports only. Our search was in line with PRISMA guidelines and the flowchart in Fig. 1 portrays the search and screening process. A total of 64 records were identified through our literature search. Two reviewers (K.S and M.A) reviewed all retrieved titles, abstracts and manuscripts and identified eight relevant manuscripts. Another four eligible cases were identified by a manual search on Google scholar, Google search engine, and the bibliographies of the primary articles which resulted in a total of 12 cases. One of the identified manuscripts reported two cases however only one of them reported a case of myocarditis and the second discussed a case of stress cardiomyopathy which was excluded. For each case identified, we collected patient demographics including age and gender and clinical information such as presentation, laboratory results, electrocardiogram, echocardiography and advanced cardiac imaging results.
Fig. 1.
PRISMA flowchart showing study screening and selection process.
3. Results
Fourteen records comprising a total of fourteen cases that report myocarditis/myopericarditis believed to have occurred secondary to COVID-19 infection were identified from December 1st 2019 to June 30th 2020 [[6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]]. There was a male predominance (58%), with the median age of the cases described being 50.4 years. A third (33%) of all cases were younger than 40 years of age. The majority of patients did not have a previously identified comorbid condition (50%), but of those with a past medical history, hypertension was most prevalent (33%). Shortness of breath and/or dyspnea were the commonest presenting features (75%) along with fever (75%).Comorbidities and presenting symptoms were summarized in table-4. Of the 11 cases with documented hemodynamic status, the majority were in shock (64%), with cardiogenic shock being the most commonly identified cause (71% cardiogenic and 29% mixed cardiogenic and septic shock). Examination findings beyond vital signs were rarely reported and so their findings were not included. Around 42% of all patients either presented in acute respiratory distress syndrome (ARDS), or developed it during their hospitalization (Table 1 ).
Table 1.
Baseline characteristics and clinical presentation.
| Case | Age and gender | Past medical history | Presenting complaint | Shock? | Acute respiratory distress syndrome? |
|---|---|---|---|---|---|
| Cizgici et al. | 78 Male | Hypertension | Chest pain and shortness of breath | No | Yes; on arrival |
| Coyle et al. | 57 Male | Hypertension | Shortness of breath, fevers, cough, nausea, diarrhea | Yes; cardiogenic, day 4 | Yes; day 3 |
| Dabbagh et al. | 67 Female | Non-ischemic cardiomyopathy; LVEF 40% | Cough, mild shortness of breath, left shoulder pain | No | No |
| Doyen et al. | 69 Male | Hypertension | Vomiting and diarrhea; fever, cough, and dyspnea 7 days later | No | Yes |
| Hu et al. | 37 Male | None reported | Chest pain and dyspnea, diarrhea | Yes; cardiogenic, day 1 | No |
| Hua et al. | 47 Female | None reported | Breathlessness, chest pain, dry cough, fevers | Yes; cardiogenic, day 1 | No |
| Inciardi et al. | 53 Female | None | Severe fatigue, preceded by cough and fever | Yes; cardiogenic, day 1 | No |
| Irabien-Ortiz et al. | 59 Female | Hypertension, lymph node tuberculosis, migraines | Fevers, squeezing chest pain | Yes; cardiogenic, day 1 | No |
| Kim et al. | 21 Female | None | Fevers, productive cough, shortness of breath, diarrhea | – | – |
| Radbel et al. | 40 Male | None | Fever, dry cough, dyspnea on exertion | Yes; septic day 4, cardiogenic day 5 | Yes; day 3 |
| Yuan et al. | 33 Male | None reported | Chest pain, fever, myalgias | No | No |
| Zeng et al. | 63 Male | Allergic cough, tobacco smoking | Productive cough, fever, shortness of breath, exertional chest tightness | Yes; cardiogenic day 11, septic day 26 | Yes; day 1 |
| Rehman et al. | 39 Male | None | Midsternal chest pain | No | No |
| Sala et al | 43 Female | None | Chest pain and dyspnea | No | No |
Electrocardiogram findings were variable and included diffuse ST-segment elevation, ST-segment depression, and T-wave inversion occurring equally at 25% each. Troponin was elevated in 91% of cases, whereas CK-MB and pro-brain natriuretic peptide (pro-BNP) were checked less frequently Endomyocardial biopsy was performed in one case which showed virus-negative lymphocytic myocarditis. Echocardiography was performed in most cases (83%) and 60% had reduced ejection fraction. Cardiac tamponade physiology was reported in 20% of all echocardiograms, with diffuse hypokinesis occurring 30% of the time. Advanced cardiac imaging with MRI was performed in 43% of cases which showed diffuse gadolinium enhancement. Coronary artery diagnostic work-up included CT angiography (17%) and invasive coronary angiography (25%). No patients were found to have any obstructive coronary disease. (Table 2 ).
Table 2.
Laboratory investigations and cardiac imaging.
| Case report | Electrocardiogram | Cardiac biomarkers | Inflammatory markers | Echocardiogram | Additional cardiac testing |
|---|---|---|---|---|---|
| Cizgici et al. | Atrial fibrillation, 150 bpm, diffuse concave ST elevation | Troponin T 998.1 ng/L | CRP 94.6 mg/L | Coronary angiography without obstructive CAD CT chest showed small pericardial effusion suggestive of pericarditis |
|
| Coyle et al. | Sinus tachycardia, no ST/T changes | Troponin I 7.33 peak (day 3), pro-BNP 1300 peak (day 5) | CRP 20.7 mg/dL peak (day 5), IL-6 18 | Diffuse hypokinesis with relative apical sparing, LVEF 35–40%, no pericardial effusion | Cardiac MRI with LVEF 82%, diffuse bi-ventricular and bi-atrial edema, and small area of late gadolinium enhancement |
| Dabbagh et al. | Low voltage limb leads, non-specific ST changes | Troponin I < 18 ng/L, pro-BNP 54 pg/mL | CRP 15.9 mg/dL, IL-6 8 pg/mL | Large circumferential pleural effusion, signs of early right ventricular diastolic collapse, dilated but collapsing inferior vena cava, LVEF 40% | – |
| Doyen et al. | Diffuse T-wave inversion, LVH | Troponin I 9002 ng/L | – | Mild LVH, LVEF normal | Coronary angiography negative Cardiac MRI with subepicardial late gadolinium enhancement (apex and inferolateral wall) |
| Hu et al. | ST elevation leads III and aVF, ST depression V4-V6 | Troponin T > 10,000 ng/L CK-MB 112.9 ng/L, pro-BNP 21,025 ng/L |
– | Enlarged heart, LVEF 27%, 2 mm pericardial effusion | CTA coronaries without stenosis |
| Hua et al. | Sinus tachycardia, concave inferolateral ST elevation | Troponin T peak 253 ng/L | – | LVEF normal, pericardial effusion 11 mm, no tamponade; repeat Echo with 20 mm effusion and tamponade | – |
| Inciardi et al. | Diffuse ST elevation, ST depression and T inversion V1 and aVR | Troponin T 0.89 ng/mL peak, CK-MB 39.9 ng/mL peak, BNP 8465 pg/mL peak | CRP 1.3 mg/dL | Diffuse hypokinesis, LVEF 40%, circumferential pericardial effusion 11 mm, no tamponade | Coronary angiography without obstructive CAD Cardiac MRI fulfilled Lake Louise criteria |
| Irabien-Ortiz et al. | Diffuse ST elevation and PR depression | Troponin T 1100 ng/dL peak, BNP 4421 ng/L | CRP 10 mg/L | Concentric hypertrophy, diminished LV volumes, normal LVEF, moderate pericardial effusion, no tamponade | – |
| Kim et al. | Non-specific IV conduction delay, multiple PVCs, T wave inversions in II, III, aVF, V3-V6 | Troponin I 1.26 ng/mL, BNP 1929 pg/mL | – | Severe LV dysfunction | Cardiac CT/CTA with normal coronary arteries; edematous myocardium and subendocardial perfusion defect lateral LV Cardiac MRI with high T2 signal intensity and increased T1 extracellular volume |
| Radbel et al. | ST depressions in V4-V6; day 5 | Troponin T < 0.01 ng/mL day 4; rose to 5.21 day 5 | CRP 18.3 mg/dL, IL-6 74.3 pg/mL | Mild global hypokinesis | – |
| Yuan et al. | Ventricular tachycardia | – | – | – | Cardiac MRI day 3 with increased T2WI signal intensity, normal early and late gadolinium enhancement |
| Zeng et al. | Sinus tachycardia, left axis deviation, no ST elevation | Troponin I 11.37 g/L peak, myoglobin >600 ng/mL peak, BNP 22,500 pg/mL peak | IL-6272.4 pg/mL peak | Enlarged LV, diffuse myocardial dyskinesia, LVEF 32%, pulmonary hypertension, normal RV function, no pericardial effusion | – |
| Rehman et al. | 1 to 2 mm ST elevations in lead I and aVL, ST depression in aVR, mild J-point elevation, and T-wave inversion in leads II, III and aVF | Troponin 5.97 ng/mL | ESR 44 mm/h, LDH 926 units/L, CRP 3.3 mg/dL, CPK 366unit/L | No wall motion abnormalities and normal ejection fraction at 55%–60% | Coronary angiography without obstructive CAD |
| Sala et al. | Mild ST-segment elevation in leads V1–V2 and aVR, reciprocal ST depression in V4–V6 | Troponin T 135 ng/L, NT-proBNP 512 pg/mL | – | Mild left ventricular systolic dysfunction (LVEF 43%) with inferolateral wall hypokinesis | Cardiac MRI showed diffuse myocardial edema and wall pseudo-hypertrophy on T1 Endomyocardial biopsy: diffuse T-lymphocytic inflammatory infiltrates (CD3+ >7/mm2) with huge interstitial edema and limited foci of necrosis |
CAD: coronary artery disease; LV: left ventricle; LVEF: left ventricular ejection fraction; MRI: magnetic resonance imaging; RV: right ventricle.
Around 50% of the patients required Vasopressor support and 25% of them requiring inotropic support. Mechanical support was also utilized (17%), with the commonest modality being extracorporeal membrane oxygenation. Medical management included therapies targeted at COVID-19, therapies for management of myocarditis/myopericarditis, and therapies targeted cytokine storm. Many treatment modalities were also utilized to manage myocarditis/myopericarditis specifically, with glucocorticoids being the most commonly used (58%), followed by immunoglobulin therapy (25%) and colchicine (17%). Of the 7 cases in which glucocorticoid therapy was used, 71% started therapy on day 1 of admission. Additional therapy targeting the cytokine storm were used such as tocilizumab (17%) and interferon (17%) (Table 3 ).
Table 3.
Management and outcomes.
| Case | Vasopressor/mechanical support | Glucocorticoid therapy | Immunoglobulin therapy | IV tocilizumab | Outcome |
|---|---|---|---|---|---|
| Cizgici et al. | – | – | – | – | Transferred back to hospital |
| Coyle et al. | Milrinone day 4, norepinephrine day 4 | IV methylprednisolone 500 mg daily x 4 days, followed by taper | – | 400 mg once, day 5 | Discharged on day 19 |
| Dabbagh et al. | – | Glucocorticoids | – | – | Discharged |
| Doyen et al. | – | IV hydrocortisone for 9 days; started day 11 | – | – | Discharged from ICU after 3 weeks |
| Hu et al. | Norepinephrine and milrinone | IV methylprednisolone 200 mg daily x 4 days | IVIG 20 g daily x 4 days | – | Improved |
| Hua et al. | Vasopressors | – | – | – | Improved/survived |
| Inciardi et al. | Dobutamine | IV methylprednisolone 1 mg/kg x 3 days | – | – | Improved |
| Irabien-Ortiz et al. | Norepinephrine; additional vasopressors unspecified IABP and ECMO day 1 |
IV methylprednisolone 500 mg daily at tapering doses x 14 days | IVIG 80 mg daily x 4 days Interferon-β 0.25 mg q48 hours |
– | Not reported |
| Kim et al. | – | – | – | – | Not reported |
| Radbel et al. | Norepinephrine day 4 | – | – | 400 mg once, day 4 | Passed away day 7 |
| Yuan et al. | – | – | – | – | Discharged |
| Zeng et al. | ECMO day 11 Vasopressors day 26 |
IV methylprednisolone; | IVIG Interferon-α1b; |
– | Passed away day 33 |
| Rehman et al. | – | – | – | – | Recovery |
| Sala et al. | – | – | – | – | Recovery |
ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump IVIG: intravenous immunoglobulin.
There was no report of outcomes whatsoever in 3 cases (25%). At the time of submission of the case reports, the majority had survived to discharge (81% of those with reported outcomes) with only a minority of cases not surviving (19% of those with reported outcomes). All of the patients that reportedly passed away were noted to have both ARDS and myocarditis (Table 4 ).
Table 4.
Grouped characteristics and outcomes identified across cases.
| N = 14 cases; mean age: 50.4 years; males 58%, females 42% | N (%) |
|---|---|
| Comorbidities | |
| None | 7 (50) |
| Cardiomyopathy | 1 (8) |
| Hypertension | 4 (33) |
| Smoking | 1 (8) |
| Other (lymph node tuberculosis, allergies) | 2 (17) |
| Presenting symptoms | |
| Chest pain | 8 (57) |
| Shortness of breath/dyspnea | 10 (71) |
| Fever | 9 (75) |
| Upper respiratory tract symptoms (cough mainly) | 8 (67) |
| Gastrointestinal symptoms | 4 (33) |
| Shock | 7 (58) |
| Purely cardiogenic | 5 (42) |
| Mixed cardiogenic and septic | 2 (17) |
| ARDS | 5 (42) |
| EKG findings | 14 (100) |
| ST elevation; in a coronary vessel distribution | 4 (28) |
| ST elevation; diffuse | 3 (25) |
| ST depression | 3 (25) |
| T-wave inversion | 3 (25) |
| Arrhythmia | 2 (17) |
| Cardiac biomarkers | 13 (93) |
| Elevated troponin (I or T) | 12 (86) |
| Elevated CK-MB | 2 (17) |
| Elevated pro-BNP | 6 (50) |
| Inflammatory markers | 7 (58) |
| Elevated CRP | 7 (50); 100% of all cases where it was reported |
| Elevated IL-6 | 4 (33); 100% of all cases where it was reported |
| Echocardiogram findings | 12 (83) |
| Reduced left ventricular ejection fraction (LVEF) | 6 (50) |
| Pericardial effusion | 5 (42) |
| Cardiac CT/CTA | 3 (21) |
| Cardiac MRI findings | 6 (43) |
| Coronary angiography | 4 (29) |
| Endomyocardial biopsy | 1 (7) |
| Management | |
| Endotracheal intubation and ventilation | 7 (50) |
| Vasopressor support | 6 (43) |
| Inotropic support | 3 (21) |
| Hydroxychloroquine | 5 (36) |
| Azithromycin | 2 (14) |
| Glucocorticoids | 7 (50) |
| Immunoglobulin | 3 (21) |
| Interferon | 2 (14) |
| Tocilizumab | 2 (14) |
| Mechanical support | 2 (14) |
| Combined therapy | |
| Corticosteroid only | 3 (21); 100% survival amongst those who received corticosteroids only |
| Corticosteroid + IVIG | 3 (21); 66% survival amongst those who received corticosteroids + IVIG therapy |
| Corticosteroid + tocilizumab | 1 (7); patient survived to discharge |
| Tocilizumab alone | 1 (7); patient ultimately died |
| Outcome at time of case submission; not reported in 3 cases (Cizgici et al., Hua et al., and Kim et al.) | |
| Survival | 9 (64); 81% of all reported outcomes |
| Death | 2 (14); 18% of all reported outcomes |
Bold illiac is the main variable and normal font is subanalysis of the variable.
4. Discussion
Our study shows several trends across all cited cases. Hypertension was the most common comorbidity noted amongst cases (33%). Steroids were used in 50% of cases and multiple second-line agents including Tocilizumab (14%), immunoglobulins (21%) and interferon (14%) were used in addition to steroids. Echocardiography showed reduced ejection fraction in the majority of cases. The overall survival rate was 81% and survival rate in those who received steroids was 85%.
SARS-CoV-2 is a beta coronavirus comprised of an enveloped positive single-stranded ribonucleic acid (RNA) structure that belongs to the Coronavirinae subfamily [17]. The virus can invade the human host cell by binding to angiotensin-converting enzyme 2 (ACE2). The ACE2 is a membrane-bound protein that is expressed in many organ tissues, including cardiovascular epithelium, renal and lung tissues. After penetration, viral RNA enters the cell nucleus for replication and apoptosis [20]. The human immune response to the virus is variable which explains the variable clinical presentation. Higher plasma level of cytokines has been found in severe cases such as ARDS [21]. To date, no definitive cure is available for COVID-19 and most of the medications used or currently being studied are targeting the activation of inflammatory cells and proinflammatory cytokines.
Cardiovascular involvement has been prominent in COVID-19 cases. However, despite rapidly developing data and information, little is known about the incidence and outcomes of cardiovascular manifestations in COVID-19. There are several cardiac presentations that have been noted, including acute myocardial infarction, acute heart failure, cardiogenic shock, myocarditis, and malignant arrhythmia [22]. Patients with underlying cardiovascular disease (CVD) have a higher risk of developing cardiac injury. In a single-center retrospective study from Wuhan including 187 patients, the mortality rate was higher in patients with underlying CVD compared to patients without CVD (54.5% vs 13.2%). It also showed that 37.5% of patients who died had cardiac injury with elevated troponin and mortality was 69.4% in those with a history of CVD [23]. In most studies, cardiac injury was evident by elevated troponin and pro-BNP. The levels of these markers were higher in critically ill patients admitted to the ICU [24]. Given that the exact mechanism of cardiac involvement is not well understood, management of this entity is more challenging. Like other viral illnesses, acute myocarditis has been reported to be associated with COVID-19 infection. Guidelines for the diagnosis of COVID-19 myocarditis have not been established. Current literature on this manifestation is limited to case reports and a small number of patients in cohort studies. However, a workup including cardiac biomarkers and electrocardiogram are initially recommended [25]. Our results revealed that ECG changes were non-specific and highly variable. This is consistent with non-specific ECG findings in myocarditis cited in the literature [26]. Serial ECGs may be a tool to provide a relatively quick, cost-effective, and non-invasive means at outlining and intervening in early stages of the disease process.
Echocardiography is an important tool in evaluating structural and functional changes secondary to myocarditis [27,28]. However, no specific echocardiographic features of myocarditis exist, but it allows the physician to exclude other causes of heart failure, pericardial effusion, and intracavitary thrombi [29]. Patterns consistent with dilated, hypertrophic, and ischemic cardiomyopathies have all described in biopsy-proven myocarditis [30]. In our study, no trend was appreciated with regards to echocardiogram findings on presentation, with 50% showing reduced ejection fraction and 42% showing evidence of pericardial effusion. Diffuse hypokinesis was also seen in 25% of cases. Cardiac MRI is the noninvasive gold standard test for myocarditis. It was done in six of the reported cases of which all showed gadolinium enhancement and two showed evidence of myocarditis as fulfilled by the Lake Louis criteria for MRI based diagnosis of myocarditis.
The mechanism of cardiac injury in COVID-19 remains poorly understood. There are several potential hypotheses on the pathogenesis of COVID-19 myocarditis including: (a) direct damage to cardiomyocytes by circulating virus through binding to ACE2 receptors [31] (b) severe cytokine release syndrome by dysregulated response by types 1 and 2 helper T cells which leads to severe systematic inflammatory response resulting in cardiomyocytes hypoxia and apoptosis, and (c) overactivation of the autoimmune system with possible interferon mediated hyperactivation of innate and adaptive immune systems [32,33].
To date, there is no clear data on the role of ACE2 receptors in the pathogenesis of COVID 19 myocarditis, but may serve as a portal for entry of COVID-19. A previous study from the SARS-CoV outbreak in Toronto showed that the virus RNA was detected in 35% of autopsied hearts [34]. Another animal study done in 2009 showed that SARS-CoV pneumonia can increase expression of ACE2 receptors and cause myocardial injury [35]. The role of these receptors has led to postulations on the potential benefits or harms of the use of angiotensin-converting enzyme inhibitors. However, given the lack of conclusive data, continuation of clinically indicated angiotensin receptor blocker medications is recommended, unless clinically contraindicated [36].
Another proposed hypothesis of the pathogenies of myocarditis in COVID-19 is severe systemic inflammation and cytokine storm. Cytokine storm is an exaggerated immune response to stimulus or pathogen and is associated with rapid deterioration and high mortality. Several studies on previous coronavirus outbreaks such as MERS-CoV and SARS-CoV revealed that serum cytokine and chemokine levels are significantly higher especially in critically ill patients and patients who developed ARDS [37]. When a host is infected with COVID-19, the primary immune system responds by secretion of interferons (IFNs) and proinflammatory cytokines. The release of interferons (the first line defense against viral infections) is delayed in the early stages infection, allowing to continue replicating and attracting inflammatory cells to tissue, lung or cardiac, which results in severe inflammation.
The current data on the use of glucocorticoids in COVID-19 infection remains controversial and to our knowledge, no current studies have been conducted to assess the efficacy of corticosteroid therapy on COVID-19 myocarditis. Corticosteroid therapy was ineffective in treating viral myocarditis according to a Cochrane systematic review published in 2013 [40]. Furthermore, it has been reported that corticosteroid therapy might delay the clearance of the virus. In a study that was recently done on COVID-19 patients, the duration of viral RNA detection for oropharyngeal swabs and feces was longer in patients who were treated with corticosteroids [41]. Moreover, there is concern for increasing secondary infection and adrenal insufficiency as a result of steroid therapy. Two studies from China showed that IV methylprednisone has no significant benefit in COVID-19 patients [42] and was associated with higher ICU admissions [43]. However, these findings might be cofounded given that steroids were used on sicker patients and likely for separate treatment purposes. On the contrary, a study from Wuhan involving 84 -patients with ARDS secondary to COVID-19, administration of corticosteroids decreased the risk of mortality [3]. Additionally, A recent press release from a large clinical trial on COVID-19 patients, the RECOVERY (Randomized Evaluation of COVid-19 thERapY), Dexamethasone was shown to reduce mortality in one third of the ventilated patients (rate ratio 0.65 [95% CI 0.48 to 0.88]; p = 0.0003). In our review, five out of seven patients who were treated with corticosteroid recovered; one passed away and the other case did not report outcomes. We cannot confirm if it is due to a true treatment effect or by chance only, yet based on recent data, steroid might be associated with favorable outcomes in critically ill COVID19 patients [44].
Intravenous immunoglobulin was used in three reported cases with variable outcomes. There is strong evidence on the efficacy of IVIG in the treatment of acute myocarditis. A meta-analysis published in 2019 comparing IVIG to corticosteroid for acute myocarditis showed that IVIG therapy improved mortality and recovery of left ventricular function [45]. It was difficult to notice a specific trend on IVIG in our review because it was used only in three patients and more studies are needed to prove its efficacy on COVID-19 myocarditis.
Tocilizumab is an IL-6 receptor antagonist that is used more commonly in rheumatoid arthritis treatment. It is also approved to be used for cytokine release syndrome based on multiple studies that proved its efficacy [46]. In a single center case series including 15 patients, 11 patients improved or stabilized after starting tocilizumab however, the study included a small number of patients and reported the outcomes after 7 days post treatment only [47]. In the reported cases, four report high IL-6 levels, and 50% of those with high IL-6 levels received Tocilizumab with variable outcomes resulting. There are currently several ongoing clinical trials [[48], [49], [50]] evaluating the efficacy of IL-6 antagonists in COVID-19 patients.
Guidelines for diagnosis and management of COVID-19 myocarditis have not been established and our knowledge on management is rapidly changing. Several treatments have been used in COVID-19 myocarditis based on our understanding of the pathogenesis and from previous experience in treating viral and fulminant myocarditis. Since hyperinflammation and cytokine release syndrome are a likely mechanism of injury in COVID-19 myocarditis, glucocorticoids have been used despite lack of proven clinical efficacy. Other possible treatments currently under study are plasma exchange therapy, immunosuppression with IVIG and cytokine inhibitors and antiviral agents such as Remdesivir. In early trial results, Remdesivir, was found to be superior to placebo in shortening the time to recovery in adults hospitalized with COVID-19 [51]. It was also found to prevent and reduce disease severity in MERS coronavirus in primates [52]. Until prospective studies and trials establish guidelines for the management of COVID-19 myocarditis, treatment has to be catered to individual case presentations.
5. Limitations
The limitations of this study are characteristic to studies based on case reports. First, our study is limited to cases reports which imparts potential selection bias since reported cases are usually unique cases in presentation and management. Since the COVID-19 pandemic impacted routine clinical practice and diagnostic approaches, many cases did not undergo further diagnostic workup done such as cardiac MRI and endomyocardial biopsy. In addition, publication bias on the part of authors reporting these cases is another factor to account for. Despite these limitations, we believe the use of case reports in our review is fundamental in detecting trends and generating hypotheses.
6. Conclusion
Our knowledge on the complications and management of COVID-19 is exponentially growing. Myocardial injury and myocarditis have been shown to be associated with higher morbidity and mortality. Despite the rapidly growing research on management of COVID-19 and its complications, there are many unanswered questions and areas to explore. Currently, most of the ongoing research is focusing on the respiratory complications of COVID-19 and little is known about myocarditis management. The use of glucocorticoids in COVID-19 myocarditis is debatable. In our review, there appears to be favorable outcomes related to myocarditis treated with steroid therapy. However, until larger scale studies are conducted, treatment approaches have to be made on an individualized case-by-case basis.
Primary funding source
None.
Declaration of competing interest
None.
References
- 1.World Health Organization Rolling updates on coronavirus disease (COVID-19) March 13, 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- 2.Badawi A., Ryoo S.G. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C., Chen X., Cai Y., Zhou X., Xu S., Huang H., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cizgici A.Y., Agus H.Z., Yildiz M. COVID-19 myopericarditis: it should be kept in mind in today’s conditions. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A recovered case of COVID-19 myocarditis and ARDS treated with corticosteroids, tocilizumab, and experimental AT-001. JACC: Case Rep. 2020;38:1547.e5–1547.e6. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dabbagh M.F., Aurora L., D’Souza P., Weinmann A.J., Bhargava P., Basir M.B. Cardiac Tamponade secondary to COVID-19. JACC: Case Rep. 2020;2:1326–1330. doi: 10.1016/j.jaccas.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyen D., Moceri P., Ducreux D., Dellamonica J. Myocarditis in a patient with COVID-19: a cause of raised troponin and ECG changes. Lancet. 2020;395:1516. doi: 10.1016/s0140-6736(20)30912-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis treated with glucocorticoid and human immunoglobulin. Eur Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hua A., O’Gallagher K., Sado D., Byrne J. Life-threatening cardiac tamponade complicating myo-pericarditis in COVID-19. Eur Heart J. 2020;41:2130. doi: 10.1093/eurheartj/ehaa253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irabien-Ortiz Á., Carreras-Mora J., Sionis A., Pàmies J., Montiel J., Tauron M. Fulminant myocarditis due to COVID-19. Revista Española De Cardiología (English Edition) 2020;73:503–504. doi: 10.1016/j.rec.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim I., Kim J.Y., Kim H.A., Han S. COVID-19-related myocarditis in a 21-year-old female patient. Eur Heart J. 2020;41:1859. doi: 10.1093/eurheartj/ehaa288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radbel J., Narayanan N., Bhatt P.J. Use of tocilizumab for COVID-19-induced cytokine release syndrome. Chest. 2020;158:e15–e19. doi: 10.1016/j.chest.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yuan W., Tang X., Zhao X. An ‘asymptomatic’ driver with COVID-19: atypical suspected myocarditis by SARS-CoV-2. Cardiovasc Diag Ther. 2020;10:242–243. doi: 10.21037/cdt.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeng J.H., Liu Y., Yuan J., Wang F., Wu W., Li J., et al. 2020. First Case of COVID-19 Infection With Fulminant Myocarditis Complication: Case Report and Insights. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brian D.A., Baric R.S. Coronavirus genome structure and replication. Curr Top Microbiol Immunol. 2005;287:1–30. doi: 10.1007/3-540-26765-4_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rehman M., Gondal A., Rehman N.U. Atypical manifestation of COVID-19-induced myocarditis. Cureus. 2020 Jun;12:e8685. doi: 10.7759/cureus.8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sala S., Peretto G., Gramegna M., Palmisano A., Villatore A., Vignale D., et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur Heart J. 2020 May 14;41:1861–1862. doi: 10.1093/eurheartj/ehaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol. 2020;215:108427. doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., et al. The variety of cardiovascular presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fung G., Luo H., Qiu Y., Yang D., McManus B. Myocarditis. Circ Res. 2016 Feb 5;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 26.Nieminen M.S., Heikkilä J., Karjalainen J. Echocardiography in acute infectious myocarditis: relation to clinical and electrocardiographic findings. Am J Cardiol. 1984 May 1;53:1331–1337. doi: 10.1016/0002-9149(84)90089-4. [DOI] [PubMed] [Google Scholar]
- 27.Felker G.M., Boehmer J.P., Hruban R.H., Hutchins G.M., Kasper E.K., Baughman K.L., et al. Echocardiographic findings in fulminant and acute myocarditis. J Am Coll Cardiol. 2000 Jul 1;36:227–232. doi: 10.1016/s0735-1097(00)00690-2. [DOI] [PubMed] [Google Scholar]
- 28.Kindermann I., Barth C., Mahfoud F., Ukena C., Lenski M., Yilmaz A., et al. Update on myocarditis. J Am Coll Cardiol. 2012 Feb 28;59:779–792. doi: 10.1016/j.jacc.2011.09.074. [DOI] [PubMed] [Google Scholar]
- 29.Cooper L.T., Baughman K.L., Feldman A.M., Frustaci A., Jessup M., Kuhl U., et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol. 2007 Nov 6;50:1914–1931. doi: 10.1016/j.jacc.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 30.Blauwet L.A., Cooper L.T. Myocarditis. Prog Cardiovasc Dis. 2010 Jan 1;52:274–288. doi: 10.1016/j.pcad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020 May 8;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang C.H., Liu C.Y., Wan Y.L., Chou C.L., Huang K.H., Lin H.C., et al. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir Res. 2005;6:42. doi: 10.1186/1465-9921-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Channappanavar R., Fehr A.R., Zheng J., C Wohlford-Lenane, Abrahante J.E., Mack M., et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the greater toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. Erratum in: JAMA. 2003;290:334. [DOI] [PubMed] [Google Scholar]
- 35.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clerkin K.J., Fried J.A., Raikhelkar J., Sayer G., Griffin J.M., Masoumi A., et al. COVID-19 and cardiovascular disease. Circulation. 2020 May 19;141(20):1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 37.Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y. Clinical progression and cytokine profiles of middle east respiratory syndrome coronavirus infection. J Korean Med Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen H.S., Wang W., Wu S.N., Liu J.P. Corticosteroids for viral myocarditis. Cochrane Database Syst Rev. 2013;10 doi: 10.1002/14651858.CD004471.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ling Y., Xu S.B., Lin Y.X., Tian D., Zhu Z.Q., Dai F.H., et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 2020;133:1039–1043. doi: 10.1097/CM9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu K., Fang Y.Y., Deng Y., Liu A., Wang M.F., Ma J.P., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133:1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China JAMA. 2020 Mar 17;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Low-Cost Dexamethasone Reduces Death by up to one Third in Hospitalised Patients With Severe Respiratory Complications of COVID-19. University of Oxford; 2020. [Google Scholar]
- 45.Huang X., Sun Y., Su G., Li Y., Shuai X. Intravenous immunoglobulin therapy for acute myocarditis in children and adults. Int Heart J. 2019;60:359–365. doi: 10.1536/ihj.18-299. [DOI] [PubMed] [Google Scholar]
- 46.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J., et al. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med. 2017 Feb;45 doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.ClinicalTrials.gov Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID19). Identifier: NCT04317092. April 7, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04317092.66 Updated.
- 49.ClinicalTrials.gov Tocilizumab for SARS-CoV2 severe pneumonitis. Identifier: NCT04315480. April 13, 2020. https://clinicaltrials.gov/ct2/show/NCT04315480.68 Updated.
- 50.ClinicalTrials.gov Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19. Identifier: NCT04315298. April 6, 2020. https://www.clinicaltrials.gov/ct2/show/NCT04315298 Updated.
- 51.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.de Wit E., Feldmann F., Cronin J., Jordan R., Okumura A., Thomas T., et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020 Mar 24;117:6771–6776. doi: 10.1073/pnas.1922083117. [DOI] [PMC free article] [PubMed] [Google Scholar]