Abstract
Background and Objectives:
Published comparisons of minimally invasive approaches to colon surgery are limited. The objective of the current study is to compare the effectiveness of robotic-assisted and laparoscopic sigmoid resection.
Methods:
A multicenter retrospective comparative analysis of perioperative outcomes from consecutive robotic-assisted and laparoscopic sigmoid resections performed between 2010 and 2015 by six general and colorectal surgeons, who are experienced in both robotic-assisted and laparoscopic surgical techniques and who had >50 annual case volumes for each approach. Baseline characteristics and surgical risk factors between the two groups were balanced using a propensity score methodology with inverse probability of treatment weighting. Mean standardized differences were reported, and in all instances, a p-value < 0.05 was considered statistically significant.
Results:
Three hundred thirty-six cases (robotic-assisted, n = 211; laparoscopic, n = 125) met eligibility criteria and were included in the study. Following weighting, patient demographics and baseline characteristics were comparable between the robotic-assisted (n = 344) and laparoscopic (n = 349) groups. The laparoscopic group was associated with shorter operating room and surgical times. The robotic-assisted group had lower estimated blood loss and shorter time to first flatus compared to the laparoscopic group. Rates of complications post discharge to 30 d tended to be lower for the RA group: 5.1% vs 8.6% [p = 0.0657]. The RA group also had lower rates of readmissions and reoperations: 4% vs 8% [p = 0.029] and 0.5% vs 5.1% [p = 0.0003], respectively.
Conclusions:
Robotic-assisted sigmoid colon resection is clinically effective and provides a minimally invasive alternative to the laparoscopic approach with improved intraoperative and postoperative outcomes for colorectal patients.
Keywords: Sigmoid, Sigmoid resection, Laparoscopy, Robotic-assisted, Minimally invasive surgery
INTRODUCTION
Comparisons of laparoscopic and open colon resection techniques and outcomes have been reported in the literature, and in some, the laparoscopic approach was comparable in terms of surgical and postoperative outcomes and was associated with shorter and less complicated recoveries.1–2 Despite these demonstrated clinical advantages, the minimally invasive approach to colon surgery has not been widely adopted, most likely due to the technical and ergonomic challenges of laparoscopic sigmoid colon resection, especially in a narrow pelvis.3 There are limited studies published on the comparative effectiveness of robotic-assisted versus laparoscopic sigmoid resection for both benign and malignant disease.4 Therefore, this multicenter retrospective study was undertaken to compare perioperative outcomes (through 30 d postoperative) between robotic-assisted (da Vinci® Surgical System, Intuitive Surgical, Inc., Sunnyvale, CA USA) and laparoscopic sigmoid resection and to explore perioperative outcomes of robotic-assisted and laparoscopic sigmoid resection in subgroups of patients with benign disease, obesity and benign disease and malignant disease.
METHODS
Study Population
A total of 336 patients underwent robotic-assisted (n = 211) or laparoscopic (n = 125) sigmoidectomy by 6 surgeons at 5 medical centers and were included in this retrospective chart review study. Each participating center provided an informed consent waiver specific to the study and provided institutional review board (IRB) approval for retrospective data collection prior to review of the charts. IRB sites (principal investigators' initials) and numbers follow: Aria Health (L.G.) AH15–284; HonorHealth Research Institute (A.A.K.) 825573–1; Morris Hospital and Silver Cross Hospital IRB (R.G.) 813348–1; WIRB (H.L. and G.P.) 1158794; BayCare Health System (C.A.S.) 2015.079-BSJ.
Patient demographics, baseline characteristics, and perioperative outcomes from sigmoid resection cases were retrospectively collected on study-specific data collection forms. International Classification of Diseases, 9th revision (ICD-9) diagnosis codes (153.3, 154.0, 211.3, 211.4, 560.2, 562.10, 562.11, 562.12, 562.13, 555.9, 556, 569.81) were used to identify cases indicated for sigmoid resection surgery. Data were collected for elective cases performed between January 2010 and December 2015 through 30 d prior to each IRB approval. Patients were considered for inclusion if they were male or female patients 18 years old or older and underwent nonemergent robotic-assisted or laparoscopic sigmoid resection for benign or malignant disease.
The participating study investigators were general and colorectal surgeons, experienced in both robotic-assisted and laparoscopic surgical techniques and had 50 annual case volumes for each robotic-assisted and laparoscopic colon resection. The study investigators were experienced with surgical management of patients with colonic lesions and with a patient population fitting the study requirements. Each surgeon had more than 5 y of experience with the robotic platform. The study was designed to exclude learning-curve cases as they could influence study outcomes in either group. Consequently, a maximum of 50 of the most recent, consecutive cases of sigmoidectomy from each arm performed by each surgeon through 30 d prior to IRB approval were considered for study inclusion. Per protocol, emergent cases were excluded. This exclusion criterion accounts for the lower number of patients ultimately included in the study (336 actual vs 500 planned).
All procedures that were performed and described in this study involving human participants were in accordance with the ethical standards of each institutional research committee and in accordance with 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Due to the retrospective nature of this study, informed consent was not required.
Surgical Technique
Robotic-assisted and laparoscopic sigmoidectomies were performed according to the surgical technique of each surgeon. No standardization of the technique was attempted. After induction of anesthesia, time out was performed in line with each institutional protocol. The trocars were placed and pneumoperitoneum obtained. Medial-to-lateral dissection or lateral-to-medial dissection was performed all the way distal at the recto-sigmoid junction. Surgeon preference guided the extent of the dissection and the length of the resected specimen. The specimen extraction occurred through either a left lower-quadrant transverse incision, a Pfannenstiel incision, or a lower-midline incision. The anastomosis was performed with the end-to-end anastomosis stapler of the surgeon's choice. Reported estimated blood loss was based on the quantitative assessment of the volume suctioned into a canister during each case.
Statistical Analysis
The retrospective data were analyzed by comparing outcomes of robotic-assisted and laparoscopic sigmoidectomy patients. All analyses were based on available data. Categorical variables were presented as numbers and percentages and were compared using the χ2 or Fisher's exact test. The continuous variables are reported as mean and standard deviation and analyzed using t-tests or Wilcoxon rank sum test. To account for treatment selection bias, we attempted to balance the baseline characteristics and surgical risk factors between the robotic and laparoscopic groups using a propensity score methodology with inverse probability of treatment weighting (IPTW).5 The IPTWs were estimated using multinomial logistic regression analysis including the following variables: age, gender, body mass index (BMI) category, surgery indication (malignant vs benign), and indicators for comorbidity conditions, previous treatment, pervious abdominal surgery, and concomitant surgery. Using this technique, the weights used for patients undergoing laparoscopic surgery were the inverse of 1 minus the propensity score, and weights used for patients receiving robotic-assisted surgery were the inverse of the propensity score alone. Patient characteristics were then reassessed for balance between surgery groups after adjusting for the IPTWs and standardized differences were reported. In all instances, a p-value < 0.05 was considered statistically significant. Data analyses were performed with SAS System version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
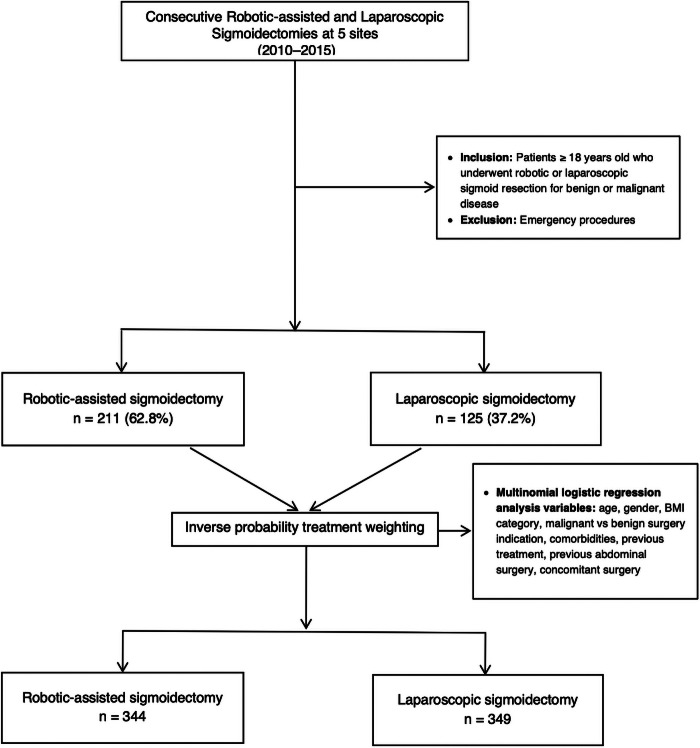
Across the five medical centers 336 cases (robotic-assisted sigmoidectomy, n = 211; laparoscopic sigmoidectomy, n = 125) met eligibility criteria and were included in the study (Figure 1). All six of the participating surgeons contributed 30–50 robotic-assisted cases and four surgeons contributed 23–58 laparoscopic cases.
Figure 1.
Patient flowchart.
For the comparison of groups based on unmatched patient demographics, baseline characteristics and perioperative outcomes (Table 1), patients who underwent robotic-assisted sigmoidectomy were significantly younger, had a higher mean BMI, had a lower preoperative malignant diagnosis, and had a higher rate of prior treatment (chemotherapy, conservative therapy “watch and wait”, and surgery) compared to laparoscopic sigmoidectomy patients. Few patients—regardless of whether they were considered within the entire subject population or only in the malignant subgroup—received chemotherapy or surgery prior to the index surgery. Most were treated conservatively. Within the malignant subgroup, one patient in each of the robotic-assisted and laparoscopic subgroups had received chemotherapy; one patient in the laparoscopic subgroup had been treated conservatively; and two and one patients in the robotic-assisted and laparoscopic subgroups, respectively, had received prior surgical treatment. Surgical and operating room times were shorter for the patients in the laparoscopic group. The robotic-assisted sigmoidectomy group demonstrated lower estimated blood loss (by approximately 12 ml), shorter time to first flatus (approximately 12 h earlier) and shorter time to first bowel movement (approximately 12 h earlier) compared to the laparoscopic group. Groups did not differ on length of hospital stay, perioperative complications, or rates of readmissions and reoperations.
Table 1.
Unmatched Baseline and Perioperative Outcomes from Robotic-Assisted and Laparoscopic Sigmoid Resection
| Variable | Robotic-assisted (n = 211) | Laparoscopic (n = 125) | p-value | Standardized Difference |
|---|---|---|---|---|
| Demographics | ||||
| Age (y), mean ± SD | 60.2 ± 13.0 | 65.1 ± 14.3 | 0.0013 | −0.3609 |
| Gender, n (%) | 0.1212 | 0.1386 | ||
| Female | 107 (50.7) | 72 (57.6) | ||
| Male | 104 (49.3) | 53 (42.4) | ||
| BMI (kg/m2), mean ± SD | 29.0 ± 6.2 | 26.9 ± 4.9 | 0.0009 | 0.3681 |
| Categorical BMI, n (%) | 0.0038 | 0.1448 | ||
| ≤24.9 kg/m2 | 57 (27) | 47 (37.6) | ||
| ≥25.0 to ≤29.9 kg/m2 | 79 (37.4) | 42 (33.6) | ||
| ≥30.0 to ≤34.9 kg/m2 | 42 (19.9) | 31 (24.8) | ||
| ≥35.0 kg/m2 | 33 (15.6) | 5 (4) | ||
| Medical/Surgical History | ||||
| Patients with ≥1 comorbidity, n (%) | 120 (56.9) | 82 (65.6) | 0.1143 | 0.1799 |
| Surgical indication, n (%) | 0.0004 | 0.3957 | ||
| Malignant disease | 34 (16.1) | 41 (32.8) | ||
| Benign disease | 177 (83.9) | 84 (67.2) | ||
| Previous treatment for current condition, n (%) | 167 (79.1) | 76 (60.8) | 0.0003 | 0.4085 |
| Previous abdominal surgery, n (%) | 115 (54.5) | 75 (60) | 0.3258 | −0.1113 |
| Surgery and Hospitalization Characteristics | ||||
| Primary procedure, n (%) | 0.3767 | 0.1015 | ||
| Left hemicolectomy | 27 (12.8) | 12 (9.6) | ||
| Sigmoidectomy | 184 (87.2) | 113 (90.4) | ||
| Cases with ≥1 concomitant procedure, n (%) | 43 (20.4) | 29 (23.2) | 0.5425 | −0.0684 |
| Surgical timea (m), mean ± SD | 164.25 ± 63.1 | 142.2 ± 53.3 | 0.0007 | 0.4348 |
| Operating room timeb (m), mean ± SD | 225.2 ± 66.6 | 198.1 ± 57.5 | 0.0002 | 0.3779 |
| Estimated blood loss (ml), mean ± SD | 48.1 ± 51.5 | 59.6 ± 51.2 | 0.0481 | −0.2242 |
| Conversion to open, n (%) | 0 (0) | 1 (0.8) | 0.372 | −0.127 |
| Length of stay (d), mean ± SD | 3.7 ± 4.6 | 4.2 ± 4.2 | 0.3609 | −0.0145 |
| Time to flatus (d), mean ± SD | 2.0 ± 0.8 | 2.5 ± 1.9 | 0.0067 | −0.3484 |
| Time to bowel movement (d), mean ± SD | 2.6 ± 1.0 | 3.1 ± 2.05 | 0.0112 | −0.3359 |
| Time to regular diet (d), mean ± SD | 3.4 ± 4.2 | 3.2 ± 2.2 | 0.4649 | 0.0814 |
| Complications and Adverse Events | ||||
| Complications, n (%) | ||||
| Intraoperative | 0 (0) | 0 (0) | not valid | |
| Postoperative prior to discharge | 5 (2.4) | 5 (4) | 0.5091 | −0.0929 |
| Post discharge to 30 d | 20 (4.7) | 7 (5.6) | 0.7986 | −0.0389 |
| Readmissionsc, n (%) | 8 (3.8) | 6 (4.9) | 0.6327 | −0.0498 |
| Reoperationsc, n (%) | 1 (0.5) | 2 (1.6) | 0.5572 | −0.1113 |
SD, standard deviation of the mean; BMI, body mass index.
Surgical time: skin to skin.
Operating room time: wheels in to wheels out.
Post discharge to 30 d.
Propensity Weighting
Following IPTW for all multinomial logistic regression analysis variables, patient demographics and baseline characteristics were comparable between the robotic-assisted (n = 344) and laparoscopic (n = 349) groups (Table 2). The laparoscopic group was associated with shorter operating room and surgical times. The robotic-assisted group maintained lower estimated blood loss and shorter time to first flatus when compared to the laparoscopic group. Rates of complications post-discharge to 30 d tended to be lower for the robotic-assisted group: 5.1% vs 8.6% [p = 0.0657]. The robotic-assisted group also had lower rates of readmissions and reoperations: 4% vs 8% [p = 0.029] and 0.5% vs 5.1% [p = 0.0003], respectively.
Table 2.
Inverse Probability Treatment Weighted Outcomes from Robotic-Assisted and Laparoscopic Sigmoid Resection
| Variable | Robotic-assisted (n = 334) | Laparoscopic (n = 349) | p-value | Standardized Difference |
|---|---|---|---|---|
| Demographics | ||||
| Age (y), mean ± SD | 61.8 ± 16.2 | 60.25 ± 25.5 | 0.3467 | 0.0718 |
| Gender, n (%) | 0.7392 | −0.0255 | ||
| Female | 177 (52.9) | 180 (51.6) | ||
| Male | 157 (47.1) | 169 (48.4) | ||
| BMI (kg/m2), mean ± SD | 28.3 ± 7.3 | 28.3 ± 9.4 | 0.9185 | 0.0078 |
| Categorical BMI, n (%) | 0.6582 | −0.0477 | ||
| ≤24.9 kg/m2 | 105 (31.4) | 105 (30) | ||
| ≥25.0 to ≤29.9 kg/m2 | 121 (36.1) | 123 (35.2) | ||
| ≥30.0 to ≤34.9 kg/m2 | 70 (21) | 70 (20) | ||
| ≥35.0 kg/m2 | 38 (11.5) | 51 (14.7) | ||
| Medical/Surgical History | ||||
| Patients with ≥ 1 comorbidity, n (%) | 201 (60.2) | 208 (59.5) | 0.8556 | 0.0139 |
| Surgical indication, n (%) | 0.7366 | −0.0257 | ||
| Malignant disease | 73 (21.8) | 72 (20.7) | ||
| Benign disease | 261 (78.2) | 277 (79.3) | ||
| Previous treatment for current condition, n (%) | 242 (72.6) | 259 (74.2) | 0.6301 | −0.0369 |
| Previous abdominal surgery, n (%) | 187 (56.1) | 179 (51.4) | 0.2168 | 0.0946 |
| Surgery and Hospitalization Characteristics | ||||
| Primary procedure, n (%) | 0.0007 | 0.2603 | ||
| Left hemicolectomy | 48 (14.3) | 23 (6.5) | ||
| Sigmoidectomy | 286 (85.7) | 327 (93.5) | ||
| Cases with ≥1 concomitant procedure, n (%) | 71 (21.3) | 67 (19.1) | 0.479 | 0.0542 |
| Surgical timea (min), mean ± SD | 224.8 ± 81.8 | 206.1 ± 94.95 | 0.0052 | 0.2112 |
| Operating room timeb (min), mean ± SD | 163.2 ± 76.7 | 149.9 ± 89.8 | 0.0388 | 0.1586 |
| Estimated blood loss (mL), mean ± SD | 45.1 ± 60.85 | 59.1 ± 80.8 | 0.0109 | −0.1959 |
| Conversion to open, n (%) | 0 (0) | 2 (0.5) | 0.1872 | −0.1022 |
| Length of stay (d), mean ± SD | 3.7 ± 6.1 | 3.7 ± 5.7 | 0.9907 | −0.0009 |
| Time to flatus (d), mean ± SD | 2.0 ± 1 | 2.4 ± 2.7 | 0.032 | −0.1692 |
| Time to bowel movement (d), mean ± SD | 2.6 ± 1.3 | 2.9 ± 3.0 | 0.1018 | −0.1349 |
| Time to regular diet (d), mean ± SD | 3.4 ± 5.1 | 3.0 ± 3.2 | 0.2234 | 0.1004 |
| Complications and Adverse Events | ||||
| Complications, n (%) | ||||
| Intraoperative | 0 (0) | 0 (0) | not valid | not valid |
| Postoperative prior to discharge | 7 (2.1) | 10 (2.9) | 0.5084 | −0.0507 |
| Post discharge to 30 d | 17 (5.1) | 30 (8.6) | 0.0657 | −0.1416 |
| Readmissionsc, n (%) | 13 (4) | 28 (8) | 0.029 | −0.1656 |
| Reoperationsc, n (%) | 2 (0.5) | 17 (5.1) | 0.0003 | −0.2824 |
SD, standard deviation of the mean; BMI, body mass index.
Surgical time: skin to skin.
Operating room time: wheels in to wheels out.
Post discharge to 30 d.
Subgroup Analyses
Subgroup analyses were performed comparing robotic-assisted and laparoscopic sigmoidectomy approaches for: all patients with a preoperative diagnosis of benign disease (Table 3), obese patients with a preoperative diagnosis of benign disease (Table 4) and all patients with a preoperative diagnosis of malignant disease (Table 5).
Table 3.
Subset of Patients Undergoing Surgery for Treatment of Benign Disease (n = 261): Demographic and Baseline Medical Characteristics, Perioperative Outcomes, and Adverse Events
| Variable | Robotic-assisted (n = 177) | Laparoscopic (n = 84) | p-value |
|---|---|---|---|
| Age (y), mean ± SD | 59.57 ± 13.26 | 64 ± 14.03 | 0.014 |
| Gender, n (%) | 0.4216 | ||
| Female | 96 (54.2) | 50 (59.5) | |
| Male | 81 (45.8) | 34 (40.5) | |
| BMI (kg/m2), mean ± SD | 28.78 ± 5.95 | 26.97 ± 4.88 | 0.0105 |
| Categorical BMI, n (%) | 0.0408 | ||
| ≤24.9 kg/m2 | 48 (27.1) | 31 (36.9) | |
| ≥25.0 to ≤29.9 kg/m2 | 64 (36.2) | 29 (34.5) | |
| ≥30.0 to ≤34.9 kg/m2 | 37 (20.9) | 20 (23.8) | |
| ≥35.0 kg/m2 | 28 (15.8) | 4 (4.8) | |
| Patients with ≥1 comorbidity, n (%) | 97 (54.8) | 52 (61.9) | 0.2788 |
| Previous treatment for current condition, n (%) | 165 (93.2) | 74 (88.1) | 0.1638 |
| Previous abdominal surgery, n (%) | 99 (55.9) | 52 (61.9) | 0.3613 |
| Primary procedure, n (%) | 0.3994 | ||
| Left hemicolectomy | 12 (6.8) | 3 (3.6) | |
| Sigmoidectomy | 165 (93.2) | 81 (96.4) | |
| Cases with ≥1 concomitant procedure, n (%) | 36 (20.3) | 17 (20.2) | 0.9849 |
| Surgical timea (min), mean ± SD | 162.67 ± 64.15 | 145.37 ± 54.73 | 0.0341 |
| Operating room timeb (min), mean ± SD | 223.24 ± 67.91 | 203.89 ± 57.77 | 0.0252 |
| Estimated blood loss (mL), mean ± SD | 50.73 ± 51.31 | 53.87 ± 44.56 | 0.6309 |
| Length of stay (d), mean ± SD | 3.85 ± 4.96 | 3.54 ± 2.15 | 0.4718 |
| Time to flatus (d), mean ± SD | 1.98 ± 0.79 | 2.22 ± 1 | 0.0598 |
| Time to bowel movement (d), mean ± SD | 2.58 ± 1.07 | 2.79 ± 1.27 | 0.2008 |
| Time to regular diet (d), mean ± SD | 3.63 ± 4.47 | 2.89 ± 1.22 | 0.0535 |
| Conversion to open, n (%) | 0 (0) | 0 (0) | |
| Complications, n (%) | |||
| Postoperative prior to discharge | 5 (2.8) | 2 (2.4) | 1 |
| Post discharge to 30 days | 7 (4) | 3 (3.6) | 1 |
| Readmissionsc, n (%) | 6 (3.4) | 2 (2.4) | 1 |
| Reoperationsc, n (%) | 1 (0.6) | 1 (1.2) | 0.5372 |
SD = standard deviation of the mean; BMI = body mass index.
First incision to closure (skin to skin).
Patient entering operating room to patient leaving operating room (wheels in to wheels out).
Discharge to 30 days.
Table 4.
Subset of Patients Who Underwent Surgery for Treatment of Benign Disease and Whose Body Mass Index ≥ 30 kg/m2 (N = 88): Demographic and Baseline Medical Characteristics, Perioperative Outcomes, and Adverse Events
| Variable | Robotic-assisted (n = 65) | Laparoscopic (n = 23) | p-value |
|---|---|---|---|
| Age (y), mean ± SD | 56.31 ± 12.62 | 59.61 ± 15.21 | 0.3104 |
| Gender, n (%) | |||
| Female | 29 (44.6) | 13 (56.5) | 0.3258 |
| Male | 36 (55.4) | 10 (43.5) | |
| BMI (kg/m2), mean ± SD | 35.12 ± 4.2 | 33.14 ± 3.0 | 0.0403 |
| Categorical BMI, n (%) | |||
| ≥30.0 to ≤34.9 kg/m2 | 37 (56.9) | 19 (82.6) | 0.0423 |
| ≥35 kg/m2 | 28 (43.1) | 4 (17.39) | |
| ≥30.0 to ≤34.9 kg/m2 | 37 (56.9) | 19 (82.6) | 0.114 |
| ≥35 to 39.9 kg/m2 | 18 (27.7) | 3 (13) | |
| ≥40 kg/m2 | 10 (15.4) | 1 (4.3) | |
| Patients with ≥1 comorbidity, n (%) | 41 (63.1) | 17 (73.9) | 0.3461 |
| Previous treatment for current condition, n (%) | 62 (95.4) | 21 (91.3) | 0.4675 |
| Previous abdominal surgery, n (%) | 40 (61.5) | 13 (56.5) | 0.6727 |
| Primary procedure, n (%) | |||
| Left hemicolectomy | 4 (6.2) | 0 (0) | 0.2233 |
| Sigmoidectomy | 61 (93.8) | 23 (100) | |
| Cases with ≥1 Concomitant procedure, n (%) | 6 (9.2) | 6 (26.1) | 0.0429 |
| Surgical timea (min), mean ± SD | 184.5 ± 73.4 | 171.13 ± 63.6 | 0.4407 |
| Operating room timeb (min), mean ± SD | 243.7 ± 72.25 | 238.87 ± 60.7 | 0.7748 |
| Estimated blood loss (mL), mean ± SD | 57.08 ± 58.7 | 68.7 ± 60.3 | 0.4203 |
| Length of stay (d), mean ± SD | 3.49 ± 3.51 | 3.22 ± 1.4 | 0.6026 |
| Time to flatus (d), mean ± SD | 1.82 ± 0.6 | 2.09 ± 0.9 | 0.1877 |
| Time to bowel movement (d), mean ± SD | 2.31 ± 1.0 | 2.19 ± 0.8 | 0.6231 |
| Time to regular diet (d), mean ± SD | 3.4 ± 3.2 | 2.56 ± 0.9 | 0.0669 |
| Conversion to open, n (%) | 0 (0) | 0 (0) | |
| Complications, n (%) | |||
| Postoperative prior to discharge | 3 (4.6) | 1 (4.3) | 1 |
| Post discharge to 30 days | 2 (3.1) | 1 (4.3) | 1 |
| Readmissionsc, n (%) | 1 (1.5) | 1 (4.3) | 0.4566 |
| Reoperationsc, n (%) | 0 (0) | 1 (4.3) | 0.2614 |
SD = standard deviation of the mean; BMI = body mass index.
First incision to closure (skin to skin).
Patient entering operating room to patient leaving operating room (wheels in to wheels out).
Discharge to 30 days.
Table 5.
Subset of Patients with Preoperative Diagnosis of Malignant Disease (n = 75): Demographic and Baseline Medical Characteristics, Perioperative, and Oncologic Outcomes and Adverse Events
| Variable | Robotic-assisted (n = 34) | Laparoscopic (n = 41) | p-value |
|---|---|---|---|
| Age (y), mean ± SD | 63.5 ± 11.2 | 67.5 ± 14.6 | 0.203 |
| Gender, n (%) | |||
| Female | 11 (32.4) | 22 (53.7) | 0.0643 |
| Male | 23 (67.6) | 19 (46.3) | |
| BMI (kg/m2), mean ± SD | 30.0 ± 7.4 | 26.8 ± 4.9 | 0.0369 |
| Categorical BMI, n (%) | |||
| ≤24.9 kg/m2 | 9 (26.5) | 16 (39) | 0.1041 |
| ≥25.0 to ≤29.9 kg/m2 | 15 (44.1) | 13 (31.7) | |
| ≥30.0 to ≤34.9 kg/m2 | 5 (14.7) | 11 (26.8) | |
| ≥35 kg/m2 | 5 (14.7) | 1 (2.4) | |
| Patients with ≥1 comorbidity, n (%) | 23 (67.6) | 30 (73.2) | 0.6009 |
| Previous treatment for current condition, n (%) | 2 (5.9) | 2 (4.9) | 1 |
| Previous abdominal surgery, n (%) | 16 (47.1) | 23 (56.1) | 0.4354 |
| Primary procedure, n (%) | |||
| Left hemicolectomy | 15 (44.1) | 9 (22) | 0.0405 |
| Sigmoidectomy | 19 (55.9) | 32 (78) | |
| ≥1 Concomitant procedure, n (%) | 7 (20.6) | 12 (29.3) | 0.3896 |
| Oncologic outcomes | |||
| Tumor size (cm), mean ± SD | 3.09 ± 1.65a | 3.57 ± 2.31b | 0.3323 |
| Harvested lymph nodes (n), mean ± SD | 15.6 ± 7.5a | 16.05 ± 7.1b | 0.8094 |
| TNM Stage | 0.465 | ||
| I | 12 (37.5) | 13 (39.4) | |
| II | 7 (21.9) | 6 (18.2) | |
| III | 13 (40.6) | 11 (33.3) | |
| IV | 0 (0) | 3 (9.1) | |
| Missing | 2 | 8 | |
| Surgical timec (min), mean ± SD | 172.5 ± 56.6 | 135.7 ± 50.4 | 0.004 |
| Operating room timed (min), mean ± SD | 235.2 ± 59.3 | 186.3 ± 55.7 | 0.0004 |
| Estimated blood loss (mL), mean ± SD | 34.6 ± 51.2 | 71.5 ± 61.7 | 0.0069 |
| Length of stay (d), mean ± SD | 3.1 ± 1.5 | 5.5 ± 6.5 | 0.0234 |
| Time to flatus (d), mean ± SD | 2.2 ± 0.8 | 3.1 ± 2.9 | 0.0602 |
| Time to bowel movement (d), mean ± SD | 2.6 ± 0.9 | 3.8 ± 2.9 | 0.0256 |
| Time to regular diet (d), mean ± SD | 2.4 ± 1.4 | 3.6 ± 3.2 | 0.0411 |
| Conversion to open, n (%) | 0 (0) | 1 (2.4) | 1 |
| Complications, n (%) | |||
| Intraoperative | 0 | 0 | — |
| Postoperative prior to discharge | 0 (0) | 3 (7.3) | 0.2465 |
| Post discharge to 30 d | 3 (8.8) | 4 (9.8) | 1 |
| Readmissionse, n (%) | 2 (5.9) | 4 (10) | 0.6809 |
| Reoperationse, n (%) | 0 (0) | 1 (2.5) | 0.3533 |
BMI, body mass index; SD, standard deviation; TNM, tumor, node, metastasis.
n = 33.
n = 41.
skin to skin.
wheels in to wheels out
Discharge to 30 days.
DISCUSSION
The literature has demonstrated the advantages of the laparoscopic approach for sigmoid colon surgery when compared with the open technique—especially in terms of length of stay, time to first flatus, time to first bowel movement, and intraoperative complications.6–7 In their meta-analysis of 22 studies involving a total of 10,898 patients, Siddiqui at al. concluded that, compared with the open approach, laparoscopic sigmoid resection for diverticular disease was associated with lower overall morbidity, earlier return of bowel function, and shorter hospital stay despite the longer operative time of the minimally invasive approach.8 Despite the growing adoption of the robotic-assisted technique in colorectal surgery, there are limited published data comparing the outcomes between the robotic-assisted and the laparoscopic approaches for sigmoid colon resection.4
The current study provides a multicenter, retrospective analysis of robotic-assisted versus laparoscopic sigmoid colon resection for benign and malignant disease. After propensity weighting, the surgical and operating room times were longer for the robotic-assisted group; whereas, estimated blood loss was lower. Rates of readmissions and reoperations were higher in laparoscopic group compared with robotic-assisted group. A literature search evidenced similar reoperation rates for laparoscopic colorectal resection. Saddiqi et al published a prospective data collection analysis of 718 patients who underwent laparoscopic colorectal resection for both benign and malignant disease.9 Group A (n = 476, 66.3%), Group B (n = 190, n = 26.5%), and Group C (n = 52, 7.2%) had, respectively: no previous abdominal surgery, previous abdominal surgery not including colonic surgery, and previous bowel surgery. Of the three groups, reoperation rates within 30 d of surgery were: 5% for Group A, 3% for Group B, and 4% for Group C.
Surgical times for robotic-assisted colorectal surgeries have been reported as comparable to10–12 or longer than those for the laparoscopic approach.13–15 Our study was designed to include surgeons experienced in both robotic-assisted and laparoscopic colorectal surgeries, thereby excluding learning curve cases, as surgical time decreases with increased robotic-assisted colorectal surgery experience.13 Upon obtaining proficiency in both robotic-assisted and laparoscopic techniques, surgeons should be able to expect decreased surgical and operating room times—even when performing challenging cases (e.g., cases involving obesity or malignancy). We thought that proficiency and comfort with the technological advantages of the robotic platform (improved surgical field visualization and exposure, greater instrument maneuverability, a higher degree of instrument articulation,16 improved cognitive and physical ergonomics and decreased surgeon fatigue13) might lead to shorter surgical times; however, our results evidenced an 18 m difference.
Although not exclusive to benign sigmoidectomy, a faster bowel recovery has been reported for robotic-assisted compared to laparoscopic colectomy patients.18–19 Performing surgery in the obese patient is technically challenging, especially during the learning period of laparoscopic surgery—largely due to difficulties with exposure and dissection.20–21 Although safe, laparoscopic colon resection in obese patients is associated with longer surgical times and higher conversion rates.21–23 The trend toward improved times and outcomes in the robotic-assisted group suggests that robotic assistance may facilitate the surgeon's ability to overcome such technical difficulties. This advantage was noticeable especially in the obese group, where we observed a trend toward less difference in surgical and operating room times.
Among patients with malignant disease, lower estimated blood loss, faster bowel recovery and shorter length of stay have been reported for robotic-assisted versus laparoscopic colectomy.18–19,24 Although statistically significant, the difference in blood loss between the robotic-assisted and laparoscopic groups was approximately 36 ml and likely reflected the technological ability of the robot for performing finer and more precise dissection as opposed to a clinically relevant outcome.25 We found that patients who underwent robotic-assisted sigmoidectomy experienced faster bowel recovery. The faster bowel recovery most likely played a role in the 2.4 d shorter hospital stay for the robotic-assisted sigmoidectomy patients. As hypothesized by Trastulli and colleagues, the robotic platform's ergonomic enhancement, “fulcrum effect” mitigation, and instrument precision may contribute to less dissection-related trauma to the viscera, tissues and mesentery—all of which may lead to faster bowel recovery and shorter hospital stay.24 Prior studies reported an earlier bowel recovery and shorter hospital stay for laparoscopy than laparotomy in malignant colectomy,2 a faster bowel recovery and shorter length of stay for robotic-assisted than laparoscopy in colectomy,18–19,24 a shorter hospital stay for robotic-assisted than laparoscopy in malignant colorectal surgery,26 and a faster bowel recovery, fewer days to soft diet and shorter hospital stay for robotic-assisted than laparoscopy in malignant anterior resection surgery.27 Our findings build on the existing literature by demonstrating an earlier recovery of bowel function and shorter hospital stay for patients who undergo robotic-assisted, compared to laparoscopic, malignant sigmoidectomy.
The current study is limited by its retrospective design. A randomized, prospective comparative study of outcomes from robotic-assisted and laparoscopic sigmoidectomy patients is optimal but difficult, as it requires patient willingness to be randomized. The study design mitigated learning curve bias by including surgical patients only after surgeons became proficient in both robotic-assisted and laparoscopic techniques. Additionally, we chose to perform inverse probability of treatment weighting of baseline variables to reduce patient selection bias and allow our analysis of demographically and clinically comparable patient populations.
CONCLUSION
Robotic-assisted sigmoid colon resection is clinically effective. When robotic-assisted surgery is utilized in more complex sigmoid colon resection scenarios, it offers technical and procedural advantages over laparoscopic surgery that could translate in less blood loss, shorter time to first flatus and bowel movement, and lower rates of readmissions and reoperations. Robotic-assisted sigmoid resection provides a minimally invasive alternative to the laparoscopic approach with improved intraoperative and postoperative outcomes for colorectal patients.
Contributor Information
Luca Giordano, Department of Minimally Invasive and Robotic Surgery, Jefferson Health Northeast Torresdale..
Andrew A. Kassir, Department of Colon and Rectal Surgery, Colon and Rectal Clinic of Scottsdale..
Reza A. Gamagami, Department General Surgery and Colon & Rectal Surgery, Progressive Surgical Associates..
Henry J. Lujan, Department of Colorectal Surgery, Jackson Health System..
Gustavio Plasencia, Department of Colorectal Surgery, Jackson Health System..
Cesar Santiago, Department of Colon and Rectal Surgery, St. Joseph Hospital..
References:
- 1. Nelson H, Sargent DJ, Wieand HS, et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med.. 2004;350(20):2050–2059. [DOI] [PubMed] [Google Scholar]
- 2. Veldkamp R, Kuhry E, Hop WC, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol.. 2005;6(7):477–484. [DOI] [PubMed] [Google Scholar]
- 3. Rea JD, Cone MM, Diggs BS, Deveney KE, Lu KC, Herzig DO. Utilization of laparoscopic colectomy in the United States before and after the clinical outcomes of surgical therapy study group trial. Ann Surg.. 2011;254(2):281–288. [DOI] [PubMed] [Google Scholar]
- 4. Liao G, Zhao Z, Lin S, et al. Robotic-assisted versus laparoscopic colorectal surgery: a meta-analysis of four randomized controlled trials. World J Surg Oncol. 2014;12:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Klarenbeek BR, Veenhof AA, Bergamaschi R, et al. Laparoscopic sigmoid resection for diverticulitis decreases major morbidity rates: a randomized control trial: short-term results of the Sigma Trial. Ann Surg.. 2009;249(1):39–44. [DOI] [PubMed] [Google Scholar]
- 7. Cirocchi R, Farinella E, Trastulli S, Sciannameo F, Audisio RA. Elective sigmoid colectomy for diverticular disease. Laparoscopic vs open surgery: a systematic review. Colorectal Dis.. 2012;14(6):671–683. [DOI] [PubMed] [Google Scholar]
- 8. Siddiqui MR, Sajid MS, Khatri K, Cheek E, Baig MK. Elective open versus laparoscopic sigmoid colectomy for diverticular disease: a meta-analysis with the Sigma trial. World J Surg.. 2010;34(12):2883–2901. [DOI] [PubMed] [Google Scholar]
- 9. Siddiqi NN, Zaman Q, Patel KM, Odermatt M, Khan J, Parvaiz A. Laparoscopic colorectal resection in patients with previous abdominal and colonic surgery. J Minim Invasive Surg Sci. 2016;4(3):e31968. [Google Scholar]
- 10. Elliott PA, McLemore EC, Abbass MA, Abbas MA. Robotic versus laparoscopic resection for sigmoid diverticulitis with fistula. J Robot Surg.. 2015;9(2):137–142. [DOI] [PubMed] [Google Scholar]
- 11. Gorgun E, Ozben V, Costedio M, Stocchi L, Kalady M, Remzi F. Robotic versus conventional laparoscopic rectal cancer surgery in obese patients. Colorectal Dis.. 2016;18(11):1063–1071. [DOI] [PubMed] [Google Scholar]
- 12. Vasudevan V, Reusche R, Wallace H, Kaza S. Clinical outcomes and cost-benefit analysis comparing laparoscopic and robotic colorectal surgeries. Surg Endosc.. 2016;30(12):5490–5493. [DOI] [PubMed] [Google Scholar]
- 13. Spinoglio G, Summa M, Priora F, Quarati R, Testa S. Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum.. 2008;51(11):1627–1632. [DOI] [PubMed] [Google Scholar]
- 14. Delaney CP, Lynch AC, Senagore AJ, Fazio VW. Comparison of robotically performed and traditional laparoscopic colorectal surgery. Dis Colon Rectum. 2003;46(12):1633–1639. [DOI] [PubMed] [Google Scholar]
- 15. Davis BR, Yoo AC, Moore M, Gunnarsson C. Robotic-assisted versus laparoscopic colectomy: cost and clinical outcomes. J Soc Laparoendosc Surg.. 2014;18(2):211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park EJ, Baik SH. Robotic surgery for colon and rectal cancer. Curr Oncol Rep. 2016;18:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee GI, Lee MR, Clanton T, Sutton E, Park AE, Marohn MR. Comparative assessment of physical and cognitive ergonomics associated with robotic and traditional laparoscopic surgeries. Surg Endosc.. 2014;28(2):456–465. [DOI] [PubMed] [Google Scholar]
- 18. Duan BS, Zhao GH, Yang H, Wang Y. A pooled analysis of robotic versus laparoscopic surgery for colon cancer. Surg Laparo Endo Per.. 2016;26(6):523–530. [DOI] [PubMed] [Google Scholar]
- 19. Chang YS, Wang JX, Chang DW. A meta-analysis of robotic versus laparoscopic colectomy. J Surg Res.. 2015;195(2):465–474. [DOI] [PubMed] [Google Scholar]
- 20. Kato JM, Iuamoto LR, Suguita FY, Essu FF, Meyer A, Andraus W. Impact of obesity and surgical skills in laparoscopic totally extraperitoneal hernioplasty. Arq Bras Cir Dig.. 2017;30(3):169–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martin ST, Stocchi L. Laparoscopic colorectal resection in the obese patient. Clin Colon Rectal Surg.. 2011;24(4):263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg.. 2003;7(4):558–561. [DOI] [PubMed] [Google Scholar]
- 23. Khoury W, Stocchi L, Geisler D. Outcomes after laparoscopic intestinal resection in obese versus non-obese patients. Br J Surg.. 2011;98(2):293–298. [DOI] [PubMed] [Google Scholar]
- 24. Trastulli S, Cirocchi R, Desiderio J, et al. Robotic versus laparoscopic approach in colonic resections for cancer and benign diseases: systematic review and meta-analysis. PLoS One. 2015;10(7):e0134062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Deutsch GB, Sathyanarayana SA, Gunabushanam V, et al. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc. 2012;26 (4):956–963. [DOI] [PubMed] [Google Scholar]
- 26. Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM. A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum.. 2011;54(2):144–150. [DOI] [PubMed] [Google Scholar]
- 27. Lim DR, Min BS, Kim MS, et al. Robotic versus laparoscopic anterior resection of sigmoid colon cancer: comparative study of long-term oncologic outcomes. Surg Endosc. 2013;27(4):1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]