Abstract
Background and aim
To conduct a systematic literature review and analyze the demographic/biochemical parameters and clinical outcomes of COVID-19 patients with diabetic ketoacidosis (DKA) and combined DKA/HHS (hyperglycemic hyperosmolar syndrome).
Methods
PubMed, Scopus, Embase, and Google Scholar databases were systematically searched till August 3, 2020 to identify studies reporting COVID-19 patients with DKA and combined DKA/HHS. A total of 19 articles reporting 110 patients met the eligibility criteria.
Results
Of the 110 patients, 91 (83%) patients had isolated DKA while 19 (17%) had DKA/HHS. The majority of the patients were male (63%) and belonged to black ethnicity (36%). The median age at presentation ranged from 45.5 to 59.0 years. Most of the patients (77%) had pre-existing type 2 diabetes mellitus. Only 10% of the patients had newly diagnosed diabetes mellitus. The median blood glucose at presentation ranged from 486.0 to 568.5 mg/dl, being higher in patients with DKA/HHS compared to isolated DKA. The volume of fluid replaced in the first 24 h was higher in patients with DKA/HHS in contrast to patients with DKA alone. The in-hospital mortality rate was 45%, with higher mortality in the DKA/HHS group than in the isolated DKA group (67% vs. 29%). pH was lower in patients who had died compared to those who were discharged.
Conclusion
DKA in COVID-19 patients portends a poor prognosis with a mortality rate approaching 50%. Differentiating isolated DKA from combined DKA/HHS is essential as the latter represents nearly one-fifth of the DKA cases and tends to have higher mortality than DKA alone.
Keywords: COVID-19, Diabetic ketoacidosis, Hyperglycemic hyperosmolar syndrome, Subcutaneous insulin therapy
Highlights
-
•
Occasional reports of diabetic ketoacidosis (DKA) have been reported in COVID-19.
-
•
DKA is more common in males and in individuals with black ethnicity and pre-existing T2DM.
-
•
DKA in COVID-19 portends a poor prognosis with mortality being higher in patients with combined DKA/HHS than isolated DKA.
1. Introduction
People with diabetes mellitus (DM) represent a highly vulnerable population at a high-risk of poor prognosis with the novel coronavirus disease (COVID-19). The presence of DM increases the probability of severe disease, admission to the intensive care unit, and mortality due to COVID-19 [[1], [2], [3], [4]]. Impaired host defense, immune dysregulation with an increased predisposition to cytokine storm, and altered angiotensin-converting enzyme 2 (ACE2) expression have been implicated as the underlying pathophysiological mechanisms [3,5].
As with other severe infections [6], diabetic ketoacidosis (DKA) has been reported in patients with COVID-19 [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. With regard to coronaviruses, it has been shown that SARS-CoV (responsible for the SARS outbreak in 2003) binds to ACE2 in the pancreatic islets leading to islet damage and acute diabetes [25]. As SARS-CoV-2 (causative organism of COVID-19) also binds to ACE2, the virus might also result in acute diabetes [26]. This theoretical pathophysiology could lead to insulinopenia and increased risk of DKA, especially in patients with pre-existing DM [13]. Besides, interleukin-6, an important cytokine of the hyper-inflammatory state in COVID-19, has also been found to be elevated in DKA and serves as a driver of ketogenesis [27]. Thus, although there is insufficient data, DKA may be more prevalent in COVID-19 and SARS-CoV-2 may pose an increased risk over other infectious diseases of equivalent severity [13]. Apart from DKA, occasional cases of combined DKA and hyperglycemic hyperosmolar syndrome (HHS) have also been reported in COVID-19 [22,28].
The clinical outcome of COVID-19 patients with DKA has been somewhat conflicting across studies; while one study found a mortality rate of 50% in COVID-19 patients with DKA [9], another study found that patients with DKA were more likely to survive compared to non-DKA patients [17]. Moreover, patients with combined DKA and HHS tend to have higher mortality than either DKA or HHS alone [29], however, similar comparative data in COVID-19 is lacking.
The aim of the study was to provide a comprehensive systematic literature review of DKA and combined DKA/HHS in patients with confirmed COVID-19 in order to analyze the demographic and biochemical parameters and the clinical outcomes.
2. Material and Methods
2.1. Search strategy and selection of studies
A systematic review of the literature was performed as per the PRISMA guidelines [30] across PubMed, Scopus, Embase and Google Scholar databases till August 3, 2020 using the following keywords: “COVID-19”, “diabetic ketoacidosis, “ketosis”, “ketonemia”, “hyperglycemic emergencies”, “hyperglycemic crises” with interposition of the Boolean operator “AND”/“OR”. The search was conducted independently by two authors (RP and MB). Articles hence identified were further screened. Duplicate articles, articles in non-English language, reviews, and comments/communications and articles not pertaining to DKA in patients with COVID-19 were excluded. Finally, a total of 19 articles met the eligibility criteria and were included (Fig. 1 ). The included articles and the number of patients in each article have been summarized in Table 1 .
Fig. 1.
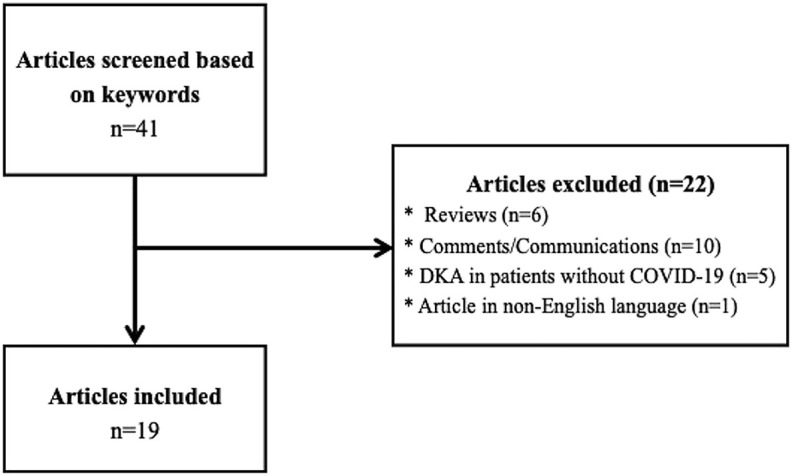
Flowchart showing the study selection process.
Table 1.
Summarizing the articles included in the systematic review.
2.2. Data extraction
The following data were extracted from the included studies: sex, age of the patient at presentation, ethnicity, type of diabetes mellitus (pre-existing T1DM vs. pre-existing T2DM vs. newly diagnosed DM), ongoing medications, body mass index (BMI), biochemical investigations (blood glucose, pH, bicarbonate, anion gap, glycated hemoglobin) and clinical outcomes.
2.3. Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) 23.0 software program (SPSS Inc., Chicago, IL, USA). Kolmogorov-Smirnov test was used to check the normality of individual patient data, wherever available. Normally distributed data were expressed as mean ± standard deviation (SD), while non-parametric data were expressed in median (interquartile range, IQR). The comparison of biochemical parameters between groups (DKA vs. combined DKA and HHS; discharged vs. deceased) were made using Mann-Whitney U test. A p value < 0.05 was considered significant.
3. Results
3.1. Demography
A total of 110 COVID-19 patients diagnosed with DKA were included in the final analysis. Amongst these 110 patients, 91 (83%) patients had DKA alone while 19 (17%) patients had combined DKA/HHS [22,28]. The demographic data have been presented in Table 2 . Notably, the majority of the patients (63%) were male. The median age at presentation ranged from 45.5 years to 59.0 years. In the 28 patients in whom individual data was available, only one patient belonged to the pediatric age group [16]. The majority of the patients were black (African-American/Black African/African/Afro-Caribbean) (n = 30, 36%), followed by Hispanic (n = 19, 23%) and White (Caucasian) (n = 10, 12%) ethnicity. The majority of the patients (77%) had pre-existing T2DM. The use of SGLT2 inhibitors was reported in 7 patients.
Table 2.
Showing demographic parameters of the COVID-19 patients with DKA (and combined DKA/HHS).
| Parameter | Value |
|---|---|
| Age (years) [Median (IQR)] | 45.5 (36.2–57.7) [7,8,[10], [11], [12], [13], [14], [15], [16],[18], [19], [20], [21],23,24,28]a |
| 57.0 (48.0–64.0) [22] b | |
| 59.0 (42.3–70.0) [9] | |
| Sex (N = 102) c | Male (n = 64, 63%) |
| Female (n = 38, 37%) | |
| Ethnicityd (N = 84) | Black (n = 30, 36%) e |
| Hispanic (n = 19, 23%) | |
| White (Caucasian) (n = 10, 12%) | |
| Asian (n = 6, 7%) | |
| Mixed (n = 4, 5%) | |
| Others (n = 8, 9%) | |
| Unknown (n = 7, 8%) | |
| Type of diabetes f (N = 97) | Pre-existing T1DM (n = 12, 12%) |
| Pre-existing T2DM (n = 74, 77%) | |
| Newly diagnosed (n = 10, 10%) | |
| Gestational DM (n = 1, 1%) | |
| Use of SGLT2 inhibitors g | 7 |
| BMI (kg/m2) [Median (IQR)] | 26.6 (23.7–32.3) [7,[11], [12], [13],16,28] h |
| 24.7 (21.3–28.5) [22] b | |
| 27.1 (23.2–33.0) [9] |
COVID-19: Novel coronavirus disease; DKA: Diabetic ketoacidosis; HHS: Hyperglycemic hyperosmolar syndrome; IQR: Interquartile range; SGLT2: Sodium-glucose transporter 2; BMI: Body mass index.
Age calculated from individual patient data available from 16 studies.
Only data of patients with isolated DKA has been shown.
Individual data on patient sex were available in 102 patients from 18 studies [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16],[18], [19], [20], [21], [22], [23], [24],28].
Patients with black ethnicity include African-American, Black African, African and Afro-Caribbean patients.
Individual data on type of diabetes were available in 97 patients from 15 studies [7,[9], [10], [11], [12], [13], [14],16,[18], [19], [20],[22], [23], [24],28].
BMI calculated from individual patient data available from 6 studies.
3.2. Biochemical parameters at presentation
The biochemical parameters at admission have been summarized in Table 3 . The median blood glucose at presentation ranged from 486.0 mg/dl to 568.5 mg/dl. On separately analyzing patients in whom individual data were available (n = 25), it was found that patients with combined DKA and HHS (n = 6) had significantly higher blood glucose than those with DKA alone (n = 22) (p = 0.004). Three patients had blood glucose <250 mg/dl at presentation (euglycemic DKA) [11,13,23]; two were on SGLT2 inhibitor therapy [11,13] while one patient had gestational DM [23]. There was no difference in glycated hemoglobin (HbA1c) (p = 0.225), pH (p = 0.144), bicarbonate (p = 0.242), and anion gap (p = 0.478) between patients with DKA vs. those with combined DKA and HHS.
Table 3.
Showing biochemical parameters at presentation in COVID-19 patients with DKA (and combined DKA/HHS).
| Biochemical parameter at presentation | Valuea |
|---|---|
| Blood glucose (mg/dl) | 568.5 (385.5–889.7) [7,8,[10], [11], [12], [13], [14], [15], [16],[18], [19], [20], [21],23,24,28] b |
| 486.0 (396.0–558.0) [22] g | |
| 506.5 (252.0–1485.0) [9] | |
| HbA1c (%) | 11.7 (9.5–13.2) [[10], [11], [12], [13],16,19,23,24,28] c |
| 12.4 (10.7–14.2) [22] g | |
| pH | 7.17 (6.99–7.24) [7,8,[10], [11], [12], [13], [14], [15], [16],18,21,23,24,28] d |
| 7.20 (6.90–7.30) [22] g | |
| Bicarbonate (mmol/l) | 8.0 (6.0–12.5) [7,8,[10], [11], [12], [13], [14],16,23,24,28] e |
| 11.8 (7.8–15.4) [22] g | |
| Anion gap (mEq/l) | 29.0 (18.0–32.0) [7,8,12,13,15,20,24,28] f |
| 14.8 (10.4–20.5) [22] g | |
| 28.1 (14.3–41.2) [9] |
COVID-19: Novel coronavirus disease; DKA: Diabetic ketoacidosis; HHS: Hyperglycemic hyperosmolar syndrome; HbA1c: Glycated hemoglobin.
Data represented as median (interquartile range).
Blood glucose calculated from individual patient data available from 16 studies.
HbA1c calculated from individual patient data available from 9 studies.
pH calculated from individual patient data available from 14 studies.
Serum bicarbonate calculated from individual patient data available from 11 studies.
Anion gap calculated from individual patient data available from 8 studies.
Only data of patients with isolated DKA has been shown.
3.3. Treatment and clinical outcomes
The majority of the patients were initially managed with a standard treatment protocol for DKA with intravenous fluids and insulin infusion. The median (IQR) volume of fluid replaced in the first 24 h was 3.8 (3.0–5.0) liters and 5.0 (4.0–6.0) liters for patients with DKA and combined DKA/HHS, respectively [22]. In another series, the median (IQR) volume of fluid replaced was 3.0 (1.0–8.0) liters in the initial 24 h [9]. Only 8 patients reported in 2 studies were treated with subcutaneous insulin [9,13]. Four patients had thromboembolic events [11,15,18,20]. Data on the final outcome (in terms of discharged, deceased, or hospitalized) was available for 78 patients [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16],[18], [19], [20], [21],23,24,28]. Among these 78 patients, 41 (52.5%) were discharged, 35 (45.0%) were deceased and 2 (2.5%) had remained hospitalized. Table 4 summarizes the clinical and biochemical predictors of final outcome in 27 patients (with individual patient data) who had either been discharged (n = 17) or deceased (n = 10) [7,8,[10], [11], [12], [13], [14], [15], [16],[18], [19], [20], [21],23,24,28]. All the deceased individuals were males. Patients with DKA/HHS had higher mortality compared to patients with isolated DKA (67% vs. 29%). In addition, deceased patients had lower pH at admission than those who were discharged (p = 0.017).
Table 4.
Showing comparison of clinical outcomes of COVID-19 patients with DKA (and combined DKA/HHS) in whom individual patient data were available (N = 27).
| Parameter | Discharged (n = 17) | Deceased (n = 10) | p value |
|---|---|---|---|
| Age (years) | 46.0 (33.5–54.0) | 42.5 (34.2–59.7) | 1.000 |
| Sex | Male = 11 | Male = 10 | – |
| Female = 6 | Female = 0 | ||
| DKA vs. Combined DKA/HHS | DKA = 15 (71%) | DKA = 6 (29%) | – |
| DKA/HHS = 2 (33%) | DKA/HHS = 4 (67%) | ||
| Blood glucose (mg/dl) | 463.0 (347.0–641.0) | 801.5 (376.5–1080.5) | 0.120 |
| pH | 7.23 (7.09–7.26) | 7.00 (6.91–7.11) | 0.017 |
| Bicarbonate (mmol/l) | 10.4 (6.0–15.0) | 7.0 (5.7–8.0) | 0.098 |
| Anion gap (mEq/l) | 25.5 (16.8–34.0) | 29.0 (28.0–30.5) | 0.806 |
COVID-19: Novel coronavirus disease; DKA: Diabetic ketoacidosis; HHS: Hyperglycemic hyperosmolar syndrome.
4. Discussion
To the best of our knowledge, this is the first comprehensive systematic review of DKA in patients with COVID-19. Amongst 110 cases hitherto described in the literature, 91 patients had DKA alone while 19 patients had combined DKA/HHS. The majority of the patients was male (63%) and had pre-existing T2DM (77%). Apart from blood glucose, none of the biochemical parameters at presentation was significantly different between the DKA and DKA/HHS groups. The in-hospital mortality rate was 45%, with higher mortality in the DKA/HHS group than in the isolated DKA group (67% vs. 29%). pH was lower in patients who had died compared to those who were discharged.
Diabetic ketoacidosis is the most common hyperglycemic crisis, which also includes HHS and the combined syndrome of DKA and HHS, often referred to as hyperosmolar ketoacidosis [31]. Diabetic ketoacidosis occurs in the setting of relative or absolute insulin deficiency that tips the insulin:glucagon ratio in favor of glucagon; this leads to reduced glucose utilization and unchecked lipolysis, causing excessive formation of ketone bodies and finally metabolic acidosis [32,33]. Although DKA is more likely to occur in T1DM, it is estimated that the majority of the DKA cases worldwide occur in patients with T2DM due to its higher prevalence [33]. Diabetic ketoacidosis is itself a pro-inflammatory state [27], but is often precipitated by an underlying illness. Infections are the commonest precipitating factors [[33], [34], [35]]. Urinary tract infections and pneumonia account for the majority of the infections [36]. Likewise, reports of DKA and DKA/HHS in patients with COVID-19 have emerged.
There are theoretical concerns that the SARS-CoV-2, just like its cousin the SARS-CoV, could bind to ACE2 expressed on the pancreatic islets, leading to islet destruction and acute diabetes [25,26]. In a patient with underlying DM (especially T2DM), the destruction of the residual β-cells by the virus would result in a state of complete insulinopenia, thereby precipitating DKA [13]. Moreover, the pro-inflammatory milieu seen even in non-severe patients with COVID-19 would theoretically promote ketogenesis, hence, predisposing an individual to ketosis [27]. In a series of 658 hospitalized patients (129 had DM) with confirmed COVID-19 from China, 42 (6.4%) presented with either urine or serum ketones. The presence of ketosis was associated with higher rates of acute respiratory distress syndrome, acute liver injury, the requirement for mechanical ventilation, a longer length of hospital stay, and mortality. Of the 42 patients with ketosis, 15 (35.7%) had diabetes mellitus; of these 15 patients, 3 had concomitant acidosis, amounting to DKA. Hence, the overall prevalence of DKA amongst patients with DM in the series was 2.3% [21]. On the contrary, in a retrospective single-center study from the United Kingdom, the prevalence of DKA in 87 COVID-19 patients with DM was 9.2% [17]. In another series from the United Kingdom, the prevalence of DKA in confirmed COVID-19 patients was 5.9% [10]. The marked dissimilarity in the prevalence of DKA may be explained based on differences in the population studied and the underlying COVID-19 disease severity.
In our systematic review, we found that the majority (63%) of the reported cases of DKA (or DKA/HHS) were male. Gender difference as a risk factor for DKA is controversial; some studies consider female sex as a risk factor [37], while others have shown no significant differences in males and females [38]. The preponderance of male cases among DKA patients could be due to the fact that COVID-19 tends to be more severe in males, resulting in more hospitalizations and deaths compared with females [39,40]. Similarly, the relative absence of pediatric COVID-19 patients with DKA also underscores the fact that children tend to have lower rates of severe COVID-19 [41]. Regarding ethnicity, we found that 36% of the patients belonged to black ethnicity, while 23% were Hispanic, 12% were Caucasian and only 7% were Asian. In general, BAME (Black, Asian and Minority Ethnic) individuals are at an increased risk of acquiring SARS-CoV-2 infection and of worse clinical outcomes from COVID-19 compared to White individuals [42]. In addition, ethnic minorities are also at a higher risk of DKA [43]. Taken together, the high prevalence of DKA in people with black ethnicity is ably explained. On the contrary, the underrepresentation of the Asian population could be fallacious as all the studies reporting patient ethnicity were either from the United Kingdom or from the United States of America [9,10,22,28].
Regarding the type of DM, we found that the majority of COVID-19 patients with DKA (77%) had underlying T2DM, likely because of the higher prevalence of T2DM worldwide [33]. In 10 patients, DM was diagnosed at admission, categorizing them as newly diagnosed DM. Of these 10 patients, 7 had HbA1c > 9.5% (ranging from 9.6% to 14.2%) [10,12,13,16,24,28], implying that these patients had underlying undiagnosed DM of significant duration prior to the present admission and unlikely to have resulted from the incident SARS-CoV-2 infection. Although data on HbA1c were not available in rest of the 3 patients, one had a BMI of 42.56 kg/m2 at the time of admission and was likely a case of hitherto undiagnosed T2DM [7].
Analyses of the biochemical parameters at admission showed that the median blood glucose ranged from 486.0 mg/dl to 568.5 mg/dl. In a series of 967 patients admitted with DKA, 176 had a viral infection (presumed). The mean blood glucose in these 176 patients was 640.0 mg/dl, not strikingly different from that seen in the COVID-19 patients [44]. However, patients presenting with DKA/HHS had significantly higher blood glucose than patients with isolated DKA, a feature that has been described in the literature [29]. DKA/HHS form a clinical entity distinct from DKA and HHS alone and some small studies have reported that up to 30% of DKA have combined features of DKA and HHS [45,46]. The diagnosis of DKA/HHS is based on the documentation of raised effective serum osmolality in a patient with biochemically confirmed DKA. The cut-off defining elevated effective serum osmolality in DKA/HHS is variable with some of the most recent large-scale studies using a threshold of 300 mOsm/kg. In general, patients with DKA/HHS tend to have higher mortality than those with isolated DKA or HHS even after adjusting for age, sex, and BMI [29]. In addition, patients with combined DKA/HHS tend to be more volume depleted and hence require more intravenous fluids in the first 24 h [22]. Therefore, early differentiation of DKA/HHS from isolated DKA is essential. In our systematic review, we found that 17% of the patients had combined DKA/HHS. This could be an underestimation as very few studies had reported effective serum osmolality.
The main tenets of DKA or DKA/HHS management include the triad of fluid resuscitation, potassium repletion, and insulin replacement [33,36]. Patients with DKA and combined DKA/HHS present with profound dehydration and fluids form the cornerstone of therapy. Administration of intravenous fluid corrects intravascular volume, improves renal perfusion, and thereby promotes glucosuria and ketonuria. The simultaneous decrease in the concentrations of circulating counter-regulatory hormones also reduces insulin resistance [33]. In adults with DKA, the American Diabetes Association and the UK guidelines recommend the use of isotonic saline (0.9% sodium chloride solution) for initial fluid replacement, administered at a rate of 500–1000 ml/h during the first 2–4 h [47,48]. However, in a patient with COVID-19, there are concerns about precipitating or exacerbating “lung leak” by liberal fluid administration resulting in increased extravascular lung water that would adversely affect gas exchange and worsen hypoxia [49]. Hence, the recently published UK guidelines by the National Inpatient Diabetes COVID-19 Response Group recommend conservative use of isotonic saline in COVID-19 patients with DKA. The guidelines suggest that after an initial fluid bolus of 250 ml over 15 min, the subsequent infusion rate should be guided by the patient’s weight and the pH at presentation. Patients with a pH ≤ 7.1 would benefit from a more aggressive fluid replacement regimen than those with lesser degrees of acidosis [50]. Apart from protecting the lungs, a conservative fluid replacement strategy would also reduce the chances of development of hyperchloremic metabolic acidosis due to excessive chloride resulting from the administration of high volumes of saline [51]. However, conservative fluid replacement can lead to delayed clearance of serum ketones resulting in protracted ketonemia. We found that the strategic use of conservative fluid replacement was reported in 12 patients in only 1 series [22].
The usual route of insulin replacement in patients with DKA or combined DKA/HHS is intravenous infusion. Patients on intravenous infusion usually require intensive care unit (ICU) admission necessitating frequent glucose monitoring and periodic insulin dose adjustments. However, during a pandemic, minimizing nursing time at the bedside, protecting healthcare workers, and preserving personal protective equipment (PPE) is a priority. In addition, the increased demand for pumps to deliver vasopressors has led to concerns of possible shortages of infusion pumps and/or 50 ml syringes being available for insulin infusions to manage these hyperglycemic emergencies [50]. Nevertheless, most of the problems can be circumvented by the use of subcutaneous insulin therapy instead of intravenous insulin infusion. Several studies have demonstrated that the use of subcutaneous rapid-acting insulin analogues is a safe and effective alternative in mild/moderate uncomplicated DKA [13]. A Cochrane review evaluating 5 randomized controlled trials concluded that there are neither advantages nor disadvantages when comparing the effects of subcutaneous rapid-acting insulin analogues versus intravenous regular insulin for treating mild or moderate DKA [52]. Thus, amid the ongoing COVID-19 pandemic, subcutaneous insulin therapy can be an effective means of treating DKA while reducing ICU utilization, inadvertant exposure and PPE use. The National Inpatient Diabetes COVID-19 Response Group recommends the use of subcutaneous rapid-acting insulin analogues at an initial dose of 0.4 units/kg body weight every 4 hourly with the aim of reducing serum ketone by at least 2 mmol/l over 4 h. The dose needs to be reduced to 0.2 units/kg body weight once the blood glucose falls below 250 mg/dl. The guidelines also recommend the initiation or continuation of long-acting insulin preparation from the very outset when using subcutaneous strategies to treat DKA [50]. In our systematic review, we found that the use of subcutaneous insulin was limited to only 8 patients as reported in 2 studies [9,13]. Although it may seem advantageous, subcutaneous strategies are not recommended for patients with severe and/or complicated DKA in whom intensive monitoring and use of intravenous insulin is required. Irrespective of the degree of acidosis, patients with combined DKA/HHS require close monitoring and hence ICU care [13,48]. Concerning the dose of insulin infusion, Armeni et al. reported that 35% of the COVID-19 patients with hyperglycemic emergencies required an increase in the fixed dose of insulin infusion above the recommended insulin dose for DKA of 0.1 units/kg/hour [22]. The pro-inflammatory milieu induced by COVID-19 can lead to a high degree of insulin resistance, thereby increasing the insulin requirement. In addition, some of the medications used in the management of COVID-19, notably, corticosteroids, remdesivir, lopinavir/ritonavir, darunavir/cobicistat and interferon-β1, can also worsen hyperglycemia [4,53].
Another aspect of DKA management that needs to be considered is appropriate electrolyte management. Almost all patients with DKA have substantial potassium deficits at presentation. As insulin treatment is initiated, ketone production is suppressed, and acidosis begins to resolve. In addition, insulin drives potassium back into the cell, and the individual can become severely hypokalemic. Thus, frequent monitoring of potassium and appropriate replacement is an integral part of the management of DKA. SARS CoV-2 binds to ACE2 with subsequent downregulation of the enzyme and reduced degradation of aldosterone, leading to hypokalemia [3]. Hence, there are concerns that patients with COVID-19 are at a higher risk of hypokalemia [54]; hence, hypokalemia can be expected to be all the more profound in COVID-19 patients with DKA. It thus becomes imperative to stringently monitor serum potassium and replenish it accordingly. Besides, patients with DKA/HHS are more prone to develop severe hypokalemia (serum potassium < 2.5 mEq/l) within first 48 h of treatment initiation compared to patients with isolated DKA, hence, monitoring serum potassium becomes all the more critical for the former group of patients [29]. In addition, hypophosphatemia is not uncommon in patients with DKA. Hypophosphatemia can lead to neuromuscular weakness and worsen respiratory failure in COVID-19 patients [55]. Hence, timely phosphate replacement is also crucial in such patients.
In the developed nations, the hospital case-fatality rates due to DKA have markedly declined, with current reported death rates being <1% [34,56]. Mortality substantially increases in those with comorbidities and with advancing age, reaching up to 8–10% in those aged >65–75 years [35,57]. In DKA patients with underlying COVID-19, we found an in-hospital mortality rate of 45% with one series reporting a mortality rate as high as 50% [9]. As has been reported in the literature [29], we found that patients with DKA/HHS had higher mortality compared to DKA alone. Low pH at presentation was found to be significantly associated with in-hospital mortality. Admission blood glucose was not associated with patient outcomes. This underscores the importance of acidosis as predictive of mortality in patients with DKA (and combined DKA/HHS) and the known role of treatment protocols to target acidosis as the main goal of management, rather than reduction of blood glucose levels alone [29]. Four patients were reported to have arterial or venous thromboembolic events [11,15,18,20]; in addition, a 60-year-old man had presented with left hemiparesis and was found to have a right middle cerebral artery infarct, consistent with acute cerebrovascular accident [24]. An important complication of DKA includes the development of a hypercoagulable state with an increased risk of deep venous thrombosis [33]. The risk is expected to increase by multiple-folds in a patient with underlying COVID-19 as the disease has been found to aggravate the chances of thrombotic complications [58,59]. Thus, it is essential that all hospitalized COVID-19 patients with DKA are kept on low-molecular weight heparin (LMWH) prophylaxis and a low threshold is kept for ruling out any suspected thromboembolic event.
We do respect the limitations of the study. First, complete individual patient data were available in only 28 patients. Second, data on serum ketones, potassium, sodium, C-reactive protein, lactate dehydrogenase, and D-dimer were infrequently reported and hence were not separately analyzed. Third, details on fluid replacement and insulin dosage were not reported by majority of the studies. Lastly, the analysis of clinical outcomes involved a limited number of patients in whom individual data were available; large-scale studies are required to validate the results.
5. Conclusions
Diabetic ketoacidosis and combined DKA/HHS are not uncommon in COVID-19 patients with pre-existing diabetes mellitus and portend a poor prognosis with a mortality rate of nearly 50%. Males and individuals belonging to black ethnicity are especially at a high-risk. Measurement of effective serum osmolality is essential to differentiate isolated DKA from combined DKA/HHS as the latter represents around one-fifth of the DKA cases and tends to have higher mortality than DKA alone. Conservative rather than liberal fluid replacement and subcutaneous instead of intravenous insulin regimen are practical and unique considerations in the management of DKA in patients with COVID-19.
Funding
None.
Data availability
Available on reasonable request to the corresponding author.
Contribution statement
Both RP and MB are the primary authors and had independently performed the literature search. UY had helped in statistical analysis. SB had edited the manuscript. All the four authors had approved the final version of the manuscript.
Declaration of competing interest
None.
Acknowledgement
None.
References
- 1.Singh A.K., Gupta R., Ghosh A., Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020 Jul;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang I., Lim M.A., Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia – a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pal R., Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020:108132. doi: 10.1016/j.diabres.2020.108132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pal R., Bhadada S.K. COVID-19 and diabetes mellitus: an unholy interaction of two pandemics. Diabetes Metab Syndr. 2020;14:513–517. doi: 10.1016/j.dsx.2020.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apicella M., Campopiano M.C., Mantuano M., Mazoni L., Coppelli A., Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 doi: 10.1016/S2213-8587(20)30238-2. https://linkinghub.elsevier.com/retrieve/pii/S2213858720302382 [cited 2020 Jul 28]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratigou T., Vallianou N., Vlassopoulou B., Tzanela M., Vassiliadi D., Ioannidis G. DKA cases over the last three years: has anything changed? Diabetes Metab Syndr. 2019;13:1639–1641. doi: 10.1016/j.dsx.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 7.Heaney A.I., Griffin G.D., Simon E.L. Newly diagnosed diabetes and diabetic ketoacidosis precipitated by COVID-19 infection. Am J Emerg Med. 2020 doi: 10.1016/j.ajem.2020.05.114. https://linkinghub.elsevier.com/retrieve/pii/S0735675720304885 [cited 2020 Jul 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabashneh S., Ali H., Alkassis S. Multi-organ failure in a patient with diabetes due to COVID-19 with clear lungs. Cureus. 2020 doi: 10.7759/cureus.8147. https://www.cureus.com/articles/32094-multi-organ-failure-in-a-patient-with-diabetes-due-to-covid-19-with-clear-lungs [cited 2020 Jul 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamorro-Pareja N., Parthasarathy S., Annam J., Hoffman J., Coyle C., Kishore P. Letter to the editor: unexpected high mortality in COVID-19 and diabetic ketoacidosis. Metabolism. 2020;110:154301. doi: 10.1016/j.metabol.2020.154301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldman N., Fink D., Cai J., Lee Y.-N., Davies Z. High prevalence of COVID-19-associated diabetic ketoacidosis in UK secondary care. Diabetes Res Clin Pract. 2020;166:108291. doi: 10.1016/j.diabres.2020.108291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oriot P., Hermans M.P. Euglycemic diabetic ketoacidosis in a patient with type 1 diabetes and SARS-CoV-2 pneumonia: case report and review of the literature. Acta Clin Belg. 2020:1–5. doi: 10.1080/17843286.2020.1780390. [DOI] [PubMed] [Google Scholar]
- 12.Chee Y.J., Ng S.J.H., Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020;164:108166. doi: 10.1016/j.diabres.2020.108166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palermo N.E., Sadhu A.R., McDonnell M.E. Diabetic ketoacidosis in COVID-19: unique concerns and considerations. J Clin Endocrinol Metab. 2020;105(8) doi: 10.1210/clinem/dgaa360. https://academic.oup.com/jcem/article/doi/10.1210/clinem/dgaa360/5857202 [cited 2020 Jul 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potier L., Julla J.B., Roussel R., Boudou P., Gauthier D.C., Ketfi C. COVID-19 symptoms masking inaugural ketoacidosis of type 1 diabetes. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.05.004. https://linkinghub.elsevier.com/retrieve/pii/S1262363620300811 [cited 2020 Jul 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaur P., Posimreddy S., Singh B., Qaqa F., Habib H.A., Maroules M. COVID-19 presenting as acute limb ischaemia. Eur J Case Rep Intern Med. 2020;7 doi: 10.12890/2020_001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel S., Gadhiya B., Parikh A., Joshi P. COVID-19 in a child with diabetic ketoacidosis: an instigator, a deviator or a spectator. Indian Pediatr. 2020 doi: 10.1007/s13312-020-2008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alkundi A., Mahmoud I., Musa A., Naveed S., Alshawwaf M. Clinical characteristics and outcomes of COVID-19 hospitalized patients with diabetes in the United Kingdom: a retrospective single centre study. Diabetes Res Clin Pract. 2020;165:108263. doi: 10.1016/j.diabres.2020.108263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavalcanti D.D., Raz E., Shapiro M., Dehkharghani S., Yaghi S., Lillemoe K. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. 2020 doi: 10.3174/ajnr.A6644. http://www.ajnr.org/content/early/2020/06/18/ajnr.A6644 [cited 2020 Jul 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim N., Ha E., Moon J.S., Lee Y.-H., Choi E.Y. Acute hyperglycemic crises with coronavirus disease-19: case reports. Diabetes Metab J. 2020;44:349. doi: 10.4093/dmj.2020.0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haider M.B., Abbas F., Hafeez W. A 46-year-old woman who presented with diabetic ketoacidosis and COVID-19 pneumonia with multiple pulmonary thromboemboli: a case report. Am J Case Rep. 2020;21 doi: 10.12659/AJCR.925794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., Wang X., Chen J., Zuo X., Zhang H., Deng A. COVID -19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metabol. 2020 doi: 10.1111/dom.14057. https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.14057 [cited 2020 Jul 26]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Armeni E., Aziz U., Qamar S., Nasir S., Nethaji C., Negus R. Protracted ketonaemia in hyperglycaemic emergencies in COVID-19: a retrospective case series. Lancet Diabetes Endocrinol. 2020;8:660–663. doi: 10.1016/S2213-8587(20)30221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smati S., Moreau P.M., Bourdiol A., Ploteau S., Hadjadj S., Cariou B. Euglycaemic ketoacidosis during gestational diabetes with concomitant COVID-19 infection. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.07.008. https://linkinghub.elsevier.com/retrieve/pii/S1262363620301002 [cited 2020 Aug 4]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reddy P.K., Kuchay M.S., Mehta Y., Mishra S.K. Diabetic ketoacidosis precipitated by COVID-19: a report of two cases and review of literature. Diabetes Metab Syndr. 2020 doi: 10.1016/j.dsx.2020.07.050. https://linkinghub.elsevier.com/retrieve/pii/S1871402120302988 [cited 2020 Aug 4]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J.-K., Lin S.-S., Ji X.-J., Guo L.-M. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010 Sep;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pal R., Banerjee M. COVID-19 and the endocrine system: exploring the unexplored. J Endocrinol Invest. 2020 doi: 10.1007/s40618-020-01276-8. http://link.springer.com/10.1007/s40618-020-01276-8 [cited 2020 May 25]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stentz F.B., Umpierrez G.E., Cuervo R., Kitabchi A.E. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes. 2004;53:2079–2086. doi: 10.2337/diabetes.53.8.2079. [DOI] [PubMed] [Google Scholar]
- 28.Chan K.H., Thimmareddygari D., Ramahi A., Atallah L., Baranetsky N.G., Slim J. Clinical characteristics and outcome in patients with combined diabetic ketoacidosis and hyperosmolar hyperglycemic state associated with COVID-19: a retrospective, hospital-based observational case series. Diabetes Res Clin Pract. 2020;166:108279. doi: 10.1016/j.diabres.2020.108279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasquel F.J., Tsegka K., Wang H., Cardona S., Galindo R.J., Fayfman M. Clinical outcomes in patients with isolated or combined diabetic ketoacidosis and hyperosmolar hyperglycemic state: a retrospective, hospital-based cohort study. Diabetes Care. 2020;43:349–357. doi: 10.2337/dc19-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gotzsche P.C., Ioannidis J.P.A. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339 doi: 10.1136/bmj.b2700. b2700–b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steenkamp D.W., Alexanian S.M., McDonnell M.E. Adult hyperglycemic crisis: a review and perspective. Curr Diabetes Rep. 2013;13:130–137. doi: 10.1007/s11892-012-0342-z. [DOI] [PubMed] [Google Scholar]
- 32.Perilli G., Saraceni C., Daniels M.N., Ahmad A. Diabetic ketoacidosis: a review and update. Curr Emerg Hosp Med Rep. 2013;1:10–17. [Google Scholar]
- 33.Dhatariya K.K., Glaser N.S., Codner E., Umpierrez G.E. Diabetic ketoacidosis. Nat Rev Dis Primer. 2020 doi: 10.1038/s41572-020-0165-1. http://www.nature.com/articles/s41572-020-0165-1 [cited 2020 Jul 30];6. Available from: [DOI] [PubMed] [Google Scholar]
- 34.Dhatariya K.K., Nunney I., Higgins K., Sampson M.J., Iceton G. National survey of the management of diabetic ketoacidosis (DKA) in the UK in 2014. Diabet Med. 2016;33:252–260. doi: 10.1111/dme.12875. [DOI] [PubMed] [Google Scholar]
- 35.Umpierrez G., Korytkowski M. Diabetic emergencies — ketoacidosis, hyperglycaemic hyperosmolar state and hypoglycaemia. Nat Rev Endocrinol. 2016;12:222–232. doi: 10.1038/nrendo.2016.15. [DOI] [PubMed] [Google Scholar]
- 36.Umpierrez G.E., Kitabchi A.E. Diabetic ketoacidosis: risk factors and management strategies. Treat Endocrinol. 2003;2:95–108. doi: 10.2165/00024677-200302020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Ehrmann D., Kulzer B., Roos T., Haak T., Al-Khatib M., Hermanns N. Risk factors and prevention strategies for diabetic ketoacidosis in people with established type 1 diabetes. Lancet Diabetes Endocrinol. 2020;8:436–446. doi: 10.1016/S2213-8587(20)30042-5. [DOI] [PubMed] [Google Scholar]
- 38.Kalscheuer H., Seufert J., Lanzinger S., Rosenbauer J., Karges W., Bergis D. Event rates and risk factors for the development of diabetic ketoacidosis in adult patients with type 1 diabetes: analysis from the DPV registry based on 46,966 patients. Diabetes Care. 2019;42:e34–e36. doi: 10.2337/dc18-1160. [DOI] [PubMed] [Google Scholar]
- 39.Klein S.L., Dhakal S., Ursin R.L., Deshpande S., Sandberg K., Mauvais-Jarvis F. Biological sex impacts COVID-19 outcomes. Coyne CB. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gebhard C., Regitz-Zagrosek V., Neuhauser H.K., Morgan R., Klein S.L. Impact of sex and gender on COVID-19 outcomes in Europe. Biol Sex Differ. 2020 doi: 10.1186/s13293-020-00304-9. https://bsd.biomedcentral.com/articles/10.1186/s13293-020-00304-9 [cited 2020 Jul 30];11. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 42.Pan D., Sze S., Minhas J.S., Bangash M.N., Pareek N., Divall P. The impact of ethnicity on clinical outcomes in COVID-19: a systematic review. EClinicalMedicine. 2020;23:100404. doi: 10.1016/j.eclinm.2020.100404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maahs D.M., Hermann J.M., Holman N., Foster N.C., Kapellen T.M., Allgrove J. Rates of diabetic ketoacidosis: international comparison with 49,859 pediatric patients with type 1 diabetes from England, Wales, the U.S., Austria, and Germany. Diabetes Care. 2015;38:1876–1882. doi: 10.2337/dc15-0780. [DOI] [PubMed] [Google Scholar]
- 44.Gillani S., Sulaiman S., Sundram S., Sari Y., Baig M., Iqbal M. Serological prediction of infections in diabetic patients with diabetes ketoacidosis in Penang, Malaysia. Trop J Pharmaceut Res. 2013 http://www.ajol.info/index.php/tjpr/article/view/85712 [cited 2020 Jul 30];11. Available from: [Google Scholar]
- 45.Umpierrez G.E., Kelly J.P., Navarrete J.E., Casals M.M., Kitabchi A.E. Hyperglycemic crises in urban blacks. Arch Intern Med. 1997;157:669–675. [PubMed] [Google Scholar]
- 46.Fadini G.P., de Kreutzenberg S.V., Rigato M., Brocco S., Marchesan M., Tiengo A. Characteristics and outcomes of the hyperglycemic hyperosmolar non-ketotic syndrome in a cohort of 51 consecutive cases at a single center. Diabetes Res Clin Pract. 2011;94:172–179. doi: 10.1016/j.diabres.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 47.Savage M.W., Dhatariya K.K., Kilvert A., Rayman G., Rees J.A.E., Courtney C.H. Joint British Diabetes Societies guideline for the management of diabetic ketoacidosis: diabetic ketoacidosis guidelines. Diabet Med. 2011;28:508–515. doi: 10.1111/j.1464-5491.2011.03246.x. [DOI] [PubMed] [Google Scholar]
- 48.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32:1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shang Y., Pan C., Yang X., Zhong M., Shang X., Wu Z. Management of critically ill patients with COVID-19 in ICU: statement from front-line intensive care experts in Wuhan, China. Ann Intensive Care. 2020 doi: 10.1186/s13613-020-00689-1. https://annalsofintensivecare.springeropen.com/articles/10.1186/s13613-020-00689-1 [cited 2020 Jul 31];10. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rayman G., Lumb A., Kennon B., Cottrell C., Nagi D., Page E. Guidance on the management of Diabetic Ketoacidosis in the exceptional circumstances of the COVID-19 pandemic. Diabet Med. 2020;37:1214–1216. doi: 10.1111/dme.14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitabchi A.E., Umpierrez G.E., Murphy M.B., Barrett E.J., Kreisberg R.A., Malone J.I. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24:131–153. doi: 10.2337/diacare.24.1.131. [DOI] [PubMed] [Google Scholar]
- 52.Andrade-Castellanos C.A., Colunga-Lozano L.E., Delgado-Figueroa N., Gonzalez-Padilla D.A. Subcutaneous rapid-acting insulin analogues for diabetic ketoacidosis. Cochrane Metabolic and Endocrine Disorders Group. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD011281.pub2. [cited 2020 Jul 31]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31022-9. https://linkinghub.elsevier.com/retrieve/pii/S0140673620310229 [cited 2020 Apr 30]; Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen D., Li X., Song Q., Hu C., Su F., Dai J. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ditzel J., Lervang H.-H. Disturbance of inorganic phosphate metabolism in diabetes mellitus: clinical manifestations of phosphorus-depletion syndrome during recovery from diabetic ketoacidosis. Diabetes, Metab Syndrome Obes Targets Ther. 2010;3:319–324. doi: 10.2147/DMSOTT.S13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benoit S.R., Zhang Y., Geiss L.S., Gregg E.W., Albright A. Trends in diabetic ketoacidosis hospitalizations and in-hospital mortality — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018;67:362–365. doi: 10.15585/mmwr.mm6712a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Azevedo L.C.P., Choi H., Simmonds K., Davidow J., Bagshaw S.M. Incidence and long-term outcomes of critically ill adult patients with moderate-to-severe diabetic ketoacidosis: retrospective matched cohort study. J Crit Care. 2014;29:971–977. doi: 10.1016/j.jcrc.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 58.The Lancet Haematology COVID-19 coagulopathy: an evolving story. Lancet Haematol. 2020;7:e425. doi: 10.1016/S2352-3026(20)30151-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bhattacharjee S., Banerjee M., Pal R. COVID-19-associated hemophagocytic lymphohistiocytosis and coagulopathy: targeting the duumvirate. Indian Pediatr. 2020 doi: 10.1007/s13312-020-1962-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available on reasonable request to the corresponding author.


