Summary
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variants with a 382-nucleotide deletion (∆382) in the open reading frame 8 (ORF8) region of the genome have been detected in Singapore and other countries. We investigated the effect of this deletion on the clinical features of infection.
Methods
We retrospectively identified patients who had been screened for the ∆382 variant and recruited to the PROTECT study—a prospective observational cohort study conducted at seven public hospitals in Singapore. We collected clinical, laboratory, and radiological data from patients' electronic medical records and serial blood and respiratory samples taken during hospitalisation and after discharge. Individuals infected with the ∆382 variant were compared with those infected with wild-type SARS-CoV-2. Exact logistic regression was used to examine the association between the infection groups and the development of hypoxia requiring supplemental oxygen (an indicator of severe COVID-19, the primary endpoint). Follow-up for the study's primary endpoint is completed.
Findings
Between Jan 22 and March 21, 2020, 278 patients with PCR-confirmed SARS-CoV-2 infection were screened for the ∆382 deletion and 131 were enrolled onto the study, of whom 92 (70%) were infected with the wild-type virus, ten (8%) had a mix of wild-type and ∆382-variant viruses, and 29 (22%) had only the ∆382 variant. Development of hypoxia requiring supplemental oxygen was less frequent in the ∆382 variant group (0 [0%] of 29 patients) than in the wild-type only group (26 [28%] of 92; absolute difference 28% [95% CI 14–28]). After adjusting for age and presence of comorbidities, infection with the ∆382 variant only was associated with lower odds of developing hypoxia requiring supplemental oxygen (adjusted odds ratio 0·07 [95% CI 0·00–0·48]) compared with infection with wild-type virus only.
Interpretation
The ∆382 variant of SARS-CoV-2 seems to be associated with a milder infection. The observed clinical effects of deletions in ORF8 could have implications for the development of treatments and vaccines.
Funding
National Medical Research Council Singapore.
Introduction
Since the emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), efforts have been made to map the genetic diversity of the virus and to identify variants with a selective advantage.1 The variations of interest include changes in immune targets, such as the spike glycoprotein; changes in primer-binding and probe-binding sites, which can reduce the sensitivity of diagnostic tests; and genetic variations that might affect transmissibility and virulence.2, 3, 4
A SARS-CoV-2 variant with a 382-nucleotide deletion (Δ382) was detected in a cluster of cases in Singapore that occurred in January and February, 2020.5 The deletion truncates open reading frame (ORF) 7b and removes the ORF8 transcription-regulatory sequence, eliminating ORF8 transcription. This variant was successfully transmitted early during the epidemic, but was not detected after March, 2020. An identical Δ382 variant was also detected in February, 2020, in a traveller who returned from Wuhan, China, to Taiwan, and other SARS-CoV-2 isolates with different deletions in ORF8 have been reported from cases in Bangladesh (345 nucleotides), Australia (138 nucleotides) and Spain (62 nucleotides).5, 6
In severe acute respiratory syndrome coronavirus (SARS-CoV), the virus responsible for the 2002–03 SARS epidemic, a characteristic 29-nucleotide deletion (Δ29) in ORF8 occurred soon after its zoonotic transmission from civets to humans in 2002, and larger deletions of 82 nucleotides and 415 nucleotides in the same genomic region were also reported.7 The effects of these deletions on the course of the SARS epidemic is unknown. However, in-vitro studies have indicated that the Δ29 variant of SARS-CoV replicates less efficiently than the wild-type virus, and consequently this variant has been hypothesised to result in a milder clinical illness than that caused by the wild-type virus.8, 9
Research in context.
Evidence before this study
Deletions in open reading frame 8 (ORF8) of severe acute respiratory syndrome coronavirus were commonly detected during the severe acute respiratory syndrome outbreak of 2002–03. These deletions reduced viral replication in vitro, and an attenuated severity of infection was hypothesised, although the effect of this deletion on clinical outcomes remains unknown. A 382-nucleotide deletion (Δ382) was detected in the genome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a cluster of infections in Singapore. A literature search was done through MEDLINE to July 27, 2020, using the keywords “coronavirus disease 2019”, “COVID-19”, “SARS-CoV-2”, “deletion”, and “ORF8”, with no language restrictions. An identical 382-nucleotide deletion in ORF8 was reported from in a traveller who returned from Wuhan to Taiwan in February, 2020. The clinical effect of this deletion was not described. Viruses with other deletions in the ORF8 region have also been described from Bangladesh (345 nucleotides), Australia (138 nucleotides) and Spain (62 nucleotides), but no accompanying clinical data are available.
Added value of this study
In this cohort study, we identified 39 patients across three transmission clusters in Singapore who were infected with the Δ382 variant of SARS-CoV-2. Ten (26%) harboured a mix of wild-type and ∆382-variant viruses, while 29 (74%) had only the ∆382 variant. A multivariable logistic regression model indicated that the variant was associated with less severe infection in terms of hypoxia requiring supplemental oxygen (adjusted odds ratio 0·07 [95% CI 0·00–0·48]). Patients infected with the Δ382 variant also had lower concentrations of proinflammatory cytokines, chemokines, and growth factors that are strongly associated with severe COVID-19.
Implications of all the available evidence
ORF8 is a hotspot for coronavirus mutation. The clinical effect of deletions in this region appears to be a milder infection with less systemic release of proinflammatory cytokines and a more effective immune response to SARS-CoV-2. Further study of these variants could have implications for development of treatments and vaccines.
The biological function of the ORF8 protein in SARS-CoV-2 remains unclear. A recent study suggested that ORF8 mediates immune evasion by downregulating MHC-I molecules.10 A previously reported interactome analysis that used affinity-purification mass spectrometry also identified 47 human proteins—mainly associated with glycoprotein metabolism—that interact with ORF8, of which 15 are known drug targets.11 In-vitro evidence has suggested that the deletion does not affect viral replicative fitness, and an analysis of subgenomic RNA has shown that transcription of the ORF6 and N genes, known SARS-CoV interferon antagonists, is altered in Δ382 variants as compared with wild-type SARS-CoV-2.5, 6 In this study, we compared the clinical outcomes and immune responses of patients infected with wild-type and Δ382 SARS-CoV-2.
Methods
Study design and participants
We retrospectively identified individuals who had been screened for the ∆382 variant and recruited to the PROTECT study. PROTECT is a prospective observational cohort study done at seven public hospitals in Singapore (the National Centre for Infectious Diseases, Singapore General Hospital, National University Hospital, Ng Teng Fong General Hospital, Changi General Hospital, Alexandra Hospital, Khoo Teck Puat Hospital). The study aimed to recruit all individuals hospitalised at one of the participating hospitals with confirmed SARS-COV-2 infection for the purpose of clinical characterisation of COVID-19.
The epidemiological investigation was implemented under the Infectious Diseases Act (Singapore). Written informed consent was obtained from participants who provided clinical data and biological samples (as part of the PROTECT study). Study protocols were approved by ethics committees of the National Healthcare Group (2012/00917) and SingHealth Centralised Institutional Review Board (2018/3045). Healthy donor samples were collected under study numbers 2017/2806 and NUS IRB 04-140. Work undertaken at the Duke–NUS Medical School ABSL3 laboratory was approved by the Singapore Ministry of Health.
Clinical data and biological sample collection
The electronic medical records of patients enrolled in the PROTECT study were reviewed and their data were entered onto a standardised collection form adapted from the International Severe Acute Respiratory and Emerging Infection Consortium's case record form for emerging severe acute respiratory infections.12 Serial blood and respiratory samples were collected during hospitalisation and post-discharge.
Clinical management
All patients with COVID-19 were isolated in hospital with airborne transmission precautions, regardless of disease severity. Supportive therapy including supplemental oxygen and symptomatic treatment were administered as required. Patients with moderate to severe hypoxia (defined as requiring a fraction of inspired oxygen ≥40%) were transferred to the intensive care unit for high-flow oxygen via nasal cannula and invasive mechanical ventilation if required. De-isolation was contingent on resolved symptoms and two consecutive nasopharyngeal swabs at least 24 h apart that were negative for SARS-CoV-2 on PCR.
Detection of ∆382 variant
To detect the 382-nucleotide deletion in the SARS-CoV-2 genome, we used two specific PCR primers (forward 5ʹ-TGTTAGAGGTACAACAGTACTTT-3ʹ; reverse 5ʹ-GGTAGTAGAAATACCATCTTGGA-3ʹ) flanking the deleted region.5 For samples with high cycle threshold (Ct) values, hemi-nested PCR was done with a second forward primer (5ʹ-TGTTTATAACACTTTGCTTCACA-3ʹ) and the same reverse primer as before. The PCR mixture contained the cDNA primers (10 μM each), 10 × Pfu reaction buffer (Promega, Madison, WI, USA), Pfu DNA polymerase (Promega), and 10 mM dNTP mix (Thermo Scientific, Waltham, MA, USA). PCR was done in a thermal cycler (Applied Biosystems Veriti, Foster City, CA, USA) with the following conditions: 95°C for 2 min, followed by 35 cycles at 95°C for 1 min, 52°C for 30 sec, and 72°C for 1 min; and a final extension at 72°C for 10 min. Deletions in the PCR products were visualised with use of a QIAxcel DNA screening cartridge on QIAxcel Advanced capillary electrophoresis system (Qiagen, Hilden, Germany).
Multiplex microbead-based immunoassay
Levels of specific immune mediators in the first plasma samples collected from patients with COVID-19 during hospitalisation were quantified by multiplex microbead-based immunoassays. Plasma samples were treated with 1% Triton X-100 solvent-detergent (SD) mix for virus inactivation. Immune mediator levels were measured with the Luminex assay using the Cytokine/Chemokine/Growth Factor 45-plex Human ProcartaPlex Panel 1 (ThermoFisher Scientific; appendix p 1). Patient samples with a concentration out of measurement range were assigned the value of the logarithmic transformation of the limit of quantification. Data analysis was done with Bio-Plex Manager 6.1.1 software. TM4-MeV Suite (version 10.2) was used to compute hierarchical clustering and generate a heatmap of immune mediators, scaling concentrations to between 0 and 1 for visualisation. Biological processes and immune pathways were predicted from the significant immune mediators with Ingenuity Pathway Analysis (version 52912811). Protein–protein interaction networks of these immune mediators and previously reported host proteins targeted by SARS-CoV-2 ORF8 were predicted and illustrated with the Search Tool for the Retrieval of Interacting Genes/Proteins (STRING) database version 11.0). All these interactions were derived from high-throughput lab experiments and previous knowledge in curated databases at a confidence threshold of 0·5.
Epidemiological investigation
As part of the COVID-19 outbreak investigation in Singapore, all patients with SARS-CoV-2 infection were interviewed to elucidate activity histories from 14 days preceding symptom onset until isolation in hospital, including recent travel history and possible contact with confirmed cases. The Singapore Ministry of Health initiated contact tracing to identify close contacts (prolonged time within 2 m of a person with confirmed COVID-19) and other contacts who had significant interactions with the infected person. Active case finding was done to detect additional COVID-19 cases among these contacts.
Outcomes
The primary endpoint for this study was the proportion of patients who developed severe COVID-19, defined by hypoxia requiring supplemental oxygen. Secondary outcomes were the concentrations of immune mediators in plasma samples. All other clinical findings and viral PCR Ct values were exploratory outcomes.
Statistical analysis
Data processing and analysis were done in the R statistical language (version 3.3.1) and Stata version 15. We compared continuous variables with Mann-Whitney U or Kruskal-Wallis tests, and categorical variables with Fisher's exact test or χ2 test as appropriate. Exact logistic regression was used to examine the association between the SARS-CoV-2 infection group and the development of hypoxia (the primary outcome). The following covariates were considered for inclusion in the multivariable exact logistic regression model: age group (<45 years, 45–64 years, or ≥65 years), sex, Charlson Comorbidity Index group (0 or ≥1) and infection group (wild-type, Δ382 variant, or mixed wild-type and Δ382 variant).
For the cytokine analysis, we compared logarithmically transformed concentrations between patients with ∆382 variant and those wild-type virus infections by use of an unpaired t-test. Plots were generated with GraphPad Prism software (version 8).
All statistical tests were two-sided, and p values less than 0·05 were considered to indicate statistical significance. Adjustment for multiple testing was not done.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. LFPN, GJDS, and BEY had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Of the 432 individuals diagnosed in Singapore with PCR-confirmed SARS-CoV-2 infection between Jan 22 and March 21, 2020, 276 (64%) had residual samples available for PCR analysis (appendix pp 2–4).13 SARS-CoV-2 was detected in samples from 251 (91%) of these individuals, among which the ∆382 variant was detected in 44 (18%).
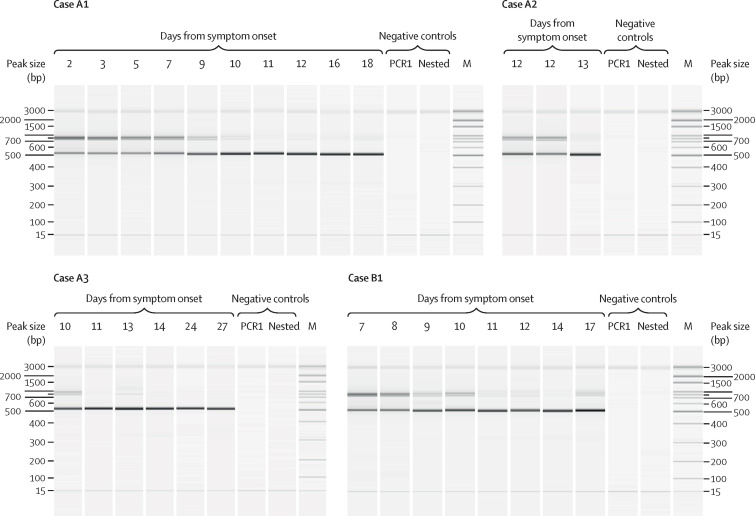
131 (52%) of the 251 individuals screened for the ∆382 variant had been enrolled in the PROTECT study and had clinical data available for further analysis (appendix pp 5–6). Among them, 92 (70%) were infected with the wild-type virus only and 39 (30%) with the ∆382 variant (29 [74%] of whom had the ∆382 variant only and ten [26%] of whom had co-infection with the wild-type virus). Serial respiratory samples were available for four individuals, and capillary electrophoresis of PCR products showed the ∆382 variant replacing wild-type virus as infection progressed into the second week from symptom onset (figure 1 ).
Figure 1.
Capillary electrophoresis of the ORF8 gene showing differences across the duration of disease in four patients co-infected with wild-type and the Δ382 variant of severe acute respiratory syndrome coronavirus 2
Approximate band sizes were 880 bp for the wild-type virus and 500 bp for the Δ382 variant. Nuclease-free water was used as a non-template negative control for PCR1. This resultant reaction was used as the negative control for the nested reaction. Δ382=382-nucleotide deletion. M=100-bp marker.
Infection groups were similar by sex and comorbidities (table 1 ). Comparing the ∆382-variant only group with the wild-type only group, those infected with the ∆382 variant were younger overall, with only one (3%) patient aged 65 years or older, in contrast to ten (11%) in the wild-type only group. Patients with ∆382-variant infection presented later after symptom onset, with a lower median temperature, and with less systemic inflammation according to baseline laboratory investigations than the wild-type only group (table 1). SARS-CoV-2 PCR Ct value from the first respiratory sample was lower from wild-type versus ∆382 infections though this difference was not apparent after adjusting for day of sample collection (appendix p 7).
Table 1.
Demographic and clinical characteristics of patients infected with Δ382 variant SARS-CoV-2, wild-type SARS-CoV-2, or both
| Δ382 variant only (n=29) | Mixed Δ382 variant and wild-type (n=10) | Wild-type only (n=92) | p value* | p value† | ||
|---|---|---|---|---|---|---|
| Patient characteristics | ||||||
| Median age, years | 37 (27–53) | 46 (39–56) | 47 (35–61) | 0·041 | 0·018 | |
| Age group, years | ·· | ·· | ·· | 0·19 | 0·17 | |
| <45 | 19 (66%) | 5 (50%) | 43 (47%) | ·· | ·· | |
| 45–64 | 9 (31%) | 5 (50%) | 39 (42%) | ·· | ·· | |
| ≥65 | 1 (3%) | 0 (0%) | 10 (11%) | ·· | ·· | |
| Sex | ·· | ·· | ·· | 0·61 | 0·52 | |
| Female | 10 (34%) | 3 (30%) | 39 (42%) | ·· | ·· | |
| Male | 19 (66%) | 7 (70%) | 53 (58%) | ·· | ·· | |
| Chinese ethnicity | 22 (76%) | 7 (70%) | 67 (73%) | 0·92 | 0·81 | |
| Charlson Comorbidity Index | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0·64 | 0·51 | |
| Diabetes | 1 (3%) | 0 (0%) | 11 (12%) | 0·22 | 0·29 | |
| Baseline symptoms and findings | ||||||
| Duration of symptoms, days | 6 (3–9) | 6 (5–98) | 4 (2–7) | 0·061 | 0·036 | |
| Fever | 17 (59%) | 1 (10%) | 72 (78%) | 0·054 | 0·05 | |
| Cough | 20 (69%) | 7 (70%) | 62 (67%) | 0·98 | 1·00 | |
| Dyspnoea | 1 (3%) | 2 (20%) | 15 (16%) | 0·18 | 0·11 | |
| Sore throat | 15 (52%) | 5 (50%) | 37 (40%) | 0·50 | 0·29 | |
| Rhinorrhoea | 10 (34%) | 2 (20%) | 21 (23%) | 0·42 | 0·22 | |
| Heart rate (beats per min) | 85 (74–97) | 80 (77–108) | 92 (83–100) | 0·26 | 0·78 | |
| Systolic blood pressure, mm Hg | 136 (122–145) | 131 (128–139) | 133 (120–146) | 0·77 | 0·49 | |
| Respiratory rate, breaths per min | 18 (18–18) | 18 (16–20) | 18 (17–19) | 0·92 | 0·68 | |
| Oxygen saturation, % | 98 (97–99) | 98·5 (96–99) | 98 (96–98) | 0·15 | 0·06 | |
| Temperature, °C | 37·1 (36·6–37·7) | 37·8 (37–38·5) | 37·7 (37·2–38·3) | 0·0064 | 0·0013 | |
| Neutrophils, ×109/L | 2·6 (2·0–3·3) | 2·6 (2·3–4·4) | 3·1 (2·1–4·1) | 0·50 | 0·24 | |
| Lymphocytes, ×109/L | 1·3 (0·9–1·9) | 1·2 (0·8–2·0) | 1·1 (0·8–1·5) | 0·22 | 0·079 | |
| Platelet, ×109/L | 193 (173–245) | 206 (159–275) | 190 (147–241) | 0·40 | 0·21 | |
| C-reactive protein concentration, mg/L | 5·6 (2·1–10·7); n=25 | 13·7 (11·7–180); n=7 | 11·6 (3·0–47·4); n=86 | 0·018 | 0·023 | |
| Lactate dehydrogenase concentration, U/L | 388 (341–509); n=27 | 375 (352–474); n=10 | 463 (368–616); n=86 | 0·10 | 0·041 | |
| Alanine aminotransferase concentration, U/L | 26 (15–36); n=22 | 36 (24–84); n=7 | 29 (19–50); n=74 | 0·29 | 0·31 | |
| Creatinine concentration, μmol | 64 (51–79); n=24 | 86 (71–93); n=8 | 68 (55–83); n=80 | 0·10 | 0·35 | |
| Ct value of first nasopharyngeal SARS-CoV-2 PCR | 29·2 (24·8–34·2) | 26·2 (21·0–29·5) | 25·6 (21·6–30·6) | 0·11 | 0·040 | |
| Outcomes | ||||||
| Pneumonia | 15 (52%) | 5 (50%) | 47 (51%) | 1·00 | 1·00 | |
| Hypoxia requiring supplemental oxygen | 0 (0%) | 3 (30%) | 26 (28%) | 0·0050 | 0·0013 | |
| Intensive care unit admission | 0 (0%) | 3 (30%) | 15 (16%) | 0·025 | 0·021 | |
| Invasive mechanical ventilation | 0 (0%) | 3 (30%) | 10 (11%) | 0·020 | 0·12 | |
| Death | 0 (0%) | 0 (0%) | 2 (2%) | 0·65 | 1·00 | |
Data are median (IQR) or n (%). p values are from Kruskal-Wallis test (for continuous variables) or χ2 test (for categorical variables). Δ382=382-nucleotide deletion. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. Ct=cycle threshold.
Δ382 variant only group versus wild-type only group versus mixed Δ382 variant and wild-type group.
Δ382 variant only group versus wild-type only group.
Clinical outcomes were considerably better in patients infected with the ∆382-variant than with the wild-type virus. Although rates of pneumonia visualised on chest radiograph were similar across all three infection groups, fewer patients required supplemental oxygen in the ∆382-variant only group (0 [0%] of 29) than in the ∆382-variant and wild-type co-infection group (three [30%] of ten) and the wild-type only group (26 [28%] of 92; absolute difference 28% [95% CI 14–28]; p=0·0050 [χ2 test]; table 1). After adjustment for age group and presence of comorbidities, patients infected with the ∆382-variant had lower odds of developing hypoxia (adjusted odds ratio 0·07 [95% CI 0·00–0·48]; table 2 ) compared with those infected with wild-type virus.
Table 2.
Exact logistic regression analysis of candidate predictors for hypoxia requiring supplemental oxygen
|
Univariable model |
Multivariable model* |
|||
|---|---|---|---|---|
| OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| Age, years | ||||
| <45 | 1 (ref) | ·· | 1 (ref) | ·· |
| 45–64 | 5·77 (1·84–21·73) | 0·0012 | 3·65 (1·04–14·79) | 0·042 |
| ≥65 | 13·95 (2·59–85·09) | 0·0012 | 8·05 (1·16–62·62) | 0·033 |
| Sex | ||||
| Female | 1 (ref) | ·· | ·· | ·· |
| Male | 1·51 (0·58–4·17) | 0·49 | ·· | ·· |
| Charlson Comorbidity Index | ||||
| 0 | 1 (ref) | ·· | 1 (ref) | ·· |
| ≥1 | 7·88 (2·67–24·31) | 0·0001 | 6·36 (1·76–25·68) | 0·0030 |
| Infection | ||||
| Wild-type only | 1 (ref) | ·· | 1 (ref) | ·· |
| Δ382 variant only | 0·07† (0·00–0·40) | 0·0008 | 0·07† (0·00–0·48) | 0·0035 |
| Mixed Δ382 and wild-type | 1·15 (0·18–5·53) | 1·00 | 1·78 (0·22–11·02) | 0·75 |
Δ382=382-nucleotide deletion. OR=odds ratio.
Adjusted for age, Charlson Comorbidity Index, and infection group.
As the conditional maximum likelihood estimate is unbounded (ie, infinite), the median unbiased estimate (ie, regression estimate that places the observed sufficient statistic at the median of the conditional distribution) is computed.
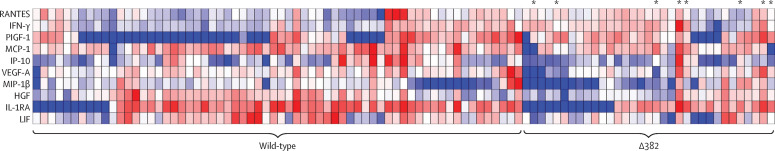
Plasma samples were available for 97 patients: 64 (66%) patients infected with wild-type virus, 25 (26%) with the ∆382-variant, and eight (8%) with mixed wild-type and ∆382-variant infection (figure 2 ). Higher concentrations of IFN-γ and lower concentrations of the chemokines IP-10 (CXCL10), MCP-1 (CCL2), and MIP-1β (CCL4) and the anti-inflammatory protein IL-1RA were detected in patients with the ∆382-variant compared with patients with the wild-type virus (figure 3 ; appendix pp 8–10) at median 8 days after symptom onset (IQR 4–11). Notably, patients infected with the ∆382 variant had lower concentrations of growth factors associated with lung injury and regeneration, including HGF, LIF, and VEGF-A, and higher concentrations of PIGF-1 (PGF) and RANTES (CCL5).
Figure 2.
Concentrations of 45 immune mediators quantified using a 45-plex microbead-based immunoassay
Heatmap of immune mediator levels in plasma samples of patients infected with either wild-type (n=64), Δ382 variant (n=25), or mixed wild-type and Δ382 variant severe acute respiratory syndrome coronavirus 2 (n=8; indicated by asterisks in figure) during the first collection timepoint upon hospital admission (median 8 days from symptom onset). Each colour represents the relative concentration of a particular analyte (blue=low concentration; red=high concentration). Δ382=382-nucleotide deletion.
Figure 3.
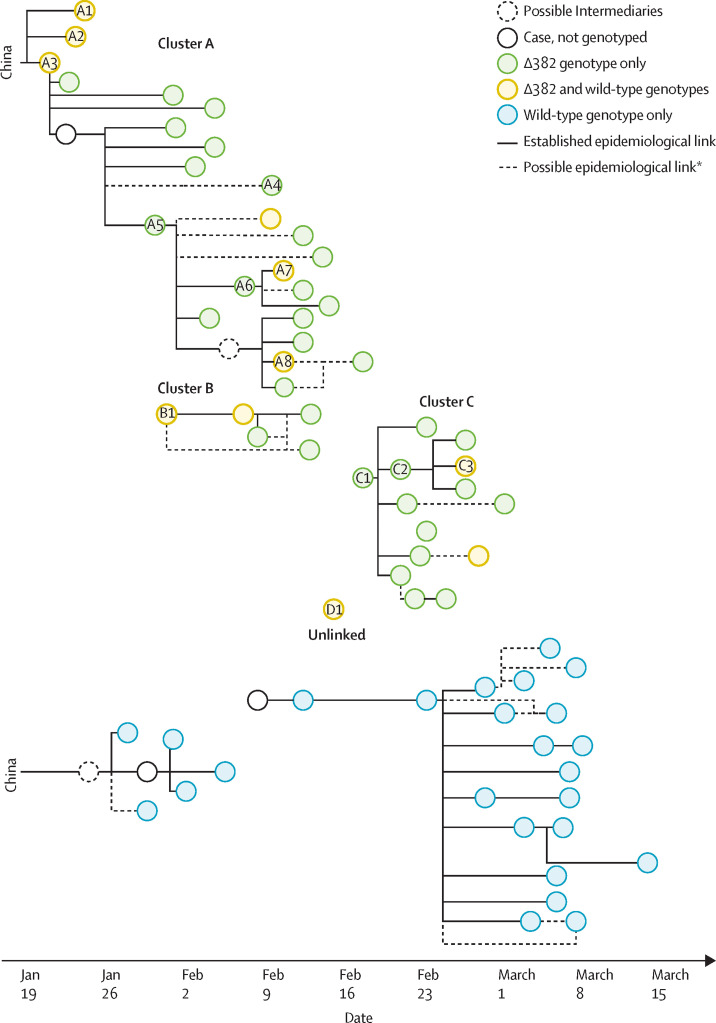
Chain of transmission between cases as established by epidemiological investigations
Δ382=382-nucleotide deletion. *Possible epidemiological links were identified when individuals with COVID-19 had a common physical location or timing but direct interaction could not be clearly established; possible epidemiological links would also reflect transmissions that have arisen from close contact between a case and a few possible sources in the same cluster.
Stratifying by disease severity, T-cell activation-associated cytokines (IFN-γ, TNF-α, IL-2, and IL-5) were upregulated in patients without pneumonia who were infected with the ∆382 variant versus wild-type virus, while growth factors associated with lung injury (HGF, LIF, and VEGF-A) were lower (appendix p 11). Ingenuity Pathway Analysis of these immune mediators highlighted several canonical pathways—including communication between immune cells, pattern recognition receptor, and T-helper cell differentiation—in the top ten ranking (appendix p 12). Further protein–protein interaction network analyses with STRING highlighted interactions of these mediators with host proteins involved in endoplasmic reticulum-associated protein degradation, focal adhesion in platelet activation, and T cell-mediated immunity (appendix p 12).
Our epidemiological investigation showed that SARS-CoV-2 ∆382 variants were first detected in three Chinese nationals who arrived in Singapore from Wuhan, China, on the same flight (cases A1–A3; figure 3). Two of these individuals (A2 and A3) were a couple, and the third was unrelated. Among 114 individuals with infections acquired overseas who were screened, only these three had the ∆382 variant, representing 15% of the 20 imported cases from China. In all three cases, both wild-type and ∆382 viruses were detected.
The ∆382 variant was detected in a further 39 individuals across three known local transmission clusters (clusters A, B, and C) as well as in two unlinked cases. These three clusters were independently established through epidemiological investigation before genotypic information was available. In all cases within those three clusters, the individuals were infected with the ∆382 variant, while those in the other contemporaneous clusters consisted only of the wild-type virus. Two of the visitors from Wuhan (cases A2 and A3) were primary cases of cluster A, in which several generations of transmission occurred across two churches and a household.14
Clusters B and C occurred during the same period at the tail-end of cluster A. No links to importation were previously established and it was uncertain how the infection was introduced to these two clusters. Cluster B occurred at a worksite and involved several foreign workers, and the individual in the first case (B1) had no known contact with other previous cases. The primary case (C1) of cluster C, in which all transmission was thought to occur in a workplace setting, had no known links to other previous cases.
In one of the unlinked cases of infection with the ∆382 variant (case A4), the individual lived in the same residential complex as an infected household unit in cluster A, but no direct interaction or exposure was identified during epidemiological investigation. The case was considered plausibly linked, but transmission could not be substantiated before genotyping was done.
In three individuals (cases A7, A8, and C3), co-infection with wild-type virus and the ∆382 variant was observed, despite four individuals earlier in these transmission chains apparently being infected only with the ∆382 variant (cases A5, A6, C1, C2). No epidemiological link to other known COVID-19 cases was uncovered to indicate that the three co-infections were due to independent infections by two viruses. However, in the four earlier cases, the individuals were diagnosed 8–16 days after symptom onset, and it is therefore plausible that a co-infection was present earlier in the infection but not detected because of delayed respiratory sampling.
Discussion
The Δ382 variant of SARS-CoV-2, which emerged in Wuhan early in the pandemic and was exported to Singapore and Taiwan,5 was transmitted as a co-infection with the wild-type virus, and became the dominant virus in the second week of illness. The ∆382 variant causes clinically significant illness, including pneumonia, but infections tended to be milder compared with those caused by the wild-type virus, with less pronounced cytokine release during the acute phase of infection. The observed attenuated clinical features further suggest that ORF8 is a possible target for therapeutic intervention and for the development of a SARS-CoV-2 controlled human infection model.
We observed that patients infected with the ∆382 variant had lower concentrations of proinflammatory cytokines, chemokines, and growth factors that are strongly associated with severe COVID-19.15, 16 Notably, patients with pneumonia from the ∆382 variant had higher concentrations of SDF-1α, which is usually downregulated during hypoxia.17 These findings corroborated our clinical observations that patients infected with the ∆382 variant had better clinical outcomes, as shown by the lower proportion of patients in the ∆382 variant group who had hypoxia requiring supplemental oxygen. The in-vitro replication kinetics of the ∆382 variant are similar to those of wild-type SARS-CoV-2, consistent with the viral loads observed in these patients, indicating that the ORF8 deletion does not reduce replicative fitness. This finding is contrary to the reduced replication observed in SARS-CoV viruses with an ORF8 deletion.9
Further analysis of immune mediator profiles in patients with mild symptoms revealed that patients infected with ∆382 variants had more effective T-cell responses and platelet regulation during the early phase of the infection. T-cell responses are severely impaired in patients with SARS-CoV-2 infection.18 Lymphopenia19 and functional exhaustion of T cells20 correlate with disease severity in COVID-19. The more robust production of IFN-γ during the early phase of the infection, which was observed in patients infected with the ∆382 variant, could promote and maintain the effector functions of T cells, which might mediate rapid and effective antibody responses against SARS-CoV-2.21, 22
SARS-CoV-2 ORF8 targets host proteins in endoplasmic reticulum quality control, extracellular matrix organisation, and glycosaminoglycan synthesis.11 Our STRING analysis showed that host proteins in endoplasmic reticulum trafficking are interacting partners pivotal to multiple pathways in T-cell-mediated immunity and platelet regulation. This finding is consistent with an interaction between the viral protein encoded by ORF8 and host MHC-I leading to downregulation of cytotoxic CD8 T-lymphocyte-mediated antiviral activity.10 Given the important roles of ORF8 in mediating SARS-CoV-2 immune evasion, inhibition of its function could be investigated as a potential therapeutic strategy.
The repeated emergence of SARS-CoV-2 viruses with a deletion in ORF8 suggests this region is important for viral adaptation to humans. Studies have reported that ORF8 is strongly immunogenic, and that antibodies are produced against ORF8 early during SARS-CoV-2 infection.23 Significant CD4-positive and CD8-positive T-cell responses to ORF8 have also been described in patients who recover from COVID-19.24 Our analysis of serial respiratory samples from patients with wild-type and ∆382 variant co-infection suggested that the ∆382 variant out-competed the wild-type virus. The disappearance of ∆382 variants in Singapore, Taiwan, and presumably China could be attributed to infection control measures. However, ∆382 variants might also be less effective at establishing infection in a new host because of the loss of the immune evasion functions of ORF8. Importantly, genomic data indicate that the ∆382 variants are not related to the D614G clade, which might or might not exhibit altered virus transmissibility,4 but belong to early outbreak sequences for which no significant difference in transmissibility is observed.25
Our study has a number of limitations. First, respiratory samples for SARS-CoV-2 PCR were collected as part of routine clinical care. We did not have samples from early in the course of the illness for many patients, and some patients' samples were not available. Second, although every effort was made to corroborate epidemiological data, the data are subject to recall bias and linkages might have been missed or incorrectly inferred. Third, we adjusted for known major determinants of severe COVID-19 in the multivariable model, but there might have been unmeasured confounders that could explain some of the differences in clinical outcomes. The presence of transmission clusters can also amplify bias, and it is possible that recruited patients were not representative of their infection groups. Fourth, some mixed wild-type and ∆382 variant infections are suspected from epidemiological linkage but were undetected by PCR. This could reflect limitations of this assay for detecting mixed infection, or virological sampling only later in the course of infection. Finally, blood samples were collected as early as possible in the course of the illness, but inevitably they were not available on the same day post-infection or symptom onset.
In summary, ORF8 is a hotspot for genetic variation in coronaviruses. The clinical effect of deletions in this region appears to be a milder infection with less systemic release of proinflammatory cytokines. Further study of these variants could improve our understanding of SARS-CoV-2 virology and pathogenesis and could have implications for the development of treatments and vaccines.
Acknowledgments
Acknowledgments
This study was funded by grants from the Singapore National Medical Research Council's COVID-19 Research Fund (grant numbers COVID19RF-001, COVID19RF-004, and COVID19RF-007), the Biomedical Research Council COVID-19 fund (H20/04/g1/006), and the A*ccelerate GAP-funded project (ACCL/19-GAP064-R20H-H) from the Agency of Science, Technology, and Research. YCFS and GJDS are supported by contract HHSN272201400006C from the National Institute of Allergy and Infectious Diseases (National Institutes of Health, US Department of Health and Human Services). We thank all clinical and nursing staff who provided care for the patients; staff at the Communicable Diseases Division of the Ministry of Health Singapore who contributed to the outbreak response and contact tracing; staff at the National Public Health and Epidemiology Unit of the National Centre for Infectious Diseases who assisted with data analysis; staff in the Singapore Infectious Disease Clinical Research Network and Infectious Disease Research and Training Office of the National Centre for Infectious Diseases for coordinating patient recruitment.
Contributors
BEY, DEA, YCFS, RTPL, SM-S, Y-SL, L-FW, LR, VJL, GJDS, DCL, and LFPN conceived and designed the study. BEY, T-MM, WEW, SK, LYAC, SP, SYT, LS, PP, YYCC, and TB collected clinical samples and data. BEY, S-WF, Y-HC, T-MM, LWA, CY-PL, SNA, BL, YSG, and WEW did the experiments and analysed the data. BEY, S-WF, Y-HC, T-MM, WEW, GJDS, and LFPN wrote the first draft of the manuscript. All authors contributed to data interpretation, critically reviewed the manuscript, and approved the final manuscript for submission.
Declaration of interests
We declare no competing interests.
Contributor Information
Gavin J D Smith, Email: gavin.smith@duke-nus.edu.sg.
Lisa F P Ng, Email: lisa_ng@immunol.a-star.edu.sg.
Supplementary Material
References
- 1.Phan T. Genetic diversity and evolution of SARS-CoV-2. Infect Genet Evol. 2020;81 doi: 10.1016/j.meegid.2020.104260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Álvarez-Díaz DA, Franco-Muñoz C, Laiton-Donato K. Molecular analysis of several in-house rRT-PCR protocols for SARS-CoV-2 detection in the context of genetic variability of the virus in Colombia. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lokman SM, Rasheduzzaman M, Salauddin A. Exploring the genomic and proteomic variations of SARS-CoV-2 spike glycoprotein: a computational biology approach. Infect Genet Evol. 2020;84 doi: 10.1016/j.meegid.2020.104389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Korber B, Fischer WM, Gnanakaran S. Tracking changes in SARS-CoV-2 spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell. 2020;20:30820–30825. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Su YCF, Anderson DE, Young BE. Discovery and genomic characterization of a 382-nucleotide deletion in ORF7b and ORF8 during the early evolution of SARS-CoV-2. MBio. 2020;11:e01610–e01620. doi: 10.1128/mBio.01610-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong Y-N, Tsao K-C, Hsiao M-J. SARS-CoV-2 genomic surveillance in Taiwan revealed novel ORF8-deletion mutant and clade possibly associated with infections in Middle East. Emerg Microbes Infect. 2020;9:1457–1466. doi: 10.1080/22221751.2020.1782271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinese SARS Molecular Epidemiology Consortium Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science. 2004;303:1666–1669. doi: 10.1126/science.1092002. [DOI] [PubMed] [Google Scholar]
- 8.Cui J, Li F, Shi Z-L. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muth D, Corman VM, Roth H. Attenuation of replication by a 29 nucleotide deletion in SARS-coronavirus acquired during the early stages of human-to-human transmission. Sci Rep. 2018;8 doi: 10.1038/s41598-018-33487-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Zhang J, Chen Y. The ORF8 protein of SARS-CoV-2 mediates immune evasion through potently downregulating MHC-I. bioRxiv. 2020 doi: 10.1101/2020.05.24.111823. published online May 24. (preprint) [DOI] [Google Scholar]
- 11.Gordon DE, Jang GM, Bouhaddou M. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Severe Acute Respiratory and Emerging Infection Consortium COVID-19 CRF. https://isaric.tghn.org/COVID-19-CRF/
- 13.Ministry of Health Singapore Nine more cases discharged; 47 new cases of COVID-19 infection confirmed. March 21, 2020. https://www.moh.gov.sg/news-highlights/details/nine-more-cases-discharged-47-new-cases-of-covid-19-infection-confirmed
- 14.Yong SEF, Anderson DE, Wei WE. Connecting clusters of COVID-19: an epidemiological and serological investigation. Lancet Infect Dis. 2020;20:809–815. doi: 10.1016/S1473-3099(20)30273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang C, Wang Y, Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Li S, Liu J. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55 doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsuzuki T, Okada H, Cho H. Hypoxic stress simultaneously stimulates vascular endothelial growth factor via hypoxia-inducible factor-1α and inhibits stromal cell-derived factor-1 in human endometrial stromal cells. Hum Reprod. 2012;27:523–530. doi: 10.1093/humrep/der405. [DOI] [PubMed] [Google Scholar]
- 18.Qin C, Zhou L, Hu Z. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan L, Wang Q, Zhang D. Correction: Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5:61. doi: 10.1038/s41392-020-0159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diao B, Wang C, Tan Y. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed JM, Branigan PJ, Bamezai A. Interferon gamma enhances clonal expansion and survival of CD4+ T cells. J Interferon Cytokine Res. 2008;28:611–622. doi: 10.1089/jir.2007.0145. [DOI] [PubMed] [Google Scholar]
- 22.Tewari K, Nakayama Y, Suresh M. Role of direct effects of IFN-gamma on T cells in the regulation of CD8 T cell homeostasis. J Immunol. 2007;179:2115–2125. doi: 10.4049/jimmunol.179.4.2115. [DOI] [PubMed] [Google Scholar]
- 23.Hachim A, Kavian N, Cohen CA. Beyond the Spike: identification of viral targets of the antibody response to SARS-CoV-2 in COVID-19 patients. medRxiv. 2020 doi: 10.1101/2020.04.30.20085670. published online May 2. (preprint) [DOI] [Google Scholar]
- 24.Grifoni A, Weiskopf D, Ramirez SI. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489. doi: 10.1016/j.cell.2020.05.015. 501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dorp L, Richard D, Tan CC, Shaw LP, Acman M, Balloux F. No evidence for increased transmissibility from recurrent mutations in SARS-CoV-2. bioRxiv. 2020 doi: 10.1101/2020.05.21.108506. published online May 21. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.