Graphical abstract
Keywords: Cancer, Chitosan, Infection, Nanoparticle, Pulmonary drug delivery
Abstract
Chitosan, as a biodegradable and biocompatible polymer, is characterized by anti-microbial and anti-cancer properties. It lately has received a widespread interest for use as the pulmonary particulate backbone materials of drug carrier for the treatment of infectious disease and cancer. The success of chitosan as pulmonary particulate drug carrier is a critical interplay of their mucoadhesive, permeation enhancement and site/cell-specific attributes. In the case of nanocarriers, various microencapsulation and micro-nano blending systems have been devised to equip them with an appropriate aerodynamic character to enable efficient pulmonary aerosolization and inhalation. The late COVID-19 infection is met with acute respiratory distress syndrome and cancer. Chitosan and its derivatives are found useful in combating HCoV and cancer as a function of their molecular weight, substituent type and its degree of substitution. The interest in chitosan is expected to rise in the next decade from the perspectives of drug delivery in combination with its therapeutic performance.
1. Introduction
The human lung is a biological system consisting of a series of tissues and organs that are responsible for the process of respiration (Leslie & Wick, 2018). Functionally, the lung is constituted of a conducting airway and a respiratory region. The conducting airway consists of nasal cavity, oral cavity and the associated sinuses, nasopharynx, oropharynx, larynx, trachea, bronchi, and bronchioles, whilst the respiratory region is divided into respiratory bronchioles, alveolar ducts, alveolar sacs and alveoli. The conducting airway conditions and conducts atmospheric air, while the respiratory region assists in the exchange of oxygen and carbon dioxide (Ruigrok et al., 2016). The lung provides a remarkably thin interface of approximately 0.2 μm to 0.7 μm between the alveolar lumen and capillary lumen, enabling fast and effective physiological respiration when combined with its large specific surface area (Ruigrok et al., 2016; Shah et al., 2012). The alveolar epithelium has type I and type II alveolar cells. Alveolar type I cell is a thin squamous cell acting as a barrier between air and internal components of the alveolar wall covering 95 % of the alveolar surfaces. Alveolar type II cell acts in the regeneration of type I and II cells, producing lung surfactant and covering 5 % of the alveolar surfaces (Chang et al., 2010). The alveoli surface contains brush cells and macrophages.
Inhalation is a non-invasive, organ-specific method of delivering the therapeutics to the lungs for the treatment of lung diseases (Kuzmov & Minko, 2015). Further, the cell- and organelle-specific pulmonary drug delivery can be introduced via decorating the drug carrier with specific targeting ligands (Majumder et al., 2019). The summative efforts reduce the exposure of therapeutics to healthy lung cells and other systemic organs, thereby allowing reduced drug doses and alleviating unnecessary adverse effects of drugs (Senapati et al., 2018). The pulmonary drug delivery is favourable specifically for protein and gene based therapeutics as the lung possesses limited intracellular and extracellular drug-metabolising enzymes unlike the gastrointestinal tract and liver (Lee, Loo, Traini, & Young, 2015a,2015b). Currently, there are four types of inhalation devices, namely pressurized metered dose inhalers, dry powder inhalers, nebulizers, and soft mist inhalers being used for the purpose of introducing pulmonary medicine by means of the inhalation route (Ruigrok et al., 2016).
The inhalation and deposition pattern of particles in lung is governed by inertial impaction, gravitational sedimentation and diffusion processes as a function of primarily the aerodynamic particle size distribution of the aerosolised therapeutics (Alhajj et al., 2020; Youngren-Ortiz et al., 2016). One of the key obstacles in pulmonary drug delivery is the high branching degree of airways with varied lengths and diameters (Fröhlich, 2019). The branched airways, gradually narrowing down from the carina of the trachea to the alveolar sac, cause the inhaled particles to hit the airway walls instead of travelling deeper into the lung as a result of an increase in the particle velocity (Lam et al., 2012). The inhaled particles fail to flow in the direction of airway branching and follow the airstream to reach the deep and peripheral lung regions (Youngren-Ortiz et al., 2016). The respiratory airway is characterized by the ciliated epithelial cells which transport mucus and alveolar fluids to the upper part of airway (Roy & Vij, 2010; Sanders et al., 2009). The mucociliary clearance is a physiological function to clear the debris, excessive secretions and any unwanted inhaled particles (Fröhlich, 2019; Yang et al., 2014). The glycosylated protein-rich mucus is constantly produced, shed and replaced to protect the lung epithelial cells from harmful particles and substances (Lai et al., 2009; Qiu et al., 2016; Yang et al., 2014). The alveolar macrophages are defence cells of the respiratory system that engulf foreign particles. The combination action of mucus and ciliary clearance with macrophage activity reduces the residence time and opportunity of particles to interact with the intended lung cell population (Ruge et al., 2013). This negates the drug delivery objective of the inhaled particles particularly when the lung cells, other than macrophages, are the target site of drug action.
2. Pulmonary disorders and pulmonary drug carriers
The most common lung diseases include asthma, pulmonary hypertension, chronic obstructive pulmonary disorder, acute respiratory distress syndrome in infants, cystic fibrosis, lung infections such as pneumonia and tuberculosis, and lung cancer (Alhajj et al., 2018; Paranjpe & Müller-Goymann, 2014). These diseases alter the normal physiology of the lung by airways constriction, mucus thickening, fibrosis and poor blood circulation, and hence affect the deposition profile of inhaled drugs (Borghardt et al., 2018). In most of the pulmonary disorders, the airways are sufficiently narrowed and this results in increased deposition of inhaled drugs in the upper airway by impaction (Darquenne, 2012). The residence time of particles in the lungs is mainly dependent on air flow rate, inhaled and exhaled volume, and end-inspiratory breath hold of patients. A deep, slow breathing allows a longer residence time and gives the inhaled particles much more time to deposit by sedimentation and diffusion especially at the peripheral lung (Löndahl et al., 2014). The residence time in the lungs can be further increased by end-inspiratory breath holding. The alveolar deposition increases with increasing inhaled volume or penetration depth of the aerosol into the lung. Fast and shallow breath on the other hand increases the deposition of particles in the extrathoracic airways by impaction.
Lactose receives a widespread application in the development of inhalation medicine specifically with respect to dry powder aerosol (Lee et al., 2018; Molina et al., 2018; Pinto et al., 2018; Zhou & Morton, 2012). This is attributed to stability, better flow properties and safety of lactose in dry powder inhaler formulations (Smyth & Hickey, 2005). The sugars such as lactose, mannitol, sorbitol and dextran are potentially applied as carriers in inhalational drug delivery (Odziomek et al., 2012). However, lactose is the only approved carrier in dry powder inhaler formulations (Molina et al., 2018).
The United States Food and Drug Administration (US FDA) stated two ‘points to consider’ in determining whether an FDA-regulated product involves the application of nanotechnology: (I) whether a material or end product is engineered to have at least one external dimension or an internal or surface structure, in the nanoscale range (approximately 1 nm–100 nm); or (II) whether a material or end product is engineered to exhibit properties or phenomena, including physical or chemical properties or biological effects, that are attributable to its dimension(s), even if these dimensions fall outside the nanoscale range, up to one μm (1000 nm) (Sheshala et al., 2019). With the advent of nanotechnology, the nanocarrier is recently advocated in pulmonary medicine design for the following advantages: (I) the carrier is characterized by an excessively large specific surface area that enables them to interact with the intended lung cells, (II) the carrier may be equipped with targeting ligand for cell- and organelle-specific drug targeting, (III) the cellular uptake of particles by lung cells is promoted, (IV) decrease drug dosages due to lower enzymatic activity and hence reduce side effects, and (V) improve the performance of imaging techniques applied for the in vivo diagnosis of lung cancer (Alhajj et al., 2018; Smola et al., 2008).
3. Chitosan
Chitin is considered as one of the major sources of nitrogen accessible to countless living terrestrial and aquatic organisms (Elieh-Ali-Komi & Hamblin, 2016). It is a major constituent of the exoskeleton of many arthropods like insects, spiders and crustaceans and the internal structures of other invertebrates (Brigham, 2018). The chitin is constituted of β (1→4) linked residues of N-acetyl-2-amino-2-deoxy-d-glucose and 2-amino-2-deoxy-d-glucose (Elieh-Ali-Komi & Hamblin, 2016). It exhibits an extremely poor aqueous solubility due to its highly crystalline characteristics which are brought about via hydrogen bonding between the acetamido moieties (Uto et al., 2018; Zhu et al., 2016). The partially deacetylated chitin is water-soluble (Muzzarelli et al., 1999; Ying et al., 2011). The lowest possible degree of deacetylation in chitin can be less than 10 % with molecular weight as high as 1–2.5 × 106 Da and parallel to a degree of polymerization of approximately 5000–10000 monomeric residues (Chawla et al., 2015). Chitosan is the most important derivative obtained by N-deacetylation of chitin with hot alkali (Fig. 1 ) (Antonino et al., 2017).
Fig. 1.
Chemical structures of (a) chitin and (b) chitosan.
The degree of deacetylation of chitosan ranges from 40 % to 98 % with its molecular weight ranging between 5 × 104 Da and 2 × 106 Da (Fig. 1) (Antonino et al., 2017; Chawla et al., 2015; Hejazi & Amiji, 2003; Mourya & Inamdar, 2008). Deacetylation of chitin provides chitosan with free amino functional groups that are readily protonated to induce polymer solubilisation or chemically reacted and grafted to produce new chitosan derivatives with specific physicochemical and biological properties (Table 1 ).
Table 1.
Selected examples of chitosan derivatives.
| Chitosan derivative | Remark | Reference |
|---|---|---|
Cationic chitosan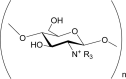 i.e. Trimethyl chitosan chloride, N-[(2-hydroxy-3-trimethyl-ammonium)-propyl] chitosan chloride, N-propyl-N,N-dimethyl chitosan, N-furfuryl-N,N dimethyl chitosan, N-diethylmethylamino chitosan. |
Highly positively charged. Strong film forming properties. Compatible with cations. Forms complexes with anions. Improved aqueous solubility at pH < 9. Enhanced intestinal permeation property. Improved mucoadhesive property. Biodegradable. Cytotoxic. Anti-bacterial. Anti-fungal. Potent hydroxyl radical scavenger. |
(Avadi et al., 2004; Cano-Cebrian et al., 2005; Ding et al., 2006; Fee et al., 2003; Guo et al., 2006, 2007; Holappa et al., 2006; Je & Kim, 2005; Jia et al., 2001; Kotze et al., 1997; Lee et al., 2002; Lim & Hudson, 2004; Rúnarsson et al., 2007; Singla & Chawla, 2001) |
N-Acylchitosan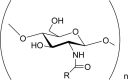 i,e. Formyl, acetyl, propionyl, butyryl, hexanoyl, octanoyl, decanoyl, carbanoyl, succinyl, acetoxybenzoyl chitosans. |
Hygroscopic. Biodegradable. Amphiphatic hydrogel with excellent water-absorption and water retention abilities. Anti-cancer drug carrier. Anti-bacterial. Cyto-compatible with chondrocytes. Excellent transfection efficiency. Facilitated endocytic uptake. |
(Guo et al., 2006; Hu et al., 2007; Lee et al., 2002; Mansouri et al., 2006; Ravi Kumar, 2000; Xiangyang et al., 2007) |
O-Acylchitosan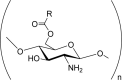 i.e. O-succinylchitosan, O-palmitoyl chitosan, O,O-didecanoylchitosan. |
Organoosoluble. Promote enzymatic hydrolysis. Fungicidal. Improved mucoadhesive and absorptive properties. Possess high cellular permeability. |
(Badawy et al., 2005; Mourya & Inamdar, 2008; Zariwala et al., 2018) |
N-Carboxyalkyl/aryl chitosans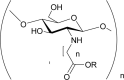 i.e. N-carboxymethyl chitosan, N- carboxypropyl chitosan, N- carboxybenzyl chitosan, N-(2-carboxyethyl)chitosan. |
Soluble in water. Show high viscosity and large hydrodynamic volume and film gel forming capacity. Possess good chelating abilities. Antioxidant. Anti-mutagenic. |
(Kogan et al., 2004; Mourya & Inamdar, 2008; Muzzarelli et al., 1999) |
O-Carboxyalkyl chitosan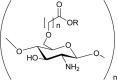 i.e. O- Carboxymethyl chitosan, cross-linked O-carboxymethyl chitosan. |
Anti-bacterial. Improved adhesive properties. Enhanced chondrocyte adhesion. Enhanced blood compatibility. |
(Mourya & Inamdar, 2008; Zhu & Fang, 2005) |
Thiolated chitosan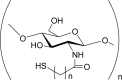 i.e. Chitosan-cysteine, chitosan-thioglycolic acid, chitosan-4-thiobutylamidine, chitosan-thioethylamidine, chitosan-2-iminothiolane conjugates. |
Improved mucoadhesiveness. Improved permeation enhancement properties. Possess cohesive and in situ gelling properties. Biodegradable. Possess enzyme inhibitory properties. |
(Bernkop-Schnürch & Kast, 2001; Föger et al., 2006; Hassan & Gallo, 1990; Kast et al., 2003; Krauland et al., 2004; Leitner et al., 2003; Maculotti et al., 2005; Roldo et al., 2004) |
Sugar linked chitosan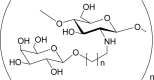 i.e. lactosaminated N-succinyl-chitosan, galactosylated chitosan, a-galactosyl chitosan, 1-deoxygalacti-1-yl-, 1-deoxyglucit-1-yl-, 1-deoxymelibiit-1-yl-, cellobiit-1-yl- chitosans. |
Water-soluble. Cell-, virus- and bacteria-specific as a function of sugars characteristics. Possess intercellular recognition and adhesion. |
(Morimoto et al., 2001; Mourya & Inamdar, 2008; Sashiwa & Aiba, 2004; Zhang et al., 2010) |
Sulfated chitosan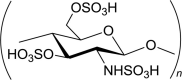 i.e. N-Sulfofurfyl chitosan, N-hexanoyl chitosan sulfate, N-propanoyl chitosan sulfate,, O-chitosan sulfate, N O-chitosan sulfate. |
Water-soluble. Anti-coagulant and hemaglutination inhibitory. Anti-viral. Anti-microbial. Possess osteogenic activity. Able to block human malignant melanoma cell adhesion. Show anti-obesity effect through anti-adipogenesis inhibition. Low cytotoxicity. |
(Huang et al., 2004; Karadeniz et al., 2011; Nishimura et al., 1998; Pires et al., 2013; Vikhoreva et al., 2005; Wang et al., 2010; Zhang et al., 2011) |
Unlike lactose disaccharide, the chitosan is a polysaccharide. The chitosan has multiple functional groups such as N—H and OH— that render it flexibly being modified for specific pharmaceutical or medical purposes (Kyzas & Bikiaris, 2015). The cationic amino moieties at the C2 position of the repeating units of chitosan readily interact electrostatically with anionic groups of other polyions to form polyelectrolyte complexes (Kulig et al., 2016). Chitosan has been complexed with various natural polyanions such as pectin, alginate, xanthan gum, carrageenan, chondroitin sulphate, carboxymethyl cellulose, hyaluronic acid, dextran sulphate or synthetic polyanions like poly (acrylic acid), poly (L-lactide) and polyphosphoric acid to confer specific drug delivery properties (Arriagada et al., 2004; Gnavi et al., 2013; Hamman, 2010; Quiñones et al., 2018). Through varying its molecular weight and degree of deacetylation, the chitosan can be transformed into nanocarrier with the intended sizes and zeta potentials (Mao et al., 2010). Chitosan is a plastic material which is mouldable into amorphous forms (Dang et al., 2017). Its molecular chains can be compacted at nanoscale as a function of formulation and processing conditions via hydrogen bonding, electrostatic interaction and/or Van der Waals forces (Zhang et al., 2015). Being polysaccharidic, chitosan is mucoadhesive and possesses viscous attribute that is essential in drug encapsulation, drug release and drug absorption kinetics modulation (Quiñones et al., 2018). As a cationic polyelectrolyte, the chitosan has a high affinity for the negatively charged mucosal interface that is rich in sialic acid and O-sulfosaccharides (Zhang et al., 2015). Chitosan is able to reduce the transepithelial electrical resistance of epithelial membranes by disrupting tight junctions between the epithelial cells (Yeh et al., 2011). It can loosen the tight junction of mucosa via the translocation of tight junction proteins from the membrane to the cytoskeleton through Protein Kinase C pathway, thereby promoting drug transport paracellularly and transcellularly (Rosenthal et al., 2012; Smith et al., 2004; Smith et al., 2005). Chitosan is biodegradable and biocompatible (Rodrigues et al., 2012; Saber et al., 2010). It is known as a therapeutic polymer which possesses anti-cancer (Azuma et al., 2015), anti-bacterial (Goy et al., 2009; Kara et al., 2014; Rabea et al., 2003), anti-fungal (Menconi et al., 2014; Tayel et al., 2010; Verlee et al., 2017), anti-oxidative (Ngo et al., 2015), anti-inflammatory (Azuma et al., 2015) and anti-diabetic activities (Liu et al., 2010; Ngo et al., 2015). On this note, the potential of chitosan for use in the development of pulmonary medicine is deemed to be higher than that of lactose. With reference to cancer nanomedicine, the physicochemical attributes of the chitosan-based drug carrier such as size, shape, surface charge, surface morphology, targeting ligand availability and its nature are found to critically dictate the effectiveness of cell targeting, internalization and anti-tumour action of cancer therapeutics (Musalli et al., 2020; Wang et al., 2017). Small, spherical and positively charged nanocarriers with reduced surface area to interrupt the cancer cell membrane and enhanced affinity for the negatively charged cancer cell surfaces favour cellular uptake. The use of a sublevel or an excessive fraction of targeting ligand translates to inadequate nanocarrier-cancer cell surface receptor interaction and poor intracellular therapeutic availability due to insufficient ligand or steric hindrance of overcrowded ligands in receptor binding. Similar findings are observed in the case of infection nanomedicine. In infectious diseases such as pulmonary tuberculosis, drug targeting at the alveolar macrophages is of paramount importance as these are the residence site of tubercle bacilli (Shah et al., 2017). The hydrophobic carrier exhibits a higher degree of opsonisation by macrophages. The nanocarrier made of high molecular weight or viscosity chitosan can hinder transmembrane drug transport into the macrophages (Mohd Chachuli et al., 2016). This nanocarrier can bind to the extracellular membrane of macrophages and develop a viscous barrier to drug diffusion or endocytosis.
4. Pulmonary infectious diseases and the applications of chitosan based carriers
Pulmonary infection represents one of the significant burdens to healthcare system worldwide, and this includes infection caused by bacteria (Mycobacterium tuberculosis, Pseudomonas aeruginosa, Streptococcus pneumonia), fungi (Aspergillus fumigates) and viruses (influenza virus A, influenza virus B, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)) (Brenner et al., 2011; Muttil et al., 2009; Wang et al., 2020; Wu et al., 2020). The infection can be fatal in immuno-compromised patients and those with underlying pulmonary dysfunctions (Merchant et al., 2016). The infection mostly involves treatment with anti-inflammatory and anti-infective agents. Conventionally, these drugs are commonly delivered via oral or parenteral routes. The systemic administration of drugs may be accompanied by inadequate drug concentration at the site of the infected lung, rapid declination of plasma drug concentration to sub-therapeutic level and development of microbial resistance. Local drug delivery by inhalation is envisaged to resolve such hurdles. Drug delivery system development using chitosan is foreseeable to benefit from its anti-microbial activity. The chitosan is mucoadhesive (Oliveira et al., 2017). It can bind to the lipopolysaccharides and phosphoryl groups of the bacterial cell membrane via its protonated amino moieties (Garg et al., 2015; Mohammed et al., 2017; Park et al., 2010). The adhesion elicits intracellular transductions of enzymes and blockage of electron transport, membrane perforation and intracellular electron leakage which eventually destroy or prevent the growth of bacterial cells (Dutta et al., 2012; Pourshahab et al., 2011; Rabea et al., 2003). The other proposed mechanism is the binding of chitosan with microbial DNA, which leads to the inhibition of the mRNA and protein synthesis via the penetration of chitosan into the nuclei of the microorganisms (Goy et al., 2009; Sebti et al., 2005). The positively charged chitosan can also interfere with the microbial growth by selectively binding with the essential nutrients of microbes (Chen et al., 2002; Jia et al., 2001). The low molecular weight chitosan with higher degrees of deacetylation and charge densities is reported to exhibit a higher level of anti-microbial activity (Goy et al., 2009; Kong et al., 2010; Park et al., 2010). The negative charge on the cell surface of the gram-negative bacteria is higher than that of gram-positive bacteria (Chung et al., 2004; Tayel et al., 2010). The former is relatively hydrophilic thereby leading to more chitosan being adsorbed onto the cell surfaces and having them experiencing a higher growth inhibitory effect.
Tuberculosis is a chronic granulomatous airborne bacterial infection caused by M. tuberculosis (Mehta et al., 2018). This contagious infectious disease occurs through inhalation of Tubercle bacilli infected droplets present in the size range of 1 μm–5 μm, that remain suspended in the air from minutes up to several hours (Fernstrom & Goldblatt, 2013; Muttil et al., 2009). The small size of droplet nuclei enables them to evade bronchial defense system and reach the alveoli (Pham et al., 2015). The bacterium can be engulfed by alveolar macrophages, replicated intracellularly and further infected the surrounding cells before the development of an immune response (Kinhikar et al., 2010). The M. tuberculosis has a tendency to infect non-phagocytic cells present in the alveolar region such as M cells, epithelial cells and alveolar endothelial cells. The disease mostly affects the lung and remains localized in 75–80 % of the cases (Sosnik et al., 2010; Yadav et al., 2009). It can also disseminate to other areas causing extra pulmonary tuberculosis. The primary infection can progress towards active disease, remain as latent infection or be eradicated by the immune system of the host (Gengenbacher & Kaufmann, 2012). The efficacy of currently available drugs (isoniazid, rifampicin, ethambutol and pyrazinamide) is complicated by constraints of drug dosage, adverse reactions and poor penetration of drugs into the sites of infection due to the poor vascularization of the lesions following drug administration by the non-inhalation route (Pham et al., 2015).
Chitosan based nano and microcarrier is an attractive choice in pulmonary delivery of anti-tubercular drugs (Table 2 ). The chitosan nanocarriers, in the form of solid nanoparticles and liquid nanoemulsion, have been investigated for their suitability of use in pulmonary anti-tubercular drug delivery. The solid nanoparticles are prepared by solvent evaporation emulsification technique or ionic gelation of oppositely charged materials in the liquid state, followed by freeze drying or spray drying process (Agnihotri et al., 2004; Rawal et al., 2017; Yildiz-Peköz et al., 2018). Alternatively, they are produced by directly spray drying a solution feed of drugs and excipients through effectively controlling the process parameters such as inlet temperature, liquid feed flow rate, atomizing gas pressure, heating air flow, nozzle geometry and cyclone performance (Noraizaan & Wong, 2017; Wong & John, 2016). The solid nanoparticles are known to be exhalation prone and highly aggregative, and these hinder their dispersion and inhalation performance (Muralidharan et al., 2015; Yang et al., 2008). They have been co-spray dried into inhalable microparticles with lactose, mannitol, maltodextrin and leucine as the bulking and/or dispersing agent, or directly blended with lactose-polyethylene glycol 3000 microparticles where the nanoparticles are adsorbed onto the surfaces of the microparticles (Agnihotri et al., 2004; Alhajj et al., 2020; Pilcer & Amighi, 2010). The co-spray dried microparticles have a mass median aerodynamic diameter of 3 μm–15 μm (Pilcer & Amighi, 2010). They are mostly aggregative in nature and are deemed to be less desirable when pulmonary inhalation is concerned (Lee et al., 2018; Smyth & Hickey, 2005). Blending of such microparticles with larger carriers of sizes between 63 and 90 μm can aid to disaggregate the microparticulate structures through the surface adsorption phenomenon (Lee et al., 2018; Smyth & Hickey, 2005). The large carrier serves as the pulmonary vehicle of microparticles and the detachment of microparticles from large carrier in lung renders drug/nanoparticles deposition at the intended target site (Grasmeijer et al., 2014; Renner et al., 2017; Zeng et al., 2000; Zeng et al., 2001; Zhou & Morton, 2012). Blending of nanoparticles with microparticles brings similar advantages in powder flow as that of the mix of microparticles and large carriers (Alhajj et al., 2020). Individually, both nanoparticles and microparticles are cohesive. Blending of them in specific weight ratios reduces the nanoparticulate aggregation through dispersing the nanoparticles over the surfaces of the microparticles. The adsorbed nanoparticles can act as a glidant. Their presence on microparticulate surfaces reduces the aggregation tendency of the microparticles, thereby producing an inhalable powder with an appropriate flow property.
Table 2.
Pulmonary chitosan carriers of drugs for the treatment of infectious diseases.
| Code | Formulation and Preparation |
Physicochemical Property | Aerodynamic Property | Cell Culture / Microbial Examination | In Vivo Performance | Remark | Reference |
|---|---|---|---|---|---|---|---|
| F1 | Second line anti-tubercular drug “prothionamide” loaded chitosan nanoparticles are prepared by ionic gelation technique, followed by freeze-drying into dry powder inhalational formulation. To increase the flow property, prothionamide nanoparticles and anhydrous inhalable grade lactose are mixed (1:0.5, 1:1, 1:1.5 weight ratio etc.) manually using a geometrical dilution process. |
Spherical particles of 314.37 ± 3.68 nm with a zeta potential value of 32.40 ± 1.04 mV are produced. The freeze dried nanoparticles are fluffy with a fair flow property. These nanoparticles are characterized by angle of repose, Carr’s index and Hausner ratio as 37.32˚, 17.43 % and 1.21 respectively. Their flowability improves through blending with lactose in 1:1 weight ratio, with angle of repose, Carr’s index and Hausner ratio of 29.25 ± 2.15˚, 9.8 ± 2.1 % and 1.11 ± 0.01 respectively. In vitro initial burst drug release followed by sustained release up to 96.91 % in 24 h is noted. |
Emitted dose of 82.37 %, aerodynamic particle size of 1.76 μm, geometric standard particle size distribution of 1.96 and fine particle fraction of 81.19 % are reflected in Anderson cascade impactor study. | Nil | Animal study using single dose administration through mono-dose inhaler indicates that prothionamide nanoparticles loaded dry powder inhalation achieves Cmax 2.90 ± 0.28 μg/mL at Tmax 3 h. The dry powder inhalation of prothionamide nanoparticles maintains the plasma drug concentration above the minimum inhibitory concentration for a period more than 12 h, unlike pure drug which could only maintain for up to 3 h. | Stability study shows no significant changes in polydispersity index, zeta potential, drug entrapment efficiency and percent drug release. In vitro drug release finding is reflected in the in vivo pharmacokinetics study. |
(Debnath et al., 2018) |
| F2 | Isoniazid (INH) and rifampin (RMP) loaded genipin-crosslinked carboxymethyl chitosan (GEN-CS) nanogel is prepared using emulsion crosslinking method, washed with ultrapure water, ultra-filtered and vacuum dried. | Spherical homogeneous particles of 60 nm–130 nm with positive zeta potential are produced. The drug loads of INH and RMP are 92.7 μg/mg and 66.1 μg/mg, respectively. The particles show sustained drug release behaviour in the simulated lung fluid. |
Nil | The nanoparticles have a relatively high anti-bacterial activity against multidrug-resistant tuberculosis at reduced cytotoxicity of drugs. | The GEN-CS/INH/RMP nanogel provides a predominant deposition of drug within the lung of animals when the nanogel particles are administered using a dried powder insufflator | To prepare GEN-CS nanogel for the encapsulation of INH and RMP, the GEN, which serves as the crosslinker, is 5000 to 10,000 times less cytotoxic than glutaraldehyde. The nanogel provides a safe and efficient administration of anti-tubercular drugs. |
(Wu et al., 2018 |
| F3 | Rifampicin loaded octanoyl chitosan nanoparticles are produced by double emulsion solvent evaporation technique. Nanoparticles obtained are then lyophilized following the addition of 1 %w/v trehalose dehydrate as the cryoprotectant. |
The octanoyl chitosan nanoparticles have smooth surface texture and spherical morphology, with diameter of 253 ± 19.06 nm, polydispersity index of 0.323 ± 0.059 and drug entrapment efficiency of 64.86 ± 7.73 %. Sustained drug release behaviour within 72 h of dissolution (73.14 ± 3.17 %) is noted. | The two-stage impinger analysis of the lyophilized rifampicin loaded octanoyl chitosan nanoparticles proceeds through dispersing the nanoparticles in 5 ml water and aerosolising from a jet nebulizer at 60 L/min. The nebulization efficiency is 77.04 ± 4.33 %, with a fine particle fraction of 43.27 ± 4.24 %. | Preliminary cytotoxicity studies of nanoparticles show no observable effect on cell viability over a period of 24 h on A549 cell lines. | Nil | The nanoparticles are physicochemically stable for 2 months. They show excellent aerosolisation profile and sustained drug release characteristics. | Petkar et al., 2018) |
| F4 | Rifampicin-oleic acid first-generation nanoemulsion and its respective chitosan- and chitosan-folate conjugate-decorated second and third generation nanoemulsions are prepared by spontaneous emulsification technique. | The nanoemulsions have average droplet sizes of 40 nm–60 nm, with narrow polydispersity indices. They exhibit desirable pH, surface tension, viscosity, refractive index, density and viscosity attributes for pulmonary administration. | Nanoemulsions demonstrate more than 95 % aerosol output with an inhalation efficiency greater than 75 %. The aerosol output and inhaled fine particle fractions are primarily governed by the size and surface tension of nanoemulsions in an inverse relationship. | A significantly higher level of internalisation of nanoemulsion by alveolar macrophages is observed with third generation nanoemulsion than second generation nanoemulsion at 2 h of incubation due to dual receptor targeting of macrophage by means of chitosan and folate. The cell viability in NR8383 cells is above 80 %. |
The intratracheal administration of the nanoemulsions depicts that first and third generation nanoemulsions attain higher plasma drug concentrations in the first hour due to higher levels of burst drug release. The second generation nanoemulsion demonstrates sustained plasma drug level in vivo. | The nanoemulsions are found to be safe with third generation nanoemulsion exhibiting higher cell internalization potential, moderately low plasma drug concentration, and higher lung drug content. | (Shah et al., 2017) |
| F5 | Rifampicin loaded chitosan nanoparticles are prepared by ionic gelation using the probe sonication method. The pre-freezing of nanoparticles is conducted using freeze dryer at −40 °C for 1 h with 10 %w/v lactose solution as the cryo-protectant. The frozen nanoparticles are subjected to secondary drying at 20 °C for 24 h at 1 Pa pressure to obtain respirable powder. Further, the freeze-dried nanoparticles are adsorbed onto the inhalable lactose pre-blend (coarse lactose: Inhalac® 230 and fine lactose: Inhalac®400) in the weight ratio of 95:5. |
Particles of 124.1 ± 0.2–402.3 ± 2.8 nm with drug entrapment efficiency of 72.0 ± 0.1 % and sustained drug release behaviour (In vitro release of 100 % pure drug within 12 h and approximately 90 % release of encapsulated rifampicin within 24 h) are produced. | Hausner ratio of nanoparticles is 1.22 ± 1.2. A fine particle fraction of 33.27 ± 0.87 % with a mass median aerodynamic diameter of 3.3 ± 0.18 μm are attained in Andersen cascade impactor analysis. |
The percentage of J774 macrophage cell viability is higher with nanoparticles (80–90 %) than pure rifampicin (75–80 %) at the drug dosage of 0.125 mg/mL. | A marked increase in maximum plasma drug concentration is attained with dry powder inhalation of nanoparticles when compared against the oral commercial formulation. | The freeze-dried rifampicin nanoparticles act as a better targeted delivery system for lung drug deposition through direct organ-specific targeting than oral drug administration. | (Rawal et al., 2017) |
| F6 | Isoniazid and rifampicin loaded chitosan nanoparticles are prepared by ionic gelation followed by spray drying processes. | Spherical particles with a size of 230 ± 4.5 nm, polydispersity index of 0.180 ± 0.021, drug encapsulation efficiency of 70.8 ± 6.6 % for rifampicin and 68.8 ± 7.0 % for isoniazid are produced. Initial burst (40 % to 50 % within 4 h) followed by late sustained drug release (90 % to 95 % up to 72 h) behaviour is attained. | Nil | Nil | Administration of nebulized nanoparticles for 28 days to M. tuberculosis infected mice results in undetectable mycobacterial colony forming unit in lung and spleen homogenates. A compressor nebulizer system is used in this study to administer drugs in aerosol to female Balb/c mice (20–30 g/mouse). Both drugs are detected in various organs (lung, liver, spleen and kidney) until 24 h post nebulization. The high lung drug concentration is attributed to drug retention via phagocytosis by macrophages. |
The chitosan nanoparticles provide an effective drug targeting to macrophage-rich organ that is essential in tuberculosis treatment. | (Garg et al., 2015) |
| F7 | Rifampicin loaded chitosan-polylactic acid-polyethylene glycol-gelatin nanoparticles are prepared by emulsion solvent evaporation technique. | Spherical and compact particles where size increases with increasing concentration of rifampicin (187 ± 10 nm to 214 ± 17.3 nm) and co-polymers (192 ± 8.5 nm to 234 ± 16.8 nm) are produced. The zeta potential increases from 21 ± 2.2 mV to 29 ± 1.6 mV with an increase in rifampicin concentration from 10 % to 50 %. The drug entrapment efficiency of nanoparticles improves with the use of copolymers where 96.8 % is achieved in the composite containing polylactic acid, polyethylene glycol and gelatin. The initial drug release is fast and declined thereafter. | Nil | Nil | Nil | The use of copolymers is deemed beneficial in drug entrapment within the nanoparticulate system, and it provides a slow late phase of drug release. | (Rajan & Raj, 2013) |
| F8 | Isoniazid loaded chitosan nanoparticles are prepared by ionic gelation method. These nanoparticles are co-spray dried with lactose, mannitol and maltodextrin with or without leucine to produce inhalable microparticles containing the drug loaded nanoparticles. |
Spherical, hexagonal and rod shaped particles with a size of 449.1 ± 0.3 nm, a polydispersity index of 0.24 ± 0.03, a zeta potential of 38.9 ± 1.0 mV, a drug loading of 6.00 ± 0.18 % and a drug encapsulation efficiency of 17.0 ± 2.0 % are produced. Initial burst release of isoniazid (40 % to 55 % in 4 h) followed by sustained release up to 6 days are noted. Co-spray drying of nanoparticles with lactose produces small particles (3 μm–5 μm). The use of mannitol and maltodextrin leads to the formation of larger particles (more than 10 μm) due to aggregation of fine particles. |
The powders were aerosolised in air stream of 60 l/min for 4 s using Cyclohaler. The in vitro deposition of the aerosolised drug is investigated using a twin stage impinge. The emitted dose of 46 % to 90 % and fine particle fraction of 7.05 % to 45 % are attained. |
The minimum inhibitory concentration of tests using isoniazid solution is about 16 times higher than that of employing isoniazid loaded nanoparticles. The nanoparticles exhibit a higher anti-bacterial activity against Mycobacterium avium Intracellulare than S. aureus and P. aeruginosa. |
Nil | The fine particle fraction of lactose containing powders is higher than mannitol and maltodextrin containing powders. The latter experience particle adhesion onto the capsule and inhaler wall thereby negating their aerosolization and inhalation processes. The introduction of leucine improves the fine particle fraction of drug aerosolized from all formulations due to the production of spherical particles with rough surface. This surface roughness could improve the aerosolisation capability of the spray dried powders by reducing cohesion between particles. |
(Pourshahab et al., 2011) |
| F9 | Isoniazid loaded chitosan microparticles, with or without prior tripolyphosphate crosslinking, are prepared by spray drying method. | The microparticles are produced in high yields (30.5 % to 46.3 %) and drug encapsulation efficiencies (> 80 %), with positive zeta potential (+17.7 mV–29.8 mV) and particle size ranging between 3.2 μm and 3.9 μm. Crosslinked chitosan microparticles exhibit a slower drug release than non-crosslinked microparticles. | Nil | The chitosan microparticles exhibit mucoadhesive property with mucin in vitro and oral mucosal fragment of pork ex vivo. 50–190 kDa chitosan microparticles do not exert cytotoxic effect against alveolar macrophages whether they are modified by drug or crosslinking agent. | Nil | Incubation of chitosan microparticles with peritoneal macrophages indicate that the cytotoxicity decreases in the presence of drug or crosslinking agent. | (Oliveira et al., 2017) |
| F10 | Doripenem loaded chitosan microparticles with different lactose, trehalose, and l-leucine concentrations are prepared by ionotropic gelation and spray drying methods. | Surface-porous particles of 3.8–6.9 μm that are spherical and corrugated in shape are produced. The drug encapsulation efficiency varies between 78 % and 86 %. The particles exhibit burst drug release in the first 3 h followed by a controlled release of doripenem over 24 h. | Emitted dose of microparticles containing leucine is higher (98 %). These particles are characterised by a mass median aerodynamic diameter of 4.11 μm, a geometric standard particle size distribution of 2.11 and a fine particle fraction of 27.6 %. | The viability of cells treated with drug loaded microparticles at 0.5 mg/mL and 10 mg/mL concentrations varies from 70 % to 90 % in human Caucasian lung adenocarcinoma (Calu-3) cell line. | Nil | The microparticles are characterised by higher fine particle fraction values than commercial dry powder inhalational products (10 % to 20 %). Doripenem loaded chitosan microparticles can be employed in the local treatment of respiratory diseases such as pneumonia. |
(Yildiz-Peköz et al., 2018) |
| F11 | Dapsone loaded solid microcapsules are prepared by pre-emulsification of dapsone with chitosan and raspberry oil in the presence of stabilizers, using a rotor-stator system followed by high-pressure homogenization and spray drying. | The mean size of the emulsion globules is 430 nm, generating spray dried microcapsules with spherical shape and diameter (D4,3) of 7 μm. Sixty % of drug are released in 3 h of dissolution, and almost all content of drug are released in 24 h. | The Anderson cascade impactor analysis of microcapsules at 60 L/min for 4 s depicts a mass median aerodynamic diameter of 4.7 μm, a span of 1.21, and a fine particle fraction of 50 %. | Nil | The acute toxicity is evaluated on the basis of lactate dehydrogenase, alkaline phosphatase and total protein in the bronchoalveolar lavage fluid of animal model, that depicts that the drug loaded microcapsules exert a lower level of toxicity than the neat drug. | The pulmonary administration of dapsone loaded microcapsules appears to be a promising treatment alternative to eradicate Pneumocystis carinii in association with pneumonia. | (Ortiz et al., 2015) |
| F12 | Levofloxacin loaded swellable chitosan microspheres are prepared by spray drying method with glutaraldehyde as crosslinker. |
The microspheres show almost immediate drug release. | The microspheres are characterised by an emitted dose of 90 %, a mass median aerodynamic diameter of 5 μm and a fine particle fraction of 30 %. | The anti-bacterial efficacy of microspheres against the bacterial isolates of P. aeruginosa is equivalent to free levofloxacin. | Nil | The microspheres are envisaged to be able to reach the conductive zone of the respiratory tract where the P. aeruginosa are located. | (Gaspar et al., 2015) |
With reference to direct blending of chitosan nanoparticles with lactose based microparticles, it is found that the size, shape and specific surface area of the nanoparticles have a strong bearing on the inhalation efficiency of the nanoparticles (Alhajj et al., 2020). Small and irregularly shaped nanoparticles with a large specific surface area tend to aggregate among themselves instead of depositing onto the microparticulate surfaces. As a result, their inhalation propensity to deep and peripheral lungs has been found to be lower than those of larger and rounder nanoparticles. Direct blending of nanoparticles with microparticles is envisaged to circumvent several drug delivery hurdles of the microencapsulated nanoparticles (Alhajj et al., 2020; Garbuzenko et al., 2014; Mehta, 2016). Unlike the microencapsulated systems, the changes in nanoparticulate sizes are less likely with blending thus the size-dependent biological performances of the nanoparticles are less affected (Alhajj et al., 2020). The blending system is characterized by nanoparticles depositing onto the surfaces of the microparticles (Alhajj et al., 2020). The nanoparticles have a high level of redispersibility. They can be detached and inhaled into the lung targets with lower risks of being captured in the core of the microencapsulated vehicle without them releasing to the lung targets.
In the case of nanoemulsion, it is delivered via the pulmonary route by means of nebulization approach (Amani et al., 2010). Liquid dosage form such as nanoemulsion commonly exhibits a high level of dispersion and inhalation to peripheral lungs (Amani et al., 2010; Shah et al., 2017). The alveolar macrophages are characterized by cell surface leptin receptor of mannose specificity (Filatova et al., 2018). The chitosan is known to be able to be recognized by alveolar macrophages. The nanoemulsion droplets have been decorated with chitosan to enable drug targeting at macrophages which harbor T. bacilli (Shah et al., 2017). The folate receptors are also expressed on the cell surfaces of activated alveolar macrophages (Jain et al., 2013). Decoration of nanoemulsion droplets by chitosan and folate in the form of a covalent conjugate has been found to enhance the endocytosis of particles into the macrophages and lung drug retention (Shah et al., 2017). The dual receptor targeting is more effective than single receptor strategy, and is expected to bring about a higher degree of bacteria eradication from the macrophages and infected lung.
The derivatives of chitosan such as carboxymethyl chitosan and octanoyl chitosan have been synthesized with the aim to prepare the nanocarrier of anti-tubercular drugs (Petkar et al., 2018). The mono-N-carboxymethyl chitosan has been reported to be able to decrease the transepithelial electrical resistance of Caco-2 cell monolayers thereby can act as a membrane permeation enhancer (Jayakumar et al., 2010). The acylation of chitosan using acyl chlorides and anhydrides confers organic solubility and increases the hydrophobicity of chitosan without incurring cytotoxicity. The hydrophobic octanoyl chitosan has been used to prepare crosslinker-free nanoparticles by means of double emulsion solvent evaporation technique for pulmonary delivery of rifampicin. The positively charged nanoparticles are equipped with drug release controlling ability of octanoyl derivative, and higher mucoadhesive and membrane permeation enhancement properties along with resistance to enzymatic degradation than that of plain chitosan (Ahmed & Aljaeid, 2016; Petkar et al., 2018). The chitosan based nanoparticles are electrostatically attracted to the negatively charged sialic acid present on the surfaces of lung alveolar macrophages. An enhancement in the binding affinity between the nanoparticles and the macrophages leads to a greater extent in uptake of drug and nanoparticles by the alveolar macrophages harboring the T. bacilli (Rawal et al., 2017). Both passive membrane adhesion and active receptor binding of nanoparticles are promotable with the use of particulate carrier characterized by positive surface charges.
Cystic fibrosis is an inherited autosomal recessive disease of the lung characterized by the production of viscid mucus which leads to the dysfunction of lung microorganism clearance system (Garbuzenko et al., 2019). The chronic inflammation and bacterial infection specifically by P. aeruginosa result in respiratory failure of the cystic fibrosis patients (Ng et al., 2014). Tobramycin, aztreonam and colistimethate, a prodrug of colistin, are available as inhalational products that represent a valuable alternative to solution for injection or oral therapy. Phase 2b trial of levofloxacin inhalation solution demonstrates that the sputum content of P. aeruginosa is reduced with the lung function improved in cystic fibrosis patients (Gaspar et al., 2015; Stockmann et al., 2014). The inhaled formulations with an aerodynamic diameter of 1 μm–5 μm are suitable for deep lung deposition, but particles of 1 μm–5 μm are also favorably phagocytosed by the resident macrophages. This poses toxicity to macrophages and could have reduced the availability of drug at the intended target site. The inhaled microparticles are designed with the ability to swell upon hydration following their deposition in the lung epithelial lining fluid in order to bypass macrophage uptake (Chaubey & Mishra, 2014). Chitosan as a swellable biopolymer, on this note, have been effectively utilized in the development of inhalable dosage forms for cystic fibrosis treatment (Table 2). Ortiz, Jornada, Pohlmann, and Guterres (2015) report that the use of chitosan in the form of microparticulate carrier of dapsone does not promote cytotoxicity in J774 macrophages and pulmonary cell damage as indicated by low levels of cytosolic lactate dehydrogenase and membrane bound alkaline phosphatase in the bronchoalveolar lavage fluid.
5. Lung cancer and the applications of chitosan based carriers
The global incidence of cancer rises to 18.1 million newly diagnosed cases, with 9.6 million deaths in 2018 (Bray et al., 2018). Lung cancer is one of the major causes in worldwide cancer related mortalities (Wang et al., 2017). It is histologically divided into small cell lung carcinoma and non-small cell lung carcinoma representing 96 % of the cases, and mesothelioma, carcinoid, and sarcoma (Alhajj et al., 2018). The small cell lung carcinoma (20 %) and non-small cell lung carcinoma (80 %) differ from each other in sizes and locations in lung. The small cell lung carcinoma is located in central lung, while non-small cell lung carcinoma is located from central to peripheral lungs. The standard treatment option of lung cancer involves chemotherapy, surgery or radiotherapy depending on the stage of malignancy. Chemotherapy with drugs like gemcitabine, cisplatin, paclitaxel, docetaxel and vinorelbine is considered one of the first options that are mostly administered systemically (Paumier & Le Péchoux, 2010). These drugs are associated with serious side effects like pain, nerve damage and skin allergic reactions (Lee et al., 2015a). With reference to the intravenous paclitaxel, the solubilizing mixture of Cremophor EL and dehydrated alcohol has also been ascribed as the cause of drug hypersensitivity reaction and neurotoxicity (Gelderblom et al., 2001). Local administration of anti-cancers drugs via inhalation can reduce the systemic adverse effects with optimum drug concentration attainable at the target site. In 1993, one of the first clinical studies on pulmonary administration of a chemotherapeutic (5-fluorouracil) solution for the treatment of non-small cell lung cancer was published (Abdelaziz et al., 2018). Pulmonary administration results in only trace amounts of 5-fluorouracil being detected in plasma with no local or systemic adverse effects. The lung tumor tissues are characterized by 5- to 15-fold higher drug concentrations than normal lung tissues. In another investigation, celecoxib solution is effectively nebulized to treat lung cancer in combination with intravenous docetaxel. The aerosolized celecoxib is therapeutically as effective as oral dosage form, with a lower dose requirement and risk of adverse effect development (Abdelaziz et al., 2018). Despite advantages of pulmonary delivery, the efficacy of inhaled anti-tumor drugs can be limited by branching pattern of the respiratory tract and bio-barriers existing in the respiratory airway systems such as mucus, ciliated cells and resident macrophages limiting the localization, penetration and adsorption of drugs in the lung (Darquenne, 2012).
Similar to anti-tubercular drugs (Table 2), the chitosan based particulate carriers of anti-cancer drugs are developed by means of ionic gelation, emulsification and/or spray drying methods (Table 3 ). Further, nanoprecipitation and chemical vapor deposition are adopted to produce chitosan nanoparticles containing lipid or carbon based composition. Microparticles or microencapsulated nanoparticles are produced to elicit aerodynamic behavior required for pulmonary inhalation (Liu et al., 2017; Rosière et al., 2018; Silva et al., 2017). Targeting ligand such as folate and hyaluronic acid, and permeation enhancer such as gallic acid are introduced to the backbone of chitosan for the purpose of raising the affinity of carrier with cancer target and cellular permeabilisation (Almutairi et al., 2019; Rosière et al., 2018). Quercetin can be used as the p-glycoprotein inhibitor, along with the drug to reduce drug efflux from the cancer cells (Liu et al., 2017). The particle sizes of the carrier for targeting lung tumor are within the range of 50 nm to 9.6 μm (Table 3). The cytotoxicity and cellular uptake of inhaled chitosan based nanoparticles are size dependent. The chitosan based nanoparticles are characterized by positive surface charges (zeta potential values approach +30 mV) and this confers physical stability through reducing particle aggregation by electrical repulsion (Lee et al., 2015a). The endothelial cells of the tumor vasculature are associated with the over-expression of negatively charged surface molecules, for example, anionic phospholipids, glycoproteins and proteoglycans (Iozzo & Schaefer, 2015; Ran et al., 2002). Electrical interaction between the positively charged chitosan particles and negatively charged endothelial cells of the tumor vasculature can potentiate the accumulation of cationic chitosan particles in the lung tumor capillaries (Honary & Zahir, 2013).
Table 3.
Pulmonary chitosan carriers of drugs for the treatment of cancer.
| Code | Formulation and Preparation |
Physicochemical Property | Aerodynamic Property | Ex Vivo / Cell Culture Performance | In Vivo Performance | Remark | Reference |
|---|---|---|---|---|---|---|---|
| F1 | Paclitaxel (PTX) and quercetin (QUE) loaded nanoparticles are prepared using oleic acid-chitosan conjugate (OA-C) as the carrier by ionic crosslinking method. The aqueous dispersion of PTX-OA-C nanoparticles, QUE-OA-C nanoparticles, hydroxypropyl-β-cyclodextrin, lactose, and mannitol are spray-dried to produce the polymeric microspheres. | The particle sizes of OA-C nanoparticles, PTX-OA-C nanoparticles, QUE-OA-C nanoparticles and polymeric microspheres are 226.1 nm, 246.5 nm, 247.4 nm and 3.373 μm respectively. The polydispersity index ranges from 0.123 to 0.456 with zeta potential of 32.9 mV, 24.2 mV and 26.0 mV for OA-C nanoparticles, PTX-OA-C nanoparticles and QUE-OA-C nanoparticles respectively. A burst drug release followed by a sustained release behaviour up to 48 h are attained with polymeric microspheres in both pH 7.4 and pH 4.5 release medium. | The aerodynamic diameter of polymeric microspheres is 1.804 ± 0.022 μm, inferring from geometric particle size and tapped density profiles. | Nil | The in vivo pharmacokinetics analysis in rats depicts that the time to reach maximum plasma drug concentration, drug half-life and AUC0–t are higher with pulmonary administration of polymeric microspheres than those of combined or single intravenous administration of PTX and QUE. The clearance and peak plasma drug concentration are lower with the use of pulmonary polymeric microspheres. The tissue distribution of PTX and QUE in the lung is significantly higher than heart, liver, spleen and kidney. |
The polymeric microspheres act as a platform to deliver the nanoparticles to lungs, with mannitol and lactose serving as disintegrant to release the nanoparticles at the target site. The OA-C nanoparticles, PTX-OA-C nanoparticles and QUE-OA-C nanoparticles are adopted to promote cellular drug uptake via nanogeometry of particles and permeation enhancement property of oleic acid. | (Liu et al., 2017) |
| F2 | 3,4,5-tribenzyloxybenzoic acid (GAOBn) loaded gold nanoparticles stabilized by quaternized chitosan-gallic acid-folic acid as a cancer-targeting drug delivery system are prepared by chemical reduction method consisting of two major steps: reduction and stabilization processes. | Spherical particles with a size of 33 ± 9 nm, a size distribution of 0.276 ± 0.050 and a zeta potential of 25.9 ± 0.4 mV are produced. | Nil | The particles exhibit a higher level of cytotoxicity (6 % cell viability) against lung cancer cells (CHAGO) and are safe (cell viability ≥ 80 %) with reference to normal fibroblast cells of skin (CRL-1947) at 20 μg/mL GAOBn. Transmission electron microscopy analysis demonstrates that particles are taken up by the lung cancer cells. | Nil | Chitosan is used to reduce and stabilize the gold nanoparticles. Chitosan is quaternized to increase its magnitude of positive charge to enhance its electrostatic interaction with the cancer cells. It is then conjugated with gallic acid as a hydrophobic moiety to increase its permeability, and with folic acid to introduce the active target element for folate receptor overexpressed on the cancer cell surfaces. | (Komenek et al., 2017) |
| F3 | Cisplatin loaded chitosan microspheres are prepared by emulsification and ionotropic gelation method. | Spherical particles with a size of 5.20 ± 1.19 μm, Carr’s index of 28.48 %, moisture and drug contents of 4.10 % and 79.2 ± 2.9 % respectively are produced. Initial burst drug release (37 % in 1 h) followed by sustained release up to 12 h are noted. | The aerodynamic diameter of the microspheres is 2.71 μm. The fine particle fraction of the microspheres is low and can be improved through employing lactose (63 μm–90 μm) as the carrier of the microspheres. | The microspheres are cytotoxic against A549 human lung cancer cells (HOP-62). | Nil | The microspheres are characterised by a higher IC50 value when compared to free drug due to a slower drug release from the matrix thus negating the drug bioavailability. | (Menon et al., 2012) |
| F4 | Raloxifene loaded hyaluronic acid and chitosan nanoparticles are prepared by single emulsion solvent evaporation method. | The nanoparticles are constituted of a core and surrounded by multilayers of hyaluronic acid- and chitosan-based shell. The nanoparticles are characterized by a size of 210.6 ± 4.4 nm, a polydispersity index of 0.05 ± 0.00, a zeta potential of -29.1 ± 4.5 mV and a drug encapsulation efficiency of 92 %. | Nil | The nanoparticles induce a higher level of cytotoxicity against A549 lung cancer cell line compared to liver cancer HepG2 and Huh-7 cell lines. | Nil | Hyaluronic acid and chitosan complexation is used to increase the half-life and activity of raloxifene through targeting cluster of differention-44 (CD 44) receptor. The significant suppression of A549 lung cancer cell viability is achieved via reducing their glucose uptake to diminish the bioenergetics of cancer cells and activation of apoptosis via nitric oxide level elevation. | (Almutairi et al., 2019) |
| F5 | POXylated strawberry-like gold-coated magnetite nanocomposites and ibuprofen are encapsulated into a chitosan matrix using the supercritical assisted spray drying technique to produce a nano-in-micro drug delivery system. | Nanocomposites with a diameter of 50 nm–200 nm are encapsulated in spherical particles having a volume-weighted mean diameter varying from 2.0 μm to 2.9 μm and a span value of 0.8 to 0.9 suitable for deep lung deposition. The drug release propensity is higher at pH 6.8 than pH 7.4. | The particles are characterized by a mass median aerodynamic diameter of approximately 1.5 μm, an emitted fraction above 96 % and a fine particle fraction of 55 %. | Nil | Nil | The higher drug release at pH 6.8 than pH 7.4 is attributed to chitosan, having a pKa value of 6.5, is characterized by partially protonated amine moieties at pH 6.8 leading to particle swelling and thus a faster drug release. The aerosolisation and inhalation of particles exceed the majority of commercial dry powder inhalation formulations. | (Silva et al., 2017) |
| F6 | Docetaxel loaded glutaraldehyde-crosslinked chitosan microspheres are prepared using a water-in-oil emulsification method. | The microspheres are spherical with smooth surface and a size of 9.6 ± 0.8 μm, a drug encapsulation efficiency and drug loading of 88.1 ± 3.5 % and 18.7 ± 1.2 % respectively. Only 23 % of drugs are released from the microspheres in the first 12 h of dissolution. | Nil | Nil | The microspheres deliver docetaxel mainly to lung following intravenous injection to mice and the concentration of drug in lung is significantly higher than other tissues (heart, liver, spleen, kidney and uterus/ovaries) and plasma. | The chitosan microspheres possess suitable physicochemical properties for lung administration and pharmacokinetics behaviour as drug delivery system to minimize the exposure of healthy tissues while increasing the accumulation of therapeutic at target sites. | (Wang et al., 2014) |
| F7 | Gemcitabine loaded surface-tailored chitosan/polyethylene glycol nanoparticles are prepared using ionic gelation method. The nanoparticles encapsulated with gemcitabine are tethered with folic acid. | The particle size and zeta potential of gemcitabine loaded chitosan nanoparticles are 157.2 ± 7.68 nm and 29.3 ± 1.91 mV respectively, whereas gemcitabine loaded surface-tailored polyethylene glycol and folate-polyethylene glycol chitosan nanoparticles are characterized by a size of 165.3 ± 11.0 nm and a zeta potential of 25.1 ± 1.8 mv, and a size of 184.3 ± 12.5 nm and a zeta potential of 21.1 ± 1.18 mV respectively. The drug encapsulation efficiency of gemcitabine loaded, gemcitabine loaded polyethylene glycol, gemcitabine folate-polyethylene glycol chitosan nanoparticles are 40.8 ± 1.5 %, 37.2 ± 2.2 % and 39.6 ± 2.7 % respectively. The extents of drug released from the nanoparticles over 10 days are nearly 87 % (gemcitabine loaded polyethylene glycol chitosan nanoparticles) and 85 % (gemcitabine loaded folate-polyethylene glycol chitosan nanoparticles) at pH 5.8 and nearly 79 % (gemcitabine loaded polyethylene glycol chitosan nanoparticles) and 75.3 % (gemcitabine loaded folate-polyethylene glycol chitosan nanoparticles) at pH 7.4. The nanoparticles exhibit a slow and sustained drug release profile. |
Nil | The higher cellular binding with eventual uptake and cytotoxicity are observed with gemcitabine loaded folate-polyethylene glycol chitosan nanoparticles, presumably facilitated by folate receptor-mediated endocytosis in lung epithelial cancer cell lines (A549 cell). | The half-life of gemcitabine increases from 0.45 ± 0.04 h (free drug) to 3.89 ± 0.13 h and 4.05 ± 0.23 h, respectively with respect to gemcitabine loaded polyethylene glycol chitosan nanoparticles and gemcitabine loaded folate-polyethylene glycol chitosan nanoparticles. The free gemcitabine is not detectable in plasma 12 h post-administration by lateral tail vein route, whereas gemcitabine shielded with a nanoparticulate system is found up to 12 h. A greater quantity of gemcitabine is found in tumour tissues followed by liver and kidney when it is delivered in the form of polyethylene glycol (11.67 ± 1.73 %) and folate-polyethylene glycol (31.33 ± 1.73 %) chitosan nanoparticles after 8 h. The incorporation of gemcitabine into nanoparticles will shield the drug from being metabolized in systemic circulation, and sustain its release from the matrix. Coupling with the use of folate, the drug targeting property of the nanoparticles is enhanced. | Gemcitabine loaded into folate-polyethylene glycol chitosan nanoparticles show a marked cytotoxicity against the A549 lung epithelial cancer cells, and A549 cell-bearing mice. | (Wang et al., 2017) |
| F8 | Paclitaxel loaded solid lipid nanoparticles are prepared by using nanoprecipitation method. The nanoparticles are then coated with folate grafted copolymer of polyethylene glycol and chitosan derivative which is N-[(2-hydroxy-3-trimethyl ammonium) propyl] chitosan chloride. | The nanoparticles are spherical in shape with encapsulation efficiency, mean diameter and zeta potential of about 100 %, 250 nm and +32 mV respectively. Cumulative drug release from the nanoparticles is about 50 % within 3 days of dissolution. After a first burst release of 13 % of drug within 7 h, the drug release rate remains constant, with approximately 15 % of drug released every 24 h. | Nil | The nanoparticles enter, or at least bind to two folate receptor-expressing cancer cell lines namely HeLa and M109-HiFR. The HeLa cells are significantly more sensitive to nanoparticles than M109-HiFR cells. The nanoparticles grafted with folate are almost five times more active than the folate-free nanoparticles in M109-HiFR cells and almost three times more active in HeLa cells. | Inhaled folate-grafted nanoparticles exhibit 7-fold and 32-fold increase in pulmonary drug concentrations compared to inhaled taxol following 1 h and 6 h of administration. These nanoparticles are able to reach and penetrate M109-HiFR murine lung carcinoma cell subline in vivo. | The nanoparticles demonstrate a favourable pharmacokinetics profile, with pulmonary exposure to drug prolonged up to 6 h with limited systemic distribution. | (Rosière et al., 2018) |
| F9 | Alginate coated chitosan hollow nanospheres of paclitaxel and doxorubicin are prepared. The paclitaxel is loaded into the nanoscale hollow structure via adsorption process. The positively charged doxorubicin is coated onto the surface of negatively charged hollow nanospheres via electrostatic adsorption. | The drug loadings of paclitaxel and doxorubicin in nanospheres are 18.4 ± 1.32 % and 74.2 ± 3.24 % respectively. The paclitaxel loaded nanospheres show a weakly crystalline diffraction pattern, whereas the doxorubicin loaded nanospheres do not display any crystalline diffraction pattern in comparison to physical mixture and pure drug. | Nil | The drug-free nanospheres are non-toxic, whereas the drug loaded nanospheres are cytotoxic against A549 lung cancer cells and induce apoptosis. | Nil | Co-delivery of paclitaxel and doxorubicin can effectively inhibit cell proliferation and promote cell apoptosis due to the nanoscale effect of particles and the synergistic effect of combined drugs. | (Tao et al., 2018) |
| F10 | A hybrid system composed of multi-walled carbon nanotubes (MWCNT) coated with chitosan is produced as a pH-responsive carrier of methotrexate. MWCNT are synthesized by fixed-bed chemical vapour deposition method. The fabrication of chitosan-MWCNT (CS-MWCNT) nanohybrid is achieved via precipitation technique. | The use of fix-bed chemical vapour deposition method produces well defined MWCNT with a narrow size distribution (length in a range of 110−980 nm, an average inner diameter of 0.7–1.5 nm, and an outer diameter of 5–8 nm corresponding to 4–7 graphene shells). The coat composition of CS-MWCNT nanohybrid is evident from chemical analysis, Raman spectroscopy and thermogravimetric evaluation. The coat content is about 20 %. The release of methotrexate from nanohybrid follows a pH-responsive behaviour with higher and faster release in acidic (pH 5.50) against neutral (pH 7.4) environments. |
Nil | Drug-free CS-MWCNT does not affect the viability of H1299 cells (non-small cell lung cancer derived from the lymph node) and MRC-5 cells (fibroblast derived from normal lung tissue). It exhibits a high biocompatibility. The drug loaded CS-MWCNT is highly selective in killing of cancer cells. They are found to possess equal or even more activity on cancer cells than free drugs. The H1299 cells viability is reduced by 15 % when free drug is used in treatment, while the drug loaded CS-MWCNT significantly increases the amount of cell death up to 44 %. |
Nil | The CS-MWCNT nanohybrid can selectively deliver the drug to H1299 lung cancer cells with negligible toxicity to healthy MRC-5 cells. This system is successful at reducing side effects of methotrexate to normal tissues and cells. | (Cirillo et al., 2019) |
The chitosan, specifically the oligosaccharide variants, exhibits anti-cancer activity (Chokraadjaroen et al., 2017; Gibot et al., 2015; Oberemko et al., 2019; Rafael et al., 2019). Its anti-cancer mechanism is generally not known, but it is presumably associated with its electrostatic charges, changes in membrane permeability of cancer cells, regulation of expression of tumor factor such as metalloproteinase-9 and/or vascular endothelial growth factor, and pro-apoptotic effects. The significance of positive charges of chitosan in combating cancer growth is reflected by quaternized chitosan derivatives being more selective and potent as a cancer therapeutic than the native chitosan (Chokraadjaroen et al., 2017). The cytotoxic effects of chitosan are characterized by apoptosis via the activation of caspase 3 and caspase 8 (Almutairi et al., 2019; Bharathi et al., 2019). The development of chitosan as pulmonary drug delivery carrier and as conjugate backbone of lung cancer therapy has been widely explored and gained successful outcomes (Bharathi et al., 2019; Chi et al., 2019; Cirillo et al., 2019; Li et al., 2019; Pandey et al., 2019; Tao et al., 2018; Wang et al., 2017). The chitosan based pulmonary drug delivery carrier has been shown to reduce systemic toxicity of anti-cancer drugs, increase drug bioavailability in lungs and improve anti-tumor efficacy of the drugs. The cellular uptake and internalization of engineered particles has been improved via targeting ligand which in turn enhances the propensity of cancer cell apoptosis. Most studies however are conducted at in vitro and/or ex vivo level (Table 3). Aerodynamic characterization and in vivo examination of the functionality of these particles through natural inhalation is required to elucidate their actual possible therapeutic performance.
The nanoparticles are prone to exhalation and susceptible to mucociliary transport (Lee, Loo, Traini, & Young, 2015a,2015b). With diameters larger than 200 nm, they have shown to induce non-specific scavenging by monocytes and reticuloendothelial system (Wang et al., 2017) with phagocytosis by the alveolar macrophages (Patel et al., 2015). The conversion of nanoparticles into microparticulate system larger than 6 μm mass median aerodynamic diameter translates to particle deposition at the upper airways and elimination by mucociliary clearance in the epithelia tissue (Dabbagh et al., 2018). Particles with mass median aerodynamic diameter of 1 μm–5 μm, though are accumulated in the narrower airways and evade mucociliary clearance, have their fraction between 2 μm and 3 μm prone to be recognized and eliminated by the resident alveolar macrophages (Lee et al., 2015a). In order to circumvent the above hurdles for the purpose of efficient drug delivery to lung cancer cells, it has been suggested to encapsulate nanocarriers with particle sizes of less than 200 nm into the microparticles with mass median aerodynamic diameters ranging from 3 μm to 5 μm (Dabbagh et al., 2018).
6. Future perspectives
Chitosan and its derivatives are excellent backbone materials of nano and microparticulate carriers for pulmonary delivery of anti-infective and anti-cancer drugs, with respect to their biodegradability, biocompatibility, mucoadhesive, anti-microbial, anti-tumour, and permeation enhancement properties. They have been investigated for their suitability of use as the pulmonary particulate carrier lately, deviating from the conventional application of lactose as the main drug carrier. The exploitation of chitosan and derivatives is relatively recent, and requires in depth analysis including in vitro aerodynamic characterisation and in vivo examination of the pharmacokinetics and functionality of these particles through natural inhalation to relate to the actual therapeutic performance.
Infection with SARS-CoV-2 brings about coronavirus disease that leads to acute respiratory distress syndrome, and the patients with cancer appear to be more likely to be diagnosed with COVID-19 (Sidaway, 2020). The late innovations of chitosan have found the therapeutic uses of this biopolymer to combat coronavirus related diseases and cancer. N-(2-hydroxypropyl)-3-trimethylammonium chitosan chloride with an average molecular weight of 250 kDa and degree of substitution between 50 % and 80 % have been shown to exert a strong interaction with the recombinant ectodomain of the S protein of HCoV, thus blocking S protein-host cellular interaction and virus infection (Milewska et al., 2016). Chito-oligosaccharides (molecular weight = 1000–12000 Da, degree of deacetylation = 65–70 %), prepared via electrical discharge plasma treatment of chitosan in the liquid state, induce apoptotic death in MCF-7 breast cancer cells (Chokradjaroen et al., 2018). The chito-oligosaccharides have also been found to be effective in killing of PC3 (prostate cancer cells), A549 (lung cancer cells) and HepG2 (hepatoma cells) with lower molecular weights being more effective than larger molecular weight counterparts. The late findings of chitosan and derivatives as therapeutic molecules to treat infection and cancer are envisaged to initiate an intense research interest combining the drug delivery as well as drug discovery attributes of this biomaterials. Their structural-activity relationships in association with coronavirus disease and lung cancer treatment are of great interest from the therapeutic and drug delivery performance viewpoints at molecular levels.
Acknowledgments
The authors wish to express their heart-felt gratitude to Universiti Teknologi MARA (0141903), and Ministry of Higher Education of Malaysia (LRGS-NanoMITe RU029-2014) for fund and facility support.
References
- Abdelaziz H.M., Gaber M., Abd-Elwakil M.M., Mabrouk M.T., Elgohary M.M., Kamel N.M., Elzoghby A.O. Inhalable particulate drug delivery systems for lung cancer therapy: Nanoparticles, microparticles, nanocomposites and nanoaggregates. Journal of Controlled Release. 2018;269:374–392. doi: 10.1016/j.jconrel.2017.11.036. [DOI] [PubMed] [Google Scholar]
- Agnihotri S.A., Mallikarjuna N.N., Aminabhavi T.M. Recent advances on chitosan-based micro- and nanoparticles in drug delivery. Journal of Controlled Release. 2004;100(1):5–28. doi: 10.1016/j.jconrel.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Ahmed T.A., Aljaeid B.M. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Design, Development and Therapy. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhajj N., Chee C.F., Wong T.W., Abd Rahman N., Abu Kasim N.H., Colombo P. Lung cancer: Active therapeutic targeting and inhalational nanoproduct design. Expert Opinion on Drug Delivery. 2018;15(12):1223–1247. doi: 10.1080/17425247.2018.1547280. [DOI] [PubMed] [Google Scholar]
- Alhajj N., Zakaria Z., Naharudin I., Ahsan F., Li W., Wong T.W. Critical physicochemical attributes of chitosan nanoparticles admixed lactose-PEG 3000 microparticles in pulmonary inhalation. Asian Journal of Pharmaceutical Sciences. 2020;15(3):374–384. doi: 10.1016/j.ajps.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almutairi F.M., Abd-Rabou A.A., Mohamed M.S. Raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles selectively induce apoptosis in lung cancer cells. Bioorganic & Medicinal Chemistry. 2019;27(8):1629–1638. doi: 10.1016/j.bmc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- Amani A., York P., Chrystyn H., Clark B.J. Evaluation of a nanoemulsion-based formulation for respiratory delivery of budesonide by nebulizers. AAPS PharmSciTech. 2010;11(3):1147–1151. doi: 10.1208/s12249-010-9486-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonino R.S.C., Fook L., Paschoal B.R., De Oliveira Lima V.A., De Farias Rached R.I., Lima E.P.N., Lia Fook M.V. Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone) Marine Drugs. 2017;15(5):141–153. doi: 10.3390/md15050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriagada R., Bergman B., Dunant A., Le Chevalier T., Pignon J.-P., Vansteenkiste J., International Adjuvant Lung Cancer Trial Collaborative Group Cisplatin-based adjuvant chemotherapy in patients with completely resected non–small-cell lung cancer. The New England Journal of Medicine. 2004;350(4):351–360. doi: 10.1056/NEJMoa031644. [DOI] [PubMed] [Google Scholar]
- Avadi M.R., Sadeghi A.M.M., Tahzibi A., Bayati K., Pouladzadeh M., Zohuriaan-Mehr M.J., Rafiee-Tehrani M. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. European Polymer Journal. 2004;40(7):1355–1361. [Google Scholar]
- Azuma K., Osaki T., Minami S., Okamoto Y. Anticancer and anti-inflammatory properties of chitin and chitosan oligosaccharides. Journal of Functional Biomaterials. 2015;6(1):33–49. doi: 10.3390/jfb6010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawy M.E.I., Rabea E.I., Rogge T.M., Stevens C.V., Steurbaut W., Höfte M., Smagghe G. Fungicidal and insecticidal activity of O-acyl chitosan derivatives. Polymer Bulletin. 2005;54(4–5):279–289. [Google Scholar]
- Bernkop-Schnürch A., Kast C.E. Chemically modified chitosans as enzyme inhibitors. Advanced Drug Delivery Reviews. 2001;52(2):127–137. doi: 10.1016/s0169-409x(01)00196-x. [DOI] [PubMed] [Google Scholar]
- Bharathi D., Ranjithkumar R., Chandarshekar B., Bhuvaneshwari V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. International Journal of Biological Macromolecules. 2019;141:476–483. doi: 10.1016/j.ijbiomac.2019.08.235. [DOI] [PubMed] [Google Scholar]
- Borghardt J.M., Kloft C., Sharma A. Inhaled therapy in respiratory disease: The complex interplay of pulmonary kinetic processes. Canadian Respiratory Journal. 2018;2018:1–11. doi: 10.1155/2018/2732017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brenner D.R., McLaughlin J.R., Hung R.J. Previous lung diseases and lung cancer risk: A systematic review and meta-analysis. PloS One. 2011;6(3):1–10. doi: 10.1371/journal.pone.0017479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham C. In: Green chemistry: An inclusive approach. Torok B., Dransfield T., editors. Elsevier; 2018. Biopolymers: Biodegradable alternatives to traditional plastics; pp. 753–770. [Google Scholar]
- Cano-Cebrian M., Zornoza T., Granero L., Polache A. Intestinal absorption enhancement via the paracellular route by fatty acids, chitosans and others: A target for drug delivery. Current Drug Delivery. 2005;2(1):9–22. doi: 10.2174/1567201052772834. [DOI] [PubMed] [Google Scholar]
- Chang L.Y., Crapo J.D., Gehr P., Rothen-Rutishauser B., Mühfeld C., Blank F. Vol. 8. Elsevier; 2010. Alveolar epithelium in lung toxicology; pp. 59–91. (Comprehensive toxicology). [Google Scholar]
- Chaubey P., Mishra B. Mannose-conjugated chitosan nanoparticles loaded with rifampicin for the treatment of visceral leishmaniasis. Carbohydrate Polymers. 2014;101:1101–1108. doi: 10.1016/j.carbpol.2013.10.044. [DOI] [PubMed] [Google Scholar]
- Chawla S.P., Kanatt S.R., Sharma A.K. In: Polysaccharides. Ramawat K., Mérillon J.M., editors. Springer International Publishing; 2015. Chitosan; pp. 219–246. [Google Scholar]
- Chen Y.M., Chung Y.C., Wang L.W., Chen K.T., Li S.Y. Antibacterial properties of chitosan in waterborne pathogen. Journal of Environmental Science and Health - Part A Toxic/Hazardous Substances and Environmental Engineering. 2002;37(7):1379–1390. doi: 10.1081/ese-120005993. [DOI] [PubMed] [Google Scholar]
- Chi J., Jiang Z., Qiao J., Peng Y., Liu W., Han B. Synthesis and anti-metastasis activities of norcantharidin-conjugated carboxymethyl chitosan as a novel drug delivery system. Carbohydrate Polymers. 2019;214:80–89. doi: 10.1016/j.carbpol.2019.03.026. [DOI] [PubMed] [Google Scholar]
- Chokraadjaroen C., Rujiravanit R., Watthanaphanit A., Theeramunkong S., Saito N., Yamashita K., Arakawa R. Enhanced degradation of chitosan by applying plasma treatment in combination with oxidizing agents for potential use as an anticancer agent. Carbohydrate Polymers. 2017;167:1–11. doi: 10.1016/j.carbpol.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Chokradjaroen C., Theeramunkong S., Yui H., Saito N., Rujiravanit R. Cytotoxicity against cancer cells of chitosan oligosaccharides prepared from chitosan powder degraded by electrical discharge plasma. Carbohydrate Polymers. 2018;201:20–30. doi: 10.1016/j.carbpol.2018.08.037. [DOI] [PubMed] [Google Scholar]
- Chung Y., Su Y., Chen C., Jia G., Wang H., Wu J.C.G., Lin J. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacologica Sinica. 2004;25(7):932–936. [PubMed] [Google Scholar]
- Cirillo G., Vittorio O., Kunhardt D., Valli E., Voli F., Farfalla A., Hampel S. Combining carbon nanotubes and chitosan for the vectorization of methotrexate to lung cancer cells. Materials. 2019;12(18):2889–2903. doi: 10.3390/ma12182889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbagh A., Abu Kasim N.H., Yeong C.H., Wong T.W., Abdul Rahman N. Critical influential parameters in particle-based pulmonary delivery of chemotherapeutics. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2018;31(3):139–154. doi: 10.1089/jamp.2017.1382. [DOI] [PubMed] [Google Scholar]
- Dang N.T.T., Chau T.T.L., Duong H.V., Le H.T., Tran T.T.V., Le T.Q., Nguyen T.-D. Water-soluble chitosan-derived sustainable materials: Towards filaments, aerogels, microspheres, and plastics. Soft Matter. 2017;13(40):7292–7299. doi: 10.1039/c7sm01292f. [DOI] [PubMed] [Google Scholar]
- Darquenne C. Aerosol deposition in health and disease. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2012;25(3):140–147. doi: 10.1089/jamp.2011.0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath S.K., Saisivam S., Debanth M., Omri A. Development and evaluation of chitosan nanoparticles based dry powder inhalation formulations of prothionamide. PloS One. 2018;13(1) doi: 10.1371/journal.pone.0190976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Xia X.H., Zhang C. Synthesis of metallic nanoparticles protected with N,N,N-trimethyl chitosan chloride via a relatively weak affinity. Nanotechnology. 2006;7(16):4156–4162. doi: 10.1088/0957-4484/17/16/027. [DOI] [PubMed] [Google Scholar]
- Dutta J., Tripathi S., Dutta P.K. Progress in antimicrobial activities of chitin, chitosan and its oligosaccharides: A systematic study needs for food applications. Food Science and Technology International. 2012;18(1):3–34. doi: 10.1177/1082013211399195. [DOI] [PubMed] [Google Scholar]
- Elieh-Ali-Komi D., Hamblin M.R. Chitin and chitosan: Production and application of versatile biomedical nanomaterials. International Journal of Advanced Research. 2016;4(3):411–427. [PMC free article] [PubMed] [Google Scholar]
- Fee M., Errington N., Jumel K., Illum L., Smith A., Harding S.E. Correlation of SEC/MALLS with ultracentrifuge and viscometric data for chitosans. European Biophysics Journal. 2003;32:457–464. doi: 10.1007/s00249-003-0317-8. [DOI] [PubMed] [Google Scholar]
- Fernstrom A., Goldblatt M. Aerobiology and its role in the transmission of infectious diseases. Journal of Pathogens. 2013;2013:1–13. doi: 10.1155/2013/493960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatova L.Y., Klyachko N.L., Kudryashova E.V. Targeted delivery of anti-tuberculosis drugs to macrophages: Targeting mannose receptors. Russian Chemical Reviews. 2018;87(4):374–391. [Google Scholar]
- Föger F., Schmitz T., Bernkop-Schnürch A. In vivo evaluation of an oral delivery system for P-gp substrates based on thiolated chitosan. Biomaterials. 2006;27(23):4250–4255. doi: 10.1016/j.biomaterials.2006.03.033. [DOI] [PubMed] [Google Scholar]
- Fröhlich E. Biological obstacles for identifying in vitro-in vivo correlations of orally inhaled formulations. Pharmaceutics. 2019;11(7):316–334. doi: 10.3390/pharmaceutics11070316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko O.B., Kbah N., Kuzmov A., Pogrebnyak N., Pozharov V., Minko T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. Journal of Controlled Release. 2019;296:225–231. doi: 10.1016/j.jconrel.2019.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuzenko O.B., Mainelis G., Taratula O., Minko T. Inhalation treatment of lung cancer: The influence of composition, size and shape of nanocarriers on their lung accumulation and retention. Cancer Biology & Medicine. 2014;11(1):44–55. doi: 10.7497/j.issn.2095-3941.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg T., Rath G., Goyal A.K. Inhalable chitosan nanoparticles as antitubercular drug carriers for an effective treatment of tuberculosis. Artificial Cells, Nanomedicine, and Biotechnology. 2015;44(3):1–5. doi: 10.3109/21691401.2015.1008508. [DOI] [PubMed] [Google Scholar]
- Gaspar M.C., Sousa J.J.S., Pais A.A.C.C., Cardoso O., Murtinho D., Serra M.E.S., Olivier J.-C. Optimization of levofloxacin-loaded crosslinked chitosan microspheres for inhaled aerosol therapy. European Journal of Pharmaceutics and Biopharmaceutics. 2015;96:65–75. doi: 10.1016/j.ejpb.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Gelderblom H., Verweij J., Nooter K., Sparreboom A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. European Journal of Cancer. 2001;37(13):1590–1598. doi: 10.1016/s0959-8049(01)00171-x. [DOI] [PubMed] [Google Scholar]
- Gengenbacher M., Kaufmann S.H.E. Mycobacterium tuberculosis: Success through dormancy. FEMS Microbiology Review. 2012;36(3):514–532. doi: 10.1111/j.1574-6976.2012.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibot L., Chabaud S., Bouhout S., Bolduc S., Auger F.A., Moulin V.J. Anticancer properties of chitosan on human melanoma are cell line dependent. International Journal of Biological Macromolecules. 2015;72:370–379. doi: 10.1016/j.ijbiomac.2014.08.033. [DOI] [PubMed] [Google Scholar]
- Gnavi S., Barwig C., Freier T., Haastert-Talini K., Grothe C., Geuna S. The use of chitosan-based scaffolds to enhance regeneration in the nervous system. International Review of Neurobiology, Tissue Engineering of the Peripheral Nerve - Biomaterials and Physical Therapy. 2013;109:1–62. doi: 10.1016/B978-0-12-420045-6.00001-8. [DOI] [PubMed] [Google Scholar]
- Goy R.C., Britto D.D., Assis O.B.G. A review of the antimicrobial activity of chitosan. Polímeros. 2009;19(3):241–247. [Google Scholar]
- Grasmeijer F., Frijlink H.W., de Boer A.H. A proposed definition of the activity of surface sites on lactose carriers for dry powder inhalation. European Journal of Pharmaceutical Sciences. 2014;56:102–104. doi: 10.1016/j.ejps.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Guo Z., Liu H., Chen X., Ji X., Li P. Hydroxyl radicals scavenging activity of N-substituted chitosan and quaternized chitosan. Bioorganic & Medicinal Chemistry Letters. 2006;16(24):6348–6350. doi: 10.1016/j.bmcl.2006.09.009. [DOI] [PubMed] [Google Scholar]
- Guo Z., Xing R., Liu S., Zhong Z., Ji X., Wang L., Li P. Antifungal properties of Schiff bases of chitosan, N-substituted chitosan and quaternized chitosan. Carbohydrate Research. 2007;342(10):1329–1332. doi: 10.1016/j.carres.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Hamman J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Marine Drugs. 2010;8(4):1305–1322. doi: 10.3390/md8041305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan E.E., Gallo J.M. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharmaceutical Research. 1990;7:491–495. doi: 10.1023/a:1015812615635. [DOI] [PubMed] [Google Scholar]
- Hejazi R., Amiji M. Chitosan-based gastrointestinal delivery systems. Journal of Controlled Release. 2003;89(2):151–165. doi: 10.1016/s0168-3659(03)00126-3. [DOI] [PubMed] [Google Scholar]
- Holappa J., Nevalainen T., Soininen P., Másson M., Järvinen T. Synthesis of novel quaternary chitosan derivatives via N -Chloroacyl-6- O -triphenylmethylchitosans. Biomacromolecules. 2006;7(2):407–410. doi: 10.1021/bm050707j. [DOI] [PubMed] [Google Scholar]
- Honary S., Zahir F. Effect of zeta potential on the properties of nano - drug delivery systems - a review (Part 2) Tropical Journal of Pharmaceutic Al Research. 2013;12(2):265–273. [Google Scholar]
- Hu Y., Du Y., Yang J., Tang Y., Li J., Wang X. Self-aggregation and antibacterial activity of N-acylated chitosan. Polymer. 2007;48(11):3098–3106. [Google Scholar]
- Huang R., Du Y., Zheng L., Liu H., Fan L. A new approach to chemically modified chitosan sulfates and study of their influences on the inhibition of Escherichia coli and Staphylococcus aureus growth. Reactive & Functional Polymers. 2004;59(1):41–51. [Google Scholar]
- Iozzo R.V., Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biology. 2015;42:11–55. doi: 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain N.K., Mishra V., Mehra N.K. Targeted drug delivery to macrophages. Expert Opinion on Drug Delivery. 2013;10(3):353–367. doi: 10.1517/17425247.2013.751370. [DOI] [PubMed] [Google Scholar]
- Jayakumar R., Prabaharan M., Nair S.V., Tokura S., Tamura H., Selvamurugan N. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Progress in Materials Science. 2010;55(7):675–709. [Google Scholar]
- Je J.-Y.Y., Kim S.-K.K. Water-soluble chitosan derivatives as a BACE1 inhibitor. Bioorganic & Medicinal Chemistry. 2005;13(23):6551–6555. doi: 10.1016/j.bmc.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Jia Z., Shen D., Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydrate Research. 2001;333(1):1–6. doi: 10.1016/s0008-6215(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Kara F., Aksoy E.A., Yuksekdag Z., Hasirci N., Akspy S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydrate Polymers. 2014;112:39–47. doi: 10.1016/j.carbpol.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Karadeniz F., Karagozlu M.Z., Pyun S.-Y., Kim S.-K. Sulfation of chitosan oligomers enhances their anti-adipogenic effect in 3T3-L1 adipocytes. Carbohydrate Polymers. 2011;86(2):666–671. [Google Scholar]
- Kast C.E., Frick W., Losert U., Bernkop-Schnürch A. Chitosan-thioglycolic acid conjugate: a new scaffold material for tissue engineering? International Journal of Pharmaceutics. 2003;256(1–2):183–189. doi: 10.1016/s0378-5173(03)00076-0. [DOI] [PubMed] [Google Scholar]
- Kinhikar A.G., Verma I., Chandra D., Singh K.K., Weldingh K., Andersen P., Laal S. Potential role for ESAT6 in dissemination of M. Tuberculosis via human lung epithelial cells. Molecular Microbiology. 2010;75(1):92–106. doi: 10.1111/j.1365-2958.2009.06959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogan G., Skorik Y.A., Zitnanová I., Krizková L., Duracková Z., Gomes C.A.R., Krajcovic J. Antioxidant and antimutagenic activity of N-(2-carboxyethyl)chitosan. Toxicology and Applied Pharmacology. 2004;201(3):303–310. doi: 10.1016/j.taap.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Komenek S., Luesakul U., Ekgasit S., Vilaivan T., Praphairaksit N., Puthong S., Muangsin N. Nanogold-gallate chitosan-targeted pulmonary delivery for treatment of lung Cancer. AAPS PharmSciTech. 2017;18(4):1104–1115. doi: 10.1208/s12249-016-0644-6. [DOI] [PubMed] [Google Scholar]
- Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. International Journal of Food Microbiology. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- Kotze A.F., Luessen H.L., De Leeuw B.J., De Boer B.G., Coos Verhoef J., Junginger H.E. N-trimethyl chitosan chloride as a potential absorption enhancer across mucosal surfaces: In vitro evaluation in intestinal epithelial cells (Caco-2) Pharmaceutical Research. 1997;14(9):1197–1202. doi: 10.1023/a:1012106907708. [DOI] [PubMed] [Google Scholar]
- Krauland A.H., Guggi D., Bernkop-Schnürch A. Oral insulin delivery: The potential of thiolated chitosan-insulin tablets on non-diabetic rats. Journal of Controlled Release. 2004;95(3):547–555. doi: 10.1016/j.jconrel.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Kulig D., Zimoch-Korzycka A., Jarmoluk A., Marycz K. Study on alginate–chitosan complex formed with different polymers ratio. Polymers. 2016;8(5):167–184. doi: 10.3390/polym8050167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmov A., Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. Journal of Controlled Release. 2015;219:500–518. doi: 10.1016/j.jconrel.2015.07.024. [DOI] [PubMed] [Google Scholar]
- Kyzas G., Bikiaris D. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Marine Drugs. 2015;13(1):312–337. doi: 10.3390/md13010312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Advanced Drug Delivery Reviews. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J.K.-W., Liang W., Chan H.-K. Pulmonary delivery of therapeutic siRNA. Advanced Drug Delivery Reviews. 2012;64(1):1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-J., Lee H.-G., Kwon Y.-B., Kim J.-Y., Rhee Y.-S., Chon J., Park C.-W. The role of lactose carrier on the powder behavior and aerodynamic performance of bosentan microparticles for dry powder inhalation. European Journal of Pharmaceutical Sciences. 2018;117:279–289. doi: 10.1016/j.ejps.2018.03.004. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Lim H.S., Kim J.H. Cytotoxic activity of aminoderivatized cationic chitosan derivatives. Bioorganic & Medicinal Chemistry Letters. 2002;12(20):2949–2951. doi: 10.1016/s0960-894x(02)00530-9. [DOI] [PubMed] [Google Scholar]
- Lee W.H., Loo C.Y., Traini D., Young P.M. Inhalation of nanoparticle-based drug for lung cancer treatment: Advantages and challenges. Asian Journal of Pharmaceutical Sciences. 2015;10(6):481–489. [Google Scholar]
- Lee W.H., Loo C.Y., Traini D., Young P.M. Nano- and micro-based inhaled drug delivery systems for targeting alveolar macrophages. Asian Journal of Pharmaceutical Sciences. 2015;10(6):1–18. doi: 10.1517/17425247.2015.1039509. [DOI] [PubMed] [Google Scholar]
- Leitner V.M., Walker G.F., Bernkop-Schnürch A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. European Journal of Pharmaceutics and Biopharmaceutics. 2003;56(2):207–214. doi: 10.1016/s0939-6411(03)00061-4. [DOI] [PubMed] [Google Scholar]
- Leslie K.O., Wick M.R. In: Practical pulmonary pathology: A diagnostic approach. Leslie K.O., Wick M.R., editors. Elsevier; 2018. Lung anatomy; pp. 1–14. e2. [Google Scholar]
- Li W., Hu X., Wang S., Xing Y., Wang H., Nie Y., Song K. Multiple comparisons of three different sources of biomaterials in the application of tumor tissue engineering in vitro and in vivo. International Journal of Biological Macromolecules. 2019;130:166–176. doi: 10.1016/j.ijbiomac.2019.02.136. [DOI] [PubMed] [Google Scholar]
- Lim S.-H.H., Hudson S.M. Synthesis and antimicrobial activity of a water-soluble chitosan derivative with a fiber-reactive group. Carbohydrate Research. 2004;339(2):313–319. doi: 10.1016/j.carres.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Liu K., Chen W., Yang T., Wen B., Ding D., Keidar M., Zhang W. Paclitaxel and quercetin nanoparticles co-loaded in microspheres to prolong retention time for pulmonary drug delivery. International Journal of Nanomedicine. 2017;12:8239–8255. doi: 10.2147/IJN.S147028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-H., Chang Y.-H., Chiang M.-T. Chitosan reduces gluconeogenesis and increases glucose uptake in skeletal muscle in streptozotocin-induced diabetic rats. Journal of Agricultural and Food Chemistry. 2010;58(9):5795–5800. doi: 10.1021/jf100662r. [DOI] [PubMed] [Google Scholar]
- Löndahl J., Möller W., Pagels J.H., Kreyling W.G., Swietlicki E., Schmid O. Measurement techniques for respiratory tract deposition of airborne nanoparticles: A critical review. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2014;27(4):229–254. doi: 10.1089/jamp.2013.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maculotti K., Genta I., Perugini P., Imam M., Bernkop-Schnürch A., Pavanetto F. Preparation and in vitro evaluation of thiolated chitosan microparticles. Journal of Microencapsulation. 2005;22(5):459–470. doi: 10.1080/02652040500162220. [DOI] [PubMed] [Google Scholar]
- Majumder J., Taratula O., Minko T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Advanced Drug Delivery Reviews. 2019;144:57–77. doi: 10.1016/j.addr.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansouri S., Cuie Y., Winnik F., Shi Q., Lavigne P., Benderdour M., Fernandes J.C. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27(9):2060–2065. doi: 10.1016/j.biomaterials.2005.09.020. [DOI] [PubMed] [Google Scholar]
- Mao S., Sun W., Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Advanced Drug Delivery Reviews. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Mehta P. Dry Powder Inhalers: A focus on advancements in novel drug delivery systems. Journal of Drug Delivery. 2016;2016:1–17. doi: 10.1155/2016/8290963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., Bothiraja C., Kadam S., Pawar A. Potential of dry powder inhalers for tuberculosis therapy: Facts, fidelity and future. Artificial Cells, Nanomedicine, and Biotechnology. 2018;46(suppl3):S791–S806. doi: 10.1080/21691401.2018.1513938. [DOI] [PubMed] [Google Scholar]
- Menconi A., Pumford N.R., Morgan M.J., Bielke L.R., Kallapura G., Latorre J.D., Tellez G. Effect of chitosan on Salmonella typhimurium in broiler chickens. Foodborne Pathogens and Disease. 2014;11(2):165–169. doi: 10.1089/fpd.2013.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon M., Singh D., Lohade A., Parmar J., Hegde D., Soni P., Samad A. Development of chitosan-based dry powder inhalation system of cisplatin for lung cancer. Indian Journal of Pharmaceutical Sciences. 2012;74(6):521–526. doi: 10.4103/0250-474X.110584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant Z., Buckton G., Taylor K.M.G., Stapleton P., Saleem I.Y., Zariwala M.G., Somavarapu S. A new era of pulmonary delivery of nano-antimicrobial therapeutics to treat chronic pulmonary infections. Current Pharmaceutical Design. 2016;22(17):2577–2598. doi: 10.2174/1381612822666160317142139. [DOI] [PubMed] [Google Scholar]
- Milewska A., Kaminski K., Ciejka J., Kosowicz K., Zeglen S., Wojarski J., Pyrc K. HTCC: Broad range inhibitor of coronavrius entry. PloS One. 2016;11(6) doi: 10.1371/journal.pone.0156552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed M., Syeda J., Wasan K., Wasan E. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics. 2017;9(4):53–78. doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Chachuli S.H., Nawaz A., Shah K., Naharudin I., Wong T.W. In vitro investigation of influences of chitosan nanoparticles on fluorescein permeation into alveolar macrophages. Pharmaceutical Research. 2016;33(6):1497–1508. doi: 10.1007/s11095-016-1893-5. [DOI] [PubMed] [Google Scholar]
- Molina C., Kaialy W., Chen Q., Commandeur D., Nokhodchi A. Agglomerated novel spray-dried lactose-leucine tailored as a carrier to enhance the aerosolization performance of salbutamol sulfate from DPI formulations. Drug Delivery and Translational Research. 2018;8(6):1769–1780. doi: 10.1007/s13346-017-0462-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto M., Saimoto H., Usui H., Okamoto Y., Minami S., Shigemasa Y. Biological activities of carbohydrate-branched chitosan derivatives. Biomacromolecules. 2001;2(4):1133–1136. doi: 10.1021/bm010063p. [DOI] [PubMed] [Google Scholar]
- Mourya V.K.K., Inamdar N.N. Chitosan-modifications and applications: Opportunities galore. Reactive & Functional Polymers. 2008;68(6):1013–1051. [Google Scholar]
- Muralidharan P., Malapit M., Mallory E., Hayes D., Jr., Mansour H.M. Inhalable nanoparticulate powders for respiratory delivery. Nanomedicine Nanotechnology Biology and Medicine. 2015;11(5):1189–1199. doi: 10.1016/j.nano.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Musalli A.H., Talukdar P.D., Roy P., Kumar P., Wong T.W. Folate-induced nanostructural changes of oligochitosan nanoparticles and their fate of cellular internalization by melanoma. Carbohydrate Polymers. 2020 doi: 10.1016/j.carbpol.2020.116488. [DOI] [PubMed] [Google Scholar]
- Muttil P., Wang C., Hickey A.J. Inhaled drug delivery for tuberculosis therapy. Pharmaceutical Research. 2009;26(11):2401–2416. doi: 10.1007/s11095-009-9957-4. [DOI] [PubMed] [Google Scholar]
- Muzzarelli R.A.A., Muzzarelli C., Cosani A., Terbojevich M. 6-Oxychitins, novel hyaluronan-like regiospecifically carboxylated chitins. Carbohydrate Polymers. 1999;39(4):361–367. [Google Scholar]
- Ng M.Y., Flight W., Smith E. Pulmonary complications of cystic fibrosis. Clinical Radiology. 2014;69(3):e153–162. doi: 10.1016/j.crad.2013.10.023. [DOI] [PubMed] [Google Scholar]
- Ngo D.-H., Vo T.-S., Ngo D.-N., Kang K.-H., Je J.-Y., Pham H.N.-D., Kim S.-K. Biological effects of chitosan and its derivatives. Food Hydrocolloids. 2015;51:200–216. [Google Scholar]
- Nishimura S.-I., Kai H., Shinada K., Yoshida T., Tokura S., Kurita K., Uryu T. Regioselective syntheses of sulfated polysaccharides: Specific anti-HIV-1 activity of novel chitin sulfates. Carbohydrate Research. 1998;306(3):427–433. doi: 10.1016/s0008-6215(97)10081-7. [DOI] [PubMed] [Google Scholar]
- Noraizaan A.N., Wong T.W. Physicochemical effects of lactose microcarrier on inhalation performance of rifampicin in polymeric nanoparticles. Powder Technology. 2017;310:272–281. [Google Scholar]
- Oberemko A., Salaberria A.M., Saule R., Saulis G., Kaya M., Labidi J., Baublys V. Physicochemical and in vitro cytotoxic properties of chitosan from mushroom species (Boletus bovinus and Laccaria laccata) Carbohydrate Polymers. 2019;221:1–9. doi: 10.1016/j.carbpol.2019.05.073. [DOI] [PubMed] [Google Scholar]
- Odziomek M., Sosnowski T.R., Gradoń L. Conception, preparation and properties of functional carrier particles for pulmonary drug delivery. International Journal of Pharmaceutics. 2012;433(1–2):51–59. doi: 10.1016/j.ijpharm.2012.04.067. [DOI] [PubMed] [Google Scholar]
- Oliveira P.M., Matos B.N., Pereira P.A.T., Gratieri T., Faccioli L.H., Cunha-Filho M.S.S., Gelfuso G.M. Microparticles prepared with 50-190 kDa chitosan as promising non-toxic carriers for pulmonary delivery of isoniazid. Carbohydrate Polymers. 2017;174:421–431. doi: 10.1016/j.carbpol.2017.06.090. [DOI] [PubMed] [Google Scholar]
- Ortiz M., Jornada D.S., Pohlmann A.R., Guterres S.S. Development of novel chitosan microcapsules for pulmonary delivery of dapsone: Characterization, aerosol performance, and in vivo toxicity evaluation. AAPS PharmSciTech. 2015;16(5):1033–1040. doi: 10.1208/s12249-015-0283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey P., Dua K., Dureja H. Erlotinib loaded chitosan nanoparticles: Formulation, physicochemical characterization and cytotoxic potential. International Journal of Biological Macromolecules. 2019;139:1304–1316. doi: 10.1016/j.ijbiomac.2019.08.084. [DOI] [PubMed] [Google Scholar]
- Paranjpe M., Müller-Goymann C.C. Nanoparticle-mediated pulmonary drug delivery: A review. International Journal of Molecular Sciences. 2014;15(4):5852–5873. doi: 10.3390/ijms15045852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J.H., Saravanakumar G., Kim K., Kwon I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Advanced Drug Delivery Reviews. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Patel B., Gupta N., Ahsan F. Particle engineering to enhance or lessen uptake by alveolar macrophages and to influence therapeutic outcomes. European Journal of Pharmaceutics and Biopharmaceutics. 2015;89:163–174. doi: 10.1016/j.ejpb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- Paumier A., Le Péchoux C. Radiotherapy in small-cell lung cancer: Where should it go? Lung Cancer. 2010;69(2):133–140. doi: 10.1016/j.lungcan.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Petkar K.C., Chavhan S., Kunda N., Saleem I., Somavarapu S., Taylor K.M.G., Sawant K.K. Development of novel octanoyl chitosan nanoparticles for improved rifampicin pulmonary delivery: Optimization by factorial design. AAPS PharmSciTech. 2018;19(4):1758–1772. doi: 10.1208/s12249-018-0972-9. [DOI] [PubMed] [Google Scholar]
- Pham D.-D., Fattal E., Tsapis N. Pulmonary drug delivery systems for tuberculosis treatment. International Journal of Pharmaceutics. 2015;478(2):517–529. doi: 10.1016/j.ijpharm.2014.12.009. [DOI] [PubMed] [Google Scholar]
- Pilcer G., Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. International Journal of Pharmaceutics. 2010;392(1-2):1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- Pinto J.T., Zellnitz S., Guidi T., Roblegg E., Paudel A. Assessment of dry powder inhaler carrier targeted design: A comparative case study of diverse anomeric compositions and physical properties of lactose. Molecular Pharmaceutics. 2018;15(7):2827–2839. doi: 10.1021/acs.molpharmaceut.8b00333. [DOI] [PubMed] [Google Scholar]
- Pires N.R., Cunha P.L.R., Maciel J.S., Angelim A.L., Melo V.M.M., de Paula R.C.M., Feitosa J.P.A. Sulfated chitosan as tear substitute with no antimicrobial activity. Carbohydrate Polymers. 2013;91(1):92–99. doi: 10.1016/j.carbpol.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Pourshahab P.S., Gilani K., Moazeni E., Eslahi H., Fazeli M.R., Jamalifar H. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. Journal of Microencapsulation. 2011;28(7):605–613. doi: 10.3109/02652048.2011.599437. [DOI] [PubMed] [Google Scholar]
- Qiu Y., Lam J.K.W., Leung S.W.S., Liang W. Delivery of RNAi therapeutics to the airways—From bench to bedside. Molecules. 2016;21(9):1249–1280. doi: 10.3390/molecules21091249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones J.P., Peniche H., Peniche C. Chitosan based self-assembled nanoparticles in drug delivery. Polymers. 2018;10(3):235–266. doi: 10.3390/polym10030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabea E.I., Badawy M.E.T., Stevens C.V., Smagghe G., Steurbaut W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules. 2003;4(6):1457–1465. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- Rafael O.-H.D., Fernando Z.-G.L., Abraham P.-T., Alberto V.-L.P., Guadalupe G.-S., Pablo P.J. Production of chitosan-oligosaccharides by the chitin-hydrolytic system of Trichoderma harzianum and their antimicrobial and anticancer effects. Carbohydrate Research. 2019;486 doi: 10.1016/j.carres.2019.107836. [DOI] [PubMed] [Google Scholar]
- Rajan M., Raj V. Formation and characterization of chitosan-polylacticacid-polyethylene glycol-gelatin nanoparticles: A novel biosystem for controlled drug delivery. Carbohydrate Polymers. 2013;98(1):951–958. doi: 10.1016/j.carbpol.2013.05.025. [DOI] [PubMed] [Google Scholar]
- Ran S., Downes A., Thorpe P.E. Increased exposure of anionic phospholipids on the surface of tumor blood vessels. Cancer Research. 2002;62(21):6132–6140. [PubMed] [Google Scholar]
- Ravi Kumar M.N.V. A review of chitin and chitosan applications. Reactive & Functional Polymers. 2000;46(1):1–27. [Google Scholar]
- Rawal T., Parmar R., Tyagi R.K., Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids and Surfaces B, Biointerfaces. 2017;154:321–330. doi: 10.1016/j.colsurfb.2017.03.044. [DOI] [PubMed] [Google Scholar]
- Renner N., Steckel H., Urbanetz N., Scherließ R. Nano- and microstructured model carrier surfaces to alter dry powder inhaler performance. International Journal of Pharmaceutics. 2017;518(1–2):20–28. doi: 10.1016/j.ijpharm.2016.12.052. [DOI] [PubMed] [Google Scholar]
- Rodrigues S., Dionísio M., López C.R., Grenha A. Biocompatibility of chitosan carriers with application in drug delivery. Journal of Functional Biomaterials. 2012;3(3):615–641. doi: 10.3390/jfb3030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roldo M., Hornof M., Caliceti P., Bernkop-Schnürch A. Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: Synthesis and in vitro evaluation. European Journal of Pharmaceutics and Biopharmaceutics. 2004;57(1):115–121. doi: 10.1016/s0939-6411(03)00157-7. [DOI] [PubMed] [Google Scholar]
- Rosenthal R., Günzel D., Finger C., Krug S.M., Richter J.F., Schulzke J.-D., Amasheh S. The effect of chitosan on transcellular and paracellular mechanisms in the intestinal epithelial barrier. Biomaterials. 2012;33(9):2791–2800. doi: 10.1016/j.biomaterials.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Rosière R., Van Woensel M., Gelbcke M., Mathieu V., Hecq J., Mathivet T., Wauthoz N. New folate-grafted chitosan derivative to improve delivery of paclitaxel-loaded solid lipid nanoparticles for lung tumor therapy by inhalation. Molecular Pharmaceutics. 2018;15(3):899–910. doi: 10.1021/acs.molpharmaceut.7b00846. [DOI] [PubMed] [Google Scholar]
- Roy I., Vij N. Nanodelivery in airway diseases: Challenges and therapeutic applications. Nanomedicine Nanotechnology Biology and Medicine. 2010;6(2):237–244. doi: 10.1016/j.nano.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruge C.C., Kirch J., Lehr C.M. Pulmonary drug delivery: From generating aerosols to overcoming biological barriers-therapeutic possibilities and technological challenges. The Lancet Respiratory Medicine. 2013;1(5):402–413. doi: 10.1016/S2213-2600(13)70072-9. [DOI] [PubMed] [Google Scholar]
- Ruigrok M.J.R., Frijlink H.W., Hinrichs W.L.J. Pulmonary administration of small interfering RNA: The route to go? Journal of Controlled Release. 2016;235:14–23. doi: 10.1016/j.jconrel.2016.05.054. [DOI] [PubMed] [Google Scholar]
- Rúnarsson Ö.V., Holappa J., Nevalainen T., Hjálmarsdóttir M., Järvinen T., Loftsson T., Másson M. Antibacterial activity of methylated chitosan and chitooligomer derivatives: Synthesis and structure activity relationships. European Polymer Journal. 2007;43(6):2660–2671. [Google Scholar]
- Saber A., Strand S.P., Ulfendahl M. Use of the biodegradable polymer chitosan as a vehicle for applying drugs to the inner ear. European Journal of Pharmaceutical Sciences. 2010;39(1–3):110–115. doi: 10.1016/j.ejps.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Sanders N., Rudolph C., Braeckmans K., De Smedt S.C., Demeester J. Extracellular barriers in respiratory gene therapy. Advanced Drug Delivery Reviews. 2009;61(2):115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sashiwa H., Aiba S.I. Chemically modified chitin and chitosan as biomaterials. Progress in Polymer Science. 2004;29(9):887–908. [Google Scholar]
- Sebti I., Martial-Gros A., Carnet-Pantiez A., Grelier S., Coma V. Chitosan polymer as bioactive coating and film against Aspergillus niger contamination. Journal of Food Science. 2005;70(2):100–104. [Google Scholar]
- Senapati S., Mahanta A.K., Kumar S., Maiti P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduction and Targeted Therapy. 2018;3(1):7–25. doi: 10.1038/s41392-017-0004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K., Chan L.W., Wong T.W. Critical physicochemical and biological attributes of nanoemulsions for pulmonary delivery of rifampicin by nebulization technique in tuberculosis treatment. Drug Delivery. 2017;24(1):1631–1647. doi: 10.1080/10717544.2017.1384298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah N.D., Shah V.V., Chivate N.D. Pulmonary drug delivery: A promising approach. Journal of Applied Pharmaceutical Science. 2012;2(6):33–37. [Google Scholar]
- Sheshala R., Anuar N.K., Abu Samah N.H., Wong T.W. In vitro drug dissolution/permeation testing of nanocarriers for skin application: A comprehensive review. AAPS PharmSciTech. 2019;20(5):164–191. doi: 10.1208/s12249-019-1362-7. [DOI] [PubMed] [Google Scholar]
- Sidaway P. COVID-19 and cancer: What we know so far. Nature Review Clinical Oncology. 2020 doi: 10.1038/s41571-020-0366-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M., Silva A., Fernandez-Lodeiro J., Casimiro T., Lodeiro C., Aguiar-Ricardo A. Supercritical CO2-assisted spray drying of strawberry-like gold-coated magnetite nanocomposites in chitosan powders for inhalation. Materials. 2017;10(1):74–89. doi: 10.3390/ma10010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla A.K., Chawla M. Chitosan: Some pharmaceutical and biological aspects - an update. The Journal of Pharmacy and Pharmacology. 2001;53(8):1047–1067. doi: 10.1211/0022357011776441. [DOI] [PubMed] [Google Scholar]
- Smith J.M., Dornish M., Wood E.J. Involvement of protein kinase C in chitosan glutamate-mediated tight junction disruption. Biomaterials. 2005;26(16):3269–3276. doi: 10.1016/j.biomaterials.2004.06.020. [DOI] [PubMed] [Google Scholar]
- Smith J., Wood E., Dornish M. Effect of chitosan on epithelial cell tight junctions. Pharmaceutical Research. 2004;21(1):43–49. doi: 10.1023/b:pham.0000012150.60180.e3. [DOI] [PubMed] [Google Scholar]
- Smola M., Vandamme T., Sokolowski A. Nanocarriers as pulmonary drug delivery systems to treat and to diagnose respiratory and non respiratory diseases. International Journal of Nanomedicine. 2008;3(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Smyth H.D.C., Hickey A.J. Carriers in drug powder delivery. American Journal of Drug Delivery. 2005;3(2):117–132. [Google Scholar]
- Sosnik A., Carcaboso Á.M., Glisoni R.J., Moretton M.A., Chiappetta D.A. New old challenges in tuberculosis: Potentially effective nanotechnologies in drug delivery. Advanced Drug Delivery Reviews. 2010;62(4–5):547–559. doi: 10.1016/j.addr.2009.11.023. [DOI] [PubMed] [Google Scholar]
- Stockmann C., Sherwin C.M.t., Ampofo K., Spigarelli M.G. Development of levofloxacin inhalation solution to treat Pseudomonas aeruginosa in patients with cystic fibrosis. Therapeutic Advances in Respiratory Disease. 2014;8(1):13–21. doi: 10.1177/1753465813508445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao L., Jiang J., Gao Y., Wu C., Liu Y. Biodegradable alginate-chitosan hollow nanospheres for codelivery of doxorubicin and paclitaxel for the effect of human lung cancer A549 Cells. BioMed Research International. 2018;2018:1–11. doi: 10.1155/2018/4607945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayel A.A., Moussa S., Opwis K., Knittel D., Schollmeyer E., Nickisch-Hartfiel A. Inhibition of microbial pathogens by fungal chitosan. International Journal of Biological Macromolecules. 2010;47(1):10–14. doi: 10.1016/j.ijbiomac.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Uto T., Idenoue S., Yamamoto K., Kadokawa J. Understanding dissolution process of chitin crystal in ionic liquids: Theoretical study. Journal of the Chemical Society Faraday Transactions. 2018;20(31):20669–20677. doi: 10.1039/c8cp02749h. [DOI] [PubMed] [Google Scholar]
- Verlee A., Mincke S., Stevens C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydrate Polymers. 2017;164:268–283. doi: 10.1016/j.carbpol.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Vikhoreva G., Bannikova G., Stolbushkina P., Panov A., Drozd N., Makarov V., Gal’braikh L. Preparation and anticoagulant activity of a low-molecular-weight sulfated chitosan. Carbohydrate Polymers. 2005;62(4):327–332. [Google Scholar]
- Wang F., Wang Y., Ma Q., Cao Y., Yu B. Development and characterization of folic acid-conjugated chitosan nanoparticles for targeted and controlled delivery of gemcitabinein lung cancer therapeutics. Artificial Cells, Nanomedicine, and Biotechnology. 2017;45(8):1530–1538. doi: 10.1080/21691401.2016.1260578. [DOI] [PubMed] [Google Scholar]
- Wang H., Xu Y., Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: In vitro and in vivo evaluation. International Journal of Molecular Sciences. 2014;15(3):3519–3532. doi: 10.3390/ijms15033519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Wang Y., Ye D., Liu Q. A review of the 2019 Novel Coronavirus (COVID-19) based on current evidence. International Journal of Antimicrobial Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R., Huang J., Wei M., Zeng X. The synergy of 6-O-sulfation and N-or 3-O-sulfation of chitosan is required for efficient inhibition of P-selectin-mediated human melanoma A375 cell adhesion. Bioscience, Biotechnology, and Biochemistry. 2010;74(8):1697–1700. doi: 10.1271/bbb.100140. [DOI] [PubMed] [Google Scholar]
- Wong T.W., John P. In: Handbook of nanoparticles. Aliofkhazraei M., editor. Springer International Publishing; 2016. Advances in spray drying technology for nanoparticle formation; pp. 329–346. [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Liao W., Wang W., Zhou J., Tan W., Xiang W., Cai X. Genipin-crosslinked carboxymethyl chitosan nanogel for lung-targeted delivery of isoniazid and rifampin. Carbohydrate Polymers. 2018;197:403–413. doi: 10.1016/j.carbpol.2018.06.034. [DOI] [PubMed] [Google Scholar]
- Xiangyang X., Ling L., Jianping Z., Shiyue L., Jie Y., Xiaojin Y., Jinsheng R. Preparation and characterization of N-succinyl-N′-octyl chitosan micelles as doxorubicin carriers for effective anti-tumor activity. Colloids and Surfaces B, Biointerfaces. 2007;55(2):222–228. doi: 10.1016/j.colsurfb.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Yadav A.B., Sharma R., Muttil P., Singh A.K., Verma R.K., Mohan M., Misra A. Inhalable microparticles containing isoniazid and rifabutin target macrophages and “stimulate the phagocyte” to achieve high efficacy. Indian Journal of Experimental Biology. 2009;47(6):469–474. [PubMed] [Google Scholar]
- Yang M.Y., Chan J.G.Y., Chan H.-K. Pulmonary drug delivery by powder aerosols. Journal of Controlled Release. 2014;193:228–240. doi: 10.1016/j.jconrel.2014.04.055. [DOI] [PubMed] [Google Scholar]
- Yang W., Peters J.I., Williams R.O. Inhaled nanoparticles-A current review. International Journal of Pharmaceutics. 2008;356:239–247. doi: 10.1016/j.ijpharm.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Yeh T.H., Hsu L.W., Tseng M.T., Lee P.L., Sonjae K., Ho Y.C., Sung H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- Yildiz-Peköz A., Akbal O., Tekarslan S.H., Sagirli A.O., Mulazimoglu L., Morina D., Cevher E. Preparation and characterization of doripenem-loaded microparticles for pulmonary delivery. Journal of Aerosol Medicine and Pulmonary Drug Delivery. 2018;31(6):347–357. doi: 10.1089/jamp.2017.1378. [DOI] [PubMed] [Google Scholar]
- Ying G.Q., Xiong W.Y., Wang H., Sun Y., Liu H.Z. Preparation, water solubility and antioxidant activity of branched-chain chitosan derivatives. Carbohydrate Polymers. 2011;83(4):1787–1796. [Google Scholar]
- Youngren-Ortiz S.R., Gandhi N.S., España-Serrano L., Chougule M.B. Aerosol delivery of siRNA to the lungs. Part 1: Rationale for gene delivery systems. KONA Powder and Particle Journal. 2016;2016(33):63–85. doi: 10.14356/kona.2016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zariwala M.G., Bendre H., Markiv A., Farnaud S., Renshaw D., Taylor K.M.G., Somavarapu S. Hydrophobically modified chitosan nanoliposomes for intestinal drug delivery. International Journal of Nanomedicine. 2018;13:5837–5848. doi: 10.2147/IJN.S166901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X.M., Martin G.P., Marriott C., Pritchard J. The influence of carrier morphology on drug delivery by dry powder inhalers. International Journal of Pharmaceutics. 2000;200(1):93–106. doi: 10.1016/s0378-5173(00)00347-1. [DOI] [PubMed] [Google Scholar]
- Zeng X., Martin G.P., Marriott C., Pritchard J. Lactose as a carrier in dry powder formulations: The influence of surface characteristics on drug delivery. Journal of Pharmaceutical Sciences. 2001;90(9):1424–1434. doi: 10.1002/jps.1094. [DOI] [PubMed] [Google Scholar]
- Zhang J., Xia W., Liu P., Cheng Q., Tahirou T., Gu W., Li B. Chitosan modification and pharmaceutical/biomedical applications. Marine Drugs. 2010;8(7):1962–1987. doi: 10.3390/md8071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K., Peschel D., Helm J., Groth T., Fischer S. FT Raman investigation of novel chitosan sulfates exhibiting osteogenic capacity. Carbohydrate Polymers. 2011;83(1):60–65. [Google Scholar]
- Zhang Y., Chan J.W., Moretti A., Uhrich K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. Journal of Controlled Release. 2015;219:355–368. doi: 10.1016/j.jconrel.2015.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.T., Morton D.A.V. Drug–Lactose binding aspects in adhesive mixtures: Controlling performance in dry powder inhaler formulations by altering lactose carrier surfaces. Advanced Drug Delivery Reviews. 2012;64(3):275–284. doi: 10.1016/j.addr.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Zhu A.-P.P., Fang N. Adhesion dynamics, morphology, and organization of 3T3 fibroblast on chitosan and its derivative: The effect of O-carboxymethylation. Biomacromolecules. 2005;6(5):2607–2614. doi: 10.1021/bm050328q. [DOI] [PubMed] [Google Scholar]
- Zhu H., Luo W., Ciesielski P.N., Fang Z., Zhu J.Y., Henriksson G., Hu L. Wood-derived materials for green electronics, biological devices, and energy applications. Chemical Reviews. 2016;116(16):9305–9374. doi: 10.1021/acs.chemrev.6b00225. [DOI] [PubMed] [Google Scholar]




