Abstract
Several viruses transmitted through saliva, such as herpes simplex virus, cytomegalovirus, and Zika virus, are capable of infecting and replicating in the oral mucosa, leading to painful oral ulcers. Few studies have described the oral manifestations of coronavirus disease 2019 (COVID-19). There is growing evidence that angiotensin-converting enzyme 2 (ACE2), the main host cell receptor of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is highly expressed on the epithelial cells of the tongue and of the salivary glands, which may explain the development of dysgeusia in patients with COVID-19. Hence, it is important to understand if SARS-CoV-2 can infect and replicate in oral keratinocytes and fibroblasts, causing oral ulcerations and superficial necrosis. Here, we report a series of 8 cases of COVID-19 infection, with oral necrotic ulcers and aphthous-like ulcerations which developed early in the course of disease after the development of dysgeusia and affected the tongue, lips, palate, and oropharynx. A short review of the literature regarding the important role of ACE2 in SARS-CoV-2 cellular entry is also provided, bringing new insights into oral keratinocytes and minor salivary glands as potential targets.
Coronavirus disease 2019 (COVID-19) has had a massive impact worldwide as a result of the mode of infection spread, the resulting severe acute respiratory syndrome, and the global death toll. Since the identification of this new airborne infectious microorganism (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]) in Wuhan, China, millions of cases have been diagnosed worldwide, with mortality rates ranging from 3% to 12%.1
In addition to fever, fatigue, dry cough, myalgias, sore throat, breathing difficulties, and respiratory complications that often deteriorate to severe acute respiratory syndrome, patients infected by SARS-CoV-2 may develop a myriad of other local and systemic complications, such as acute cardiac damage, acute renal failure, gastrointestinal complications, dysgeusia, anosmia, and neurologic symptoms, including Guillain-Barré syndrome.2, 3, 4
SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor, which is detected in the cell membrane of numerous human organs and tissues, including the lungs, kidneys, liver, epithelial cells of the tongue and salivary glands, upper respiratory tract, nervous system, and skeletal muscle, among others.5, 6, 7, 8
Although SARS-CoV-2 can be detected in saliva and oropharyngeal secretions,9, 10, 11, 12 the routes of infection remain elusive, and little is known about the routes of transmission through the oral mucosa. Thus, more clinical evidence and research are needed to confirm the ability of SARS-CoV-2 to infect the oral tissues and its pathogenic mechanisms in the oral and oropharyngeal mucosae. This report on a case series presents 2 patterns of oral ulcerations—aphthous-like and superficial necrosis—affecting multiple oral sites in patients diagnosed with COVID-19. These lesions develop in oral sites known to express ACE2 receptors, as recently described in the tongue epithelium and the salivary glands tissue,6 , 7 after the manifestation of dysgeusia.
Case 1
An 81-year-old man with cough and progressive chest tightness present for 10 days was admitted to the Emergency Department of the Hospital Sírio-Libanês, São Paulo, Brazil, on March 26, 2020. The patient had a history of well-controlled hypertension and chronic obstructive pulmonary disease. Fifteen days earlier, on March 11, 2020, he had come in contact with a family member who had traveled to Israel and had been recently diagnosed with SARS-CoV-2 pneumonia. In our patient, dysgeusia appeared on March 16, 2020. Five days later, the patient developed chills and fever, and a maximum body temperature of 37.7°C. On March 25, 2020, the patient developed a dry cough and mild dyspnea. Physical examination revealed normal body temperature (36°C); blood pressure 108/67 mm Hg; heart rate 83 beats per minute; respiratory rate 25 breaths per minute; and oxygen saturation of 97%. He was conscious and had no dyspnea at the time of hospitalization. Biochemical examination showed that leukocytes and the neutrophils/lymphocytes ratio were all in the normal range (420/mm3); the C-reactive protein level was 23.4 mg/L; and the glucose level was 97 mg/L. The diagnosis of COVID-19 infection was based on real-time reverse transcriptase polymerase chain reaction (rRT-PCR) amplification of the viral DNA from a pharyngeal sample. Computed tomography (CT) revealed multiple “ground glass” images in both lungs.
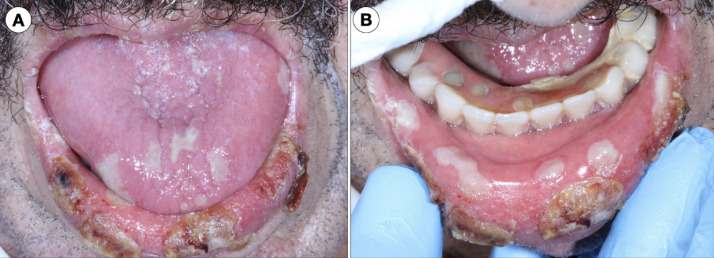
The patient was placed in a special isolation ward and was treated with azithromycin and ceftriaxone for 7 days. Head and neck examination did not identify asymmetries, swellings, or enlarged cervical lymph nodes. Oral examination revealed multiple shallow aphthous-like ulcers of varying sizes and irregular margins covered with mucopurulent membrane, suggesting superficial necrosis in the upper and lower lip mucosa as well as the anterior dorsal tongue (Figures 1 A and 1B). The lesions were painful on palpation and believed to have developed in the last few days at the time of hospital admission. Herpes simplex virus (HSV-1) was detected in the saliva sample by performing PCR, and the patient was immediately started on intravenous acyclovir 250 mg/m2 3 times a day for 10 days. There was no clinical improvement. As an adjuvant measure to manage the pain associated with the oral ulcers, a trained dentist administered daily photobiomodulation therapy (PBMT) for 10 consecutive days. The PBMT device (Twin Flex, MMOptics, São Carlos, Brazil) was positioned perpendicular to the surface of the oral ulcers, for 10 seconds per site, operating at 660 nm wavelength, 40 mW average power, 0.04 cm2 beam area, 1 W/cm2 irradiance, 0.4 J energy, and 10 J/cm2 fluence. This is our protocol of PBMT used for patients with oral mucositis associated with cancer therapy. The patient reported symptom relief after 2 days of PBMT, and the oral lesions completely resolved after 11 days of PBMT. After 16 days in the intensive care unit (ICU) and 14 additional days in a critical care unit, the patient's clinical course and respiratory status showed improvement. He was discharged and is currently recovering well at home.
Fig. 1.
A, Clustered ulcers 1 to 1.5 cm in diameter covered with crusts occurring on the lower lip (vermilion). Ulcerative painful lesions with superficial necrosis affecting the anterior dorsal tongue. B, Lower lip mucosal ulcers covered with a mucopurulent membrane and the so-called aphthous-like pattern.
Case 2
A 71-year-old female was admitted to the Emergency Department of Hospital Sírio-Libanês, São Paulo, Brazil, on April 8, 2020, with symptoms of severe acute respiratory syndrome. Four days before hospitalization, cough, dysgeusia, fever, and mild dyspnea had developed in the patient. Past medical history was significant for hypertension, diabetes, obesity, and renal failure. In addition, the patient reported a history of bariatric surgery and fibromyalgia. On physical examination, the body temperature was 38.5°C, blood pressure 130/66 mm Hg, heart rate 92 beats per minute, respiratory rate 20 breaths per minute, and oxygen saturation of 90% on room air. Laboratory examinations confirmed serum glucose level 156 mg/dL, creatinine 0.69 mg/dL, white blood cell count 6930 cells per microliter (neutrophils = 83.2%; lymphocytes = 15%), C-reactive protein 16.5 mg/dL, and hemoglobin 10.7 g/dL. Lung CT demonstrated diffuse “ground glass” hyperdense images in both lungs. rRT-PCR for SARS-CoV-2 was positive, and the patient was treated with azithromycin for 20 days and ceftriaxone for 3 days.
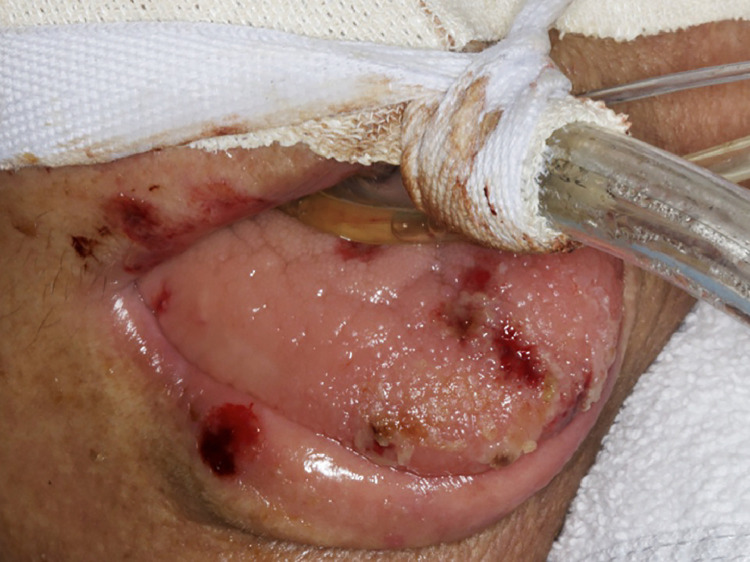
Examination of the head and neck region was negative for lymphadenopathy. Intraoral examination identified small hemorrhagic ulcerations affecting the upper and lower lips, as well as focal areas of shallow necrosis on the anterior dorsal tongue (Figure 2 ). These lesions developed at the time of hospital admission. Herpes simplex virus (HSV-1) was detected by performing PCR of the saliva sample. The patient was immediately started on intravenous acyclovir 250 mg/m2 3 times a day for 7 days but showed no clinical improvement. Because of the severe pain associated with the ulcers, the patient received daily treatments of PBMT, following the same protocol described above. The intraoral lesions presented signs of clinical regression after 10 days of light therapy; however, the lip ulcerations did not respond even after 15 days of PBMT. Because of progression of the respiratory symptoms, on April 10, 2020, the patient was placed on mechanical ventilator support, with the aim to prevent acute respiratory failure; however, he remains in critical condition in the ICU.
Fig. 2.
Small hemorrhagic ulcerations affecting the upper and lower lips (vermilion) and focal ulcerative areas of necrosis affecting the anterior dorsal tongue.
Case 3
An 83-year-old female patient was admitted to the Emergency Department of Hospital Sírio-Libanês, São Paulo, Brazil, on May 20, 2020, due to abdominal distension and mild dyspnea. Her past medical history was significant for obesity, Parkinson disease, hypertension, pancreatitis, and chronic obstructive pulmonary disease. On physical examination, her body temperature was 37.5°C, blood pressure 189/71 mm Hg, heart rate 82 beats per minute, respiratory rate 17 breaths per minute, and oxygen saturation 98% on room air. Her laboratory results showed serum glucose 134 mg/dL, creatinine 0.85 mg/dL, white blood cell count 10,620 cells per microliter (neutrophils = 86%; lymphocytes = 7.3%); C-reactive protein 3.45 mg/dL, and hemoglobin 12 g/dL. Lung CT showed discrete hyperdense areas in both lungs. rRT-PCR for SARS-CoV-2 yielded positive results, and the patient was placed in an isolation ward and treated with piperacillin/tazobactam and ceftriaxone.
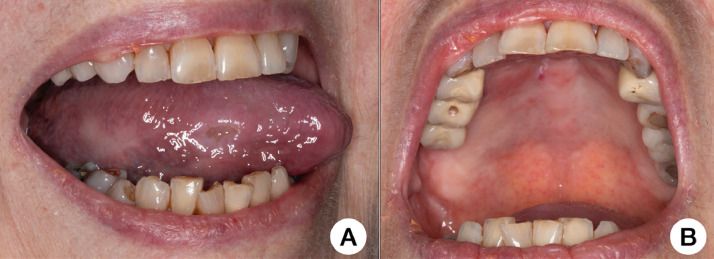
Head and neck examination results were negative. The intraoral examination identified a 1.5 × 1.5 cm ulcer on the right lateral border of the tongue as well as a discrete area in the anterior hard palate affected by a petechia and a shallow necrotic area (Figures 3 A and 3B). Both lesions were painful and developed simultaneously to hospital admission. PCR of saliva was negative for HSV-1. The patient started receiving PBMT according to the standard protocol of the hospital. Complete pain control was achieved after 5 days of light therapy. Because of the mild respiratory symptoms, the patient was placed in a critical care unit and is currently showing improvement after 10 days of hospitalization.
Fig. 3.
A, Painful ulcer on the right lateral border of the tongue. B, Focal erythema/petechia and a shallow necrotic area on the anterior hard palate.
Case 4
A 72-year-old male patient checked into the emergency room (ER) complaining of fever and dyspnea lasting for several days. He was admitted to the Emergency Department of Hospital Sírio-Libanês, São Paulo, Brazil, on April 29, 2020, with a past medical history of diabetes and hypertension. On physical examination, his body temperature was 36.0°C, blood pressure 150/91 mm Hg, heart rate 75 beats per minute, respiratory rate 23 breaths per minute, and oxygen saturation 91% on room air. The laboratory results were as follows: serum glucose 170 mg/dL, creatinine 0.93 mg/dL, white blood cell count 8450 cells per microliter (neutrophils = 83.1%; lymphocytes = 9.9%), C-reactive protein 19.39 mg/dL, and hemoglobin 15.3 g/dL. Lung CT demonstrated diffuse hyperdense areas in both lungs, affecting approximately 50% of both lungs. rRT-PCR of the sample from a nasopharyngeal swab was positive for SARS-CoV-2. The patient was treated with piperacillin/tazobactam, azithromycin, and ceftriaxone (Rocefin).
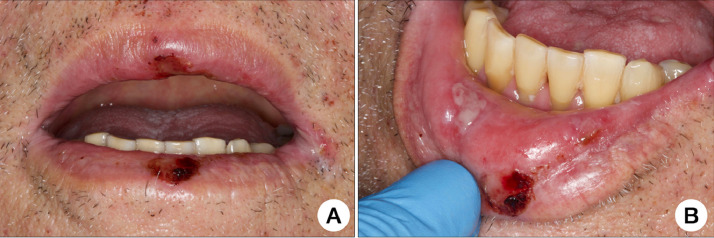
Regional head and neck examination did not reveal relevant changes. Intraoral examination identified small hemorrhagic ulcerations affecting the upper and lower lips, as well as a painful necrotic ulceration on the right lower lip mucosa (Figures 4 A and 4B). These lesions were detected a few days after hospital admission. HSV-1 was detected on PCR of a saliva sample, and the patient was started on intravenous acyclovir 250 mg/m2 3 times a day for 7 days but showed no clinical improvement. The oral necrotic ulcerations were also managed with PBMT. The intraoral lesions presented signs of pain and clinical regression after 7 days of light therapy. The respiratory symptoms, however, did not improve. The patient remains in critical condition in the ICU after 30 days of hospitalization.
Fig. 4.
A, Hemorrhagic ulcerations affecting the upper and lower lip vermilions. B, Painful “aphthous-like” necrotic ulceration affecting the right lower lip mucosa.
Case 5
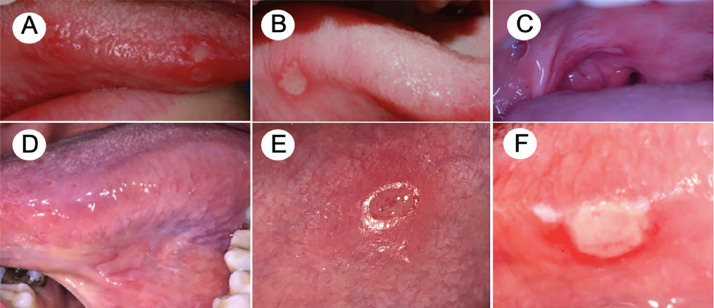
A 32-year-old female patient checked into the ER complaining of fever, cough, and headache lasting for 5 days. She was otherwise healthy, and her medical history was noncontributory. Physical examination failed to show cardiovascular, pulmonary, abdominal, or neurologic alterations. Her body temperature was 38.5°C, and oxyhemoglobin saturation was 98%. Blood and urine test results were normal, with C-reactive protein and white blood cell count within the normal range. The nasopharyngeal swab for amplification of the SARS-CoV-2 RNA was collected, and the results were positive. The patient was diagnosed with mild COVID-19, discharged from the hospital, and sent home for 14 days of quarantine, and dipyrone 1 g, 4 times per day, to control the fever. Eight days after the onset of the symptomatic phase, the patient's condition worsened with anosmia, and on the 10th day, she observed multiple ulcers on the apex and lateral borders of the tongue. On teleconsultation with an oral medicine specialist, she denied any previous history of recurrent oral ulcers, oral inflammatory disease, or allergy. Clinical pictures were provided by the patient, revealing the lesions to be shallow and circular with a whitish center and surrounded by an erythematous halo, ranging from 3 to 4 mm (Figures 5 A and 5B). The patient recovered well from COVID-19 after 14 days. The oral lesions showed clinical regression approximately 8 days later, and no additional lesions were noted.
Fig. 5.
Lesions presenting an aphthous-like pattern. A, B, Patient 3 presenting with multiple shallow ulcers at the apex and anterior lateral border of the tongue. C, Patient 4 with an isolated peritonsillar major aphthous-like ulcer. D, Patient 5. Isolated painful ulcer in the ventral portion of the tongue. E, Patient 6. Ulcerated lesion with slightly elevated and a marked erythematous halo. F, Patient 6. Aphthous-like ulcer covered by a necrotic membrane on the lateral border of the tongue.
Case 6
A 35-year-old male patient went to the ER with complaints of fever, cough, sore throat, and general malaise for the past 3 days. He was otherwise healthy and did not report chronic use of any medication. On physical examination at the ER, no significant changes were observed, and laboratory tests were within the normal range. On the basis of the patient's clinical presentation, a nasopharyngeal swab test for amplification of the SARS-CoV-2 RNA was performed, and the results were positive for mild COVID-19. After discharge from the hospital, the patient was instructed to strictly follow the quarantine protocol at home. Six days after the onset of symptoms, he continued to have persistent fever and experienced primarily hyposmia and ageusia and then an oral ulcer in the tonsillar pillar, causing mild odynophagia. On teleconsultation, the patient reported it was the first onset of oral ulcers, denying past history of recurring aphthous stomatitis or any other ulcerative disease of the mouth. The lesion was shallow and had a circular pattern, covered by a fibrinopurulent membrane and surrounded by an erythematous halo, measuring 0.5 cm (Figure 5C). The patient made a full recovery at the end of the quarantine, with complete resolution of the oral lesion as well.
Case 7
A 29-year-old male patient went to the ER complaining of fever, cough, headache, dyspnea on exertion, and general malaise lasting for 3 days. His medical history was unremarkable, with no report of associated diseases or medication use. No organ involvement was noted on physical examination, and oxyhemoglobin saturation was 96% in ambient air. The patient's body temperature was 38°C, and laboratory test results were within the normal range. The nasopharyngeal swab test for amplification of the SARS-CoV-2 RNA showed positive results. The patient was started on ipratropium bromide and fenoterol hydrochloride and was discharged from the hospital with instructions to follow the quarantine protocol at home. On the day 6 of the symptomatic phase, anosmia and ageusia developed. Two days later, the patient observed a painful ulcer in the ventral portion of the tongue. On teleconsultation, the provider noted a shallow ulcer, measuring 1 cm in diameter, with a whitish pseudomembrane surrounded by an erythematous halo (Figure 5D). The patient denied previous history of recurrent oral ulcers and refused any drug treatment. The lesion lasted 6 days, followed by uneventful recovery.
Case 8
A 28-year-old male patient presented to the emergency department with cough, fever (38.5°C), headache, myalgia, and chills of 4 days’ duration. Physical examination was unremarkable, the oxyhemoglobin saturation was 99% in ambient air, and the laboratory test results were within normal limits. The patient denied any relevant diseases or chronic use of any medication. The nasopharyngeal swab was tested for amplification of the SARS-CoV-2 RNA and showed positive results; the patient was instructed to follow the quarantine protocol at home. After 2 days, he developed anosmia and ageusia, and on day 8, he observed aphthous-like ulcers in the upper and lower labial mucosae. Another ulcer was observed on the right lateral border of the tongue after 2 days (Figures 5E and 5F). The patient was started on 0.12% nonalcoholic chlorhexidine mouthwash and recovered after 4 days. No complications of COVID-19 were noted, and complete healing of the oral lesions was observed 9 days after the onset.
Discussion
This report of a case series presented several cases of SARS-Cov-2 infection, with oral manifestations developing during the infectious period of the disease. Of importance, the oral manifestations appeared concomitant with the loss of taste and smell. The oral lesions were more severe and widespread in older patients with more severe COVID-19 infection. The observed lesions presented 2 well-defined and distinct patterns, one resembling aphthous-like ulcers in young patients with mild cases of COVID-19 and another with more widespread patterns resembling HSV-1 necrotic ulcers in the more severe and immunosuppressed older individuals (Table I ).
Table I.
Clinical features of the oral ulcers in patients with COVID-19
| Patient | Gender | Age | COVID-19 severity | Anosmia | Dysgeusia/Ageusia | Oral lesion | Time to onset (days) | Duration |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 81 | Severe | No | Yes | Aphthous-like + necrosis | 5 | 11 |
| 2 | F | 71 | Severe | No | Yes | Hemorrhagic ulcerations with necrotic areas | 4 | > 15 |
| 3 | F | 83 | Mild | No | No | Aphthous-like + necrosis | 2 | 5 |
| 4 | M | 72 | Mild | No | No | Aphthous-like + necrosis | 5 | 7 |
| 5 | F | 32 | Mild | Yes | Yes | Aphthous-like | 10 | 5 |
| 6 | M | 35 | Moderate | Hyposmia | Yes | Aphthous-like | 6 | 8 |
| 7 | M | 29 | Mild | Yes | Yes | Aphthous-like | 8 | 5 |
| 8 | M | 28 | Mild | Yes | Yes | Aphthous-like | 8 | 6 |
COVID-19, coronavirus disease 2019; M, male; F, female.
The numerous signs and symptoms that have been associated with COVID-19 include dysgeusia and anosmia even in the absence of respiratory symptoms.13 The recently published tropism of SARS-CoV-2 to the tongue and salivary gland epithelium6 , 7 is of importance, suggesting that the oral mucous membrane may be targeted by the virus.14 Although we did not obtain samples of the oral lesions to test for the presence of COVID-19, we observed that the evolution of the oral lesions and the healing process occurred in parallel with the resolution of the COVID-19 infection. This leads us to speculate that the development of oral manifestations in these patients may be directly associated with the COVID-19 infection. Whether the lesions are caused directly by the virus or are an associated manifestation resulting from the severe compromised state of the patient remains to be determined. Nevertheless, considering that the distribution of ACE2 receptors may determine the route of SARS-CoV-2 infection, the presence of ACE2 receptors on the tongue and salivary glands, as recently described, suggests that the epithelial cells of the tongue and of the salivary glands may be involved in COVID-19 infection and the dysfunction caused by it. This could lead to the development of dysgeusia and oral mucosal ulcerations and necrosis.14 Thus, the interaction between SARS-CoV-2 and ACE2 might disrupt the function of oral keratinocytes and the epithelial lining of salivary glands ducts, resulting in painful oral ulcers. Therefore, understanding the pathogenesis of COVID-19 infection is vital for the development of robust infection control in dental offices and hospitals.
On the basis of the findings of this study, we propose that the incidence of the oral manifestations of COVID-19 may have been underestimated in previous investigations2, 3, 4 and that ACE2-expressing epithelial cells of the tongue and of the salivary glands may be susceptible to SARS-CoV-2 infection. After infection of the oral keratinocytes/glandular tissues, there is an increase in the permeability of the cell walls to foreign pathogens and viral replication in the cells lining the oral mucosa, leading to ulcers and necrosis.
The limitations of this study are obvious. The sample did not include cases to show the presence of SARS-CoV-2 in oral lesions or in saliva. In future studies, the characterization of the oral manifestations of COVID-19 in infected individuals should include incisional biopsy, followed by direct viral testing for SARS-CoV-2. Because we did not obtain oral tissue samples from our patients, we could not investigate the immunoexpression of ACE2 either. Therefore, we have to consider whether these lesions are herpetic, immunologic, or erythema multiforme–like. Last, although the use of PBMT for relieving pain secondary to oral ulcerative lesions of inflammatory nature is well accepted, the fast onset and progression of the pandemic has not allowed sufficient time for validating specific light therapy protocols for patients with COVID-19. Therefore, the current approach should be considered empirical, although it was effective in controlling pain in the cases presented here.
The duration of anosmia and ageusia in the patients in the current series could not be characterized in depth because of their critical medical condition and hospitalization in the ICU, which impaired their perception in terms of signs and symptoms. COVID-19–related olfactory and gustatory dysfunctions have been described as a possible surrogate marker of the SARS-CoV-2 infection, sometimes presenting as the only symptom of the disease. A meta-analysis has shown that olfactory dysfunction is observed in 52.73% of patients with COVID-19, and gustatory changes are noted in 43.93% of these individuals; the use of validated instruments to asses these alterations seems to show even higher frequencies.15 Awareness of these symptoms is important to initiate early diagnosis and treatment of the infection, but currently, it is not considered to have any other significance. Considering that the length of symptom burden is often short (approximately 9 days) and that most of the patients will experience complete resolution in 14 days,16 no specific treatment has been used in the context of the pandemic. In fact, anosmia and dysgeusia treatments are often conducted by ENT or oral medicine specialists, who are not consulted because of the short duration of the symptoms and the challenging medical scenario of the pandemic.
Conclusions
To our knowledge, these are the first reported cases of oral manifestations in COVID-19. We suggest that a new etiopathogenic mechanism between ACE2 and SARS-CoV-2 may exist in the oral cavity. Additional clinical and epidemiologic data are required to validate this hypothesis. Awareness of such oral manifestation is important because the lesions may precede the typical respiratory symptoms by several days, and worsening of the oral lesions may precede a more serious clinical scenario. Additional studies need to investigate whether SARS-CoV-2 infection directly causes oral ulcerations or whether oral lesions are a coincidental event with COVID-19 progression.
The findings of this case series have highlighted2, 3, 4 the possible development of dysgeusia and anosmia early in the course of SARS-CoV-2 infection; therefore, these manifestations should be considered a disease marker by dentists working in the frontlines of the pandemic. Patients complaining of lack of taste and smell may need to be evaluated preferably through telemedicine17 or in person. The presence of dysgeusia or anosmia should be considered a potential indication of COVID-19. Those with these symptoms should be referred for further investigation, be isolated, and receive appropriate medical care. Dentists working in the hospital setting or in ICUs should perform careful oral and oropharyngeal examinations and document suspicious oral lesions in patients with COVID-19, especially in those who complain of loss of taste and smell.
References
- 1.https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed May 3, 2020.
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395:497‐506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sungnak W., Huang N., Bécavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681‐687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu H., Zhong L., Deng J. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu J., Li Y., Gan F., Du Y., Yao Y. Salivary glands: potential reservoirs for COVID-19 asymptomatic infection. J Dent Res. 2020;99:989. doi: 10.1177/0022034520918518. [DOI] [PubMed] [Google Scholar]
- 8.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14:185‐192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu R., Cui B., Duan X., Zhang P., Zhou X., Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.To KK., Tsang OT., Chik-Yan Yip C. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71:841–843. doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson RN., Cunniffe NJ. The probability of detection of SARS-CoV-2 in saliva. Stat Methods Med Res. 2020;29:1049–1050. doi: 10.1177/0962280220915049. [DOI] [PubMed] [Google Scholar]
- 12.Wyllie AL, Fournier J, Casanovas-Massana A., et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. MedRxiv. Available at: https://doi.org/10.1101/2020.04.16.20067835.
- 13.Lao WP., Imam SA., Nguyen SA. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol Head Neck Surg. 2020 Apr 17 doi: 10.1016/j.wjorl.2020.04.001. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariz BALA., Brandão TB., Ribeiro ACP., Lopes MA., Santos-Silva AR. New insights for the pathogenesis of COVID-19-related dysgeusia. J Dent Res. 2020 Jun 12 doi: 10.1177/0022034520936638. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 15.Tong JY., Wong A., Zhu D., Fastenberg JH., Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2020;163:3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- 16.Klopfenstein T., Kadiane-Oussou NJ., Toko L. Features of anosmia in COVID-19. Med Mal Infect. 2020;50:436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes MA., Santos-Silva AR., Vargas PA., Kowalski LP. Virtual assistance in oral medicine for prioritizing oral cancer diagnosis during the COVID-19 pandemic. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130:127–128. doi: 10.1016/j.oooo.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]