Abstract
Methicillin-resistant Staphylococcus aureus USA300, belonging to sequence type (ST) 8, is a rare cause of necrotizing fasciitis in the USA. We herein report a case of monomicrobial Fournier's gangrene caused by an ST8, methicillin-susceptible Staphylococcus aureus (designated ksw1). Whole-genome sequencing and analyses for virulence determinants revealed that, unlike USA300, ksw1 lacked virulence genes, such as Panton-Valentine leukocidin and SCCmec, while harboring the toxic shock syndrome toxin-1 gene. These genomic features correlate with ST8 CA-MRSA/J, which is the major genotype of ST8 in Japan.
Keywords: Fournier's gangrene, methicillin-susceptible Staphylococcus aureus ST8, Panton-Valentine leukocidin, toxic shock syndrome toxin-1
Introduction
Fournier's gangrene is characterized by necrotizing fasciitis of the perineal, perianal or genital regions (1, 2). Diabetes mellitus is well known to be a particularly important risk factor for Fournier's gangrene (2, 3). Fournier's gangrene is typically caused by polymicrobial infection (2). It has been increasingly reported that monomicrobial infection of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) can cause necrotizing fasciitis (4).
In the USA, the most predominant CA-MRSA strain, USA300, belongs to multilocus sequence type (ST) 8 and typically harbors virulence genes, such as the Panton-Valentine leukocidin genes lukF-PV and lukS-PV (lukPVSF), and staphylococcal cassette chromosome mec (SCCmec) type IV carrying penicillin and cephem resistance gene mecA (4).
In Japan, the strain ST8 has not been well characterized (5) despite several studies showing evidence of USA300 infection (6-10). We herein report a case of monomicrobial Fournier's gangrene caused by a strain of methicillin-susceptible S. aureus ST8 (designated ksw1) that lacked the Panton-Valentine leukocidin gene while harboring the toxic shock syndrome toxin-1 gene.
Case Report
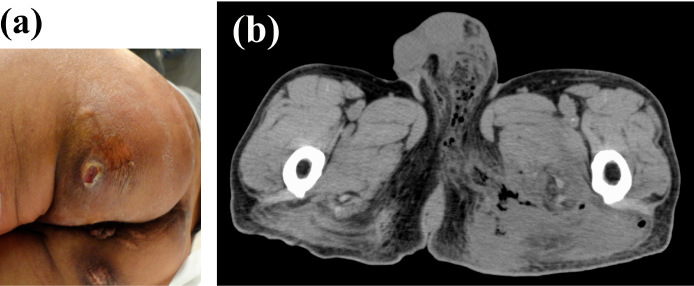
A 66-year-old man presented with a 1-month history of left buttock pain. His medical records indicated type 2 diabetes mellitus that had not been treated for 30 years. The patient had never been abroad. A clinical examination revealed tachycardia (100 beats per minute) with a normal blood pressure (105/55 mmHg) and slight pyrexia (38.1°C). His body mass index was 18.64 kg/m2 (height, 167 cm; body weight, 52.0 kg). A physical examination revealed an open wound with purulent discharge on his left buttock (Figure a). A laboratory examination indicated leucocytosis (white blood cell count, 23,820 /μL), elevated level of C-reactive protein (C-reactive protein, 25.08 mg/dL), mildly elevated level of muscle-derived enzymes (aspartate aminotransferase, 90 U/L; lactate dehydrogenase, 433 U/L; creatine kinase, 322 U/L), normal level of serum creatinine (creatinine, 0.59 mg/dL) and hyperglycemia (blood glucose, 238 mg/dL; hemoglobin A1c, 8.6%). Furthermore, computed tomography (CT) demonstrated subcutaneous emphysema extending from the left scrotum and inguinal region to the left thigh (Figure b). The CT findings indicated the possibility of Fournier's gangrene. In accordance with this observation, intravenous tazobactam/piperacillin (4.5 g, thrice daily) was initiated, and exploratory surgery was conducted. Concurrently, insulin therapy was started to manage his hyperglycemia. Intraoperative findings revealed subcutaneous abscess in the left buttock with necrotizing muscle fascia, symptomatically confirming Fournier's gangrene. Accordingly, extensive debridement was conducted.
Figure.
(a) Open wound on the left buttock of the patient. (b) CT scan of subcutaneous emphysema. CT: computed tomography
Gram staining of the intraoperative purulence demonstrated clusters of Gram-positive cocci, indicating the involvement of S. aureus. Therefore, intravenous vancomycin was added. Monomicrobial S. aureus was isolated from the blood and intraoperative purulence samples. This isolate was tested for antimicrobial susceptibility by the microliquid dilution method using an autoSCAN-4 (Beckman Coulter, Brea, USA) and the CLSI M100-S26 interpretive criteria and was found to be resistant to penicillin and ampicillin while retaining susceptibility to the other 14 antimicrobials examined (sulbactam/ampicillin, cefazolin, cefotiam, cefmetazole, flomoxef, gentamicin, arbekacin, erythromycin, minocycline, vancomycin, sulfamethoxazole/trimethoprim, fosfomycin, imipenem/cilastatin and levofloxacin). Based on the results of an antimicrobial susceptibility test, antimicrobials were replaced with intravenous cefazolin (1 g thrice daily) and clindamycin (600 mg twice daily). Repeated CT revealed a residual lesion in the left scrotum. Accordingly, additional debridement was performed on day 19 of admission. After the second debridement, antimicrobials were replaced with levofloxacin (500 mg once daily) administered orally and discontinued 3 days after the surgery.
The genome of the S. aureus strain isolated from the intraoperative purulence (ksw1) was sequenced with an MiSeq sequencer (illumina, San Diego, USA), and genome assembly was performed with a CLC Genomics Workbench 11. Multilocus sequence typing (11) and spa (protein A gene) typing (12) were conducted, and the genotype was determined to be ST8/spa1 (t622). We further performed gene annotation with the Pathosystems Resource Integration Center (PATRIC, http://www.patricbrc.org) (13) and searched for SCCmec using the SCCmecFinder (http://cge.cbs.dtu.dk/services/SCCmecFinder) (14). We compared the virulence factors between ksw1, USA300 strain FPR3757 (GenBank accession number: CP000255) (15) and ST8 CA-MRSA/J strain NN50 (Genbank accession number: BAEA01000000) (16), which is the major genotype of ST8 in Japan (Table). The ksw1 lacked lukPVSF and harbored the toxic shock syndrome toxin-1 gene, like ST8 CA-MRSA/J, but lacked SCCmec. For further confirmation, we conducted polymerase chain reaction (PCR) for lukPVSF genes using two primer sets: Fw (5'-CATCAACAGGAGGTAATGG) and Rv (5'-CAGAATAAATGTTTCCAGCAGC). We did not detect a 751-bp PCR product that could have been generated if the strain harbors lukPVSF genes. This confirms that the strain lacks lukPVSF genes. The genome sequence of the isolated strain has been deposited in the public sequence repository DNA Data Bank of Japan, DDBJ (http://www.ddbj.nig.ac.jp), under the accession number: PRJDB8899 (DDBJ BioProject), SAMD00193980 (ksw1) (DDBJ BioSample).
Table.
The Comparison of Multilocus Sequence Types and Virulence Factors between Ksw1, ST8 CA-MRSA/J and USA300.
| Type, virulence gene, drug resistance |
ksw1 | ST8 CA-MRSA/J -MM50 | USA300 -FPR3757 |
|---|---|---|---|
| Type | |||
| ST | 8 | 8 | 8 |
| spa | 1 (t622) | 1 (t1767) | 1 (t008) |
| SCCmec | - | IV | IVa |
| virulence genes | |||
| leukocidins | |||
| lukPVSF | - | - | + |
| lukED | + | + | + |
| phenol-soluble modulin | |||
| hld | |||
| hemolysins | + | + | + |
| hla | + | + | + |
| hlb | + | + | + |
| hlg | + | + | + |
| tsst-1 | + | + | - |
| enterotoxins | sec, sel, sep | sec, sel, sep | sek, seq |
| adhesins |
ebpS, sdrC, sdrD, sdrE, icaA, icaB, icaC, icaD, icaR, clfB |
ebpS, sdrC, sdrD, sdrE, icaA, icaB, icaC, icaD, icaR, clfB |
ebpS, sdrC, sdrD, sdrE, icaA, icaB, icaC, icaD, icaR, clfB |
| others | sak, scn | sak, scn | sak, scn, chp |
| drug resistance | PCG, ABPC | PCG, DMPPC, EM, GM 1 | β lactams, EM, CLDM, TC, CPFX, MUP |
1 Computational Prediction. PCG: benzylpenicillin, ABPC: ampicillin, DMPPC: methicillin, EM: erythromycin, GM: gentamicin, CLDM: clindamycin, TC: tetracycline, CPFX: ciprofloxacin, MUP: mupirocin
Discussion
We encountered a case of monomicrobial Fournier's gangrene caused by Panton-Valentine leukocidin-negative methicillin-susceptible S. aureus ST8 in a patient with type 2 diabetes mellitus. The clinical course of necrotizing fasciitis caused by CA-MRSA is reported to be subacute, with symptoms presenting an average of six days before admission (4). In the present case, left buttock pain had been noticed one month before admission, and a residual lesion in the left scrotum developed over two weeks after the first debridement. Therefore, the clinical course of the present case is considerably persistent.
The isolated strain ksw1 was found to share its ST8 with USA300, the most predominant CA-MRSA strain in the USA and known to cause skin and soft tissue infection. Epidemiological studies have demonstrated an association between CA-MRSA and the presence of lukPVSF genes and SCCmec type IV (17-19). Panton-Valentine leukocidin is a cytotoxin that induces apoptosis of human neutrophils, and intradermal injection of purified Panton-Valentine leukocidin to rabbits reportedly induces tissue necrosis (20, 21). Therefore, Panton-Valentine leukocidin has been considered to be responsible for the enhanced virulence of CA-MRSA strains. However, several experimental studies in animal models of skin and soft tissue infection have shown a minimal effect of Panton-Valentine leukocidin (22-24). Furthermore, five cases of invasive infection reported in South Korea were found to be caused by Panton-Valentine leukocidin-negative CA-MRSA (25). This evidence negates the significant role of Panton-Valentine leukocidin as a virulence factor in skin and soft tissue infections caused by CA-MRSA. Interestingly, our observations also indicate that Panton-Valentine leukocidin is dispensable in causing necrotizing fasciitis.
The virulence factor composition of ksw1 differed considerably from that of the USA300 strain. Besides lacking Panton-Valentine leukocidin, ksw1 was found to harbor the toxic shock syndrome toxin-1 gene. Regarding the virulence factor composition, ksw1 showed high similarities to ST8 CA-MRSA/J, a major genotype of ST8 CA-MRSA in Japan (16). Furthermore, the spa type of ksw1, 1 (t622) includes variant strains of ST8 CA-MRSA/J (16). More detailed studies involving comparative genomics, especially of the gene structure including SCCmec, are desired.
This research was conducted in Kashiwa Municipal Hospital, Chiba, Japan.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sorensen MD, Krieger JN, Rivara FP, et al. Fournier's gangrene: population based epidemiology and outcomes. J Urol 181: 2120-2126, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eke N. Fournier's gangrene: a review of 1726 cases. Br J Surg 87: 718-728, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Nisbet AA, Thompson IM. Impact of diabetes mellitus on the presentation and outcomes of Fournier's gangrene. Urology 60: 775-779, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med 352: 1445-1453, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Kawaguchiya M, Urushibara N, Ghosh S, et al. Genetic diversity of emerging Panton-Valentine leukocidine/arginine catabolic mobile element (ACME)-positive ST8 SCCmec-IVa meticillin-resistant Staphylococcus aureus (MRSA) strains and ACME-positive CC5 (ST5/ST764) MRSA in northern Japan. J Med Microbiol 62: 1852-1863, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Shibuya Y, Hara M, Higuchi W, et al. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in Japan. J Infect Chemother 14: 439-441, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Higashiyama M, Ito T, Han X, et al. Epidural abscess caused by community-associated methicillin-resistant Staphylococcus aureus strain USA300 in Japan. J Infect Chemother 16: 345-349, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Higuchi W, Takano T, Iwao Y, et al. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in a Japanese child, demonstrating multiple divergent strains in Japan. J Infect Chemother 16: 292-297, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Yabe S, Takano T, Higuchi W, et al. Spread of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone among family members in Japan. J Infect Chemother 16: 372-374, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Nagao M, Iimura Y, Suzuki M, et al. First outbreak of methicillin-resistant Staphylococcus aureus USA300 harboring the Panton-Valentine leukocidin genes among Japanese health care workers and hospitalized patients. Am J Infect Control 38: e37-e39, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Enright MC, Day NP, Davis CE, et al. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol 38: 1008-1015, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartels MD, Petersen A, Worning P, et al. Comparing whole-genome sequencing with Sanger sequencing for spa typing of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 52: 4305-4308, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wattam AR, Brettin T, Davis JJ, et al. Assembly, annotation, and comparative genomics in PATRIC, the all bacterial bioinformatics resource center. Methods Mol Biol 1704: 79-101, 2018. [DOI] [PubMed] [Google Scholar]
- 14.Kaya H, Hasman H, Larsen J, et al. SCCmecFinder, a web-based tool for typing of staphylococcal cassette chromosome mec in Staphylococcus aureus using whole-genome sequence data. mSphere 3: e00612-e00617, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diep BA, Gill SR, Chang RF, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired methicillin-resistant Staphylococcus aureus. Lancet 367: 731-739, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Iwao Y, Ishii R, Tomita Y, et al. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: associated infections, genetic diversity, and comparative genomics. J Infect Chemother 18: 228-240, 2012. [DOI] [PubMed] [Google Scholar]
- 17.Vandenesch F, Naimi T, Enright MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis 9: 978-984, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naimi TS, LeDell KH, Como-Sabetti K, et al. Comparison of community-and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA 290: 2976-2984, 2003. [DOI] [PubMed] [Google Scholar]
- 19.Diep BA, Sensabaugh GF, Somboona NS, et al. Widespread skin and soft-tissue infections due to two methicillin-resistant Staphylococcus aureus strains harboring the genes for Panton-Valentine leucocidin. J Clin Microbiol 42: 2080-2084, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cribier B, Prévost G, Couppie P, et al. Staphylococcus aureus leukocidin: a new virulence factor in cutaneous infections? An epidemiological and experimental study. Dermatology 185: 175-180, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Genestier AL, Michallet MC, Prevost G, et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondoria and induces Bax-independent apoptosis of human neutrophils. J Clin Invest 115: 3117-3127, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoong P, Torres VJ. The effects of Staphylococcus aureus leukotoxins on the host: cell lysis and beyond. Curr Opin Microbiol 16: 63-69, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto M. MRSA virulence and spread. Cell Microbiol 14: 1513-1521, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Otto M. Community-associated MRSA: what makes them special? Int J Med Microbiol 303: 324-330, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee SS, Kim YJ, Chung DR, et al. Invasive infection caused by a community-associated methicillin resistant Staphylococcus aureus strain not carrying Panton-Valentine leukocidin in South Korea. J Clin Microbiol 48: 311-313, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]