Abstract
Objective
We aimed to investigate the association between the digit symbol test (DST) and clinical characteristics, including the nutritional status of liver cirrhosis patients.
Methods
Fifty-nine cirrhotic patients without a history of overt hepatic encephalopathy were retrospectively evaluated. We examined neuropsychological abnormalities (NPAs) using the DST. We also estimated the detailed nutritional status using the Food Frequency Questionnaire (FFQ). The patients were divided into two groups according to their DST status: patients with normal DST scores (DST-Nor group, n=45) and those with abnormal DST scores (DST-Abn group, n=14). The clinical and nutritional findings of the two groups were compared.
Results
Overall, 14 (23.7%) patients had a DST abnormality. There were significant differences between the two groups in serum albumin (Alb; p=0.0043), valine (Val; p=0.0016), leucine (Leu; p=0.0078), isoleucine (Ile; p=0.0022), the molar ratio of total branched-chain amino acids to tyrosine (BTR; p=0.00025), total-bilirubin (T-Bil; p=0.0071), prothrombin time(%) (PT; p=0.028), and serum sodium (Na; p=0.035). A multivariate analysis found the BTR to be the only independent predictor of a DST abnormality (hazard ratio, 9.24; p<0.031). An FFQ analysis, revealed that the nutritional findings of patients with and without a DST abnormality, were similar.
Conclusion
The BTR was useful for predicting the risk of NPAs, as defined by a DST abnormality. The risk of NPAs may be estimated by monitoring the BTR.
Keywords: liver cirrhosis, digit symbol test, BTR, FFQ, hepatic encephalopathy
Introduction
Various symptoms and clinical findings are indicative of the progression of liver cirrhosis (LC). Among these symptoms, the development of hepatic encephalopathy (HE) is related to a poor prognosis in patients with LC (1-3). Minimal hepatic encephalopathy (MHE), which causes cognitive problems in LC patients, impairs the patient's ability to perform activities of daily living and their quality of life. MHE is an early stage of overt hepatic encephalopathy (OHE) (4-6). The serum ammonia level is commonly measured, and electroencephalography and magnetic resonance spectroscopy are used to evaluate patients with MHE (7).
Some researchers have reported that the results of neuropsychiatric tests (NPTs) are useful for accurately diagnosing MHE (8). The Committee of the Working Party at the Eleventh World Congress of Gastroenterology Vienne (WCOG), the International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN), and the Japanese Society of Hepatology (JSH) have recommended the use of NPTs for diagnosing MHE (8-10). The WCOG has recommended that at least two of four tests [Number Connection Test-A (NCT-A), Number Connection Test-B (NCT-B), Digit Symbol Test (DST) and Block Design Test (BDT)] be used for the diagnosis of MHE (8, 10). In previous studies, MHE was diagnosed when at least two test results were abnormal (11, 12). In other studies, MHE was diagnosed when the result of at least one of the two tests was abnormal (13, 14). Among the several NPTs available, the DST has been reported to have a high sensitivity and specificity for detecting neuropsychological problems (14-16). However, the association between the DST and clinical characteristics, including the nutritional status, of LC patients has not been fully clarified.
In the present study, we investigated the association between the DST and clinical characteristics, including the nutritional status of LC patients.
Materials and Methods
Study design
Fifty-nine cirrhotic patients without a history of OHE who had been treated at the Division of Gastroenterology and Hepatology, the Jikei University School of Medicine between May 2014 and November 2016 were enrolled in this study. All medical records were retrospectively reviewed for the patients' demographic and clinical data, including the nutritional status. Patients whose condition was complicated with mental disorders, renal dysfunction, bacterial infection, gastrointestinal hemorrhaging, or severe constipation or those treated with an anticonvulsant drug that might affect the cognitive function were excluded from the analysis.
LC was diagnosed based on physical findings, laboratory data, and clinical imaging characteristics. Irregularity and deformity of the shape of the liver was detected via ultrasonography and computed tomography.
The present study was approved by the institutional review board of Jikei University School of Medicine (26-006 7511) and complied with the provisions of the Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. All enrolled patients provided their written informed consent for participation in the present study.
Physical status and laboratory findings
Demographic and clinical data were extracted or calculated from patient medical records. The body mass index (BMI) and ideal body weight were calculated. According to the assessment criteria for sarcopenia in liver cirrhosis established by the JSH, sarcopenia associated with liver disease is known to reduce the muscle mass and muscle strength. The skeletal muscle mass index (SMI), calculated by dividing the left-right sum (cm2) of the long axis (cm)×short axis (cm) of the iliopsoas muscles at the level of third lumbar vertebra by the height squared (m2) (the so-called “simple method”), correlated well with the SMI calculated using a muscle mass measurement software program (cut-off value of the SMI calculated by the simple method: 6.0 cm2/m2 in men, 3.4 cm2/m2 in women) (17). While we have no data on the handgrip strength in our LC patients, we evaluated the SMI using a simple method for the evaluation of the muscle mass.
Hematological and biochemical tests were performed after fasting for more than 8 hours. The hematological analyses included the white and red blood cell counts, and platelet counts; in addition, the hematocrit and hemoglobin levels were determined. The levels of serum alanine transaminase (ALT), serum albumin (Alb), total bilirubin (T-Bil), creatinine, serum ammonia (NH3), branched-chain amino acids to tyrosine ratio (BTR), prothrombin time (PT), and C-reactive protein (CRP) were determined. As an amino acids analysis, the levels of valine (Val), leucine (Leu), isoleucine (Ile), methionine (Met), tyrosine (Tyr), phenylalanine (Phe), histidine (His), tryptophan (Trp), ornithine (Orn), lysine (Lys), and arginine (Arg) were also determined.
Performance of the DST
The DST was performed using a tablet-type device developed by Otsuka Pharmaceutical (Tokyo, Japan), Kokuyo (Osaka, Japan), and ISB (Tokyo, Japan); the tablet and NPT program were provided by the JSH. The DST values were estimated based on deviations from standard age- and sex-dependent values. Each patient's DST data were compared with the 10th and 90th percentile cut-off values for healthy individuals, with 5-year quartile ranges (5, 18). The DST is performed after several days of alcohol abstinence for patients with alcoholic liver cirrhosis.
The evaluation of the nutritional status
We calculated the total daily energy expenditure (TEE) using the Harris-Benedict equation. The activity factor was 1.3, and the injury/stress factor was 1.0, as enrolled patients had chronic liver disease but were ambulatory and were able to visit the hospital outpatient clinic by themselves (19, 20). We estimated the detailed nutritional status using the Food Frequency Questionnaire (FFQ), which was administered at the initiation of nutritional support and performed under the supervision of a nationally registered dietician, according to our study schedule. The nutrient intake, estimated intake energy per day (EIE), and the usual daily energy ratio of nutrients (PFC ratio) were calculated by the FFQ. The estimated intake amount of dietary fiber, n-3/n-6 unsaturated fatty acids and salt were calculated. The intake amount of each nutrient was estimated using a software program adapted to Japanese eating and living habits (Excel Eiyou-kun version 6.0; Kenpakusha, Tokyo, Japan). We compared the detailed nutritional data of our patients to the estimated energy requirements (EER), the estimated average requirements (EAR), and adequate intake values (AI), as determined by the Japanese Ministry of Health, Labor and Welfare (21, 22).
Oral branched-chain amino acid (BCAA) supplementation
Patients ingested BCAA granules (LIVACT; EA Pharma, Tokyo, Japan) that contained 952 mg of L-isoleucine, 1,904 mg of L-leucine, and 1,144 mg of L-valine per sachet or BCAA powder mix (Aminoleban EN; Otsuka Pharmaceutical, Tokyo, Japan) that contained 2,037 mg of L-leucine, 1,922.5 mg of L-isoleucine, 1,602 mg of L-valine, 242.5 mg of L-lysine, 302 mg of arginine, 187.5 mg of histidine, and 73.5 mg of tryptophan per package. Patients ingested one sachet after each meal or one to three packages daily.
Statistical analyses
We used EZR (23), a graphical user interface for R, version 1.29, to perform the statistical analysis (The R Foundation for Statistical Computing, Vienna, Austria). For individual variables, all data were expressed as the mean and standard deviation (SD), unless stated otherwise. Categorical variables were compared using the chi-squared test, while continuous variables were compared using the Mann-Whitney U test. To assess the factors predicting a DST abnormality, logistic regression analyses were used to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Only variables with p values of <0.05 in a univariate analysis were included in the multivariate analysis.
A receiver operating characteristics (ROC) was also generated to determine the optimal cut-off value of continuous variables. P value of <0.05 is considered to indicate statistical significance.
Results
Etiology and clinical findings
The patient demographics and clinical characteristics are shown in Table 1. The mean age of the patients was 66 years old. Thirty-six (61%) patients were men, and 23 (39%) were women. The mean BMI was 24.0±4.7 kg/m2. The most common etiology of LC was hepatitis C (n=24, 40.7%), followed by alcohol-associated hepatitis (n=17, 28.8%), NASH (n=6, 10.1%), and hepatitis B (n=4, 6.8%). Of the 59 patients enrolled, none showed portosystemic shunt on dynamic-enhanced computed tomography scans or ultrasonography. Thirty-one patients (52.5%) had a history of hepatocellular carcinoma (HCC) treatment. Twenty-seven patients (45.8%) used BCAA supplementation. Thirteen patients took 1 BCAAs granule sachet after each meal, whereas BCAA powder mix was used by 14 patients (11 patients took 1 package daily as a late-evening snack, 1 patient took 2 packages per day, and 2 patients took 3 packages per day). The Child-Pugh stages of the patients were as follows: stage A (n=37), B (n=14), and C (n=8). The mean Child-Pugh score (CP-S) was 6.8±2.1. The mean Alb (3.4±0.5 g/dL), BTR (3.9±1.6), and platelet counts (11.5±5.9×104/μL) were lower than the reference values.
Table 1.
The Clinical Characteristics and Laboratory Findings of the Enrolled Patients.
| Characteristics | All patients (n=59) Mean±SD or n (%) |
|---|---|
| Age (years) | 66.1±10.7 |
| Sex (Man/Woman) | 36/23 |
| BMI (kg/m2) | 24.0±4.7 |
| BMI>25 | 19 (32.2) |
| Number of cases with reduced muscle volume | 10 (16.9) |
| Etiology | |
| HBV | 4 (6.8) |
| HCV | 24 (40.7) |
| Alcohol | 17 (34.7) |
| NASH | 6 (10.2) |
| others | 8 (13.6) |
| Child-Pugh score | 6.8±2.1 |
| Child-Pugh classification (A/B/C) | 37/14/8 |
| MELD score | 9.3±4.8 |
| Complication | |
| HCC | 31 (52.5) |
| Ascites | 12 (20.3) |
| Number of cases using BCAA supplementation | 27 (45.8) |
| BCAA granules, three sachets daily | 13 (22.0) |
| BCAA powder mix, one/two/three packages daily | 11 (18.6)/1 (1.7)/2 (3.4) |
| Concomitant medications | |
| Lactulose | 5 (8.5) |
| Antibiotics | 5 (8.5) |
| Zinc sulfate | 3 (5.1) |
| L-Carnitine | 0 (0.0) |
| Molecular-targeted agents for HCC | 0 (0.0) |
| <Laboratory findings> | |
| ALT (U/L) | 39.1±31.2 |
| Albumin (g/dL) | 3.4±0.5 |
| Total-bilirubin (mg/dL) | 1.5±1.1 |
| Platelet (×104/μL) | 11.5±5.9 |
| Prothrombin time (%) | 73.3±17.0 |
| Na (mmol/L) | 139.4±3.0 |
| NH3 (μg/dL) | 56.2±27.4 |
| CRP (mg/dL) | 0.44±0.64 |
| Amino acids, standard value | |
| Valine (nmol/L), 158.4-287.7 | 212.6±57.4 |
| Leucine (nmol/L), 80.9-154.3 | 113.7±35.5 |
| Isoleucine (nmol/L), 41.3-84.9 | 64.2±19.3 |
| Methionine (nmol/L), 19.2-32.7 | 37.0±14.3 |
| Tyrosine (nmol/L), 50.2-82.6 | 106.3±31.1 |
| Phenylalanine (nmol/L), 45.7-76.5 | 88.3±26.6 |
| Histidine (nmol/L), 67.9-97.1 | 88.9±15.8 |
| Tryptophan (nmol/L), 41.4-65.5 | 50.5±14.6 |
| Ornithine (nmol/L), 43.2-95.7 | 99.5±31.2 |
| Lysine (nmol/L), 118.7-257.0 | 177.1±44.4 |
| Arginine (nmol/L), 46.0-121.7 | 80.3±28.4 |
| BTR | 3.9±1.6 |
SD: standard deviation, BMI: body mass index, HBV: hepatitis B virus, HCV: hepatitis C virus, NASH: nonalcoholic steatohepatitis, MELD score: model for end-stage liver disease score, HCC: hepatocellular carcinoma, BCAAs: branched-chain amino acids, ALT: alanine transaminase, Na: serum sodium, NH3: serum ammonia, CRP: C-reactive protein, BTR: branched-chain amino acid to tyrosine ratio
DST results and their correlation with clinical characteristics
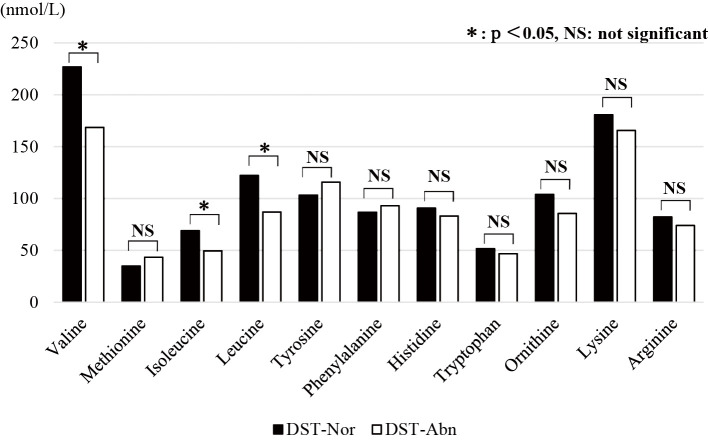
We divided patients into two groups based on the reference values (DST-Abn group, n=14, DST-Nor group, n=45). The correlation between the DST status and the clinical characteristics is shown in Table 2 and Figure. The CP-S of the DST-Abn group was significantly higher than that of the DST-Nor group (6.3±1.3 vs. 8.8±2.9, p=0.0052). The DST-Abn group also had markedly higher T-Bil levels than the DST-Nor group (2.6±1.7 vs. 1.2±0.5, p=0.0071). In addition, the levels of Alb (3.0±0.5 vs. 3.5±0.5, p=0.0043), PT (62.4±20.9 vs. 76.7±14.3, p=0.028), serum sodium (137.6±3.5 vs. 140.0±2.6, p=0.035), Val (168.6±60.9 vs. 226.9±49.0, p=0.0016), Leu (87.1±36.2 vs. 122.3±31.2, p=0.0078), and Ile (49.5±19.1 vs. 69.0±17.0, p=0.0022) as well as the BTR values (2.8±1.1 vs. 4.3±1.6, p=0.00025) were notably lower in the DST-Abn group than in the DST-Nor group. There were no significant differences between the two groups regarding the use or non-use of BCAA supplementation (p=0.776).
Table 2.
The Clinical Characteristics of Patients with Normal and Abnormal Digit Symbol Test Results.
| Characteristics | DST-Nor Mean±SD or n (%) |
DST-Abn Mean±SD or n (%) |
p value |
|---|---|---|---|
| No. of cases | 45 | 14 | |
| Age (years) | 66.5±11.3 | 64.6±8.8 | p=0.52 |
| Sex (Man/Woman) | 29/16 | 7/7 | |
| BMI (kg/m2) | 23.9±4.8 | 24.5±4.4 | p=0.65 |
| Number of cases with reduced muscle volume | 6 (13.3) | 4 (28.6) | p=0.24 |
| Child-Pugh score | 6.1±1.3 | 8.8±2.9 | p=0.0052 |
| Child-Pugh classification (A/B/C) | 33/10/2 | 4/4/6 | |
| MELD score | 8.6±4.3 | 11.6±5.7 | p=0.095 |
| Complication | |||
| HCC | 25 (55.6) | 6 (42.9) | p=0.542 |
| Ascites | 6 (13.3) | 6 (42.9) | p=0.026 |
| Number of cases using BCAA supplementation | 20 (44.4) | 7 (50.0) | p=0.776 |
| BCAA granules, three sachets daily | 10 (22.2) | 3 (21.4) | |
| BCAA powder mix, one/two/three packages daily | 9 (20.0)/0 (0)/0 (0) | 2 (14.3)/1 (7.1)/2 (14.3) | |
| Concomitant medications | |||
| Lactulose | 2 (4.4) | 3 (21.4) | p=0.081 |
| Antibiotics | 2 (4.4) | 3 (21.4) | p=0.081 |
| Zinc sulfate | 3 (6.7) | 0 (0.0) | p=1.00 |
| <Laboratory findings> | |||
| ALT (U/L) | 36.2±25.1 | 48.3±45.7 | p=0.36 |
| Albumin (g/dL) | 3.5±0.5 | 3.0±0.5 | p=0.0043 |
| Total-bilirubin (mg/dL) | 1.2±0.5 | 2.6±1.7 | p=0.0071 |
| Platelet (×104/μL) | 11.4±5.5 | 11.9±7.4 | p=0.80 |
| Prothrombin time (%) | 76.7±14.3 | 62.4±20.9 | p=0.028 |
| Na (mmol/L) | 140.0±2.6 | 137.6±3.5 | p=0.035 |
| NH3 (μg/dL) | 56.8±28.5 | 54.1±24.7 | p=0.73 |
| CRP (mg/dL) | 0.4±0.6 | 0.7±0.7 | p=0.122 |
| Amino acids, standard value | |||
| Valine (nmol/L), 158.4-287.7 | 226.9±49.0 | 168.6±60.9 | p=0.0016 |
| Leucine (nmol/L), 80.9-154.3 | 122.3±31.2 | 87.1±36.2 | p=0.0078 |
| Isoleucine (nmol/L), 41.3-84.9 | 69.0±17.0 | 49.5±19.1 | p=0.0022 |
| Methionine (nmol/L), 19.2-32.7 | 34.9±10.6 | 43.4±21.6 | p=0.215 |
| Tyrosine (nmol/L), 50.2-82.6 | 103.3±29.5 | 115.8±35.3 | p=0.284 |
| Phenylalanine (nmol/L), 45.7-76.5 | 86.8±22.1 | 93.0±38.2 | p=0.825 |
| Histidine (nmol/L), 67.9-97.1 | 90.7±15.4 | 83.2±16.3 | p=0.175 |
| Tryptophan (nmol/L), 41.4-65.5 | 51.8±13.2 | 46.8±18.6 | p=0.364 |
| Ornithine (nmol/L), 43.2-95.7 | 104.0±32.7 | 85.6±21.9 | p=0.112 |
| Lysine (nmol/L), 118.7-257.0 | 180.8±46.9 | 165.7±34.8 | p=0.376 |
| Arginine (nmol/L), 46.0-121.7 | 82.3±28.6 | 74.1±28.1 | p=0.339 |
| BTR | 4.3±1.6 | 2.8±1.1 | p=0.00025 |
DST-Nor: normal digit symbol test, DST-Abn: abnormal digit symbol test, BMI: body mass index, MELD: model for end-stage liver disease, HCC: hepatocellular carcinoma, BCAAs: branched-chain amino acids, ALT: alanine transaminase, Na: serum sodium, NH3: serum ammonia, CRP: C-reactive protein, BTR: branched-chain amino acids to tyrosine ratio
Figure.
The results of the amino acid analysis according to the DST status. Significant differences between the two groups were noted in valine (p=0.0016), leucine (p=0.0078), and isoleucine (p=0.0022). The tyrosine value was similar between the two groups. DST: digit symbol test
The results of the multivariate analysis are shown in Table 3. While univariate analyses identified significant differences in CP-S, the CP-S is composed of Alb, T-Bil, PT, ascites, and hepatic encephalopathy and was therefore excluded from the multivariate analysis. As a result, a BTR of <2.92 was found to be an independent predictor of a DST abnormality [hazard ratio (HR), 9.24; p<0.031].
Table 3.
The Multivariate Analysis of Factors Associated with a DST Abnormality.
| Parameter | Odds ratio | 95% CI | p value |
|---|---|---|---|
| BTR <2.92 | 9.24 | 1.23-69.2 | 0.031 |
| Alb <2.90 | 1.63 | 0.175-15.2 | 0.669 |
| T-Bil >1.70 | 3.10 | 0.581-16.6 | 0.185 |
| PT <70 | 0.772 | 0.101-5.90 | 0.803 |
| Na <138 | 3.34 | 0.519-21.5 | 0.204 |
| The presence of ascites | 1.13 | 0.100-13.0 | 0.923 |
DST: digit symbol test, 95% CI: 95% confidence interval, BTR: branched-chain amino acids to tyrosine ratio, Alb: albumin, T-Bil: total-bilirubin, PT: prothrombin time, Na: serum sodium
Given the possible effect of BCAA supplementation on the DST and BTR, LC patients not taking BCAA supplementation were divided into two groups according to the DST result. The clinical characteristics of the LC patients without BCAA supplementation who had normal and abnormal DST results are shown in Table 4. There was a significant difference in the BTR between the two groups in a univariate analysis (p=0.0385). A multivariate analysis was not performed due to the small number of subjects.
Table 4.
The Clinical Characteristics of LC Patients That Do Not Take BCAA Supplementation with Normal and Abnormal Digit Symbol Test Results.
| Characteristics | DST-Nor Mean±SD or n (%) |
DST-Abn Mean±SD or n (%) |
p value |
|---|---|---|---|
| Number of cases | 25 | 7 | |
| Age (years) | 66.9±10.9 | 61.7±10.1 | p=0.266 |
| Sex (Man/Woman) | 18/7 | 6/1 | |
| BMI (kg/m2) | 24.2±4.9 | 24.4±3.4 | p=0.915 |
| Number of cases with reduced muscle volume | 1 (4.0) | 2 (28.6) | p=0.12 |
| Child-Pugh score | 5.9±1.3 | 8.4±3.6 | p=0.121 |
| Child-Pugh classification (A/B/C) | 21/3/1 | 3/1/3 | |
| MELD score | 7.6±4.8 | 9.9±7.3 | p=0.469 |
| Complication | |||
| HCC | 18 (72.0) | 2 (28.6) | p=0.074 |
| Ascites | 5 (20.0) | 3 (42.9) | p=0.327 |
| Concomitant medications | |||
| Lactulose | 0 (0.0) | 0 (0.0) | |
| Antibiotics | 0 (0.0) | 0 (0.0) | |
| Zinc sulfate | 1 (4.0) | 0 (0.0) | p=1.00 |
| <Laboratory findings> | |||
| ALT (U/L) | 35.6±25.1 | 46.6±58.1 | p=0.641 |
| Albumin (g/dL) | 3.6±0.5 | 3.0±0.6 | p=0.05 |
| Total-bilirubin (mg/dL) | 1.0±0.5 | 2.8±2.0 | p=0.06 |
| Platelet (×104/μL) | 11.8±5.9 | 15.9±8.1 | p=0.251 |
| Prothrombin time (%) | 80.3±13.3 | 69.4±26.3 | p=0.326 |
| Na (mmol/L) | 140.2±2.1 | 137.0±4.7 | p=0.125 |
| NH3 (μg/dL) | 52.3±25.2 | 50.9±16.6 | p=0.862 |
| CRP (mg/dL) | 0.31±0.37 | 0.92±0.77 | p=0.08 |
| Amino acids, standard value | |||
| Valine (nmol/L), 158.4-287.7 | 222.6±42.6 | 169.7±77.6 | p=0.127 |
| Leucine (nmol/L), 80.9-154.3 | 119.5±27.9 | 93.5±45.9 | p=0.195 |
| Isoleucine (nmol/L), 41.3-84.9 | 69.4±14.8 | 50.6±24.3 | p=0.09 |
| Methionine (nmol/L), 19.2-32.7 | 32.6±9.3 | 29.2±8.8 | p=0.403 |
| Tyrosine (nmol/L), 50.2-82.6 | 100.2±33.0 | 97.5±26.0 | p=0.824 |
| Phenylalanine (nmol/L), 45.7-76.5 | 84.6±22.9 | 74.4±21.0 | p=0.288 |
| Histidine (nmol/L), 67.9-97.1 | 90.0±13.3 | 73.8±11.0 | p=0.00691 |
| Tryptophan (nmol/L), 41.4-65.5 | 52.4±15.5 | 43.0±15.3 | p=0.185 |
| Ornithine (nmol/L), 43.2-95.7 | 105.1±29.9 | 81.3±21.8 | p=0.036 |
| Lysine (nmol/L), 118.7-257.0 | 188.7±49.9 | 158.4±39.3 | p=0.117 |
| Arginine (nmol/L), 46.0-121.7 | 83.1±26.2 | 57.2±19.0 | p=0.012 |
| BTR | 4.58±1.83 | 3.24±1.21 | p=0.0385 |
LC: liver cirrhosis, DST-Nor: normal digit symbol test, DST-Abn: abnormal digit symbol test, BMI: body mass index, MELD: model for end-stage liver disease, HCC: hepatocellular carcinoma, BCAAs: branched-chain amino acids, ALT: alanine transaminase, Na: serum sodium, NH3: serum ammonia, CRP: C-reactive protein, BTR: branched-chain amino acids to tyrosine ratio
The estimation of the nutritional status using the FFQ
Overall, 41 enrolled LC cases completed the FFQ. The remaining 18 patients were unable to complete the FFQ because of the amount of time required. We estimated the nutritional status of 41 patients using physical measurements and the FFQ (Table 5). The TEE was not significantly different from the EIE (men: 1,769.4±309.3 kcal vs. 1,932.7±533.4 kcal, p>0.05; women: 1,515.7±149.7 kcal vs. 1,636.9±455.8 kcal, p>0.05). The protein ingestion values (men: 1.2±0.3 g/kg; women: 1.1±0.4 g/kg) were not significantly different from the recommended values. The fat energy ratio (men: 28.6±5.2%; women: 30.2±4.5%) was significantly higher than the AI (20.0-25.0%). We found that 36.6% of the patients had a fat energy ratio of >30% (n=15/41). The dietary fiber intake of men (16.2±6.0 g/day; vs. women, 16.2±4.4 g/day) was significantly less than the AI (>19 g/day, p=0.03; vs. women, >17 g/day, p=0.60).
Table 5.
Estimation of the Usual Daily Nutritional Condition Using the FFQ.
| Man (n=22) Mean±SD |
Woman (n=19) Mean±SD |
EAR or AI (man, woman) |
|
|---|---|---|---|
| Age (years) | 68.5±9.1 | 63.5±13.3 | |
| Height (cm) | 166.0±8.5 | 154.4±4.1 | |
| Body weight (kg) | 63.9±14.3 | 58.6±11.9 | |
| Ideal body weight (kg) | 60.8±6.1 | 52.5±2.8 | |
| BMI | 23.0±3.9 | 24.7±5.6 | |
| TEE (kcal) | 1,769.4±309.3* | 1,515.7±149.7** | |
| TEE/body weight (kcal/kg) | 26.5±1.6 | 28.1±3.4 | |
| <Results of FFQ> | |||
| EIE (kcal/day) | 1,932.7±533.4* | 1,636.9±455.8** | 2,200-2,650, 1,700-2,000 |
| Ratio of EIE to EER | 0.94±0.29 | 0.93±0.29 | |
| EIE/body weight (kcal/kg) | 30.9±8.0 | 28.9±8.5 | |
| TEE/EIE | 1.0±0.5 | 1.0±0.3 | |
| Protein intake (g/kg) | 1.2±0.3 | 1.1±0.4 | |
| <Usual daily energy ratio> | |||
| Protein (%) | 15.1±1.8 | 14.8±2.5 | 20-30 |
| Fat (%) | 28.6±5.2 | 30.2±4.5 | 20-25 |
| Carbohydrates (%) | 56.6±5.7 | 54.9±6.2 | 50-70 |
| Dietary fiber (g/day) | 16.2±6.0 | 16.2±4.4 | 19<, 17< |
| n-3 USFA (g/day) | 2.3±1.0 | 1.8±1.7 | 2.2<, 1.8< |
| n-6 USFA (g/day) | 9.6±3.9 | 8.9±3.5 | 8-10, 7-9 |
| n-6 USFA/n-3 USFA | 4.5±1.6 | 5.1±1.4 | |
| Salt (g/day) | 9.8±2.8 | 8.9±2.9 | 9>, 7.5> |
*/**: There was no significant difference for the comparison of TEE and EIE, based on sex. FFQ: Food Frequency Questionnaire, SD: standard deviation, TEE: total daily energy expenditure, EIE: estimated intake energy per day, EER: estimated energy requirement, EAR: estimated average requirement, n-3 USFA: n-3 unsaturated fatty acid, n-6 USFA: n-6 unsaturated fatty acid, AI: adequate intake
The nutritional condition according to the DST status
In the 41 LC patients who completed the FFQ, we compared the nutritional condition between the DST-Abn and DST-Nor groups. There were only 7 patients (2 men and 5 women) in the DST-Abn group. We therefore judged that it was impossible to consider man and woman patients separately. Nutritionally, the DST-Abn and DST-Nor groups were similar (Table 6). Based on the FFQ analysis, both groups had fat energy ratios that were above the AI, and both groups demonstrated insufficient dietary fiber intake.
Table 6.
The Nutritional Findings of Patients with Normal and Abnormal Digit Symbol Test Results.
| Parameter | DST-Nor (n=34) Mean±SD |
DST-Abn (n=7) Mean±SD |
p value |
|---|---|---|---|
| Sex (Man:Wonam) | 19:15 | 2:5 | |
| <Results of FFQ> | |||
| EIE (kcal/day) | 1,814.4±553.7 | 1,704.7±136.4 | p=0.826 |
| Ratio of EIE to EER | 0.94±0.31 | 0.94±0.16 | p=0.852 |
| EIE/body weight (kcal/kg) | 29.8±8.3 | 33.0±8.2 | p=0.466 |
| Protein intake (g/kg) | 1.1±0.4 | 1.2±0.4 | p=0.834 |
| <Usual daily energy ratio> | |||
| Protein (%) | 15.2±2.0 | 13.8±2.1 | p=0.141 |
| Fat (%) | 29.3±5.0 | 28.6±4.2 | p=0.533 |
| Carbohydrates (%) | 55.5±6.1 | 57.7±4.3 | p=0.200 |
| Dietary fiber (g/day) | 16.5±5.4 | 14.6±3.2 | p=0.703 |
| n-3 USFA (g/day) | 2.1±0.9 | 1.9±0.4 | p=0.599 |
| n-6 USFA (g/day) | 9.4±3.9 | 8.8±2.2 | p=0.795 |
| n-6 USFA/n-3 USFA | 4.8±1.5 | 4.9±1.5 | p=0.876 |
| Salt (g/day) | 9.6±2.9 | 8.4±1.7 | p=0.267 |
DST-Nor: normal digit symbol test, DST-Abn: abnormal digit symbol test, FFQ: Food Frequency Questionnaire, EIE: estimated intake energy per day, EER: estimated energy requirements, n-3 USFA: n-3 unsaturated fatty acid, n-6 USFA: n-6 unsaturated fatty acid
Discussion
HE serves as a prognostic factor in patients with LC. Although studies have reported that half of MHE patients develop OHE within three years (1, 3, 4), the diagnostic methods and detailed pathogenesis of MHE remain to be clarified. Based on these limitations, as well as the adverse effects on the patient's quality of life, we consider the establishment of diagnostic criteria for MHE to be imperative.
Most LC patients have protein energy malnutrition (PEM) and accelerated resting energy expenditure. Drastic changes in the metabolic nutrient ratio are observed in patients with liver dysfunction, in addition to a decreased carbohydrate ratio and increased fat ratio (24, 25). The absorption of aromatic amino acids into the nerve cells is also stimulated due to an amino acid imbalance, which leads to higher-brain-function restrictions and the development of HE (26-28). Practical nutritional support, which can help overcome nutritional problems, improves the prognosis of LC patients (25, 29, 30).
In the present study, we selected the DST as an indicator of earlier neurophysiological problems in cirrhotic patients. The approach used in this study conformed to the WCOG report, which requires that a neurophysiological assessment be used to diagnose MHE after excluding any other brain disorders (8). The NPTs, which are recommended by the WCOG, ISHEN, and JSH, are a valuable and useful method for diagnosing MHE (8-10). Michitaka et al. reported that abnormalities in 2 NPT items has 80% specificity for MHE, while abnormalities in ≥3 items has 95% specificity (12). Some studies have reported that more than two abnormal test items in the NCT-A/B, DST, and BDT are indicative of MHE (31). Li et al. reported that MHE could be diagnosed with 76.9% sensitivity and 96.3% specificity using the combination of the NCT-A and DST (15). Weissenborn et al. reported that, among the NPTs, the DST has high sensitivity (80.0%) and specificity (96.5%) for the diagnosis of MHE (16). We therefore selected the DST to evaluate early neurophysiological problems.
In our study, 14 (23.7%) patients were classified into the DST-Abn group, indicating that patients in this group are at risk of developing MHE. Although the CP-S in the DST-Abn group was higher than that in the DST-Nor group, four patients in the DST-Abn group had a Child-Pugh classification of A. Researchers are particularly interested in such groups of patients, as these patients have a relatively good clinical liver function. The values of Val, Leu, Ile, and the BTR in the DST-Abn group were significantly lower than in the DST-Nor group. Furthermore, a multivariate analysis demonstrated that a BTR <2.92 was independently associated with DST abnormality. Recently, Hanai et al. reported that the presence of sarcopenia and a low BCAAs level (<327 nmol/mL) was independently associated with MHE in LC patients, supporting our findings (32). Previous studies reported that the serum BCAA values might be remarkably decreased in patients with compensated LC in Child-Pugh class A; in particular, lower valine values were closely associated with the liver prognosis and mortality (33-35). The ammonia detoxification pathway includes the urea cycle in the liver and the glutamine synthesis pathway in the skeletal muscle and brain. In cirrhotic patients, while the ability to process ammonia by the urea cycle is reduced, the detoxification of ammonia to glutamine in skeletal muscle, brain, and likely the lungs is activated. When glutamate and ammonia react to form glutamine, ammonia is processed. However, an increase in the concentration of glutamine induces hepatic encephalopathy by causing astrocyte swelling and cerebral hypertension in the brain. Furthermore, in skeletal muscle, BCAAs (Val, Leu, Ile) are metabolized to acetyl-CoA and succinyl-CoA and converted to α-ketoglutarate in the Krebs cycle. α-ketoglutarate itself is also a source of glutamate, generating ammonia through the process of producing glutamine. However, the produced glutamine is metabolized in the intestine and kidney to ammonia and glutamate, and glutamate in also decomposed into α-ketoglutarate and ammonia. Even if the ammonia concentration decreases in a certain organ, ammonia can be consequently produced in another organ, resulting in a vicious cycle in which the ammonia level in the body does not decrease (34, 36). We hypothesize that the neuropsychological dysfunction and MHE associated with LC is related to the fractional imbalance of amino acids in these patients. In fact, Akahoshi et al. demonstrated that NPT scores (including those of the NCT, BDT, and DST) were negatively associated with the BTR in patients undergoing liver transplantation and that the BTR in patients with MHE was significantly lower than that in those without MHE (37). We therefore suggest that the BTR is a useful parameter for predicting the development of cognitive problems similar to MHE.
The FFQ have been used in nutritional epidemiological studies to investigate the association between food and chronic diseases (38-44). Evaluating the dietary intake helps physicians and dieticians understand poor food habits, dietary choice, and excessive consumption. Suitable management of malnutrition involves establishing daily a diet that includes a restricted calorie intake and diverse nutrient consumption (45). Using the FFQ, the quantity of daily intakes was calculated based on the portion size and consumption frequency. The TEE/EIE ratios in men and women in the present study were 1.0±0.5 and 1.0±0.3, respectively. The EIE as estimated by the FFQ and the TEE as calculated by the Harris-Benedict equation were almost same value. Furthermore, the EIE/BW and protein intake in this study were similar to the ideal values established in the European Society for Parenteral and Enteral Nutrition (ESPEN) guidelines and in the ISHEN recommendations (29, 30).
The ideal AI for the fat energy ratio is reported to be 20-25% (21, 22). In the present study, regardless of the presence of an abnormal DST, the fat energy ratio (men: 28.6±5.2%; women: 30.2±4.5%) exceeded that of the ideal AI (21, 22). Consistent with our results, Tajika et al. showed that the rate of fat oxidation in cirrhotic patients (47.8%), as determined by an indirect calorimeter, was higher than that in the control group (26.6%) (24). In addition, the lipid uptake rate of NASH patients estimated using a 4-day diet diary was reported to be 35% of the total energy calories (38). The findings of these reports are similar to our results, indicating the accuracy of the FFQ results in our study.
In the present study, the intake of dietary fiber by the enrolled patients was lower than the AI. Dietary fiber reduces gut digestion, passage time, and ammonia absorption. A previous study reported a close relationship between gut flora and the ingestion of dietary fiber, with a reduced fiber consumption resulting in gut flora changes (46-48). Nutrient ratios and dietary fiber ingestion should be further investigated as a means of preventing cognitive problems, including DST abnormalities and even HE.
There were no marked nutritional differences between the DST-Abn and DST-Nor groups in our study. In contrast, a recent report, by Zhu et al. showed that the total protein intake, total unsaturated fatty acid intake, and total carbohydrate intake were associated with changes in the mean total latency and number of errors in a symbol digit substitution test (49). The small sample size of our study may have affected the statistical analyses.
This study was associated with several limitations. First, the study was retrospective in nature and included a relatively small number of patients who were managed in a single center. Although the multivariate analysis showed the BTR to be an independent marker for detecting a DST abnormality, this method is of limited value in a retrospective, small-scale study. These methodological drawbacks may reduce the strength of our statistical conclusions. Second, we did not directly compare the FFQ and the measurement results, including the oxidation rates of carbohydrate, fat, and protein, respiratory quotient and resting energy expenditure, as determined by an indirect calorimeter. Third, liver cancer may affect the nutritional status. Nevertheless, LC patients with liver cancer and a good performance status are often encountered in actual clinical practice and participated in the current study. Thus, a large-scale, prospective study is required to confirm our findings.
In conclusion, we found that the pathophysiology of a DST abnormality is closely related to decreases in the BTR level. The BTR is the most useful predictor of a DST abnormality in LC patients. The NPA risk may be estimated by monitoring the BTR.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We acknowledge the members of the University Hospital Nutrition Support Team for their advice and assistance.
References
- 1.Stewart CA, Malinchoc M, Kim WR, Kamath PS. Hepatic encephalopathy as a predictor of survival in patients with end-stage liver disease. Liver Transpl 13: 1366-1371, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bustamante J, Rimola A, Ventura PJ, et al. . Prognostic significance of hepatic encephalopathy in patients with cirrhosis. J Hepatol 30: 890-895, 1999. [DOI] [PubMed] [Google Scholar]
- 3.Garg H, Kumar A, Garg V, Sharma P, Sharma BC, Sarin SK. Clinical profile and predictors of mortality in patients of acute-on-chronic liver failure. Dig Liver Dis 44: 166-171, 2012. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Gomez M, Boza F, Garcia-Valdecasas MS, Garcia E, Aguilar-Reina J. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol 96: 2718-2723, 2001. [DOI] [PubMed] [Google Scholar]
- 5.Kato A, Tanaka H, Kawaguchi T, et al. . Nutritional management contributes to improvement in minimal hepatic encephalopathy and quality of life in patients with liver cirrhosis: a preliminary, prospective, open-label study. Hepatol Res 43: 452-458, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj JS, Pinkerton SD, Sanyal AJ, Heuman DM. Diagnosis and treatment of minimal hepatic encephalopathy to prevent motor vehicle accidents: a cost-effectiveness analysis. Hepatology 55: 1164-1171, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhiman RK, Saraswat VA, Sharma BK, et al. . Minimal hepatic encephalopathy: Consensus statement of a working party of the indian national association for study of the liver. J Gastroenterol Hepatol 25: 1029-1041, 2010. [DOI] [PubMed] [Google Scholar]
- 8.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th world congresses of gastroenterology, vienna, 1998. Hepatology 35: 716-721, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Randolph C, Hilsabeck R, Kato A, et al. . Neuropsychological assessment of hepatic encephalopathy: ISHEN practice guidelines. Liver Int 29: 629-635, 2009. [DOI] [PubMed] [Google Scholar]
- 10.Kato A, Kato M, Ishii H, et al. . Development of quantitative neuropsychological tests for diagnosis of subclinical hepatic encephalopathy in liver cirrhosis patients and establishment of diagnostic criteria-multicenter collaborative study in japanese. Hepatol Res 30: 71-78, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology 45: 549-559, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Michitaka K, Tokumoto Y, Uesugi K, et al. . Neuropsychiatric dysfunction in patients with chronic hepatitis and liver cirrhosis. Hepatol Res 38: 1069-1075, 2008. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj JS, Saeian K, Verber MD, et al. . Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol 102: 754-760, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Maric D, Klasnja B, Filipovic D, Brkic S, Ruzic M, Bugarski V. Minimal hepatic encephalopathy in patients with decompensated liver cirrhosis. Acta Clin Croat 50: 375-380, 2011. [PubMed] [Google Scholar]
- 15.Li SW, Wang K, Yu YQ, Wang HB, Li YH, Xu JM. Psychometric hepatic encephalopathy score for diagnosis of minimal hepatic encephalopathy in China. World J Gastroenterol 19: 8745-8751, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34: 768-773, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan society of hepatology guidelines for sarcopenia in liver disease (1st edition): Recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res 46: 951-963, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Kawaguchi T, Konishi M, Kato A, et al. . Updating the neuropsychological test system in japan for the elderly and in a modern touch screen tablet society by resetting the cut-off values. Hepatol Res 47: 1335-1339, 2017. [DOI] [PubMed] [Google Scholar]
- 19.Long CL, Schaffel N, Geiger JW, Schiller WR, Blakemore WS. Metabolic response to injury and illness: Estimation of energy and protein needs from indirect calorimetry and nitrogen balance. JPEN J Parenter Enteral Nutr 3: 452-456, 1979. [DOI] [PubMed] [Google Scholar]
- 20.Barak N, Wall-Alonso E, Sitrin MD. Evaluation of stress factors and body weight adjustments currently used to estimate energy expenditure in hospitalized patients. JPEN J Parenter Enteral Nutr 26: 231-238, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Tsuboyama-Kasaoka N, Takizawa A, Tsubota-Utsugi M, et al. . Dietary intake of nutrients with adequate intake values in the dietary reference intakes for japanese. J Nutr Sci Vitaminol (Tokyo) 59: 584-595, 2013. [DOI] [PubMed] [Google Scholar]
- 22.Tsubota-Utsugi M, Imai E, Nakade M, Tsuboyama-Kasaoka N, Morita A, Tokudome S. Dietary reference intakes for japanese -2010-. [Google Scholar]
- 23.Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48: 452-458, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tajika M, Kato M, Mohri H, et al. . Prognostic value of energy metabolism in patients with viral liver cirrhosis. Nutrition 18: 229-234, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Tsiaousi ET, Hatzitolios AI, Trygonis SK, Savopoulos CG. Malnutrition in end stage liver disease: recommendations and nutritional support. J Gastroenterol Hepatol 23: 527-533, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht J, Norenberg MD. Glutamine: a trojan horse in ammonia neurotoxicity. Hepatology 44: 788-794, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Plauth M, Merli M, Kondrup J, et al. . ESPEN guidelines for nutrition in liver disease and transplantation. Clin Nutr 16: 43-55, 1997. [DOI] [PubMed] [Google Scholar]
- 28.Muller MJ, Bottcher J, Selberg O, et al. . Hypermetabolism in clinically stable patients with liver cirrhosis. Am J Clin Nutr 69: 1194-1201, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Plauth M, Cabre E, Riggio O, et al. . ESPEN guidelines on enteral nutrition: liver disease. Clin Nutr 25: 285-294, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Amodio P, Bemeur C, Butterworth R, et al. . The nutritional management of hepatic encephalopathy in patients with cirrhosis: International society for hepatic encephalopathy and nitrogen metabolism consensus. Hepatology 58: 325-336, 2013. [DOI] [PubMed] [Google Scholar]
- 31.Hirano H, Saito M, Yano Y, et al. . Chronic liver disease questionnaire would be a primary screening tool of neuropsychiatric test detecting minimal hepatic encephalopathy of cirrhotic patients. Hepatol Res 45: 994-1003, 2015. [DOI] [PubMed] [Google Scholar]
- 32.Hanai T, Shiraki M, Watanabe S, et al. . Sarcopenia predicts minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Res 47: 1359-1367, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Kinny-Koster B, Bartels M, Becker S, et al. . Plasma amino acid concentrations predict mortality in patients with end-stage liver disease. PLoS One 11: e0159205, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holecek M. Ammonia and amino acid profiles in liver cirrhosis: effects of variables leading to hepatic encephalopathy. Nutrition 31: 14-20, 2015. [DOI] [PubMed] [Google Scholar]
- 35.Kato A, Suzuki K. How to select BCAA preparations. Hepatol Res 30S: 30-35, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Manoli I, Venditti CP. Disorders of branched chain amino acid metabolism. Transl Sci Rare Dis 1: 91-110, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akahoshi M, Ichikawa T, Taura N, et al. . Sleep disturbances and quality of life in patients after living donor liver transplantation. Transplant Proc 46: 3515-3522, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Bredin C, Naimimohasses S, Norris S, et al. . Development and relative validation of a short food frequency questionnaire for assessing dietary intakes of non-alcoholic fatty liver disease patients. Eur J Nutr 59: 571-580, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cade JE, Burley VJ, Warm DL, Thompson RL, Margetts BM. Food frequency questionnaires: a review of their design, validation and utilisation. Nutr Res Rev 17: 5-22, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Denissen KFM, Boonen A, Nielen JTH, et al. . Consumption of dairy products in relation to the presence of clinical knee osteoarthritis: the maastricht study. Eur J Nutr 58: 2693-2704, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eng JY, Moy FM. Validation of a food frequency questionnaire to assess dietary cholesterol, total fat and different types of fat intakes among malay adults. Asia Pac J Clin Nutr 20: 639-645, 2011. [PubMed] [Google Scholar]
- 42.Kanehara R, Goto A, Kotemori A, et al. . Validity and reproducibility of a self-administered food frequency questionnaire for the assessment of sugar intake in middle-aged Japanese adults. Nutrients 11: 554, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marshall SJ, Livingstone KM, Celis-Morales C, et al. . Reproducibility of the online Food4Me food-frequency questionnaire for estimating dietary intakes across europe. J Nutr 146: 1068-1075, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shim JS, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health 36: 1-8, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perumpail BJ, Li AA, Cholankeril G, Kumari R, Ahmed A. Optimizing the nutritional support of adult patients in the setting of cirrhosis. Nutrients 9: 1114, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shawcross DL. Is it time to target gut dysbiosis and immune dysfunction in the therapy of hepatic encephalopathy? Expert Rev Gastroenterol Hepatol 9: 539-542, 2015. [DOI] [PubMed] [Google Scholar]
- 47.Malaguarnera G, Giordano M, Nunnari G, Bertino G, Malaguarnera M. Gut microbiota in alcoholic liver disease: pathogenetic role and therapeutic perspectives. World J Gastroenterol 20: 16639-16648, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 39: 1441-1449, 2004. [DOI] [PubMed] [Google Scholar]
- 49.Zhu S, Zhao J, Chen Z, Wang Y. Influential factors on cognitive performance in middle-aged cohort: Third national health and nutrition examination survey-based study. Medicine (Baltimore) 97: e12033, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]