Abstract
Background
Stricture is a common presentation of Crohn’s disease with the site of prevalence being the distal ileum. This study aimed to compare the efficacy and safety of patients with primary distal ileum stricture treated with endoscopic stricturotomy (ESt) vs ileo-colonic resection (ICR).
Methods
All consecutive patients with primary distal ileum stricture that were treated with ESt and/or ICR were extracted from the interventional inflammatory bowel disease (i-IBD) unit from 2001 to 2016. All patients with a stricture >5 cm or those with anastomotic strictures were excluded from the study. The primary outcomes were surgery-free survival and post-procedural complications.
Results
A total of 13 patients receiving ESt and 32 patients receiving ICR were included in this study. Although the length of the stricture is comparable between the two groups (2.4 ± 0.9 vs 3.0 ± 1.1 cm, P = 0.17), patients who received surgery had a more complicated obstruction presented by the high pre-stenosis proximal dilation rate (67.7% vs 9.1%, P = 0.001). All patients in both groups achieved immediate technical success after treatment. The median follow-up durations were 1.8 and 1.5 years in the ESt and ICR groups, respectively. The subsequent surgery rates were similar between the two groups (15.4% vs 18.8%, P = 0.79) and the overall surgery-free survival was also comparable between the two groups (P = 0.98). Post-procedural adverse events were seen in 2/29 ESt procedures (6.9% per procedure) and 8/32 (25.0%) patients receiving ICR (P = 0.05).
Conclusions
ESt achieved comparable stricture-related surgery-free survival as ICR, while ESt had a numerically lower post-operative complication rate.
Keywords: Crohn’s disease, stricture, endoscopy, stricturotomy, resection
Introduction
One-third of Crohn’s disease (CD) patients would develop a stricture within 10 years of diagnosis [1–4]. The majority of CD patients present with stricture located at the terminal ileum or ileocecal valve; and the most commonly performed surgical treatment is ileo-colonic resection (ICR) with an ileo-colonic anastomosis. Unlike in inflammatory strictures associated with underlying primary CD, medical therapy has a limited role in the treatment of fibrostenotic anastomotic strictures and ‘mechanical’ approaches are often required, such as endoscopic balloon dilation (EBD) and further surgical resection [3, 5]. However, the reported rate of post-operative complications ranged from 8.8% to 31% and the rate of recurrent anastomotic stricture ranged from 30% to 80% [2, 3]. EBD has emerged as a safe and effective treatment option for CD strictures, particularly anastomotic strictures [6, 7]. However, our group recently reported that the surgery appeared to the more durable option for treating primary CD strictures than EBD [8].
Endoscopic stricturotomy (ESt) has been routinely used in the treatment of strictures in the biliary tract [9, 10] and occasionally in the esophagus [11]. When treating secondary (i.e. anastomotic) strictures, our group described a subsequent surgery rate of 11.3% in patients receiving ESt and 10.0% in patients receiving ICR [12]. However, outcomes of ESt and ICR in the treatment of primary (i.e. disease-associated) distal ileum strictures have not been directly compared. The current study is the natural extension of our previous investigations. The aim of this study was to compare the efficacy and safety of ESt and ICR in the treatment of primary distal ileum strictures in CD patients.
Patients and methods
Data sources
All consecutive eligible patients with CD stricture located at the distal ileum were identified from our i-IBD database or CD surgery database from 2001 to 2016. Demographic, clinical, endoscopic, and imaging features together with the management and outcomes were carefully reviewed. This study was approved by the Cleveland Clinic Institutional Review Board. Written informed consent was obtained from patients prior to the endoscopic or surgical procedures.
Inclusion and exclusion criteria
The inclusion criteria were patients with: (i) a primary diagnosis of CD that developed disease-associated, primary strictures; (ii) strictures located at the distal ileum within 15 cm from the ileocecal valve and/or at the ileocecal valve; (iii) strictures with a length ≤5 cm; and (iv) the strictures treated with primary ESt or surgical ICR.
The exclusion criteria were patients with: (i) concurrent penetrating CD; (ii) secondary or anastomotic strictures; (iii) treated strictures beyond 15 cm proximally from the ileocecal valve; (iv) surgical history of strictureplasty or bowel resection in the area due to CD; or (v) follow-up time shorter than 3 months.
Data collection
Patients were classified into two groups, based on to the inception procedure, which was either ESt or ICR, whichever came first. Demographic information, such as age, gender, ethnicity, height, weight, and body mass index, were extracted from the database. Clinical histories were also extracted from the data along with previous surgical history and medication history. The information on current smoker and ex-smoker was obtained from medical charts at the time of procedures. Family histories recorded were those of the patient’s first-degree relatives.
Patients included in this study were those classified as L1 or L3 B2 according to the Montreal Classification [13]. Pre-procedural use of medications was defined as medications used within the last 3 months preceding the inception procedure and were categorized into 5-aminosalicylates (ASA) or mesalamine, antibiotics, corticosteroids, immunomodulators, and biologics. An escalation of medications to the use of immunomodulators/biologics was also examined [14].
The diagnosis of stricture was obtained by either endoscopy and/or abdominal imaging, regardless of the presence or absence of stricture-related clinical symptoms. Multiple strictures were defined as additional stricture(s) within 15 cm of the treated stricture. The degree of the strictures was classified as traversable and non-traversable. The length of the stricture was measured based on endoscopy reports. Due to the incomparable difference between patients receiving ESt or ICR, we included only patients with a stricture length ≤5 cm measured under endoscopy. The radiographic presentation was evaluated according to records within 30 days prior to the procedure.
Indications and techniques of ESt and ICR
The decision for whether to perform ESt or ICR for primary strictures was at the discretion of the treating IBD interventionalist (B.S.) and/or colorectal surgeons, based on a combined assessment of clinical, endoscopic, and imaging presentations.
All ESt procedures were performed by an experienced endoscopist (B.S.). The stricture was treated with either a Boston Scientific triple-lumen needle-knife (Boston Scientific, Marlborough, MA) or later on an Olympus single-use electrosurgical IT knife 2 (Olympus Medical Systems, Tokyo, Japan) under the setting of an ERCP Endocut (ERBE USA Incorporated Surgical Systems, Marietta, GA). Strictures were incised in a circumferential or radial manner until the adequate passage of the scope was achieved [15]. The decision on endoscopic therapy (ESt vs EBD) or surgical referral was at the discretion of the IBD interventionalist. ESt was considered as being appropriate if the distal ileum stricture was: (i) shorter than 5 cm; (ii) fibrotic; (iii) free of current fistulas or abscesses; and (iv) free of current anticoagulation.
Surgical resection of distal ileum stricture was generally performed using an open or laparoscopic surgical approach. The diseased segment was identified after lysis of the adhesion, after which the bowel segment containing the stricture was resected. The type of anastomosis performed (stapled vs hand-sewn), its configuration (end-to-end, end-to-side, side-to-side), and possible added diverting loop ileostomy was left at the discretion of the operating surgeon.
Outcome measurements
The primary outcome was surgery-free survival. The term ‘salvage surgery’ refers to surgery after ESt and the term ‘secondary surgery’ refers to surgery after ICR. The salvage/secondary surgery only indicated stricture-related bowel resection. Follow-up time was defined as the time from the inception procedure to the latest Gastroenterology clinical/telephone follow-up or stricture-related surgery, whichever came first. Surgery due to post-ESt or post-ICR adverse events was also included as salvage/secondary surgery.
The secondary outcomes were an immediate technical success (defined as the passage of endoscope without resistance), post-procedural complications, and symptomatic improvements. Post-procedural complications were classified using Clavien-Dindo’s classification [16]. Post-ESt complications were collected from emergency department (ED) visits or hospitalization notes immediately after the procedure. Post-operative complications were collected within 30 days after the inception surgery. Disease-related ED visits and hospitalizations were also recorded.
Statistical analysis
Descriptive statistics were computed for all variables. Categorical variables were summarized as percentages. Quantitative variables with a normal distribution were summarized as mean ± standard deviation. Quantitative variables with paranormal distribution were summarized in median and interquartile range (IQR). Tests for the association between the groups and categorical variables were performed using the chi-square method and Fisher’s exact test. For quantitative variables, the means were compared by Student’s t-tests or Wilcoxon rank-sum tests. Surgery-free survival was constructed using the Kaplan–Meier curve. A Cox proportional-hazard model was used if appropriate, and the results were concluded using hazard ratios and 95% confidence intervals. A P-value <0.05 was considered as being statistically significant. All analyses were performed using SPSS software version 20.0 (SPSS, Chicago, IL).
Results
A total of 45 patients met the inclusion criteria, for whom ESt was performed in 13 patients and ICR in 32 patients. No patient was diagnosed with intestinal malignancy while being treated for primary distal ileum stricture or during follow-up.
Demographic and clinical features of underlying CD
Patients in both groups presented with a similar demographic background (Table 1). Perianal disease was observed in four (30.8%) patients and seven (21.9%) patients in the ESt and ICR groups, respectively (P = 0.53). However, no patients had concurrent fistula, sinus, or abscess at the ileo-colonic anastomosis site at the time of the inception procedure. The use of mesalamine (46.2% vs 81.2%, P = 0.02) and corticosteroid (30.8% vs 68.8%, P = 0.02) was more prevalent in patients receiving ICR.
Table 1.
Demographic and clinical characteristics in Crohn’s-disease patients with primary strictures receiving endoscopic stricturotomy (ESt) and ileo-colonic resection (ICR)
| Characteristic | ESt |
ICR |
P-value | ||
|---|---|---|---|---|---|
| N | Statistics | N | Statistics | ||
| Female gender | 13 | 9 (69.2) | 32 | 22 (68.8) | 0.97 |
| Caucasian | 13 | 13 (100) | 32 | 31 (96.9) | 0.52 |
| Smoking | 0.34 | ||||
| Current | 13 | 2 (15.4) | 32 | 7 (21.9) | |
| Ex | 13 | 4 (30.8) | 32 | 4 (12.5) | |
| Family history | |||||
| Inflammatory bowel disease | 13 | 1 (7.7) | 32 | 3 (9.4) | 0.86 |
| Colon cancer | 13 | 4 (30.8) | 32 | 2 (6.2) | 0.03 |
| Weight, kg | 13 | 68.0 ± 25.9 | 29 | 64.9 ± 15.1 | 0.12 |
| Body mass index, kg/m2 | 13 | 22.7 ± 7.2 | 29 | 23.2 ± 4.7 | 0.11 |
| Age at Crohn’s diagnosis, year | 13 | 29.1 ± 9.9 | 32 | 25.6 ± 16.2 | 0.86 |
| Extra-intestinal manifestations | 13 | 4 (30.8) | 32 | 7 (21.9) | 0.53 |
| History of perianal involvement | 13 | 4 (30.8) | 32 | 7 (21.9) | 0.53 |
| Medication | |||||
| 5-Aminosalicylic acid | 13 | 6 (46.2) | 32 | 26 (81.2) | 0.02 |
| Corticosteroids | 13 | 4 (30.8) | 32 | 22 (68.8) | 0.02 |
| Immunomodulator | 13 | 5 (38.5) | 32 | 17 (53.1) | 0.37 |
| Biologics | 13 | 5 (38.5) | 32 | 12 (37.5) | 0.95 |
| Symptoms | 11 | 9 (81.8) | 30 | 30 (100) | 0.91 |
| Diarrhea/urgency | 11 | 6 (54.5) | 30 | 20 (66.7) | 0.48 |
| Constipation | 11 | 6 (54.5) | 30 | 3 (10.0) | 0.002 |
| Abdominal pain | 11 | 6 (54.5) | 30 | 24 (80.0) | 0.10 |
| Bloating | 11 | 1 (9.1) | 30 | 3 (10.0) | 0.93 |
| Nausea/vomiting | 11 | 5 (45.5) | 30 | 12 (40.0) | 0.75 |
| Bleeding | 11 | 1 (9.1) | 30 | 7 (23.3) | 0.31 |
| Radiological presentation | |||||
| Pre-stenosis proximal dilation | 11 | 1 (9.1) | 31 | 21 (67.7) | 0.001 |
| Bowel wall thickness | 11 | 10 (90.9) | 32 | 32 (100) | 0.26 |
| Mucosal enhancement | 11 | 8 (72.7) | 26 | 23 (88.5) | 0.24 |
| Age at stricture diagnosis, year | 13 | 37.9 ± 8.7 | 32 | 33.5 ± 15.3 | 0.94 |
| Duration from Crohn’s diagnosis to stricture diagnosis, year | 13 | 5.1 (0.8–14.9) | 32 | 8.1 (2.8–12.4) | 0.57 |
| Age at treatment, year | 13 | 40.3 ± 9.8 | 32 | 33.7 ± 15.2 | 0.73 |
| Duration from stricture diagnosis to treatment, year | 13 | 0.5 (0–5.5) | 32 | 0.2 (0.1–0.6) | 0.90 |
| Length of stricture, cm | 9 | 2.4 ± 0.9 | 32 | 3.0 ± 1.1 | 0.17 |
| Ulceration | 13 | 3 (23.1) | 23 | 9 (39.1) | 0.33 |
| Multiple strictures | 13 | 6 (46.2) | 32 | 9 (28.1) | 0.25 |
| Degree of stricture | 0.26 | ||||
| Mild resistance to scope | 13 | 0 (0) | 15 | 1 (6.7) | |
| Moderate resistance to scope | 13 | 0 (0) | 15 | 1 (6.7 ) | |
| Severe resistance to scope | 13 | 0 (0) | 15 | 2 (13.3) | |
| Not transversable to scope | 13 | 13 (100) | 15 | 11 (73.3) | |
Values are presented as mean ± standard deviation, median (interquartile range), or n (%).
Characteristics of the strictures
The mean ages of diagnosis were 37.9 ± 8.7 years for the ESt group and 33.5 ± 15.3 years for the ICR group (P = 0.94). The characteristics of strictures were comparable between the two groups (Table 1). Symptomatic strictures were reported in nine (81.8%) patients receiving ESt and all patients (100%) receiving ICR (P = 0.91). The average length of the stricture was also similar in patients receiving ESt (2.4 ± 0.9 cm) vs those receiving ICR (3.0 ± 1.1 cm) (P = 0.17). Most of the patients treated had a tight stricture not traversable to endoscope in either group (100% vs 73.3%, P = 0.26). After reviewing the patients with available imaging reports, pre-stenotic proximal dilations were more prevalent in patients receiving ICR than those with ESt (67.7% vs 9.1%, P = 0.001). Multiple strictures were seen in six (46.2%) patients in the ESt group and nine (28.1%) patients in the ICR group (P = 0.25).
Outcomes of ESt treatment
A total of 29 ESt were carried out in the 13 patients of the ESt group with a median of 1.0 (IQR: 1.0–3.0) procedure per patient. Immediate technical success was noted in all 13 (100%) patients and symptomatic improvement was documented in 3/6 (50.0%) patients (Table 2). The majority of patients tolerated the procedure well and post-procedural adverse events were seen in 2/29 (6.9% per procedure) occasions (Table 3). Perforation occurred in one patient, who underwent an emergency exploratory laparotomy with ICR and creation of neoanastomosis. The other patient was hospitalized for abdominal pain, nausea, and fever. The patient had a repeat colonoscopy and an abdominal X-ray, which both showed no sign of perforation at the treated site and five clips were deployed empirically for precautionary purposes.
Table 2.
Outcome of patients undergoing endoscopic stricturotomy (ESt) and ileo-colonic resection (ICR)
| Outcome | ESt (n = 13) | ICR (n = 32) | P-value |
|---|---|---|---|
| Follow-up, year, median (IQR) | 1.8 (1.1–2.4) | 1.5 (0.8–4.1) | 0.84 |
| Post-procedural medication, n (%) | |||
| 5-Aminosalicylic acid | 5 (38.5) | 9 (28.1) | 0.50 |
| Corticosteroids | 0 (0) | 15 (46.9) | 0.002 |
| Immunomodulators | 5 (38.5) | 11 (34.4) | 0.80 |
| Biologics | 6 (46.2) | 6 (18.8) | 0.06 |
| Escalation of medication, n (%) | 3 (23.1) | 6 (18.8) | 0.74 |
| Immediate technical success, n (%) | 13 (100) | 32 (100) | – |
| Healing of stricture on scope, n (%) | 6 (46.2) | 32 (100) | <0.001 |
| Symptom improvement, n (%) | 3/6 (50.0) | 27/30 (90.0) | 0.07 |
| Subsequent surgery, n (%) | 2 (15.4) | 6 (18.8) | 0.79 |
| Stricture-related hospitalizations, n (%) | 5 (38.5) | 5 (15.6) | 0.09 |
| Stricture-related emergency department visits, n (%) | 0 (0) | 3 (9.4) | 0.25 |
Table 3.
Post-procedural complications of patients undergoing endoscopic stricturotomy (ESt) and ileo-colonic resection (ICR)
| Complication | ESt (n = 29)* | ICR (n = 32) | P-value |
|---|---|---|---|
| Complication | |||
| Ileus | 0 (0) | 1 (3.1) | 1.00 |
| Anatomists leak/ perforation | 2 (6.9)** | 2 (6.2) | 0.57 |
| Abscess | 0 (0) | 1 (3.1) | 1.00 |
| Surgical site infection | 0 (0) | 2 (6.2) | 1.00 |
| Urinary tract infection | 0 (0) | 2 (6.2) | 1.00 |
| Total | 2 (6.9) | 8 (25.0) | 0.05 |
| Clavien-Dindo classification | 0.061 | ||
| 1 | 0 (0) | 1 (3.1) | |
| 2 | 1 (3.5) | 3 (9.4) | |
| 3 | 1(3.5) | 4 (12.5) |
A total of 29 ESt procedures were carried out with the 13 patients of the ESt group.
One case confirmed perforation, one case unconfirmed with perforation symptoms.
Outcomes of the ICR treatment
The overall morbidity rate in patients undergoing ICR was 25.0% (Table 3). There were four patients requiring diverting ileostomy, two of whom underwent stoma reversal after a mean of 3.6 months. The remaining two patients had a subsequent surgery with ICR with neoanastomosis. During a median follow-up of 1.5 (0.8–4.1) years, symptom improvement was documented in 27 (90.0%) patients (Table 2). Recurrence of stricture at the neoanastomosis site was seen in six (18.8%) patients. All six patients required another surgical resection.
Comparison of outcomes of ESt vs ICR
Follow-up time was comparable in the patients receiving ESt and ICR (P = 0.84). In comparison to patients receiving index ESt, patients undergoing upfront ICR seemed to have greater symptom improvement (90.0% vs 50.0%, P = 0.07) and a lower CD-medication escalation rate (18.8% vs 23.1%, P = 0.74) (Table 2). However, patients undergoing ICR were found to have more post-procedural complications than those in the ESt group (25.0% vs 6.9% per procedure, P = 0.05) (Table 3). The frequency of the subsequent stricture-related surgery was comparable between the two procedures (18.8% vs 15.4%, P = 0.79). Kaplan–Meier survival curves showed that the surgery-free survivals were comparable between the two groups (P = 0.98; Figure 1). A Cox univariable analysis was conducted to evaluate factors associated with surgery-free survival. Due to the small sample size and the fact only that eight patients in the whole cohort underwent subsequent surgery, no factor was found to be significant in univariable analysis.
Figure 1.
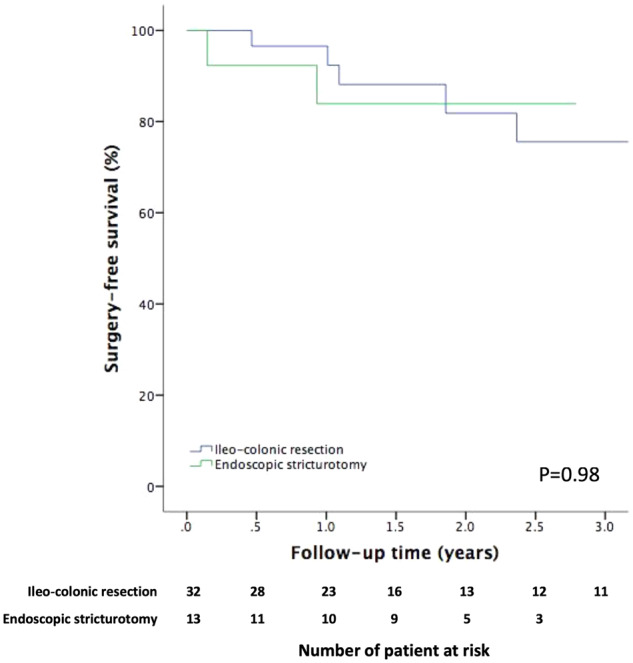
Kaplan–Meier curve of surgery-free survival in patients receiving ileo-colonic resection and endoscopic stricturotomy.
Discussion
In this historical cohort, we evaluated 43 CD patients with primary distal ileum strictures treated with ESt or ICR. The majority of the strictures were not traversable with an endoscope. Symptom improvement was higher in patients with ICR than those with ESt, and the rate of medication escalation was higher in patients with ESt. However, the salvage/secondary surgery rate and surgery-free survival were comparable between the two groups. Post-operative complication rates were numerically higher in patients undergoing ICR than that treated with ESt.
Our center and the Global Interventional Inflammatory Bowel Disease Group have previously described the classification of IBD-associated strictures, which included the primary (de novo) and secondary (anastomotic) strictures [17–19]. Previous data published indicate that ICR is a more durable option in the treatment of primary distal ileum stricture in CD patients [8]. The surgery-free survival in patients receiving ICR was significantly longer in patients receiving EBD even after subgroup analysis of strictures of length ≤5 cm. Regardless, the complication rate remained higher in patients with ICR in subgroup analysis. There were patients undergoing EBD who could successfully continue avoiding surgery during long-term follow-up. However, surgery with resection had been the mainstay of treatment, due to the nature of the primary stricture in CD.
Endoscopic stricturotomy has been used in the treatment of various strictures ranging from the biliary system to upper and lower Gastroenterology tract [20–22]. The use of ESt in the treatment of long fibrotic strictures of the ileal pouch refractory to multiple EBD therapy was previously published from our center [23]. Subsequently, we reported three cases of ESt in the treatment of nipple-valve stenosis in the continent ileostomy [24], outlet stricture of diverted colostomy [25], and ileo-rectal anastomotic stricture [26]. A case series previously published had shown ESt to be effective in treating patients with primary and secondary strictures in patients with underlying IBD [27]. ESt had also proven its potential in treating CD anastomotic strictures in comparison to ICR and EBD [12, 15]. ESt is technically more demanding than EBD, as the former requires complete stabilization of the tip of the knife and endoscope, and placement of the treatment-targeted area at the center of the screen. On the other hand, post-procedural bleeding had also been a challenging issue for patients undergoing ESt. Nevertheless, the endoscopic procedures are preferred as the first-line treatment modalities in patients with anastomotic strictures, while ICR had been a more durable option in patients with primary strictures. ESt in the treatment of primary strictures had not been independently investigated, especially in comparison with ICR.
This study is a natural extension of our previous studies, focusing on the treatment of primary distal ileum stricture in CD patients treated with ESt vs ICR. The subsequent surgery rate in CD patients undergoing ICR for primary strictures (≤5 cm) was comparable with the results from our previous study (15.0% vs 15.4%) [8]. However, in that study, the complication rate was higher, probably due to the small number of patients involved after subgroup analysis (35% vs 25%). As for patients treated with ESt, the subsequent surgery rate in patients with primary stricture was higher than patients with anastomotic strictures (18% vs 11%). The complication rates were comparable between primary and anatomic strictures treated by ESt [12]. Regarding primary strictures, when compared to EBD, the subsequent surgery rate was much lower in patients treated with ESt (18.8% vs 45.3%) during a similar median follow-up time of around 2 years. However, the complication rate in the ESt group was higher than that published for EBD (6.9% vs 4.7%). Overall, the efficacy of ESt was more sustainable than EBD and was comparable with patients receiving ICR for short primary CD strictures. A longer follow-up with a larger patient base is needed to conclude the algorism in the treatment of primary CD strictures.
The findings of this study have clinical implications. The results showed that ESt appeared to be effective in treating short (≤5 cm) primary distal ileum strictures in CD and had a comparable surgery-free survival to the ICR approach. Patients receiving ESt were shown to have a lower overall complication rate, making it a possible first-line treatment for CD patients with short primary strictures in experienced hands. The procedure is not considered to be being as technically challenging as endoscopic papillotomy. In the authors’ opinion, most endoscopists with a background of diagnosis and medical management of IBD and most IBD specialists with some training in advanced endoscopy can reach competence for ESt after performing 15–20 procedures. Full control of the motion of the tip of the endoscope and an approach with precision are the key requirements regarding the technical expertise of the endoscopist. In contrast to patients with secondary strictures, patients with a primary stricture have a higher rate of perforation, and no patients had presented with post-procedural bleeding in the present study. Nevertheless, the endoscopist should always anticipate perforation or bleeding and always be ready to utilize endoscopic clipping or surgical intervention. Surgical resection may be reserved for patients with a stricture not amendable to ESt or those who fail the endoscopic treatment. As for patients with a stricture longer than 5 cm, it would be difficult to assess which procedure is superior, since we do not routinely treat those strictures endoscopically. In our practice, ESt is not preferred in patients with long, angulated strictures.
There are limitations to this study. Referral and selection bias were inevitable as our hospital is a tertiary center and our i-IBD Unit and department of colorectal surgery are subspecialized in managing complex cases with a skilled endoscopist, surgeons, and supporting personnel. This study was also a historical, non-randomized cohort study—the decision to treat the patient with ESt vs ICR was at the discretion of treating endoscopists or surgeons. Stricture characteristics measured by imaging may not be accurate [28] and therefore the choice of procedure may not reflect the severity of the stricture. The performance of ESt requires the expertise of the endoscopist, and it is important that the endoscopist is fully trained in ESt and other skills in interventional IBD in order to achieve the best results. Lastly, ESt is a novel procedure. Thus, the follow-up time was short, the sample size was small, and the technique remains to be optimized. A further large, randomized–controlled trial with a longer follow-up is needed for relatively unbiased results.
In conclusion, ESt appeared to be as effective in treating short primary distal ileum strictures as ICR. ESt may be considered as the first-line treatment in experienced hands, although multiple treatments are often required and there is a small risk of perforation. ESt may be able to delay or avoid surgical resection for a short non-traversable stricture. However, ICR should be considered if the patient is not responsive to ESt. The treatment strategy should be individualized.
Authors’ contributions
Study design: N.L. and B.S. Data acquisition and analysis: N.L. Manuscript drafting: N.L. and B.S. Critical review of the manuscript: T.H. and B.S.
Acknowledgements
Dr Bo Shen is supported by the Ed and Joey Story Endowed Chair. This study was presented as a poster at the Digestive Disease Week, May 2019, San Diego, CA, USA.
Conflicts of interest
The authors declared no conflict of interest.
References
- 1. Cosnes J, Cattan S, Blain A. et al. Long-term evolution of disease behavior of Crohn's disease. Inflamm Bowel Dis 2002;8:244–50. [DOI] [PubMed] [Google Scholar]
- 2. Li Y, Stocchi L, Shen B. et al. Salvage surgery after failure of endoscopic balloon dilatation versus surgery first for ileocolonic anastomotic stricture due to recurrent Crohn's disease. Br J Surg 2015;102:1418–25. [DOI] [PubMed] [Google Scholar]
- 3. Rutgeerts P, Geboes K, Vantrappen G. et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984;25:665–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bharadwaj S, Fleshner P, Shen B.. Therapeutic Armamentarium for stricturing Crohn's disease: medical versus endoscopic versus surgical approaches. Inflamm Bowel Dis 2015;21:2194–213. [DOI] [PubMed] [Google Scholar]
- 5. Van Assche G, Geboes K, Rutgeerts P.. Medical therapy for Crohn's disease strictures. Inflamm Bowel Dis 2004;10:55–60. [DOI] [PubMed] [Google Scholar]
- 6. Lian L, Stocchi L, Remzi FH. et al. Comparison of endoscopic dilation vs surgery for anastomotic stricture in patients with Crohn's disease following ileocolonic resection. Clin Gastroenterol Hepatol 2017;15:1226–31. [DOI] [PubMed] [Google Scholar]
- 7. Bharadwaj S, Narula N, Tandon P. et al. Role of endoscopy in inflammatory bowel disease. Gastroenterol Rep (Oxf) 2018;6:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lan N, Stocchi L, Ashburn JH. et al. Outcomes of endoscopic balloon dilation vs surgical resection for primary ileocolic strictures in patients with Crohn's disease. Clin Gastroenterol Hepatol 2018;16:1260–7. [DOI] [PubMed] [Google Scholar]
- 9. Katsinelos P, Mimidis K, Paroutoglou G. et al. Needle-knife papillotomy: a safe and effective technique in experienced hands. Hepatogastroenterology 2004;51:349–52. [PubMed] [Google Scholar]
- 10. Fukatsu H, Kawamoto H, Harada R. et al. Quantitative assessment of technical proficiency in performing needle-knife precut papillotomy. Surg Endosc 2009;23:2066–72. [DOI] [PubMed] [Google Scholar]
- 11. Hordijk ML, Siersema PD, Tilanus HW. et al. Electrocautery therapy for refractory anastomotic strictures of the esophagus. Gastrointest Endosc 2006;63:157–63. [DOI] [PubMed] [Google Scholar]
- 12. Lan N, Stocchi L, Delaney CP. et al. Endoscopic stricturotomy versus ileocolonic resection in the treatment of ileocolonic anastomotic strictures in Crohn's disease. Gastrointest Endosc 2019;90:259–68. [DOI] [PubMed] [Google Scholar]
- 13. Silverberg MS, Satsangi J, Ahmad T. et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005;19:5A–36A. [DOI] [PubMed] [Google Scholar]
- 14. Lian L, Stocchi L, Shen B. et al. Prediction of need for surgery after endoscopic balloon dilation of ileocolic anastomotic stricture in patients with Crohn's disease. Dis Colon Rectum 2015;58:423–30. [DOI] [PubMed] [Google Scholar]
- 15. Lan N, Shen B.. Endoscopic stricturotomy vs. balloon dilation in the treatment of anastomotic strictures in Crohn’s disease. Inflamm Bowel Dis 2018;24:897–907. [DOI] [PubMed] [Google Scholar]
- 16. Katayama H, Kurokawa Y, Nakamura K. et al. Extended Clavien-Dindo classification of surgical complications: Japan Clinical Oncology Group postoperative complications criteria. Surg Today 2016;46:668–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Paine E, Shen B.. Endoscopic therapy in inflammatory bowel diseases (with videos). Gastrointest Endosc 2013;78:819–35. [DOI] [PubMed] [Google Scholar]
- 18. Shen B, Kochhar G, Navaneethan U. et al. Role of interventional inflammatory bowel disease in the era of biological therapy: a position statement from the Global Interventional IBD Group. Gastrointest Endosc 2019;89:215–37. [DOI] [PubMed] [Google Scholar]
- 19. Chen M, Shen B.. Endoscopic therapy in Crohn's disease: principle, preparation, and technique. Inflamm Bowel Dis 2015;21:2222–40. [DOI] [PubMed] [Google Scholar]
- 20. Gao D, Hu B, Pan Y. et al. Feasibility of using wire-guided needle-knife electrocautery for refractory biliary and pancreatic strictures. Gastrointest Endosc 2013;77:752–8. [DOI] [PubMed] [Google Scholar]
- 21. Samanta J, Dhaka N, Sinha SK. et al. Endoscopic incisional therapy for benign esophageal strictures: technique and results. World J Gastrointest Endosc 2015;7:1318–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee JK, Van Dam J, Morton JM. et al. Endoscopy is accurate, safe, and effective in the assessment and management of complications following gastric bypass surgery. Am J Gastroenterol 2009;104:575–82. [DOI] [PubMed] [Google Scholar]
- 23. Shen B, Lian L, Kiran RP. et al. Efficacy and safety of endoscopic treatment of ileal pouch strictures. Inflamm Bowel Dis 2011;17:2527–35. [DOI] [PubMed] [Google Scholar]
- 24. Chen M, Shen B.. Endoscopic needle-knife stricturotomy for nipple valve stricture of continent ileostomy (with video). Gastrointest Endosc 2015;81:1287–8. [DOI] [PubMed] [Google Scholar]
- 25. Nyabanga CT, Veniero JC, Shen B.. Rendezvous computed tomography-assisted endoscopic needle-knife stricturotomy for sealed outlet of diverted large bowel. Endoscopy 2015;47:E625–6. [DOI] [PubMed] [Google Scholar]
- 26. Li Y, Shen B.. Doppler ultrasound-guided endoscopic needle knife treatment of an anastomotic stricture following subtotal colectomy. Endoscopy 2011;43:E343.. [DOI] [PubMed] [Google Scholar]
- 27. Lan N, Shen B.. Endoscopic stricturotomy with needle knife in the treatment of strictures from inflammatory bowel disease. Inflamm Bowel Dis 2017;23:502–13. [DOI] [PubMed] [Google Scholar]
- 28. Vogel J, Moreira A, Baker M. et al. CT enterography for Crohn's disease: accurate preoperative diagnostic imaging. Dis Colon Rectum 2007;50:1761–9. [DOI] [PubMed] [Google Scholar]


