Abstract
Esophageal adenocarcinoma (EAC) is a major cause of cancer-related death, particularly in Western populations, and is rapidly rising in Asian populations at this time. Virtually all EACs develop from the precursor lesion Barrett’s esophagus (BE), which is the most significant risk factor for EAC. However, the rates of progression from BE to EAC are low and patients with BE are asymptomatic. Thus, any strategy for EAC prevention must carry a low risk of harm in order to be clinically useful. Since current EAC-screening and BE-surveillance methods carry some procedural risk and are burdensome, there is an opportunity for chemoprevention, i.e. medications or dietary factors that may prevent BE from progressing to EAC. A variety of candidate chemoprevention therapies have been assessed to date. Proton-pump inhibitors (PPIs) are the best studied and have modest EAC-chemoprevention efficacy in BE patients, with a recent randomized trial showing that high-dose PPI may be more effective than low-dose PPI. Aspirin and other non-steroidal anti-inflammatory drugs have moderate quality observational and randomized-trial evidence for preventing progression of BE to EAC, but their risks for harm have precluded their routine clinical use. Other therapies (statins, metformin, female sex hormones) generally do not have strong evidence to support their use in EAC chemoprevention. Although progress has been made in this field, there is still a need for more effective and safe chemoprevention therapies for EAC.
Keywords: Barrett’s esophagus, esophageal adenocarcinoma, chemoprevention
Introduction
Barrett’s esophagus and esophageal adenocarcinoma
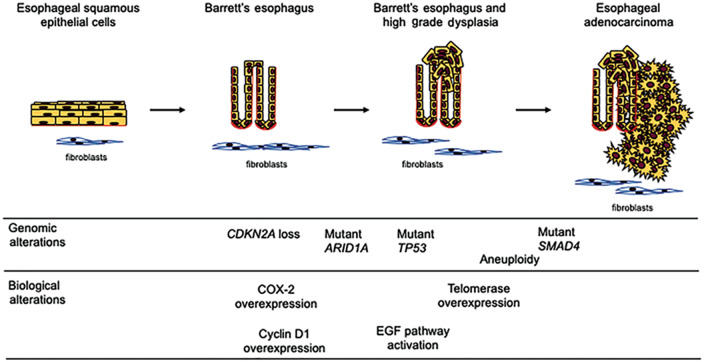
Esophageal adenocarcinoma (EAC) has shown a dramatic increase in incidence in the last 50 years in Western populations and is rising substantially in developing countries. It currently affects 18,000 people per year in the USA [1–3]. EAC is often asymptomatic until locally invasive, with 5-year case overall survival rates among the lowest of all cancers, despite recent improvements in surveillance and treatment [1, 4]. There is therefore significant interest in predicting and preventing the development of EAC in patients with Barrett’s esophagus (BE), the premalignant precursor of virtually all EACs (Figure 1).
Figure 1.
Schematic diagram of the Barrett’s esophagus-to-esophageal adenocarcinoma progression sequence. Normal esophageal epithelium gives rise to specialized intestinal metaplasia (Barrett’s esophagus), which can transform into dysplastic tissue and then cancer. Some of the common genetic and biologic alterations seen during this process are shown. HCl, hydrochloric acid; EGF, epidermal growth factor; COX-2, cyclooxygenase 2.
BE affects 1%–3% of people in Western societies and is found most commonly in individuals with certain risk factors [1]. Chronic gastroesophageal reflux disease (GERD) is the strongest single clinical risk factor for the development of BE and/or EAC [5]. Caucasian race, male gender, obesity, advanced age (>60 years), tobacco use, and alcohol use are other well-recognized risk factors [6]. Helicobacter pylori infection is associated with a reduced risk of BE [1, 6, 7].
Although BE is relatively common, progression of BE to EAC is low, with recent epidemiologic studies showing rates from 0.1% to 0.5% of non-dysplastic BE patients per year being diagnosed with EAC [8–10]. Importantly, because BE is asymptomatic, the vast majority of BE carriers are unaware of having BE, which has led to the advent of BE-screening programs as a way to identify patients at high risk of progression to EAC. Although there is controversy surrounding the clinical utility and cost-effectiveness of BE screening and surveillance, current society guidelines recommend endoscopic-based screening of individuals at high risk of BE and EAC and endoscopic surveillance of those people with BE [11–13]. The risk of progression of BE is higher in people with increasing degrees of dysplasia detected in esophageal biopsies of the BE tissue [4]. Therefore, current guidelines recommend that patients with dysplasia undergo more frequent surveillance exams and that individuals are offered endoscopic eradication therapy (radiofrequency ablation ± endoscopic mucosal resection) if high-grade dysplasia is found [11].
Although effective, this strategy carries the risk of procedural complications and has a high healthcare-cost burden because of the use of frequent endoscopic exams [14]. The relatively high prevalence of BE but low progression rate of BE to EAC coupled with the costs, semi-invasive nature, and inconvenience of endoscopy-based screening and surveillance methods has created a need both for non-invasive, inexpensive, accurate, and convenient screening technologies and for chemoprevention therapies. Because advanced EAC carries a poor prognosis, effective prevention of EAC is highly desirable, but any preventative measure must either carry minimal clinical risk to the general population or be applied only to selected high-risk populations in order to have an acceptable risk-to-benefit ratio.
Chemoprevention
The use of medications or supplements for the prevention and/or mitigation of risk of disease (i.e. chemoprevention) is well established across many fields of medicine. For example, aspirin is commonly used for either the primary or secondary prevention of the development of atherosclerotic coronary vascular disease and its complications [15]. Well established roles for cancer chemoprevention exist for the use of selective-estrogen receptor modulation in women at high risk of breast cancer or for the use of 5-alpha reductase inhibitors in men at high risk of prostate cancer [16, 17]. Chemoprevention also has a potential role in the prevention of certain gastrointestinal cancers;aspirin has shown a protective benefit against the development of colon cancer in populations at risk [18, 19].
Patients with BE frequently have symptomatic GERD and require treatment with acid-suppressive medications such as proton-pump inhibitors (PPIs). They are also frequently prescribed medications for other co-morbid conditions such as coronary artery disease or diabetes because of the high prevalence of these conditions in elderly, obese, Caucasian males [20]. Medications prescribed for such common conditions may have potential anticancer mechanisms of action and have potential to be used as chemoprevention agents, in part because their safety has already been demonstrated by their approval for clinical use. Epidemiological and cross-sectional studies have described associations of several of these medications with a reduced risk of esophageal adenocarcinoma, although few randomized trials have been performed to determine the clinical efficacy of these medications when used prospectively to prevent BE and EAC. This review will summarize the current evidence from observational cohort studies, case–control studies, and clinical trials for the use of medications to reduce the risk of EAC in patients with BE.
Candidate chemoprevention therapies for esophageal adenocarcinoma in BE patients
Agents that suppress gastric hydrochloric acid
PPIs
Reflux of acidic and bilious stomach contents into the lower esophagus leads to chronic inflammation and the formation of reactive oxygen species, which have been shown to contribute to the carcinogenesis of EAC [21–23]. It therefore follows that raising the pH of the stomach or preventing the reflux of gastric fluid should prevent at least some of these effects and may have a cancer-preventing effect in the clinical setting. (Candidate chemopreventive mechanisms for the PPIs as well as other agents are shown in Figure 2.)
Figure 2.
Barrett’s esophagus-to-esophageal adenocarcinoma tumorigenic factors and mechanisms of action of chemoprevention agents. Chemoprevention agents act to potentially inhibit a variety of mechanisms that contribute to carcinogenesis of esophageal adenocarcinoma. HCl, hydrochloric acid; PPI, proton-pump inhibitor; COX-2, cyclooxygenase 2; ROS, reactive oxygen species; IGF, insulin-like growth factor; EGF, epidermal growth factor; AKT/PKB, protein kinase B; ERK, extracellular signal-related kinases; NF-KB, nuclear factor kappa-light-chain-enhancer of activated B-cells; HER2, human epidermal growth factor receptor 2.
The preponderance of early studies of PPIs in patients with BE or EAC favored a protective effect of PPIs on the development of EAC [24]. Published data from the mid-2000s to early 2010s consistently showed a reduced odds ratio (OR) of high-grade dysplasia (HGD)/EAC in patients using PPIs, with meta-analyses at that time demonstrating a 71% risk reduction in HGD/EAC in BE patients using PPIs (OR 0.29, 95% confidential interval [CI] 0.12–0.79) [24–30]. However, two recently published high-quality observational trials conflict with this prior data, casting doubt on this premise. Hvid-Jensen et al. [31], in their case–control study of 1,440 patients with BE from a national cohort in Denmark, demonstrated an increased OR of EAC in patients on PPIs (OR 2.39, 95% CI 1.03–5.54). Masclee et al. [32] also studied a large national cohort of BE patients in the UK and observed an increased odds ratio of HGD/EAC in patients using PPIs (OR 1.95, 95% CI 1.00–3.81). Proposed reasons for these conflicting results compared with other published studies include differences in the epidemiology and risk factors in American vs European patients with BE and the possibility of confounding by indication (e.g. patients with more symptomatic or severe reflux were more likely to be prescribed PPIs) [24]. A more recent meta-analysis, including the recent negative studies as well as previous positive data, demonstrated a trend toward a protective effect of PPIs on the development of HGD/EAC, but the effect no longer reached statistical significance (OR 0.43, 95% CI 0.17–1.08) [33].
Adding to the uncertainty and controversy surrounding the use of PPIs for chemoprevention of BE/EAC, data from a recent randomized trial supports the use of PPIs for chemoprevention of EAC. The AspECT trial randomized 2,557 patients with confirmed BE to high- vs low-dose PPI therapy (esomeprazole 40 mg twice daily vs 20 mg once daily). At 8.9 years (mean follow-up), high-dose PPI was superior to low-dose PPI in the primary composite endpoint of time to mortality or development of HGD or EAC (time ratio 1.27, 95% CI 1.01–1.58) [34].
Notably, PPIs are currently the only medications for the prevention of progression of BE that are included in clinical guidelines, with recommendations for PPI use at least once daily in all BE patients, although the evidence supporting this recommendation is not felt to be strong [11, 13]. From a practical standpoint, high-dose PPI use in BE patients is common in clinical practice; however, this is currently only recommended in BE patients with breakthrough symptoms on low-dose PPIs. Given that these guidelines were published before results of the AspECT trial were available, it remains to be seen whether a strategy of ‘minimal effective dosing’ will continue to be recommended in individuals with BE for the management of GERD and HGD/EAC chemoprevention. An important consideration if implementing a high-dose PPI strategy in BE patients includes the associated medication costs and potential dose-related adverse effects from chronic gastric acid suppression, including infectious complications (pneumonia; Clostridium difficile), micronutrient deficiencies, osteoporosis, and renal insufficiency [35, 36]. These issues are important for clinicians and population health experts to consider when determining the risks vs benefits of a chemoprevention strategy for BE.
Histamine-2 receptor antagonists
Histamine-2 receptor antagonists (H2RAs) are the other major class of medication used in the treatment of GERD, but they have not been shown to affect the rate of neoplastic progression in observational trials of BE. Thota et al. [37] demonstrated in a single-center cohort that H2RA use was not protective against HGD/EAC (RR 0.62 95% CI 0.27–1.41), which is generally consistent with previously published trials [24, 28, 30], although some studies have shown a protective effect [38]. Additionally, H2RAs do not appear to have any synergistic protective effect when combined with PPIs [30]. This lack of chemoprotective effect from H2RAs has been attributed to the development of tachyphylaxis and therefore a lack of long-term acid-suppressive benefit. H2RAs are currently not recommended for risk reduction or chemoprophylaxis in BE patients in clinical guidelines [11, 13].
Aspirin and non-steroidal anti-inflammatory drugs
Aspirin and many other non-steroidal anti-inflammatory drugs (NSAIDs) have been associated with a reduced risk of many gastrointestinal-tract cancers, including cancers of the upper gastrointestinal tract [39]. Given their ready availability as a generic over-the-counter medication, there has been substantial interest in their chemoprevention effects. Notably, patients with BE are often prescribed long-term aspirin for co-morbid cardiovascular conditions. Aspirin use has been associated with a decreased risk of HGD/EAC in several observational trials, as described below.
Cyclooxygenase 2 (COX-2)-mediated inflammation has been shown to be associated with the progression of BE metaplasia to dysplasia [40]. Aspirin and other NSAIDs are cyclooxygenase inhibitors and reduce tissue levels of pro-tumorigenic prostaglandins, such as prostaglandin E2 (PGE2). They have been shown to inhibit the progression of BE in preclinical trials and animal models through this mechanism [41, 42]. Aspirin also modulates the carcinogenic activation of nuclear factor kappa-light-chain-enhancer of activated B-cells (NF-κB) and CDX2 expression in patients with BE [43]. Aspirin use has been well studied for chemoprevention of colorectal cancer and its use for this indication is recommended by the US Preventative Task Force for adults aged 50–59 years, although this depends on a calculation of the patient’s cardiovascular risk and concurrent need for cardiovascular disease risk reduction [44–46].
In a 2014 meta-analysis of four studies comprising 2,152 patients from the USA, the UK, and the Netherlands, Zhang et al. [47] showed a protective effect of aspirin on the risk of HGD/EAC in BE patients (RR 0.63, 95% CI 0.43–0.94). Based on these data, a cost-effectiveness analysis demonstrated that the addition of aspirin chemoprevention to regular endoscopic surveillance would result in both decreased healthcare costs and improved quality-adjusted life years for BE patients [48]. However, results of observational data since that time have not shown consistent protective effects of aspirin on EAC risk. Masclee et al. [32], in their nested case–control study of a large cohort of BE patients from the Netherlands and the UK, showed no benefit of either long-term or any use of low-dose aspirin (OR 0.9, 95% CI 0.4–1.8; OR 0.9, 95% CI 0.4–2.1). In patients diagnosed with EAC, aspirin use had no effect on mortality [49]. It is also important to recognize that regular aspirin use carries a significant clinical risk of gastrointestinal and cerebral hemorrhage [50], but the nature of the observational trials performed to date has not allowed an analysis of these risks.
The AspECT trial, as described above in the discussion of PPI use, also randomized participants into aspirin and non-aspirin use, excluding patients with a contraindication to aspirin use or who were already prescribed aspirin for cardiovascular indications [34]. Overall, individuals receiving aspirin (300 mg daily) had a trend toward improved time to mortality or development of HGD/EAC that did not reach statistical significance (TR 1.24, 95% CI 0.98–1.57). However, in the subgroup of patients not already taking other NSAIDs, aspirin use did achieve a statistically significant protective effect (TR 1.29, 95% CI 1.01–1.66). Additionally, aspirin use appeared to provide additive benefits when combined with high-dose PPI as compared with low-dose PPI and no aspirin (TR 1.59, 95% CI 1.14–2.23). Patients in the aspirin-therapy arm had higher rates of serious gastrointestinal hemorrhage than patients taking concurrent PPI therapy with no aspirin, but overall numbers of events were very low (n = 14 vs n = 6, P not reported).
Non-aspirin NSAIDs are often included in studies investigating aspirin and have a similar purported mechanism of action. However, given that NSAIDs are generally less likely to be prescribed for long-term use and are frequently associated with unrecorded, over-the-counter use, the effect of these medications is challenging to evaluate in retrospective analyses. A meta-analysis published in 2014 showed a relative risk of 0.50 for HGD/EAC (95% CI 0.32–0.78) in NSAID users, but the frequency of use, dosage, and magnitude of protective effect varied widely among studies [47]. Masclee et al. [32], when examining NSAIDs in their cohort, did not show a protective effect on the progression of BE to HGD/EAC (OR 0.9, 95% CI 0.5–1.8). In contrast, in a prospective cohort of 570 Dutch patients, non-aspirin NSAIDs did demonstrate a protective effect against BE progression that was independent of aspirin use (HR 0.47, P = 0.03) [51].
One small randomized trial of celecoxib in 100 patients with low-grade dysplasia or HGD showed no benefit in terms of dysplasia progression after 48 weeks [52]. Because chronic non-aspirin NSAID use is associated with significant clinical adverse events (peptic ulcer disease, acute or chronic kidney disease, etc.) without any of the positive cardioprotective effects of aspirin, in the absence of higher-quality evidence, they are not recommended for routine use for EAC chemoprevention [13].
Statins
HMG-coA reductase inhibitors, or statins, are a medication class commonly prescribed for the treatment of dyslipidemia in patients at risk of cardiovascular disease. Because of the common co-occurrence of BE and coronary artery disease risk factors, the impact of HMG-coA reductase inhibitors on BE can be readily assessed in observational cohort studies. Statin use has been shown to be associated with reduced overall cancer mortality and multiple observational studies have shown a reduced incidence of EAC in statin users [28, 32, 53].
The anti-carcinogenic effect of statins does not appear to be dependent solely on cholesterol-biosynthesis pathways. In vitro studies of statins demonstrated inhibition of proliferation and induction of apoptosis in EAC cell lines through the extracellular signaling regulated kinase (ERK) and AKT signaling pathways, as well as through the enzyme-dependent farnesylation of cholesterol precursors [54]. Statins have also been shown to decrease the malignant potential of EAC cells in in vitro cell-line studies through decreasing the expression of intracellular adhesion molecule 1 (ICAM-1) [55].
A 2017 meta-analysis of 11 studies, comprising almost 19,000 patients with BE, showed an OR of 0.59 of EAC among statin users, with consistent effects seen across all included studies (95% CI 0.50–0.68) [56]. This benefit might be dose-related, as Beales et al. [57] demonstrated a reduced OR of EAC in patients taking high-dose statin therapy (equivalent to simvastatin 40 mg daily or higher) compared with that in those taking low-dose therapy (OR 0.31, 95% CI 0.05–0.97 vs OR 0.54, 95% CI 0.27–0.98).
The duration of therapy required to benefit from statins is unclear at this time, although increased time of exposure has been shown to correlate with a greater magnitude of protective effect across multiple studies [28, 32, 58]. The protective effect of statins on EAC incidence appears to be potentiated by COX-2 inhibition, with additive effects seen with both aspirin and non-aspirin NSAIDs use [51, 57]. At this time, prospective clinical trials are needed to assess the efficacy of these agents in populations with BE.
Metformin
Metformin is a medication that decreases hepatic glucose production, decreases intestinal absorption of glucose, and improves insulin sensitivity by increasing peripheral glucose uptake and utilization. It is commonly prescribed for the treatment of adult-onset diabetes mellitus and has demonstrated protective anti-carcinogenic effects for a variety of gastrointestinal cancers, particularly colorectal cancer [59]. Metformin has also been shown to reduce the growth of EAC xenografts and suppress tumor formation in mouse models of EAC through reduced activation of a wide variety of oncogenes, including epidermal growth factor receptor, vascular endothelial growth factor, and insulin-like growth factor (IGF), and by inhibiting the phosphorylation of S6 kinase 1 (S6K1), which mediates the mTOR signaling pathway [60]. However, observational population-based studies on the association of metformin with EAC incidence are limited and generally have demonstrated no association with reduced EAC risk. A retrospective analysis of 138 male BE patients with type II diabetes did not show any significant association of metformin use with the risk of EAC (P = 0.138) [61]. Furthermore, a small randomized trial of 74 patients with BE given metformin or placebo did not show any difference in its primary endpoint of a reduction in tissue pS6K1 levels at 12 weeks, suggesting that metformin would not inhibit the progression of BE to EAC [62].
Sex hormones
The high male-to-female ratio of BE and EAC incidence, which is not entirely explained by differences in the prevalence of other clinical risk factors for EAC, has led to the hypothesis that female sex hormones may be protective against the development of EAC [63]. Exogenous estrogen use most commonly occurs in postmenopausal women using hormone replacement therapy (HRT) or in premenopausal women using oral contraceptives. A number of small population-based studies of women taking exogenous estrogens have demonstrated a trend towards a protective effect against EAC. A meta-analysis of these studies showed a slight statistically significant protective effect against EAC of HRT and a trend toward protection with oral contraceptives in premenopausal women (OR 0.75, 95% CI 0.58–0.98 and OR 0.76, 95% CI 0.57–1.00) [64]. The largest retrospective analysis of the effect of HRT on EAC utilized the Women’s Health Initiative database of 160,080 postmenopausal women and assessed the incidence of EAC in a subset of the subjects who were randomized to HRT. In this cohort, there was no statistically significant association between a diagnosis of EAC and hormone use (either estrogen or estrogen plus progesterone) [65]. This study, like others investigating the risk of EAC in female patients, had relatively small total case numbers, with only 23 total cases of EAC in the cohort, which limits the power to detect small effect sizes. Data from studies specifically evaluating the effect of estrogens on EAC incidence in previously diagnosed female BE patients are currently lacking.
Other candidate prevention agents
A multitude of other medications and dietary agents have been assessed in preclinical studies and small clinical trials for their potential roles for EAC chemoprevention, but there is generally a lack of significant clinical data to support their use at this time. Some of the more promising agents and medications will be discussed in this section.
Difluoromethylornithine (DFMO) is an inhibitor of ornithine decarboxylase (OTC), which has been shown to promote oncogenesis through its effects on polyamine metabolism in preclinical studies [66]. High levels of OTC and polyamines have been shown to be expressed in BE and EAC tissues [67]. A single-arm trial of 10 non-dysplastic BE patients given DFMO showed reduced levels of tissue polyamines after 6 months, but studies measuring EAC development in BE patients given DFMO are lacking at this time [68].
Studies assessing the association of vitamin D deficiency with EAC have provided conflicting evidence for the potential role of vitamin D for EAC chemoprevention. Some observational studies have shown an association between vitamin D deficiency and an increased risk of EAC, whereas others have found no such association [69, 70]. A small, non-randomized prospective trial of 18 BE patients did not demonstrate any change in mucosal expression of a set of tumor-suppressor genes after 12 weeks of high-dose vitamin D supplementation [71].
Ursodeoxycholic-acid supplementation has been hypothesized to alter the acid and bile composition of gastroesophageal refluxate and its use in preclinical studies has shown reduced rates of EAC in a rat model of BE [72]. However, a non-randomized pilot study of 29 patients with BE showed altered bile-acid composition after 6 months of ursodeoxycholic-acid supplementation but no change in the primary study endpoint of altered tissue biomarkers indicative of oxidative damage and apoptosis [73].
Omega-3 polyunsaturated fatty acids have been shown to reduce systemic inflammation through COX-2-mediated mechanisms, which may play a role in EAC carcinogenesis [74, 75]. A small randomized trial of omega-3 fatty-acid supplementation in 52 BE patients showed a reduction in tissue COX-2 protein levels but no reduction in the tissue levels of inflammatory cytokines, such as PGE2 and LTB4 [76].
Folate, given its role in DNA synthesis and repair, has been proposed to play an anti-carcinogenic role in EAC. Observational population-based trials have shown an inconsistent association between dietary folate intake or serum folate levels and reduced incidence of esophageal adenocarcinoma, but no specific trials of folate supplementation in BE patients have been conducted to date [77].
Finally, curcumin, an active phenol derivative of the spice turmeric, has been hypothesized to have anti-carcinogenic effects and has demonstrated in vitro activity against oncogenic signaling pathways active in BE progression, such as NF-κB; however, no clinical trial of human curcumin supplementation has been conducted at this time [78].
Conclusions
BE is relatively common and is the major risk factor for EAC. In light of the high mortality rates of advanced EAC, BE screening and surveillance are currently the standard of care and recommendation by organizations like the American Gastroenterological Association. However, the clinical effectiveness of screening and surveillance in BE patients for preventing EAC is not clear at this time and is a source of controversy. This has led to interest in other approaches for preventing EAC in individuals with BE, including chemoprevention. Barriers to the use of chemoprevention agents have included not only the lack of a therapy with substantial clinical effectiveness, but also a need for agents that have very low or no potential for adverse effects. The rates of progression of BE to EAC are low enough that careful consideration must be paid to the side effects and medical risks of any strategy of chemoprevention.
Endoscopic ablative strategies are now recommended for patients with low-grade or HGD and have been proved to be very effective for eradicating dysplastic tissue, which has relegated the use of primary chemoprevention to non-dysplastic BE. Of interest, because BE does recur and EAC does develop in some patients after endoscopic ablation, the role of chemoprevention after ablation therapy is also under investigation at this time [13].
Despite recent controversy regarding their efficacy, PPIs remain the mainstay for the chemoprevention of EAC for patients with BE. The results of the AspECT trial suggest, contrary to current recommendations, that aspirin and high-dose PPIs are beneficial for patients with non-dysplastic BE, but further studies are needed before they can be recommended for routine clinical use. Statins also deserve ongoing consideration for Barrett’s chemoprevention, although, at this point, their role is limited to those patients who also carry a cardiovascular indication for their use. Overall, PPIs and aspirin appear to have the greatest potential for the chemoprevention of EAC. However, only PPIs are generally recommended for BE patients at this time, and the evidence for this recommendation is modest. In summary, there remains a need for effective and safe chemoprevention agents for EAC and ongoing investigation of promising but clinically unproven prevention therapies.
Authors’ contributions
WG: study concept and design, drafting of manuscript, revision of manuscript. ES: study concept and design, drafting of manuscript, revision of manuscript. JI: critical revision of manuscript for important intellectual content. AK: critical revision of manuscript for important intellectual content.
Funding
Support for this work was provided by National Institutes of Health (NIH) National Cancer Institute (NCI) [RO1CA220004, RO1CA194663, P30CA15704, U01086402, UO1CA152756, U54CA163060, and P01CA077852] (to W.M.G.) and the Cottrell Family Fund and the Listwin Family Foundation.
Conflicts of interest
WM Grady is on the advisory boards of Guardant Health, Freenome and SEngine and is a consultant for Diacarta. He is also an investigator for a clinical trial sponsored by Janssen Pharmaceuticals and receives services for investigator inititated research from Tempus and Lucid Technologies.
References
- 1. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med 2014;371:2499–509. [DOI] [PubMed] [Google Scholar]
- 2. Abrams JA, Sharaiha RZ, Gonsalves L et al. Dating the rise of esophageal adenocarcinoma: analysis of Connecticut Tumor Registry data, 1940-2007. Cancer Epidemiol Biomarkers Prev 2011;20:183–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA A Cancer J Clin 2019;69:7–34. [DOI] [PubMed] [Google Scholar]
- 4. Rubenstein JH, Shaheen NJ. Epidemiology, diagnosis, and management of esophageal adenocarcinoma. Gastroenterology 2015;149:302–17 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Taylor JB, Rubenstein JH. Meta-analyses of the effect of symptoms of gastroesophageal reflux on the risk of Barrett's esophagus. Am J Gastroenterol 2010;105:1729; 1730–7; quiz 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am 2015;44:203–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubenstein JH, Inadomi JM, Scheiman J et al. Association between Helicobacter pylori and Barrett's esophagus, erosive esophagitis, and gastroesophageal reflux symptoms. Clin Gastroenterol Hepatol 2014;12:239–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hvid-Jensen F, Pedersen L, Drewes AM et al. Incidence of adenocarcinoma among patients with Barrett's esophagus. N Engl J Med 2011;365:1375–83. [DOI] [PubMed] [Google Scholar]
- 9. Rastogi A, Puli S, El-Serag HB et al. Incidence of esophageal adenocarcinoma in patients with Barrett's esophagus and high-grade dysplasia: a meta-analysis. Gastrointest Endosc 2008;67:394–8. [DOI] [PubMed] [Google Scholar]
- 10. Thrift AP, Whiteman DC. The incidence of esophageal adenocarcinoma continues to rise: analysis of period and birth cohort effects on recent trends. Ann Oncol 2012;23:3155–62. [DOI] [PubMed] [Google Scholar]
- 11. Spechler SJ, Sharma P, Souza RF et al. American Gastroenterological Association medical position statement on the management of Barrett's esophagus. Gastroenterology 2011;140:e18–91. [DOI] [PubMed] [Google Scholar]
- 12. Fitzgerald RC, di Pietro M, Ragunath K et al. British Society of Gastroenterology guidelines on the diagnosis and management of Barrett's oesophagus. Gut 2014;63:7–42. [DOI] [PubMed] [Google Scholar]
- 13. Shaheen NJ, Falk GW, Iyer PG et al. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 2016;111:30–50; quiz 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Inadomi JM, Saxena N. Screening and surveillance for Barrett's Esophagus: is it cost-effective? Dig Dis Sci 2018;63:2094–104. [DOI] [PubMed] [Google Scholar]
- 15. Arnett DK, Khera A, Blumenthal RS. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Part 1, Lifestyle and Behavioral Factors. JAMA Cardiol 2019;4:1043. [DOI] [PubMed] [Google Scholar]
- 16. Vogel VG, Costantino JP, Wickerham DL et al. Effects of tamoxifen vs raloxifene on the risk of developing invasive breast cancer and other disease outcomes: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA 2006;295:2727–41. [DOI] [PubMed] [Google Scholar]
- 17. Thompson IM, Goodman PJ, Tangen CM et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003;349:215–24. [DOI] [PubMed] [Google Scholar]
- 18. Burn J, Gerdes AM, Macrae F et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet 2011;378:2081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cao Y, Nishihara R, Wu K et al. Population-wide impact of long-term use of aspirin and the risk for cancer. JAMA Oncol 2016;2:762–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Drahos J, Ricker W, Pfeiffer RM et al. Metabolic syndrome and risk of esophageal adenocarcinoma in elderly patients in the United States: an analysis of SEER-Medicare data. Cancer 2017;123:657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang HY, Hormi-Carver K, Zhang X et al. In benign Barrett's epithelial cells, acid exposure generates reactive oxygen species that cause DNA double-strand breaks. Cancer Res 2009;69:9083–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang HY, Zhang Q, Zhang X et al. Cancer-related inflammation and Barrett's carcinogenesis: interleukin-6 and STAT3 mediate apoptotic resistance in transformed Barrett's cells. Am J Physiol Gastrointest Liver Physiol 2011;300:G454–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunbar KB, Souza RF, Spechler SJ. The effect of proton pump inhibitors on Barrett's esophagus. Gastroenterol Clin North Am 2015;44:415–24. [DOI] [PubMed] [Google Scholar]
- 24. Singh S, Garg SK, Singh PP et al. Acid-suppressive medications and risk of oesophageal adenocarcinoma in patients with Barrett's oesophagus: a systematic review and meta-analysis. Gut 2014;63:1229–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hillman LC, Chiragakis L, Shadbolt B et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett's oesophagus. Med J Aust 2004;180:387–91. [DOI] [PubMed] [Google Scholar]
- 26. de Jonge PJ, Steyerberg EW, Kuipers EJ et al. Risk factors for the development of esophageal adenocarcinoma in Barrett's esophagus. Am J Gastroenterol 2006;101:1421–9. [DOI] [PubMed] [Google Scholar]
- 27. Nguyen DM, El-Serag HB, Henderson L et al. Medication usage and the risk of neoplasia in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2009;7:1299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett's esophagus. Gastroenterology 2010;138:2260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jung KW, Talley NJ, Romero Y et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett's esophagus: a population-based study. Am J Gastroenterol 2011;106:1447–55; quiz 1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kastelein F, Spaander MC, Steyerberg EW et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2013;11:382–8. [DOI] [PubMed] [Google Scholar]
- 31. Hvid-Jensen F, Pedersen L, Funch-Jensen P et al. Proton pump inhibitor use may not prevent high-grade dysplasia and oesophageal adenocarcinoma in Barrett's oesophagus: a nationwide study of 9883 patients. Aliment Pharmacol Ther 2014;39:984–91. [DOI] [PubMed] [Google Scholar]
- 32. Masclee GM, Coloma PM, Spaander MC et al. NSAIDs, statins, low-dose aspirin and PPIs, and the risk of oesophageal adenocarcinoma among patients with Barrett's oesophagus: a population-based case-control study. BMJ Open 2015;5:e006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hu Q, Sun TT, Hong J et al. Proton pump inhibitors do not reduce the risk of esophageal adenocarcinoma in patients with Barrett's esophagus: a systematic review and meta-analysis. PLoS One 2017;12:e0169691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jankowski JAZ, de Caestecker J, Love SB et al. Esomeprazole and aspirin in Barrett's oesophagus (AspECT): a randomised factorial trial. Lancet 2018;392:400–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwok CS, Arthur AK, Anibueze CI et al. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol 2012;107:1011–9. [DOI] [PubMed] [Google Scholar]
- 36. Hess MW, Hoenderop JG, Bindels RJ et al. Systematic review: hypomagnesaemia induced by proton pump inhibition. Aliment Pharmacol Ther 2012;36:405–13. [DOI] [PubMed] [Google Scholar]
- 37. Thota PN, Hajifathalian K, Benjamin T et al. Lack of incremental effect of histamine receptor antagonists over proton pump inhibitors on the risk of neoplastic progression in patients with Barrett's esophagus: a cohort study. J Dig Dis 2017;18:143–50. [DOI] [PubMed] [Google Scholar]
- 38. Tan MC, El-Serag HB, Yu X et al. Acid suppression medications reduce risk of oesophageal adenocarcinoma in Barrett's oesophagus: a nested case-control study in US male veterans. Aliment Pharmacol Ther 2018;48:469–77. [DOI] [PubMed] [Google Scholar]
- 39. Cuzick J. Preventive therapy for cancer. Lancet Oncol 2017;18:e472–82. [DOI] [PubMed] [Google Scholar]
- 40. Shirvani VN, Ouatu-Lascar R, Kaur BS et al. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology 2000;118:487–96. [DOI] [PubMed] [Google Scholar]
- 41. Falk GW, Buttar NS, Foster NR et al. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett's esophagus. Gastroenterology 2012;143:917–26 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Buttar NS, Wang KK, Leontovich O et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology 2002;122:1101–12. [DOI] [PubMed] [Google Scholar]
- 43. Huo X, Zhang X, Yu C et al. Aspirin prevents NF-kappaB activation and CDX2 expression stimulated by acid and bile salts in oesophageal squamous cells of patients with Barrett's oesophagus. Gut 2018;67:606–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rothwell PM, Wilson M, Elwin CE et al. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet 2010;376:1741–50. [DOI] [PubMed] [Google Scholar]
- 45. Drew DA, Cao Y, Chan AT. Aspirin and colorectal cancer: the promise of precision chemoprevention. Nat Rev Cancer 2016;16:173–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bibbins-Domingo K, Force U. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836–45. [DOI] [PubMed] [Google Scholar]
- 47. Zhang S, Zhang XQ, Ding XW et al. Cyclooxygenase inhibitors use is associated with reduced risk of esophageal adenocarcinoma in patients with Barrett's esophagus: a meta-analysis. Br J Cancer 2014;110:2378–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi SE, Perzan KE, Tramontano AC et al. Statins and aspirin for chemoprevention in Barrett's esophagus: results of a cost-effectiveness analysis. Cancer Prev Res (Phila) 2014;7:341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spence AD, Busby J, Johnston BT et al. Low-dose aspirin use does not increase survival in 2 independent population-based cohorts of patients with esophageal or gastric cancer. Gastroenterology 2018;154:849–60 e1. [DOI] [PubMed] [Google Scholar]
- 50. Whitlock EP, Burda BU, Williams SB et al. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:826–35. [DOI] [PubMed] [Google Scholar]
- 51. Kastelein F, Spaander MC, Biermann K et al. Nonsteroidal anti-inflammatory drugs and statins have chemopreventative effects in patients with Barrett's esophagus. Gastroenterology 2011;141:2000–8. quiz e13–4. [DOI] [PubMed] [Google Scholar]
- 52. Heath EI, Canto MI, Piantadosi S et al. Secondary chemoprevention of Barrett's esophagus with celecoxib: results of a randomized trial. J Natl Cancer Inst 2007;99:545–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012;367:1792–802. [DOI] [PubMed] [Google Scholar]
- 54. Ogunwobi OO, Beales IL. Statins inhibit proliferation and induce apoptosis in Barrett's esophageal adenocarcinoma cells. Am J Gastroenterology 2008;103:825–37. [DOI] [PubMed] [Google Scholar]
- 55. Sadaria MR, Reppert AE, Yu JA et al. Statin therapy attenuates growth and malignant potential of human esophageal adenocarcinoma cells. J Thorac Cardiovasc Surg 2011;142:1152–60. [DOI] [PubMed] [Google Scholar]
- 56. Thomas T, Loke Y, Beales I. Systematic review and meta-analysis: use of statins is associated with a reduced incidence of oesophageal adenocarcinoma. J Gastrointest Canc 2018;49:442–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beales IL, Vardi I, Dearman L. Regular statin and aspirin use in patients with Barrett's oesophagus is associated with a reduced incidence of oesophageal adenocarcinoma. Eur J Gastroenterol Hepatol 2012;24:917–23. [DOI] [PubMed] [Google Scholar]
- 58. Nguyen T, Duan Z, Naik AD et al. Statin use reduces risk of esophageal adenocarcinoma in US veterans with Barrett's esophagus: a nested case-control study. Gastroenterology 2015;149:1392–8. [DOI] [PubMed] [Google Scholar]
- 59. Joo MK, Park JJ, Chun HJ. Additional benefits of routine drugs on gastrointestinal cancer: statins, metformin, and proton pump inhibitors. Dig Dis 2018;36:1–14. [DOI] [PubMed] [Google Scholar]
- 60. Fujihara S, Kato K, Morishita A et al. Antidiabetic drug metformin inhibits esophageal adenocarcinoma cell proliferation in vitro and in vivo. Int J Oncol 2015;46:2172–80. [DOI] [PubMed] [Google Scholar]
- 61. Agrawal S, Patel P, Agrawal A et al. Metformin use and the risk of esophageal cancer in Barrett esophagus. South Med J 2014;107:774–9. [DOI] [PubMed] [Google Scholar]
- 62. Chak A, Buttar NS, Foster NR et al. Metformin does not reduce markers of cell proliferation in esophageal tissues of patients with Barrett's esophagus. Clin Gastroenterol Hepatol 2015;13:665–72 e1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xie SH, Lagergren J. The male predominance in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2016;14:338–47 e1. [DOI] [PubMed] [Google Scholar]
- 64. Lagergren K, Lagergren J, Brusselaers N. Hormone replacement therapy and oral contraceptives and risk of oesophageal adenocarcinoma: a systematic review and meta-analysis. Int J Cancer 2014;135:2183–90. [DOI] [PubMed] [Google Scholar]
- 65. Bodelon C, Anderson GL, Rossing MA et al. Hormonal factors and risks of esophageal squamous cell carcinoma and adenocarcinoma in postmenopausal women. Cancer Prev Res (Phila) 2011;4:840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Meyskens FL Jr., Gerner EW. Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res 1999;5:945–51. [PubMed] [Google Scholar]
- 67. Brabender J, Lord RV, Danenberg KD et al. Upregulation of ornithine decarboxylase mRNA expression in Barrett's esophagus and Barrett's-associated adenocarcinoma. J Gastrointest Surg 2001;5:174–81. discussion 182. [DOI] [PubMed] [Google Scholar]
- 68. Sinicrope FA, Broaddus R, Joshi N et al. Evaluation of difluoromethylornithine for the chemoprevention of Barrett's esophagus and mucosal dysplasia. Cancer Prev Res (Phila) 2011;4:829–39. [DOI] [PubMed] [Google Scholar]
- 69. Giovannucci E, Liu Y, Rimm EB et al. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98:451–9. [DOI] [PubMed] [Google Scholar]
- 70. Dong J, Gharahkhani P, Chow WH et al. No association between vitamin d status and risk of Barrett's esophagus or esophageal adenocarcinoma: a Mendelian randomization study. Clin Gastroenterol Hepatol 2019;17: 2227–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cummings LC, Thota PN, Willis JE et al. A nonrandomized trial of vitamin D supplementation for Barrett's esophagus. PLoS One 2017;12:e0184928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rizvi S, Demars CJ, Comba A et al. Combinatorial chemoprevention reveals a novel smoothened-independent role of GLI1 in esophageal carcinogenesis. Cancer Res 2010;70:6787–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Banerjee B, Shaheen NJ, Martinez JA et al. Clinical study of ursodeoxycholic acid in Barrett's esophagus patients. Cancer Prev Res (Phila) 2016;9:528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wilson KT, Fu S, Ramanujam KS et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinomas. Cancer Res 1998;58:2929–34. [PubMed] [Google Scholar]
- 75. Irun P, Lanas A, Piazuelo E. Omega-3 polyunsaturated fatty acids and their bioactive metabolites in gastrointestinal malignancies related to unresolved inflammation: a review. Front Pharmacol 2019;10:852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mehta SP, Boddy AP, Cook J et al. Effect of n-3 polyunsaturated fatty acids on Barrett's epithelium in the human lower esophagus. Am J Clin Nutr 2008;87:949–56. [DOI] [PubMed] [Google Scholar]
- 77. Ni Y, Du J, Yin X et al. Folate intake, serum folate, and risk of esophageal cancer: a systematic review and dose-response meta-analysis. Eur J Cancer Prev 2019;28:173–80. [DOI] [PubMed] [Google Scholar]
- 78. Rawat N, Alhamdani A, McAdam E et al. Curcumin abrogates bile-induced NF-kappaB activity and DNA damage in vitro and suppresses NF-kappaB activity whilst promoting apoptosis in vivo, suggesting chemopreventative potential in Barrett's oesophagus. Clin Transl Oncol 2012;14:302–11. [DOI] [PubMed] [Google Scholar]