Abstract
Background
Acute mountain sickness (AMS) usually occurs among non‐acclimated individuals after rapid ascending to high‐altitude environments (generally ≥2,500 m). However, the precise molecular mechanism of AMS remains unclear. Our study aimed to investigate the relationship between several single nucleotide polymorphisms (SNPs) and AMS susceptibility.
Methods
In this work, sequencing data were obtained from 69 AMS patients and 95 matched acclimated Han Chinese individuals from southwest China. Five SNPs (rs1008438, rs150877473, rs1799983, rs2153364, and rs3025039) were systematically investigated in all the participants.
Results
In our study, we found that allele frequencies of “A” (AMS 69.57% vs. non‐AMS 54.74%) and “C” (AMS 30.43% vs. non‐AMS 45.26%) in the HSPA1A gene rs1008438 were significantly different between the AMS and non‐AMS groups (p = .01). Genotypes “CC” and “CA” of the HSPA1A gene (rs1008438) were associated with lower risk of developing AMS than the genotype “AA.” Comparing the genotypes “CC + CA” and “AA,” we also observed that the “CC + CA” genotype of rs1008438 was associated with lower AMS risk.
Conclusions
In our case‐control study, there was a significant association between the rs1008348 polymorphism and AMS susceptibility, suggesting that this particular SNP might be a Han‐specific risk factor for AMS. We believe that this study establishes a foundation for further elucidation of the genetic mechanisms underlying AMS.
Keywords: acute mountain sickness, HSPA1A, single nucleotide polymorphism, susceptibility
In our case‐control study, there was a significant association between the rs1008348 polymorphism and acute mountain sickness (AMS) susceptibility, suggesting that this particular single nucleotide polymorphism might be a Han‐specific risk factor for AMS. We believe that this study establishes a foundation for further elucidation of the genetic mechanisms underlying AMS.

1. INTRODUCTION
Acute mountain sickness (AMS) is a medical condition resulting from acute exposure to high‐altitude plateau, which develops mainly at above 2,500 m when the human body can no longer adapt to acute hypoxia (Koirala, Wolpin, & Peterson, 2018; Ogilvie, 2001; Wang, Chen, Li, Fu, & Yao, 2018). Many factors have been identified that contribute to AMS during rapid ascent to high altitudes (Koirala et al., 2018; Shen et al., 2013; Singh, 2017), including oxygen deficiency (Shen et al., 2013; Zhang et al., 2014), low temperature (Loeppky et al., 2003; Seys et al., 2013; Singh, 2017), and intense ultraviolet rays. Among these factors, oxygen deficiency plays a vital role in the development of AMS (Gonggalanzi et al., 2017). AMS can progress to life‐threatening high‐altitude pulmonary edema (HAPE) (Akunov et al., 2017; Du, Zhao, Su, Liu, & Yang, 2018) or to high‐altitude cerebral edema (HACE) (Li, Zhang, & Zhang, 2018; West, 2015), which have a detrimental impact on an individual's health, thereby reducing the physical capability. As the modern community is increasingly undertaking activities in high plateau environments, AMS has become a public health problem. Generally, AMS is considered as a complication linked to hypoxia. However, the pathophysiology of AMS is not fully illustrated.
Some biochemical medium genetic mutations have been identified and characterized in different populations, which may affect the oxygen sensing mechanism, such as polymorphisms in the endothelial nitric oxide synthase (eNOS) (Altundag et al., 2014; Ding et al., 2011; Mansoor et al., 2005; Rossetti et al., 2017; Sun, Wang, Xi, & Hu, 2013; Tissot van Patot et al., 2005; Wang, Ha, Kidd, Koehle, & Rupert, 2010). eNOS plays a significant role in the production of nitric oxide (NO) gaseous hormone and is an essential regulator of physiological functions (Wang et al., 2013). Variations of several nitric oxide synthase 3 (NOS3) genes can affect the expression and activity of eNOS enzyme, as well as the levels of circulating and expired NO in the organism, which suggests that NOS3 variations may influence the high‐altitude acclimatization (MacInnis, Wang, Koehle, & Rupert, 2011). The heat shock protein 70 (HSP70) gene family is associated with homeostatic adjustments to stress (Qi et al., 2009). One gene of the HSP70 superfamily that was described to be divergent between lowland and highland populations is HSPA1A (Dulin, Garcia‐Barreno, & Guisasola, 2012). Endothelial PAS domain protein 1 (EPAS1) and egl nine homolog 1 (EGLN1) are the products of the genes involved in hypoxia adaptation. EPAS is a transcription factor and EGLN1 is a vital oxygen sensor, which plays a key role in the hypoxia‐inducible factor pathway (Buroker et al., 2012; Guo et al., 2015; Hackinger et al., 2016; Yasukochi, Nishimura, Motoi, & Watanuki, 2018; Zhang et al., 2014). Additionally, vascular endothelial growth factor (VEGF) has been shown to be associated with the development of pulmonary edema under hypoxic conditions (Ding et al., 2012).
These genes have been proved to be related to the high‐altitude adaptation or the development of AMS. Thus, these genes might play a key role in AMS pathogenesis. Although several previous findings emphasized the molecular mechanisms underlying gene polymorphisms associated with adaptation to high‐altitude hypoxia, most investigations were carried out on European populations or non‐Han Asian populations. However, China has a population of 1.3 billion, which is one‐fifth of the world's population, and the vast majority of them are Han Chinese. Thus, previous studies could not thoroughly explain the occurrence of AMS in Han Chinese individuals.
To better understand the molecular mechanisms associated with AMS risk in Han Chinese, five single nucleotide polymorphisms (SNPs) of the above‐mentioned genes, including HSPA1A (rs1008438), EPAS1 (rs150877473), NOS3 (rs1799983), EGLN1 (rs2153364), and VEGF (rs3025039), were selected to investigate their association with AMS susceptibility.
2. MATERIALS AND METHODS
2.1. Ethical statements
This study was approved by the Ethics Committee of the Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China. Additionally, this work has been registered in the Chinese Clinical Trial Register (CHiCTR‐PRC‐16008441). All participants provided written informed consent to join this study, and the individual information was documented strictly confidential to protect the participants’ privacy rights.
2.2. Data collection
For this study, we selected 250 eligible healthy Han Chinese males and aged <30 years. Meanwhile, those with high‐altitude (>2,500 m) exposure history in the past year, or severe organic diseases, such as congenital heart disease, dysrhythmia, liver or kidney dysfunction, or psychological and neurological disorders; or other unsuitable conditions were excluded. Subjects were ascended to 4,000 m altitude (Litang County, Sichuan Province, China) from a low‐altitude area (2,000 m, Malong County, Yunnan Province, China) by car. The AMS group consisted of 69 individuals detected by the Lake Louise scoring system (LLS) results. Meanwhile, 95 healthy control individuals without symptoms of AMS were randomly selected for the non‐AMS group.
We collected the clinical characteristics of the AMS and non‐AMS groups, including age, weight, height, body mass index (BMI), and pulse oxygen saturation (SpO2). Blood pressure was detected using electronic sphygmomanometers (OMRON Healthcare Co. Ltd.). SpO2 levels of participants were detected using pulse oximeters (Nonin Medical Co. Ltd.). Blood pressure and SpO2 levels were detected three times after the participants rested in a quiet environment for above 15 min, respectively.
2.3. DNA extraction and index determination
Venous blood (4–5 ml) was drawn from all participants for further genetic analysis. Genomic DNA was extracted from whole blood cells of the participants using the Gentra Puregene Blood Kit (Qiagen Co. Ltd.) and the process was carried out according to the manufacturer's protocol (Yang et al., 2018).
2.4. Genotyping
Single nucleotide polymorphisms genotyping was characterized by the single base extension detecting technology (iPLEX). The single‐base extension primers and SNP loci‐tested polymerase chain reaction (PCR) primers were designed by the Sequenom mass array assay design genotyping software (Sequenom). The purified extension products were added into the 384‐element SpectroCHIP bioarray (Sequenom) by the mass array nanodispenser RS 1000 (Capital Bio Corporation). The data were analyzed using the TYPER 4.0 software (Sequenom).
2.5. Statistical analysis
Allele frequencies and genotype distribution were calculated in the AMS and non‐AMS groups and analyzed for an association with the development of AMS via the Chi‐square test. Odds ratios (ORs) and 95% confidence intervals (CI) were calculated to test the association between AMS risk and genotype. Statistical analysis was conducted using SPSS 17.0 (SPSS Co. Ltd.) and significance was set at p < .05.
3. RESULTS
3.1. Participant characteristics
The characteristics of participants are summarized in Table 1. Comparing the AMS and non‐AMS groups, our results indicated no significant differences in terms of age, height, weight, BMI, and SpO2 at 2,000 m among the study subjects. The SNP selection information is presented in Table 2.
TABLE 1.
Characteristics of the subjects in AMS and Non‐AMS groups at 2,000 m
| Characteristics | AMS (n = 69) | Non‐AMS (n = 95) | p‐Value |
|---|---|---|---|
| Age (mean ± SD, years) | 28.20 ± 0.80 | 27.00 ± 1.20 | .805 |
| Weight (mean ± SD, kg) | 65.48 ± 8.14 | 65.05 ± 7.17 | .723 |
| Height (mean ± SD, cm) | 172.00 ± 4.00 | 173.00 ± 5.00 | .687 |
| BMI (kg/m2) | 22.19 ± 2.16 | 21.96 ± 1.99 | .502 |
| SpO2 (%) | 96.78 ± 1.04 | 96.71 ± 1.24 | .707 |
Abbreviations: AMS, acute mountain sickness; BMI, body mass index; SpO2, pulse oxygen saturation.
TABLE 2.
Basic single nucleotide polymorphism (SNPs) information
| Polymorphisms | Gene name | Allele | Chromosome position |
|---|---|---|---|
| rs1008438 | HSPA1A | A/C | Chr 6: 31815431 |
| rs150877473 | EPAS1 | G/C | Chr 2: 46360880 |
| rs1799983 | NOS3 | T/G | Chr 7: 150999023 |
| rs2153364 | EGLN1 | A/G | Chr 1: 231424474 |
| rs3025039 | VEGF | C/T | Chr 6: 43784799 |
Abbreviations: EGLN1, egl nine homolog 1; EPAS1, endothelial PAS domain protein 1; VEGF, vascular endothelial growth factor, HSPA1A, heat shock proteins A1ANOS3, nitric oxide synthase 3.
3.2. Genotyping of target SNPs associated with AMS risk
In the assay, dominant, codominant, and recessive models were applied to assess the association of SNPs within HSPA1A, EPAS1, NOS3, EGLN1, and VEGF with the risk of AMS. For HSPA1A (rs1008438), the distribution of the allele and overall genotype frequency was significantly different between the AMS and non‐AMS groups (p = .01). However, there was no significant difference in the distribution of both the variant genotypes (p > .05) and alleles (p > .05) for the other four genes between the AMS and non‐AMS groups (Table 3).
TABLE 3.
SNPs genetic models and analyses of the association between SNPs and AMS
| SNP No. | Genotype | Total | Non‐AMS (%) | AMS (%) | Β | SE | χ 2 | p | OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| rs1008438 | C | 128 (39.02) | 86 (45.26) | 42 (30.43) | |||||
| A | 200 (60.98) | 104 (54.74) | 96 (69.57) | 7.39 | .01* | ||||
| CC | 29 (17.68) | 20 (21.05) | 9 (13.04) | −1.01 | 0.47 | 4.61 | .03 | 0.36 (0.14, 0.92) | |
| CA | 70 (42.68) | 46 (48.42) | 24 (34.78) | −0.87 | 0.35 | 5.98 | .01 | 0.42 (0.21, 0.84) | |
| AA | 65 (39.63) | 29 (30.53) | 36 (52.17) | — | — | — | — | — | |
| CC + CA | 99 (60.37) | 66 (69.47) | 33 (47.83) | −0.91 | 0.33 | 7.68 | .01 | 0.40 (0.21, 0.77) | |
| AA | 65 (39.63) | 29 (30.53) | 36 (52.17) | — | — | — | — | ||
| CC | 29 (17.68) | 20 (21.05) | 9 (13.04) | 0.58 | 0.44 | 1.73 | .19 | 0.56 (0.24, 1.33) | |
| CA + AA | 135 (82.32) | 75 (78.95) | 60 (86.96) | — | — | — | — | — | |
| rs150877473 | G | 9 (2.74) | 5 (2.63) | 4 (2.90) | |||||
| C | 319 (97.26) | 185 (97.37) | 134 (97.10) | 0.02 | .88 | ||||
| GG | 0 (0.00) | 0 | 0 | — | — | — | — | — | |
| GC | 9 (5.49) | 5 (5.26) | 4 (5.80) | 0.10 | 0.69 | 0.02 | .88 | 1.11 (0.29, 4.29) | |
| CC | 155 (94.51) | 90 (94.74) | 65 (94.20) | — | — | — | — | — | |
| GG + GC | 9 (5.49) | 5 (5.26) | 4 (5.80) | 0.10 | 0.69 | 0.02 | .88 | 1.11 (0.29, 4.29) | |
| CC | 155 (94.51) | 90 (94.74) | 65 (94.20) | — | — | — | — | — | |
| GG | 0 (0.00) | 0 | 0 | — | — | — | — | — | |
| GC + CC | 164 (100.0) | 95 (100.00) | 69 (100.00) | — | — | — | — | — | |
| rs1799983 | T | 38 (11.59) | 21 (11.05) | 17 (12.32) | |||||
| G | 290 (88.41) | 169 (88.95) | 121 (87.68) | 0.13 | .72 | ||||
| TT | 3 (1.83) | 1 (1.05) | 2 (2.90) | 1.02 | 1.24 | 0.68 | .41 | 2.78 (0.25, 31.42) | |
| GT | 32 (19.51) | 19 (20.00) | 13 (18.84) | −0.05 | 0.40 | 0.02 | .90 | 0.95 (0.43, 2.09) | |
| GG | 129 (78.66) | 75 (78.95) | 54 (78.26) | — | — | — | — | — | |
| TT + GT | 35 (21.34) | 20 (21.05) | 15 (21.74) | 0.04 | 0.39 | 0.01 | .92 | 1.04 (0.49, 2.22) | |
| GG | 129 (78.66) | 75 (78.95) | 54 (78.26) | — | — | — | — | — | |
| TT | 3 (1.83) | 1 (1.05) | 2 (2.90) | −1.03 | 1.24 | 0.70 | .40 | 2.81 (0.25, 31.58) | |
| GT + GG | 161 (98.17) | 94 (98.95) | 67 (97.10) | — | — | — | — | — | |
| rs2153364 | A | 169 (51.84) | 104 (55.32) | 65 (47.10) | |||||
| G | 157 (48.16) | 84 (44.68) | 73 (52.90) | 2.15 | .14 | ||||
| AA | 40 (24.39) | 27 (28.42) | 13 (18.84) | −0.73 | 0.48 | 2.31 | .13 | 0.48 (0.19, 1.24) | |
| AG | 89 (54.27) | 50 (52.63) | 39 (56.52) | −0.25 | 0.40 | 0.38 | .54 | 0.78 (0.35, 1.72) | |
| GG | 35 (21.34) | 18 (18.95) | 17 (24.64) | — | — | — | — | — | |
| AA + AG | 129 (78.66) | 77 (81.05) | 52 (75.36) | −0.39 | 0.39 | 1.03 | .31 | 0.68 (0.32, 1.44) | |
| GG | 35 (21.34) | 18 (18.95) | 17 (24.64) | — | — | — | — | — | |
| AA | 40 (24.39) | 27 (28.42) | 13 (18.84) | 0.55 | 0.38 | 2.07 | .15 | 0.58 (0.27 ,1.22) | |
| AG + GG | 124 (75.61) | 68 (71.58) | 56 (81.16) | — | — | — | — | — | |
| rs3025039 | T | 52 (15.95) | 29 (15.43) | 23 (16.67) | |||||
| C | 274 (84.05) | 159 (84.57) | 115 (83.33) | 0.09 | .76 | ||||
| TT | 6 (3.66) | 4 (4.21) | 2 (2.90) | −0.33 | 0.89 | 0.14 | .71 | 0.72 (0.13, 4.08) | |
| CT | 40 (24.39) | 21 (22.11) | 19 (27.54) | 0.26 | 0.37 | 0.51 | .48 | 1.30 (0.63 ,2.68) | |
| CC | 118 (71.95) | 70 (73.68) | 48 (69.57) | — | — | — | — | — | |
| TT + CT | 46 (28.05) | 25 (26.32) | 21 (30.43) | 0.19 | 0.35 | 0.29 | .59 | 1.21 (0.61,2.40) | |
| CC | 118 (71.95) | 70 (73.68) | 48 (69.57) | — | — | — | — | — | |
| TT | 6 (3.68) | 4 (4.21) | 2 (2.90) | 0.40 | 0.88 | 0.20 | .65 | 0.67 (0.12, 3.78) | |
| CT + CC | 158 (96.34) | 91 (95.79) | 67 (97.10) | — | — | — | — | — |
Abbreviations: AMS, acute mountain sickness; CI, confidence intervals; OR, Odds ratio; SNP, single nucleotide polymorphism.
Indicates statistically significant after the false discovery rate (FDR) correction.
The allele frequencies of “A” (AMS 69.57% vs. non‐AMS 54.74%) and “C” (AMS 30.43% vs. non‐AMS 45.26%) of HSPA1A rs1008438 were significantly different between the AMS and non‐AMS groups (p = .01). The frequency of the “AA” genotype was significantly higher among the AMS group (52.17%) than the non‐AMS group (30.53%) (p = .01). The genotype frequencies of HSPA1A rs1008438 “AA,” “CA,” and “CC” were 52.17% (n = 36), 34.78% (n = 24), and 13.04% (n = 9) in the AMS group, respectively, and 30.53% (n = 29), 48.42% (n = 46), and 21.05% (n = 20) in the non‐AMS group, respectively. Additionally, genotypes “CC” (OR, 0.36; 95% CI, 0.14–0.92) and “CA” (OR, 0.42; 95% CI, 0.21–0.84) of rs1008438 were associated with a significantly lower AMS risk than genotypes “AA” (p = .03 and p = .01, respectively; Figure 1‐a). Comparing the genotype of “CC + CA” with “AA” (Figure 1‐b), the results illustrated that the “CC + CA” genotype of rs1008438 was associated with a lower AMS risk (OR, 0.40; 95% CI, 0.21–0.77; p = .01). However, genotype “CC” (OR, 0.56; 95% CI, 0.24–1.33) of rs1008438 presents no significant differences when compared with genotypes “CA + AA” (p = .19; Figure 1‐c). These results illustrated that rs1008438 “AA” homozygous genotype was significantly associated with an elevated morbidity due to AMS compared with the homozygous “CC” and heterozygous “CA” genotypes. Taken together, HSPA1A rs1008438 was associated with AMS risk.
FIGURE 1.
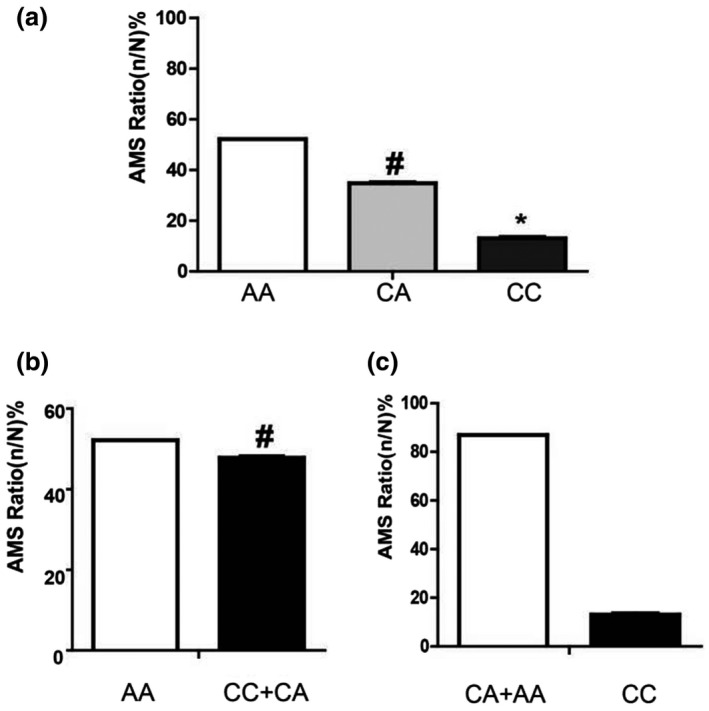
HSPA1A genotypes for rs1008438 and their association with AMS risk. (A) The association of genotypes “AA,” “CA,” and “CC” with AMS risk; (B) The association of genotypes “AA” and “CC + CA” with AMS risk; (C) The association of genotypes “CA + AA” and “CC” with AMS risk. *, p < 0.05;#, p < .01. AMS, acute mountain sickness
4. DISCUSSION
Acute mountain sickness is considered a multifaceted disease whose occurrence and development are influenced by genetic and environmental factors (Koirala et al., 2018; Singh, 2017). An individual's susceptibility to AMS under the specific environmental conditions may be related to its genetic makeup. Currently, 23 genes have been reported as putative genetic susceptibility factors for AMS. Among them, the most frequently reported are NOS3, HSPA1A, EPAS1, EGLN1, and VEGF.
NO is an effective vasodilator and plays an important role in acclimatization and adaptation to hypoxia. In the vasculature, NO acts locally via relaxing smooth muscles and thereby causing vasodilatation and is produced by NO synthases (NOSs) encoded by NOS3 (Altundag et al., 2014; MacInnis et al., 2011; Mansoor et al., 2005; Wang et al., 2010, 2013). Heat shock protein 70 (HSP70) family is a critical component of cytoprotective gene products (Qi et al., 2009). HSPA1A, one of the polymorphic genes of the HSP70 family, has been widely considered in association with a variety of disease symptoms. EPAS1 encodes a transcription factor that is involved in the hypoxia‐inducible factor pathway, which is the key regulator of responses to hypoxia (Buroker et al., 2012; Dulin et al., 2012; Guo et al., 2015). The EGLN1 gene encodes a vital oxygen sensor that negatively regulates hypoxia‐inducible factor‐1 alpha (HIF‐1A) activity (Yasukochi et al., 2018; Zhang et al., 2014). The inactivation of EGLN1 caused by hypoxia leads to an increase of HIF activity, which induces the expression of adaptive responses genes. This pathway suggests that EGLN1 may be an important factor for high‐altitude adaptation in the population. Under hypoxic conditions, VEGF expression is significantly upregulated and the high levels of VEGF in the lungs can cause pulmonary vascular permeability increase, leading to the occurrence of pulmonary edema (Ding et al., 2012). In most studies, it has been reported that VEGF is a key factor in the pathogenesis of high‐altitude adaptation and sickness (Bian et al., 2016; Buroker et al., 2013; Yu et al., 2016). Thus, we selected 5 SNPs (rs1008438, rs150877473, rs1799983, rs2153364, and rs3025039) in these genes to investigate their association with AMS.
Recent genomic surveys of SNPs among humans in high‐altitude environments have uncovered evidence for a history of positive selection of these genes. The most reliable signals include several genes with known roles in oxygen transport and regulation. One of these genes, HSPA1A was proved to be divergent between lowland and highland populations. Within the superfamily of HSP, HSPA1A has anti‐inflammatory and anti‐apoptotic functions. Two studies have associated variants of HSP gene polymorphisms with AMS in Chinese populations. Li et al. detected polymorphisms in the HSP70‐1 (b1/b2; +190G/C) and HSP70‐2 (A/B; +1267A/G) genes in their study; however, no association was found between the HSP70‐1 polymorphism and AMS, whereas the HSP70‐2 B/B genotype was over‐represented in individuals with AMS (Li et al., 2004). Additionally, Zhou et al. also detected the same polymorphisms in HSP70‐1 and HSP70‐2, and another polymorphism (A/B; 2437G/C) in the HSP70‐hom gene and their study confirmed the findings of Li et al. (Zhou et al., 2005).
Although five SNPs were investigated in the present study, the results demonstrated that only the HSPA1A polymorphism (rs1008438) might be a potential factor for the development of AMS. In our study, genotypes “CC” and “CA” of rs1008438 were associated with lower AMS risk than the genotypes “AA” (p = .03 and p = .01, respectively). Comparing with “AA” genotypes, the results also indicated that the “CC + CA” genotype of rs1008438 was associated with lower AMS risk (p = .01). Our analysis demonstrated that the “A” allele carriers were at a 2.29‐fold higher risk of developing AMS than the “C” allele carriers. Thus, the “A” allele of the HSPA1A (rs1008348) polymorphism was associated with an increased risk of AMS, having implications for Han populations in particular.
Previous studies have reported that several genetic variants of EPAS1 are associated with high‐altitude adaptation in Tibetans. Among these variants, the derived G allele of rs150877473 was 78% of a higher frequency in Tibetans than in Han Chinese (Yi et al., 2010). However, in our study, the EPAS1 rs150877473 polymorphism was not found to be related to AMS development; the reason may be that our subjects were Han Chinese only.
Additionally, our study has several limitations that may influence our results. First,the participants of this study are young Han Chinese under 30 years and in good health. For that reason, they may be better adapted to the plateau than the general population and this fact might explain the low incidence of AMS in our study. Second, the sample size of the original case‐control genetic association study was small. Further studies elaborating higher number of samples are required to confirm our findings.
CONFLICT OF INTEREST
All authors declared that there was no conflict of interest.
AUTHOR CONTRIBUTIONS
Xiaomei Wang and Chunyan Yao performed the experiment; Zhicheng Liu and Ting Xu analyzed the data and written manuscript; Hong Chen and Chunyan Yao designed the research and revised the manuscript. All authors have approved the final manuscript to submission.
ACKNOWLEDGMENTS
We are very grateful to all the volunteers for participating in this study.
Liu Z, Chen H, Xu T, Wang X, Yao C. HSPA1A gene polymorphism rs1008438 is associated with susceptibility to acute mountain sickness in Han Chinese individuals. Mol Genet Genomic Med. 2020;8:e1322 10.1002/mgg3.1322
Zhicheng Liu and Hong Chen have contributed equally to this work.
Funding information
This study was supported by the Foundation of Major Project of Military Science and Technology during the Twelfth Five‐Year Plan Period (AWS14L005), Special Project of the Central Military Commission (17BJZ12), and the Youth Development Projects from the Southwest Hospital of Third Military Medical University (SWH2018QNLC‐08).
DATA AVAILABILITY STATEMENT
The data were not shared to protect participants' privacy.
REFERENCES
- Akunov, A. C. , Sartmyrzaeva, M. A. , Maripov, A. M. , Muratali Uulu, K. , Mamazhakypov, A. T. , Sydykov, A. S. , & Sarybaev, A. S. (2017). High altitude pulmonary edema in a mining worker with an abnormal rise in pulmonary artery pressure in response to acute hypoxia without prior history of high altitude pulmonary edema. Wilderness & Environmental Medicine, 28, 234–238. 10.1016/j.wem.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Altundag, A. , Salihoglu, M. , Cayonu, M. , Cingi, C. , Tekeli, H. , & Hummel, T. (2014). The effect of high altitude on nasal nitric oxide levels. European Archives of Oto‐rhino‐laryngology, 271, 2583–2586. 10.1007/s00405-014-3170-8 [DOI] [PubMed] [Google Scholar]
- Bian, S.‐Z. , Jin, J. , Dong, J.‐Q. , Li, Q.‐N. , Yu, J. , Tang, C.‐F. , … Huang, L. (2016). A higher baseline somatization score at sea level as an independent predictor of acute mountain sickness. Physiology & Behavior, 167, 202–208. 10.1016/j.physbeh.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Buroker, N. E. , Ning, X.‐H. , Zhou, Z.‐N. , Li, K. , Cen, W.‐J. , Wu, X.‐F. , … Chen, S.‐H. (2012). EPAS1 and EGLN1 associations with high altitude sickness in Han and Tibetan Chinese at the Qinghai‐Tibetan Plateau. Blood Cells Molecules and Diseases, 49, 67–73. 10.1016/j.bcmd.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Buroker, N. E. , Ning, X.‐H. , Zhou, Z.‐N. , Li, K. , Cen, W.‐J. , Wu, X.‐F. , … Chen, S.‐H. (2013). VEGFA SNPs and transcriptional factor binding sites associated with high altitude sickness in Han and Tibetan Chinese at the Qinghai‐Tibetan. Plateau. Journal of Cellular Physiology, 63, 183–193. 10.1007/s12576-013-0257-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, H. , Liu, Q. , Hua, M. , Ding, M. , Du, H. , Zhang, W. , … Zhang, J. (2011). Polymorphisms of hypoxia‐related genes in subjects susceptible to acute mountain sickness. Respiration, 81, 236–241. 10.1159/000322850 [DOI] [PubMed] [Google Scholar]
- Ding, H. , Liu, Q. , Hua, M. , Ding, M. , Du, H. , Zhang, W. , … Zhang, J. (2012). Associations between vascular endothelial growth factor gene polymorphisms and susceptibility to acute mountain sickness. Journal of International Medical Research, 40, 2135–2144. 10.1177/030006051204000611 [DOI] [PubMed] [Google Scholar]
- Du, H. , Zhao, J. , Su, Z. , Liu, Y. , & Yang, Y. (2018). Sequencing the exons of human glucocorticoid receptor (NR3C1) gene in Han Chinese with high‐altitude pulmonary edema. Journal of Physiological Anthropology, 37(1), 7 10.1186/s40101-018-0168-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulin, E. , Garcia‐Barreno, P. , & Guisasola, M. C. (2012). Genetic variations of HSPA1A, the heat shock protein levels, and risk of atherosclerosis. Cell Stress & Chaperones, 17, 507–516. 10.1007/s12192-012-0328-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonggalanzi, , Labasangzhu, , Bjertness, E. , Wu, T. , Stigum, H. , & Nafstad, P. (2017). Acute mountain sickness, arterial oxygen saturation and heart rate among Tibetan students who reascend to Lhasa after 7 years at low altitude: A prospective cohort study. British Medical Journal Open, 7, e016460 10.1136/bmjopen-2017-016460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L. I. , Zhang, J. , Jin, J. , Gao, X. , Yu, J. , Geng, Q. , … Huang, L. (2015). Genetic variants of endothelial PAS domain protein 1 are associated with susceptibility to acute mountain sickness in individuals unaccustomed to high altitude: A nested case‐control study. Experimental and Therapeutic Medicine, 10, 907–914. 10.3892/etm.2015.2611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackinger, S. , Kraaijenbrink, T. , Xue, Y. , Mezzavilla, M. , Asan, van Driem, G. , … Ayub, Q. (2016). Wide distribution and altitude correlation of an archaic high‐altitude‐adaptive EPAS1 haplotype in the Himalayas. Human Genetics, 135, 393–402. 10.1007/s00439-016-1641-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala, P. , Wolpin, S. E. , & Peterson, J. T. (2018). High altitude illness: Knowledge, practice, and attitudes of porters in Nepal. Wilderness & Environmental Medicine, 29, 431–436. 10.1016/j.wem.2018.06.002 [DOI] [PubMed] [Google Scholar]
- Li, F. Z. , Zhou, F. , Jiang, C. Z. , Sun, S. Y. , He, M. A. , Zhang, S. Y. , … Wu, T. C. (2004). Relationship between heat stress protein 70 gene polymorphisms and the risk of acute mountain sickness [Chinese]. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi, 22, 413–415. 10.1016/j.csr.2003.12.006 [DOI] [PubMed] [Google Scholar]
- Li, Y. , Zhang, Y. , & Zhang, Y. (2018). Research advances in pathogenesis and prophylactic measures of acute high altitude illness. Respiratory Medicine, 145, 145–152. 10.1016/j.rmed.2018.11.004 [DOI] [PubMed] [Google Scholar]
- Loeppky, J. A. , Icenogle, M. V. , Maes, D. , Riboni, K. , Scotto, P. , & Roach, R. C. (2003). Body temperature, autonomic responses, and acute mountain sickness. High Altitude Medicine & Biology, 4, 367–373. 10.1089/152702903769192322 [DOI] [PubMed] [Google Scholar]
- MacInnis, M. J. , Wang, P. , Koehle, M. S. , & Rupert, J. L. (2011). The genetics of altitude tolerance: The evidence for inherited susceptibility to acute mountain sickness. Journal of Occupational and Environmental Medicine, 53, 159–168. 10.1097/JOM.0b013e318206b112 [DOI] [PubMed] [Google Scholar]
- Mansoor, J. K. , Morrissey, B. M. , Walby, W. F. , Yoneda, K. Y. , Juarez, M. , Kajekar, R. , … Schelegle, E. S. (2005). L‐arginine supplementation enhances exhaled NO, breath condensate VEGF, and headache at 4,342 m. High Altitude Medicine & Biology, 6, 289–300. 10.1089/ham.2005.6.289 [DOI] [PubMed] [Google Scholar]
- Ogilvie, R. I. (2001). High‐altitude illness. New England Journal of Medicine, 345, 1279–1280. 10.1056/NEJM200110253451713 [DOI] [PubMed] [Google Scholar]
- Qi, Y. , Niu, W. Q. , Zhu, T. C. , Liu, J. L. , Dong, W. Y. , Xu, Y ., … Qiu, C. C. (2009). Genetic interaction of Hsp70 family genes polymorphisms with high‐altitude pulmonary edema among Chinese railway constructors at altitudes exceeding 4000 meters. Clinica Chimica Acta, 405, 17–22. 10.1016/j.cca.2009.03.056 [DOI] [PubMed] [Google Scholar]
- Rossetti, G. M. K. , Macdonald, J. H. , Wylie, L. J. , Little, S. J. , Newton, V. , Wood, B. , … Oliver, S. J. (2017). Dietary nitrate supplementation increases acute mountain sickness severity and sense of effort during hypoxic exercise. Journal of Applied Physiology, 123, 983–992. 10.1152/japplphysiol.00293.2017 [DOI] [PubMed] [Google Scholar]
- Seys, S. F. , Daenen, M. , Dilissen, E. , Van Thienen, R. , Bullens, D. M. , Hespel, P. , … Dupont, L. J. (2013). Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax, 68, 906–913. 10.1136/thoraxjnl-2013-203280 [DOI] [PubMed] [Google Scholar]
- Shen, G. , Wu, X. , Tang, C. , Yan, Y. , Liu, J. , Guo, W. , … Zhang, J. (2013). An oxygen enrichment device for lowlanders ascending to high altitude. BioMedical Engineering OnLine, 12, 100 10.1186/1475-925X-12-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, L. C. (2017). High altitude dermatology. Indian Journal of Dermatology, 62, 59–65. 10.4103/0019-5154.198050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, N. L. , Wang, L. Y. , Xi, Y. , & Hu, Q. Z. (2013). Effect of renin‐angiotensin system on arterial function in persons with acute mountain sickness. International Journal of Cardiology, 167, 1641–1642. 10.1016/j.ijcard.2012.11.029 [DOI] [PubMed] [Google Scholar]
- Tissot van Patot, M. C. , Leadbetter, G. , Keyes, L. E. , Bendrick‐Peart, J. , Beckey, V. E. , Christia, U. , & Hackett, P. (2005). Greater free plasma VEGF and lower soluble VEGF receptns Uor‐1 in acute mountain sickness. Journal of Applied Physiology, 1985(98), 1626–1629. 10.1152/japplphysiol.00589.2004 [DOI] [PubMed] [Google Scholar]
- Wang, P. , Ha, A. Y. , Kidd, K. K. , Koehle, M. S. , & Rupert, J. L. (2010). A variant of the endothelial nitric oxide synthase gene (NOS3) associated with AMS susceptibility is less common in the Quechua, a high altitude Native population. High Altitude Medicine & Biology, 11, 27–30. 10.1089/ham.2009.1054 [DOI] [PubMed] [Google Scholar]
- Wang, Q.‐Q. , Yu, L. , Huang, G.‐R. , Zhang, L. U. , Liu, Y.‐Q. , Wang, T.‐W. , … Xiong, H.‐Y. (2013). Polymorphisms of angiotensin converting enzyme and nitric oxide synthase 3 genes as risk factors of high‐altitude pulmonary edema: A case‐control study and meta‐analysis. Tohoku Journal of Experimental Medicine, 229, 255–266. 10.1620/tjem.229.255 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Chen, H. , Li, R. , Fu, W. , & Yao, C. (2018). The effects of respiratory inhaled drugs on the prevention of acute mountain sickness. Medicine, 97, e11788 10.1097/MD.0000000000011788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West, J. B. (2015). High‐altitude medicine. The Lancet Respiratory Medicine, 3, 12–13. 10.1016/S2213-2600(14)70238-3 [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Xu, J. , Tang, F. , Ga, Q. , Li, Y. , Guan, W. , & Ge, R. L. (2018). NR3C2 gene is associated with susceptibility to high‐altitude pulmonary edema in Han Chinese. Wilderness & Environmental Medicine, 29, 488–492. 10.1016/j.wem.2018.07.006 [DOI] [PubMed] [Google Scholar]
- Yasukochi, Y. , Nishimura, T. , Motoi, M. , & Watanuki, S. (2018). Association of EGLN1 genetic polymorphisms with SpO2 responses to acute hypobaric hypoxia in a Japanese cohort. Journal of Physiological Anthropology, 37, 9 10.1186/s40101-018-0169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, X. , Liang, Y. , Huerta‐Sanchez, E. , Jin, X. , Cuo, Z. X. P. , Pool, J. E. , … Wang, J. (2010). Sequencing of 50 human exomes reveals adaptation to high altitude. Science, 329, 75–78. 10.1126/science.1190371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J. , Zeng, Y. , Chen, G. , Bian, S. , Qiu, Y. , Liu, X. I. , … Huang, L. (2016). Analysis of high‐altitude syndrome and the underlying gene polymorphisms associated with acute mountain sickness after a rapid ascent to high‐altitude. Scientific Reports, 6, 38323 10.1038/srep38323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, E. , Zhang, J. , Jin, J. , Qin, J. , Li, H. , & Huang, L. (2014). Variants of the low oxygen sensors EGLN1 and HIF‐1AN associated with acute mountain sickness. International Journal of Molecular Sciences, 15, 21777–21787. 10.3390/ijms151221777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, F. , Wang, F. , Li, F. , Yuan, J. , Zeng, H. , Wei, Q. , … Wu, T. (2005). Association of hsp70‐2 and hsp‐hom gene polymorphisms with risk of acute high‐altitude illness in a Chinese population. Cell Stress & Chaperones, 10, 349–356. 10.1379/csc-156.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data were not shared to protect participants' privacy.


