Abstract
Individuals with both post-traumatic stress disorder and major depressive disorder (PTSD + MDD) often show greater social and occupational impairment and poorer treatment response than individuals with PTSD alone. Increasing evidence reveals that the amygdala, a brain region implicated in the pathophysiology of both of these conditions, is a complex of structurally and functionally heterogeneous nuclei. Quantifying the functional connectivity of two key amygdala subregions, the basolateral (BLA) and centromedial (CMA), in PTSD + MDD and PTSD-alone could advance our understanding of the neurocircuitry of these conditions. 18 patients with PTSD + MDD, 28 with PTSD-alone, and 50 trauma exposed healthy controls (TEHC), all from a cohort who survived the same large earthquake in China, underwent resting-state functional magnetic resonance imaging. Bilateral BLA and CMA functional connectivity (FC) maps were created using a seed-based approach for each participant. The analysis of covariance of FC was used to determine between-group differences. A significant interaction between amygdala subregion and diagnostic group suggested that differences in connectivity patterns between the two seeds were mediated by diagnosis. Post-hoc analyses revealed that PTSD + MDD patients showed weaker connectivity between right BLA and (a) left anterior cingulate cortex/supplementary motor area, and (b) bilateral putamen/pallidum, compared with PTSD-alone patients. Higher CMA connectivities left ACC/SMA were also observed in PTSD + MDD compared with PTSD-alone. An inverse relationship between the connectivity of right BLA with right putamen/pallidum and MDD symptoms was found in PTSD + MDD. These findings indicate a relationship between the neural pathophysiology of PTSD + MDD compared with PTSD-alone and TEHC and may inform future clinical interventions.
Keywords: Post-traumatic stress disorder, Major depressive disorder, Amygdala, Fear processing, Resting-state functional connectivity
1. Introduction
Post-traumatic stress disorder (PTSD) is a debilitating disease characterized by re-experiencing, avoidance, emotional numbing, and hyperarousal (Association, 2013), which has become a major worldwide public health problem (Breslau, 2001; Kessler et al., 2005). PTSD is frequently comorbid with major depressive disorder (MDD), with approximately half of people with PTSD also suffering from MDD across diverse epidemiological samples (Caramanica et al., 2014; Kessler et al., 1995; Rytwinski et al., 2013). Patients with both PTSD and MDD (PTSD + MDD) show greater social, occupational, and neurocognitive impairment (Campbell et al., 2007; Nijdam et al., 2013), and are more likely to attempt suicide (Cougle et al., 2009; Morina et al., 2013). They also show poorer treatment response than patients with PTSD or depression alone (Campbell et al., 2007; Chan et al., 2009). It is therefore important to determine the neurobiological overlap and differences between PTSD + MDD, PTSD-alone, and healthy controls.
The amygdala has been associated with the neural pathophysiology of both PTSD and MDD in numerous studies (Kemp et al., 2007; Price and Drevets, 2010; Ross et al., 2017). Neuroimaging studies of PTSD suggest the presence of disrupted neural circuits between amygdala and other regions related to fear processing (e.g., anterior cingulate cortex (ACC), striatum, hippocampus, insular cortex, and medial prefrontal cortex (PFC)) (Fonzo et al., 2010; Kim et al., 2011; Lazarov et al., 2017; Linnman et al., 2011; Rabinak et al., 2011). While most of these studies analyze data from the entire amygdala, the amygdala is a complex of structurally and functionally heterogeneous nuclei rather than a single homogeneous unit (Ball et al., 2007; Morris et al., 2001). Specialized roles of basolateral (BLA) and centromedial amygdala (CMA) complexes have been identified during fear conditioning in PTSD (Jovanovic and Ressler, 2010; Mahan and Ressler, 2012). The BLA receives inputs from many cortical and subcortical regions, including PFC, thalamus, and hippocampus, and facilitates associative learning processes such as fear conditioning (LeDoux, 2003; Phelps and LeDoux, 2005). The CMA, in contrast, receives mostly modulatory inputs from the BLA and medial PFC, and has a critical role in fear expression via its projections to the brainstem, thalamus, forebrain, as well as to cortical and striatal regions (Duvarci and Pare, 2014; LeDoux, 2003).
Four recent studies examined amygdala subregion-based functional networks in PTSD patients compared with healthy controls (HC) or trauma-exposed healthy controls (TEHC) using resting-state functional MRI. All of these studies revealed dissociable connectivity profiles of the BLA and CMA subregions (Aghajani et al., 2016; Brown et al., 2014; Nicholson et al., 2015; Zhu et al., 2017). One study observed stronger resting-state functional connectivity (rsFC) between BLA and ACC in the PTSD vs. the TEHC group, but no group differences were found in CMA connectivity (Brown et al., 2014). In contrast, similar studies in adolescent PTSD reported that PTSD patients had weaker right BLA rsFC with ACC and PFC cortices compared with HCs, but stronger connectivity between left CMA and orbitofrontal and subcallosal cortices (Aghajani et al., 2016). Another study showed widespread cortical and subcortical differences in the functional connectivity with BLA and CMA when comparing both the dissociative subtype of PTSD and non-dissociative PTSD patients to HCs (Nicholson et al., 2015). Many factors may explain these inconsistencies, including differences in control groups (i.e., HC or TEHC group), comorbidity of PTSD (i.e., different proportions of patients comorbid with MDD in each of the aforementioned studies), and different trauma type (childhood or adulthood, repeated or a single massive event).
To date, only one study has investigated directly whether there are differences in amygdala functional connectivity at the subregional level between PTSD-alone and PTSD + MDD using a between-region-of-interest (ROI) connectivity analysis (Zhu et al., 2017). That study showed that the PTSD + MDD group exhibited weaker functional connectivity between BLA and orbitalfrontal cortex than either PTSD-alone or TEHC subjects, suggesting the presence of deficits in amygdala pathways confined to PTSD + MDD comorbid patients. Nonetheless, a whole-brain group analysis, beyond these predefined ROIs, is needed to comprehensively understand the functional brain networks involving the amygdala at a subregional level in PTSD + MDD. Moreover, the neural circuits in PTSD within a single trauma type remains to be addressed, as differences in the sources of trauma and in the timing of traumatic events between PTSD and TEHC subjects could explain different results reported in previous studies (Deering et al., 1996; Hull, 2002).
The present study sought to examine connectivity patterns of the amygdala subregions in PTSD with and without MDD, with respect to matched TEHC in a cohort of earthquake survivors from the 2008 Wenchuan, Richter Scale 8.0-magnitude earthquake (the same type of trauma at the same time for all subjects). First, we hypothesized that PTSD + MDD subjects will show weaker connectivity between BLA and regulatory prefrontal regions, such as PFC and ACC, compared with PTSD-alone and TEHC subjects, and stronger connectivity between CMA and fear expression regions such as striatum and thalamus, according to previous findings (Aghajani et al., 2016; Duvarci and Pare, 2014; Zhu et al., 2017). Second, based on earlier work implicating distinctive roles of BLA and CMA within the amygdala-centered network dysfunction in abnormal fear processing and excessive fear responses (Cisler et al., 2014; Etkin et al., 2009; Jovanovic and Ressler, 2010; LeDoux, 2003; Roy et al., 2009; Shin and Liberzon, 2010), we hypothesized that dissociable BLA and CMA connectivity profiles will be revealed in each patient group and in the TEHC group, with largely cortical connectivity patterns expected for the BLA and subcortical connectivity patterns expected for the CMA. Finally, as exploratory analyses, we investigated whether amygdala connectivity would be related to depressive symptoms in PTSD + MDD patients.
2. Experimental procedures
2.1. Participants
Participants were recruited between 2015 and 2016 from one of the most devastated areas affected by the 2008 Wenchuan 8.0-magnitude earthquake (Stone, 2009). Inclusion criteria were as follows: aged 18 to 60 years, right-handed, experienced the earthquake, witnessed people buried and suffered heavy property losses in the disaster. Exclusion criteria were contraindication to MRI imaging. We did not prospectively recruit participants based on the presence or absence of PTSD or MDD; these diagnoses were assessed following enrollment of all individuals who met enrollment criteria and consented to study participation. We recruited 107 participants who completed functional magnetic resonance imaging (MRI) and clinical assessments, which were completed within two days of enrollment for each participant.
Participants were assessed using the DSM-IV Structured Clinical Interview (SCID) (First et al., 1997), the Clinical Administered PTSD Scale (CAPS) (Blake et al., 1995), Hamilton Depression Rating Scale-24 item (HAMD-24) and Hamilton Rating Scale for Anxiety (HAMA-14). All PTSD participants were required to meet the diagnostic criteria of PTSD in DSM-IV and have a CAPS total score of > 40 to ensure at least moderate symptom severity. MDD diagnosis was determined by SCID DSM-IV criteria for a major depressive episode. The exclusion criteria for PTSD patients included any previously serious traumatic events, any history of psychiatric medication or psychological therapy, any history of Axis I psychiatric diagnosis other than comorbid depressive and anxiety disorders, any history of neurological disease, mental retardation, major head injury involving loss of consciousness for more than 10 min, any history of alcohol and/or other substance abuse/dependence, metal implants (e.g., surgical clips or pacemakers), and pregnancy. For TEHCs, the exclusion criteria were the same as PTSD patients except that a CAPS total score of < 20 was required.
We excluded a group with intermediate symptoms (CAPS >20 but <40, n = 7) from the participants. In addition, three patients with PTSD and one TEHC were excluded due to excessive head movement during the MRI scan. We analyzed a final sample of functional MRI (fMRI) data from 18 participants with PTSD + MDD, 28 with PTSD-alone, and 50 TEHCs. In the PTSD + MDD group, two patients were diagnosed with a comorbid panic disorder (full remission), one with general anxiety disorder and one patient had comorbid dysthymia, but these participants were not excluded.
This study was approved by the Medical Ethics Committee of West China Hospital, Sichuan University, and all subjects gave written informed consent.
2.2. Image acquisition and data preprocessing
For every participant, both resting-state blood-oxygen-level-dependent (BOLD) fMRI images and T1-weighted images were acquired using a 3.0-T MRI imaging system (Siemens 3.0 T Trio, Erlangen, Germany) with a 12-channel phased-array head coil, as described in our previous study (Zhu et al., 2015). Participants were instructed to relax with eyes closed; without falling asleep; and without directed, systematic thought during the 6.8 min (205 vol) scan. Details of the scanning parameters are provided in the Supplementary Materials.
MRI data preprocessing was performed using the Data Processing Assistant for Resting-State fMRI (DPARSF_V4.3) in DPABI (http://rfmri.org/dpabi) (Yan et al., 2016), which is based on SPM (https://www.fil.ion.ucl.ac.uk/spm/). Considering the magnetization saturation effects and participants’ adaptation to the scanning conditions, the first 5 vol of each data set were discarded. The remaining 200 consecutive functional volumes were first slice-time corrected and then motion corrected. As described above, data from three PTSD patients and one TEHC were discarded due to excessive head motion (translational or rotational parameters exceeded ± 1.5 mm or ± 1.5° or the mean framewise displacement (FD) exceeded 0.3 mm). Nuisance covariates were regressed out, including linear trends, white matter signal, cerebrospinal fluid signal, the Friston 24-parameter model (Friston et al., 1996), and spike regression (Satterthwaite et al., 2013; Yan et al., 2013) (for more details see the Supplementary Materials). Global signal regression (GSR) was not performed as it was demonstrated that GSR may induce network-specific negative biases in connectivity measures (Glasser et al., 2016; Yang et al., 2016) and distort group differences and correlation patterns (Saad et al., 2012). Then, the T1 images were registered to the averaged EPI image and spatial normalization was performed to a 3-mm Montreal Neurological Institute template. Smoothing was performed using a 6-mm, full-width half maximum (FWHM) Gaussian kernel inconsistent with previous studies (Wang et al., 2015; Zhao et al., 2018). Finally, band-pass filtering with a frequency window of 0.01 to 0.1 Hz was performed to reduce the effects of low-frequency machine magnetic field drifts and high-frequency respiratory and cardiac noise.
2.3. Region of interest definition and functional connectivity analysis
Amygdala subregion masks were derived from the Juelich Histological Atlas (Amunts et al., 2005). In accordance with previous studies (Aghajani et al., 2016; Qiu et al., 2018), voxels were included in the subregion masks only if the probability of their assignment to the BLA or CMA was higher than that for other nearby structures and greater than 40% likelihood. Each voxel was exclusively assigned to a single subregion, resulting in four seed regions (left BLA: 2160 mm3 , right BLA: 2295 mm3 , left CMA: 378 mm3 , right CMA: 486 mm3 ) for subsequent functional connectivity (FC) analyses. The average time series for each seed was computed across all voxels and correlated with the time series of every voxel in the brain in order to create four FC maps per participant. Both positive and inverse correlations were examined. FC maps were standardized using a Fisher z transformation, resulting in individual z-maps for second-level group analysis.
2.4. Statistical analyses
The second-level group analysis was conducted using SPM12. A whole-brain 3 (group) × 2 (subregion) full-factorial analysis of covariance (ANCOVA) was conducted for each hemisphere, with age, sex, education level and mean FD included as covariates.
The group × subregion interaction, main effects of group (PTSD + MDD, PTSD-alone and TEHC groups) and subregion (BLA and CMA seed regions) on rsFC were determined. The statistical F-maps were corrected for multiple comparisons using family-wise error (FWE) cluster-corrected (cluster-level p < 0.05) when using a primary cluster determining threshold of p < 0.001. The FC values (average z-values) were extracted from the voxel clusters showing significant differences in the group × subregion interaction using Marsbar (Brett et al., 2002). To test our first hypothesis, post-hoc, two-sample between-group contrasts were explored for each seed region based on these FC values, comparing both PTSD patient groups, and each patient group to TEHC group. For the comparison between PTSD + MDD and PTSD alone, baseline CAPS scores were used as covariates to ensure that between-group differences in rsFC were attributable to group effect as opposed to an effect of symptom severity. In addition, one-sample within-group analyses were conducted for each of the three diagnostic groups, individually for each amygdala seed region to test our second hypothesis, which produced thresholded z-maps of both positively and negatively correlated voxels associated with each amygdala subregion in each group for each hemisphere.
In addition, correlation analyses were performed between the FC values in PTSD + MDD and PTSD-alone and PTSD and MDD symptom severity (i.e., CAPS score and HAMD score) respectively. We also examined the association between the whole-brain FC maps and CAPS and HAMD scores respectively in a linear regression model in SPM 12, without grouping the participants (n = 96) to better explore the relationship between FC values and dimensional PTSD and depression severity. Details of the analyses were presented in Supplementary Materials.
3. Results
3.1. Demographics and clinical variables
As shown in Table 1, differences were found between all patients with PTSD and TEHCs in CAPS, HAMD, HAMA scores (p < 0.001), while no group difference was found with respect to age, sex or education level (p > 0.05). When comparing PTSD + MDD with PTSD-alone groups, as expected, we observed greater symptom severity in the PTSD + MDD group with respect to depression (p < 0.001), PTSD (p = 0.003), and anxiety symptoms (p < 0.001). In addition, there were more females in the PTSD + MDD group than the PTSD-alone group (p < 0.05).
Table 1.
Demographic and clinical information.
| Characteristics | PTSD all (n = 46) |
TEHC (n = 50) |
p (PTSD vs. TEHC) |
PTSD + MDD (n = 18) |
PTSD-alone (n = 28) |
p (MDD vs. PTSD-a) |
|---|---|---|---|---|---|---|
| Age (years) | 45.6 ±6.7 | 45.0 ±6.6 | 0.657 | 45.3 ±5.9 | 45.8 ±7.3 | 0.786 |
| Sex (female/male) | 34/12 | 32/18 | 0.295 | 17/1 | 17/11 | 0.028a |
| Education (years) | 8.5 ±3.5 | 8.8 ±3.1 | 0.689 | 7.6 ±3.9 | 9.0 ±3.2 | 0.157 |
| HAMD-24 | 15.5 ±7.9 | 4.1 ±4.0 | <0.001 | 22.4 ±6.1 | 11.0 ±5.2 | <0.001 |
| HAMA-14 | 13.7 ±7.0 | 3.3 ±3.7 | <0.001 | 18.7 ±5.9 | 10.5 ±5.6 | <0.001 |
| CAPS-total | 73.9 ±22.2 | 6.9 ±6.4 | <0.001 | 85.6 ±20.7 | 66.3 ±20.0 | 0.003 |
Abbreviations: HAMD, Hamilton Depression Scale; HAMA, Hamilton Anxiety Scale; CAPS, Clinician-Administered PTSD Scale; TEHC, trauma-exposed healthy controls; MDD, major depressive disorder.
Chi-Square Test Continuity Correction.
3.2. Group differences in BLA and CMA functional connectivity
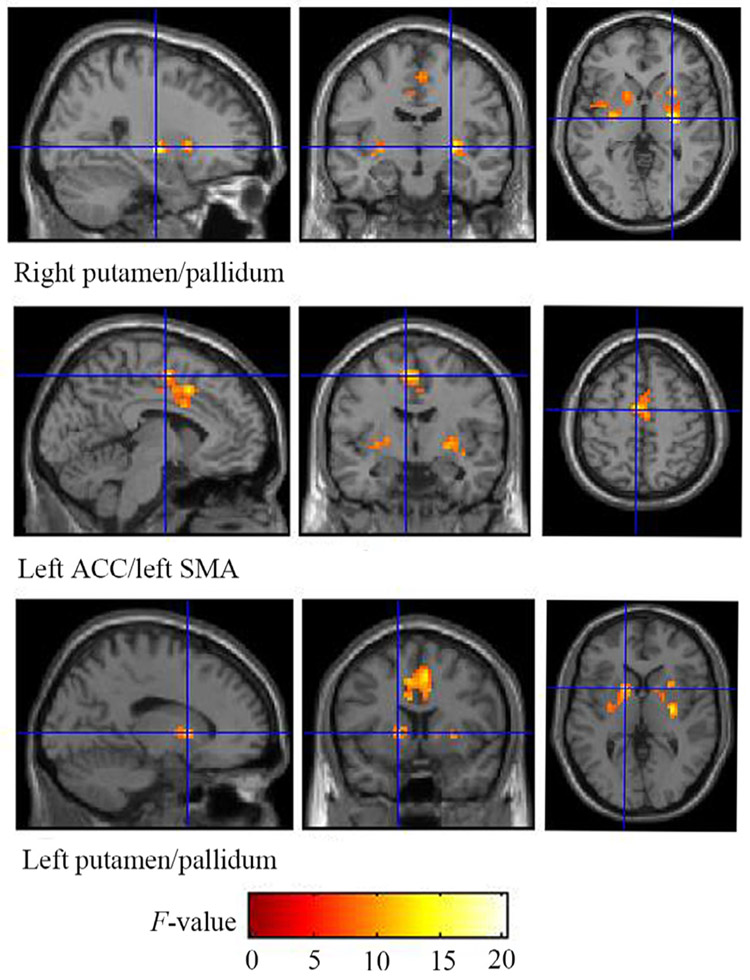
No main effect of group on the functional connectivity profiles was observed when considering both subregions of amygdala together. A significant group × subregion interaction for BLA and CMA seed regions in the right hemisphere on rsFC was observed from the ANCOVA, yielding three significant (FWE cluster-corrected threshold, p < 0.05) gray matter clusters (Table 2, Fig. 1). No group × subregion interaction was observed for the seed regions in the left hemisphere. For the post-hoc group comparisons, the PTSD + MDD group showed weaker BLA connectivities with left ACC/SMA and bilateral putamen/pallidum, and higher CMA connectivities left ACC/SMA, generated from the group × subregion interaction, compared with the PTSD-alone group (p < 0.05, FDR corrected). The following statistical directions were also observed, but none surviving FDR correction (p < 0.05, uncorrected): PTSD + MDD also showed higher CMA-left putamen/pallidum connectivities compared with PTSD-alone; PTSD-alone exhibited higher BLA- left ACC/SMA connectivities and BLA-right putamen/pallidum connectivities compared with TEHC (Fig. 2).
Table 2.
Brain regions of significance from group ×Subregion interaction.
| Hemisphere of seed region |
Brain region | Cluster size | MNI coordinate |
F (2, 184) | Z-score | p FWE | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Right | Right putamen/pallidum | 72 | 27 | −12 | 0 | 20.33 | 5.60 | 0.016 |
| Left ACC/left SMA | 302 | −6 | −6 | 54 | 15.13 | 4.79 | < 0.001 | |
| Left putamen/pallidum | 71 | −15 | 9 | 3 | 12.25 | 4.26 | 0.017 | |
| Left | None | |||||||
Abbreviations: ACC, anterior cingulate cortex; SMA, supplementary motor area; FWE, family-wise error cluster-corrected threshold.
Fig. 1.
Brain regions of significance from the group × subregion interaction for the BLA and CMA seed regions in the right hemisphere (FWE cluster-corrected threshold, cluster-level p < 0.05 when using a primary cluster determining threshold of p < 0.001) on resting-state functional connectivity. Three gray matter clusters were identified: the right putamen/pallidum, the left ACC/SMA and the left putamen/pallidum. ACC, anterior cingulate cortex; SMA, supplementary motor area; BLA, basolateral amygdala; CMA, centromedial amygdala.
Fig. 2.
Box and Whisker plot showing group differences of the mean zFC values and standard deviations extracted from the BLA and CMA pathways. * p < 0.05 (FDR corrected); zFC, Fisher z transformed functional connectivity; BLA, basolateral amygdala; CMA, centromedial amygdala; ACC, anterior cingulate cortex; SMA, supplementary motor area.
3.3. BLA and CMA connectivity profiles within PTSD + MDD, PTSD-alone and tehc groups
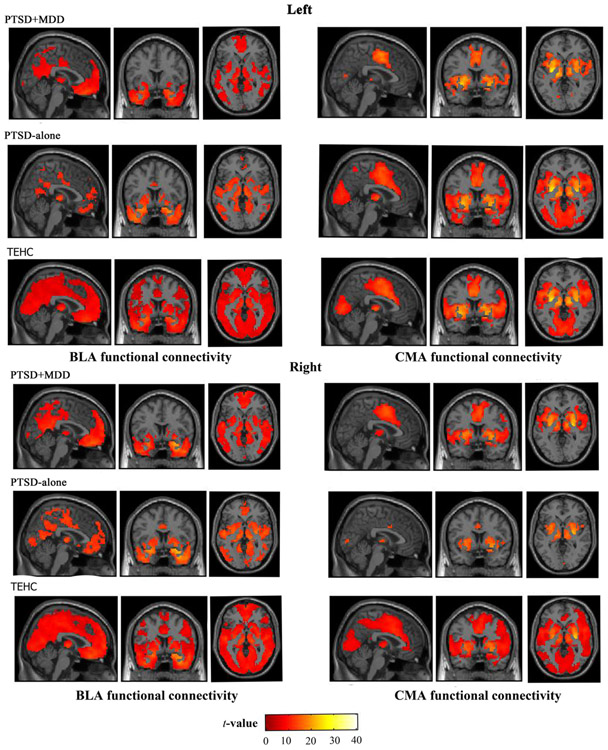
A main effect of subregion on rsFC was observed from the ANCOVA (p < 0.05, FWE corrected, k = 10), indicating distinct BLA and CMA connectivity profiles across all PTSD and TEHC subjects. As shown in Fig. 3, whole-brain within-group rsFC analysis revealed different BLA and CMA connectivity profiles with cortical and subcortical regions in each group p < 0.05, FWE corrected, k = 10). No significant negative correlations with the amygdala subregions were observed for within-group connectivity maps. The BLA and CMA connectivity profiles, observed in PTSD + MDD and PTSD-alone group, are generally consistent with established models of amygdalar circuitry in PTSD (Aghajani et al., 2016; Brown et al., 2014; LeDoux, 2007).
Fig. 3.
Whole-brain voxel-wise resting-state function connectivity profiles with left basolateral amygdala (BLA) and centromedial amygdala (CMA) seeds and right and BLA and CMA seeds are displayed in the PTSD + MDD group, the PTSD-alone group and TEHC group, separately (FWE corrected, p < 0.05).
3.4. Relationship between functional connectivity and clinical measures
Post-hoc correlation analyses between the FC values extracted from the ANCOVA interaction clusters of significance and CAPS and HAMD scores were performed within both patient groups separately. A negative relationship between BLA-right putamen/ pallidum connectivity and HAMD (r = −0.570, p = 0.014, uncorrected) was observed in the PTSD + MDD group (Fig. 4). No significant correlations were observed between the FC values and clinical measures in PTSD-alone patients. For dimensional depression severity (HAMD scores across groups), a negative correlation with rsFC between left BLA and bilateral putamen/pallidum (FWE cluster-corrected, p < 0.05) (Table S1, Fig. S1) was also observed. We did not observe any correlation with FC values for PTSD symptom across groups.
Fig. 4.
Correlations between HAMD scores and zFC values of BLA- right putamen/ pallidum connectivity (r = −0.570, p = 0.014, uncorrected). HAMD, Hamilton Depression Rating Scale; zFC, Fisher z transformed functional connectivity; BLA, basolateral amygdala.
4. Discussion
The present study compares the whole-brain connectivity patterns of basolateral and centromedial subnuclei of the amygdala between PTSD patients with or without MDD and TEHCs. The entire study population is derived from a cohort exposed to the same massive earthquake. Group differences were found in three functional connectivity pathways. The connectivities between right BLA and left ACC/SMA, and between right BLA and bilateral putamen/pallidum, were weaker in PTSD + MDD compared with PTSD-alone. We also found distinct BLA and CMA connectivity profiles in each group, which complemented and extended previous research into the circuitry of anxiety and depression (LeDoux, 2007; Nicholson et al., 2015; Roy et al., 2009). The negative correlation between BLA-right putamen/pallidum connectivity values and HAMD score in PTSD + MDD patients, further links MDD comorbidity in the context of PTSD to this stress pathway.
We found weaker rsFC between the BLA and bilateral putamen/pallidum in PTSD + MDD versus PTSD-alone, along with an inverse correlation between depressive symptoms (i.e., HAMD scores) and BLA-right putamen/pallidum connectivity. Analyses of association between dimensional depression severity and rsFC confirmed this finding. Because we did not find any correlation between the connectivity values of any of the significant pathways with PTSD symptoms (i.e., CAPS scores) in either PTSD + MDD or PTSD-alone, this indicated that the between-group differences in rsFC may be more closely related to severity of MDD comorbidity, as opposed to greater PTSD symptom severity in PTSD + MDD. The BLA connects with striatal areas in addition to connecting with the central nucleus (LeDoux, 2007). Previous fMRI studies found that individuals with depression have lower activation in the putamen during the perception of happy faces (Lawrence et al., 2004; Phan et al., 2002). Moreover, females with MDD displayed attenuated functional connectivity between amygdala and the cortico-striatal-pallidal-thalamic circuit (Yang et al., 2017), which is involved in the maintenance of information in working memory (Levy et al., 1997). There is an association between working memory as a main cognitive deficit and PTSD (McNally, 2006; Wisdom et al., 2014). Therefore, the weaker BLA-putamen/pallidum connectivity in PTSD + MDD in our study may subserve emotionally-mediated working memory impairment.
We also found weaker functional connectivity between right BLA and a cluster including mostly the dorsal portion of left ACC (dACC), extending into the left SMA, in PTSD + MDD compared with PTSD-alone. Although the post-hoc differences between PTSD + MDD and TEHCs were not significant after correction for multiple comparison, PTSD +MDD patients showed less connectivity between BLA and ACC than either PTSD-alone or TEHC groups. The dACC has been recognized as key nodes of the salience network (SN), which is responsible for detecting both interoceptive and external salient changes in the environment (Biswal et al., 2010; Seeley et al., 2007). Studies have observed intrinsic connectivity of the dACC with subcortical nodes consisting of the sublenticular extended amygdala (Menon, 2011; Seeley et al., 2007). Less BLA-dACC connectivity may underlie the difficulty of PTSD + MDD patients in distinguishing relevant salient cues and avoidance of situations that could generate interoceptive or environmental stimulus overload (Williams, 2016). Moreover, imbalances of amygdala and ACC/PFC activation, as well as impaired amygdala-ACC connectivity have also been consistently observed in MDD (Carballedo et al., 2011; Disner et al., 2011; Matthews et al., 2008). The SMA was believed to be among the network of neural regions mediating top-down control of negative affect (Ray and Zald, 2012) and has previously been implicated in emotion regulation success (Wager et al., 2008). Taken together, our findings indicated that the impairment in the frontal-limbic circuit is aggravated in PTSD with comorbid MDD, reflecting more severe problems with detecting relevant salient cues and emotion regulation in this group.
We found higher CMA connectivities with left ACC/SMA in PTSD + MDD versus PTSD-alone, suggesting exaggerated fear expression in PTSD + MDD, which is in accordance with previous findings showing higher CMA connectivity with regulatory prefrontal regions in adolescents with PTSD compared with HCs (Aghajani et al., 2016). However, other studies observed no areas showing altered connectivity with the CMA (Brown et al., 2014; Zhu et al., 2017). This disagreement in the literature may be due to diversity of the study population, such as different trauma type of PTSD, medication history of PTSD patients, PTSD comorbidity, and different control groups in different studies. Nevertheless, it should be pointed out that the relatively small size of the CMA, as the seed region for functional connectivity, could also contribute to the lack of CMA connectivity differences found between groups. Studies comparing the connectivity patterns of different trauma sources of PTSD to both TEHCs and HCs are needed to replicate these findings and better define the role of CMA in PTSD.
The BLA and CMA connectivity profiles observed in each PTSD patient group are in agreement with functional connectivity patterns of the amygdala subregions shown previously in another PTSD group (Aghajani et al., 2016; Brown et al., 2014), specifically in that the BLA connectivity network targeted largely prefrontal cortex as well as some subcortical areas. Additionally, the CMA connectivity network targets mainly subcortical regions involved in fear expression, such as striatum and thalamus (LeDoux, 1998), as well as other brain regions including ACC and medial PFC. Moreover, our findings extend previous work (Brown et al., 2014) by showing different BLA and CMA connectivity profiles in different PTSD subgroups, which can lead to further understanding of the distinct roles of the BLA and CMA amygdala subregions in PTSD. The BLA and CMA functional connectivity patterns observed in the TEHC group were more widespread compared with previously reported results (Brown et al., 2014), possibly due to different trauma type of the participants and different motion correction methods (i.e. either removing or regressing out motion-corrupted time points) (Power et al., 2012; Yan et al., 2013).
Limitations of this study include the small number of males in PTSD + MDD group (1 male out of 18 patients), which may limit the generalizability of our results, as others report sex-related differences in amygdala functional connectivity (Kilpatrick et al., 2006). Furthermore, we did not perform multiple comparison correction for the relationship between the BLA-right putamen/ pallidum connectivity and HAMD in the PTSD + MDD group, as these were exploratory analyses. Nevertheless, this finding was confirmed by analyses of association between dimensional depression severity and rsFC. Future studies with larger sample size, testing these specific hypotheses, may better clarify the relationships. Four PTSD + MDD patients also had comorbidity other than depression, which may potentially affect the specificity of our results. As such, we repeated the main statistical analysis excluding the four patients and found that the main results remained the same (Supplementary Materials Table S2). Last, a MDD-only group was not included in the current study, preventing direct comparison of the functional connectivity in PTSD + MDD with only MDD.
In conclusion, the present study revealed differences between PTSD + MDD and PTSD-alone in resting-state functional connectivity of the amygdala subnuclei BLA and CMA, and differences from TEHC. Weaker BLA-right putamen/pallidum connectivity was more closely related to severity of MDD comorbidity, as opposed to greater PTSD symptom severity in PTSD + MDD, indicating an important role of MDD comorbidity in the neural pathophysiology in PTSD. Weaker BLA-ACC/SMA connectivity in PTSD + MDD may be related to difficulties in distinguishing relevant salient cues and avoidance of situations that could generate interoceptive or environmental stimulus overload and deficits in emotion regulation.
These findings indicate a relationship between the neural pathophysiology of PTSD + MDD compared with PTSD-alone and TEHC and may inform future clinical interventions.
Supplementary Material
Acknowledgments
We thank the participants in this study. Minlan Yuan is supported by a scholarship from the China Scholarship Council. This work was also supported by the National Institute of Mental Health K01MH108721 (SPP).
Role of funding source
This study was supported by National Key Research & Development Program of China (Grant no. 2016YFC1307200) and the Special Project on Natural Chronic Non-infectious Diseases (Grant no. 2016YFC1307201); The National Natural Science Foundation of China (Grant nos. 81701328, 81871061 and 81371484); China Postdoctoral Science Foundation (Grant no. 2017M612972); Department of Science & Technology of Sichuan Province (Grant no. 2018SZ0131) and Postdoctoral Foundation of Sichuan University (Grant no. 2018SCU12042) to Dr. H.Z. The funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Declaration of Competing Interest
Drs. Mann receives royalties for commercial use of the Columbia-Suicide Severity Rating Scale (C-SSRS) from the Research Foundation for Mental Hygiene. Other authors have no conflicts of interest to report.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.euroneuro.2019.07.238.
References
- Aghajani M, Veer IM, van Hoof MJ , Rombouts SA, van der Wee NJ, Vermeiren RR, 2016. Abnormal functional architecture of amygdala-centered networks in adolescent posttraumatic stress disorder. Hum. Brain Mapp 37, 1120–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, Habel U, Schneider F, Zilles K, 2005. Cytoarchi-tectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol 210, 343–352. [DOI] [PubMed] [Google Scholar]
- Association, A.P. , 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Publishing. [Google Scholar]
- Ball T, Rahm B, Eickhoff SB, Schulze-Bonhage A, Speck O, Mutschler I, 2007. Response properties of human amygdala subregions: evidence based on functional mri combined with probabilistic anatomical maps. PLoS One 2, e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, 2010. Toward discovery science of human brain function. Proc. Natl. Acad. Sci 107, 4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW , Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM, 1995. The development of a clinician-administered ptsd scale. J. Trauma Stress 8, 75–90. [DOI] [PubMed] [Google Scholar]
- Breslau N, 2001. The epidemiology of posttraumatic stress disorder: what is the extent of the problem? J. Clin. Psychiatry 62 (Suppl 17), 16–22. [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline J-B, 2002. Region of interest analysis using an spm toolbox, 8th international conference on functional mapping of the human brain. Sendai 16, 497. [Google Scholar]
- Brown VM, LaBar KS, Haswell CC, Gold AL, Workgroup M-AM, Beall SK, Van Voorhees E, Marx CE, Calhoun PS, Fairbank JA, 2014. Altered resting-state functional connectivity of basolateral and centromedial amygdala complexes in posttraumatic stress disorder. Neuropsychopharmacology 39, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DG, Felker BL, Liu C-F, Yano EM, Kirchner JE, Chan D, Rubenstein LV, Chaney EF, 2007. Prevalence of depression-PTSD comorbidity: implications for clinical practice guidelines and primary care-based interventions. J. Gen. Intern. Med. 22, 711–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramanica K, Brackbill RM, Liao T, Stellman SD, 2014. Comorbidity of 9/11-Related ptsd and depression in the world trade center health registry 10-11 years postdisaster. J. Trauma Stress 27, 680–688. [DOI] [PubMed] [Google Scholar]
- Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Möller H-J, Doyle M, Wiesmann M, Frodl T, 2011. Functional connectivity of emotional processing in depression. J. Affect. Disord 134, 272–279. [DOI] [PubMed] [Google Scholar]
- Chan D, Cheadle AD, Reiber G, Unützer J, Chaney EF, 2009. Health care utilization and its costs for depressed veterans with and without comorbid ptsd symptoms. Psychiatr. Serv 60, 1612–1617. [DOI] [PubMed] [Google Scholar]
- Cisler JM, Steele JS, Lenow JK, Smitherman S, Everett B, Messias E , Kilts CD, 2014. Functional reorganization of neural networks during repeated exposure to the traumatic memory in posttraumatic stress disorder: an exploratory fMRI study. J. Psychiatr. Res 48, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR , Resnick H, Kilpatrick DG, 2009. PTSD, depression, and their comorbidity in relation to suicidality: cross-sectional and prospective analyses of a national probability sample of women. Depress. Anxiety 26, 1151–1157. [DOI] [PubMed] [Google Scholar]
- Deering CG, Glover SG, Ready D, Eddleman HC, Alarcon RD, 1996. Unique patterns of comorbidity in posttraumatic stress disorder from different sources of trauma. Compr. Psychiatry 37, 336–346. [DOI] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT, 2011. Neural mechanisms of the cognitive model of depression. Nat. Rev. Neurosci 12, 467. [DOI] [PubMed] [Google Scholar]
- Duvarci S, Pare D, 2014. Amygdala microcircuits controlling learned fear. Neuron 82, 966–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD, 2009. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry 66, 1361–1372 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M , Spitzer RL, Gibbon M, 1997. Structured Clinical Interview For DSM-IV Axis I disorders. American Psychiatric Press, Washington. [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB, 2010. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol. Psychiatry 68, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS , Turner R, 1996. Movement-related effects in fMRI time-series. Magn. Reson. Med 35, 346–355. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Smith SM, Marcus DS, Andersson JL, Auerbach EJ, Behrens TE, Coalson TS , Harms MP, Jenkinson M, Moeller S, 2016. The human connectome project’s neuroimaging approach. Nat. Neurosci 19, 1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull AM, 2002. Neuroimaging findings in post-traumatic stress disorder: systematic review. Br. J. Psychiatry 181, 102–110. [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ, 2010. How the neurocircuitry and genetics of fear inhibition may inform our understanding of ptsd. Am. J. Psychiatry 167, 648–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Felmingham K, Das P, Hughes G, Peduto AS, Bryant RA, Williams LM, 2007. Influence of comorbid depression on fear in posttraumatic stress disorder: an fMRI study. Psychiatry Res.: Neuroimaging 155, 265–269. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR , Walters EE, 2005. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry 62, 617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB, 1995. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry 52, 1048–1060. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Zald D, Pardo J, Cahill L, 2006. Sex-related differences in amygdala functional connectivity during resting conditions. Neuroimage 30, 452–461. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA , Palmer AL, Brown AC, Solomon KM, Marchante AN , Whalen PJ, 2011. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav. Brain Res 223, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS , Williams AM, Surguladze S , Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML, 2004. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol. Psychiatry 55, 578–587. [DOI] [PubMed] [Google Scholar]
- Lazarov A, Zhu X, Suarez-Jimenez B, Rutherford BR , Neria Y, 2017. Resting-state functional connectivity of anterior and posterior hippocampus in posttraumatic stress disorder. J. Psychiatr. Res 94, 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 1998. Fear and the brain: where have we been, and where are we going? Biol. Psychiatry 44, 1229–1238. [DOI] [PubMed] [Google Scholar]
- LeDoux J, 2003. The emotional brain, fear, and the amygdala. Cell. Mol. Neurobiol 23, 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J, 2007. The amygdala. Curr. Biol 17, R868–R874. [DOI] [PubMed] [Google Scholar]
- Levy R, Friedman HR, Davachi L, Goldman-Rakic PS, 1997. Differential activation of the caudate nucleus in primates performing spatial and nonspatial working memory tasks. J. Neurosci 17, 3870–3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeffiro TA, Pitman RK, Milad MR, 2011. An fMRI study of unconditioned responses in post-traumatic stress disorder. Biol. Mood Anxiety Disord 1, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ, 2012. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 35, 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP, 2008. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J. Affect. Disord 111, 13–20. [DOI] [PubMed] [Google Scholar]
- McNally RJ, 2006. Cognitive abnormalities in post-traumatic stress disorder. Trends Cogn. Sci. (Regul. Ed.) 10, 271–277. [DOI] [PubMed] [Google Scholar]
- Menon V, 2011. Large-scale brain networks and psychopathology: a unifying triple network model. Trends. Cogn. Sci 15, 483–506. [DOI] [PubMed] [Google Scholar]
- Morina N, Ajdukovic D, Bogic M, Franciskovic T, Kucukalic A, Lecic-Tosevski D, Morina L, Popovski M, Priebe S, 2013. Co-occurrence of major depressive episode and posttraumatic stress disorder among survivors of war: how is it different from either condition alone? J. Clin. Psychiatry 74, e212–e218. [DOI] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ, 2001. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage 13, 1044–1052. [DOI] [PubMed] [Google Scholar]
- Nicholson AA, Densmore M , Frewen PA, Théberge J, Neufeld RW, McKinnon MC, Lanius RA, 2015. The dissociative subtype of posttraumatic stress disorder: unique resting-state functional connectivity of basolateral and centromedial amygdala complexes. Neuropsychopharmacology 40, 2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijdam MJ, Gersons BP, Olff M, 2013. The role of major depression in neurocognitive functioning in patients with posttraumatic stress disorder. Eur. J. Psychotraumatol 4, 19979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I , 2002. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in pet and fMRI. Neuroimage 16, 331–348. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE, 2005. Contributions of the amygdala to emotion processing: from animal models to human behavior. Neuron 48, 175–187. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE, 2012. Spurious but systematic correlations in functional connectivity mri networks arise from subject motion. Neuroimage 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL , Drevets WC, 2010. Neurocircuitry of mood disorders. Neuropsychopharmacology 35, 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Xia M , Cheng B, Yuan L, Kuang W, Bi F , Ai H , Gu Z, Lui S, Huang X, He Y, Gong Q, 2018. Abnormal dynamic functional connectivity of amygdalar subregions in untreated patients with first-episode major depressive disorder. J. Psychiatry Neurosci 43, 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinak CA, Angstadt M, Welsh RC, Kennedy A, Lyubkin M, Martis B, Phan KL, 2011. Altered amygdala resting-state functional connectivity in post-traumatic stress disorder. Front Psychiatry 2, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Zald DH, 2012. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev 36, 479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DA, Arbuckle MR, Travis MJ, Dwyer JB, van Schalk-wyk GI, Ressler KJ, 2017. An integrated neuroscience perspective on formulation and treatment planning for posttraumatic stress disorder: an educational review. JAMA Psychiatry 74, 407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS , Kelly AC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP, 2009. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45, 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytwinski NK, Scur MD, Feeny NC, Youngstrom EA, 2013. The co-occurrence of major depressive disorder among individuals with posttraumatic stress disorder: a meta-analysis. J. Trauma Stress 26, 299–309. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW, 2012. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Elliott MA, Gerraty RT, Ruparel K , Loughead J, Calkins ME, Eickhoff SB, Hakonarson H , Gur RC, Gur RE, 2013. An improved framework for confound regression and filtering for control of motion artifact in the preprocessing of resting-state functional connectivity data. Neuroimage 64, 240–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD, 2007. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Liberzon I, 2010. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology 35, 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone R, 2009. A Deeply Scarred Land. American Association for the Advancement of Science. [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Xia M, Li K, Zeng Y, Su Y, Dai W, Zhang Q, Jin Z, Mitchell PB, Yu X, 2015. The effects of antidepressant treatment on resting-state functional brain networks in patients with major depressive disorder. Hum. Brain Mapp 36, 768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, 2016. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry 3, 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisdom NM, Pastorek NJ, Miller BI, Booth JE , Romesser JM, Linck JF, Sim AH, 2014. PTSD and cognitive functioning: importance of including performance validity testing. Clin. Neuropsychol. 28, 128–145. [DOI] [PubMed] [Google Scholar]
- Yan C-G, Cheung B, Kelly C, Colcombe S, Craddock RC, Di Martino A, Li Q, Zuo X-N , Castellanos FX, Milham MP, 2013. A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. Neuroimage 76, 183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Wang X-D, Zuo X-N, Zang Y-F, 2016. DPABI: data processing & analysis for (resting-state) brain imaging. Neuroinformatics 14, 339–351. [DOI] [PubMed] [Google Scholar]
- Yang GJ , Murray JD, Glasser M, Pearlson GD, Krystal JH, Schleifer C, Repovs G , Anticevic A, 2016. Altered global signal topography in schizophrenia. Cereb. Cortex 27, 5156–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Yin Y, Svob C, Long J, He X, Zhang Y , Xu Z, Li L, Liu J, Dong J, Zhang Z, Wang Z, Yuan Y, 2017. Amygdala atrophy and its functional disconnection with the cortico-striatal-pallidal-thalamic circuit in major depressive disorder in females. PLoS One 12, e0168239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao N, Yuan L-X, Jia X-Z, Zhou X-F, Deng X-P, He H-J, Zhong J , Wang J, Zang Y-F, 2018. Intra-and inter-scanner reliability of voxel-wise whole-brain analytic metrics for resting state fMRI. Front Neuroinform. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Qiu C, Meng Y, Cui H, Zhang Y, Huang X, Zhang J, Li T, Gong Q, Zhang W, 2015. Altered spontaneous neuronal activity in chronic posttraumatic stress disorder patients before and after a 12-week paroxetine treatment. J. Affect. Disord 174, 257–264. [DOI] [PubMed] [Google Scholar]
- Zhu X, Helpman L, Papini S, Schneier F, Markowitz JC, Van Meter PE, Lindquist MA, Wager TD, Neria Y, 2017. Altered resting state functional connectivity of fear and reward circuitry in comorbid PTSD and major depression. Depress. Anxiety 34, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.