Dear Editor,
Kunutsor and Laukkanen have written to this journal regarding elevated admission levels of markers of liver injury (alanine aminotransferase and aspartate aminotransferase, gamma-glutamyltransferase, alkaline phosphatase and total bilirubin) may be associated with progression to severe disease or death in COVID-19.1 On the other hand, serum cholinesterase plays an important role in the inflammatory response and may be associated with prognosis in sepsis.2, 3, 4 We focused on the similarities between severe COVID-19 pneumonia and sepsis.
We examined associations between cholinesterase levels on admission and the severity, and mortality of patients with COVID-19 pneumonia, as well as the interaction between cholinesterase and the previously reported factors of severity and mortality. We included patients who had tested positive for severe acute respiratory syndrome coronavirus 2 from February to May 2020 at Yokohama City University Hospital and Yokohama City University Medical Center. Ultimately, 26 patients were included in the study. Outcomes were aggravation of symptoms and in-hospital death.
The clinical characteristics of the patients grouped by severity are shown in Table 1 . There was no significant difference in patient characteristics between the groups. Supplementary Materials 1 shows the time course of cholinesterase and other factors in critically ill patients with good outcome and death. In critically ill patients with favorable outcome, cholinesterase, lymphocytes, albumin, and PaO2/FiO2 ratio decreased but C-reactive protein increased toward the peak of inflammation. Later, C-reactive protein decreased with improvement in inflammation, but there was a tendency for cholinesterase, lymphocytes, albumin, and PaO2/FiO2 ratio to increase. In contrast, in the severely ill patient who died, C-reactive protein poorly decreased, and cholinesterase, lymphocytes, albumin, and PaO2/FiO2 ratio were not elevated.
Table 1.
Clinical characteristics grouped by severity.
| Mild-to-moderate cases* (n = 11) | Severe cases⁎⁎ (n = 15) | p-value | ||||
|---|---|---|---|---|---|---|
| Median (interquartile range)/frequency (%) | Median (interquartile range)/frequency (%) | |||||
| Age | 70 | (49–75) | 69 | (61–77) | 0.878 | |
| Male | 7 | (64) | 14 | (93) | 0.128 | |
| Nationality – no. (%) | ||||||
| Japan | 8 | 12 | ||||
| United States | 1 | 2 | ||||
| China | 0 | 1 | ||||
| Canada | 1 | 0 | ||||
| Republic of the Philippines | 1 | 0 | ||||
| Past history – no. (%) | ||||||
| Diabetes | 2 | 8 | ||||
| Hypertension | 4 | 5 | ||||
| Chronic kidney disease | 0 | 3 | ||||
| Ischemiac heart disease | 0 | 2 | ||||
| Asthma | 1 | 1 | ||||
| Dyslipidemia | 1 | 0 | ||||
| Anything | 2 | 2 | ||||
| Oxygen-support therapy – no. (%) | ||||||
| Oxygen support | 7 | (64) | 15 | (100) | ||
| Mechanical ventilation | 0 | 13 | (87) | |||
| Extracorporeal membrane oxygenation | 0 | 4 | (27) | |||
| Treatment – no. (%) | ||||||
| Antibaiotics | 8 | (73) | 15 | (100) | ||
| Ciclesonide | 5 | (45) | 12 | (80) | ||
| Lopinavir/Ritonavir | 3 | (27) | 11 | (73) | ||
| Steroid | 3 | (27) | 3 | (20) | ||
| Favipiravir | 1 | (9) | 5 | (33) | ||
| Peramivir | 1 | (9) | 2 | (13) | ||
| Remdesivir | 0 | (0) | 2 | (13) | ||
| Nafamostat | 0 | (0) | 1 | (7) | ||
| Median laboratory values (IQR) | ||||||
| ChE (U/L) | 326 | (228–394) | 218 | (185–279) | 0.006 | |
| CRP (mg/dL) | 2.23 | (1.04–4.28) | 14.63 | (7.04–18.00) | <0.001 | |
| WBC (/μL) | 7100 | (5200–9300) | 7100 | (5900–11,200) | 0.574 | |
| Lymphocytes (%) | 16.9 | (7.6–22.3) | 7.0 | (5.8–10.8) | 0.047 | |
| Alb (g/dL) | 3.8 | (3.4–4.1) | 3.1 | (2.7–3.3) | 0.001 | |
| D-dimer (μg/dL) | 1.2 | (0.6–5.1) | 2.1 | (0.9–5.0) | 0.384 | |
| AST (U/L) | 29 | (24–39) | 56 | (38–94) | 0.025 | |
| ALT (U/L) | 20 | (16–56) | 30 | (18–46) | 0.467 | |
| P/F ratio (mmHg) | 308 | (300–380) | 154 | (113–209) | <0.001 | |
| Death | 0 | (0) | 6 | (40) |
0.051 | |
The mild-to-moderate group was defined based on the need for oxygen inhalation or no oxygen inhalation.
The severe group was defined as having a respiratory condition requiring ventilator management (PaO2/FiO2 ratio <200 mmHg to respiratory rate >30/min).
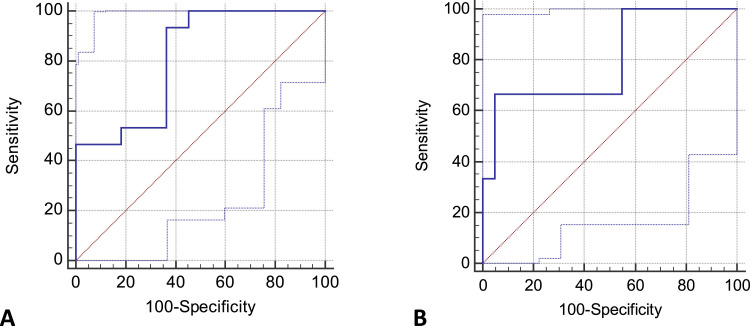
Fig. 1 A and Supplementary Materials 2 show the association between severity and cholinesterase levels in COVID-19 patients. Cholinesterase levels on admission were significantly lower in the severe group than in the mild-to-moderate group (326 vs. 218 IU/L, p = 0.006). The optimal cut-off value for cholinesterase on severe cases was 301 U/L using the ROC curve, sensitivity was 93.3, and specificity was 63.6. The area under the ROC curve (AUC) in this case was 0.81. The positive and negative likelihood ratios were 2.6 and 0.1, respectively. In addition, ROC curves of C-reactive protein, albumin, lymphocytes, D-dimer, and PaO2/FiO2 ratio were prepared, and their AUCs were determined to be 0.94, 0.87, 0.73, 0.61, and 0.94, respectively. Fig. 1B and Supplementary Materials 2 show the association between mortality and cholinesterase levels in COVID-19 patients. Cholinesterase levels on admission were significantly lower in the death group than in the survival group (274 vs. 187.5 IU/L, p = 0.028). The optimal cutoff value for cholinesterase on mortality was 190 U/L using the ROC curve, sensitivity was 66.7, and specificity was 95.0. The AUC in this case was 0.79. The positive and negative likelihood ratios were 12.7 and 0.4, respectively. ROC curves of C-reactive protein, albumin, lymphocytes, D-dimer, and PaO2/FiO2 ratio were prepared, and their AUCs were 0.84, 0.77, 0.66, 0.70 and 0.79, respectively. Supplementary Materials 3A and 3B show analyses of the interaction between cholinesterase and the previously established factors of severity and mortality.
Fig. 1.
Prediction of severity (A) and mortality (B) based on cholinesterase level on admission.
Our results demonstrate that the potential of cholinesterase levels and their interactions were significantly associated with severity and mortality in COVID-19 pneumonia patients. Cholinesterase is an enzyme produced in the liver that hydrolyzes cholinesters, and measured as a liver function test. Cholinesterase levels are high in patients with nephrotic syndrome, diabetes, hyperthyroidism, fatty liver, dyslipidemia, and obesity and low in patients with liver cirrhosis, hepatitis, malignant tumor, malnutrition, sepsis, and organophosphate poisoning.5 Although the mechanism underlying cholinesterase reduction in sepsis has not yet been determined, it is thought to be affected by acute-phase infections and inflammatory processes.6 It has been hypothesized that cholinesterase synthesis decreases owing to hepatic dysfunction with disease progression, capillary permeability enhancement, dilution with fluid challenges, cholinesterase catabolism enhancement, and cholinesterase inhibition by inflammatory mediators (cytokines).7
Levels of "positive" acute-phase proteins such as C-reactive protein, amyloid A, and ferritin generally increase in patients with inflammatory diseases. In contrast, levels of "negative" acute-phase proteins such as albumin, prealbumin, and transferrin decrease in response to inflammation and increase during the recovery period.2 , 8 Cholinesterase and lymphocytes behave similar to the "negative" acute-phase proteins in response to inflammation.9 , 10 Even in patients with COVID-19 pneumonia, amyloid A, which is classified as a "positive" acute-phase protein; albumin, which is classified as a "negative" acute-phase protein; and lymphocytes showing similar reactions as those of "negative" acute-phase proteins against inflammation have been suggested to be related to severity.4
Our study suggests that cholinesterase, which responds similar to the "negative" acute-phase proteins in response to inflammation, is reduced even in the acute phase of severe COVID-19 pneumonia. Following the changes in cholinesterase over time, we found that it decreased with deterioration of the condition and increased with improvement. Cholinesterase level on admission is suggested to be an independent predictor of severity and mortality for COVID-19 pneumonia. Cholinesterase levels on admission were significantly lower in the severe group than in the mild-to-moderate group, and they were also significantly lower in the death group than in the survival group. Cholinesterase was comparable to other markers, such as C-reactive protein, PaO2/FiO2 ratio, albumin, lymphocytes, and D-dimer regarding associations with the severity and mortality of COVID-19 pneumonia.
Limitations of this study include individual variances in cholinesterase, limited sample size, and potential bias owing to the confounding factors due to the retrospective nature of the study. Multiple factors may have been involved and multivariate analysis might have yielded more detailed results. Finally, owing to the limited number of facilities and regions, close attention should be paid to the generalization of the results.
In conclusion, cholinesterase may reflect the disease state of COVID-19 pneumonia, suggesting that a patient's cholinesterase level on admission may be useful as one of predictors of severity and prognosis. It has potential to be used as an indicator of severity or death and for recommending therapeutic interventions including intensive care during early stages of the disease.
Declaration of Competing Interest
None.
Acknowledgments
Acknowledgments
We express our deep appreciation to all of the staff at Yokohama City University Hospital and Yokohama City University Medical Center.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.08.021.
Appendix. Supplementary materials
References
- 1.Kunutsor S.K., Laukkanen J.A. Markers of liver injury and clinical outcomes in COVID-19 patients: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.05.045. published online ahead of print, 2020 May 28. S0163-4453(20)30325-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santarpia L., Grandone I., Contaldo F., Pasanisi F. Butyrylcholinesterase as a prognostic marker: a review of the literature. J Cachexia Sarcopenia Muscle. 2013;4(1):31–39. doi: 10.1007/s13539-012-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zivkovic A.R., Decker S.O., Zirnstein A.C., Sigl A., Schmidt K., Weigand M.A. A sustained reduction in serum cholinesterase enzyme activity predicts patient outcome following sepsis. Mediators Inflamm. 2018;2018 doi: 10.1155/2018/1942193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao R., Zhang X., Wang H., Zhang R., Duan X., Liu S. Value of serum cholinesterase in the prognosis of septic shock. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(1):44–49. doi: 10.3760/cma.j.cn121430-20191219-00008. [DOI] [PubMed] [Google Scholar]
- 5.Ramachandran J., Sajith K.G., Priya S., Dutta A.K., Balasubramanian K.A. Serum cholinesterase is an excellent biomarker of cirrhosis. Trop Gastroenterol. 2014;35(1):15–20. doi: 10.7869/tg.158. [DOI] [PubMed] [Google Scholar]
- 6.Das U.N. Acetylcholinesterase and butyrylcholinesterase as possible markers of low-grade systemic inflammation. Med Sci Monit. 2007;13(12):Ra214–Ra221. [PubMed] [Google Scholar]
- 7.Bahloul M., Baccouch N., Chtara K., Turki M., Turki O., Hamida C.B. Value of serum cholinesterase activity in the diagnosis of septic shock due to bacterial infections. J Intensive Care Med. 2017;32(5):346–352. doi: 10.1177/0885066616636549. [DOI] [PubMed] [Google Scholar]
- 8.Soeters P.B., Schols A.M. Advances in understanding and assessing malnutrition. Curr Opin Clin Nutr Metab Care. 2009;12:487–494. doi: 10.1097/MCO.0b013e32832da243. [DOI] [PubMed] [Google Scholar]
- 9.Camarero González E., Muñoz Leira V., Iglesias Guerrero M., Fernández Alvarez J.A., Cabezas-Cerrato J. Protein-energy malnutrition: its effects on 4 metabolic parameters. Nutr Hosp. 1995;10:158–160. [PubMed] [Google Scholar]
- 10.Hubbard R.E., O'Mahony M.S., Calver B.L., Woodhouse K.W. Plasma esterases and inflammation in ageing and frailty. Eur J Clin Pharmacol. 2008;64:895–900. doi: 10.1007/s00228-008-0499-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.