Abstract
Previous studies have found an inverse relation between serum concentrations of interleukin (IL)-6 and physical performance in seniors, however this was limited to higher functioning older adults with low to moderate levels of inflammation.
We explored the consistency of this association in a cohort of mobility limited older adults with chronic low-grade inflammation. This study included 289 participants (≥ 70 years old) with IL-6 level between 2.5 and 30 pg/ml and a walking speed < 1.0 m/sec from the ENRGISE Pilot study. Physical performance was assessed using the short physical performance battery (SPPB), usual gait speed over 400 meters, grip strength, and knee extensor and flexor strength measured by isokinetic dynamometry at 60 and 180°/sec.
There was a significant inverse correlation between log IL-6 and knee extensor strength at 60°/sec (r= −0.20, p= 0.002), at 180°/sec (r = −0.14, p= 0.037), and knee flexor strength at 60°/sec (r = −0.15, p= 0.021). After adjustment for potential confounders, the values of knee extensor strength at 60°/sec showed a trend toward a progressive reduction across IL-6 tertiles as IL-6 levels increased (p= 0.024). No significant association was found between IL-6 and other objectively measured physical performance.
The findings were generally of smaller magnitude and less consistent than previously reported, which suggests that the associations are attenuated in those with both elevated inflammation and mobility limitations. These results have implications for planning and interpreting future intervention studies in older adults with low-grade inflammation and mobility limitations.
Keywords: interleukin-6, physical performance, knee muscle strength, chronic low-grade inflammation
1. Introduction
Aging is associated with a progressive functional decline in both cognitive and physical performance (Guralnik et al., 1996). The appearance of a motor limitation commonly represents a risk factor for mobility disability in older adults (Wolinsky et al., 2005). Indeed, low physical performance as measured by lower extremity function and muscle strength, independently predict adverse outcomes such as physical disability, falls, hospitalization, institutionalization and mortality in older adults (Hicks et al., 2012; Legrand et al., 2014).
Accumulating evidence indicates that a chronic low-grade and systemic pro-inflammatory state occurs with increased age. It is primarily driven by a naturally progressive deterioration of the immune system, a process also known as immunosenescence, along with contributing factors (e.g. redox state, hormonal changes, adiposity, impaired autophagy) to this pro-inflammatory imbalance (Chung et al., 2009; Franceschi et al., 2017). Chronic low-grade inflammation (LGI) is intimately correlated with age-related decline in physical performance, with morbidity and mortality (Chung et al., 2009). In particular, older adults with low physical performance possess poorer muscle strength and higher levels of pro-inflammatory cytokine and chemokine, compared to their higher functioning counterparts (Calvani et al., 2017).
Among the plethora of inflammatory biomarkers, interleukin (IL)-6 is one of the most well accepted indicators of chronic systemic inflammation in older adults (Maggio et al., 2006). IL-6 has been shown to be positively associated with markers of physical frailty, such as slow gait speed, poor lower extremity performance and low muscle strength (Cesari et al., 2004; Taaffe et al., 2000; Visser et al., 2002), sarcopenia (Haddad et al., 2005), and also predict future disability in non-disabled older persons (Ferrucci et al., 1999). Circulating levels of IL-6 greater than 2.5 pg/ml have been identified as a reliable cut-off level indicating chronic LGI and are associated with an almost two-fold risk of functional decline (Ferrucci et al., 1999).
The causative link between elevated IL-6 and poor physical performance has not yet been fully established. Preclinical studies indicate that chronic inflammation overexpresses the ubiquitin-proteasome pathway, reduces insulin-like growth factors 1 and increases synthesis of muscle cortisol, all factors promoting skeletal muscle protein breakdown (Wilson et al., 2017). Epidemiological data suggest that the relationship between higher IL-6 levels and incident mobility disability might be mediated by a parallel decline of muscle strength (Ferrucci et al., 2002). Schaap et al., in a prospective population based study, showed that higher circulating IL-6 levels (> 4.9 pg/ml) predicted 3-fold higher risk of strength loss during three year follow-up (Schaap et al., 2006). Moreover, in a cohort of high-functioning elders (70–79 years old) a standard deviation increase in IL-6 was associated with up to 2.4 kg reduction of grip strength (Visser et al., 2002). There is also evidence that the association between inflammation and physical performance is attenuated at higher levels indicating a “risk threshold” (Ferrucci et al., 1999).
We sought to investigate the association between inflammation and physical performance in mobility limited older adults who already possess low grade chronic inflammation. Such knowledge would aid in understanding the degree to which inflammation remains relevant for predicting physical function. In turn, it provides evidence of the estimated effect that lowering inflammation would have on physical function improvements in a high risk group.
2. Materials and methods
2.1. Study Participants
The current study included baseline data from 289 participants from the ENRGISE (ENabling Reduction of low-Grade Inflammation in SEniors) Pilot study, a multi-center, double-blind, placebo-controlled randomized pilot trial designed to target older adults with chronic LGI, determined by IL-6 levels ≥ 2.5 pg/mL, and at risk for mobility impairment (Manini et al., 2017). Comprehensive details about the design and rationale of the ENRGISE Pilot study have been described elsewhere (Manini et al., 2017). The trial is registered at clinicaltrials.gov (identifier NCT01072500).
Participants were recruited from five different centers across the United States (Northwestern University, Tufts University, University of Florida, University of Pittsburgh, and Wake Forest School of Medicine) between April 2016 and July 2017. The ENRGISE Pilot study eligibility criteria were designed to target older adults (age 70 years and older) who: self-reported difficulty walking one-quarter of a mile or climbing a flight of stairs, had a slow walking speed (less than 1 m/s on a 4-m walk), were able to walk 400 meters in ≤15 minutes and had chronic-low grade inflammation defined as IL-6 levels between 2.5 pg/ml and 30 pg/ml on two occasions. Furthermore, participants were excluded if they had medical conditions characterized by very high levels of inflammation (e.g. acute infections, autoimmune diseases, cancer, renal failure on hemodialysis, terminal illnesses), major cognitive impairment as defined by Mini-Mental State Examination (MMSE) score <24, or with neurological or orthopedic conditions causing impaired mobility. More specific criteria about eligibility criteria for this study have been described elsewhere (Manini et al., 2017).
2.2. Ethics
The study protocol was approved by the Institutional Review Boards of each study site, and all participants provided written informed consent.
2.3. Measurements
2.3.1. Interleukin-6
Two assays were used to improve the detection sensitivity in the lower bound of IL-6 levels— where most values lie in a population of immune stable older adults. For the first test, IL-6 determined using a sandwich immunoassay (Human IL-6 Quantikine ELISA Kit, catalog #HS600B, R & D Systems, Minneapolis, MN) with detection range from 0.2 to 10 pg/mL. Levels greater than 10 pg/mL were evaluated using another assay (Human IL-6 QuantiGlo ELISA Kit, catalog #Q6000B, R & D Systems) with greater dynamic range (0.5 – 1,500 pg/mL). To increase the sensitivity and specificity of the test and confirm the chronic nature of inflammation, assessment of IL-6 levels was performed in two different sessions. Blood tests were done 1–3 three weeks apart, with the first being required to be between 2.3 and 30 pg/mL and the average of the two measures required to be between 2.5 and 30 pg/mL.
2.3.2. Short Physical Performance Battery (SPPB)
Short Physical Performance Battery (SPPB) consists of timed measures of gait speed, repeated chair stand and standing balance test. For the walking test, participants were asked to walk 4 m at usual pace. For the chair–stand test, participants were asked to fold their arms across their chest and to stand up from a sitting position and sit down five times as quickly as possible. For the balance test, participants were asked to stand in tandem as long as possible. Each task was assigned a score from 0 (inability to complete the task) to 4 (best performance, corresponding to the highest quartiles of time needed to complete the task). A summary score ranging from 0 to 12 was computed (Guralnik et al., 1994).
2.3.3. Gait Speed
Gait speed was evaluated based on the time to complete the 400 meter walk test within 15 minutes (Newman et al., 2006). Participants were asked to walk ten laps of a 20-meter course at their usual pace. Subjects were able to use a cane, but not to receive help of a person or use a walker. If needed, participants were allowed to stop and rest for up to 1 min, but not to sit down.
2.3.4. Hand Grip Strength
Maximal grip strength force was assessed two times in kilograms in both hands using a handheld dynamometer (Jamar, Lafayette Instruments, Lafayette, IN) (Hamilton et al., 1992). With their forearm resting on a table and elbow bent, participants were asked to hold the dynamometer and “squeeze as hard as they can”. The maximum value recorded from either hand was used for statistical analysis. Any participants who reported having a surgical procedure of the hand or pain in the wrist/hand did not undergo the test with that afflicted hand.
2.3.5. Knee Extension and Flexion Isokinetic Strength
Maximal isokinetic leg extension and flexion torque were measured in both legs using an isokinetic dynamometer (Biodex Inc., Shirley, NY) set at angular velocities of 60 and 180 degrees per second over 90 degrees pain-free range of motion (Taylor et al., 1991). Participants were placed in a comfortable seated position and secured using torso, pelvic, thigh, and shin stabilization straps in order to minimize extraneous body movements. The axis of rotation of the dynamometer was aligned with the axis of rotation of the subject’s knee joint considering as landmark the point at the center of a line that passes transversely through the femoral condyles. Two sets of five repetitions were performed for each velocity. The peak torque in newton-meters at 60 and 180 degrees/second for leg extension and flexion were used for statistical analyses.
2.4. Statistical Analyses
Baseline characteristics of participants in the ENRGISE Pilot study were summarized using means and standard deviations for continuous variables or percentages for categorical variables. We categorized IL-6 scores into distributional-free tertiles to explore whether increasing levels of IL-6 were associated with performance on the SPPB, 4- and 400-meter gait speed, isometric hand grip strength, isokinetic dynamometry of the knee extensors and flexors. All associations between physical function and IL-6 relied on log-transformed IL-6 to account for skewness. Relationships among plasma IL-6 concentrations and physical performance (measured by SPPB, gait speed) and muscle strength (isometric hand grip strength, isokinetic dynamometry of the leg extensors and flexors) were assessed using Pearson correlation coefficients for continuous associations and analysis of variance for function estimates across IL-6 tertiles.
Partial correlation coefficients were produced to examine whether IL-6 levels were independently associated to physical performance: Model 1) adjusted for age and sex; Model 2) Model 1 plus race (non-hispanic white vs. non-white), body mass index (BMI), smoking status (former vs. never), comorbidities >2 (type 2 diabetes, heart failure, hypertension, ischaemic heart diseases, stroke, chronic obstructive lung disease); Model 3) Model 2 plus use of renin angiotensin system (RAS) inhibitors, use of lipid-lowering drugs, and use of anti-inflammatory medications. Tests for model-adjusted relationships used partial Pearson correlations for continuous associations and analysis of covariance for function estimates across IL-6 tertiles.
All analyses were conducted with SAS v9.4 (SAS Institute, Cary, NC) using an overall level of significance of 0.05. For significant overall differences across IL-6 tertiles, post-hoc comparisons among the groups were performed at a Bonferroni-adjusted 0.0167 level.
3. Results
The characteristics of study participants (N= 289) are summarized in Table 1. Participants were predominantly male (52.6%) and white (77.9%) with a mean age of 78.3 ± 5.4 years, mean BMI of 31.5 ± 5.7 kg/m2, and mean waist circumference of 107.3 ± 14.9 cm. Approximately half the study sample (47.8%) reported two or more comorbidities, with a high representation from hypertension (69.2%), type 2 diabetes (23.5%), ischaemic heart disease (13.1%), history of cancer (37.7%). Analyzing medication use, 72.3% of participants took anti-inflammatory drugs, 60.9% lipid lowering agents and 43.9% antihypertensives of renin-angiotensin system inhibitors class. Overall the mean levels of IL-6 in the study sample was 4.8 ± 3.2 pg/ml. Mean SPPB score was 8.7 ± 2.1 and among the SPPB items, the most compromised test was the chair rise (mean score: 2.3) evaluating strength in lower limbs.
Table 1.
Characteristics of participants, overall and by IL-6 category (N=289)
| Description | Overall (N=289) | 1st IL-6 Tertile (N=96) | 2nd IL-6 Tertile (N=97) | 3rd IL-6 Tertile (N=96) | p-value |
|---|---|---|---|---|---|
| Age, years | 78.3 (5.4) | 78.1 (5.1) | 77.7 (5.6) | 79.1 (5.4) | 0.178 |
| Male | 152 (52.6) | 49 (51.0) | 42 (43.3) | 61 (63.5) | 0.018 |
| Race White | 225 (77.9) | 79 (82.3) | 74 (76.3) | 72 (75.0) | 0.411 |
| BMI, kg/m2 | 31.4 (5.7) | 30.8 (5.1) | 32.1 (6.2) | 31.4 (5.7) | 0.321 |
| Weight, kg | 87.0 (17.8) | 86.2 (16.7) | 87.2 (18.6) | 87.7 (18.1) | 0.841 |
| Waist Circumference, cm | 107.3 (14.9) | 107.4 (15.3) | 106.3 (15.1) | 108.3 (14.2) | 0.656 |
| High BP self-report | 200 (69.2) | 58 (60.4) | 74 (76.3) | 68 (70.8) | 0.053 |
| Type 2 Diabetes | 68 (23.5) | 21 (21.9) | 26 (26.8) | 21 (21.9) | 0.647 |
| Ischemic Heart Disease | 38 (13.1) | 10 (10.4) | 13 (13.4) | 15 (15.6) | 0.563 |
| Stroke | 13 (4.5) | 7 (7.3) | 2 (2.1) | 4 (4.2) | 0.211 |
| Cancer History | 109 (37.7) | 40 (41.7) | 31 (32.0) | 38 (39.6) | 0.341 |
| Pulmonary Disease | 6 (2.1) | 2 (2.1) | 2 (2.1) | 2 (2.1) | 0.999 |
| Congestive Heart Failure | 8 (2.8) | 2 (2.1) | 2 (2.1) | 4 (4.2) | 0.593 |
| 2 or more comorbidities | 138 (47.8) | 44 (45.8) | 47 (48.5) | 47 (49.0) | 0.897 |
| Former Smoker | 151 (52.8) | 50 (52.6) | 47 (49.5) | 54 (56.3) | 0.644 |
| IL-6, pg/mL | 4.77 (3.19) | 3.03 (0.27) | 3.98 (0.32) | 7.32 (4.52) | <.0001 |
| Time engaged in physical activity, min | 327.6 (647.3) | 354.3 (540.5) | 344.1 (909.4) | 284.2 (373.4) | 0.721 |
| Handgrip Strength, kg | 26.5 (8.8) | 26.6 (9.5) | 26.0 (8.5) | 27.0 (8.3) | 0.736 |
| Knee Extensor Strength 60°/sec, Nm | 82.4 (32.9) | 87.6 (33.2) | 81.5 (32.7) | 77.9 (32.5) | 0.154 |
| Knee Extensor Strength 180°/sec, Nm | 55.9 (21.4) | 58.0 (22.1) | 54.8 (21.7) | 54.8 (20.5) | 0.541 |
| Knee Flexor Strength 60°/sec, Nm | 43.4 (17.0) | 44.8 (16.2) | 43.7 (17.8) | 41.7 (17.1) | 0.505 |
| Knee Flexor Strength 180°/sec, Nm | 38.5 (17.1) | 39.4 (18.2) | 37.0 (17.0) | 38.9 (16.1) | 0.635 |
| Gait Speed 4 m walk, m/s | 0.805 (0.120) | 0.806 (0.133) | 0.798 (0.121) | 0.810 (0.106) | 0.782 |
| Gait Speed 400 m walk, m/s | 0.826 (0.165) | 0.850 (0.175) | 0.825 (0.168) | 0.803 (0.148) | 0.137 |
| SPPB total score | 8.7 (2.1) | 8.8 (2.1) | 8.3 (2.1) | 8.8 (2.0) | 0.186 |
| SPPB - Balance score | 3.0 (1.1) | 3.0 (1.1) | 2.8 (1.1) | 3.1 (1.1) | 0.234 |
| SPPB - Chair Rise score | 2.3 (1.3) | 2.5 (1.2) | 2.2 (1.4) | 2.3 (1.2) | 0.276 |
| SPPB - 4-meter gait score | 3.4 (0.7) | 3.4 (0.7) | 3.3 (0.7) | 3.4 (0.7) | 0.959 |
| Lipid Lowering Drugs | 176 (60.9) | 56 (58.3) | 61 (62.9) | 59 (61.5) | 0.803 |
| RAS Inhibitors | 127 (43.9) | 32 (33.3) | 48 (49.5) | 47 (49.0) | 0.037 |
| Anti-inflammatory Drugs | 209 (72.3) | 64 (66.7) | 75 (77.3) | 70 (72.9) | 0.251 |
All data are Mean (SD) or Count (%); IL-6 = Interleukin-6; BMI = body mass index; BP = blood pressure; SPPB = Short Physical Performance Battery; RAS = renin angiotensin system
Participants were relatively homogeneous in demographic, anthropometric, comorbidities, medication use and reported health behaviors across tertiles of IL-6 (lowest tertile: 2.5–3.48 pg/mL, n= 96; middle tertile: 3.48–4.61 pg/ml, n= 97; highest tertile: 4.61–30 pg/ml, n= 96).
3.1. IL-6 levels, muscle strength and physical performance
Table 2 contains adjusted correlation coefficients between IL-6 and muscle strength and physical performance. Adjusted partial correlations indicated a significant inverse relationship between IL-6 concentrations and knee extensor strength at 60°/sec (r = −0.20, p=0.002), 180°/sec (r = −0.14, p= 0.037) and knee flexor strength at 60°/sec (r = −0.15, p= 0.021). IL-6 levels were also negatively correlated to 400-meter gait speed (r = −0.12, p=0.038) in model 1, but the association was nullified after adjusting for model 2 covariates.
Table 2.
Adjusted associations between muscle strength and physical performance and log-transformed IL-6
| Variable | Model* | Log-IL6 Partial correlation | p-value |
|---|---|---|---|
| SPPB total score | 1 | −0.03 | 0.668 |
| 2 | 0.00 | 0.998 | |
| 3 | −0.01 | 0.861 | |
| SPPB - Balance score | 1 | 0.00 | 0.936 |
| 2 | 0.00 | 0.943 | |
| 3 | 0.00 | 0.954 | |
| SPPB - Chair Rise score | 1 | −0.04 | 0.548 |
| 2 | −0.01 | 0.891 | |
| 3 | −0.02 | 0.724 | |
| SPPB - 4-meter gait score | 1 | −0.02 | 0.757 |
| 2 | 0.01 | 0.899 | |
| 3 | 0.00 | 0.981 | |
| Handgrip Strength, kg | 1 | −0.03 | 0.660 |
| 2 | −0.03 | 0.605 | |
| 3 | −0.03 | 0.680 | |
| Knee Extensor Strength 60°/sec, Nm | 1 | −0.18 | 0.004 |
| 2 | −0.19 | 0.002 | |
| 3 | −0.20, | 0.002 | |
| Knee Extensor Strength 180°/sec, Nm | 1 | −0.11 | 0.087 |
| 2 | −0.1 | 0.034 | |
| 3 | −0.1 | 0.037 | |
| Knee Flexor Strength 60°/sec, Nm | 1 | −0.14 | 0.024 |
| 2 | −0.15 | 0.023 | |
| 3 | −0.15 | 0.021 | |
| Knee Flexor Strength 180°/sec, Nm | 1 | −0.04 | 0.539 |
| 2 | −0.05 | 0.464 | |
| 3 | −0.05 | 0.488 | |
| Gait Speed 4 m walk, m/s | 1 | 0.02 | 0.679 |
| 2 | 0.04 | 0.539 | |
| 3 | 0.04 | 0.560 | |
| Gait Speed 400 m walk, m/s | 1 | −0.12 | 0.038 |
| 2 | −0.10 | 0.083 | |
| 3 | −0.1 | 0.064 |
IL-6 = Interleukin-6; SPPB = Short Physical Performance Battery
Model 1: values adjusted for age at randomization and gender; Model 2: values adjusted for model 1 covariates, race (non-hispanic white vs. minority), body mass index, former smoker status, and having ≥ 2 comorbidities; Model 3: values adjusted for model 2 covariates, use of lipid-lowering drugs, anti-inflammatory drugs, and RAS inhibitors.
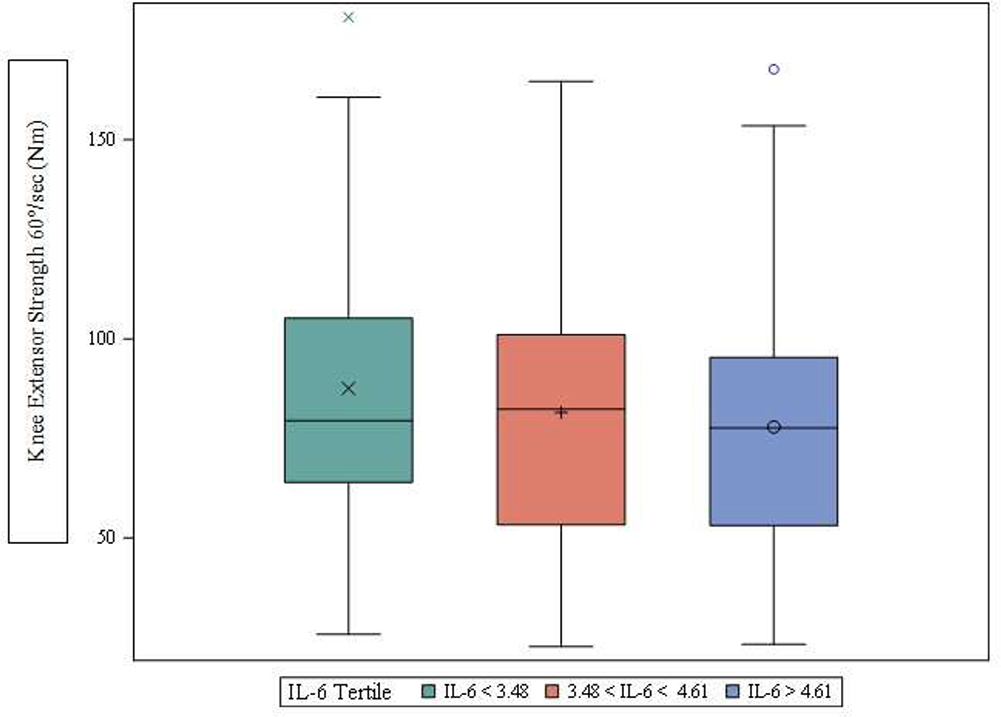
Table 3 contains least-squared means of muscle strength and physical performance values across IL-6 tertiles. After statistically adjusting for all covariates in model 3, participants in the highest tertile of IL-6 had approximately 13% lower leg extension muscle strength (p=0.024) (Figure 1). Other measures of leg strength and physical performance did not differ across IL-6 tertiles.
Table 3.
Least-squared means of muscle strength and physical performance values across IL-6 tertiles
| Variable | Model* | 1st Tertile IL-6 | 2nd Tertile IL-6 | 3rd Tertile IL-6 | p-value |
|---|---|---|---|---|---|
| SPPB total score | 1 | 8.8 (8.4, 9.2) | 8.3 (7.9, 8.7) | 8.8 (8.3, 9.2) | 0.198 |
| 2 | 8.7 (8.2, 9.2) | 8.3 (7.8, 8.8) | 8.8 (8.3, 9.3) | 0.206 | |
| 3 | 8.7 (8.2, 9.2) | 8.2 (7.7, 8.7) | 8.7 (8.2, 9.2) | 0.154 | |
| SPPB - Balance score | 1 | 3.0 (2.7, 3.2) | 2.8 (2.5, 3.0) | 3.1 (2.9, 3.3) | 0.086 |
| 2 | 3.0 (2.7, 3.2) | 2.7 (2.5, 3.0) | 3.1 (2.8, 3.4) | 0.088 | |
| 3 | 3.0 (2.7, 3.3) | 2.7 (2.4, 3.0) | 3.1 (2.8, 3.4) | 0.067 | |
| SPPB - Chair Rise score | 1 | 2.5 (2.2, 2.7) | 2.2 (2.0, 2.5) | 2.3 (2.0, 2.6) | 0.359 |
| 2 | 2.6 (2.2, 2.9) | 2.3 (2.0, 2.6) | 2.4 (2.1, 2.7) | 0.460 | |
| 3 | 2.5 (2.2, 2.8) | 2.3 (2.0, 2.6) | 2.4 (2.1, 2.7) | 0.436 | |
| SPPB - 4-meter gait score | 1 | 3.4 (3.2, 3.5) | 3.3 (3.2, 3.5) | 3.3 (3.2, 3.5) | 0.980 |
| 2 | 3.2 (3.0, 3.4) | 3.2 (3.0, 3.4) | 3.2 (3.1, 3.4) | 0.949 | |
| 3 | 3.2 (3.0, 3.4) | 3.2 (3.0, 3.4) | 3.2 (3.1, 3.4) | 0.961 | |
| Handgrip Strength, kg | 1 | 26.4 (25.1, 27.7) | 26.5 (25.2, 27.9) | 25.7 (24.4, 27.0) | 0.664 |
| 2 | 27.0 (25.4, 28.6) | 27.0 (25.4, 28.5) | 26.1 (24.5, 27.6) | 0.558 | |
| 3 | 27.0 (25.4, 28.7) | 27.0 (25.4, 28.6) | 26.1 (24.5, 27.7) | 0.549 | |
| Knee Extensor Strength 60°/sec (Nm) | 1 | 86.3 (80.1, 92.5) | 81.8 (75.6, 88.0) | 73.9 (67.6, 80.3) | 0.023 |
| 2 | 89.2 (81.3, 97.0) | 84.0 (76.4, 91.7) | 75.9 (68.3, 83.5) | 0.016 | |
| 3 | 88.6 (80.7, 96.4) | 83.7 (75.8, 91.5) | 76.0 (68.4, 83.7) | 0.024 | |
| Knee Extensor Strength 180°/sec (Nm) | 1 | 57.5 (53.4, 61.6) | 55.1 (51.0, 59.2) | 52.4 (48.2, 56.5) | 0.223 |
| 2 | 59.5 (54.4, 64.6) | 56.4 (51.4, 61.4) | 53.6 (48.7, 58.5) | 0.146 | |
| 3 | 59.2 (54.1, 64.4) | 56.9 (51.8, 62.1) | 54.1 (49.1, 59.1) | 0.228 | |
| Knee Flexor Strength 60°/sec (Nm) | 1 | 44.0 (40.8, 47.1) | 43.7 (40.5, 46.8) | 39.5 (36.3, 42.7) | 0.095 |
| 2 | 42.5 (38.6, 46.4) | 41.9 (38.1, 45.8) | 37.9 (34.1, 41.7) | 0.098 | |
| 3 | 42.3 (38.4, 46.3) | 42.2 (38.3, 46.2) | 38.1 (34.3, 42.0) | 0.122 | |
| Knee Flexor Strength 180°/sec, Nm | 1 | 39.2 (35.8, 42.6) | 37.0 (33.6, 40.4) | 37.1 (33.6, 40.5) | 0.585 |
| 2 | 39.6 (35.4, 43.9) | 37.0 (32.8, 41.1) | 37.2 (33.1, 41.3) | 0.480 | |
| 3 | 39.3 (35.1, 43.5) | 37.6 (33.4, 41.9) | 37.6 (33.4, 41.7) | 0.729 | |
| Gait Speed 4 m walk, m/s | 1 | 0.804 (0.780, 0.828) | 0.799 (0.775, 0.823) | 0.805 (0.781, 0.830) | 0.923 |
| 2 | 0.796 (0.766, 0.826) | 0.792 (0.762, 0.821) | 0.800 (0.771, 0.829) | 0.895 | |
| 3 | 0.794 (0.764, 0.825) | 0.790 (0.760, 0.820) | 0.800 (0.771, 0.830) | 0.849 | |
| Gait Speed 400 m walk, m/s | 1 | 0.847 (0.815, 0.879) | 0.829 (0.796, 0.861) | 0.797 (0.764, 0.829) | 0.095 |
| 2 | 0.824 (0.785, 0.863) | 0.815 (0.777, 0.853) | 0.787 (0.749, 0.824) | 0.246 | |
| 3 | 0.820 (0.781, 0.859) | 0.810 (0.771, 0.848) | 0.784 (0.746, 0.822) | 0.284 |
All data are Mean (95% confidence interval); IL-6 = Interleukin-6; SPPB = Short Physical Performance Battery
Model 1: values adjusted for age at randomization and gender; Model 2: values adjusted for model 1 covariates, race (non-hispanic white vs. minority), body mass index, former smoker status, and having ≥ 2 comorbidities; Model 3: values adjusted for model 2 covariates, use of lipid-lowering drugs, anti-inflammatory drugs, and RAS inhibitors.
Figure 1.
Adjusted knee extensor strength at 60°/sec across IL-6 tertiles from ANCOVA model adjusted for age, gender, race, BMI, former smoker status, ≥ 2 comorbidities, use of renin angiotensin system inhibitors, use of lipid-lowering drugs, and use of anti-inflammatory medications. The box indicates the 25th to 75th percentile; the line inside the box, the median; the symbol inside the box, the mean; the bars, the largest and smallest non-outlier values; the symbols outside the bars, the outliers.
4. Discussion
This study explored whether chronic LGI, measured by levels of IL-6, were associated with lower extremity muscle strength in mobility-limited older adults. Specifically, our findings showed that higher IL-6 levels were associated with poor knee extension muscle strength at 60° and 180°/sec, knee flexion muscle strength at 60°/sec, and slower walking speed on 400-m. These associations, however, were reduced after adjusting for confounders that are associated with higher inflammation and general physical function. These data suggest the associations between inflammation and physical performance are somewhat attenuated in mobility-limited older adults with chronic LGI when compared to previous work in the general older adult population with and without LGI.
In line with our findings, Ferrucci et al. demonstrated that a decline in knee extensor muscle strength may partially explain the higher risk of progression of physical disability and impairment in walking ability in older women with moderate to severe disability and high IL-6 levels. They found that leg strength was lower only in the subgroup with higher IL-6 levels with a cut point of 3.10 pg/ml which is close to the cut point used for defining chronic LGI in our study (Ferrucci et al., 2002). This study, along with others, was used to define cutoffs for LGI in the ENRGISE study (> 2.5 pg/ml). Given the low-to-moderate correlations found in the current study, the results support the notion that the window of IL-6 risk occurs when transitioning from less than to greater than 2.5 pg/ml. Additional physical function impairment with higher IL-6 levels, beyond 2.5 pg/ml, is marginal.
Higher IL-6 levels were not associated with slower 400-m and 4-m gait speed after adjusting for considered confounders. This finding is in contrast with previous works (Brinkley et al., 2009; Hsu et al., 2009; Verghese et al., 2011) as the Einstein Aging Study, a cohort of community-dwelling elderly subjects where higher IL-6 levels were associated with slow gait at baseline and faster decline in gait speed over 2.3-years of follow-up (Verghese et al., 2011). This discrepancy could be explained by truncating the variability in walking speeds in ENRGISE Pilot study by enrolling only those with 4-m walk speeds < 1.0 m/s who already possess LGI. In turn, this likely restricted the potential association with the IL-6 levels found in previous studies. Also, while 400-m gait speed was significant in the age and gender adjusted model, the association was reduced after adding clinical and behavioral conditions. This suggests that adjusting for these confounders likely had a large impact on the association with IL-6 particularly in a more vulnerable sample as ENRGISE.
Muscle weakness— or dynapenia— is a risk factor for mobility disability and a myriad of other health factors in older adults (Goodpaster et al., 2006; Manini and Clark, 2012). Despite its high relevance, there remains a dearth of knowledge about its causes. The current study and others found that inflammation may be a causal factor in muscle weakness (Brinkley et al., 2009; Cesari et al., 2004; Ferrucci et al., 2002; Hsu et al., 2009; Schaap et al., 2006; Visser et al., 2002). The basic science supports this notion — pro-inflammatory cytokines like IL-6 stimulate proteolytic pathways causing muscle degradation through ubiquitination. Additionally, inflammation impacts muscular support systems that lead to microvascular impairments, hormonal changes, insulin resistance, and impairment in the central nervous system motor control which can lead to muscle weakness (Fougere et al., 2017). Pro-inflammatory cytokines induce excessive generation of free-radical species in multiple organs including skeletal muscle (Reid, 2001; Supinski and Callahan, 2007). There is overwhelming evidence that imbalance of cellular redox system in the muscle can lead to reduction in muscle force generation. In vitro studies demonstrated that exposure, of isolated muscle or muscle cell lines, to pro-inflammatory cytokines reduces muscle-specific force without changing muscle mass or protein levels (Reid et al., 2002).
Our findings from a cohort of older adults at risk for mobility disability showed that a state of chronic elevation of IL-6 levels was associated with muscle weakness in lower limbs— specifically poor knee extensor strength. It is plausible that chronic LGI could be mechanistically linked, not only with sarcopenia (Wang et al., 2017; Wilson et al., 2017), but also, in an early stage, with dynapenia. There are a few potential mechanisms that could explain the observed connection between high levels of inflammation and lower muscle strength (Bodine et al., 2001; Li et al., 2000; Reid et al., 2002; Supinski and Callahan, 2007; Wang et al., 2017). Bodine et al. demonstrated that pro-inflammatory cytokines, such as TNF-α or interleukin-1, stimulate the expression of muscle ring finger 1 (MuRF-1) and muscle atrophy F-box (MAFbx), increase protein ubiquitination, and thereby accelerate ubiquitin-proteasome system-mediated protein degradation leading to loss and/or shrinkage of muscle fibers (Bodine et al., 2001). Additionally, cytokines with pro-inflammatory characteristics may also impact muscle strength by disrupting intrinsic force producing properties not related to fiber size—namely excitation-contraction coupling and regulation of calcium channels (Li et al., 2000; Reid et al., 2002).
This study has a few notable strengths and weakness. Regarding the former, the work represents a comprehensive study investigating the connection between IL-6 and multiple indices of physical functioning in older adults. Furthermore, the work was conducted in a large sample of older men and women (N=279) who underwent a rigorous assessment of IL-6 levels to confirm the presence of chronic LGI. The weaknesses include its cross-sectional nature which does not permit causal inferences between IL-6 levels and lower body strength. The measurement of chronic LGI was based only on IL-6 when many other exist (e.g. TNF-α, interleukin-1β). IL-6 was chosen, in part, because it is among the most stable inflammatory biomarkers when time of day and food intake (e.g. fasting condition) are standardized (Rao et al., 1994). It also appears to be one of the best predictors of gerontological health outcomes (Brinkley et al., 2009; Hsu et al., 2009). Lastly, our findings are not generalizable to higher functioning older adults and those with IL-6 levels outside the range of 2.5 and 30 pg/ml.
In conclusion, IL-6 levels are inversely associated with leg extension muscle strength in older adults with chronic LGI and existing mobility limitations. Contrary to the literature, an independent association with tests of physical function and performance was not detected in this unique population. These results are expected to help plan and interpret future intervention studies in older adults with low-grade inflammation and mobility limitations.
Highlights.
We explored the association between circulating IL-6 levels and physical performance among mobility limited older adults with chronic LGI
Higher IL-6 levels were associated with lower leg extensor muscle strength
Associations between inflammation and physical performance are attenuated in mobility-limited older adults with chronic LGI
Acknowledgment
Funding
The ENRGISE Pilot Study is funded by National Institutes of Health (NIH), National Institute on Aging (NIA) Cooperative Agreement U01 AG050499 and sponsored in part by the Intramural Research Program, NIA, NIH. The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), and University of Pittsburgh (P30 AG024827). Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture under agreement 58–1950–0–014. The views of the authors do not reflect those of the Department of Agriculture. Abbott Laboratories provided funding for the purchase of the study drug and matching placebo.
Footnotes
Conflict of Interest
No conflicts of interest to declare.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ, 2001. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708. [DOI] [PubMed] [Google Scholar]
- Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, Marsh AP, Kritchevsky SB, Nicklas BJ, 2009. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 64, 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvani R, Marini F, Cesari M, Buford TW, Manini TM, Pahor M, Leeuwenburgh C, Bernabei R, Landi F, Marzetti E, 2017. Systemic inflammation, body composition, and physical performance in old community-dwellers. J Cachexia Sarcopenia Muscle 8, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Pahor M, Lauretani F, Corsi AM, Rhys Williams G, Guralnik JM, Ferrucci L, 2004. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 59, 242–248. [DOI] [PubMed] [Google Scholar]
- Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, Carter C, Yu BP, Leeuwenburgh C, 2009. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev 8, 18–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, Penninx B, Pahor M, Wallace R, Havlik RJ, 1999. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc 47, 639–646. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Penninx BW, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, Leveille SG, Fried LP, Md JM, 2002. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 50, 1947–1954. [DOI] [PubMed] [Google Scholar]
- Fougere B, Boulanger E, Nourhashemi F, Guyonnet S, Cesari M, 2017. Chronic Inflammation: Accelerator of Biological Aging. J Gerontol A Biol Sci Med Sci 72, 1218–1225. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Garagnani P, Vitale G, Capri M, Salvioli S, 2017. Inflammaging and ‘Garb-aging’. Trends Endocrinol Metab 28, 199–212. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB, 2006. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61, 1059–1064. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Fried LP, Salive ME, 1996. Disability as a public health outcome in the aging population. Annu Rev Public Health 17, 25–46. [DOI] [PubMed] [Google Scholar]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB, 1994. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 49, M85–94. [DOI] [PubMed] [Google Scholar]
- Haddad F, Zaldivar F, Cooper DM, Adams GR, 2005. IL-6-induced skeletal muscle atrophy. J Appl Physiol (1985) 98, 911–917. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, McDonald C, Chenier TC, 1992. Measurement of grip strength: validity and reliability of the sphygmomanometer and jamar grip dynamometer. J Orthop Sports Phys Ther 16, 215–219. [DOI] [PubMed] [Google Scholar]
- Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, Lauretani F, Simonsick EM, Ferrucci L, 2012. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 67, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Kritchevsky SB, Liu Y, Kanaya A, Newman AB, Perry SE, Visser M, Pahor M, Harris TB, Nicklas BJ, Health ABCS, 2009. Association between inflammatory components and physical function in the health, aging, and body composition study: a principal component analysis approach. J Gerontol A Biol Sci Med Sci 64, 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand D, Vaes B, Mathei C, Adriaensen W, Van Pottelbergh G, Degryse JM, 2014. Muscle strength and physical performance as predictors of mortality, hospitalization, and disability in the oldest old. J Am Geriatr Soc 62, 1030–1038. [DOI] [PubMed] [Google Scholar]
- Li X, Moody MR, Engel D, Walker S, Clubb FJ Jr., Sivasubramanian N, Mann DL, Reid MB, 2000. Cardiac-specific overexpression of tumor necrosis factor-alpha causes oxidative stress and contractile dysfunction in mouse diaphragm. Circulation 102, 1690–1696. [DOI] [PubMed] [Google Scholar]
- Maggio M, Guralnik JM, Longo DL, Ferrucci L, 2006. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci 61, 575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Anton SD, Beavers DP, Cauley JA, Espeland MA, Fielding RA, Kritchevsky SB, Leeuwenburgh C, Lewis KH, Liu C, McDermott MM, Miller ME, Tracy RP, Walston JD, Radziszewska B, Lu J, Stowe C, Wu S, Newman AB, Ambrosius WT, Pahor M, investigators E.P.s., 2017. ENabling Reduction of Low-grade Inflammation in SEniors Pilot Study: Concept, Rationale, and Design. J Am Geriatr Soc 65, 1961–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manini TM, Clark BC, 2012. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 67, 28–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Simonsick EM, Naydeck BL, Boudreau RM, Kritchevsky SB, Nevitt MC, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB, 2006. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295, 2018–2026. [DOI] [PubMed] [Google Scholar]
- Rao KM, Pieper CS, Currie MS, Cohen HJ, 1994. Variability of plasma IL-6 and crosslinked fibrin dimers over time in community dwelling elderly subjects. Am J Clin Pathol 102, 802–805. [DOI] [PubMed] [Google Scholar]
- Reid MB, 2001. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc 33, 371–376. [DOI] [PubMed] [Google Scholar]
- Reid MB, Lannergren J, Westerblad H, 2002. Respiratory and limb muscle weakness induced by tumor necrosis factor-alpha: involvement of muscle myofilaments. Am J Respir Crit Care Med 166, 479–484. [DOI] [PubMed] [Google Scholar]
- Schaap LA, Pluijm SM, Deeg DJ, Visser M, 2006. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med 119, 526 e529–517. [DOI] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA, 2007. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol (1985) 102, 2056–2063. [DOI] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE, 2000. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur studies of successful aging. J Gerontol A Biol Sci Med Sci 55, M709–715. [DOI] [PubMed] [Google Scholar]
- Taylor NA, Sanders RH, Howick EI, Stanley SN, 1991. Static and dynamic assessment of the Biodex dynamometer. Eur J Appl Physiol Occup Physiol 62, 180–188. [DOI] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C, 2011. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci 66, 1083–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB, 2002. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 57, M326–332. [DOI] [PubMed] [Google Scholar]
- Wang J, Leung KS, Chow SK, Cheung WH, 2017. Inflammation and age-associated skeletal muscle deterioration (sarcopaenia). J Orthop Translat 10, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D, Jackson T, Sapey E, Lord JM, 2017. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res Rev 36, 1–10. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Miller DK, Andresen EM, Malmstrom TK, Miller JP, 2005. Further evidence for the importance of subclinical functional limitation and subclinical disability assessment in gerontology and geriatrics. J Gerontol B Psychol Sci Soc Sci 60, S146–151. [DOI] [PubMed] [Google Scholar]