Abstract
Fibrosis is characterized by excessive deposition of extracellular matrix components such as collagen in tissues or organs. Fibrosis can develop in the heart, kidneys, liver, skin or any other body organ in response to injury or maladaptive reparative processes, reducing overall function and leading eventually to organ failure. A variety of cellular and molecular signaling mechanisms are involved in the pathogenesis of fibrosis. The renin-angiotensin-aldosterone system (RAAS) interacts with the potent Transforming Growth Factor β (TGFβ) pro-fibrotic pathway to mediate fibrosis in many cell and tissue types. RAAS consists of both classical and alternative pathways, which act to potentiate or antagonize fibrotic signaling mechanisms, respectively. This review provides an overview of recent literature describing the roles of RAAS in the pathogenesis of fibrosis, particularly in the liver, heart, kidney and skin, and with a focus on RAAS interactions with TGFβ signaling. Targeting RAAS to combat fibrosis represents a promising therapeutic approach, particularly given the lack of strategies for treating fibrosis as its own entity, thus animal and clinical studies to examine the impact of natural and synthetic substances to alter RAAS signaling as a means to treat fibrosis are reviewed as well.
Keywords: angiotensin II, extracellular matrix, fibroblast, myofibroblast, therapeutics, wound healing
Fibrosis as a Response to Injury
All mammalian organs respond to tissue injury, whether from trauma or various diseases, by initiating a cascade of cellular and molecular events that can eventually lead to tissue fibrosis. The tissue response to injury usually involves an integrated sequence of cellular events that include tissue inflammation, proliferation and regeneration [1]. The regenerative stage aims to restore the tensile strength of the organ, and scar formation occurs during this stage [2–4]. Tissue injury stimulates different effector cells such as inflammatory cells, epithelial cells, endothelial cells and fibrogenic cells to activate and deactivate subsets of intracellular signaling pathways that are common in different body organs, for example the transforming growth factor beta (TGFβ) pathway.
Early in the proliferative phase, fibroblasts migrate to the site of the wound and acquire bundles of microfilaments composed of α- and β-cytoplasmic actin to form stress fibers, at which time they are variously described as activated fibroblasts or proto-myofibroblasts based on their ability to generate small tractional forces [5]. Eventually, α-smooth muscle actin (αSMA) becomes expressed and incorporated into these microfilaments, marking the conversion of protomyofibroblasts to myofibroblasts [1, 6]. Myofibroblasts synthesize the elements of the extracellular matrix (ECM) and restore the tensile strength of the organ [7, 8]. ECM proteins that contribute to fibrosis are similar in different tissues, and include collagens (primarily types I and III), fibronectin, basement membrane proteins such as laminin and other less common proteins and glycoproteins, however the relative amount of these proteins may vary by tissue [9]. As myofibroblasts express the contractile protein αSMA, they are responsible for wound contraction and maturation of the granulation tissue [10, 11]. Once the wound closes, myofibroblasts may undergo regulated cell death, apoptosis, to resolve the wound healing process [12]. If myofibroblasts persist after wound healing is otherwise complete, they may contribute to tissue deformation by contracture, as well as by maintaining elevated production of ECM constituents including type I collagen [13].Contractures in the skin manifest as hypertrophic scars [14]. The hallmark of scleroderma, also referred to as ―stiff skin disease,‖ is progressive fibrosis and contracture of multiple organs. Myofibroblast persistence can result in devastating outcomes when contractures and fibrosis affect vital organs such as the heart, lung, liver and kidney [15].
The molecular signaling pathways of fibrosis comprise a complex and sophisticated network that involves myriad inflammatory mediators such as cytokines, chemokines, and growth factors that recruit and activate a wide range of inflammatory cells that in turn lead to activation of stromal fibrogenic effector cells including fibroblasts [2].These signaling pathways include growth factors that alter fibrogenic cell behavior, including TGFβ, connective tissue growth factor (CTGF, now referred to as CCN2), and platelet-derived growth factor (PDGF), which generally exert pro-fibrosis effects [16]. However, mechanical force is also an important regulator of cell activation in fibrosis, thus the ECM and its interaction with cells via adhesion molecules such as integrins and cadherins also provide alternative signaling mechanisms [17]. Vasoactive peptides including endothelin-1 but in particular angiotensin II (Ang II) appear capable of tuning these responses, in part by impacting TGFβ signaling [18]. TGFβ is synthesized and secreted from inflammatory and fibrogenic cells, yielding both paracrine and autocrine modes of action [19, 20]. The TGFβ signaling pathway interacts with other cellsignaling mechanisms, including in particular the renin-angiotensin-aldosterone system (RAAS), which has been extensively shown to contribute to the development of fibrosis in multiple organs [20, 21]. This review will discuss the role of RAAS in the development and progression of fibrosis in various organs, with a focus on RAAS crosstalk with TGFβ, and highlights recent efforts to target RAAS to ameliorate fibrosis.
The Renin-Angiotensin-Aldosterone System
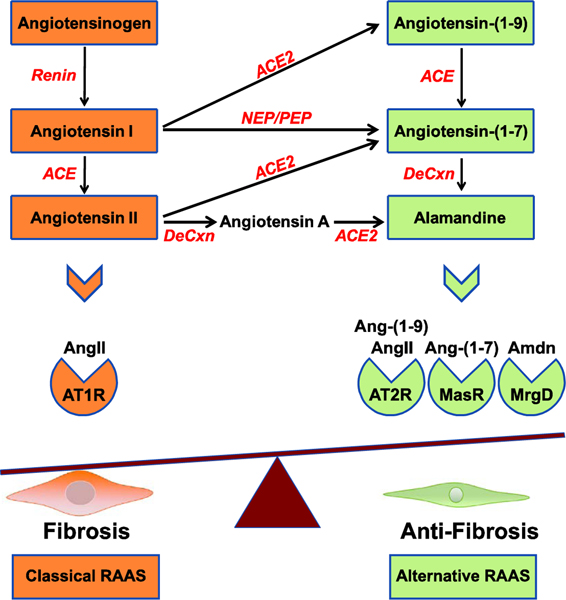
RAAS is best recognized for its essential role in the physiological regulation of blood volume, blood pressure, and sodium homeostasis [22]. Given this critical role, it is not surprising that altered RAAS signaling is also involved in the pathogenesis of several significant clinical conditions such as hypertension, heart failure, extracellular matrix remodeling and fibrosis [23]. Beside the systemic RAAS, detailed studies have revealed that local, tissue-level RAAS is active in many body organs [24]. Ang II, a major hormone produced in the RAAS, acts through specific receptors to exert a wide range of biological actions affecting almost every tissue in the body including the kidneys, cardiovascular system, liver, brain, skin and immune system [25, 26]. The components of RAAS can be considered to arise from two individual pathways: the classical, and the alternative pathway (Figure 1).
Figure 1. The Renin-Angiotensin-Aldosterone System.
RAAS consists of two parallel axes, representing the classical and alternative signaling pathways. The classical RAAS includes angiotensinogen that is converted into angiotensin I by the action of renin. Angiotensin I is subsequently converted into angiotensin II (AngII) by the action of Angiotensin Converting Enzyme (ACE). The classical RAAS induces its pro-fibrotic actions by activating the angiotensin II type 1 receptor (AT1R). The alternative RAAS axis comprises angiotensin-(1–9), angiotensin-(1–7) and alamandine (Amdn), generated by decarboxylation (DeCxn) of angiotensin-(1–7) or step-wise conversion of angiotensin II to angiotensin A by decarboxylation followed by processing by ACE2. The alternative RAAS pathway exerts anti-fibrotic actions through the angiotensin II type 2 receptor (AT2R), Mas receptor (MasR) and Mas-related GPCR, member D (MrgD). Angiotensin I can also be converted to angiotensin-(1–7) by the action of neutral endopeptidase (NEP) or prolylendopeptidase (PEP).
The Classical RAAS pathway
Activation of the classical RAAS pathway begins with the synthesis of the aspartyl protease renin. Renin is produced primarily from juxtaglomerular cells of the renal afferent arteriole [27]. Low body fluid volume, high sodium, decreased renal perfusion and sympathetic activity act as stimuli for renal renin release [28]. In the circulation, renin converts liver-derived angiotensinogen into angiotensin I (Ang I). Ang I in turn is converted into the biologically active octapeptide Ang II by the action of angiotensin converting enzyme (ACE) [29].
It is noteworthy that the renin precursor prorenin can also be released by the kidneys and has a receptor that is expressed in the kidneys as well as other tissues. Binding of prorenin to its receptor amplifies the classical pathway by increasing the efficiency of angiotensinogen cleavage by renin [30]. However, receptor-bound prorenin may induce distinct signaling pathways. Prorenin activates the extracellular signal-regulated kinase (ERK) 1/2 and p38 pathways, and increases the production of TGFβ and the matrix proteins plasminogen activator inhibitor (PAI1) and fibronectin [31–33].
Ang II is a versatile molecule that acts in intracrine/autocrine/paracrine fashions in almost all body tissues, performing its biological functions via interaction with two G protein-coupled receptors (GPCR), the Ang type 1 (AT1R) and Ang type 2 (AT2R) receptors [34]. Most biological functions of Ang II have been attributed to its binding to AT1R, which leads to the activation of phospholipases C, A2 and D, and inhibition of adenylate cyclase [35, 36]. AT1R binding of Ang II also activates tyrosine kinase phosphorylation and activation of phospholipase C-γ, leading to the activation of several downstream signals such as Janus kinases and mitogenactivated protein kinases (MAPKs), and eventually changes in gene transcription resulting in other cellular effects. Ang II activation of AT1R results in the opening of Ca2+ channels and the influx of extracellular Ca2+ into the cell [37, 38]. Ang II also stimulates the Smad signaling pathway similar to the action of TGFβ, activating the receptor-regulated R-Smads Smad2 and Smad3 even in the presence of antagonists of TGFβ, leading to downstream pro-fibrotic effects [39, 40] (Figure 2).
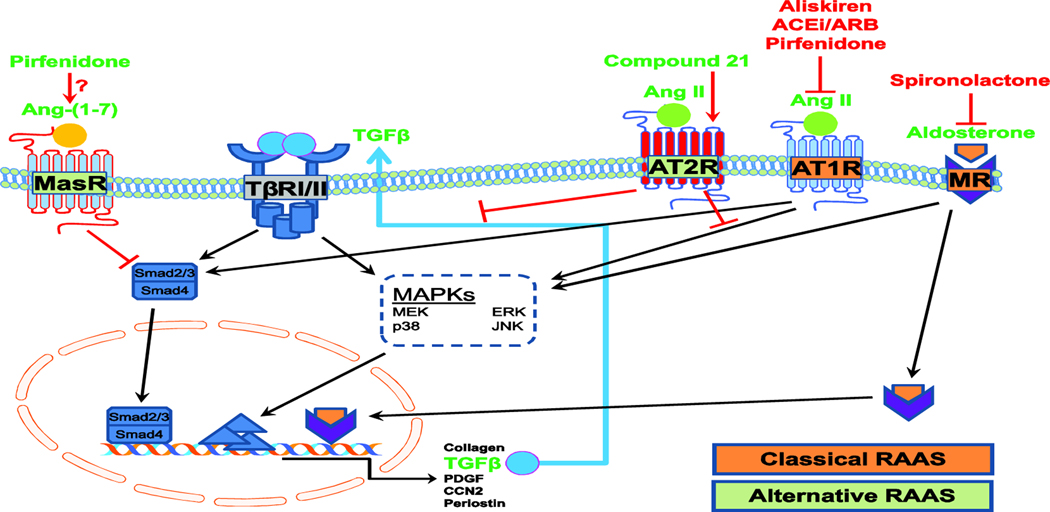
Figure 2. Schematic representation of the interactions of TGFβ and RAAS signaling.
Ligand activation of TGFβ receptors leads to Smad2/Smad3 phosphorylation, leading to recruitment of Smad4 and translocation to the nucleus to activate transcription of fibrotic genes such as collagen, TGFβ, PDGF, CCN2 and periostin. TGFβ also activates non-canonical pathways via MAPKs such as ERK, JNK and p38.Binding of AngII to its type 1 receptor (AT1R) activates MAPKs and Smads, leading to synthesis and release of TGFβ and creating a positive feedback loop to amplify fibrotic TGFβ signaling. Aldosterone activation of mineralocorticoid receptor (MR) induces transcriptional activation of pro-fibrotic proteins. Inhibition of AT1R and MR attenuates receptor activation and inhibits pro-fibrotic signaling. Activation of AT2R by Compound 21 or Ang II, or of MasR by Ang-(1–7), inhibits TGFβ fibrotic signaling and counteracts the effects of the classical RAAS pathway. Pirfenidone may both inhibit AT1R and activate MasR.
The Alternative RAAS Pathway
The main components of the alternative RAAS pathway are ACE2, the heptapeptide Ang-(17), and the Ang-(1–7) Mas receptor (MasR/MAS1) (Figure 2) [41]. Interestingly, the effects of the alternative pathway typically antagonize those of the classical pathway. ACE2 is a close homologue of ACE with 42% similarity in the catalytic domain [42]. ACE2 cleaves one amino acid at the carboxy-terminal end of Ang I to produce Ang-(1–9), and cleaves Ang II to produce Ang-(1–7) [43]. Ang-(1–7) can also be generated by the ACE-mediated cleavage of Ang-(1–9) [44], or by cleavage of Ang I by neutral endopeptidase or prolylendopeptidase [45, 46]. Ang-(17) and Ang-(1–9) mediate their patho/physiological functions by binding to MasR and AT2R, respectively [47]. MasR is an oncogenic GPCR with a ubiquitous expression pattern; it is abundant in the brain and gonads, but under physiological conditions is expressed at low levels in the lungs, heart, blood vessels, kidney and skeletal tissues [48]. Recently, a new member of the RAAS family named Ala1-Ang-(1–7), or alamandine, has been identified as a decarboxylation product of Ang-(1–7) [49]. Alamandine is also produced by Ang II decarboxylation to form angiotensin A which is converted to alamandine by the action of ACE2 [49, 50]. This peptide exerts anti-hypertensive and anti-fibrotic actions through its binding to the Mas-related GPCR, member D (MrgD) [49, 51].
The Ang II/AT2R axis produces actions that counteract those stimulated by Ang II/AT1R. In this regard, Ang II/AT1R usually activates phosphorylation and protein synthesis, while Ang II/AT2R dephosphorylates signaling molecules and inhibits protein synthesis. However, both axes produce actions that are dose- and location-dependent. ACE/ACE2 activity is essential for both RAAS axes, and regulates local shifts in the balance of the two pathways [52].
ACE2/Ang-(1–7)/MasR may regulate several important functions whose perturbation may cause various diseases. For example, ACE2 knockout in mice resulted in impaired cardiac contraction and increased plasma Ang II levels [52]. ACE2 mRNA and protein were reduced in rat hearts with hypertension [52]. Renal fibrosis was attenuated through ACE2 upregulation in long-term renal denervation in spontaneously hypertensive rats [53]. This RAAS axis is mediated by diverse signaling pathways including nitric oxide (NO), stimulation of cyclic AMPdependent protein kinase, and reduced MAP kinase activities [54–56]. Moreover, activation of this pathway leads to anti-inflammatory, anti-fibrotic, and anti-thrombotic activities, and inhibition of cell growth [56].
RAAS Physiological Functions
As noted above, RAAS components are expressed by almost all tissues of the body and are involved in myriad homeostatic functions. In the cardiovascular system, Ang II increases blood pressure through direct and indirect actions that target vascular tone and sodium/water balance [57]. Ang II activates the sympathetic nervous system leading to increased heart rate and contractility and induces general vasoconstriction, resulting in an increase in total peripheral resistance. Ang II also increases the pro-thrombotic potential of the blood by stimulating plasminogen activator inhibitor protein PAI-1 and PAI-2 [58]. In the renal system, Ang II promotes sodium reabsorption in the proximal tubule and induces the secretion of aldosterone from the adrenal cortex, which in turn regulates salt homeostasis by stimulating sodium reabsorption and potassium secretion in the kidney distal tubules [59]. Furthermore, RAAS stimulates the thirst reflex and prevents fluid urinary loss through its action on the hypothalamus to stimulate the antidiuretic hormone (ADH/vasopressin) release from the posterior pituitary gland [60]. Ang II stimulates the release of adrenocorticotropic hormone (ACTH) from the anterior pituitary to regulate cortisol secretion from the adrenal gland [61]. Ang II has been shown to influence prostaglandin release, amplifying the vasoconstriction effect of Ang II [62]. In the adipose tissues, Ang II increases lipogenesis and the risk of adipose inflammation, and is linked to glucose homeostasis and insulin release [63].
Importantly, Ang II and aldosterone influence the synthesis of extracellular matrix proteins such as collagen I, fibronectin and plasminogen activator inhibitor proteins PAI-1 and PAI-2. These actions suggest crucial roles for RAAS in tissue fibrosis [64, 65].
TGFβ Signaling
Ang II has been widely associated with fibrosis due to its ability to activate TGFβ signaling pathways in addition to its stimulatory effect on TGFβ synthesis and secretion [66]. Overactivation of TGFβ is a key feature of fibrosis in almost all tissues and is responsible for fibroblast to myofibroblast activation and ECM protein synthesis. TGFβ can inhibit the activity of matrix metalloproteinases (MMPs) by stimulating synthesis of tissue inhibitors of metalloproteinases (TIMPs) [67].The impact of TGFβ on MMP/TIMP balance favors ECM production, and thus amplifies the fibrotic signals, although the specific effect of TGFβ on the expression of individual MMPs and TIMPs can vary, and in fact TGFβ can up-regulate some MMPs to promote ECM remodeling [68, 69]. Additionally, TGFβ enhances the synthesis of the fibrotic mediators CCN2 and PDGF [70, 71].
TGFβ mediates its biological function by binding to its receptor, TGFβ type II receptor (TβRII). Subsequently, TβRII complexes with and trans-phosphorylates the TGFβ type I receptor (TβRI) [72]. In turn, TβRI activates intracellular Smad signaling pathways by phosphorylating Smad2 and Smad3 (Figure 2), which bind to a common mediator Smad4 and translocate to the nucleus where this complex acts as transcription factor for genes encoding ECM proteins such as collagen 1α1, αSMA, periostin, and fibronectin. Smad3 can activate Smad7 as well, which acts as a negative feedback mechanism to inhibit TGFβ-mediated downstream effects [73].
In addition to the canonical Smad signaling pathway, TGFβ stimulates several non-canonical pathways by activating multiple kinases, including the MAPKs ERK, p38, c-Jun N-terminal kinase (JNK), phosphatidylinositol-3-kinase (PI3K), Akt/PKB, and ROCK [74, 75]. Activated kinases in turn regulate the Smad signaling pathways positively or negatively, depending on cell type [76]. Non-canonical TGF pathways play several roles in fibrosis of different organs [77]. Additionally, TGFβ induces the nuclear translocation of serum response factor/myocardinrelated transcription factors (SRF/MRTF). A number of SRF/MRTF target genes such as αSMA and CCN2 are involved in tissue fibrosis [78].
RAAS in Liver Fibrosis
Hepatic fibrosis is a multifactorial process that involves hepatic stellate cells (HSC) and Kupffer cells (KC), growth factors, chemokines and cytokines that eventually results in disruption of homeostatic functions and organ failure. Similar to fibrosis in other tissues, hepatic fibrosis proceeds by HSC and KC activation and proliferation, resulting in the generation of myofibroblasts or the production of TGFβ, respectively [8, 79]. These actions lead to the synthesis of increased amounts of ECM components, ECM remodeling and the contraction of scar tissues [79]. Ample evidence demonstrates the involvement of RAAS over-production at different stages of liver fibrosis [80]. Ang II leads to increases in intrahepatic resistance and ECM protein production via its vasoconstriction effect, enhances oxidative stress, and acts as a local and systemic cytokine, thus enhancing pro-inflammatory mediators [81, 82].
Renin and aldosterone levels have been shown to be elevated in patients with liver cirrhosis [83]. In healthy human liver, quiescent HSCs do not express RAAS components, but activation of these cells in the cirrhotic liver or in culture models of liver damage leads to substantial expression of renin, ACE, and Ang II [84]. This increase could be a physiological reflex to systemic vasodilatation that takes place in patients with cirrhosis, or due to a local activation of the RAAS [85]. Hepatic fibrosis results, in part, from Ang II-mediated conversion of HSC to myofibroblasts and subsequent contraction through TGFβ stimulation[86]. Moreover, Ang II induces the production of vascular endothelial growth factor (VEGF), which is one of the major angiogenic factors upregulated during fibrosis [87]. VEGF stimulates HSC activation and exacerbates hepatic fibrosis [87].
Ang II acts on AT1R to activate HSCs via several signaling molecules, including MAPK pathways, Janus kinase 2 (JAK2), the phosphoinositide/Ca2+ pathway, and the generation of reactive oxygen species via phosphorylation of the p47phox subunit of Nox [88, 89]. In quiescent and activated HSCs, Ang II-produced TGFβ mRNA and protein expression is dependent on ERK1/2- and Nox-dependent pathways, but independent of protein kinase C [90]. AT1R stimulation in wild type rats and mice resulted in activation of HSCs and production of liver fibrosis by the activation of intracellular JAK2, which in turn activated RhoA/Rho-kinase [89]. Liver fibrosis was prevented by deleting the AT1R gene in vivo, and by pharmacological inhibition of JAK2 [89]. Ang II enhances periostin expression in HSCs, and periostin in turn upregulates both TGFβ and collagen 1α1expression to drive fibrosis [91]. Furthermore, Ang II induced the expression of TIMP-1 via AT1R and phosphokinase C [91].
Conversely, the alternative RAAS pathway counterbalances the effects of the classical RAAS pathway through its components ACE2, Ang-(1–7), and MasR [41]. This ‘alternate axis’ produces anti-fibrotic effects, thus protecting tissues from the potentially harmful effects of classical RAAS activation [41]. In animal models of cirrhosis, ACE2 and Ang-(1–7) expression levels were upregulated [92]. Ang-(1–7) in turn reduced the vascular tone in the mesenteric bed of cirrhotic animals [92]. Moreover, the MasR agonist AVE 0991 reduced expression of the profibrotic proteins collagen 1α1, αSMA, TGFβ, and ACE [93]. Furthermore, loss of ACE2 exacerbated fibrosis in BALB/c mice subjected to bile duct ligation, while recombinant ACE2 inhibited key features of liver fibrosis in chronic liver injury models, suggesting a protective role of the alternative RAAS pathway in liver fibrosis [94].
RAAS in Cardiac and Renal Fibrosis
Cardiac fibrosis is a significant contributor to mortality and morbidity worldwide [95, 96]. It is characterized by the excessive production and deposition of ECM in the cardiac wall or septum, and is associated with most cardiac pathologic conditions such as acute myocardial infarction, pressure overload with hypertension and aortic stenosis, volume overload due to valvular diseases, hypertrophic cardiomyopathy, post-viral dilated cardiomyopathy, and aging [97]. Cardiac fibrosis is variable with different pathological insults; however, the cellular mechanisms involved in fibrosis are similar. The RAAS is a major pathway that can contribute to cardiac fibrosis, with TGFβ being a key downstream signaling molecule of Ang II/AT1R that acts in an autocrine or paracrine manner to induce fibrosis in the heart, although the full mechanisms involved are not fully understood [98]. The Ang II/AT1R/p38 MAPK pathway is activated in cardiac and renal fibrosis, leading to an imbalance in the ratio of ACE/ACE2 which, if restored, could provide a novel approach to treat fibrotic diseases [99].
After cardiomyocyte injury, local RAAS components are dramatically elevated in the heart [100]. Ang II regulates the expression of integrins – transmembrane receptors that play important roles in the physiology and pathophysiology of the heart, including fibrosis [101]. Acting on AT1R, Ang II upregulates αv, β1, β3, β5,and α8β1 integrins at the mRNA and protein levels [101–103]. Ang II enhances the binding of cardiac fibroblasts to a variety of ECM proteins, including collagen I, fibronectin, vitronectin, and laminin, and this binding activates focal adhesion kinase (FAK) [104]. Additionally, β3 integrin plays an important role in Ang IIinduced attachment of these ECM proteins [104]. Conversely, β1 integrin regulates angiotensinogen expression in cardiac fibroblasts by activating and inhibiting the intracellular kinases Rac1 and RhoA, respectively [105]. Ang II induces c-Fos, Early Growth Response Protein 1 and MAPK activities [106]. Furthermore, Ang II up-regulates the expression of profibrotic TGFβ, the ECM proteins laminin and fibronectin, collagen I and II, as well as PAI-1 through AT1R [101]. Further, Ang II upregulates Tenascin-C which is a matricellular protein that exacerbates the fibrotic process by activating macrophages and fibroblasts to generate more pro-fibrotic cytokines in the myocardium [107]. Angiotensin-mediated activation of Tenascin-C occurs via activation of the integrin αvβ3 and nuclear translocation of phosphorylated NF-κB, and eventually increased production of collagen I by cardiac fibroblasts [107].
Osteopontin [108] is an adhesion molecule that is important for renal and cardiac fibrosis [108]. Ang II augments its expression in cardiac fibroblasts, and osteopontin depletion has been shown to prevent Ang II-mediated induction of cardiac fibroblast collagen gel contraction by an integrin-dependent mechanism [109–111]. Osteopontin promotes fibrosis by enhancing macrophage activation and fibroblast proliferation stimulated by Ang II [112].
Ang II also upregulates CCN2 in renal and cardiac tissues. Ang II-induced CCN2 expression via AT1R occurs in a protein kinase C (PKC)-dependent manner [113]. Ang II stimulates TGFβ and CCN2 in cultured fibroblasts by activating p38 MAPK. AT1R inhibition using losartan and a p38 MAPK inhibitor attenuates CCN2 expression [114].
Aldosterone, via the mineralocorticoid receptor (MR), induces cardiac and renal fibrosis that can be prevented by the MR antagonists spironolactone and eplerenone [115–117] (Figure 2). Aldosterone recruits and activates macrophages, leading to increased expression of inflammatory cytokines such as intercellular adhesion molecule 1, and activates fibroblast conversion to myofibroblasts [118]. Aldosterone potentiates cardiac and renal fibrosis signaling by directly stimulating pro-fibrotic proteins TGFβ, CCN2, endothelin 1, placental growth factor, PAI-1, galectin-3, and osteopontin [119, 120]. Generation of reactive oxygen species (ROS) is another mechanism by which Ang II and aldosterone contribute to fibrosis [120, 121]. Ang II and aldosterone both stimulate several enzymes required for ROS production such as nicotinamide adenine dinucleotide phosphate oxidase through AT1R- and MR-dependent mechanisms, respectively [122]. In cardiac and renal fibrosis, the effects of aldosterone are in part due to the inhibition of NO and cGMP pathways and the subsequent activation of NF-κB signaling, which in turn may induce the expression of pro-fibrotic proteins [123, 124].
In addition to the transcriptional activation of MR, aldosterone can induce rapid non-genomic effects that are insensitive to transcriptional and translational inhibitors [125]. These nongenomic effects may include the activation of various intracellular signaling pathways such as ERK1/2, PI3K, diacylglycerol, PKC, and JNK. These affects might be due to aldosterone activation of the newly characterized G protein-coupled receptor 30 [125–127]. The interactions between Ang II and aldosterone are evident in a variety of experimental settings and diseases. For example, Ang II directly induces the nuclear translocation of MR, which may partially account for the gene expression induced by Ang II stimulation. Moreover, Ang II-induced cardiac fibrosis has been shown to depend on the ability of Ang II to induce local aldosterone synthesis in heart tissues by activating AT1R, steroidogenic acute regulatory protein and aldosterone synthase protein expression [128].
While the classical RAAS pathway described above produces pro-fibrotic responses, the alternative RAAS components act in the heart and kidney to counteract these pathological actions. The key enzyme of alternative RAAS activation, ACE2, is expressed in the heart, kidney and other tissues [52, 92, 94, 129]. Several studies have reported that ACE2/Ang-(1–7)/MasR pathway activation has anti-fibrotic effects in the heart and kidney [130]. Genetic deletion of ACE2 in mice results in severe cardiac dysfunction and up-regulation of hypoxia-induced genes with a concomitant increase of Ang II expression [52]. Moreover, loss of ACE2 in the left anterior descending coronary artery ligation model of myocardial infarction leads to increased infarct size that is associated with oxidative stress, increased MMP expression and greater inflammation [131]. These changes were associated with increased classical RAAS pathway activity that mediated the up-regulation of nicotinamide adenine dinucleotide phosphate oxidase and enhanced ROS formation [131]. Similarly, ACE2 deletion or inhibition leads to Ang IIdependent oxidative renal damage in aged mice [131]. Furthermore, these effects could be due to Ang II-dependent activation of the MAPK pathway as phosphorylation of ERK1/2 and JNK1/2 was increased in the infarct area of ACE2 knockout mice and AT1R blockade prevented these adverse effects [131].Over-expression of ACE2 using lentiviral delivery in rats attenuates the deleterious cardiac effects and fibrosis induced by Ang II [132, 133]. Moreover, recombinant ACE2 treatment in Col4a3 knockout mice results in reduction in ECM protein accumulation, inhibition of TGFβ signaling and less fibrosis in the kidney [129]. More recently, Wang et al. showed that ischemic post-conditioning could serve as an adjunct to treat fibrosis after MI. Postconditioning was associated with reduced ACE and AT1R and increased ACE2 and AT2R expression in addition to a coincident reduction in the TGFβ/Smad fibrotic pathway [134]. Similar to the cardiac role of ACE2, loss of this enzyme resulted in a four-fold increase in the ratio of local renal Ang II/Ang-(1–7) that was associated with increased expression of fibrotic proteins, including αSMA and collagen I [135]. These changes were due to activation of Ang II and the ERK1/2, TGFβ/Smad2/3, and NF-κB signaling pathways [135]. Moreover, ubiquitin degradation of inhibitory Smad7, mediated by the E3 ligase Smurf2, was reported in the same study, and could act to amplify TGFβ/Smad2/3-mediated renal fibrosis [135].
RAAS in Skin Fibrosis
RAAS components are present in normal skin, suggesting that local RAAS may be a homeostatic regulator of the skin [136]. However, the expression level of RAAS components is not abundant. AT1R is the predominant Ang II receptor present in unwounded skin, with distribution in epidermis keratinocytes and blood vessels, and low expression in dermis fibroblasts [137]. Angiotensinogen, renin, MasR, ACE and ACE2 are also present in normal skin, and colocalized with AT1R [137, 138].
Upon injury, RAAS is a key regulator of all steps of wound healing in the skin, including hemostasis, recruitment and activation of keratinocytes and fibroblasts, wound contraction, and ECM remodeling, and it does so by stimulating synthesis and release of various pro-fibrotic proteins [139]. Injury of the skin induces time-dependent expression changes in various RAAS components [139]. Inflammatory cells and resident cell byproducts such as cytokines and chemokines act locally to increase or decrease RAAS players in the skin. Inhuman skin, both AT1R and AT2R are up-regulated 24 hours after cutaneous injury, with higher expression of AT2R in the latter stages of the healing process [140, 141]. The roles of AT1R and AT2R in skin healing have been demonstrated in loss-of-function knockout animals. AT1R deletion in mice delayed skin healing, while deleting AT2R halted healing at early stages but accelerated the healing process at later stages [142]. AT2R knockout mice showed weaker healed skin compared to wild type animals [142]. Ang-(1–7) promotes wound healing via activation of MasR, leading to stem cell proliferation and eventually re-epithelialization [143]. ACE expression is upregulated in the skin after injury, and inhibition of ACE is correlated with a reduction in TGFβ and tissue necrosis factor α suggesting that ACE is involved in skin healing [144]. Furthermore, ACE deletion or inhibition with ramipril reduced scar formation and expression of fibrotic markers [145]. ACE appeared to induce these effects via TGFβ-induced-Smad2/3 phosphorylation and a TGFβ-activated kinase 1-dependent mechanism [145]. These observations suggest that the temporal balance in the expression of local AT1R and AT2R regulates proper skin wound healing.
Several debilitating diseases such as diabetes, aging and chronic burns induce disturbances in the local skin RAAS that lead to dysregulated wound healing [146]. Ang II induces collagen synthesis in skin fibroblasts via AT1R and augments insulin-like growth factor 1 (IGF-1)induced collagen synthesis. Conversely, activation of AT2R by Ang II inhibits collagen production and IGF-1-induced collagen synthesis [146]. Moreover, Ang II opposes MMPs activity by regulating the level of TIMP-1 [147]. AT1R activation enhances TIMP-1 expression and thus inhibits collagen degradation, whereas AT2R activation inhibits TIMP-1 expression to promote collagen degradation [146]. The inhibitory effect of AT2R could be partially due to activation of protein tyrosine phosphatase SHP-1 [146].
Targeting RAAS to Treat and Prevent Fibrosis
Fibrotic disorders and resultant organ failure account for an increasing and significant socioeconomic burden on patients worldwide, yet with few exceptions, there are no specific and potent medications available for the treatment of fibrosis [148]. The molecular mechanisms responsible for fibrosis exhibit both overlap and distinction across body organs, however in almost all tissues, activation of the classical RAAS pathway leads to pro-fibrotic effects through AT1R while activation of the alternative pathway Ang-(1–7)/ACE2/AT2R or MasR leads to inhibition of fibrosis [149]. There is a growing body of evidence that fibrosis in various body organs, at a minimum, can be attenuated, and may even be reversible to some degree, which would have significant positive effects on tissue/organ function and recovery from disease (Table 1). In this regard, targeting RAAS represents a novel and promising approach.
Table 1.
Studies of RAAS blockade and fibrosis inhibition
| Drug | Study Type | Target Organ | Mechanism of Action | Outcome | References |
|---|---|---|---|---|---|
| Candesartan | Randomized, open-label controlled study | Liver | ARB | Reduction in αSMA, TGFβ1, Col-1, TIMP-1 and MMP2 | [150] |
| Olmesartan, candesartan, losartan | Meta-analysis of 4 randomized controlled trials | Liver | ARB | Reduction in liver fibrosis score and area | [152] |
| A-779 | Animal | Liver | Mas receptor antagonist | Aggravation of liver fibrosis and elevation of TGFβ | [93] |
| Aliskiren | In vitro | Heart, kidney | Renin inhibitor | Reduction in fibroblast proliferation, αSMA, TGFβ and collagen | [156, 159] |
| Candesartan | Clinical trial | Heart | ARB | Reduction of type I procollagen-Npeptide and type III procollagenN-peptide | [160] |
| Spironolactone | Animal | Heart, liver | Aldosterone antagonist | Reduction of TGFβ and reactive oxygen species | [151, 162, 167] |
| Lisinopril | Randomized clinical trial | Heart | ACE inhibitor | Reduction of myocardial fibrosis and collagen volume | [163] |
| Enalapril | Animal | Heart, kidney | ACE inhibitor | Reduction in fibrosis and long-term protection against organ damage | [164, 165] |
| Pirfenidone | Animal | Heart | Classical/alternative RAAS balance | Reduction in fibrosis | [99] |
| Compound 21 and A8011 | In vitro | Heart, skin | AT2R agonist | Reduction in fibrosis, collagen, TIMP1, MMP1–9 and TGFβ | [171-173, 175] |
| Ramipril | Animal and in vitro | Skin | ACE inhibitor | Decreased collagen, TGFβ1 expression and attenuated TGFβ-activated kinase-1 phosphorylation | [145] |
| Topical losartan and valsartan | Pilot placebocontrolled single blinded study | Skin | ARB | Reduction in scar score | [174, 175] |
Targeting RAAS in Liver Fibrosis
The efficacy of RAAS blockade in preventing and treating liver fibrosis has been tested in several in vitro and in vivo investigations. AT1R blockers (ARBs), ACE inhibitors, and selective aldosterone blockers have been reported to be effective in suppressing hepatic fibrosis [150, 151]. The ARB candesartan, administered for 6 months in patients with compensated alcoholic liver disease, ameliorated liver fibrosis and significantly reduced fibrotic areas, scores and related markers such as αSMA and hydroxyproline levels [150]. A recent meta-analysis of randomized controlled trials in patients with liver fibrosis revealed that RAAS inhibition significantly reduced measures of liver fibrosis, and that the therapeutic agents were welltolerated [152]. Ang-(1–7) reduces hepatic fibrosis by inhibiting activation of hepatic stellate cells [153, 154]. These data suggest that RAAS antagonism represents a promising therapeutic approach for liver fibrosis, however additional translational and controlled randomized clinical trials are needed for adoption as front-line therapy.
Targeting RAAS in Cardiac and Renal Fibrosis
Inhibiting renin could represent a successful approach to combat cardiac and renal fibrosis as it is the rate limiting step in the synthesis of Ang II [155]. Aliskiren is an oral renin inhibitor approved by the United States Food and Drug Administration for the treatment of hypertension [155]. It has been shown that aliskiren attenuates collagen deposition, oxidative stress, TGFβ synthesis and the infiltration of inflammatory cells in gingival tissues of both normal and diabetic mice [156–158]. Aliskiren may exert its anti-fibrotic effects independently of Ang II via activation of the prorenin receptor [159].
Blocking ACE/AT1R/MR has also been shown to improve cardiac and renal fibrosis. Candesartan reduces markers of cardiac fibrosis such as type I procollagen-N-peptide and type III procollagen-N-peptide in patients with atrial fibrillation [160]. Whether blood pressure reduction alone is sufficient to attenuate cardiac fibrosis has not been fully determined. However, it is noteworthy that the beneficial effect of RAAS inhibition on fibrosis can be independent of blood pressure regulation [151]. Queisser et al. have reported that blocking the MR receptor with spironolactone ameliorated hepatic fibrosis in hypertensive rats [151]. At sub-therapeutic doses, spironolactone prevented heart fibrosis as well [161]. The beneficial effects of RAAS inhibition could be due to reduction in reactive oxygen species in cardiac tissues [162]. In one prospective clinical trial, the ACE inhibitor lisinopril significantly reduced collagen volume fraction in participating patients, suggesting its potential use as an anti-fibrotic agent [163]. Reports have indicated that even transient ACE inhibition can protect against fibrotic stimuli [164]. Two weeks of enalapril treatment of adult spontaneously hypertensive rats produced persistent physiological changes in cardiac fibroblasts that lasted beyond the treatment course [164]. These changes were able to protect the heart from future noxious stimuli such as nitric oxide synthase inhibition [164]. Similarly, this transient treatment approach also produced long-term protection against renal interstitial fibrosis in response to nitric oxide synthase inhibition [165]. The AT1R antagonist telmisartan inhibited cardiac fibrosis in TGFβ over-expressing mice by modulating the MMP/TIMP ratio [166]. Aldosterone antagonists have also been reported to inhibit cardiac fibrosis. Treatment with spironolactone attenuates collagen deposition, inflammation and increases antioxidants in hyperthyroid rats [167].
Pirfenidone is an anti-fibrotic agent that could ameliorate fibrosis in various tissues [99]. The mechanism of action of pirfenidone is not fully understood, however recent studies have shown that this small molecule converges at the RAAS pathway in cardiac fibrosis. Pirfenidone seems to balance the ACE/ACE2 ratio to favor the anti-fibrotic RAAS axis [99]. Moreover, pirfenidone inhibited AT1R/p38 MAPK, corrected the ACE/ACE2 ratio and shifted the RAAS balance that eventually ameliorated MI-induced cardiac fibrosis [99]. The ARB losartan similarly inhibited AT1R/p38 MAPK and increased the expression of Liver X Receptor α (LXRα) which exerts anti-fibrotic effects as well [99, 168]. In support of these data, deletion of the small GTPase Rho A in activated fibroblasts using a tamoxifen-inducible periostin-Cre promoter (RhoAfl/fl::PSTNCre/+) attenuated cardiac fibrosis and was associated with reduced TGFβ and non-canonical p38-MAPK signaling [169].
A recent study suggests a combination therapy of anti-oxidants and serelaxin, a recombinant form of the human ovarian hormone relaxin-2, may be effective to combat cardiac fibrosis without affecting the progression of related cardiac diseases. This combination therapy in a normotensive mouse model completely abrogated cardiac fibrosis to levels comparable to the fibrosis measured in control mice [170].
Targeting AT2R is a tempting approach to treat fibrosis. However, at present there are few selective AT2R agonists. Compound 21 (C21) is a highly selective AT2R agonist that has been widely used in preclinical research for this purpose. Treatment of myocardial infarction in rats with C21 significantly reduced scar size, and this was associated with reduced phosphorylation of the p44/42 and p38 MAPKs [171]. Additionally, C21 treatment of induced myocardial infarction for 6 weeks resulted in a substantial reduction in collagen content in peri-infarct myocardium, elevation of TIMP-1 expression and reduction in MMPs 1 and 9 [172]. Recently, Wang et al. were able to produce selective AT2R ligands through single β-amino acid substitutions into various angiotensin fragments, producing a β-substituted angiotensin peptide MU37 and comparing its efficiency as an anti-fibrotic agent to C21. Both reagents produced a dose-dependent reduction in TGFβ-induced collagen and αSMA expression, and significantly reduced high salt-induced cardiac fibrosis [173].
Targeting RAAS in Skin Fibrosis
As noted above, skin expresses local RAAS components that may undergo dysregulation during wound healing and in skin pathologies, resulting in the formation of hypertrophic scars and keloids. Blocking ACE/AT1R and/or activating ACE2/AT2R may thus be beneficial in these conditions. Deletion of ACE in mice or treatment with the ACE inhibitor ramipril reduced scar formation after full thickness skin wounding [145]. AT1R inhibition with losartan significantly reduced scar formation, but took more time than ramipril. ACE inhibition resulted in decreased fibroblast proliferation, and decreased collagen and TGFβ1 expression [145]. A pilot singleblinded placebo-controlled clinical trial was designed to test the effect of topical losartan on patients with hypertrophic scars and keloids [174]. Results of this study showed that scar scores were significantly reduced in the losartan group compared to placebo, suggesting that AT1Rselective antagonism using losartan 5% can alleviate the keloid and hypertrophic scar [174].
In mouse and porcine models of chronic diabetic wounds, and in aging diabetic pigs, application of 1% losartan gel resulted in faster wound healing and enhanced the quality of healing skin compared to other tested medications and controls [175]. The roles of AT1R and AT2R in skin healing are phase-dependent, and it is the late application of AT1R blocker that enhanced the wound closure time and the quality of healing. Notably, AT2R deletion abolished the effects of losartan on wound healing through AT1R, which may explain why ACE inhibition failed to produce beneficial effects similar to losartan. That is, ACE inhibitors reduce Ang II production resulting in a lack of stimulation of both AT1R and AT2R while losartan selectively blocks AT1R allowing unopposed binding of AT2R.
In a xenograft model of Dupuytren’s Disease, C21 significantly reduced fibrotic markers such as CCN2, TGFβ, Smad3,Smad4and αSMA both in vitro and in vivo [176]. C21 may thus represent a novel therapeutic for this condition, which can be challenging to treat, and suggests that RAAS modulation in general may be a useful strategy in this setting.
Conclusion
Organ fibrosis is associated with increased mortality and morbidity worldwide. A large body of literature has demonstrated that, in the pathogenesis of fibrosis in different systems of the body, including the liver, heart, kidney, skin and others, TGFβ signaling represents a central mechanism. The RAAS pathway interacts with TGFβ at multiple levels to enhance or retard tissue fibrosis. The classical RAAS pathway, including renin, ACE, Ang II, AT1R and aldosterone, acts to exacerbate fibrosis. Conversely, ACE2, Ang-(1–7), AT2R and MasR, constituting the alternative RAAS pathway, act to ameliorate and prevent fibrosis.
Although targeting the RAAS pathways has been shown to be a promising approach to combat fibrosis, more investigation is needed to precisely understand the RAAS molecular and cellular mechanisms at different stages of the disease in various body organs, particularly since the timing of any intervention in RAAS signaling may be critical to success. Moreover, additional clinical studies are needed to confirm RAAS-based treatment effectiveness in patients at various stages of disease progression, and to establish the long-term safety of this approach in treating different forms of fibrosis, since such strategies are likely to involve extended periods of treatment.
Highlights.
The classical renin-angiotensin-aldosterone system (RAAS) has been implicated as an inducer of fibrosis in multiple tissue and organ types.
Recent evidence demonstrates that the alternative RAAS counteracts the classical RAAS to attenuate fibrosis.
Augmentation of the alternative RAAS, or altering the balance of the classical and alternative RAAS, holds promise for the therapeutic treatment of fibrosis.
Acknowledgements
This work was supported by a Project Grant (PJT-162422) to MPC from the Canadian Institutes of Health Research and by funding to TMH from the American Heart Association (19AIREA34460000) and National Institutes of Health (R56HL141165).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Bergmeier V, Etich J, Pitzler L, Frie C, Koch M, Fischer M, Rappl G, Abken H, Tomasek JJ, Brachvogel B, Identification of a myofibroblast-specific expression signature in skin wounds, Matrix Biology 65 (2018) 59–74. [DOI] [PubMed] [Google Scholar]
- [2].Rockey DC, Bell PD, Hill JA, Fibrosis—a common pathway to organ injury and failure, New England Journal of Medicine 372(12) (2015) 1138–1149. [DOI] [PubMed] [Google Scholar]
- [3].Darby I, Laverdet B, Bonte F, Desmouliere A, Fibroblasts and myofibroblasts in wound healing, Clinical, Cosmetic and Investigational Dermatology 7 (2014) 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gonzalez A.C.d.O., Costa TF, Andrade Z.d.A., Medrado ARAP, Wound healing-A literature review, Anais Brasileiros de Dermatologia 91(5) (2016) 614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA, Myofibroblasts and mechano-regulation of connective tissue remodelling, Nature Reviews Molecular Cell Biology 3(5) (2002) 349–363. [DOI] [PubMed] [Google Scholar]
- [6].Shinde AV, Humeres C, Frangogiannis NG, The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1863(1) (2017) 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].JJ HBCGT, Gabbiani G Chaponnier C. Alpha-smooth muscle actin expression upregulates fibroblast contractile activity, Molecular Biology of the Cell 12 (2001) 2730–2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Molokanova O, Schönig K, Weng S-Y, Wang X, Bros M, Diken M, Ohngemach S, Karsdal M, Strand D, Nikolaev A, Inducible knockdown of procollagen I protects mice from liver fibrosis and leads to dysregulated matrix genes and attenuated inflammation, Matrix Biology 66 (2018) 34–49. [DOI] [PubMed] [Google Scholar]
- [9].Galie PA, Westfall MV, Stegemann JP, Reduced serum content and increased matrix stiffness promote the cardiac myofibroblast transition in 3D collagen matrices, Cardiovascular Pathology 20(6) (2011) 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Roche PL, Nagalingam RS, Bagchi RA, Aroutiounova N, Belisle BM, Wigle JT, Czubryt MP, Role of scleraxis in mechanical stretch-mediated regulation of cardiac myofibroblast phenotype, American Journal of Physiology-Cell Physiology 311(2) (2016) C297C307. [DOI] [PubMed] [Google Scholar]
- [11].Schnieder J, Mamazhakypov A, Birnhuber A, Wilhelm J, Kwapiszewska G, Ruppert C, Markart P, Wujak L, Rubio K, Barreto G, Loss of LRP1 promotes acquisition of contractilemyofibroblast phenotype and release of active TGF-β1 from ECM stores, Matrix Biology (2019). [DOI] [PubMed] [Google Scholar]
- [12].Aarabi S, Bhatt KA, Shi Y, Paterno J, Chang EI, Loh SA, Holmes JW, Longaker MT, Yee H, Gurtner GC, Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis, The FASEB Journal 21(12) (2007) 3250–3261. [DOI] [PubMed] [Google Scholar]
- [13].Qi J, Liu Y, Hu K, Zhang Y, Wu Y, Zhang X, MicroRNA-205-5p regulates extracellular matrix production in hyperplastic scars by targeting Smad2, Experimental and Therapeutic Medicine 17(3) (2019) 2284–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Redmond RW, Kochevar IE, Medical Applications of Rose Bengal-and Riboflavin-Photosensitized Protein Crosslinking, Photochemistry and Photobiology 95(5) (2019) 10971115. [DOI] [PubMed] [Google Scholar]
- [15].Castelino FV, Varga J, Emerging cellular and molecular targets in fibrosis: implications for scleroderma pathogenesis and targeted therapy, Current Opinion in Rheumatology 26(6) (2014) 607–614. [DOI] [PubMed] [Google Scholar]
- [16].Cabello-Verrugio C, Córdova G, Vial C, Zúñiga LM, Brandan E, Connective tissue growth factor induction by lysophosphatidic acid requires transactivation of transforming growth factor type β receptors and the JNK pathway, Cellular Signalling 23(2) (2011) 449–457. [DOI] [PubMed] [Google Scholar]
- [17].Zheng G, Zhang J, Zhao H, Wang H, Pang M, Qiao X, Lee SR, Hsu T-T, Tan TK, Lyons JG, α3 Integrin of cell-cell contact mediates kidney fibrosis by integrin-linked kinase in proximal tubular E-cadherin deficient mice, The American Journal of Pathology 186(7) (2016) 1847–1860. [DOI] [PubMed] [Google Scholar]
- [18].Zhang Y, Huang X-R, Wei L-H, Chung AC, Yu C-M, Lan H-Y, miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling, Molecular Therapy 22(5) (2014) 974–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vanhee D, Gosset P, Wallaert B, Voisin C, Tonnel A, Mechanisms of fibrosis in coal workers’ pneumoconiosis. Increased production of platelet-derived growth factor, insulin-like growth factor type I, and transforming growth factor beta and relationship to disease severity, American Journal of Respiratory and Critical Care Medicine 150(4) (1994) 1049–1055. [DOI] [PubMed] [Google Scholar]
- [20].Solini A, Santini E, Ferrannini E, Enhanced angiotensin II-mediated effects in fibroblasts of patients with familial hypercholesterolemia, Journal of Hypertension 23(2) (2005) 367–374. [DOI] [PubMed] [Google Scholar]
- [21].Peters H, Rückert M, Gaedeke J, Liefeldt L, Ketteler M, Sharma AM, Neumayer H-H, Angiotensin-converting enzyme inhibition but not β-adrenergic blockade limits transforming growth factor-β overexpression in acute normotensive anti-thy1 glomerulonephritis, Journal of Hypertension 21(4) (2003) 771–780. [DOI] [PubMed] [Google Scholar]
- [22].Lijnen P, Petrov V, Induction of cardiac fibrosis by aldosterone, Journal of Molecular and Cellular Cardiology 32(6) (2000) 865–879. [DOI] [PubMed] [Google Scholar]
- [23].Gan W, Ren J, Li T, Lv S, Li C, Liu Z, Yang M, The SGK1 inhibitor EMD638683, prevents Angiotensin II–induced cardiac inflammation and fibrosis by blocking NLRP3 inflammasome activation, Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1864(1) (2018) 1–10. [DOI] [PubMed] [Google Scholar]
- [24].Camelo L, de Souza Marinho T, Águila MB, Souza-Mello V, Barbosa-da-Silva S, Intermittent fasting exerts beneficial metabolic effects on blood pressure and cardiac structure by modulating local renin-angiotensin system in the heart of mice fed high-fat or high-fructose diets, Nutrition Research 63 (2019) 51–62. [DOI] [PubMed] [Google Scholar]
- [25].Su L, Yao Y, Song W, Downregulation of miR-96 suppresses the profibrogenic functions of cardiac fibroblasts induced by angiotensin II and attenuates atrial fibrosis by upregulating KLF13, Human Cell (2020) 1–10. [DOI] [PubMed] [Google Scholar]
- [26].Cat AND, Touyz RM, A new look at the renin–angiotensin system—focusing on the vascular system, Peptides 32(10) (2011) 2141–2150. [DOI] [PubMed] [Google Scholar]
- [27].Shigemura N, Takai S, Hirose F, Yoshida R, Sanematsu K, Ninomiya Y, Expression of Renin-Angiotensin System Components in the Taste Organ of Mice, Nutrients 11(9) (2019) 2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zanchetti AS, Neural regulation of renin release: experimental evidence and clinical implications in arterial hypertension, Circulation 56(5) (1977) 691–698. [DOI] [PubMed] [Google Scholar]
- [29].van den Heuvel M, Batenburg WW, Jainandunsing S, Garrelds IM, van Gool JM, Feelders RA, van den Meiracker AH, Danser AJ, Urinary renin, but not angiotensinogen or aldosterone, reflects the renal renin–angiotensin–aldosterone system activity and the efficacy of renin–angiotensin–aldosterone system blockade in the kidney, Journal of Hypertension 29(11) (2011) 2147–2155. [DOI] [PubMed] [Google Scholar]
- [30].Batenburg WW, Krop M, Garrelds IM, de Vries R, de Bruin RJ, Burcklé CA, Müller DN, Bader M, Nguyen G, Danser AJ, Prorenin is the endogenous agonist of the (pro) renin receptor. Binding kinetics of renin and prorenin in rat vascular smooth muscle cells overexpressing the human (pro) renin receptor, Journal of Hypertension 25(12) (2007) 2441–2453. [DOI] [PubMed] [Google Scholar]
- [31].Zhou G, Wu J, Gu C, Wang B, Abel ED, Cheung AK, Huang Y, Prorenin independently causes hypertension and renal and cardiac fibrosis in cyp1a1-prorenin transgenic rats, Clinical Science 132(12) (2018) 1345–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Chappell MC, Nonclassical renin-angiotensin system and renal function, Comprehensive Physiology 2(4) (2012) 2733–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer J-D, Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin, The Journal of Clinical Investigation 109(11) (2002) 1417–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang N-P, Erskine J, Zhang W-W, Zheng R-H, Zhang L-H, Duron G, Gendreau J, Zhao Z-Q, Recruitment of macrophages from the spleen contributes to myocardial fibrosis and hypertension induced by angiotensin II, Journal of the Renin-Angiotensin-Aldosterone System 18(2) (2017) 1470320317706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Watson S, Burnside T, Carver W, Angiotensin II-stimulated collagen gel contraction by heart fibroblasts: Role of the AT1 receptor and tyrosine kinase activity, Journal of Cellular Physiology 177(2) (1998) 224–231. [DOI] [PubMed] [Google Scholar]
- [36].Hou J, Kato H, Cohen RA, Chobanian AV, Brecher P, Angiotensin II-induced cardiac fibrosis in the rat is increased by chronic inhibition of nitric oxide synthase, The Journal of Clinical Investigation 96(5) (1995) 2469–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gao N, Wang H, Yin H, Yang Z, Angiotensin II induces calcium-mediated autophagy in podocytes through enhancing reactive oxygen species levels, Chemico-Biological Interactions 277 (2017) 110–118. [DOI] [PubMed] [Google Scholar]
- [38].Marrero MB, Paxton WG, Duff JL, Berk BC, Bernstein KE, Angiotensin II stimulates tyrosine phosphorylation of phospholipase C-gamma 1 in vascular smooth muscle cells, Journal of Biological Chemistry 269(14) (1994) 10935–10939. [PubMed] [Google Scholar]
- [39].Ikeda Y, Aihara K.-i., Sato T, Akaike M, Yoshizumi M, Suzaki Y, Izawa Y, Fujimura M, Hashizume S, Kato M, Androgen receptor gene knockout male mice exhibit impaired cardiac growth and exacerbation of angiotensin II-induced cardiac fibrosis, Journal of Biological Chemistry 280(33) (2005) 29661–29666. [DOI] [PubMed] [Google Scholar]
- [40].Wong CKS, Falkenham A, Myers T, Légaré J-F, Connective tissue growth factor expression after angiotensin II exposure is dependent on transforming growth factor-β signaling via the canonical Smad-dependent pathway in hypertensive induced myocardial fibrosis, Journal of the Renin-Angiotensin-Aldosterone System 19(1) (2018) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hu Q, Hu Z, Chen Q, Huang Y, Mao Z, Xu F, Zhou X, BML-111 equilibrated ACEAngII-AT1R and ACE2-Ang-(1–7)-Mas axis to protect hepatic fibrosis in rats, Prostaglandins & Other Lipid Mediators 131 (2017) 75–82. [DOI] [PubMed] [Google Scholar]
- [42].Watermeyer JM, Sewell BT, Schwager SL, Natesh R, Corradi HR, Acharya KR, Sturrock ED, Structure of testis ACE glycosylation mutants and evidence for conserved domain movement, Biochemistry 45(42) (2006) 12654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D, Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes, Journal of the American Society of Nephrology 17(11) (2006) 3067–75. [DOI] [PubMed] [Google Scholar]
- [44].Vickers C, Hales P, Kaushik V, Dick L, Gavin J, Tang J, Godbout K, Parsons T, Baronas E, Hsieh F, Hydrolysis of biological peptides by human angiotensin-converting enzymerelated carboxypeptidase, Journal of Biological Chemistry 277(17) (2002) 14838–14843. [DOI] [PubMed] [Google Scholar]
- [45].Welches WR, Santos R, Chappell MC, Brosnihan KB, Greene LJ, Ferrario CM, Evidence that prolyl endopeptidase participates in the processing of brain angiotensin, Journal of Hypertension 9(7) (1991) 631–638. [DOI] [PubMed] [Google Scholar]
- [46].Yamamoto K, Chappell MC, Brosnihan KB, Ferrario CM, In vivo metabolism of angiotensin I by neutral endopeptidase (EC 3.4. 24.11) in spontaneously hypertensive rats, Hypertension 19(6_pt_2) (1992) 692–696. [DOI] [PubMed] [Google Scholar]
- [47].Santos RA, e Silva ACS, Maric C, Silva DM, Machado RP, de Buhr I, HeringerWalther S, Pinheiro SVB, Lopes MT, Bader M, Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas, Proceedings of the National Academy of Sciences 100(14) (2003) 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gaidarov I, Adams J, Frazer J, Anthony T, Chen X, Gatlin J, Semple G, Unett DJ, Angiotensin (1–7) does not interact directly with MAS1, but can potently antagonize signaling from the AT1 receptor, Cellular Signalling 50 (2018) 9–24. [DOI] [PubMed] [Google Scholar]
- [49].Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A, Discovery and characterization of alamandine: a novel component of the renin–angiotensin system, Circulation Research 112(8) (2013) 11041111. [DOI] [PubMed] [Google Scholar]
- [50].Jankowski V, Vanholder R, van der Giet M, T lle M, Karadogan S, Gobom J, Furkert J, Oksche A, Krause E, Anh Tran TN, Mass-spectrometric identification of a novel angiotensin peptide in human plasma, Arteriosclerosis, Thrombosis, and Vascular Biology 27(2) (2007) 297302. [DOI] [PubMed] [Google Scholar]
- [51].Etelvino GM, Peluso AAB, Santos RAS, New components of the renin-angiotensin system: alamandine and the MAS-related G protein-coupled receptor D, Current Hypertension Reports 16(6) (2014) 433. [DOI] [PubMed] [Google Scholar]
- [52].Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, Oliveirados-Santos AJ, da Costa J, Zhang L, Pei Y, Angiotensin-converting enzyme 2 is an essential regulator of heart function, Nature 417(6891) (2002) 822–828. [DOI] [PubMed] [Google Scholar]
- [53].Wang M, Han W, Zhang M, Fang W, Zhai X, Guan S, Qu X , Long-term renal sympathetic denervation ameliorates renal fibrosis and delays the onset of hypertension in spontaneously hypertensive rats, American Journal of Translational Research 10(12) (2018) 4042. [PMC free article] [PubMed] [Google Scholar]
- [54].Inaba S, Iwai M, Furuno M, Kanno H, Senba I, Okayama H, Mogi M, Higaki J, Horiuchi M, Role of angiotensin-converting enzyme 2 in cardiac hypertrophy induced by nitric oxide synthase inhibition, Journal of Hypertension 29(11) (2011) 2236–2245. [DOI] [PubMed] [Google Scholar]
- [55].Tallant EA, Clark MA, Molecular mechanisms of inhibition of vascular growth by angiotensin-(1–7), Hypertension 42(4) (2003) 574–579. [DOI] [PubMed] [Google Scholar]
- [56].Liu Q, Tian J, Xu Y, Li C, Meng X, Fu F, Protective effect of RA on myocardial infarction-induced cardiac fibrosis via AT1R/p38 MAPK pathway signaling and modulation of the ACE2/ACE ratio, Journal of Agricultural and Food Chemistry 64(35) (2016) 6716–6722. [DOI] [PubMed] [Google Scholar]
- [57].Harris P, Munro J, Reversal by angiotensins II and III of the effects of converting enzyme inhibition on renal electrolyte excretion in rats, The Journal of Physiology 351(1) (1984) 491500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Feener EP, Northrup JM, Aiello LP, King GL, Angiotensin II induces plasminogen activator inhibitor-1 and-2 expression in vascular endothelial and smooth muscle cells, The Journal of Clinical Investigation 95(3) (1995) 1353–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bai J, Chow BKC, Secretin is involved in sodium conservation through the reninangiotensin-aldosterone system, The FASEB Journal 31(4) (2017) 1689–1697. [DOI] [PubMed] [Google Scholar]
- [60].Katayama Y, Sakamoto T, Saito K, Tsuchimochi H, Kaiya H, Watanabe T, Pearson JT, Takei Y, Drinking by amphibious fish: convergent evolution of thirst mechanisms during vertebrate terrestrialization, Scientific Reports 8(1) (2018) 625–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Patel S, Rauf A, Khan H, Abu-Izneid T, Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies, Biomedicine & Pharmacotherapy 94 (2017) 317–325. [DOI] [PubMed] [Google Scholar]
- [62].Alanazi WA, Fakhruddin S, Jackson K, Angiotensin II Induces Prostaglandin E2 Production and Oxidative Stress in the Renal Cortex, The FASEB Journal 30(1_supplement) (2016) 1196–1198. [Google Scholar]
- [63].Menikdiwela KR, Ramalingam L, Allen L, Scoggin S, Kalupahana NS, MoustaidMoussa N, Angiotensin II Increases endoplasmic Reticulum stress in Adipose tissue and Adipocytes, Scientific Reports 9(1) (2019) 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Xu Z, Li W, Han J, Zou C, Huang W, Yu W, Shan X, Lum H, Li X, Liang G, Angiotensin II induces kidney inflammatory injury and fibrosis through binding to myeloid differentiation protein-2 (MD2), Scientific Reports 7 (2017) 44911–44911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yang Y, Chen S, Tao L, Gan S, Luo H, Xu Y, Shen X, Inhibitory Effects of Oxymatrine on Transdifferentiation of Neonatal Rat Cardiac Fibroblasts to Myofibroblasts Induced by Aldosterone via Keap1/Nrf2 Signaling Pathways In Vitro, Medical Science Monitor 25 (2019) 5375–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kagami S, Border WA, Miller DE, Noble NA, Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells, The Journal of Clinical Investigation 93(6) (1994) 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY, Smad2 protects against TGF-β/Smad3-mediated renal fibrosis, Journal of the American Society of Nephrology 21(9) (2010) 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Zhang Q, Liu C, Hong S, Min J, Yang Q, Hu M, Zhao Y, Hong L, Excess mechanical stress and hydrogen peroxide remodel extracellular matrix of cultured human uterosacral ligament fibroblasts by disturbing the balance of MMPs/TIMPs via the regulation of TGF-β1 signaling pathway, Molecular Medicine Reports 15(1) (2017) 423–430. [DOI] [PubMed] [Google Scholar]
- [69].Zheng Y, Xu QF, Chen HY, Ye CX, Lai W, Maibach HI, Inhibition of MMPs Cat G and downregulates the signaling of TGF-beta/Smad in chronic photodamaged human fibroblasts, European Review for Medical Pharmacological Sciences 21(22) (2017) 5160–5165. [DOI] [PubMed] [Google Scholar]
- [70].Rupérez M, Lorenzo Ó, Blanco-Colio LM, Esteban V, Egido J, Ruiz-Ortega M, Connective tissue growth factor is a mediator of angiotensin II–induced fibrosis, Circulation 108(12) (2003) 1499–1505. [DOI] [PubMed] [Google Scholar]
- [71].Borkham-Kamphorst E, van Roeyen CRC, Ostendorf T, Floege J, Gressner AM, Weiskirchen R, Pro-fibrogenic potential of PDGF-D in liver fibrosis, Journal of Hepatology 46(6) (2007) 1064–1074. [DOI] [PubMed] [Google Scholar]
- [72].Khalil H, Kanisicak O, Prasad V, Correll RN, Fu X, Schips T, Vagnozzi RJ, Liu R, Huynh T, Lee S-J, Fibroblast-specific TGF-β–Smad2/3 signaling underlies cardiac fibrosis, The Journal of Clinical Investigation 127(10) (2017) 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bhandary B, Meng Q, James J, Osinska H, Gulick J, Valiente-Alandi I, Sargent MA, Bhuiyan MS, Blaxall BC, Molkentin JD, Robbins J, Cardiac Fibrosis in Proteotoxic Cardiac Disease is Dependent Upon Myofibroblast TGF-beta Signaling, Journal of the American Heart Association 7(20) (2018) e010013-e010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chaturvedi S, Misra DP, Prasad N, Rastogi K, Singh H, Rai MK, Agarwal V, 5-HT2 and 5-HT2B antagonists attenuate pro-fibrotic phenotype in human adult dermal fibroblasts by blocking TGF-beta1 induced non-canonical signaling pathways including STAT3 : implications for fibrotic diseases like scleroderma, International Journal of Rheumatic Diseases 21(12) (2018) 2128–2138. [DOI] [PubMed] [Google Scholar]
- [75].Tsoyi K, Chu SG, Patino-Jaramillo NG, Wilder J, Villalba J, Doyle-Eisele M, McDonald J, Liu X, El-Chemaly S, Perrella MA, Rosas IO, Syndecan-2 Attenuates Radiationinduced Pulmonary Fibrosis and Inhibits Fibroblast Activation by Regulating PI3K/Akt/ROCK Pathway via CD148, American Journal of Respiratory Cell and Molecular Biology 58(2) (2018) 208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Murphy AM, Wong AL, Bezuhly M, Modulation of angiotensin II signaling in the prevention of fibrosis, Fibrogenesis & Tissue Repair 8(1) (2015) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Pannu J, Nakerakanti S, Smith E, ten Dijke P, Trojanowska M, Transforming growth factor-β receptor type I-dependent fibrogenic gene program is mediated via activation of Smad1 and ERK1/2 pathways, Journal of Biological Chemistry 282(14) (2007) 10405–10413. [DOI] [PubMed] [Google Scholar]
- [78].Meng C, He Y, Wei Z, Lu Y, Du F, Ou G, Wang N, Luo X-G, Ma W, Zhang T-C, He H, MRTF-A mediates the activation of COL1A1 expression stimulated by multiple signaling pathways in human breast cancer cells, Biomedicine & Pharmacotherapy 104 (2018) 718–728. [DOI] [PubMed] [Google Scholar]
- [79].Tao L, Wu L, Zhang W, Ma W-T, Yang G-Y, Zhang J, Xue D-Y, Chen B, Liu C, Peroxisome proliferator-activated receptorgammainhibits hepatic stellate cell activation regulated by miR-942 in chronic hepatitis B liver fibrosis, Life Sciences (2020) 117572–117572. [DOI] [PubMed] [Google Scholar]
- [80].Li S, Zhao W, Tao Y, Liu C, Fugan Wan alleviates hepatic fibrosis by inhibiting ACE/Ang II/AT-1R signaling pathway and enhancing ACE2/Ang 1–7/Mas signaling pathway in hepatic fibrosis rat models, American Journal of Translational Research 12(2) (2020) 592–601. [PMC free article] [PubMed] [Google Scholar]
- [81].Ning Z-W, Luo X-Y, Wang G-Z, Li Y, Pan M-X, Yang R-Q, Ling X-G, Huang S, Ma X-X, Jin S-Y, Wang D, Li X, MicroRNA-21 Mediates Angiotensin II-Induced Liver Fibrosis by Activating NLRP3 Inflammasome/IL-1beta Axis via Targeting Smad7 and Spry1, Antioxidants & Redox Signaling 27(1) (2017) 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Tandon P, Abraldes JG, Berzigotti A, Garcia-Pagan JC, Bosch J, Renin-angiotensinaldosterone inhibitors in the reduction of portal pressure: a systematic review and meta-analysis, Journal of Hepatology 53(2) (2010) 273–282. [DOI] [PubMed] [Google Scholar]
- [83].Pereira RM, dos Santos RAS, da Costa Dias FL, Teixeira MM, e Silva ACS, Reninangiotensin system in the pathogenesis of liver fibrosis, World Journal of Gastroenterology 15(21) (2009) 2579–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bataller R, Sancho-bru P, Ginès P, Lora JM, Al-garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N, Activated human hepatic stellate cells express the reninangiotensin system and synthesize angiotensin II, Gastroenterology 125(1) (2003) 117–125. [DOI] [PubMed] [Google Scholar]
- [85].Fialla AD, De Muckadell OBS, Bie P, Thiesson HC, Activation of RAAS in a rat model of liver cirrhosis: no effect of losartan on renal sodium excretion, BMC Nephrology 19(1) (2018) 238–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Rajapaksha IG, Gunarathne LS, Asadi K, Cunningham SC, Sharland A, Alexander IE, Angus PW, Herath CB, Liver-Targeted Angiotensin Converting Enzyme 2 Therapy Inhibits Chronic Biliary Fibrosis in Multiple Drug-Resistant Gene 2-Knockout Mice, Hepatology Communications 3(12) (2019) 1656–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Yoshiji H, Kuriyama S, Yoshii J, Ikenaka Y, Noguchi R, Nakatani T, Tsujinoue H, Fukui H, Angiotensin-II type 1 receptor interaction is a major regulator for liver fibrosis development in rats, Hepatology 34(4) (2001) 745–750. [DOI] [PubMed] [Google Scholar]
- [88].Li X, Meng Y, Jiang B, Yang X-S, Wang W-W, Guo D, Lai Z-S, Zhang Z-S, [Effects of angiotensin II and aldosterone on NF-kappaB binding activity in hepatic stellate cells], Zhonghua Yi Xue Za Zhi 85(6) (2005) 374–380. [PubMed] [Google Scholar]
- [89].Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, Mazar IGR, Görtzen J, Vogt A, Schildberg FA, Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis, Hepatology 60(1) (2014) 334–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Moreno-Alvarez P, Sosa-Garrocho M, Briones-Orta MA, Gonzalez-Espinosa C, Medina-Tamayo J, Nicolás E, Jiménez W, Molina-Jijon N, Pedraza-Chaverri J, Macias-Silva M, Angiotensin II increases mRNA levels of all TGF-beta isoforms in quiescent and activated rat hepatic stellate cells, Cell Biology International 34(10) (2010) 969–978. [DOI] [PubMed] [Google Scholar]
- [91].Takeda K, Noguchi R, Kitade M, Namisaki T, Moriya K, Kawaratani H, Okura Y, Kaji K, Aihara Y, Douhara A, Periostin cross-reacts with the renin-angiotensin system during liver fibrosis development, Molecular Medicine Reports 16(5) (2017) 5752–5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Casey S, Schierwagen R, Mak KY, Klein S, Uschner F, Jansen C, Praktiknjo M, Meyer C, Thomas D, Herath C, Activation of the Alternate Renin-Angiotensin System Correlates with the Clinical Status in Human Cirrhosis and Corrects Post Liver Transplantation, Journal of Clinical Medicine 8(4) (2019) 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lubel JS, Herath CB, Tchongue J, Grace J, Jia Z, Spencer K, Casley D, Crowley P, Sievert W, Burrell LM, Angus PW, Angiotensin-(1–7), an alternative metabolite of the reninangiotensin system, is up-regulated in human liver disease and has antifibrotic activity in the bile-duct-ligated rat, Clinical Science 117(11) (2009) 375–386. [DOI] [PubMed] [Google Scholar]
- [94].Grace JA, Klein S, Herath CB, Granzow M, Schierwagen R, Masing N, Walther T, Sauerbruch T, Burrell LM, Angus PW, Trebicka J, Activation of the MAS receptor by angiotensin-(1–7) in the renin-angiotensin system mediates mesenteric vasodilatation in cirrhosis, Gastroenterology 145(4) (2013) 874–884.e5. [DOI] [PubMed] [Google Scholar]
- [95].Almehmadi F, Joncas SX, Nevis I, Zahrani M, Bokhari M, Stirrat J, Fine NM, Yee R, White JA, Prevalence of myocardial fibrosis patterns in patients with systolic dysfunction: prognostic significance for the prediction of sudden cardiac arrest or appropriate implantable cardiac defibrillator therapy, Circulation. Cardiovascular imaging 7(4) (2014) 593–600. [DOI] [PubMed] [Google Scholar]
- [96].Leyva F, Taylor RJ, Foley PWX, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith REA, Prasad SK, Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy, Journal of the American College of Cardiology 60(17) (2012) 1659–1667. [DOI] [PubMed] [Google Scholar]
- [97].Kong P, Christia P, Frangogiannis NG, The pathogenesis of cardiac fibrosis, Cellular and Molecular Life Sciences 71(4) (2014) 549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Lu M, Qin Q, Yao J, Sun L, Qin X, Induction of LOX by TGF-beta1/Smad/AP-1 signaling aggravates rat myocardial fibrosis and heart failure, IUBMB Life 71(11) (2019) 17291739. [DOI] [PubMed] [Google Scholar]
- [99].Li C, Han R, Kang L, Wang J, Gao Y, Li Y, He J, Tian J, Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-alpha in myocardial infarction-induced cardiac fibrosis, Scientific Reports 7 (2017) 40523–40523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu Q, Zhang Q, Wang K, Wang S, Lu D, Li Z, Geng J, Fang P, Wang Y, Shan Q, Renal denervation findings on cardiac and renal fibrosis in rats with isoproterenol induced cardiomyopathy, Scientific Reports 5 (2015) 18582–18582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Flevaris P, Khan SS, Eren M, Schuldt AJT, Shah SJ, Lee DC, Gupta S, Shapiro AD, Burridge PW, Ghosh AK, Vaughan DE, Plasminogen Activator Inhibitor Type I Controls Cardiomyocyte Transforming Growth Factor-beta and Cardiac Fibrosis, Circulation 136(7) (2017) 664–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Bouzeghrane F, Mercure C, Reudelhuber TL, Thibault G, Alpha8beta1 integrin is upregulated in myofibroblasts of fibrotic and scarring myocardium, Journal of Molecular and Cellular Cardiology 36(3) (2004) 343–353. [DOI] [PubMed] [Google Scholar]
- [103].Ji Y, Qiu M, Shen Y, Gao L, Wang Y, Sun W, Li X, Lu Y, Kong X, MicroRNA-327 regulates cardiac hypertrophy and fibrosis induced by pressure overload, International Journal of Molecular Medicine 41(4) (2018) 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Kawano H, Cody RJ, Graf K, Goetze S, Kawano Y, Schnee J, Law RE, Hsueh WA, Angiotensin II enhances integrin and α-actinin expression in adult rat cardiac fibroblasts, Hypertension 35(1) (2000) 273–279. [DOI] [PubMed] [Google Scholar]
- [105].Verma SK, Lal H, Golden HB, Gerilechaogetu F, Smith M, Guleria RS, Foster DM, Lu G, Dostal DE, Rac1 and RhoA differentially regulate angiotensinogen gene expression in stretched cardiac fibroblasts, Cardiovascular Research 90(1) (2011) 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Tharaux PL, Chatziantoniou C, Fakhouri F, Dussaule JC, Angiotensin II activates collagen I gene through a mechanism involving the MAP/ER kinase pathway, Hypertension 36(3) (2000) 330–336. [DOI] [PubMed] [Google Scholar]
- [107].Shimojo N, Hashizume R, Kanayama K, Hara M, Suzuki Y, Nishioka T, Hiroe M, Yoshida T, Imanaka-Yoshida K, Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin αVβ3/nuclear factor–κB/interleukin-6 axis, Hypertension 66(4) (2015) 757–766. [DOI] [PubMed] [Google Scholar]
- [108].Pohjolainen V, Rysa J, Napankangas J, Koobi P, Eraranta A, Ilves M, Serpi R, Porsti I, Ruskoaho H, Left ventricular periostin gene expression is associated with fibrogenesis in experimental renal insufficiency, Nephrology, Dialysis, Transplantation 27(1) (2012) 115–122. [DOI] [PubMed] [Google Scholar]
- [109].Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA, Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart, Journal of the American College of Cardiology 43(9) (2004) 1698–1705. [DOI] [PubMed] [Google Scholar]
- [110].Chen C, Li R, Ross RS, Manso AM, Integrins and integrin-related proteins in cardiac fibrosis, Journal of Molecular and Cellular Cardiology 93 (2016) 162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T, Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy, Hypertension 43(6) (2004) 1195–1201. [DOI] [PubMed] [Google Scholar]
- [112].Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan T-L, Hsueh WA, Osteopontin is produced by rat cardiac fibroblasts and mediates A (II)-induced DNA synthesis and collagen gel contraction, The Journal of Clinical Investigation 98(10) (1996) 2218–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].He Z, Way KJ, Arikawa E, Chou E, Opland DM, Clermont A, Isshiki K, Ma RCW, Scott JA, Schoen FJ, Differential regulation of angiotensin II-induced expression of connective tissue growth factor by protein kinase C isoforms in the myocardium, Journal of Biological Chemistry 280(16) (2005) 15719–15726. [DOI] [PubMed] [Google Scholar]
- [114].Gu J, Liu X, Wang Q.-x., Tan H.-w., Guo M, Jiang W.-f., Zhou L, Angiotensin II increases CTGF expression via MAPKs/TGF-β1/TRAF6 pathway in atrial fibroblasts, Experimental Cell Research 318(16) (2012) 2105–2115. [DOI] [PubMed] [Google Scholar]
- [115].Azibani F, Fazal L, Chatziantoniou C, Samuel J-L, Delcayre C, Aldosterone mediates cardiac fibrosis in the setting of hypertension, Current Hypertension Reports 15(4) (2013) 395400. [DOI] [PubMed] [Google Scholar]
- [116].Brown NJ, Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis, Nature Reviews Nephrology 9(8) (2013) 459–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Syed M, Ball JP, Mathis KW, Hall ME, Ryan MJ, Rothenberg ME, Yanes Cardozo LL, Romero DG, MicroRNA-21 ablation exacerbates aldosterone-mediated cardiac injury, remodeling, and dysfunction, American Journal of Physiology-Endocrinology and Metabolism 315(6) (2018) E1154-E1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Qi G, Jia L, Li Y, Bian Y, Cheng J, Li H, Xiao C, Du J, Angiotensin II Infusion–Induced Inflammation, Monocytic Fibroblast Precursor Infiltration, and Cardiac Fibrosis are Pressure Dependent, Cardiovascular Toxicology 11(2) (2011) 157–167. [DOI] [PubMed] [Google Scholar]
- [119].Martín-Fernández B, Rubio-Navarro A, Cortegano I, Ballesteros S, Alía M, CannataOrtiz P, Olivares-Álvaro E, Egido J, de Andrés B, Gaspar ML, Aldosterone induces renal fibrosis and inflammatory M1-macrophage subtype via mineralocorticoid receptor in rats, PLoS One 11(1) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM, Johar S, Cave AC, Narayanapanicker A, Grieve DJ, Shah AM, Aldosterone mediates angiotensin II-induced interstitial cardiac fibrosis via a Nox2-containing NADPH oxidase, The FASEB Journal 20(9) (2006) 1546–1548. [DOI] [PubMed] [Google Scholar]
- [121].Rodríguez-Ayala E, Ávila-Díaz M, Foyo-Niembro E, Amato D, Ramirez-San-Juan E, Paniagua R, Effect of parathyroidectomy on cardiac fibrosis and apoptosis: possible role of aldosterone, Nephron Physiology 103(3) (2006) p112–p118. [DOI] [PubMed] [Google Scholar]
- [122].Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y, Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin-angiotensin system, Endocrinology 148(4) (2007) 1688–1696. [DOI] [PubMed] [Google Scholar]
- [123].Ogawa Y, Mukoyama M, Yokoi H, Kasahara M, Mori K, Kato Y, Kuwabara T, Imamaki H, Kawanishi T, Koga K, Ishii A, Tokudome T, Kishimoto I, Sugawara A, Nakao K, Natriuretic peptide receptor guanylyl cyclase-A protects podocytes from aldosterone-induced glomerular injury, Journal of the American Society of Nephrology : JASN 23(7) (2012) 11981209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Kimura K, Ohkita M, Koyama M, Matsumura Y, Reduced NO production rapidly aggravates renal function through the NF-kappaB/ET-1/ETA receptor pathway in DOCA-saltinduced hypertensive rats, Life Sciences 91(13–14) (2012) 644–650. [DOI] [PubMed] [Google Scholar]
- [125].Eiam-Ong S, Chaipipat M, Manotham K, Eiam-Ong S, Aldosterone rapidly activates pPKC delta and GPR30 but suppresses p-PKC epsilon protein levels in rat kidney, Endocrine Regulations 53(3) (2019) 154–164. [DOI] [PubMed] [Google Scholar]
- [126].De Giusti VC, Orlowski A, Ciancio MC, Espejo MS, Gonano LA, Caldiz CI, Vila Petroff MG, Villa-Abrille MC, Aiello EA, Aldosterone stimulates the cardiac sodium/bicarbonate cotransporter via activation of the g protein-coupled receptor gpr30, Journal of Molecular and Cellular Cardiology 89(Pt B) (2015) 260–267. [DOI] [PubMed] [Google Scholar]
- [127].Ren Y, D’Ambrosio MA, Garvin JL, Leung P, Kutskill K, Wang H, Peterson EL, Carretero OA, Aldosterone sensitizes connecting tubule glomerular feedback via the aldosterone receptor GPR30, American Journal of Physiology-Renal Physiology 307(4) (2014) F427–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Zhang W-W, Zheng R-H, Bai F, Sturdivant K, Wang N-P, James EA, Bose HS, Zhao ZQ, Steroidogenic acute regulatory protein/aldosterone synthase mediates angiotensin IIinduced cardiac fibrosis and hypertrophy, Molecular Biology Reports (2019) 1–16. [DOI] [PubMed] [Google Scholar]
- [129].Bae EH, Fang F, Williams VR, Konvalinka A, Zhou X, Patel VB, Song X, John R, Oudit GY, Pei Y, Murine recombinant angiotensin-converting enzyme 2 attenuates kidney injury in experimental Alport syndrome, Kidney International 91(6) (2017) 1347–1361. [DOI] [PubMed] [Google Scholar]
- [130].Rodrigues Prestes TR, Rocha NP, Miranda AS, Teixeira AL, Simoes-e-Silva AC, The anti-inflammatory potential of ACE2/angiotensin-(1–7)/mas receptor axis: evidence from basic and clinical research, Current Drug Targets 18(11) (2017) 1301–1313. [DOI] [PubMed] [Google Scholar]
- [131].Kassiri Z, Zhong J, Guo D, Basu R, Wang X, Liu PP, Scholey JW, Penninger JM, Oudit GY , Loss of angiotensin-converting enzyme 2 accelerates maladaptive left ventricular remodeling in response to myocardial infarction, Circulation: Heart Failure 2(5) (2009) 446–455. [DOI] [PubMed] [Google Scholar]
- [132].Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, Ferrario CM, Raizada MK, Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats, Experimental Physiology 90(5) (2005) 783–790. [DOI] [PubMed] [Google Scholar]
- [133].Díez-Freire C, Vázquez J, Correa de Adjounian M.a.F., Ferrari MFR, Yuan L, Silver X, Torres R, Raizada MK, ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR, Physiological Genomics 27(1) (2006) 12–19. [DOI] [PubMed] [Google Scholar]
- [134].Wang Z-F, Wang N-P, Harmouche S, Philip T, Pang X-F, Bai F, Zhao Z-Q, Postconditioning attenuates coronary perivascular and interstitial fibrosis through modulating angiotensin II receptors and angiotensin-converting enzyme 2 after myocardial infarction, The Journal of Surgical Research 211 (2017) 178–190. [DOI] [PubMed] [Google Scholar]
- [135].Liu Z, Huang XR, Chen H-Y, Penninger JM, Lan HY, Loss of angiotensinconverting enzyme 2 enhances TGF-β/Smad-mediated renal fibrosis and NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy, Laboratory Investigation 92(5) (2012) 650–650. [DOI] [PubMed] [Google Scholar]
- [136].Hao S.y., Ren M, Yang C, Lin D.z., Chen L.h., Zhu P, Cheng H, Yan L, Activation of skin renin–angiotensin system in diabetic rats, Endocrine 39(3) (2011) 242–250. [DOI] [PubMed] [Google Scholar]
- [137].Steckelings UM, Wollschläger T, Peters J, Henz BM, Hermes B, Artuc M, Human skin: source of and target organ for angiotensin II, Experimental Dermatology 13(3) (2004) 148–154. [DOI] [PubMed] [Google Scholar]
- [138].Steckelings UM, Artuc M, Paul M, Stoll M, Henz BM, Angiotensin II Stimulates Proliferation of Primary Human Keratinocytes via a Non-AT1, Non-AT2Angiotensin Receptor, Biochemical and Biophysical Research Communications 229(1) (1996) 329–333. [DOI] [PubMed] [Google Scholar]
- [139].Akershoek JJ, Vlig M, Brouwer K, Talhout W, Beelen RHJ, Middelkoop E, Ulrich MMW, The presence of tissue renin-angiotensin system components in human burn wounds and scars, Burns Open 2(3) (2018) 114–121. [Google Scholar]
- [140].Steckelings UM, Henz BM, Wiehstutz S, Unger T, Artuc M, Differential expression of angiotensin receptors in human cutaneous wound healing, British Journal of Dermatology 153(5) (2005) 887–893. [DOI] [PubMed] [Google Scholar]
- [141].Bernasconi R, Nyström A, Balance and circumstance: The renin angiotensin system in wound healing and fibrosis, Cellular Signalling 51 (2018) 34–46. [DOI] [PubMed] [Google Scholar]
- [142].Faghih M, Hosseini SM, Smith B, Ansari AM, Lay F, Ahmed AK, Inagami T, Marti GP, Harmon JW, Walston JD, Knockout of Angiotensin AT2 receptors accelerates healing but impairs quality, Aging (Albany NY) 7(12) (2015) 1185–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Rodgers K, Xiong S, Felix J, Roda N, Espinoza T, Maldonado S, Dizerega G, Development of angiotensin (1-7) as an agent to accelerate dermal repair, Wound Repair and Regeneration 9(3) (2001) 238–247. [DOI] [PubMed] [Google Scholar]
- [144].Fang Q-Q, Wang X-F, Zhao W-Y, Chen C-Y, Zhang M-X, Shi B-H, Zhang L-Y, Tan W-Q, The source of ACE during scar formation is from both bone marrow and skin tissue, The FASEB Journal 32(9) (2018) 5199–5208. [DOI] [PubMed] [Google Scholar]
- [145].Tan WQ, Fang QQ, Shen XZ, Giani JF, Zhao TV, Shi P, Zhang LY, Khan Z, Li Y, Li L, Angiotensin-converting enzyme inhibitor works as a scar formation inhibitor by downregulating Smad and TGF-β-activated kinase 1 (TAK1) pathways in mice, British Journal of Pharmacology 175(22) (2018) 4239–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Min L-J, Cui T-X, Yahata Y, Yamasaki K, Shiuchi T, Liu H-W, Chen R, Li J-M, Okumura M, Jinno T, Wu L, Iwai M, Nahmias C, Hashimoto K, Horiuchi M, Regulation of collagen synthesis in mouse skin fibroblasts by distinct angiotensin II receptor subtypes, Endocrinology 145(1) (2004) 253–260. [DOI] [PubMed] [Google Scholar]
- [147].Mizoue S, Iwai M, Ide A, Suzuki J, Horiuchi M, Shiraishi A, Ohashi Y, Role of angiotensin II receptor subtypes in conjunctival wound healing, Current Eye Research 31(2) (2006) 129–136. [DOI] [PubMed] [Google Scholar]
- [148].Wynn TA, Common and unique mechanisms regulate fibrosis in various fibroproliferative diseases, The Journal of Clinical Investigation 117(3) (2007) 524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Chappell MC, Al Zayadneh EM, Angiotensin-(1–7) and the Regulation of Anti-Fibrotic Signaling Pathways, Journal of Cell Signaling 2(1) (2017) 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kim MY, Cho MY, Baik SK, Jeong PH, Suk KT, Jang YO, Yea CJ, Kim JW, Kim HS, Kwon SO, Beneficial effects of candesartan, an angiotensin-blocking agent, on compensated alcoholic liver fibrosis-A randomized open-label controlled study, Liver International 32(6) (2012) 977–987. [DOI] [PubMed] [Google Scholar]
- [151].Queisser N, Happ K, Link S, Jahn D, Zimnol A, Geier A, Schupp N, Aldosterone induces fibrosis, oxidative stress and DNA damage in livers of male rats independent of blood pressure changes, Toxicology and Applied Pharmacology 280(3) (2014) 399–407. [DOI] [PubMed] [Google Scholar]
- [152].Zhu Q, Li N, Li F, Zhou Z, Han Q, Lv Y, Sang J, Liu Z, Therapeutic effect of renin angiotensin system inhibitors on liver fibrosis, Journal of the Renin-Angiotensin-Aldosterone System 17(1) (2016) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].de Macêdo SM, Guimarães TA, Feltenberger JD, Santos SHS, The role of reninangiotensin system modulation on treatment and prevention of liver diseases, Peptides 62 (2014) 189–196. [DOI] [PubMed] [Google Scholar]
- [154].Pereira RM, dos Santos RAS, Teixeira MM, Leite VHR, Costa LP, da Costa Dias FL, Barcelos LS, Collares GB, e Silva ACS, The renin–angiotensin system in a rat model of hepatic fibrosis: evidence for a protective role of angiotensin-(1–7), Journal of Hepatology 46(4) (2007) 674–681. [DOI] [PubMed] [Google Scholar]
- [155].Wiggins KJ, Kelly DJ, Aliskiren: a novel renoprotective agent or simply an alternative to ACE inhibitors?, Kidney International 76(1) (2009) 23–31. [DOI] [PubMed] [Google Scholar]
- [156].Wang LP, Fan SJ, Li SM, Wang XJ, Sun N, Aliskiren inhibits proliferation of cardiac fibroblasts in AGT-REN double transgenic hypertensive mice in vitro, Sheng Li Xue Bao:[Acta Physiologica Sinica] 68(5) (2016) 684–690. [PubMed] [Google Scholar]
- [157].Oliveira SHP, Brito VGB, Frasnelli SCT, da Silva Ribeiro B, Ferreira MN, Queiroz DP, Beltan CT, Lara VS, Santos CF, Aliskiren attenuates the inflammatory response and wound healing process in diabetic mice with periodontal disease, Frontiers in Pharmacology 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Park S, Nguyen NB, Pezhouman A, Ardehali R, Cardiac fibrosis: potential therapeutic targets, Translational Research 209 (2019) 121–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Gross O, Girgert R, Rubel D, Temme J, Theissen S, Müller G-A, Renal protective effects of aliskiren beyond its antihypertensive property in a mouse model of progressive fibrosis, American Journal of Hypertension 24(3) (2011) 355–361. [DOI] [PubMed] [Google Scholar]
- [160].Kawamura M, Ito H, Onuki T, Miyoshi F, Watanabe N, Asano T, Tanno K, Kobayashi Y, Candesartan decreases type III procollagen-N-peptide levels and inflammatory marker levels and maintains sinus rhythm in patients with atrial fibrillation, Journal of Cardiovascular Pharmacology 55(5) (2010) 511–517. [DOI] [PubMed] [Google Scholar]
- [161].Schmidt BMW, Schmieder RE, Aldosterone-induced cardiac damage: focus on blood pressure independent effects, Oxford University Press, 2003. [DOI] [PubMed] [Google Scholar]
- [162].Habibi J, DeMarco VG, Ma L, Pulakat L, Rainey WE, Whaley-Connell AT, Sowers JR, Mineralocorticoid receptor blockade improves diastolic function independent of blood pressure reduction in a transgenic model of RAAS overexpression, American Journal of Physiology-Heart and Circulatory Physiology 300(4) (2011) H1484-H1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Brilla CG, Funck RC, Rupp H, Lisinopril-mediated regression of myocardial fibrosis in patients with hypertensive heart disease, Circulation 102(12) (2000) 1388–1393. [DOI] [PubMed] [Google Scholar]
- [164].D’Souza KM, Biwer LA, Madhavpeddi L, Ramaiah P, Shahid W, Hale TM, Persistent change in cardiac fibroblast physiology after transient ACE inhibition, American Journal of Physiology-Heart and Circulatory Physiology 309(8) (2015) H1346-H1353. [DOI] [PubMed] [Google Scholar]
- [165].Hale TM, Robertson SJ, Burns KD, deBlois D, Short-term ACE inhibition confers long-term protection against target organ damage, Hypertension Research 35(6) (2012) 604–610. [DOI] [PubMed] [Google Scholar]
- [166].Seeland U, Schaffer A, Selejan S, Hohl M, Reil JC, Muller P, Rosenkranz S, Bohm M, Effects of AT1- and beta-adrenergic receptor antagonists on TGF-beta1-induced fibrosis in transgenic mice, European Journal of Clinical Investigation 39(10) (2009) 851–859. [DOI] [PubMed] [Google Scholar]
- [167].Mayyas FA, Aljohmani AI, Alzoubi KH, Impact of spironolactone on markers of myocardial oxidative status, inflammation and remodeling in hyperthyroid rats, Current Molecular Pharmacology (2019). [DOI] [PubMed] [Google Scholar]
- [168].Cannon MV, Yu H, Candido WM, Dokter MM, Lindstedt EL, Silljé HHW, van Gilst WH, de Boer RA, The liver X receptor agonist AZ876 protects against pathological cardiac hypertrophy and fibrosis without lipogenic side effects, European Journal of Heart Failure 17(3) (2015) 273–282. [DOI] [PubMed] [Google Scholar]
- [169].Kaikkonen LA, Sätzler V, Roy A, Contreras M, Xu B, Stroll S, McCarthy J, Molkentin JD, Das S, Kontaridis M, Activated Fibroblast-specific Deletion of RhoA Reduces Cardiac Fibrosis Through Regulation of the Non-canonical p38-MAPK Signaling Pathway, Circulation Research 125(Suppl_1) (2019) A252–A252. [Google Scholar]
- [170].Wang C, Kemp-Harper BK, Samuel CS, Abstract P2012: Combining the Anti-Fibrotic Actions of Serelaxin With the Anti-Oxidant, N-Acetylcysteine, Abrogates Cardiac Fibrosis in an Isoprenaline-Induced Murine Model of Cardiomyopathy, Hypertension 74(Suppl_1) (2019) AP2012-AP2012. [Google Scholar]
- [171].Kaschina E, Grzesiak A, Li J, Foryst-Ludwig A, Timm M, Rompe F, Sommerfeld M, Kemnitz UR, Curato C, Namsolleck P, Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction?, Circulation 118(24) (2008) 2523–2532. [DOI] [PubMed] [Google Scholar]
- [172].Lauer D, Slavic S, Sommerfeld M, Thone-Reineke C, Sharkovska Y, Hallberg A, Dahlof B, Kintscher U, Unger T, Steckelings UM, Kaschina E, Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor beta1 in the rat heart, Hypertension 63(3) (2014) e60–7. [DOI] [PubMed] [Google Scholar]
- [173].Wang Y, DelBorgo M, Aguilar M, Denton K, Samuel C, Widdop R, A8011 A novel angiotensin AT2 receptor ligand for the treatment of cardiac fibrosis, Journal of Hypertension 36 (2018) e50–e51. [Google Scholar]
- [174].Hedayatyanfard K, Ziai SA, Niazi F, Habibi I, Habibi B, Moravvej H, Losartan ointment relieves hypertrophic scars and keloid: A pilot study, Wound Repair and Regeneration 26(4) (2018) 340–343. [DOI] [PubMed] [Google Scholar]
- [175].Abadir P, Hosseini S, Faghih M, Ansari A, Lay F, Smith B, Beselman A, Vuong D, Berger A, Tian J, Topical reformulation of valsartan for treatment of chronic diabetic wounds, Journal of Investigative Dermatology 138(2) (2018) 434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Chisholm J, Gareau AJ, Byun S, Paletz JL, Tang D, Williams J, LeVatte T, Bezuhly M, Effect of Compound 21, a Selective Angiotensin II Type 2 Receptor Agonist, in a Murine Xenograft Model of Dupuytren Disease, Plastic and Reconstructive Surgery 140(5) (2017) 686e-696e. [DOI] [PubMed] [Google Scholar]