Abstract
Change in coronary artery plaque on serial catheter intravascular ultrasound (IVUS) is an established technique to monitor the therapeutic effect of drugs on coronary atherosclerosis. Recent advances in coronary computed tomography angiography (CTA) now allow for non-invasive assessment of change in coronary plaque. Because coronary CTA is noninvasive, it enables clinical trials with lower-risk populations, higher retention rates, and lower costs. This review presents an overview of serial coronary CTA as a noninvasive imaging technique to gauge the therapeutic effect of anti-atherosclerotic therapies. Furthermore, it reviews the increasing use of serial CTA as an imaging endpoint in completed and ongoing clinical trials.
Keywords: Serial computed tomography angiography, Coronary artery plaque, Anti-atherosclerotic drugs
Introduction
Cardiovascular disease (CVD) is the most common cause of death worldwide [1]. Although current lipid-lowering treatment is effective, high residual risk leads to increasing demand for new potent therapies. The path from bench to bedside is lined with failed drugs, with half not advancing past Phase II, despite an average Phase II trial cost exceeding $23 million [2]. The problem is especially acute for coronary artery disease (CAD), as major adverse cardiovascular events (MACE) are rare and so event-driven trials must enroll a large number of patients [3]. Thus there is a high demand for imaging biomarkers to identify the most efficacious drugs in an efficient manner [3].
Coronary plaque is an important marker as the amount of plaque is associated with MACE [4–9]. Using change in coronary artery plaque volume as a surrogate imaging endpoint allows smaller clinical trials, which can inform the important decision whether larger event-driven trials are likely to be successful [10]. Recently there has been an increasing number of trials using serial imaging methods for surrogate endpoint measures, with the gold standard being intravascular ultrasound (IVUS) or Optical Coherence Tomography (OCT). Yet, invasive imaging is practically only possible in persons already having invasive coronary angiography for suspected symptomatic stenosis, neglecting the vast majority of persons in the asymptomatic primary prevention population [11]. Coronary CTA, on the other hand, provides information on the entire coronary tree and has rapidly matured into an alternative to invasive imaging modalities.
This review presents an overview of coronary CTA as a noninvasive imaging technique for plaque assessment, its strengths and limitations, and its use in clinical trials assessing anti-atherosclerotic treatment effects.
Plaque imaging techniques
IVUS is the current reference standard for in vivo plaque imaging [12, 13]. Its strengths include the possibility to quantify coronary artery disease and obtain “in vivo” information on plaque composition [14] making it an important diagnostic tool to accurately assess changes in coronary plaque [12, 15–17] (Table 1).
Table 1.
List of selected studies using intravascular ultrasound (IVUS) to monitor changes in coronary plaque on anti-atherosclerotic therapies
| Study | Year | Design | Number of participants | Dropout rate (%) | Follow-up period | Therapy or comparison | What is measured | Treatment effect* |
|---|---|---|---|---|---|---|---|---|
| REVERSAL | 2004 | Prospective interventional randomized controlled | 654 | 23 | 18 months | Moderate versus intensive statin therapy (pravastatin vs. atorvastatin) | Percent change in atheroma volume | Median change 2.7% versus − 0.4%; p = 0.02 |
| CAMELOT | 2004 | Prospective interventional randomized controlled | 431 | 36 | 24 months | Amlodipine versus enalapril versus placebo | Change in percent atheroma volume | Mean change 0.5% versus 0.8% versus 1.3% (amlodipine vs. enalapril p = 0.59, amlodipine vs. placebo p = 0.12, enalapril vs. placebo p = 0.32) |
| ASTEROID | 2006 | Prospective interventional | 507 | 31 | 24 months | Rosuvastatin | Change in percent atheroma volume | Median change − 0.79%; p < 0.001 versus baseline |
| SATURN | 2011 | Prospective interventional randomized | 1385 | 25 | 24 months | Atorvastatin versus rosuvastsatin | Change in Percent atheroma volume | Median change − 0.99% versus − 1.22%; p = 0.17 |
| PRECISE-IVUS | 2015 | Prospective interventional randomized controlled | 246 | 28 | 9 to 12 months | Atorvastatin versus atorvastatin + ezetimibe | Absolute change in percent athroma volume | Median change − 0.3% versus − 1.4%; p = 0.001 |
p values given for cohort versus control. If not available, p values given for baseline versus follow-up
Such accuracy derives from high-resolution images of the vessel lumen, its contours and adjacent media-adventitia interface [10, 18]. Precisely, IVUS has a spatial resolution of 150–200 μm and a penetration depth of 5–8 mm [19], allowing to characterize the composition of potential atherosclerotic plaques and calculate the diameter of lumen and vessel [10, 18]. Yet, IVUS is an invasive test that is performed during catheter invasive coronary angiography (ICA). In practice, this limits its application to high-risk persons already undergoing ICA and secondary prevention populations. Moreover, studies using serial IVUS have traditionally suffered from high dropout rates approaching 20–30%, presumably because participants do not want to undergo a second invasive test for research [12, 20, 21]. For instance, in the ASTEROID trial, Nissen et al. investigated the effect of very high-intensity statin therapy on regression of atherosclerotic plaque using serial IVUS examinations. The study delivered encouraging results (with a median reduction in total atheroma volume of 6.8% compared to baseline). However, only 349 out of 507 patients completed the trail, which translated into a 31% drop-out rate [16].
OCT is a second invasive plaque imaging test that is also performed during ICA. With its tenfold higher resolution compared to that of IVUS (approx. 10–15 μm) [19, 22], OCT is especially useful in imaging thin cap fibroatheroma and is considered the clinical reference of fibrous cap thickness measurements and evaluation of necrotic core [22–24]. Fibrous cap thickness as a marker of plaque stability in lipid-rich plaques was used in a trial by Habara et al. investigating the effect of supplemental ezetimibe to fluvastatin therapy on coronary artery plaque in patients with prior myocardial infarction. After 9 months of follow-up, the change in fibrous cap thickness was significantly greater in the group receiving combination therapy versus fluvastatin alone (0.08 mm vs. 0.04 mm, p < 0.001), suggesting the benefit of more extensive medication in this patient cohort [25]. OCT’s disadvantage relative to IVUS is its reduced penetration of 2 mm depth, due to which it fails to reach the outer vessel boundary. Like IVUS, it is an invasive technique performed during coronary catheterization and thus is limited to high-risk patients [19, 22].
Coronary CTA is a noninvasive plaque imaging modality that has emerged as a safe and widely accessible alternative to IVUS and OCT [26]. The noninvasive nature of this technique not only allows studies to enroll low-risk patients but also allows for a significant reduction in costs. For example, the outpatient Medicare cost for diagnostic ICA is $2854 while that for coronary CTA is $341 [27]. Furthermore, coronary CTA provides information that is not attainable in IVUS or OCT. The image acquisition of coronary CTA covers the entire coronary tree, enabling the assessment of overall plaque burden and extracting information from pericoronary structures. In contrast, IVUS and OCT are typically only performed in a small portion of the coronary tree. The major limitation of coronary CTA is its lower spatial resolution of 300–600 μm [19, 28] and variability in image quality, which may limit its ability to see small changes in plaque.
Quantitative plaque assessment
Plaque quantification has been shown to improve risk stratification and prediction of future events beyond disease detection alone [13, 29–31]. Such quantitative analyses most commonly comprise information on stenosis, plaque volume, plaque composition, and presence of high-risk plaque features (HRP) [29, 32–37] (Fig. 1). In serial imaging, the delta of these parameters (i.e., change in plaque volume, etc.) is another common measure to reflect differences from baseline to follow-up.
Fig. 1.
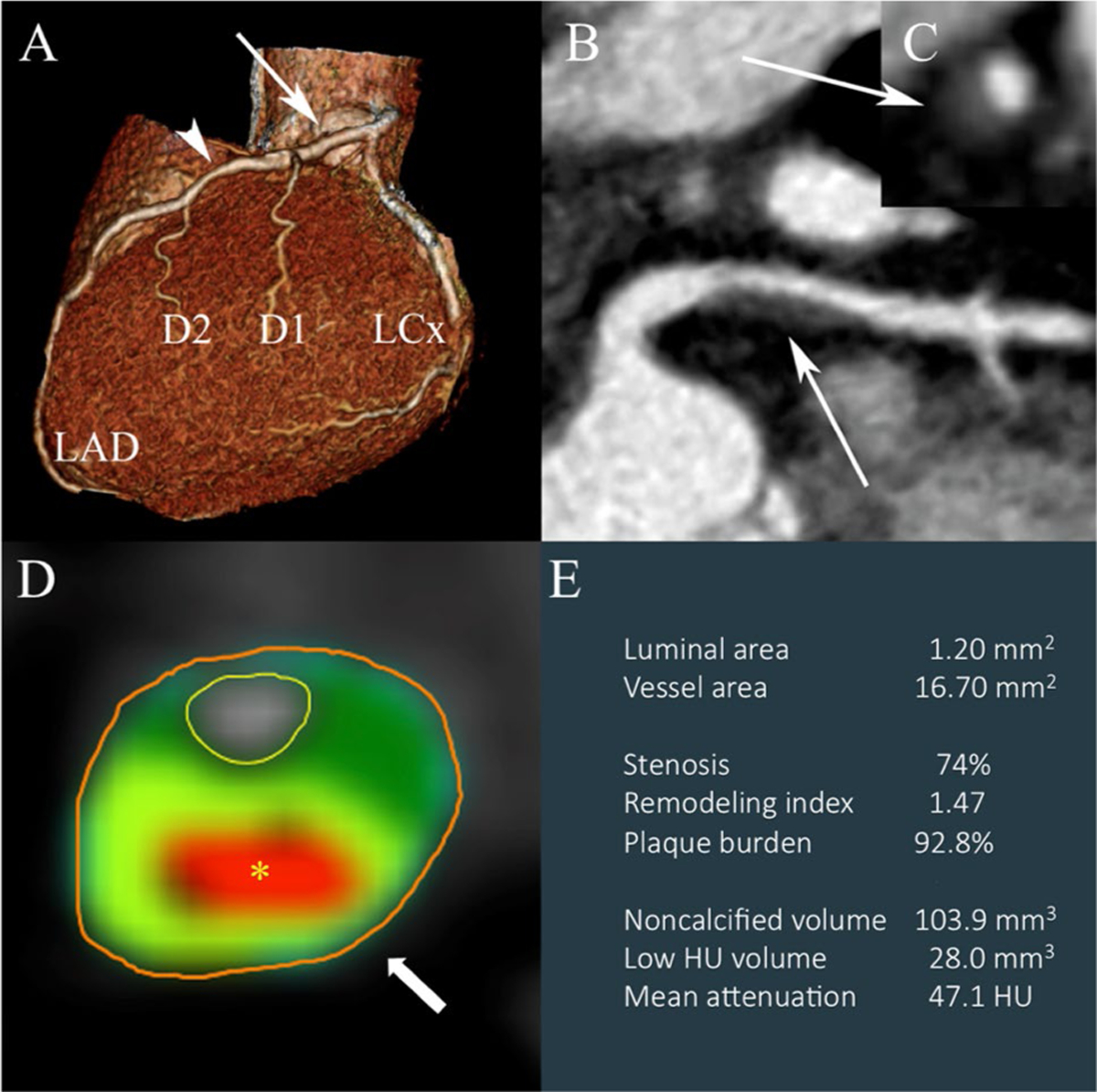
Qualitative and quantitative analysis of coronary CTA. Panels a–c Standard qualitative analysis of coronary CTA data for the presence of obstructive CAD (stenosis ≥ 50%): a 3D volume-rendered image of the coronary tree with potential narrowing in the proximal (arrow) and mid LAD (arrowhead). b multiplanar reformatted long and c short axis images of the proximal LAD area (white arrows) demonstrating luminal narrowing < 50%. This patient would be classified as having non-obstructive CAD unlikely to be hemodynamically significant as a result of routine diagnostic assessment. Panels d, e Advanced quantitative analysis of another patient demonstrating a plaque with high-risk features. d Cross section of a vessel depicting central low attenuation core in red (asterisk) with ring-like peripheral high attenuation in light green (arrow)—the napkin ring sign. e Output of quantitative analysis with derived minimal luminal area, degree of stenosis, remodeling index, plaque burden, noncalcified and low HU volume, and mean plaque attenuation
Stenosis
Stenosis quantification serves as an important predictor of coronary events [38] and overall mortality [39]. While invasive and non-invasive imaging techniques alike assess the degree of luminal narrowing caused by coronary atherosclerotic plaques, ICA serves as gold standard and reference for coronary CTA. Coronary CTA’s accuracy compared to ICA is high. This is due to technical aspects such as image noise or lower spatial and temporal resolution with subsequent artifacts [40]. For that matter, most coronary CTA studies have used visually estimated binary cut-off values to define clinically significant obstruction—either using ≥ 50% or ≥ 70% stenosis [41–43]. Alternatively, categorical values (0%, 1–24%, 25–49%, 50–69%, 70–89%, 90–100%) have been introduced with good agreement to ICA [44].
Plaque volume
Total plaque volume (TPV) has shown a strong correlation with traditional risk factors such as diabetes and obesity [45, 46], as well as acute coronary syndrome (ACS) [47] and chronic inflammatory diseases (such as HIV) [48]. Plaque volume per se is calculated as the difference in vessel and lumen volume including all plaque components [23]. TPV includes all of the patient′s plaque. It is most commonly used to measure treatment efficacy on serial CT studies [23] and encompasses more than one diseased segment (i.e., a vessel or the entire coronary tree). Relative plaque volume measures assessed as percent atheroma volume (PAV) (i.e., the ratio of plaque to vessel volume × 100) are also commonly described [49].
Plaque composition and high-risk plaque features
CT allows for identification of different plaque components based on their density (attenuation). Plaque can be divided into calcified and noncalcified components. The noncalcified portion can be further divided into fibrous, fibrofatty, and lipid-rich components based on attenuation. Generally, we think of the noncalcified part as being the biologically active component of plaque, with the lipid-rich component carrying an increased risk of rupture [31].
Half of culprit plaques that cause MACE arise from plaques that had previously caused a stenosis < 50% [50]. Thus, there is considerable interest in identifying which plaques are prone to rupture – the high-risk plaque (HRP). HRP features on CT have been associated with incidence ACS [51], an increase in future cardiovascular events [31], and add value beyond stenosis [11]. HRP features identified by CT include positive remodeling, low Hounsfield Unit (HU) attenuation, napkin-ring sign (NRS), and spotty calcium [52]. Positive remodeling describes the phenomenon where the vessel increases its outer diameter to compensate for luminal narrowing [53]. A low attenuation plaque (< 30HU) is the CT-equivalent to lipid-rich components [54]. The combination of central low attenuation (< 30HU) with a surrounding rim of higher attenuation resembles a necrotic core with fibrous cap – also referred to as the NRS [28]. Lastly, spotty calcifications are defined as areas of calcification (> 130HU) measuring < 3mm on one side of the artery wall, within an otherwise noncalcified plaque [31].
Comparison of IVUS and coronary CTA in plaque assessment
Several studies have compared IVUS and coronary CTA in their assessment of coronary artery plaque using measures for lesion and stenosis (i.e., TPV, PAV, minimal diameter/ area, or percent stenosis), but also identifying plaque composition (i.e., calcified/noncalcified/mixed plaque or HRP features) [29, 32–37].
Despite coronary CTA’s tendency to overestimate calcified plaque volume due to blooming, with an associated underestimate of noncalcified plaque, a high correlation between coronary CTA and IVUS in the quantitative assessment of TPV has been published [29, 32, 33].
In a recently published meta-analysis correlating coronary CTA and IVUS, coronary CTA provided excellent diagnostic accuracy for plaque detection comparable to that in IVUS (AUC 0.94; CI 0.92–0.96). In quantitative comparison, differences in plaque area (mean difference 0.09 mm2, p = 0.88), plaque volume (mean difference 5.30 mm3, p = 0.21) and area stenosis (weighted mean difference − 1.81%, p = 0.12) were not significant between measurements done in coronary CTA and IVUS. However, it is noteworthy that coronary CTA overestimated luminal area, which may be due to partial volume effects [34].
Furthermore, multiple studies have shown coronary CTA’s ability to differentiate noncalcified and calcified plaque components with a high correlation to IVUS results [29, 35–37] or histology [55] as the gold standard as well as the ability for a detailed evaluation of NCP components [28, 56].
In a head-to-head comparison of multislice CT and virtual-histology IVUS (VH-IVUS), Pundziute et al. observed a good correlation in the quantification of calcified, non-calcified, and mixed plaque using both modalities. They further demonstrated that mixed plaques characterized by multislice CT were associated with high-risk features in VH-IVUS and that there was a significant correlation in coronary calcium score in multislice CT and calcified plaque volumes in VH-IVUS (r = 0.69, p < 0.0001). However, higher spatial resolution in VH-IVUS yielded more precise results when determining plaque composition, with the highest precision observed in mixed and noncalcified plaques [36].
In another study, Marwan et al. sought to separate non-calcified plaque composition into predominantly ‘fibrous’ or ‘lipid-rich’ using each plaque’s mean HU attenuation with IVUS serving as the gold standard. CT attenuation between both plaque subtypes was significantly different, reaching a 95% sensitivity and 80% specificity for predominantly lipid plaques when using a threshold of 5.5% of pixels ≤ 30HU. However, they described a vast overlap in attenuation values of fibrous or lipid-rich plaques, which led them to recommend an additional histogram analysis for further characterization [56].
Anti-atherosclerotic drug studies using serial coronary CTA
An increasing number of studies have assessed drug efficacy using serial coronary CTA. In 2007, Burgstahler et al. published the New Age II study, in which they found that a combination therapy of atorvastatin and aspirin led to regression of NCPV [15]. While this study included only 27 patients, subsequent large-scale clinical trials followed confirming these findings.
One of the early large-scale prospective observational trials by Li et al. addressed the effect of statin therapy on NCP. 206 patients were grouped into intensive, moderate, or no statin treatment; coronary CTA was performed at baseline and after a median follow-up of 18 months. Results demonstrated a significant reduction in NCPV as well as TPV between the intensive-statin and no-statin group (with annualized changes of − 7.1 vs. 0.9mm3, p < 0.001; − 16.4 vs. 12.3 mm3, p < 0.001), and an attenuation of plaque progression when comparing moderate-statin to no-statin treatment (annualized changes NCPV: − 2.8 vs. 0.9 mm3, p = 0.041; − 0.1 vs. 12.3 mm3, p = 0.014). Both moderate, as well as intensive statin treatment, were independent predictors of plaque regression, which led to the conclusion that statins reduce growth and induce regression in patients with mild noncalcified plaque [57].
The most notable reduction of NCPV through statin therapy as measured by serial coronary CTA illustrated Lo et al.’s study in 2015 [48]. In this prospective randomized controlled trial, the effect of atorvastatin on TPV, NCPV and HRP features in people living with HIV (PLWH) was examined. The investigators concluded that among 37 participants receiving atorvastatin, the median NCPV decreased by 19% whereas the NCPV increased by 20% in participants receiving placebo (p = 0.009). TPV likewise was significantly reduced in the atorvastatin group with a 5% decrease versus an 18% increase in the placebo group (p = 0.02) (Fig.2). Similarly, the number of plaques with high-risk features, namely low attenuation, and positive remodeling, significantly decreased in the statin therapy group [48]. In a subanalysis of this trial investigating the natural history of plaque change, statins were found to reduce fatty and fibrotic components in progressing lesions causing plaque stabilization [58].
Fig. 2.
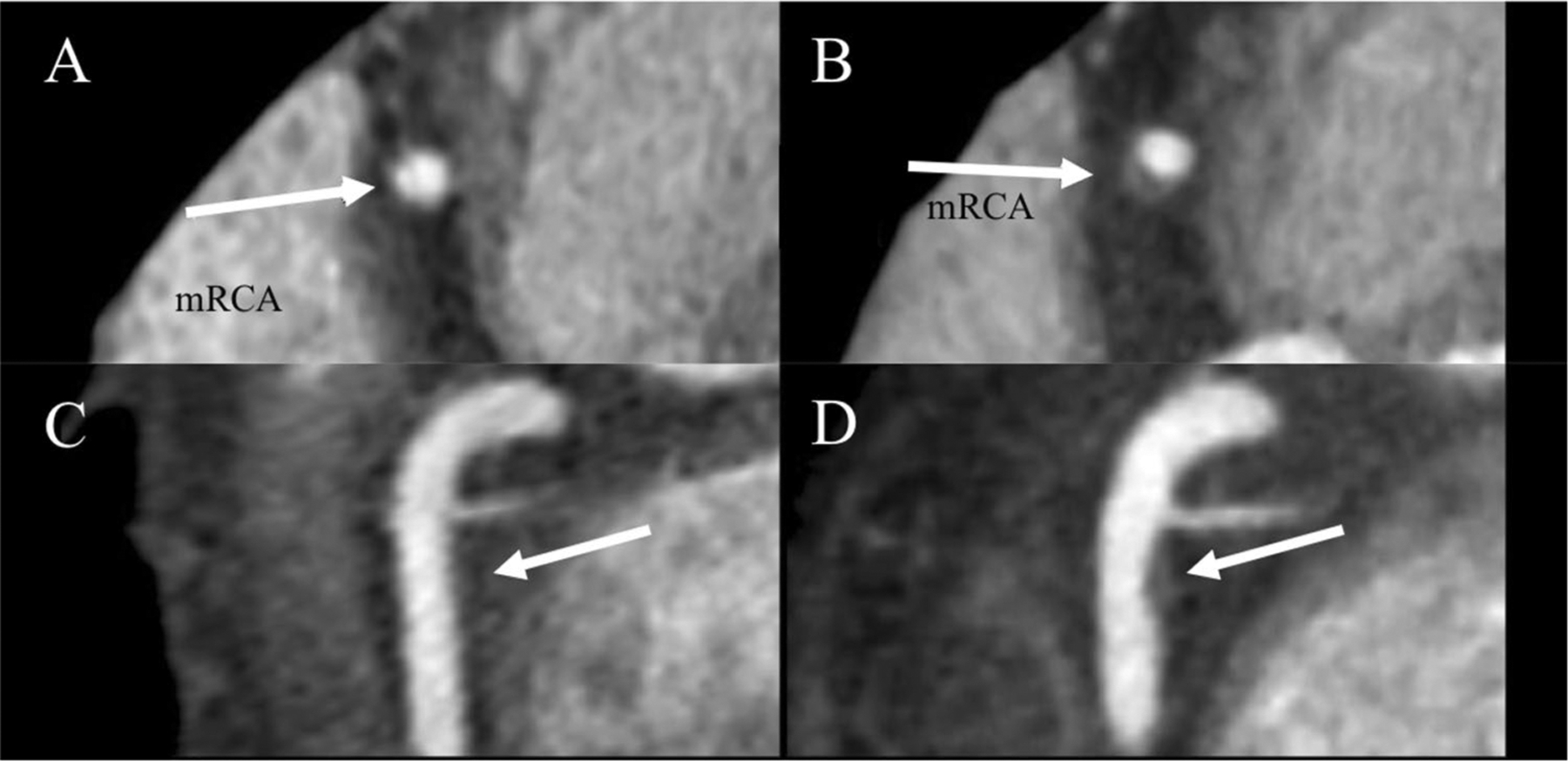
Increasing non-calcified plaque volume in mid right coronary artery (mRCA) in a patient on placebo. Coronary CTA of mRCA (arrow) at baseline a, c and 12 months follow-up b, d in patient with HIV. High-risk morphology features including positive remodeling and low attenuation lipid core developed on follow-up. Multi-planar reformations in short (a, b) and long (c, d) axis
The success of serial coronary CTA studies in evaluating the efficacy of statins in changing coronary plaque volume encouraged investigators to evaluate other anti-atherosclerotic therapies using the same imaging modality. In 2010, Tardif et al. demonstrated that 12 weeks of atreleuton, a 5-lipoxygenase inhibitor, managed to decrease NCPV in patients with recent ACS by an average of 2.33 mm3, whereas that in patients treated with placebo increased by an average of 2.83mm3 [59]. Similar results were described in a study by Matsumoto et al. in 2016, which also evaluated the effect of atreleuton on plaque progression in patients with recent ACS. After a follow-up of 24 weeks, atreleuton treatment resulted in a significant reduction of plaque progression as well as a reduction of non-calcified plaque components (i.e., low attenuation, fibrous, and fibro-fatty plaque) compared to the placebo group [60].
While a novel drug agent such as atreleuton successfully reduced or stabilized coronary plaque volume, not all drugs led to similar results. For example, in 2016, Hauser et al. investigated the effect of non-steroidal anti-inflammatory treatment on NCPV in overweight or obese patients. The trial enrolled a total of 257 patients which were randomized to salasate treatment versus. placebo; change in NCPV as measured by CCTA was defined as the primary outcome. However, when compared to baseline no significant increase in NCPV in each group nor a difference in between groups was detected [61].
Another study testing the effect of testosterone treatment in older men with hypogonadism on coronary artery plaque likewise did not lead to a regression in plaque volume. In fact, NCPV, as well as TPV, were significantly increased (with an estimated change of 41 mm3, p = 0.003, and 47mm3, p = 0.006) in subjects who received testosterone gel as treatment compared to the placebo group during a follow-up of 12 months [62].
Similarly, other treatment approaches such as garlic extract [63] or different anticoagulant therapies [64] did not lead to a regression of coronary plaque volume.
In addition to many completed investigations, there are a number of promising ongoing clinical trials utilizing serial coronary CTA to validate the efficacy of novel treatments.
One novel pharmaceutical approach to atherosclerotic treatment is MEDI6012—a recombinant human lecithin-cholesterol acyltransferase (rhLCAT) [65]. LCAT plays an important role in cholesterol metabolism by stabilizing high-density lipoprotein and promoting the transport of excess cholesterol from the periphery to the liver [66]. A recently launched randomized, placebo-controlled phase IIb study (REAL-TIMI 63B) is aiming to enroll 414 participants with acute ST-elevation myocardial infarction to undergo serial coronary CTA to examine the efficacy of this new drug. With the estimated completion date in March 2020, results are highly anticipated [65].
Apart from novel drug developments in patients with traditional cardiovascular risk factors, the focus has moved to patients with chronic inflammatory conditions such as HIV marking a risk enhancing factor in the development of atherosclerotic cardiovascular disease. In the treatment of HIV, the focus has shifted from preventing the spread to preventing major cardiovascular events in PLWH because of the improvements in antiretroviral therapy and medical care. In 2015, the National Institutes of Health (NIH) launched the REPRIEVE trial—the first primary prevention trial for HIV. The REPRIEVE trial is a prospective randomized controlled clinical trial using pitavastatin to prevent vascular events in PLWH. A mechanistic substudy, recruiting approximately 800 participants to undergo serial coronary CTA, will enable the investigators to determine drug-efficacy through the change in plaque volume and the number of observed vascular events in this cohort [67].
Another ongoing randomized, placebo-controlled trial (EPIC-HIV Study) is evaluating the impact of a PCSK9-inhibitor called alirocumab on cardiovascular risk in PLWH. This study, which started in April 2018 and is estimated to be completed by November 2021, intents to recruit 140 patients with risk factors for CVD or known CVD and evidence of vascular inflammation. One study endpoint is to assess the effect of PCSK9-inhibition on NCP, which will be measured using serial CCTA in association with inflammatory markers [68].
These above-mentioned studies only represent a selection of published and ongoing trails using serial coronary CTA for surrogate imaging endpoint studies between Phase II and III. A complete overview is displayed in Tables 2 and 3. It is interesting to note, that most of these investigations started after January 2015, which can mainly be traced back to technical improvements in cardiac imaging. However, with higher retention rates and a better cost-effectiveness profile compared to invasive imaging studies, serial coronary CTA is expected to gain further popularity in future clinical trials.
Table 2.
Published studies that used anti-atherosclerotic therapies
| Study | Design | Population | Number of participants | Follow-up period | Therapy or comparison | What is measured | Treatment effect* |
|---|---|---|---|---|---|---|---|
| Burgstahler et al. Invest Radiol. 2007 | Prospective interventional | Patients with elevated risk for CAD | 27 | 12 months | Atorvastatin + aspirin | Noncalcified plaque volume | Mean change−0.012 mL; p<0.05 versus baseline |
| Hoffmann H et al. Eur Radiol 2010 | Retrospective observational | Patients with suspected CAD | 63 | 25 ± 3 months | Statins | Noncalcified plaque volume | Plaque growth slowed by statin therapy (p = 0.01) |
| Inoue K et al. JACC Cardiovasc Imaging. 2010 | Prospective interventional controlled | Patients with suspected CAD | 32 | 12 months | Fluvastatin versus control | Total and Low-attenuation plaque volume | Mean change −15.9 versus 4 mm3; p = 0.01,−3.7 versus 0.2 mm3; p<0.01 |
| Tardif JC et al. Circ Cardiovasc Imaging. 2010 | Prospective interventional randomized controlled | Patients with MI or unstable angina | 93 | 24 weeks | Atreleuton versus placebo | Noncalcified plaque volume | Mean change −2.33 versus 2.83 mm3; p<0.01 |
| Soeda T et al. Circ J. 2011 | Prospective interventional | Patients with ACS | 11 | 24 weeks | Rosuvastatin | Total plaque volume | Mean change −24.7mm3; p = 0.07 |
| Zeb I et al. Atherosclerosis. 2013 | Retrospective observational | Patients with no prior heart disease or revascularization | 100 | 406 ± 92 days | Statin versus no statin | Total & Noncalcified plaque volume | Mean change: −33.3 versus 31.0 mm3; p = 0.0006, − 47.7 versus 13.8mm3; p<0.001 |
| Lo J, Lu MT et al. Lancet HIV. 2015 | Prospective interventional randomized controlled | People living with HIV | 40 | 12 months | Atorvastatin versus placebo | Noncalcified plaque volume | Median change − 8.2 versus 6.7 mm3; p = 0.03 (−19.4% vs.+ 20.4%; p = 0.009) |
| Auscher S et al. Atherosclerosis. 2015 | Prospective interventional randomized controlled | Patients with acute MI | 140 | 12 months | Intensive statin (Rosuvastatin) versus standard statin | Total plaque volume & dense calcium volume | Mean change 43.5 versus 19.1 mm3; p = 0.57,+ 11.1 versus−0.4 mm3; p< 0.001 |
| Hauser et al. JAMA Cardiol. 2016 | Prospective interventional randomized controlled | Patients with CAD | 257 | 30 months | Salsalate versus placebo | Total & Noncalcified plaque volume | Mean change 7 versus 13 mm3; p = 0.35, 0 versus 0 mm3; p = 0.87 |
| Li Z et al. Am Heart J. 2016 | Prospective observational | Patients with mild noncalcified plaque | 206 | 18 months | Intensive versus moderate versus no statin | Total & Low-attenuation plaque volume | Mean change − 16.4 versus −0.1 versus 12.3; p<0.001,−7.1 versus −2.8 versus 0.9 mm3; p< 0.001 |
| Matsumoto S et al. J Nutr. 2016 | Prospective interventional randomized controlled | Patients with metabolic syndrome | 55 | 354 ±41 days | Aged Garlic Extract versus placebo | Total, Non-calcified and Low-attenuation plaque volume | % change mean Total: 0.3 versus 1.6, p = 0.13; Noncalcified: 0.2 versus 1.4, p = 0.14; LAP: −1.5 versus 0.2, p = 0.0049 |
| Alfaddagh et al. J Aha. 2017 | Prospective interventional randomized controlled | Patients with stable CAD on statins | 285 | 30 months | Omega-3 ethyl-ester versus control | Total & Noncalcified plaque volume | % change median 6.5 versus 10.0; p = 0.11,−2.4 versus 4.5; p − 0.14 |
| Budoff Metal. JAMA 2017 | Prospective interventional randomized controlled | Older men with low testosterone | 170 | 12 months | Testosterone versus placebo | Total & Noncalcified plaque volume | Least squares Mean change 75 versus 28mm3; p = 0.006, 54 versus 14 mm3; p = 0.003 |
| Lee DH et al. Atherosclerosis. 2017 | Prospective interventional randomized controlled | Diabetic patients | 40 | 6 months | Sarpogrelate + aspirin versus aspirin | Total & Noncalcified plaque volume | Mean change −7.8 versus 3.7 mm3; p<0.05,−4.4 versus 1.6 mm3; p<0.01 |
| Matsumoto S et al. Clinical Cardiology 2017 | Prospective interventional randomized controlled | Patients with ACS | 60 | 6 months | Atreleuton versus control | Low-attenuation plaque, Fibro-fatty tissue, Fibro-calcified plaque & Dense calcium plaque volume | Mean change LAP − 9.7 versus 5.9 mm3; p<0.05, FF−0.9 versus 11.1 mm3; p<0.05, FC − 14.3 versus − 0.1mm3; p<0.05, DC 0.2 versus 3.9 mm3; p<0.05 |
| Vaidya K et al. JACC Cardiovasc Imaging. 2017 | Prospective observational controlled | Patients with ACS | 80 | 12 months | Colchicine + OMT versus OMT | Low-attenuation & Noncalcified plaque volume | Mean change − 15.9 versus −6.6mm3; p = 0.008,−26.3 versus− 18.2mm3; p = 0.62 |
| Lee J et al. Am Heart J. 2018 | Prospective interventional randomized | Patients with nonvalvular atrial fibrillation | 120 | 12 months | Warfarin versus Rivaroxaban | Total, Noncalcified & Low-attenuation plaque volume | Median change: 40.5 versus 26.3 mm3; p = 0.123, 30.1 versus 20.1mm3; p = 0.259, 0.2 versus 1.2mm3; p = 0.475 |
| Win T et al. Am Heart J. 2019 | Prospective interventional randomized | Patients with nonvalvular atrial fibrillation | 66 | 12 months | Warfarin versus Apixaban | Total, Noncalcified & Low-attenuation plaque volume | Mean change 53.8 versus 46.8 mm3; p = 0.40, 36 versus 31.5 mm3; p = 0.43, 2.3 versus 0.3mm3; p = 0.97 |
| Lee SE et al. Eur Heart J Cardiovasc Imaging. 2019 | Retrospective observational | Patients from PARADIGM registry | 654 | 3.9 ± 1.5 years | Statins versus no statins | Total & Noncalcified plaque volume | Annualized change in normalized plaque volumes: 20.2 versus 13.0mm3/year; p< 0.001, 6.4 versus 7.0mm3/year; p = 0.702 |
We conducted a systematic review using PubMed and ClinicalTrials.gov to identify published and ongoing studies using CCTA to assess therapies’ efficacy in reducing coronary plaque volume untill February 2019. We included studies if they performed serial CCTA to assess the efficacy of anti-atherosclerotic therapies and examined changes in plaque volume. Studies were excluded if (1) the full article was not in English, (2) recruitment status is not yet recruiting, suspended, terminated, withdrawn, or unknown
p values given for cohort versus control. If not available, p values given for baseline versus follow-up
Table 3.
Ongoing studies using anti-atherosclerotic therapies
| Study | Design | Population | Number of participants | Follow-up period | Therapy or comparison | What is measured |
|---|---|---|---|---|---|---|
| Evaluating the Use of Pitavastatin to Reduce the Risk of Cardiovascular Disease in HIV-Infected Adults (REPRIEVE) | Prospective interventional randomized controlled | People living with HIV | 800 | 24 months | Pitavastatin versus placebo | Noncalcified plaque volume |
| Effect of PCSK9 Inhibition on Cardiovascular Risk in Treated HIV Infection (EPIC-HIV Study) (EPIC-HIV) | Prospective interventional randomized controlled | People living with HIV | 140 | 13 months | Alirocumab versus Placebo | Noncalcified plaque volume |
| A Study to Evaluate the Safety and Efficacy of MEDI6012 in Acute ST Elevation Myocardial Infarction (REAL-TIMI 63B) | Prospective interventional randomized controlled | Patients with acute STEMI | 540 | 10 to 12 weeks post-MI | MEDI6012 versus Placebo | Noncalcified plaque volume |
| CT COMPARE: CT Coronary Angiography to Measure PlAque Reduction | Prospective interventional randomized | Good candidates for statin treatment | 190 | 12 months, 24 months, 36 months | moderate atorvastatin versus high intense rosuvastatin or atorvastatin | Noncalcified plaque volume |
| Effects of Eplerenone on Cardiovascular Disease in HIV (MIRACLE HIV Study) | Prospective interventional randomized controlled | People living with HIV | 60 | 12 months | Eplerenone versus placebo | Plaque volume |
| Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy (EVAPORATE) | Prospective interventional randomized controlled | Subjects with elevated triglycerides (200–499 mg/dl) | 80 | 18 months | Vascepa versus placebo | Noncalcified plaque volume |
| Effect of Evolocumab on Coronary Artery Plaque Volume and Composition by CCTA and Microcalcificiation by F18-NaF PET | Prospective interventional | Patients with cardiovascular disease | 55 | 18 months | Evolocumab | Noncalcified plaque volume |
| Assessment of Change in Atherosclerotic Plaque by Serial CCTA (ACROSS) | Prospective observational | Patients with CAD | 400 | 24 months | Atorvastatin | Total atheroma volume |
| A Study of the Gut Barrier and Blood Vessel Inflammation in Individuals With and Without HIV | Prospective interventional randomized controlled | People living with HIV | 60 | 6 months | Teduglutide versus Placebo | Plaque volume (not specified) |
We conducted a systematic review using PubMed and ClinicalTrials.gov to identify published and ongoing studies using CCTA to assess therapies’ efficacy in reducing coronary plaque volume before February 2019. We included studies if they performed serial CCTA to assess the efficacy of anti-atherosclerotic therapies and examined changes in plaque volume. Studies were excluded if (1) the full article was not in English, (2) recruitment status is not yet recruiting, suspended, terminated, withdrawn, or unknown
p values given for cohort versus control. If not available, p values given for baseline versus follow-up
Image quality assurance, reproducibility and sample size estimations
When assessing plaque progression as a surrogate endpoint of anti-atherosclerotic therapies, diagnostic image quality and reproducibility are key—affecting hardware and software alike.
In terms of hardware, the scan-rescan variation using different CT-vendors is a critical point. This is especially relevant in multi-center investigations but also needs to be kept in mind in a multi-vendor facility. A systematic comparison of scanner variability in coronary CTA was recently performed by Symons et al. comparing coronary plaque volume measurements acquired with the same versus a different scanner within 30 days. The authors chose a vessel- as well as a lesion-based approach to assess plaque burden of the entire coronary tree and plaque burden in most diseased segments (as both have been reported in current trials) to quantify TPV, NCPV and calcified plaque volume. Intra -scan reproducibility for NCPV for all segments and the most diseased segments was good (± 18.4% and ± 16.0% coefficient of variation), yet, inter-scan variability in follow-up imaging largely differed for NCPV in all coronaries as well as in those with the highest plaque burden (± 29.9% and ± 26.5%). After adjusting for within-patient correlation of segments, an effect of scanner on calcified plaque volume remained (with a 12% difference, while TPV and NCPV were similar). The authors concluded, that variability in plaque volume would increase the sample size needed to detect a 5% change in NCPV in a lesion-based analysis from 217 (same scanner) to 587 patients (two different scanners). An even larger increase in sample size would results for a per-vessel analysis of change in NCPV from 286 (using the same scanner) to 753 subjects (when using different vendors) [69], both of which might not be achievable for many proposed studies.
This scenario also highlights the importance of the correct choice of primary endpoints (along with their respective standard deviations) used for sample size calculations which was recently demonstrated in a study assessing the effect of testosterone treatment on coronary artery plaque. Due to a smaller standard deviation, Budoff et al. were able to reduce the sample size from 400 to 140 by changing the primary outcome from TPV to NCPV [62].
Sample sizes in coronary CTA studies are similar to those using IVUS [16, 70]. For instance, in the ASTEROID trial, Nissen et al. reported an estimated sample size of 313 patients for their primary endpoint, change in PAV, to detect a difference of − 0.7% with an 80% power and a 2-sided alpha-level of 0.025 (assuming a standard deviation of 4%). For their secondary endpoint, change TPV in 10-mm subsegments with highest plaque at baseline, however, a sample size of only 171 patients was calculated for an expected difference of − 3mm3 (standard deviation 12.6mm3) reaching the same power and alpha-levels [16].
This leads to another challenge, which is the choice of the most appropriate endpoint to capture an expected effect. A number of endpoint measures have been proposed, some of which were adapted from IVUS trials (such as TPV or PAV), others which are unique to CT (such as overall plaque burden or HRP features). Yet, there is no consensus on the best approach or guidelines for standardized quantification causing a wide range of different endpoints which can impede study comparison. For instance, in trials assessing the effect of statin treatment on coronary artery plaque volume change, Lo et al. used NCPV as a surrogate [48], while Inoue et al. chose TPV and low-attenuation plaque volume [71], and Auscher et al. evaluated TPV and dense calcium volume [72]. Furthermore, treatment effect on coronary artery plaque of many—especially novel—therapies is unknown and most effective endpoints need yet to be defined. Data from the coronary CTA PARADIGM study indicate an accelerated transformation from non-calcified towards more calcified lesions in patients on statin therapy [73], whereas an earlier study described a slower plaque progression [74] or even regression [71]. Furthermore, there is currently no established threshold for a ‘clinically relevant’ change, which is why studies have relied on prior observations from CTA or IVUS studies to estimate the expected delta.
Ensuring constant high image quality is another important aspect. Image quality and CT attenuation values can vary greatly depending on radiation dose [23, 55, 75], reconstruction algorithm [76], and concentration of intraluminal contrast material [77, 78]. Variations in tube potential (i.e., kVp) are known to cause shifts in measured CT density not only in intra-scan but also in inter-scan comparison [79]. Keeping the x-ray energy at a constant is important for comparability when using HU attenuation values for plaque quantification on two consecutive data sets. Another critical aspect in serial imaging is constant high image quality as a non-diagnostic baseline or follow-up scan can lead to an exclusion of the patient per se. Such a scenario occurred in a CT-substudy of a placebo-controlled trial using coronary artery plaque as a secondary endpoint. In this study, 28 out of 88 qualified patients had to be excluded due to insufficient image quality, which was impaired by motion, noise, or artifacts [59].
When quantifying plaque, reliable software and reproducibility are warranted. For intra-software comparison, plaque quantification has generally resulted in good inter-reader variability [31, 80]. Inter-software reproducibility, on the other hand, still greatly varies and requires standardization across vendors. This concern was raised when variability of NCPV measured with three different commercially available software packages demonstrated significantly different results with Pearson correlation coefficients ranging from 0.550 to 0.677 (p < 0.001) [75].
In general, to ensure high image quality as well as reproducibility, serial coronary CTA image acquisition parameters (i.e., pre-scan medication, ECG-gating, tube potential, use of reconstruction techniques, application of intravenous contrast), need to be pre-specified. To account for inter-scan variations, baseline, and follow-up imaging should be performed using one scanner. For quantitative image analysis assessment of an entire dataset (i.e., baseline and follow-up images of one subject) by the same reader has proven effective to calculate inter-observer variability. However, variability in data resulting from serial CTA studies, especially for multi-center trials, alongside with hard- and software limitations remain the Achilles’ heel of CTA. To establish coronary CTA as a tool to monitor drug effects, pre-specification of acquisition parameters, choice of scanner type for baseline and follow-up (the same scanner for both timepoints) and readers for quantitative image analysis (ideally one reader per dataset) are mandatory to ensure high image quality and reproducibility. For future trials, consensus on CT metrics and evaluation standards as well as an accepted threshold for a clinically significant change would be desirable.
Conclusion
Change in coronary plaque volume on CTA is a noninvasive test that is increasingly used to demonstrate the efficacy of anti-atherosclerotic treatments. Several trials have successfully utilized serial CTA to measure coronary plaque progression, and several trials are ongoing.
Footnotes
Conflict of interest Dr. Lu reported research funding as a co-investigator to MGH from Kowa Company Limited and Medimmune/Astrazeneca and receiving personal fees from PQBypass unrelated to this work. He reports a research grant from the Nvidia Corporation Academic Program. Dr. Hoffmann reported receiving research support on behalf of his institution from Duke University (Abbott), HeartFlow, Kowa Company Limited, and MedImmune/Astrazeneca; and receiving consulting fees from Duke University (NIH), and Recor Medical unrelated to this research. Dr. Taron was funded by the Deutsche Forschungsge-meinschaft (DFG, German Research Foundation) -TA 1438/1–1.
Informed consent For this type of study formal consent is not required.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Murray CJ, Barber RM, Foreman KJ, Ozgoren AA, Abd-Allah F, Abera SF, Aboyans V, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet (London, England) 385:117–171. 10.1016/S0140-6736(14)61682-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DiMasi JA, Hansen RW, Grabowski HG (2003) The price of innovation: new estimates of drug development costs. J Health Econ 22:151–185. 10.1016/S0167-6296(02)00126-1 [DOI] [PubMed] [Google Scholar]
- 3.Tardif J-C, Heinonen T, Orloff D, Libby P (2006) Vascular biomarkers and surrogates in cardiovascular disease. Circulation 10.1161/CIRCULATIONAHA.105.598987 [DOI] [PubMed] [Google Scholar]
- 4.Nicholls SJ, Hsu A, Wolski K et al. (2010) Intravascular ultrasound-derived measures of coronary atherosclerotic plaque burden and clinical outcome. J Am Coll Cardiol 55:2399–2407. 10.1016/j.jacc.2010.02.026 [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann U, Ferencik M, Udelson JE et al. (2017) Prognostic value of noninvasive cardiovascular testing in patients with stable chest pain: insights from the PROMISE trial (prospective multicenter imaging study for evaluation of chest pain). Circulation 135:2320–2332. 10.1161/CIRCULATIONAHA.116.024360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S-E, Sung JM, Rizvi A et al. (2018) Quantification of coronary atherosclerosis in the assessment of coronary artery disease. Circ Cardiovasc Imaging 11:e007562 10.1161/CIRCIMAGING.117.007562 [DOI] [PubMed] [Google Scholar]
- 7.Andreini D, Magnoni M, Conte E et al. (2019) Coronary plaque features on CTA can identify patients at increased risk of cardiovascular events. JACC Cardiovasc Imaging 10.1016/j.jcmg.2019.06.019 [DOI] [PubMed] [Google Scholar]
- 8.Deseive S, Straub R, Kupke M et al. (2018) Quantification of coronary low-attenuation plaque volume for long-term prediction of cardiac events and reclassification of patients. J Cardiovasc Comput Tomogr 12:118–124. 10.1016/j.jcct.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 9.Hell MM, Motwani M, Otaki Y et al. (2017) Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging 18:1331–1339. 10.1093/ehjci/jex183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huisman J, Hartmann M, von Birgelen C et al. (2011) Serial intravascular ultrasound assessment of changes in coronary atherosclerotic plaque dimensions and composition: an update. Eur J Echocardiogr 12:313–321. 10.1093/ejechocard/jer017 [DOI] [PubMed] [Google Scholar]
- 11.Maurovich-Horvat P, Ferencik M, Voros S et al. (2014) Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol 11:390–402. 10.1038/nrcardio.2014.60 [DOI] [PubMed] [Google Scholar]
- 12.Tsujita K, Sugiyama S, Sumida H et al. (2015) Impact of dual lipid-lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE-IVUS trial. J Am Coll Cardiol 66:495–507. 10.1016/j.jacc.2015.05.065 [DOI] [PubMed] [Google Scholar]
- 13.Stone GW, Maehara A, Lansky AJ et al. (2011) A prospective natural-history study of coronary atherosclerosis. N Engl J Med 364:226–235. 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 14.Garcìa-Garcìa HM, Gogas BD, Serruys PW, Bruining N (2011) IVUS-based imaging modalities for tissue characterization: similarities and differences. Int J Cardiovasc Imaging 27:215–224. 10.1007/s10554-010-9789-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burgstahler C, Reimann A, Beck T et al. (2007) Influence of a lipid-lowering therapy on calcified and noncalcified coronary plaques monitored by multislice detector computed tomography: results of the New Age II Pilot Study. Invest Radiol 42:189–195. 10.1097/01.rli.0000254408.96355.85 [DOI] [PubMed] [Google Scholar]
- 16.Nissen SE, Nicholls SJ, Sipahi I et al. (2006) Effect of very high-intensity statin therapy on regression of coronary atherosclerosis: the ASTEROID trial. JAMA 295:1556–1565. 10.1001/jama.295.13.jpc60002 [DOI] [PubMed] [Google Scholar]
- 17.Wakabayashi K, Nozue T, Yamamoto S et al. (2016) Efficacy of statin therapy in inducing coronary plaque regression in patients with low baseline cholesterol levels. J Atheroscler Thromb 23:1055–1066. 10.5551/jat.34660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mintz GS, Nissen SE, Anderson WD et al. (2001) American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus)31When citing this document, the American College of Cardiology would appreciate the follow. J Am Coll Cardiol 37:1478–1492. 10.1016/S0735-1097(01)01175-5 [DOI] [PubMed] [Google Scholar]
- 19.Noguchi T, Nakao K, Asaumi Y et al. (2018) Noninvasive coronary plaque imaging. J Atheroscler Thromb 25:281–293. 10.5551/jat.RV17019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nissen SE, Tuzcu EM, Libby P et al. (2004) Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA 292:2217–2225. 10.1001/jama.292.18.2217 [DOI] [PubMed] [Google Scholar]
- 21.Nicholls SJ, Ballantyne CM, Barter PJ et al. (2011) Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med 365:2078–2087. 10.1056/NEJMoa1110874 [DOI] [PubMed] [Google Scholar]
- 22.Kashiwagi M, Tanaka A, Kitabata H et al. (2009) Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging 2:1412–1419. 10.1016/j.jcmg.2009.09.012 [DOI] [PubMed] [Google Scholar]
- 23.Sandfort V, Lima JAC, Bluemke DA (2015) Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ Cardiovasc Imaging 8:e003316–e003316. 10.1161/CIRCIMAGING.115.003316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yabushita H, Bouma BE, Houser SL et al. (2002) Characterization of human atherosclerosis by optical coherence tomography. Circulation 106:1640–1645 [DOI] [PubMed] [Google Scholar]
- 25.Habara M, Nasu K, Terashima M et al. (2014) Impact on optical coherence tomographic coronary findings of fluvastatin alone versus fluvastatin + ezetimibe. Am J Cardiol 113:580–587. 10.1016/j.amjcard.2013.10.038 [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann U, Truong QA, Schoenfeld DA et al. (2012) Coronary CT angiography versus standard evaluation in acute chest pain. N Engl J Med 367:299–308. 10.1056/NEJMoa1201161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hospital outpatient prospective payment system centers for medicare & medicaid services no title [Google Scholar]
- 28.Maurovich-Horvat P, Schlett CL, Alkadhi H et al. (2012) The napkin-ring sign indicates advanced atherosclerotic lesions in coronary CT angiography. JACC Cardiovasc Imaging 5:1243–1252. 10.1016/j.jcmg.2012.03.019 [DOI] [PubMed] [Google Scholar]
- 29.Schepis T, Marwan M, Pflederer T et al. (2010) Quantification of non-calcified coronary atherosclerotic plaques with dual-source computed tomography: comparison with intravascular ultrasound. Heart 96:610–615. 10.1136/hrt.2009.184226 [DOI] [PubMed] [Google Scholar]
- 30.Liu T, Maurovich-Horvat P, Mayrhofer T et al. (2018) Quantitative coronary plaque analysis predicts high-risk plaque morphology on coronary computed tomography angiography: results from the ROMICAT II trial. Int J Cardiovasc Imaging 34:311–319. 10.1007/s10554-017-1228-6 [DOI] [PubMed] [Google Scholar]
- 31.Ferencik M, Schlett CL, Ghoshhajra BB et al. (2012) A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol 110:183–189. 10.1016/j.amjcard.2012.02.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakazato R, Shalev A, Doh J-H et al. (2013) Quantification and characterisation of coronary artery plaque volume and adverse plaque features by coronary computed tomographic angiography: a direct comparison to intravascular ultrasound. Eur Radiol 23:2109–2117. 10.1007/s00330-013-2822-1 [DOI] [PubMed] [Google Scholar]
- 33.Leber AW, Knez A, Becker A et al. (2004) Accuracy of multidetector spiral computed tomography in identifying and differentiating the composition of coronary atherosclerotic plaques: a comparative study with intracoronary ultrasound. J Am Coll Cardiol 43:1241–1247. 10.1016/j.jacc.2003.10.059 [DOI] [PubMed] [Google Scholar]
- 34.Voros S, Rinehart S, Qian Z et al. (2011) Coronary atherosclerosis imaging by coronary CT angiography: current status, correlation with intravascular interrogation and meta-analysis. JACC Cardiovasc Imaging 4:537–548. 10.1016/j.jcmg.2011.03.006 [DOI] [PubMed] [Google Scholar]
- 35.Schroeder S, Kopp AF, Baumbach A et al. (2001) Noninvasive detection and evaluation of atherosclerotic coronary plaques with multislice computed tomography. J Am Coll Cardiol 37:1430–1435 [DOI] [PubMed] [Google Scholar]
- 36.Pundziute G, Schuijf JD, Jukema JW et al. (2008) Head-to-head comparison of coronary plaque evaluation between multislice computed tomography and intravascular ultrasound radiofrequency data analysis. JACC Cardiovasc Interv 1:176–182. 10.1016/j.jcin.2008.01.007 [DOI] [PubMed] [Google Scholar]
- 37.Achenbach S, Moselewski F, Ropers D et al. (2004) Detection of calcified and noncalcified coronary atherosclerotic plaque by contrast-enhanced, submillimeter multidetector spiral computed tomography: a segment-based comparison with intravascular ultrasound. Circulation 109:14–17. 10.1161/01.CIR.0000111517.69230.0F [DOI] [PubMed] [Google Scholar]
- 38.Falk E, Shah PK, Fuster V (1995) Coronary plaque disruption. Circulation 92:657–671 [DOI] [PubMed] [Google Scholar]
- 39.Min JK, Dunning A, Lin FY et al. (2011) Age-and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the International Multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicen). J Am Coll Cardiol 58:849–860. 10.1016/j.jacc.2011.02.074 [DOI] [PubMed] [Google Scholar]
- 40.Achenbach S (2008) Quantification of coronary artery stenoses by computed tomography. JACC Cardiovasc Imaging 1:472–474. 10.1016/j.jcmg.2008.05.008 [DOI] [PubMed] [Google Scholar]
- 41.Hoffmann U, Moselewski F, Cury RC et al. (2004) Predictive value of 16-slice multidetector spiral computed tomography to detect significant obstructive coronary artery disease in patients at high risk for coronary artery disease: patient-versus segment-based analysis. Circulation 110:2638–2643. 10.1161/01.CIR.0000145614.07427.9F [DOI] [PubMed] [Google Scholar]
- 42.Mollet NR, Cademartiri F, Krestin GP et al. (2005) Improved diagnostic accuracy with 16-row multi-slice computed tomography coronary angiography. J Am Coll Cardiol 45:128–132. 10.1016/j.jacc.2004.09.074 [DOI] [PubMed] [Google Scholar]
- 43.Kuettner A, Beck T, Drosch T et al. (2005) Image quality and diagnostic accuracy of non-invasive coronary imaging with 16 detector slice spiral computed tomography with 188 ms temporal resolution. Heart 91:938–941. 10.1136/hrt.2004.044735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng V, Gutstein A, Wolak A et al. (2008) Moving beyond binary grading of coronary arterial stenoses on coronary computed tomographic angiography: insights for the imager and referring clinician. JACC Cardiovasc Imaging 1:460–471. 10.1016/j.jcmg.2008.05.006 [DOI] [PubMed] [Google Scholar]
- 45.De Rosa R, Vasa-Nicotera M, Leistner DM et al. (2017) Coronary atherosclerotic plaque characteristics and cardiovascular risk factors insights from an optical coherence tomography study. Circ J 81:1165–1173. 10.1253/circj.CJ-17-0054 [DOI] [PubMed] [Google Scholar]
- 46.Nozue T, Takamura T, Fukui K et al. (2018) Changes in coronary atherosclerosis, composition, and fractional flow reserve evaluated by coronary computed tomography angiography in patients with type 2 diabetes. Int J Cardiol Hear Vasc 19:46–51. 10.1016/j.ijcha.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambrose JA, Tannenbaum MA, Alexopoulos D et al. (1988) Angiographic progression of coronary artery disease and the development of myocardial infarction. J Am Coll Cardiol 12:56–62 [DOI] [PubMed] [Google Scholar]
- 48.Lo J, Lu MT, Ihenachor EJ et al. (2015) Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV 2:e52–63. 10.1016/S2352-3018(14)00032-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nakazato R, Shalev A, Doh J-H et al. (2013) Aggregate plaque volume by coronary computed tomography angiography is superior and incremental to luminal narrowing for diagnosis of ischemic lesions of intermediate stenosis severity. J Am Coll Cardiol 62:460–467. 10.1016/j.jacc.2013.04.062 [DOI] [PubMed] [Google Scholar]
- 50.Fleg JL, Stone GW, Fayad ZA et al. (2012) Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging 5:941–955. 10.1016/j.jcmg.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kitagawa T, Yamamoto H, Horiguchi J et al. (2009) Characterization of noncalcified coronary plaques and identification of culprit lesions in patients with acute coronary syndrome by 64-slice computed tomography. JACC Cardiovasc Imaging 2:153–160. 10.1016/j.jcmg.2008.09.015 [DOI] [PubMed] [Google Scholar]
- 52.Puchner SB, Liu T, Mayrhofer T et al. (2014) High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol 64:684–692. 10.1016/j.jacc.2014.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glagov S, Weisenberg E, Zarins CK et al. (1987) Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316:1371–1375. 10.1056/NEJM198705283162204 [DOI] [PubMed] [Google Scholar]
- 54.Motoyama S, Kondo T, Sarai M et al. (2007) Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50:319–326. 10.1016/j.jacc.2007.03.044 [DOI] [PubMed] [Google Scholar]
- 55.Galonska M, Ducke F, Kertesz-Zborilova T et al. (2008) Characterization of atherosclerotic plaques in human coronary arteries with 16-slice multidetector row computed tomography by analysis of attenuation profiles. Acad Radiol 15:222–230. 10.1016/j.acra.2007.09.007 [DOI] [PubMed] [Google Scholar]
- 56.Marwan M, Taher MA, El Meniawy K et al. (2011) In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis 215:110–115. 10.1016/j.atherosclerosis.2010.12.006 [DOI] [PubMed] [Google Scholar]
- 57.Li Z, Hou Z, Yin W et al. (2016) Effects of statin therapy on progression of mild noncalcified coronary plaque assessed by serial coronary computed tomography angiography: a multicenter prospective study. Am Heart J 180:29–38. 10.1016/j.ahj.2016.06.023 [DOI] [PubMed] [Google Scholar]
- 58.Foldyna B, Lo J, Mayrhofer T et al. (2019) Individual coronary plaque changes on serial CT angiography: within-patient heterogeneity, natural history, and statin effects in HIV. J Cardiovasc Comput Tomogr. 10.1016/j.jcct.2019.08.011 [DOI] [PubMed] [Google Scholar]
- 59.Tardif J-C, Lallier PL, Ibrahim R et al. (2010) Treatment with 5-lipoxygenase inhibitor VIA-2291 (Atreleuton) in patients with recent acute coronary syndrome. Circ Cardiovasc Imaging 3:298–307. 10.1161/CIRCIMAGING.110.937169 [DOI] [PubMed] [Google Scholar]
- 60.Matsumoto S, Ibrahim R, Grégoire JC et al. (2017) Effect of treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) on coronary plaque progression: a serial CT angiography study. Clin Cardiol 40:210–215. 10.1002/clc.22646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hauser TH, Salastekar N, Schaefer EJ et al. (2016) Effect of targeting inflammation with salsalate: the TINSAL-CVD randomized clinical trial on progression of coronary plaque in overweight and obese patients using statins. JAMA Cardiol 1:413–423. 10.1001/jamacardio.2016.0605 [DOI] [PubMed] [Google Scholar]
- 62.Budoff MJ, Ellenberg SS, Lewis CE et al. (2017) Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA 317:708–716. 10.1001/jama.2016.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsumoto S, Nakanishi R, Li D et al. (2016) Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with metabolic syndrome in a prospective randomized double-blind study. J Nutr 146:427S–432S. 10.3945/jn.114.202424 [DOI] [PubMed] [Google Scholar]
- 64.Lee J, Nakanishi R, Li D et al. (2018) Randomized trial of rivaroxaban versus warfarin in the evaluation of progression of coronary atherosclerosis. Am Heart J 206:127–130 [DOI] [PubMed] [Google Scholar]
- 65.ClinicalTrials.gov (2019) MedImmune LLC T in MI (TIMI) SG No Title. https://clinicaltrials.gov/ct2/show/NCT03578809. Accessed 13 Feb 2019
- 66.Rousset X, Shamburek R, Vaisman B et al. (2011) Lecithin cholesterol acyltransferase: an anti or pro-atherogenic factor? Curr Atheroscler Rep 13:249–256. 10.1007/s11883-011-0171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gilbert JM, Fitch KV, Grinspoon SK (2015) HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med 23:146–149 [PMC free article] [PubMed] [Google Scholar]
- 68.Effect of PCSK9 Inhibition on Cardiovascular Risk in Treated HIV Infection (EPIC-HIV Study) (EPIC-HIV). https://clinicaltrials.gov/ct2/show/NCT03207945. Accessed 12 Feb 2019
- 69.Symons R, Morris JZ, Wu CO et al (2016) Coronary CT angiography: variability of CT scanners and readers in measurement of plaque volume. Radiology 281:737–748. 10.1148/radiol.2016161670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nissen SE, Tuzcu EM, Schoenhagen P, Brown GC (2004) Effect of intensive compared with moderate lipid-lowering therapy on progression of coronary atherosclerosis. Evid Based Eye Care 5:228–229. 10.1097/01.ieb.0000142773.91809.e5 [DOI] [PubMed] [Google Scholar]
- 71.Inoue K, Motoyama S, Sarai M et al. (2010) Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging 3:691–698. 10.1016/j.jcmg.2010.04.011 [DOI] [PubMed] [Google Scholar]
- 72.Auscher S, Heinsen L, Nieman K et al. (2015) Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: assessment with serial coronary CT angiography. Atherosclerosis 241:579–587. 10.1016/j.atherosclerosis.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 73.Lee S-E, Chang H-J, Sung JM et al. (2018) Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging 11:1475–1484. 10.1016/j.jcmg.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann H, Frieler K, Schlattmann P et al. (2010) Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol 20:2824–2833. 10.1007/s00330-010-1880-x [DOI] [PubMed] [Google Scholar]
- 75.Oberoi S, Meinel FG, Schoepf UJ et al. (2014) Reproducibility of noncalcified coronary artery plaque burden quantification from coronary CT angiography across different image analysis platforms. AJR Am J Roentgenol 202:W43–W49. 10.2214/AJR.13.11225 [DOI] [PubMed] [Google Scholar]
- 76.Achenbach S, Boehmer K, Pflederer T et al. (2010) Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J Cardiovasc Comput Tomogr 4:110–115. 10.1016/j.jcct.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 77.Cademartiri F, Mollet NR, Runza G et al. (2005) Influence of intracoronary attenuation on coronary plaque measurements using multislice computed tomography: observations in an ex vivo model of coronary computed tomography angiography. Eur Radiol 15:1426–1431. 10.1007/s00330-005-2697-x [DOI] [PubMed] [Google Scholar]
- 78.Suzuki S, Furui S, Kuwahara S et al. (2006) Accuracy of attenuation measurement of vascular wall in vitro on computed tomography angiography: effect of wall thickness, density of contrast medium, and measurement point. Invest Radiol 41:510–515. 10.1097/01.rli.0000209662.24569.c7 [DOI] [PubMed] [Google Scholar]
- 79.Sande EPS, Martinsen ACT, Hole EO, Olerud HM (2010) Interphantom and interscanner variations for Hounsfield units–establishment of reference values for HU in a commercial QA phantom. Phys Med Biol 55:5123–5135. 10.1088/0031-9155/55/17/015 [DOI] [PubMed] [Google Scholar]
- 80.Versteylen MO, Kietselaer BL, Dagnelie PC et al. (2013) Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol 61:2296–2305. 10.1016/j.jacc.2013.02.065 [DOI] [PubMed] [Google Scholar]


