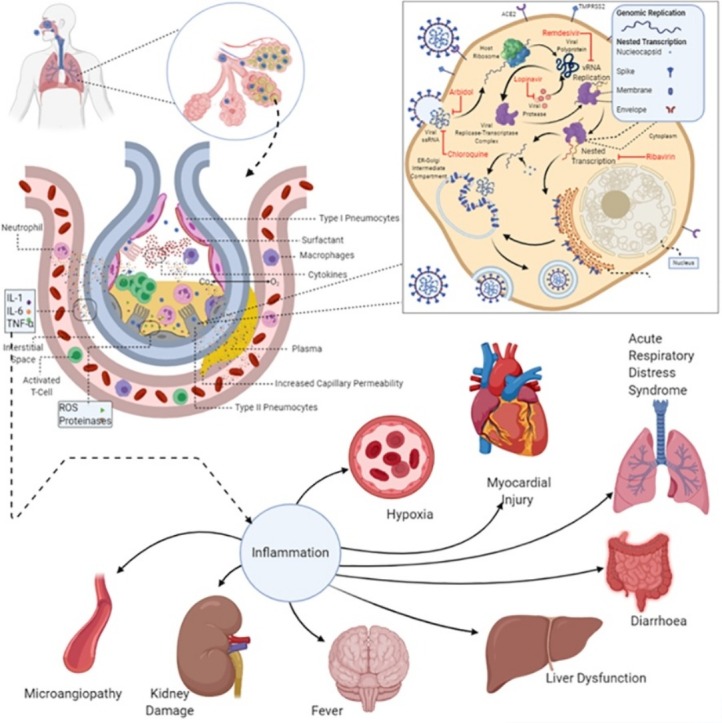
Graphical abstract
Keywords: COVID-19, SARS-CoV-2, Life-cycle, Pathophysiology, Co-morbidities, Pregnancy, Vaccine, Anti-virals
Abstract
The current coronavirus pandemic, COVID-19, is the third outbreak of disease caused by the coronavirus family, after Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome. It is an acute infectious disease caused by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). This severe disease is characterised by acute respiratory distress syndrome, septic shock, metabolic acidosis, coagulation dysfunction, and multiple organ dysfunction syndromes. Currently, no drugs or vaccines exist against the disease and the only course of treatment is symptom management involving mechanical ventilation, immune suppressants, and repurposed drugs. The severe form of the disease has a relatively high mortality rate. The last six months have seen an explosion of information related to the host receptors, virus transmission, virus structure-function relationships, pathophysiology, co-morbidities, immune response, treatment and the most promising vaccines. This review takes a critically comprehensive look at various aspects of the host-pathogen interaction in COVID-19. We examine the genomic aspects of SARS-CoV-2, modulation of innate and adaptive immunity, complement-triggered microangiopathy, and host transmission modalities. We also examine its pathophysiological impact during pregnancy, in addition to emphasizing various gaps in our knowledge. The lessons learnt from various clinical trials involving repurposed drugs have been summarised. We also highlight the rationale and likely success of the most promising vaccine candidates.
1. Introduction
Coronavirus (CoV) have the largest known genomes among RNA viruses and belong to the family of Coronaviridae. They are known to infect a range of animals, including humans, and cause respiratory, gastrointestinal, and neurological diseases (Weiss and Leibowitz, 2020). Coronaviruses are further classified into four groups, namely Alpha-coronavirus (α-CoV), Beta-coronavirus (β-CoV), Gamma-coronavirus (γ-CoV) and Delta-coronavirus (δ-CoV) (Yang and Leibowitz, 2015). α-CoV and β-CoV use bats and rodents as animal reservoirs, while γ-CoV and δ-CoV mostly use members of the avian species as reservoirs (Columbus et al., 2019). A total of seven human infecting coronaviruses that cause respiratory distress of various severity have been identified. These include two α-CoV (HCoV-NL63 and HCoV-229E) and two β-CoV (HCoV−OC43, HCoV-HKU1), which cause mild respiratory illness in immunocompetent individuals (Drosten et al., 2003). In addition, three highly pathogenic β-CoV [Severe Acute Respiratory Syndrome-CoV (SARS-CoV), Middle East respiratory syndrome-CoV (MERS-CoV) and Severe acute respiratory syndrome CoV-2 (SARS-CoV-2)] have caused outbreaks in the last two decades (Drosten et al., 2003; Zaki et al., 2012; Zhu et al., 2020a). The initial SARS-CoV outbreak occurred in China in late 2002 (Xu et al., 2004a). The virus was traced to horseshoe bats through civets that acted as an intermediate amplifying and transmitting host; the jump for a viral pathogen from the animal reservoir to human host is a crucial aspect in the emergence of the virus and helps in controlling the spread of the pathogen (Hu et al., 2017). The disease had a case fatality rate of −10 %, and around 8000 confirmed cases were reported in the two years of the outbreak as of December 2003 (World Health Organisation, 2004). This was followed by the MERS-CoV outbreak that started in Jeddah, Saudi Arabia in late 2012 (Zaki et al., 2012). The virus is believed to have been originated in bats and subsequently transmitted to camels in the distant past (Widagdo et al., 2019). The MERS-CoV has a case fatality rate of −35 % and −2500 confirmed cases were reported until January 2020 across the planet (World Health Organization, 2020a). Sporadic identification of MERS-CoV cases have been reported across countries since then, unlike SARS-CoV (no reported cases since 2004).
The latest of the CoV outbreak, COVID-19, which started in Wuhan, China in late 2019, is caused by SARS-CoV-2 (Gorbalenya et al., 2020; Zhou et al., 2020a). This virus is classified in the order Nidovirales, family Coronaviridae, subfamily Coronavirinae and the Beta-coronavirus genus.
1.1. Molecular and genomic characteristics of SARS-CoV-2
The genome of SARS-CoV-2 was sequenced and characterised early in the COVID-19 pandemic and is a large positive sense, single-stranded, non-segmented RNA genome of 29,903 nucleotides in length (Zhu et al., 2020a; Wu et al., 2020a). The genome of SARS- CoV-2 shows 80 % similarity to SARS-CoV and 50 % to MERS-CoV (Zhu et al., 2020a; Zhou et al., 2020a; Kim et al., 2020a). The genome of SARS- CoV-2, like other Beta-coronaviruses, is complex and tightly packed with 2 open-reading frames (ORFs), ORF1a and ORF1b, which code for non-structural proteins (nsps), other structural proteins, as well as viral regulators and transcription factors (Zhu et al., 2020a) (Fig. 1 ). ORF1a specifically codes for polypeptide 1a (pp1a) of approximately 500 kDa, which is subsequently cleaved into 11 nsps. ORF1b is translated as part of a larger polypeptide (pp1ab), which is produced through a continuous translation of ORF1a and ORF1b, because of a ribosomal frameshift that is proximal to the stop codon of ORF1 (Fig. 1). This results in a larger polypeptide of approximately 800 kDa that is cleaved into 15 nsps by viral proteases, nsp3 and nsp5 (Kim et al., 2020b). Another key nsp, viral RNA-dependent RNA polymerase (nsp12), is responsible for replication and transcription of the viral genome. The SARS-CoV-2 genome also contains negative sense RNA species that are transcribed into the positive-sense genomic RNA (gRNA) and sub-genomic RNA (sgRNA) types (Kim et al., 2020b). The sgRNA code for the structural proteins, viral spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N), as well as several putative accessory proteins (3a, 6, 7a, 7b, 8, and 10) (Kim et al., 2020a; Zhu et al., 2020a).
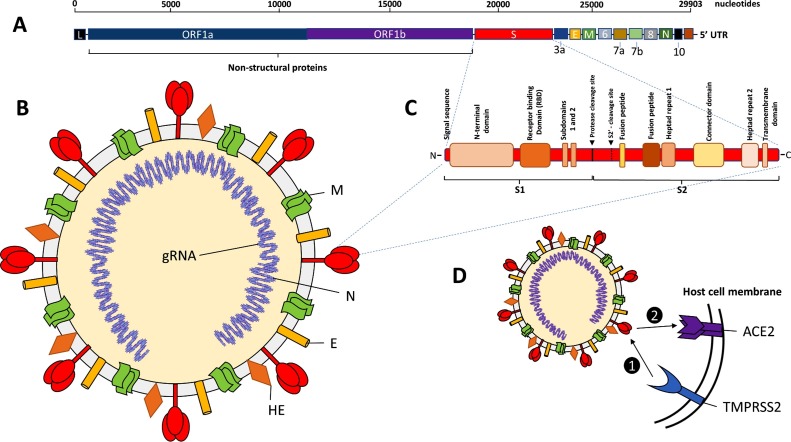
Fig. 1.
Genomic and molecular characteristics of SARS-CoV-2 virus.
A. The genome of SAR-CoV-2: large positive sense, single-stranded, non- segmented RNA genome of 29,903 nucleotides in length. Two open-reading frames (ORFs), ORF1a and ORF1b code for non-structural proteins (nsps). The sgRNA code for the structural proteins, viral spike protein (S), envelope protein (E), membrane protein (M), and nucleocapsid protein (N), as well as several putative accessory proteins (3a, 6, 7a, 7b, 8, and 10). L: Leader 3′ sequence. UTR: 5′ untranslated region. B. Structure of SARS-CoV-2 viron: An enveloped virus containing the major surface antigens including, hemagglutinin-esterase (HE) and the spike (S) protein trimer, surrounding the genomic RNA that has been packaged in the nucleocapsid (N). C. Protein structure of the spike (S) protein monomer showing the key molecular domains involved in pathogenesis. D. Primary cellular host receptor and co-receptor for SAR-CoV-2. 1) Attachment and entry of SAR2- CoV-2 requires priming by transmembrane serine protease 2 (TMPRSS2) which cleaves the S protein into S1 and S2 portions, facilitating 2) S1 targeting and binding of the receptor angiotensin-Converting Enzyme 2 (ACE2), followed by receptor-mediated endocytosis of the virion into the host cell.
1.2. Molecular evolution of SARS-CoV-2
The comparison of the genome of SARS-CoV-2 with genomes of other Beta-coronaviruses suggests possible frequent recombination and rapid evolution that has enabled the virus to adapt to the human host for transmission, tissue specificity and pathogenesis. As in the case for SARS and MERS, COVID-19 infection is thought to have originated as a zoonotic infection, probably from bat to human, via an intermediary species (suspected to be pangolin). Genomic analysis has shown that the SARS-CoV-2 genome is most similar to Bat-CoV and pangolin-CoV, with the receptor-binding domain (RBD) of the S protein, showing high similarity to bat-CoV and pangolin-CoV (Zhu et al., 2020a; Zhou et al., 2020a; Xiao et al., 2020b). The evolution of the S protein is particularly vital for the host and tissue tropism of the virus. The SARS-CoV-2 S protein is critical in targeting and invasion of host cells. It is coded by the sgRNA (S) and is a trimeric type I membrane glycoprotein of 1255 amino acids, composed of an N-terminal S1 subunit and a C-terminal S2 portion (Ksiazek et al., 2003; Lu et al., 2015).
1.3. Major receptors for SARS-CoV-2
The principal host cell receptor for SARS-CoV-2 (as for SARS-CoV) is the angiotensin-converting enzyme 2 (ACE2) receptor (Kuhn et al., 2004). ACE2 is recognised by S protein after priming by transmembrane Serine Protease 2 (TMPRSS2) on the primary target host cell (Matsuyama et al., 2010; Glowacka et al., 2011; Shulla et al., 2011; Hoffmann et al., 2020; Walls et al., 2020). TMPRSS2 cleaves the S protein into S1 and S2 portions, facilitating S1-mediated targeting and receptor-mediated early fusion pathway driven by S2 subunit (Fig. 1) (Shulla et al., 2011; Grove and Marsh, 2011; Kuba et al., 2005). Furin and other host proteases may also be involved in cleaving the SARS-CoV-2 S protein (Walls et al., 2020; Bugge et al., 2009; Böttcher-Friebertshäuser et al., 2013; Coutard et al., 2020). The affinity for binding of the SARS-CoV-2 S protein to ACE2 is around 10–20 fold higher than SARS-CoV, suggesting that this is a significant reason for higher human-to-human transmission in COVID-19 (Hoffmann et al., 2020; Loganathan et al., 2020). A recent study on gene expression of ACE2 in multiple single cell RNA (scRNA)-seq datasets suggests that it is expressed in multiple tissues, such as airways, oesophagus, ileum, colon, liver, cornea, heart, kidney and testis (Sungnak et al., 2020). Study of single-cell gene expression matrices from 13 relatively healthy human tissues showed that ACE2 was mainly expressed in lung type II pneumocytes, liver cholangiocytes, colon colonocytes, oesophagus keratinocytes, ileum endothelial cells (ECs), rectum ECs, stomach epithelial cells, and kidney proximal tubules (Qi et al., 2020). In normal physiology, ACE2 has an essential role in tissue protection during severe acute lung injury (Kuba et al., 2005). ACE2 expression is found in the upper respiratory tract but is less abundant than the lower respiratory tract (Sungnak et al., 2020). ACE2 is also commonly found as a receptor on enterocytes in the small intestine and is consistent with clinical reports of gastrointestinal symptoms and viral shedding in faeces (Ong et al., 2020; Xu et al., 2020a). This has been further resolved with the comprehensive identification of host cells/tissues expressing both ACE2 and TMPRSS2 (Fig. 2 ). Thus, likely targets for SARS-CoV-2 primarily include secretory goblets of the nasal mucosa, lung type II pneumocytes and absorptive erythrocytes of the small intestine (Ziegler et al., 2020). Of note, this study also showed that the ACE2 receptor is an interferon-stimulated gene in SARS-CoV-2 infection in the cells of the human upper nasal epithelium and lung, predominatly mediated by Interferon (IFN)-α2 and IFN-γ (Ziegler et al., 2020). Moreover, bystander cells are subject to interferon-mediated effects (upregulation of ACE2 receptor) rather than SARS-CoV-2 infected cells, suggesting a mechanism of enhanced viral targeting and entry during pathogenesis and a possible avenue for therapeutic intervention (Ziegler et al., 2020).
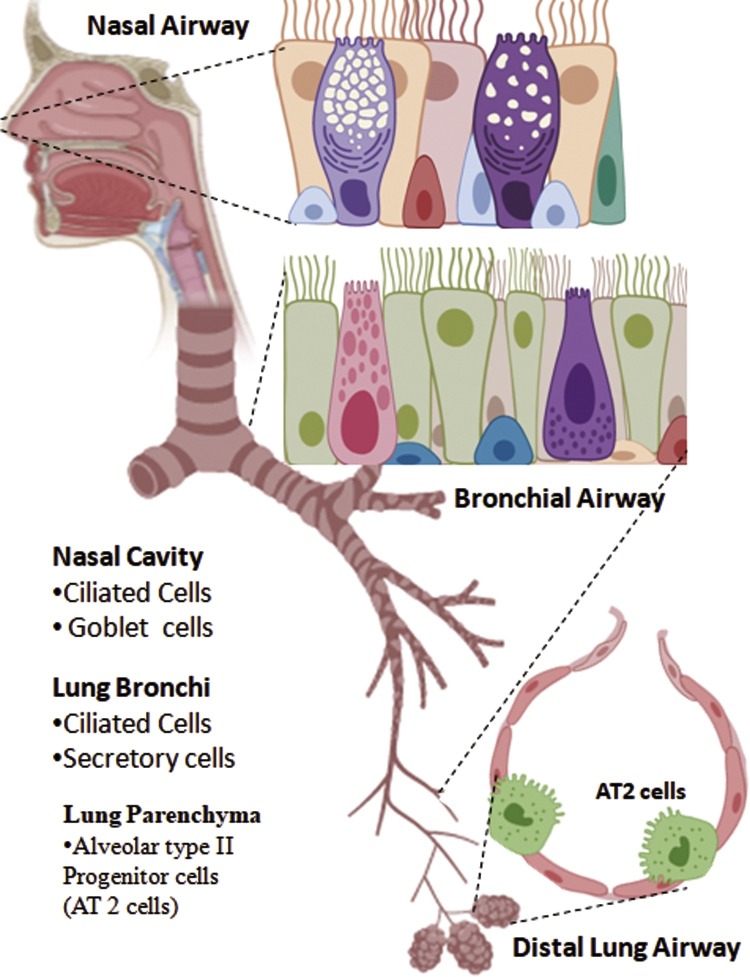
Fig. 2.
Co-expression of ACE2 and TMPRSS2 in Respiratory Airways.
TMPRSS2 is the key protease involved in priming SARS-CoV-2, which forms a receptor-protease complex with ACE2 on the host cell surface, thus facilitating viral targeting and entry to the host cell. Co-expression of ACE2 and TMPRSS2 has been found in proximal as well in distal airways. The nasal cavity has the highest expression of both the receptors in ciliated and secretory (goblet) cells compared to lung bronchi (ciliated and secretory cells) and lung parenchyma (alveolar type 2 progenitor cells, AT2).
Analysis of genetic variation in the ACE2 gene has identified single nucleotide polymorphisms (SNPs) that differ in frequency globally among the human population, particularly between males and females (Cao et al., 2020a). Characterising these SNPs more fully with epidemiological and clinical data on COVID-19 will in time shed light on the precise molecular mechanisms of transmission and disease. Furthermore, in the SARS-CoV-2 viral S protein, 27 amino acid substitutions have been described, although these occurred outside the RBD that directly interacts with ACE2 (Wu et al., 2020a). Of paramount importance is characterising the genetic variation and its consequences in the S protein and its RBD, as this will determine whether the SARS-CoV-2 virus is evolving and is likely to be a seasonal infection with new variants for the human population. Undoubtedly, variation in the S protein and ACE2, the central interface of host- pathogen interaction in COVID-19 will have evolved from natural selection contributing to the pathogenesis of this disease.
RBD region of SARS-CoV-2 has been found to have higher binding affinity for ACE2 receptor than SARS-CoV. There is an additional main-chain hydrogen bond in the conformation of RBM loops being involved in ACE2 binding ridge, thus making a favourable conformation than SARS-CoV (Shang et al., 2020a). RBD has been found to have two different conformations: standing-up state which favours receptor binding, and lying-down state which does not allow binding with host receptors (Fig. 3 ). Interestingly, it has been found that RBD of SARS-CoV-2 is often in lying-down state and thus being less accessible to binding (Shang et al., 2020b). This hidden RBD could also possibly be a masking strategy by SARS-CoV-2 as poor pseudovirus capabilities have been observed in spite of the high affinity of RBD towards host ACE2 (Shang et al., 2020b).
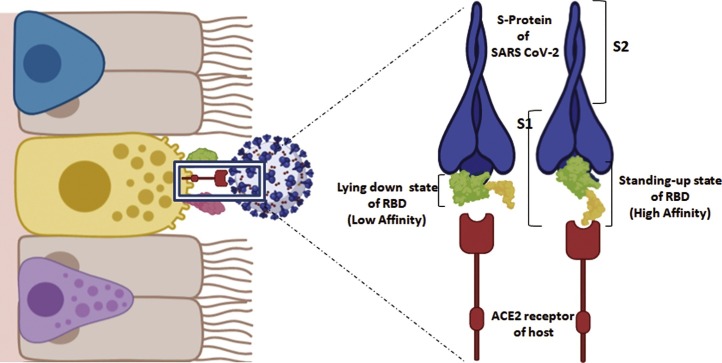
Fig. 3.
Affinity of Receptor Binding Domain (RBD) of Spike protein for ACE2.
Structural conformation of receptor-binding domain (RBD) present in S1 region of SARS-CoV-2 spike protein is capable of influencing the ACE2-binding affinity. In case of SARS-CoV-2, the RBD contains a four-residue motif glycine- valine/glutamine-glutamate/threonine-glycine which enables the binding loop to take a different conformation. It can undergo two possible conformational changes, a “lying down state” which has low affinity towards ACE2 and a “standing up state” with high binding affinity. SARS-CoV-2 RBD is found mostly in lying down state, and thus being less accessible to ACE2. This hidden conformation of RBD in the spike protein can possibly be a masking strategy for immune evasion by SARS-CoV-2.
1.4. SARS-CoV-2 life cycle
1.4.1. Priming viral S protein, binding, and entry into target host cell
There is still only a rudimentary understanding of the specific life cycle of the SARS-CoV-2 virus. Much of the current understanding has been extrapolated from studies on SARS-CoV and MERS-CoV. This is undoubtedly the case for the viral ligand-host cell receptor binding and viral entry of SARS-CoV-2. Much of the steps are well understood, but the chronological sequence of events remains to be fully determined. The N-terminal S1 portion of the viral S protein has the crucial role of targeting the host-cell receptor ACE2. Receptor binding is facilitated by the C-terminal RBD domain on the S1 portion (Li et al., 2005; Wu et al., 2009; Li, 2012; Reguera et al., 2012). There is also a separate N-terminal domain in the S1 portion which may also target specific carbohydrate moieties, facilitating the initial binding of the SARS-CoV-2 to the host cell (Schwegmann-Weßels and Herrler, 2006; Krempl et al., 1997). After receptor binding via the S1 portion, the S2 portion facilitates the fusion between the viral and the host cell membranes (Lu et al., 2015). The S2 portion has several fusion peptides and two conserved heptad repeats (HRs), which are essential in the steering and fusion of the virus through the cell membrane (Lu et al., 2015). The fusion peptides also have a key role in attaching and disrupting the host cell membrane (Epand, 2003; Peisajovich and Shai, 2003). At the same time, the HRs form a trimeric coiled (six-helix bundle) structure that contracts the viral envelope and host cell membrane together to facilitate fusion (Eckert and Kim, 2001; Xu et al., 2004b; Zhu et al., 2004; Gao et al., 2013). It is thought that the viral S protein is cleaved in a two-step process called ‘priming’ cleavage and ‘activation’ cleavage (Belouzard et al., 2009; Mille and Whittaker, 2014). ‘Priming’ cleavage of the S protein can be achieved by several host proteases. In SARS-CoV, the S protein can be cleaved by cathepsin L in the endosome during viral entry, enabling infection to occur via the endosomal route (Simmons et al., 2005). It has been observed that cathepsin B/L may also substitute for TMPRSS2 in case of SARS CoV-2 (Hoffmann et al., 2020). Low pH activates endosomal protease, cathepsin, which further facilitates endosomal entry of the virus (Fig. 4 ) (Tang et al., 2020a). The viral S protein can also be cleaved by several other extracellular enzymes, including trypsin, thermolysin and elastase, which have been reported to enhance viral infection via syncytia formation (Matsuyama et al., 2005). However, as has been discussed previously, TMPRSS2, which is highly expressed in the human respiratory tract, is the main co-receptor host protease involved in SARS- CoV and SARS-CoV-2 entry (Glowacka et al., 2011; Ziegler et al., 2020; Kam et al., 2009; Bertram et al., 2011). Other proteases which may also be involved are TMPRSS11a and human airway trypsin-like protease (HAT) (Glowacka et al., 2011; Kam et al., 2009; Bertram et al., 2011). Through recent studies, TMPRSS2 has been shown to be most important protease involved in priming SARS-CoV, and presumably SARS-CoV-2, since it can form a receptor-protease complex with ACE2 on the host cell surface, thus greatly facilitating viral targeting and entry (Shulla et al., 2011). Moreover, TMPRSS2, a disintegrin and metalloproteinase 17 (ADAM17) and HAT can induce the shedding of soluble ACE2 receptor, which has been observed to facilitate uptake of SARS-CoV (Haga et al., 2008; Heurich et al., 2014). The S protein of SARS-CoV and presumably SARS-CoV-2 has an additional site called the ‘activation’ cleavage site and is located at the S2′ position, near the S1-S2 border (Belouzard et al., 2009; Watanabe et al., 2008). It is thought to be critical for the final priming of the S protein. The region between the C-terminus and S2′ cleavage site shows similarities to a viral-fusion peptide that plays a key role in viral-host cell fusion (Madu et al., 2009). However, the exact order of events of priming of the SARS-CoV-2 S protein is still unclear, particularly the cleavage of S protein at the S1-S2 border (‘priming’) and the S2′ site (‘activation’), and precisely when the insertion of the fusion peptides and assembly of HR regions occur in order to initiate and accomplish viral-host cell membrane fusion.
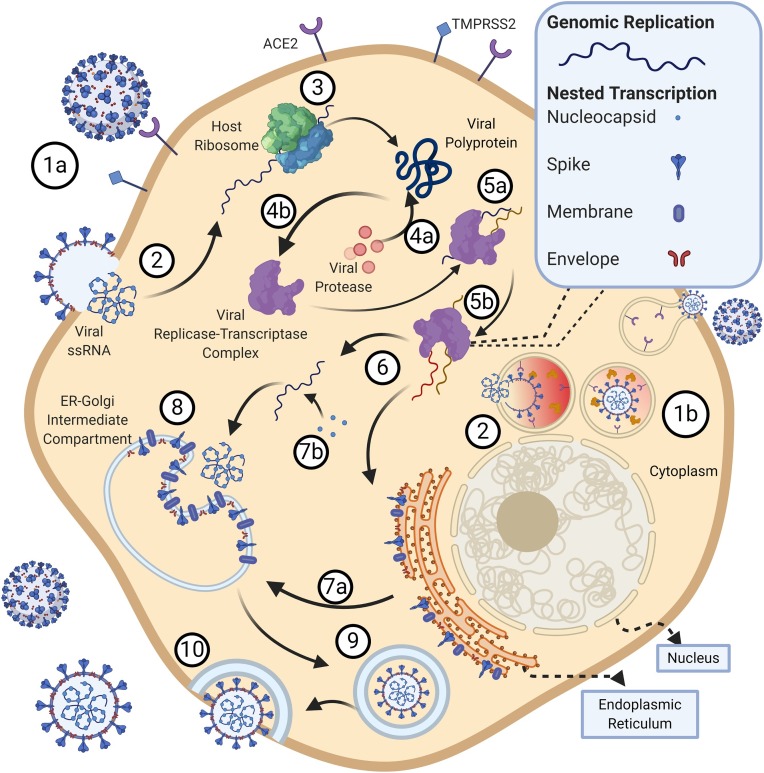
Fig. 4.
SARS CoV-2 Life cycle.
(1) The SARS-CoV-2 binds to the cell via the ACE2 receptor using the S1 subunit of the spike protein. Once bound, the S2 subunit facilitates virus-cell membrane fusion by two tandem domains, heptad repeats 1 (HR1) and heptad repeats 2 (HR2) to form a six-helix bundle (6-HB) fusion core, bringing viral and cellular membranes into close proximity for fusion and infection. (1b) Cathepsin B/L may also facilitate endosomal entry of the virus in TMPRSS2− cells. (Yang and Leibowitz, 2015) Post fusion, the virus releases its 30 kilobase (kb) positive sense single stranded RNA (ssRNA) into the host cytoplasm. (Columbus et al., 2020) Using the host ribosomal machinery, the 5′ end of the ssRNA is translated into a viral poly-protein. (4a) The poly-protein is auto-proteolytically cleaved by virus-encoded proteinases into 16 non-structural proteins that form the (4b) replicase-transcriptase complex, which includes multiple enzymes like the viral RNA-dependent RNA polymerase and endo- and exonucleases essential for nucleic acid metabolism. (5a) The 3′ end of the genome expressing 13 ORFs and encoding the four major viral structural proteins: Spike (S), Envelope (E), Membrane (M) and Nucleocapsid (N) are also expressed using host ribosomal machinery. (5b) Concurrently, the ssRNA undergoes replication using viral RNA-Dependent RNA Polymerase. (Zhu et al., 2020a) S, E, and M structural viral proteins are then inserted into the endoplasmic reticulum (ER). (7a) These proteins then move to the ER-Golgi intermediate compartment via the secretory pathway. (7b) The viral RNA encapsulated by N protein buds into membranes of the ER-Golgi intermediate compartment. (Hu et al., 2017) The N protein encapsulated viral RNA and the S, E, and M structural viral proteins are assembled together to form a mature virion. (World Health Organisation, 2004) Following assembly, virions are transported to the cell surface in vesicles. (Widagdo et al., 2019) The SARS-CoV-2 virions fuse with the plasma membrane of the host cell for exocytosis; a large number of virions are released.
1.4.2. Replication, assembly, and release
Upon the release of the SARS-CoV-2 genome into the cytoplasm of the host cell, the viral replicase is translated from the gRNA (Fig. 4). The resulting polypeptide is processed and cleaved by viral proteases. Following this, several nsps gather to form the replicase-transcriptase complex (RTC), in double-membrane vesicles (DMV), to enable RNA synthesis that is critical for RNA transcription and replication of the sgRNAs (Snijder et al., 2006). Viral RNA is synthesised, producing both gRNAs and sgRNAs. The sgRNA are positive sense and are effectively mRNA molecules for the structural and accessory genes which reside downstream of the replicase polypeptides. The sgRNAs share common 5′ and 3′ leader sequences on their termini with the full-length SARS-CoV-2 genome, and hence, can create nested RNAs, a characteristic of all viruses in the order, Nidovirales (Sawicki et al., 2007; Perlman and Netland, 2009). The gRNA and sgRNA species are synthesised via negative sense intermediates, which are only about 1/100th the amount compared to the positive sense RNA species and have poly-uridylate and anti- leader sequences (Sethna et al., 1991).
The coronavirus structural and accessory proteins, e.g. HE, 3a/b, and 4a/b, are all translated from sgRNAs species (Snijder et al., 2006). Coronaviruses can utilise both homologous and non- homologous recombination, which is primarily a consequence of the strand switching capability of the RNA-dependent RNA polymerase (nsps12), enabling efficient assembly of the genome and is probably a major mechanism for the genetic evolution of the virus (Lai et al., 1985; Keck et al., 2020). Finally, after replication and synthesis of sgRNA, the S, E, and M genes are translated into viral structural proteins and transported into the endoplasmic reticulum (ER). These proteins are processed via the secretory pathway and are transported into the ER-Golgi intermediate compartment (Tooze et al., 1984; Krijnse-Locker et al., 1994), where the full- length viral genomes are packaged with the nucleocapsid N protein, budding from the membrane, and thus forming the enveloped mature virion (de Haan and Rottier, 2020). The N protein has two domains that can bind the RNA genome, with the aid of nsp3 protein, and attaching it to the RTC, facilitating the packaging of the virus (Chang et al., 2006; Hurst et al., 2009; Fehr and Perlman, 2020). The viral M protein has three transmembrane domains and is responsible for the majority of protein-protein interactions needed for virus assembly, including membrane curvature and binding the nucleocapsid (Neuman et al., 2011; Nal et al., 2005). Pseudo-virus particles can also only be formed when there is a co-expression of M protein and E protein, indicating the requirement of both these two proteins to form the coronavirus envelope (Bos et al., 1996). The viral E protein is also involved in structural shaping of the viral membrane envelope and in inhibiting M protein aggregation, as well as a role in pathogenesis (Fischer et al., 1998; Corse and Machamer, 2000; Boscarino et al., 2008; Raamsman et al., 2000). After the assembly of the mature virions, they are transported in vesicles, where they are released from the infected cell via exocytosis (Du et al., 2009).
1.5. Viral load and antibody titre
Unlike SARS, COVID-19 patients had the highest viral load near presentation, which could account for the fast-spreading nature of this epidemic. A study involving COVID-19 patients in Hong Kong, they recorded high viral load on presentation with the onset of symptoms, even when the symptoms were mild (To et al., 2020). SARS CoV-2 viral RNA load was detected in the deep throat (posterior oropharyngeal) saliva samples for 20 days or even longer. The peak of the viral load correlated positively with age. Viral load in posterior oropharyngeal saliva samples was higher during the first week of symptom onset, which gradually declined. Thus, the location of sample collection and the timing for the onset of symptoms both are important factors to be considered for the detection of SARS CoV-2 positive cases. In the same study, most of the patients showed rising antibody titres 10 days after symptom onset, though the serum antibody levels did not show correlation with clinical severity (To et al., 2020).
Antibody responses to SARS-CoV-2 viral nucleocapsid protein, using infected cell lysates, was identified on the 10th day after symptom onset by western blot (Lee et al., 2020). In another study involving 285 patients with COVID-19, all tested positive for antiviral IgG within 19 days after symptom onset. Both IgG and IgM titres reached a plateau within 6 days after seroconversion (Long et al., 2020). In Wuhan Tongji Hospital, around 60 convalescent patients tested positive for the IgG against the virus, while 13 patients tested negative for IgM, where IgG titre was higher comparatively. Both the antibody titres showed a decrease when tested weeks apart (Du et al., 2020). Thus, titres of SARS‐CoV‐2 antibodies can reflect the progress of viral infection and can be a vital component to understand the development and prognosis of the disease. Similarly, the timing of antibody seroconversion is also crucial for determining the optimum duration for collecting serum specimens for antibody diagnosis. As previously mentioned, several other studies also confirmed the presence of SARS-CoV-2 nucleic acids in the fecal, urine samples and rectal swabs of COVID-19 patients and thus it becomes essential to ascertain viral load dynamics in such samples too (Guan et al., 2020; Young et al., 2020; He et al., 2020).
1.6. Transmission
SARS-CoV-2 is transmitted through “respiratory droplets”, which are large droplets of virus-laden mucus or through close contact with infected individuals (Jin et al., 2020a; Ghinai et al., 2020; Li et al., 2020a; Chan et al., 2020). At the same time virus has also been reported to spread via asymptomatic but infected individuals in several countries, including China, Germany, USA, and India (Ghinai et al., 2020; Mazumder et al., 2020; Pan et al., 2020; Ling et al., 2020; Rothe et al., 2020). A systematic review and meta-analysis of 172 observational studies with no randomised controlled trials and 44 relevant comparative studies in health-care and non-health-care settings revealed transmission of virus decreased as physical distancing increased to 1 m or more (Chu et al., 2020a). Eye protection, N95 or similar respirators in health-care settings and 12–16-layer cotton or surgical masks in the community were found to greatly control the transmission (Chu et al., 2020a). Bandiera et al. also reported similar findings, i.e. the usage of masks reduced the dispersion of respiratory droplets by 1000 x from a distance of 5 cm when tested on human volunteers under speaking or coughing conditions (Bandiera et al., 2020). However, the direct protective effect of surgical and non-surgical masks for the wearer in non-healthcare conditions are yet to be evaluated; systematic review conducted on this topic have reported little or no protective effects for the wearer (Brainard et al., 2020; Jefferson et al., 2020; Xiao et al., 2020a). Hence, the usage of mask, when widely adopted, seem to best reduce the transmission of the virus by restricting the dispersion of respiratory droplets. Studies have also established that the median half-life of the aerosolised virus is ∼1.2 h under lab conditions, similar to the SARS-CoV. However currently, no evidence supports real-world airborne transmission of the virus through aerosols (Van Doremalen et al., 2020). SARS-CoV-2 was found to remain viable for up to 4 h on copper surfaces, up to 24 h on cardboard surfaces, and up to 72 h on plastic and stainless-steel surfaces. Thus, there exists a possibility of contact transmission to occur, although no confirmed cases of contact transmission have been reported (Van Doremalen et al., 2020). The virus was also found in the faeces of infected patients showing that the virus can survive and replicate in the digestive system (Zang et al., 2020). This suggests that there may be a possibility of an oral-faecal route of transmission, though again no confirmed cases have been reported (Holshue et al., 2020). The Royal College of Obstetricians and Gynaecologists UK have reported that transmission from mother to baby antenatally or intrapartum is possible although this requires further study for confirmation; there appears to be no evidence supporting vertical transmission to the foetus (Royal College of Obstetricians and Gynaecologists, 2020; Chen et al., 2020a; Zhu et al., 2020b). Additionally, as reported by WHO and CDC, the virus has not been found to be transmitted by breastfeeding and has not been found in breastmilk of COVID-19 mothers (World Health Organization, 2020b; CDC, 2020). COVID-19 was found to have a lower severity and mortality than SARS, but it is highly contagious and affecting comparatively more men than women (Mazumder et al., 2020; Jin et al., 2020b; Huang et al., 2020). The difference in fatality rate between males and females may probably be explained by the fact that as ACE2 is located on the X chromosome. There may be alleles that confer resistance to COVID-19, at the same time, oestrogen and testosterone sex hormones have different immunoregulatory functions that may contribute to protection or severity of the disease (Tay et al., 2020; Taneja, 2018). The disease has also been found to disproportionately affect older aged persons and people suffering from social deprivation, diabetes, severe asthma, cardiovascular disease, obesity, haematological malignancy, recent cancer, kidney, liver, neurological or autoimmune conditions (Williamson et al., 2020). Studies have also reported that members of minority communities such as the black and south Asian populations, are at a higher risk of the disease (Williamson et al., 2020).
1.7. Pathophysiology in COVID-19
1.7.1. Contributions of innate immunity and cytokine storm
The incubation period of the disease ranges between 2–14 days and the median incubation period is approximately 4–5 days before symptom onset (Guan et al., 2020; Li et al., 2020a; Wang et al., 2020a; Backer et al., 2020). During the onset of the illness, the common symptoms that most patients exhibited were fever and cough. Other symptoms include conjunctivitis, myalgia (muscle pain) or fatigue, headache, dyspnoea (short of breath), chest pain, diarrhoea, nausea, rhinorrhoea (runny nose), vomiting, loss of appetite, abdominal pain, gastrointestinal bleeding, autoimmune haemolytic anaemia, and sometimes haemoptysis (coughing of blood) (Huang et al., 2020; D’Amico et al., 2020; Wang et al., 2020b; Chen et al., 2020b; Lazarian et al., 2020; Dockery et al., 2020). Patients have also reported anosmia (loss of smell), dysgeusia (distortion of the sense of taste) (Aziz et al., 2020; Mermelstein, 2020; Hopkins et al., 2020; Nunan, 2020). For SARS-CoV-2 asymptomatic patients, anosmia, hyposmia, or dysgeusia are symptoms that were suggestive of screening (Lao et al., 2020). In addition to these, neurological manifestations such as dizziness, headache, impaired consciousness, acute cerebrovascular disease, ataxia, seizure, nerve pain, skeletal muscular injury manifestations, intracerebral haemorrhage, central nervous system vasculitis, encephalopathy, encephalitis, cranial neuropathies and psychosis were reported predominantly in older people (Darlenski and Tsankov, 2020; Mao et al., 2020; Varatharaj et al., 2020). In paediatric patients, an autoimmune and autoinflammatory disease, Paediatric Inflammatory Multisystem Syndrome (PIMS), also known as Multisystem Inflammatory Syndrome in Children (MIS-C), has been reported to occur after SARS-CoV-2 infection (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020).
Cutaneous manifestations of COVID-19 have also been reported (Galván Casas et al., 2020; Fahmy et al., 2020; Zulfiqar et al., 2020). A case report from Strasbourg, France reported purpuric lesions in the lower extremity (Zulfiqar et al., 2020). An Italian study reported patients presenting with an erythematous rash, urticaria and chickenpox‐like vesicles mainly in the trunk with little or no itching that did not correspond to disease severity (Recalcati, 2020). The prolonged use of personal protective equipment and repeated washing have also led to an increase in dermal conditions such as pressure injury, contact dermatitis, itch, pressure urticaria, and exacerbation of pre-existing skin diseases (Darlenski and Tsankov, 2020).
The first step of infection is the inhalation of viral particles present in respiratory droplets from an infected host. Once inhaled, the virion enters the nasal cavity of a healthy host and likely binds to goblet and ciliated cells in the nose that express ACE2 (Sungnak et al., 2020). At this time, a limited innate immune response may occur, and the virus replicates and moves further down the respiratory tract via the conducting airways.
As the virions proliferate and spread towards the upper respiratory tract, usually a robust innate immune response is triggered by the detection of the virions by pattern recognition receptors (PRRs) like Toll-Like Receptors, RIG-1, and MDA-5. This may present several symptoms starting from dysphonia (hoarseness), ulceration of the epiglottis and subglottis, and profound oedema and granulations in the subglottis and also in the upper trachea (Oliver et al., 2020). In a few patients, mild tachypnoea and coarse breath sounds were also observed while the virus is in the upper airway (Ghinai et al., 2020). Furthermore, the detection by PRRs leads to the expression of type 1 IFN in the early stages of infection, which helps establish an anti-viral state in the cells by producing inflammatory cytokines and chemokines. The SARS-CoV produces an enzyme that adds 2′ O-methyl group to viral RNA, which helps it evade detection by MDA-5, thereby delaying the induction of IFN. Studies have established that unlike an early IFN response, a delayed IFN response causes an inability to control viral replication, leading to cellular damage of airway epithelia and the lung parenchyma and an eventual lethal inflammatory cytokine storm (Züst et al., 2011; Decroly et al., 2008; Nikolich-Zugich et al., 2020).
The SARS-CoV-2 papain-like protease, which is essential to generate the replicase-transcriptase complex has been shown to preferentially cleave the ubiquitin-like protein ISG15 from interferon responsive factor 3 (IRF3), attenuating type I IFN responses (Shin et al., 2020). The C- terminus of the SARS-CoV-2 non-structural protein 1 was reported to bind to the 40S ribosomal subunit and block the mRNA entry tunnel (Thoms et al., 2020). This obstruction effectively inhibits the RIG-I-dependent innate immune response (Thoms et al., 2020). Accordingly, no significant expression of IFN was detected up to 48 h post-infection with SARS-CoV-2. Only Interleukin (IL) 6, which correlates with respiratory failure, Acute Respiratory Distress Syndrome (ARDS), and adverse clinical outcomes were upregulated. Monocyte Chemoattractant Protein-1 (MCP1), C-X-C motif chemokine (CXCL) 1, CXCL5, and CXLC10, were also upregulated 48 h post-infection with SARS-CoV-2 (Chu et al., 2020b). The suppression of innate immune activation and annihilation of T cells can help explain the mild or even the lack of symptoms in many infected patients. The increased viral replication efficiency in the respiratory tract early on leads to the highly efficient person-to-person transmission of the virus in the community (Chu et al., 2020b).
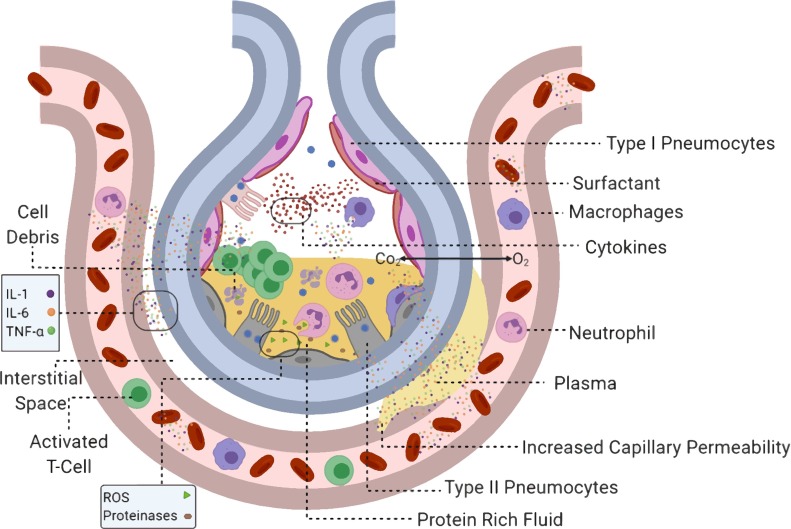
The virions further migrate towards the lower respiratory tract and reach the alveoli where they bind to the type 2 pneumocytes and begin replication. As the type 2 pneumocytes undergo apoptosis after viral release, they secrete inflammatory mediators like CXLC proteins that attract macrophages and neutrophils (Fig. 5 ) (Rockx et al., 2020). The stimulated macrophages further secrete cytokines such as IL-1β, IL-6 and Tumor Necrotic Factor α (TNF-α). The released cytokines trigger a “cytokine storm”, which stimulates the release of vascular endothelial growth factor (VEGF), monocyte chemoattractant protein-1 (MCP-1), IL-8, and additional IL-6, as well as reduced E-cadherin expression on endothelial cells causing vasodilation and increase capillary permeability (Moore and June, 2020). This causes the plasma to leak into the interstitial spaces and alveoli, increasing interstitial and alveolar oedema. The increased alveolar oedema decreases the level of surfactant in the alveoli. This causes an increase in the surface tension in the alveoli, which leads to alveolar collapse. Oedema and alveolar collapse may present as multiple peripheral ground-glass opacities in subpleural regions of both lungs, which is observed in many patients (Lei et al., 2020). Chest CT scan of patients also revealed bilateral multifocal infiltrates and mediastinal and hilar lymphadenopathy in some patients (Ghinai et al., 2020). These decrease the gas exchange efficiency causing hypoxemia and increased work of breathing presenting as dyspnoea (shortness of breath), culminating in ARDS (Xu et al., 2020b). Abnormal coagulation parameters, mainly elevated d-dimers seem to be associated with a higher risk of development of ARDS in COVID-19 patients (Tang et al., 2020b). The aberrant wound healing may even lead to fibrosis than other forms of ARDS (Mason, 2020). Stimulated neutrophils secrete Reactive Oxygen Species (ROS) and proteases which destroy both infected and uninfected type 1 and type 2 pneumocytes, leading to further reduced gas exchange and alveolar collapse, respectively (Barnes et al., 2020).
Fig. 5.
Pathophysiology orchestrated by SARS-CoV-2.
Type II pneumocytes infected with SARS-CoV 2 trigger the release of cytokines, chemokines and interferons. The secreted inflammatory mediators recruit macrophages, neutrophils and activated T cells. The stimulated macrophages secrete IL-1, IL-6 and TNF-α. This increases capillary permeability, causing plasma to leak into the interstitial space and the alveolus. The stimulated neutrophils release reactive oxygen species and proteinases, which destroy infected cells. The cell debris and the plasma combine to form a protein-rich fluid. The increasing fluid leads to dyspnoea and pneumonia. It also dilutes the surfactant lining of the alveolus causing alveolar collapse, which leads to hypoxaemia and acute respiratory distress syndrome. The sustained inflammation leads to systemic inflammatory response syndrome, which develops into septic shock causing multi-organ failure and death.
Furthermore, the dead pneumocytes slough off into alveoli filling them up with fluid, protein deposits, cell debris, macrophages, and neutrophils. This causes pulmonary consolidation, which leads to altered gas exchange and causes hypoxemia (Sarkar et al., 2017; Wong et al., 2019). The consolidation also leads to productive cough. The hypoxemia can further trigger chemoreceptors that stimulate the Sympathetic Nervous System (SNS) that causes tachycardia (increased heart rate) and tachypnoea (increased respiratory rate) (Ottestad et al., 2020; Zhang et al., 2014). The Central Nervous System (CNS) is also affected by the high concentrations of IL-1β, IL-6 and TNF-α in the blood, as these cytokines stimulate the hypothalamus to release prostaglandins such as PGE2, which cause an increased body temperature leading to fever (Davidson et al., 2001).
Studies have also reported elevated levels of myeloperoxidase (MPO)-DNA and citrullinated histone H3 (Cit-H3), which are markers used to detect neutrophil extracellular traps (NETs), in the serum of COVID-19 patients (Zuo et al., 2020). Furthermore, control neutrophils treated with COVID-19 patient serum exhibited NETosis (Zuo et al., 2020). NETs, while protecting the host from invasive pathogens, have been attributed to play a role in many autoimmune and vascular diseases. For example, NETs are known to contribute to ARDS, pathogen- induced acute lung injury, thrombosis and can contribute to further cytokine release leading to the inflammation (Zuo et al., 2020).
An increased frequency of neutrophils, eosinophils and monocytes was reported in severe COVID-19 positive patients; severe patients showed further increase in neutrophils though their activation status had not altered. There was no significant change in the immature granulocyte frequencies. However, there was an inverse correlation between frequency of immature granulocytes in moderate and severe patients with the duration since the appearance of symptoms. Severe patients exhibited lower percentages of both conventional and plasmacytoid dendritic cells (DC) (Kuri-Cervantes et al., 2020).
The increased inflammation of the lungs can further lead to Systemic Inflammatory Response Syndrome (SIRS). The spread of the inflammation from the lungs into the circulatory system causes increased capillary permeability within the systemic circulation. This leads to a decrease in blood volume along with increased vasodilation of systemic arteries, leading to decreased peripheral resistance. The decreased blood volume, along with peripheral resistance, causes hypotension (decreased blood pressure), which decreases perfusion to other organs leading to Multisystemic Organ Failure (MOF) (Cao, 2020; Xiong et al., 2020a; Zheng et al., 2020). The cytokine storm has also been shown to trigger autoimmune haemolytic anaemias (AIHA) (with warm or cold antibodies) (Capes et al., 2020; Lazarian et al., 2020; Zagorski et al., 2020). Most of the studies report manifestation of AIHA early, during the active phase of COVID-19 (within 4–13 days), a timeframe matching that of the cytokine storm (Lazarian et al., 2020; Galeotti and Bayry, 2020; Zagorski et al., 2020; Capes et al., 2020). As a result of ARDS, SIRS and MOF, patients suffering from severe SARS-CoV-2 infection exhibit significantly elevated levels of, IL-2, IL-8, IL-17, G-CSF, GM-CSF, MIP-1α, CRP, and d-dimer, in addition to IL-6, IL-1β and TNF-α (Chu et al., 2020b). There are reports suggesting that in addition to the lungs, SARS-CoV-2 infection may induce the multiorgan injury in patients involving brain, heart, liver, kidney, intestine and eyes (Klok et al., 2020a; Li et al., 2020b).
1.7.2. COVID-19 associated neurological complications
The neurological pathologies observed in COVID-19 are similar to those observed in previous coronavirus epidemics (Paterson et al., 2020). Myoclonus and demyelination are reported in a few cases (Varatharaj et al., 2020; Zanin et al., 2020; Rábano-Suárez et al., 2020). A study conducted in Wuhan, China involving 214 COVID-19 patients, reported that 78 patients developed neurological manifestations (Mao et al., 2020). In another study from Strasbourg, France where effectively 58 patients were recruited for an observational study, reported agitation in 69 % of the patients, confusion in 65 %, and 67 % of the patients had corticospinal tract signs (Helms et al., 2020). A systematic review and meta-analysis of literature databases for psychiatric and neuropsychiatric presentations in coronavirus infections reported transient encephalopathies with features of delirium and psychosis (Rogers et al., 2020). The study also reported cognitive dysexecutive syndrome and delirium with agitation in a few cases (Rogers et al., 2020). There is also a reported case of autoimmune encephalitis with the typical clinical features of opsoclonus and myoclonus, and another case of autoimmune encephalitis with a radiological imagery showing typical limbic encephalitis (Paterson et al., 2020). The exact mechanism for encephalopathy may be multifactorial (effect of sepsis, hypoxia, and/or cytokine storm) (Mehta et al., 2020). A few cases of Guillain–Barré Syndrome (GBS) associated with SARS-CoV-2 have been reported from Italy (Toscano et al., 2020). However, further epidemiological and mechanistic study is required to confirm the incidents of GBS in COVID-19. The binding of the virus to the ACE-2 receptors on endothelial cells causes extravasation of red blood cells leading to cerebral microbleeds (Zulfiqar et al., 2020; Paterson et al., 2020). There have also been reports of severe strokes in COVID-19 patients, but further study is required to determine its association with COVID-19 (Paterson et al., 2020). Magnetic resonance imaging (MRI) revealed abnormalities such as meningeal enhancement, ischaemic stroke, perfusion changes, microhaemorrhages, medial temporal lobe signal abnormalities similar to that seen in viral or autoimmune encephalitis (Helms et al., 2020; Kremer et al., 2020). Very few cases have been reported where SARS-CoV-2 was detected in CSF and its supportive histopathological features; no reports of the virus in the brain exist yet (Paterson et al., 2020; von Weyhern et al., 2020; Reichard et al., 2020). Thus, it is important to establish whether the above-described syndromes may be caused due to either direct viral injury, hyperinflammation, vasculopathy and/or coagulopathy, autoantibody production to neuronal antigens, sepsis and hypoxia, or a combination of these (Mehta et al., 2020).
1.7.3. COVID-19 associated cardiac complications
Out of the first 41 patients diagnosed with COVID-19 in Wuhan, 5 of them had myocardial injury associated with the SARS-CoV-2, which mainly manifested as an increase in high-sensitivity cardiac troponin I (Wang et al., 2020b). The hemogas analysis showed hypoxia; laboratory tests showed elevation of C- reactive protein (CRP), transaminases and lactate dehydrogenase, and lymphopenia (Bonomi et al., 2020). Several patients showed abnormal myocardial zymogram, showing high levels of creatine kinase (Wang et al., 2020a). Because of an excessive inflammation, hypoxia, immobilisation and diffuse intravascular coagulation (DIC), COVID-19 patients may predispose to both venous and arterial thromboembolic disease (Guan et al., 2020; Wang et al., 2020b; Chen et al., 2020c). It has also been observed that concomitant acute thrombosis of the abdominal aorta and pulmonary embolism induces cardiovascular complications in COVID-19 patients, suggesting an association of hypercoagulable condition with the disease (Le Berre et al., 2020).
1.7.4. COVID-19 associated gastrointestinal complications
COVID-19 patients with abnormal liver function were also documented, where patients had alanine aminotransferase (ALT) or aspartate aminotransferase (AST), bilirubin, acute phase reactants like CRP, fibrinogen and IL-6 above the normal range (Wang et al., 2020a; Bangash et al., 2020). Sepsis, hypovolaemia, and nephrotoxins were found to be important contributors to kidney damage in COVID-19 patients. Cardiorenal syndrome, particularly right ventricular failure, might lead to kidney congestion and acute kidney injury in COVID-19 patients (Wang et al., 2020a). Symptoms such as olfactory and gustatory dysfunctions were also found to be related to COVID-19 (Vaira et al., 2020). SARS-CoV-2, facilitated by TMPRSS2 and TMPRSS4, was found to infect and reproduce in ACE-2+ mature enterocytes (Zang et al., 2020). However, the virions released into the intestinal lumen were inactivated by stimulated human colonic fluid and no infectious virions were recovered in stool samples, in spite of the presence of viral RNA in stools. This study thus established the intestine as a site of viral replication and its effect on local and systemic illness and overall COVID-19 progression (Zang et al., 2020).
1.7.5. COVID-19 associated Ophthalmological complications
As in the case respiratory infections by Respiratory syncytial virus and SARS-CoV, the eyes have been shown to act as a portal of entry for the virus. While there have been no reports of SARS-CoV-2 transmission in humans via ocular tissues, further studies are required to exclude the eyes as a source of infection and as a portal of entry. Moderate conjunctivitis could be the first sign of severe respiratory distress in COVID-19 patients (Daruich et al., 2020). Studies from China on patients with COVID-19 reported conjunctivitis and other ocular manifestations, such as epiphora, conjunctival congestion, or chemosis in patients with severe COVID-19 (Wu et al., 2020b; Zhou et al., 2020b; Sun et al., 2020; Chen et al., 2020g). The studies also reported a few patients with positive conjunctival swab for COVID- 19 determined by RT-PCR (Wu et al., 2020b; Zhou et al., 2020b; Chen et al., 2020d). Similar results were also reported in a study conducted by the National Institute for Infectious Diseases in Rome, Italy (Colavita et al., 2020). In addition to conjunctivitis, the ocular swabs were positive for SARS-CoV-2 even when nasopharyngeal swabs tested negative for the virus. This suggests that the conjunctiva may sustain viral replication for an extended period of time (Dockery et al., 2020).
1.7.6. COVID-19 associated complications in paediatric patients
There are reports from France, Italy, United Kingdom and the USA, suggesting the presentation of autoimmune and auto inflammatory diseases in children, especially in children of African descent, such as paediatric inflammatory multisystemic syndrome (PIMS), also known as, multisystemic inflammatory syndrome in children (MIS-C) (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). This syndrome includes Kawasaki-like disease, Kawasaki disease shock syndrome, toxic shock syndrome, myocarditis and macrophage activation syndrome (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). The exact cause for Kawasaki disease remains unknown; however, it is believed that it is caused by an apparent atypical immune response to pathogens in genetically predisposed individuals (Shulman and Rowley, 2015; Nakamura, 2018; Nakamura et al., 2012). Previous studies have implicated the pathogenesis of Kawasaki disease with the infection of certain members of the coronavirus family (Esper et al., 2005; Shirato et al., 2014). The temporal association between the beginning of COVID-19, SARS-CoV-2 infection and the onset of PIMS suggest a causal link (Toubiana et al., 2020). This is further supported by the fact that in most cases, the patients exhibiting PIMS tested positive for IgM or IgG SARS-CoV-2 antibodies (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). The presence of IgG antibodies clearly indicates a delayed onset of PIMS following SARS-CoV-2 infection (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). The onset of PIMS occurred 4–5 weeks after acute COVID-19 (Belot et al., 2020). The patients presented with fever, diffused skin rashes, rash/oedema of hands and feet, conjunctivitis, dry cracked lips, cervical lymphadenopathy and arthritis. The Kawasaki-like disease caused by COVID-19 exhibited a few differences in both clinical and biochemical features from patients suffering from Kawasaki disease without SARS-CoV-2 infection. Clinically, the patients suffering from COVID-19 associated Kawasaki-like disease were older and the disease occurred in both sexes, unlike the classical Kawasaki disease that occurs in younger male children (Belot et al., 2020). The COVID-19 associated Kawasaki-like disease also had a higher incidence of abdominal pain and/or more frequent diarrhoea, meningeal and respiratory involvement, and a strikingly different myocarditis severity and frequency when compared to classical Kawasaki disease (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). Biochemically the patients exhibited leukopenia with thrombocytopenia, increased ferritin, elevated myocarditis markers and high levels of procalcitonin, CRP and cytokines were observed when compared to classical Kawasaki disease (Galeotti and Bayry, 2020; Pouletty et al., 2020; Toubiana et al., 2020; Verdoni et al., 2020; Whittaker et al., 2020; Belhadjer et al., 2020; Feldstein et al., 2020; Dufort et al., 2020). Nearly 62 % patients also showed resistance to the initial treatment with intravenous immunoglobulin (IVIg) infusion, and required a second infusion for successful treatment (Pouletty et al., 2020; Belhadjer et al., 2020). While the children exhibited the devastating effects of the cytokine storm associated with COVID-19, such as heart failure, pneumonia, gastrointestinal, neurological and renal manifestations, the paediatric patients in the French study rarely had respiratory manifestations (Pouletty et al., 2020). This suggests a different host immune response in children compared to adults. Treatment for PIMS involves the administration of IL-1 receptor antagonist, IL-6 receptor blockers such as Tocilizumab or Sarilumab, IVIg, and steroids or biologics to control inflammation (Pouletty et al., 2020; Belhadjer et al., 2020).
1.7.7. COVID-19 associated complications in geriatric patients
COVID-19 is known to affect older members of the population disproportionately, with adults over the age of 65 years making up to 80 % of hospitalization and having a 23-fold greater risk of death (Williamson et al., 2020; Mueller et al., 2020). One possible explanation for this could be the increased baseline inflammation, called inflammaging, commonly observed in individuals over the age of 60 (Akbar and Gilroy, 2020). Studies have shown increased baseline serum concentrations of CRP and cytokines such as IL-6 and IL-8 (Franceschi et al., 2017). Inflammaging could be the result of the accumulation of mis-folded proteins, compromised gut barrier, obesity and impaired clearance of dead or dying cells (Franceschi et al., 2017; De Maeyer et al., 2020). Senescent nonlymphoid cells have been known to secrete inflammatory cytokines, chemokines, growth factors, and matrix metalloproteinases (Akbar and Gilroy, 2020; Campisi et al., 2019). This increased baseline inflammation inhibits antigen-specific immunity affecting the efficacy of many vaccines (Parmigiani et al., 2013). Studies have shown that treatment with rapamycin, MAPK inhibitor or steroids reduces this excessive inflammation and enhances vaccine efficacy (Akbar and Gilroy, 2020; Mannick et al., 2018; Vukmanovic-Stejic et al., 2018). In case of COVID-19, this baseline inflammation may itself not be detrimental but contributes to the initiation of an inflammatory cascade that ends in the deadly cytokine storm (Akbar and Gilroy, 2020). Furthermore the accumulation of senescent cells in the lungs of older patients could inhibit T cell response, induce NKR ligand expression, which marks the cells for elimination by infiltrating T cells expressing NKRs (Akbar and Gilroy, 2020). As observed in the case of vaccines against other respiratory viruses, inflammaging may reduce the efficacy of COVID-19 vaccinations in this already disproportionately affected group.
1.8. SARS-CoV-2 targeting of the adaptive immunity
As with any infection, both the innate and adaptive arms of the immune system are required to mount a successful defence against a viral incursion. In case of COVID-19, a decrease in the circulating T helper cells (CD4+ cells), Cytotoxic T cells (CD8+ cells), B cells, natural killers cells, lymphocytes, monocytes, eosinophils and basophils has been reported (Huang et al., 2020; Xu et al., 2020b; Kuri-Cervantes et al., 2020; Wu et al., 2020c). A retrospective, single-centre study involving 452 patients revealed a significant decrease in the total number of regulatory T cells, memory T cells and suppressor T cells (Qin et al., 2020). The study also reported an increase in the percentage of naïve T cells (Qin et al., 2020). As naïve T cells help respond to novel pathogens that the immune system has not yet encountered by managing release of cytokines, this may help explain the hyperinflammation (Catanzaro et al., 2020). The lower levels of memory T cells reported may also explain the relapses reported in COVID-19 convalescent individuals (Catanzaro et al., 2020). Direct infection of THP-1 cells, human peripheral blood monocyte-derived macrophages and dendritic cells by MERS-CoV and infection of T cells and macrophages by SARS-CoV has been reported (Zhu et al., 2020a; Perlman and Dandekar, 2005). Hence, it can be speculated that SARS-CoV-2 may also infect monocytes and macrophages by a mechanism that is yet to be elucidated (Catanzaro et al., 2020). Receptors such as CD147 on the surface of T cells and other immune cells may mediate viral entry (Radzikowska et al., 2020). The clinical trial with anti-CD147 monoclonal antibody, Meplazumab, showed promising efficacy and safety in COVID-19 patients (Bian et al., 2020). However, CD147 did not show a direct interaction with the S protein of SARS-CoV-2 (Shilts and Wright, 2020). Similarly, lymphopenia can be attributed to SARS-CoV-2 direct infection and lymphocyte death, destruction of the lymphatic organs, and/or high levels of the programmed cell death protein 1 (PD-1) on CD8+ T cells (which is known to trigger T cell exhaustion) (Catanzaro et al., 2020; Jiang et al., 2015; Moon, 2020). Lymphocytopenia, neutrophilia and neutrophil-to-lymphocyte ratio are being used as predictors for the severity of the illness during early stages of infection and poor outcomes in COVID-19 (Kuri-Cervantes et al., 2020; Wu et al., 2020c; Zhou et al., 2020c; Liu et al., 2020a). This further alludes to the hyper-inflammatory nature of COVID-19. Furthermore, COVID-19 patients were reported to have elevated serum levels of high-sensitivity C-reactive protein and procalcitonin, whose levels have been associated with high risks of mortality and organ injury (Li et al., 2020c). Lower percentage and count of CD3+, CD4+, and CD8+ lymphocytes populations serve as prognostic markers for mortality, organ injury, and severe pneumonia (Li et al., 2020c). SARS-CoV exposed as well as a subset of non-exposed people exhibit a cross-reactive T cell repertoire (Le Bert et al., 2020). Studies have also reported the presence of SARS-CoV-2 spike glycoprotein-reactive CD4+ T cells in peripheral blood of a subset of donor who were not infected with SARS-CoV-2 (Pia, 2020; Braun et al., 2020). These S reactive CD4+ T cells were found to primarily react with the C-terminal of the S epitope (Braun et al., 2020). This binding preference could be attributed to the presence of overlapping human coronavirus MHC-II epitopes in the C-terminal domain. Hence, these CD4+ T cells are cross reactive clones generated during previous infections with endemic human coronavirus (Braun et al., 2020). A Long-term Information and Knowledge for Ageing – Camden’ (LINKAGE) sub-study is currently underway to investigate if pre-existing antibodies and specific T cells contribute to the devastating effect observed in old people (Linkage, 2020).
The B cell response occurs alongside the T helper cell response (∼1 week post infection) in COVID-19 patients and helps mount a humoral response via antibodies that would help neutralise the virus (Tay et al., 2020). Characterisation of the transcriptome during the recovery stage of the disease revealed significantly lower levels of naive B cells, while plasma B cell levels had increased in peripheral blood mononuclear cells (Kuri-Cervantes et al., 2020; Wen et al., 2020). It was found that a certain subset of patients who contract the disease may not develop long-lasting antibodies against the pathogen; it is possible that these patients may be susceptible to the re-infection (Tay et al., 2020).
Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing has identified several new B cell-receptor changes such as IGHV3−23 and IGHV3−7, and isotypes used earlier for vaccine development including IGHV3−15, IGHV3−30, and IGKV3−11 (Wen et al., 2020). The strongest pairing frequencies, IGHV3−23-IGHJ4, has been suggested to indicate a monoclonal state associated with SARS-CoV-2 specificity (Wen et al., 2020). Antibodies analysed from the serum of COVID-19 patients revealed no cross-reactivity with the S1 subunit of the SARS-CoV spike antigen, while some reactivity was observed between the nucleocapsid antigens of SARS-CoV and SARS-CoV-2 (Long et al., 2020). The RBD-specific IgM and IgG antibodies were significantly elevated in the severe and recovered patients (Kuri-Cervantes et al., 2020). Investigations conducted on COVID-19 recuperating rhesus macaque models, re-infected with SARS-CoV-2, reported no measurable viral spreading, clinical manifestations, or histopathological changes associated with COVID-19 (Li et al., 2020d). The study found lower viral loads in nasopharyngeal or anal swabs 5 or 7 days after reinfection, compared to the recorded viral loads 5 or 7 days after the initial infection with SARS-CoV-2 at similar sites. Similarly, increased levels of leukocytes and neutrophils were recorded 14 days after reinfection, compared to the levels measured during the initial infection. Significantly higher specific antibody titres were recorded 14 day post re-infection. There were also increased activation of CD8+ T cells, changes in CD4+ T cells and memory B cells. Thus, increased production of neutralising antibodies protected the primates against COVID-19 re-infection (Chandrashekar et al., 2020; Deng et al., 2020). A study on 149 COVID-19 convalescent individuals revealed that plasma collected after 39 days of symptom manifestation had a variable half-maximal pseudovirus neutralizing titres of less than 1:50 in 33 %, below 1:1,000 in 79 %, and only 1 % showed titres above 1:5,000 (Robbiani et al., 2020). Interestingly, in spite of the low titres reported, antibodies specific to three distinct epitopes on the RBD of the SARS-CoV-2 S protein neutralized at half-maximal inhibitory concentrations as low as single digit ng/mL (Robbiani et al., 2020). Hence, a vaccine that can elicit the production of such highly potent antibodies, or monoclonal antibodies raised against the RBD of the SARS-CoV-2 S protein, may be highly protective. However, studies on SARS-CoV and MERS-CoV revealed that neutralizing antibodies to S protein can potentially augment severe lung injury by exacerbating inflammatory responses (Tay et al., 2020; Drosten et al., 2014; Liu et al., 2019; Arabi et al., 2016). Hence, therapeutic antibodies should be carefully studied to minimise any unwanted pro-inflammatory activity while retaining maximum virus neutralizing capacity.
1.9. Genomic insights into host-pathogen interaction
Additional specific insights on the intracellular life cycle have also been gained from next-generation sequencing (NGS) studies on the transcriptome and epi-transcriptome profile of SARS-CoV-2 virus and infected host cell. This fundamental approach has given an insight into the specific molecular dialogue between the pathogen and the host cell. This dialogue is complex. The SARS-CoV-2 transcriptome has been studied in high resolution. It has revealed its highly complex nature, mainly as a result of numerous discontinuous transcription events, revealing canonical and non-canonical RNA transcripts with RNA modifications (Kim et al., 2020b). In addition to the canonical full-length genome and other 9 sgRNAs, this study also found numerous non-canonical RNA transcripts of unknown ORFs that contained RNA modifications. 41 putative RNA modifications were identified at an AAGAA motif. These previously unknown ORFs represent the epi-transcriptome of SARS-CoV-2 and has revealed numerous viral transcripts that may be involved in pathogenesis (Kim et al., 2020b).
Another study looked at transcriptome profiling in the primary human lung epithelium and compared differences between SARS-CoV-2 and SARS-CoV infection and identified several pathways potentially involved in pathogenesis and gender-specific differences in clinical presentation (Fagone et al., 2020). Among the genes that were upregulated were a cluster involved in the cytokine-mediated signalling pathways, and in particular, the IL-17 signalling pathway (Fagone et al., 2020). Specifically, cytokine pathways driven by nuclear factor kappa-light-chain- enhancer of activated B cell (NF-κB), toll-like receptors (TLRs), mitogen-activated protein kinase (MAPK), bone marrow stromal cell antigen 2 (BST2), IL-32, TNF alpha induced protein 3 (TNFAIP3), TNFAIP3 interacting protein 1 (TN1P1), intercellular adhesion molecule 1 (ICAM-1), intercellular adhesion molecule 2 (ICAM-2), intercellular adhesion molecule 9 (MMP9), baculoviral IAP repeat containing 3 (BIRC3), and Rho family GTPase 1 (RND1), were significantly upregulated during SARS-CoV-2 infection, suggesting a significant role in pathogenesis (Fagone et al., 2020). Moreover, RELA (NF-κB p65 subunit) seems to be significantly upregulated in SARS-CoV-2 infection, leading to IL-8 involvement (Fagone et al., 2020). Of note is the expression of oestrogen receptor 1 (ESR1), which was also enhanced under SARS-CoV-2 infection, suggesting sex hormones may be involved in differential expression during viral infection and may have implications for the differences in clinical severity seen between genders (Fagone et al., 2020). Additionally, over 24 and 48 h post-infection, CXCL-2 was significantly upregulated in SARS-CoV-2 infection compared to SARS-CoV (Fagone et al., 2020). A recent study using single-cell RNA-Seq in human, non-human primate and mouse tissues/cells was able to resolve further the host cellular targets for SARS-CoV-2 and their abundance in specific tissue/cell types (Ziegler et al., 2020). The study identified ACE2 and TMPRSS2 co-expressing cells (lung type II pneumocytes, ileal absorptive enterocytes and nasal goblet secretory cells) and also determined that that ACE2 is induced by interferon-stimulated genes (IGS), suggesting a possible mechanism for enhanced viral infection (Ziegler et al., 2020).
The clinical pathways of COVID-19 disease severity may also depend on host-specific factors that may contribute to the ‘cytokine storm’, or Cytokines Release Syndrome (CRS), which is the massive release of pro- inflammatory cytokines including cytokines (IL-1β, IL-2, IL-6, IL-7, IL-8, and TNF-α) and chemokines such as CXCL10 and CCL2 in the lungs (Mehta et al., 2020; Xu et al., 2020c). These genomic approaches also shed light on the specific genetic host factors that predispose individuals to this severe clinical presentation. Proteomic and transcriptomic studies on bronchoalveolar lavage (BAL) samples from COVID-19 patients have also revealed considerable insights into the expression of SARS-CoV-2 receptors, co-receptors, immune responses, as well as risk factors for severe disease e.g. age and co-morbidities. Asthma, chronic obstructive pulmonary disease (COPD), hypertension, smoking, obesity, and male gender status were all associated with higher expression of ACE2 and CD147 in BAL, as well as bronchial biopsy and blood from COVID-19 patients (Radzikowska et al., 2020). Furthermore, there was a positive correlation between the expression of CD147-related genes in BAL and the age and body mass index (BMI) of COVID-19 patients (Radzikowska et al., 2020). In another study on BAL from COVID-19 patients, an association was observed between COVID-19 severity and enhanced levels of certain cytokines, e.g. CCL2/MCP-1, CXCL10/IP-10, CCL3/MIP-1A, and CCL4/MIP1B (Xiong et al., 2020b). This study also found that SARS-CoV-2 triggered apoptosis and the p53 signalling pathway in lymphocytes, probably causing additional lymphopenia in these patients (Xiong et al., 2020b). A comparison of transcriptome profiles between patients with COVID-19 and influenza A virus infection revealed an absence of significant type I interferon/antiviral responses with SARS-CoV-2 infection, with enhanced expression of genes involved in metabolic pathways e.g. haem biosynthesis, oxidative phosphorylation and tryptophan metabolism, suggesting an important role for mitochondria during SARS-CoV-2 infection (Gardinassi et al., 2020). Furthermore, a meta-analysis on BAL data from COVID-19 patients also revealed an excess for neutrophils and chemokines (Gardinassi et al., 2020).
In meta-transcriptomic sequencing of BAL from 8 COVID-19 patients, the expression of pro- inflammatory genes, especially chemokines, was significantly elevated in these patients compared to community-acquired pneumonia patients and healthy controls, suggesting hypercytokinemia (Zhou et al., 2020d). It also revealed enhanced dendritic cell and neutrophil activity (Zhou et al., 2020d). In contrast to SARS-CoV, which induces an ineffective interferon response, SARS-CoV-2 was found to strongly initiate expression of numerous interferon stimulated genes, which are thought to significantly contribute to immunopathogenesis (Zhou et al., 2020d). Similarly, an analysis of RNA-seq data sets of BAL from COVID-19 patients identified upregulation of neutrophil, inflammatory genes and chemokines, which may be involved in immunopathology, e.g. TNFR, IL-8, CXCR1, CXCR2, ADAM10, GPR84, MME, ANPEP, and LAP3 (Didangelos, 2020).
1.10. Chronic co-morbidities in COVID-19 patients
Chronic co-morbidities for COVID-19 patients include cardiovascular disease, hypertension, diabetes, stroke and malignant tumour (Wang et al., 2020a). It was also found that parameters such as older age, underlying hypertension, high cytokine levels (IL-6, IL-10, and TNF-α), and high lactate dehydrogenase level were significantly associated with severe COVID-19 during hospital admission (Li et al., 2020b). In a study involving 184 ICU patients with COVID-19 pneumonia, all of them showed an incidence of thrombotic complications such as symptomatic acute pulmonary embolism (PE), deep vein thrombosis, ischemic stroke, myocardial infarction or systemic arterial embolism (Klok et al., 2020a). Approximately, one-third of patients experienced gastrointestinal symptoms. During hospitalization, a substantial proportion of patients presented cardiac injury, liver, and kidney dysfunction, and hyperglycaemia. ICU COVID-19 patients had higher plasma levels of IL-2, IL-7, IL-10, GSCF, IP10, MCP-1, MIP-1α, and TNF-α, compared to non- ICU patients. Majority of ICU patients diagnosed with COVID-19 were found to be at highest thrombotic risk (Klok et al., 2020a). Patients with severe COVID-19 likely developed ARDS and died of respiratory failure. Biopsy samples at autopsy from a patient who died from severe COVID-19 showed bilateral diffuse alveolar damage with cellular fibromyxoid exudates, and mononuclear inflammatory lymphocytes in both lungs (Xu et al., 2020b; Bonomi et al., 2020). Diffuse alveolar damage with fibrin rich hyaline membranes are pathological results of COVID-19. In a study, 12 COVID-19-infected cancer patients were found to have underlying diseases, such as hypertension, diabetes and chronic obstructive pulmonary disease (Ma et al., 2020). Cancer patients with accompanying COVID-19 infection showed deteriorating conditions and poor outcomes, and thus it was recommended to avoid treatments causing immunosuppression (Zhang et al., 2020a).
1.11. Complement, neutrophil NET and microangiopathy in COVID-19
The complement system is an integral part of the innate immune response. It consists of a group of plasma proteins produced mainly by the liver or membrane proteins expressed on cell surface. These proteins interact in a cascade that leads to the opsonization of pathogens and the induction of inflammatory responses. The complement system comprises of three distinct activation pathways, i.e. Classical, Alternative or Lectin (MBL). The activation of these pathways is based on different molecules present on the pathogen surfaces. The classical pathway is initiated by the binding of C1q to the pathogen surface or antibody complex. The initiation of the alternative pathway is triggered by the binding of a spontaneously activated complement component to pathogen surface. The binding of the MBL to mannose-containing carbohydrates on pathogens triggers the initiation of the lectin pathway. The early events of three pathways eventually converge to generate a protease called, C3 convertase, which is covalently bound to the pathogen. The C3 convertase then cleaves C3, present in plasma, into C3a and C3b. The C3b binds to the pathogen and targets it for destruction by phagocytes. Furthermore, C3b binds with the C3 convertase to form C5 convertase, which produces C5a and C5b. C5b triggers the late events of the complement cascade, which are a series of polymerization reactions where C6, C7, C8 and C9 interact with each other to form the Membrane Attack Complex (MAC). The MAC can damage the membrane of certain pathogens by creating a pore in it. The C5a and C3a produced are important small peptide mediators of inflammation [Reviewed in (Merle et al., 2015)].
Studies in C3−/− (gene-deficient) mice infected with SARS-CoV revealed the presence of C3 activation products such as C3a, C3b, iC3b, C3c, and C3dg 1 day post infection (Gralinski et al., 2018). The C3 deficient mice showed significantly less respiratory dysfunction and lower weight loss as compared to control. The mice also showed significantly lower levels of neutrophils and monocytes compared to the control. Lower IL-6, TNF-α and IL-1α levels were reported in the lungs of the C3 deficient mice (Gralinski et al., 2018). The study also reported lower weight loss in mice deficient in Factor B or C4. In view of the critical role of the complement system in SARS-CoV infection since the first day of infection, it raised possibility for complement involvement in SARS-CoV-2. Levels of the terminal component of the complement system (MAC) and C5a are increased in patients with ARDS (Langlois and Gawryl, 1988; Hammerschmidt et al., 1980). MAC is known to damage endothelial cells, and thus, regulation or inhibition of MAC by its known regulators such as CD59 or clusterin could be a potential treatment for endothelial dysfunction/damage in ARDS or COVID-19 (Chang, 2019a; Kerr and Richards, 2012; Boom et al., 1989).
Considering the lectin pathway of the complement system, MBL was shown to bind SARS-CoV in vitro and inhibit its infectivity (Ip et al., 2005). The N-protein of SARS-CoV and SARS-CoV-2 has been shown to interact with MBL-associated serine proteases-2 (MASP2), which is known to initiate the lectin pathway (Gao et al., 2020a), leading to over-activation of the complement system. This same study also highlighted excess complement proteins found in post-mortem COVID-19 patient lungs (Gao et al., 2020a). Furthermore, deletion of the masp-2 gene or perturbance of the MASP-2–N protein interaction was found to reduce lung injury. These studies, along with human proteomic studies, demonstrate the activation of multiple complement pathways during a coronavirus infection. In case of COVID-19, the alternative and lectin pathways of the complement system seem to be preferentially activated (Java et al., 2020). Increased levels of plasma C5a and MAC were recorded in patients with moderate and severe COVID-19 (Cugno et al., 2020). A post-mortem study of lung and skin vasculature in 5 COVID-19 patients showed significant deposits of MAC and C4d that colocalized with the SARS-CoV-2 S-protein, and MASP-2 in the micro-vasculature. This study did not find prominent classical features of ARDS such as hyaline membranes and inflammation in the histopathological examination (Magro et al., 2020). A recent study reported an increase in levels of C5a, which correlated with increased COVID-19 disease severity, as well as high levels of expression of C5aR1 in blood and pulmonary myeloid cells of COVID-19 patients (Carvelli et al., 2020). Furthermore, use of anti-C5aR1 monoclonal antibodies in human C5aR1 knock-in mice was found to successfully prevent C5a-mediated myeloid cell recruitment and activation, thereby inhibiting acute lung injury (Carvelli et al., 2020). A recent genetic study in COVID-19 patients has reported that gene variants associated with complement regulatory protein, CD55 (decay-accelerating factor, which accelerates the decay of complement proteins, and thus inhibits complement activation) is associated with increased risk in clinical outcome (odds ratio 2.34–2.4); gene variants that map to C3 showed some protective effect (odds ratio 0.66−0.68) (Ramlall et al., 2020).
Neutrophils along with the complement system are another important component in the defence of the host against invading pathogens. Neutrophil infiltration in pulmonary capillaries, acute capillaritis with fibrin deposition, extravasation of neutrophils into the alveolar space, and neutrophilic mucositis have been reported in the case of SARS-CoV-2 infection (Zuo et al., 2020). The neutrophilic extracellular traps (NETs) and the neutrophils activated by SARS- CoV-2 infection contain C3, factor B and properdin, triggering the alternative pathway of the complement system (Java et al., 2020). While this is usually beneficial, the sustained activation and NETs formation leads to a hyper-inflammatory immune response that damages and destroys surrounding tissue. This aberrant behaviour, in concert with the abnormal complement activation, leads to the well recorded clinical manifestations observed in the case of COVID-19 such as ARDS and pulmonary inflammation (de Bont et al., 2019). Additionally, NETs have been reported to induce the production of excessive thrombin and subsequently generate C5a (de Bont et al., 2019). Hence, it has been speculated that feedback loop that begins with complement activation leading to NETosis causing an increases in thrombin production, that further stimulates the complement activation causing enhanced NET formation (Java et al., 2020).
Microangiopathy refers to a disease of the small blood vessels. The term is used when small blood vessels pathologically thicken or weaken, which leads to impaired flow of blood as well as leaking of cells and proteins. Sustained inflammation in the vascular system due to the SARS-CoV-2 infection leads to thrombosis and microangiopathy (Tay et al., 2020; Liu et al., 2020b). This is supported by reports of increased lactate dehydrogenase, bilirubin, activation of platelets, elevated d-dimer levels and hyper-fibrinolysis (Feng et al., 2020). A post-mortem case series of 4 patients with COVID-19 found thrombotic microangiopathy, which was restricted to the lungs, along with diffuse alveolar damage could have contributed to causing death (Fox et al., 2020). Another such study of 21 cases found similar diffuse alveolar damage with significant capillary congestion and microthrombi despite anti-coagulation therapy (Menter et al., 2020).
Due to the presence of severe pulmonary vascular dysfunction in ARDS, it has been argued that ARDS is a type of vascular microthrombotic disease with lung phenotype involvement. This argument is supported by the association of mortality in ARDS with thrombocytopenia and MOF as a result of disseminated intravascular coagulation (Gando et al., 2020; Dias et al., 2002; Wang et al., 2014). In recent times, a couple of theories on the pathogenesis of ARDS in sepsis have evolved: the ‘two-path unifying theory’ in which certain homeostasis mechanisms lead to microthrombogenesis, and the ‘two-activation theory of the endothelium’ in which the complement MAC leads to inflammation via cytokines and microthrombogenesis via platelet activation (Chang, 2018, 2019b). The complement system plays a key role in the pathogenesis of thrombotic microangiopathy. This is a syndrome characterised by thrombocytopenia (low platelet count), microangiopathic haemolytic anaemia and systemic organ damage. Atypical haemolytic uremic syndrome (aHUS) is an example of such a disorder that typically leads to kidney damage. It is caused by excessive activation of the alternative pathway due to mutations in complement regulators factor H (common), factor I, membrane- cofactor protein, or C3. Analysis of renal tissue morphology from autopsies of COVID-19 patients revealed strong C5b-9 staining (via the alternative pathway) on the apical brush border of tubular epithelial cells with minimal deposition on glomeruli and capillaries of the kidney (Diao et al., 2020). Treatment with eculizumab (C5 inhibitor) dramatically improved outcomes of survival in aHUS. Features similar to complement-mediated thrombotic angiopathy such as kidney and cardiac injury increased lactate dehydrogenase and d-dimer, and decreased platelets were observed in COVID-19 (Zulfiqar et al., 2020; Zhao et al., 2020). Eculizumab was used successfully as part of management of four COVID-19 patients with severe pneumonia or ARDS in the intensive care unit, and this preliminary data is being used to conduct further full-fledged clinical trials with eculizumab (Diurno et al., 2020).
Considering the overlap with complement-mediated thrombotic angiopathy in COVID-19, few studies are underway to test the effectiveness of complement regulators. A recent case study demonstrated a favourable outcome for the compstatin-based C3 inhibitor AMY-101. The study, which involved a 71-year-old Caucasian male with severe pneumonia and systemic inflammation, found that AMY-101 was safe and had a favourable outcome in improving the clinical presentation of the patient significantly (Mastaglio et al., 2020). Furthermore, treatment with a recombinant C5a antibody on 2 male COVID-19 patients aged 54 and 67 years showed significant benefit in suppressing complement hyperactivation, which contributes to the excessive immune response causing aggravated inflammatory lung injury, a hallmark of SARS-CoV-2 pathogenesis and lethality (Gao et al., 2020a). One of the many challenges includes determining patients who have a dysregulated complement activation. C3 bound to erythrocytes has been detected in patients with COVID-19 (Lam et al., 2020), which may prove to be a useful blood marker as well as in identifying patients who potentially merit intervention with complement regulators (Lo et al., 2020).
In COVID-19, endothelial injury has been found to be a key pathophysiological feature. A case series found direct evidence of viral infection of endothelial cells and endothelial inflammation, leading to endothelial cell death (Varga et al., 2020). In COVID-19 patients, endothelial cell abnormalities were recorded in the kidney, lung, heart, small bowel, and liver. 5 of 26 deceased COVID-19 patients were found to have suffered endothelial cell swelling with variable foamy degeneration in the glomeruli and an additional 3 patients were found to have severe injury to the endothelium due to segmental fibrin thrombi in glomerular capillary loops (Varga et al., 2020; Bryce et al., 2020; Su et al., 2020). MAC deposition has been observed in the endothelium of COVID-19 patients (Magro et al., 2020). Such studies have led to notion that in COVID-19, there are strong vascular and inflammatory components as well, which play a significant role in the pathophysiology of the illness (Connors and Levy, 2020). Consistent with endothelial injury, the significantly elevated levels of von Willebrand factor found in the patient with severe COVID-19 has led to the idea that the infection of the ACE2 expressing endothelium by SARS-CoV-2 induces injury and activates the complement, which sets up a feedback loop that maintains a state of inflammation (Java et al., 2020; Escher et al., 2020; Gavriilaki and Brodsky, 2020; Panigada et al., 2020). It is worth noting that ARDS may occur in COVID-19 despite well-preserved lung gas volume, which could indicate a key role for inflammatory processes, leading to vascular constriction and subsequent low oxygen levels in the blood (Gattinoni et al., 2020). Furthermore, d-dimer (a fibrin degradation product) levels are also found to be elevated in COVID-19 and are associated with poorer prognosis (Escher et al., 2020; Zhou et al., 2020e). These factors add to the importance of understanding vascular changes in this disease, including microangiopathic processes and coagulopathies in patients with COVID-19.
1.12. Pregnancy and COVID-19
Pregnancy is associated with several maternal adaptations in both immune function (immunosuppression) and cardiovascular physiology (increased cardiac output, physiological anaemia, cardiac hypertrophy) that would likely alter susceptibility to viral respiratory infections including SARS-CoV-2. Maternal death occurred in 15 % of patients admitted to the ICU for COVID-19 and in 25 % of those who required invasive mechanical ventilation (Blitz et al., 2020). To date, the literature consists of case reports, case series and retrospective studies.
The most common presenting symptoms of maternal COVID-19 are fever, cough, dyspnoea, and gastrointestinal symptoms (Trad et al., 2020). Clinical findings of respiratory manifestations were similar to those seen in the non-pregnant populations, with similar CT findings together with elevations in C-Reactive protein with decreased white blood cell counts (Zaigham and Andersson, 2020). Although the portal of entry is inhalational, there are widespread systemic effects. The immobility, hypoxia and acute inflammation lead to a prothrombotic hypercoagulable state, and indeed, elevated d-dimers are correlated with disease severity (Zhang et al., 2020b). COVID-19 is thus associated with venous or arterial thromboembolism (Klok et al., 2020b). The mechanism by which this occurs is currently thought to be as a result of inflammatory cytokines (Qin et al., 2020) inducing production of tissue factor with subsequent thrombin activation. The elevations of d-dimer (often seen in acute phases of infection) may be related to this increased thrombin generation.
While serious maternal morbidity has been seen, the vast majority of pregnant women with SARS- CoV-2 infection remained asymptomatic for respiratory symptoms (Breslin et al., 2020; Li et al., 2020e; Lu et al., 2020). Pregnancy is coupled to physiological changes in cardiorespiratory status (O’Day, 1997) which might be expected to alter susceptibility to a respiratory upset. Nevertheless, the evidence suggests that this is less prevalent than thought. However, the changes in immune function and coagulation in pregnancy appear to increase some complications. Similarly, the coagulopathies seen in COVID-19 in the non-pregnant population might be expected to have deleterious effects in pregnancy, which is already a prothrombotic state.
COVID-19 has been seen to be associated with preeclampsia (Trad et al., 2020; Chen et al., 2020e) with one report suggesting a causal link (Hosier et al., 2020). The placenta has also been reported as having vascular malperfusion and thrombosis (Baergen and Heller, 2020; Shanes et al., 2020), which may provide an underlying explanation for the preeclampsia, a disease, associated with poor placental perfusion and altered vascular function (Flint et al., 2019). Evidence of increased clotting at the placental surface suggests that this mechanism may be responsible (in part) for the increased incidence of preeclampsia. SARS-CoV-2 virions have been seen in the Syncytiotrophoblast, the part of the placenta responsible for the angiogenic imbalance seen in preeclampsia and effects on the release of known factors associated with the disease (sFlt-1 – soluble Fms like tyrosine kinase 1 and PlGF –placental growth factor) are unknown. The disease is also linked to preterm premature rupture of membrane (PPROM) (Zhu et al., 2020b; Chen et al., 2020e), and preterm labour (Yan et al., 2020), both of which are linked to inflammation. The underlying mechanism by which PPROM occurs is not entirely elucidated. However, reports have suggested that activation of the coagulation system and thrombin causes fetal membrane weakening and subsequent rupture of membranes (Chaiworapongsa et al., 2002; Draper et al., 1995). The alterations in clotting and thrombin seen in COVID-19 may well provide a mechanism for this. Similarly, thrombin has been related to the induction of preterm labour and weakened fetal membranes by induction of decidual colony-stimulating factor (CSF)-2 (Sinkey et al., 2020).
1.13. Anti-viral Drugs and treatments in COVID-19: variable efficacy and repurposing
At present, there are no drugs, therapeutics, or vaccines approved for curing, preventing, or treating SARS-CoV-2 specifically. As of June 2020, a total of 239 (147 in human trails, 92 in preclinical development) therapeutic drugs are under development against COVID-19. The current treatment for SARS-CoV-2 patients involves the management of clinical symptoms and providing supportive care. While research into developing new drugs and treatments against SARS-CoV-2 are ongoing, much of the effort currently focuses on the repurposing existing medicines used against viruses, multiple sclerosis, arthritis, blood plasma derivatives and malaria. Moreover, although immunosuppressive treatments, e.g. corticosteroids have shown promise for COVID-19, there is considerable concern about possible side effects. Other immunotherapeutic approaches given as adjunct therapy and based on neutralizing inflammatory cytokines and other immunomodulators, passive viral neutralization to reduce lung pathology and viral load, could be a promising approach (Bonam et al., 2020). A number of these approaches are discussed below.
1.13.1. Remdesivir
The antiviral drug Remdesivir, developed by Gilead Sciences, is an adenosine analogue, which incorporates into nascent viral RNA chains and results in premature termination, effectively inhibiting viral RNA synthesis (Warren et al., 2016). It was developed for the treatment of Ebola and Marburg virus infections (Mulangu et al., 2019), and animal studies have shown that it is effective against the other coronavirus (Sheahan et al., 2017). In vitro studies have established its efficacy against SARS- CoV-2 (Wang et al., 2020c). An open-label trial across the United States, Europe, Canada and Japan showed clinical improvement of 36 of the 53 COVID-19 patients who were treated with a 10-day course of Remdesivir on a compassionate basis (Grein et al., 2020). However, a follow-up multi-centre, randomized, double- blinded, placebo-controlled trial of 237 patients showed that the drug was not associated with a difference in time to clinical improvement. Compared to the placebo, the drug was found to have a non-significant but, numerically faster time to clinical improvement in patients with a symptom duration of 10 days or lower (Wang et al., 2020d). Currently, Japan and the USA have allowed the use of the drug under emergency use authorization for the treatment of COVID-19. In a randomized, open-label, multi-centre Phase 3 clinical trials, a 5-day course Remdesivir brought about a significant clinical improvement compared to standard alone in patients with moderate COVID-19. This clinical study assessed the effect of 5-day (n = 191) and 10-day (n = 193) investigational Remdesivir courses plus standard of care, versus standard of care alone (n = 200) on clinical improvement on Day 11 (Maffei, 2020). In case of patients with severe disease, both 5 day and 10 courses of the drug have been found to have similar clinical outcomes, but as the study lacked placebo control, the magnitude of benefit cannot be determined (Goldman et al., 2020).
1.13.2. Umifenovir
Umifenovir, marketed as Arbidol, is a derivative of indole carboxylic acids used for the treatment of influenza A and B virus infection, and other arboviruses (Boriskin et al., 2008). It functions by incorporating into cell membranes and interfering with the hydrogen bonding network of phospholipids, blocking both the fusion of the virus to the cell membrane and the virus-endosome fusion (Villalaín, 2010). in vitro studies have established anti-viral efficacy against Ebola virus, human herpesvirus 8, hepatitis C virus, Tacaribe arena virus, SARS-CoV and SARS-CoV-2 (Pécheur et al., 2016; Dong et al., 2020; Lian et al., 2020). A retrospective study on 81 SARS-CoV-2 patients treated with umifenovir did not reveal any improvement in clinical prognosis or accelerated viral clearance (Lian et al., 2020). Currently, two randomized and open-label trials to determine the safety and efficiency of the drug are ongoing in China.
1.13.3. Favipiravir
Favipiravir, another anti-viral drug, developed by Fujifilm Toyama Chemical (as Avigan) and Zhejiang Hisun Pharmaceutical, is a pyrazinecarboxamide derivative. It is converted into an active phosphoribosylated form (favipiravir-RTP) in cells and is recognized as a substrate by viral RNA polymerase, thereby blocking the activity of RNA-dependent RNA polymerase. It was developed as a treatment against influenza. The drug is currently approved for the treatment of SARS-CoV-2 in China and Italy. A study with 80 SARS-CoV-2 patients treated using the drug has reported that better efficacy was observed in anti-viral activity and lower adverse reactions compared to the control group that was treated with lopinavir/ritonavir (Dong et al., 2020). Another prospective, multi-centre, open-label, randomized superiority study with 240 SARS-CoV-2 infected patients was conducted at three hospitals. They showed faster recovery from clinical symptoms when compared to the controls that were treated with umifenovir, even though similar numbers required the use of ventilators and oxygen (Chen et al., 2020f). There are currently six trials ongoing in China evaluating the efficiency of this drug against other antivirals for the treatment of COVID-19 and a Phase 3 clinal trial to assess its effectiveness and safety is scheduled in Japan and USA.
1.13.4. Chloroquine and hydroxychloroquine
Anti-malaria drugs, Chloroquine and Hydroxychloroquine, are lysosomotropic agents that function by increasing late endosomal and lysosomal pH, which results in impaired viral release from the endosome or lysosome (Ohkuma and Poole, 1978; Al-Bari, 2017; Fredericksen et al., 2002). in vitro studies have shown antiviral activity against SARS-CoV-2 with Hydroxychloroquine, a weak diprotic base, to have higher potency against the virus (Wang et al., 2020c; Liu et al., 2020c). In SARS-CoV-2, Chloroquine, along with its lysosomotropic activity, is believed to reduce glycosylation of ACE2 affecting the binding of the virus to the cells (Devaux et al., 2020). Furthermore, Chloroquine is also shown to block the production of pro-inflammatory cytokines such as IL-6 preventing ARDS (Savarino et al., 2003); Hydroxychloroquine was found to possess an anti- inflammatory effect on Th17-related cytokines (IL-6, IL-17 and IL-22) (da Silva et al., 2013). Initial clinical studies in China involving 100 SARS-CoV-2 infected patients, who were treated with Chloroquine, showed amelioration of pneumonia, shortened disease progression, increased resolution of lung lesions on CT, and a better virus-negative conversion (Cortegiani et al., 2020; Gao et al., 2020b). Hydroxychloroquine and combination therapy with azithromycin was found to reduce viral load in a French open-label non-randomised clinical trial and in an observational pilot study (Gautret et al., 2020a, b). Nevertheless, these studies were plagued with several limitations, such as small sample size, very short observation period, no randomisation, lack of reports on clinical progression, poorly described inclusion and exclusion criteria, and low National Early Warning Score (Guan et al., 2020; Gautret et al., 2020a, b). Another trial with 30 SARS-CoV-2 infected patients treated with Hydroxychloroquine for seven days in China and a study with effectively 10 SARS-CoV-2 patients, revealed no significant difference in the nasopharyngeal viral carriage when compared to the controls that were provided with the local standard care (Molina et al., 2020; Chen et al., 2020g). A third randomized clinical trial conducted in China with 62 patients exhibiting mild SARS-CoV-2 when treated with Hydroxychloroquine were found to have recovered faster from cough and fever when compared to the placebo. However, the result of this study cannot be extrapolated to patients with severe SARS-CoV-2 (Chen et al., 2020h). A retrospective cohort study of 1438 random sample of in-patients with laboratory confirmed SARS-CoV-2 admitted to hospitals in the New York City was conducted. It did not find any significant differences in in-hospital mortality associated with the treatment with Hydroxychloroquine, azithromycin, or both, compared to the controls where the patients were given neither of the drugs (Rosenberg et al., 2020). The US FDA and European Medicines Agency (EMA) and many other countries like India and Poland have authorized emergency use of Hydroxychloroquine to treat SARS−COV-2 infected patients. However, the FDA and EMA have issued warnings against the reported side effects of the drugs. These include abnormal electrical activity that affects the heart rhythm (QT interval prolongation, ventricular tachycardia, and ventricular fibrillation), particularly when taken at high doses or in combination with the antibiotic azithromycin. Other side effects reported are liver and kidney problems, nerve cell damage that can lead to seizures and hypoglycaemia (U.S. Food and Drug Administration, 2020; Borba et al., 2020). Around 30 clinical trials have been registered to study the effects of Hydroxychloroquine and Chloroquine independently or in combination with each other on SARS-CoV-2 have been registered in the USA and China (Sinha and Balayla, 2020). Another anti-parasitic drug, ivermectin, has been shown to be effective against SARS-CoV-2 in vitro (Caly et al., 2020). A clinical trial to assess the efficiency of ivermectin against SARS-CoV-2 has been planned to take place in Japan soon.
1.13.5. Dexamethasone
The corticosteroid, Dexamethasone, functions as an immunosuppressant. The drug is believed to modulate the lung injury caused by a dysregulated immune system, thereby reducing the progression to respiratory failure and death (Horby et al., 2020). In a randomized, controlled, open-label, adaptive, platform trial, 2104 patients treated with 6 mg of Dexamethasone (orally or intravenously) for 10 days were found to have a significantly reduced 28 day mortality rate among those receiving mechanical ventilation by 33.33 %, and by 20 % among those receiving oxygen without mechanical ventilation, compared to 4321 patients treated with standard care (Horby et al., 2020). Treatment with the drug did not provide any benefit to patients who did not require oxygen or mechanical ventilation, hinting at possible harm. The use of corticosteroid in the case of severe respiratory infections requires the use of “the right dose, at the right time, in the right patient” (Horby et al., 2020). This is because a high or an early dose may help the virus proliferate by suppressing the immune system, instead of reducing inflammation. In case of COVID-19, the peak of viral shedding is higher early in the disease. The benefit of dexamethasone when patients require respiratory support or after the first week of the disease suggest that this stage is dominated by an irrepressible immune response versus active viral replication (Horby et al., 2020). Dexamethasone is the first drug found to reduce mortality in COVID-19 (National Institute for Health Research, 2020).
1.13.6. Lopinavir/ritonavir
Lopinavir/ritonavir is a drug combination. Lopinavir is a protease inhibitor, developed by Abbott Laboratories against HIV-1 that functions by blocking essential viral proteases (Uchida et al., 2010). Due to poor pharmacokinetics, it is administered exclusively in combination with ritonavir which increases Lopinavir’s plasma half-life through inhibition of CYP3A-mediated metabolism of Lopinavir, thereby increasing its exposure and improving the anti-viral activity of the drug (Uchida et al., 2010). in vitro studies have revealed that Lopinavir inhibited the replication of the SARS-CoV-2 virus in Vero E6 cell (Choy et al., 2020). In a randomized, controlled, open-label trial with 199 patients with laboratory-confirmed SARS-CoV-2 infection, no benefit was observed with Lopinavir-ritonavir treatment beyond standard care (Cao et al., 2020b). Another single-blind randomised controlled trial in China treated 44 patients with mild/moderate COVID-19 for 14 days, or Umifenovir or standard care with no antiviral (Li et al., 2020d). In the study, no differences were found in the time taken for viral clearance, as assessed by PCR of nasopharyngeal swabs, fever, cough, or lung CT findings. Clinical status deterioration to severe/critical from mild or moderate clinical status and gastrointestinal side effects was seen highest in patients treated with Lopinavir/ritonavir when compared to Umifenovir treated or those treated with standard care and no antivirals (Li et al., 2020d). Both these randomised clinical trials suffer from small sample sizes and lack of blinding. A multi-centre, prospective, open-label, randomised, phase 2 trial in Hong Kong with 127 SARS-CoV-2 infected patients involved treatment for 14 days with only Lopinavir-Ritonavir (Control), or with a combination of Lopinavir–Ritonavir, ribavirin, an oral hepatitis C virus drug, and IFN-β1. It was found that the combination treatment was effective in reducing symptoms and viral shedding faster, as well as duration of hospital stay (Hung et al., 2020). Currently, about a dozen trials are studying the effect of the drug against SARS-CoV-2. One such study is a Phase 4 randomized controlled trial in China in which the effectiveness of lopinavir-ritonavir against influenza drugs, Umifenovir and oseltamivir, is to be studied. Another South Korean trial is looking to compare the efficacy of Lopinavir–Ritonavir against Hydroxychloroquine. The WHO SOLIDARITY trial and UK-based RECOVERY trial is looking to study the effectiveness of Lopinavir–Ritonavir independently; the WHO SOLIDARITY trial also looks to explore the drug in combination with interferon-β.
1.13.7. Darunavir
Another second-generation protease inhibitor against HIV-1, Darunavir, has shown significant inhibition of SARS-CoV-2 replication (in vitro). According to a press release by Johnson & Johnson, an unpublished single-centre, open-label, randomized, and controlled trial in China in which 30 SARS−COV-2 patients were treated with darunavir and cobicistat was not effective in treating SARS−COV-2 (Johnson and Johnson, 2020). However, a further three clinical studies in China are scheduled.
1.13.8. SNG001
SNG001, is an inhaled formulation of interferon beta, a naturally occurring anti-viral protein, which is delivered using a nebuliser (Balfour, 2020). SARS COV 2 is known to block the production of interferon beta and interferon beta production was found to be reduced in older people, this treatment aims to correct this deficiency thwarting viral strategies to evade the host immune system (University Hospital Southampton, 2020a). The SG016 clinical trial which was conducted across nine different UK hospitals in which 101 patients were randomly treated with either a placebo or SNG001, alongside the regular COVID-19 treatment, found that those who received the drug reported reduced breathlessness and where more than twice as likely to recover from COVID-19 during the duration of the study (Balfour, 2020; University Hospital Southampton, 2020a). The study also reported that patients treated with the drug and 79% lower chance of developing severe COVID-19 symptoms compared with those treated with a placebo (University of Southampton, 2020). In addition three patients died within the control group, while no deaths were reported in patients treated with the drug (Balfour, 2020). The study has now been expanded to include an additional 120 patients who have had symptoms for less than 72 hours and are aged 50 or over with a high risk of co-morbidity or aged 65 and over in their home environment (University Hospital Southampton, 2020b).
1.13.9. Tocilizumab
Other drugs currently being tested against SARS-CoV-2 include Tocilizumab, a monoclonal antibody against IL-6 developed by Roche, which is used for the treatment of moderate to severe rheumatoid arthritis by blocking IL-6 activity. The drug was found to have helped cure 19 of 20 COVID-19 patients in a trial conducted in China (Zhang et al., 2020c). Another open multi-centre randomized controlled trial French study awaits publication, in which 129 patients were split into two groups, i.e. routine treatment with and without Tocilizumab: in the group treated with Tocilizumab, the combination of ventilation requirement (mechanical or non-invasive) or death was achieved in a significantly lower proportion of patients (Assistance publique - Hôpitaux de Paris, 2020). A phase 3 trial to test its efficacy in treating patients with severe COVID-19 has been authorised by the FDA. Moreover, an Italian multi-centre, retrospective study of 544 patients with severe COVID-19 pneumonia, revealed that the use of Tocilizumab given either intravenously or subcutaneously was associated with reduced risk of mechanical ventilation and death (Guaraldi et al., 2020).
1.13.10. Anakinra
Anakinra is a recombinant IL-1 receptor antagonist that has shown promise in treating severe COVID-19 disease. In a retrospective cohort study of patients with COVID-19 and ARDS that were managed with non-invasive ventilation (outside the ICU), their treatment with high-dose anakinra was observed to be safe and associated with clinical improvement in 72 % of patients (Cavalli et al., 2020). Another study has also described the early use of anakinra in COVID-19 patients with cytokine storm syndrome (CSS) and acute hypoxic respiratory failure (AHRF) which may be beneficial in preventing the need for mechanical ventilation (Navarro‐Millán et al., 2020). These results have encouraged further clinical trials to validate its safety and efficacy (Salvi and Patankar, 2020).
1.13.11. Inhibition of Bruton tyrosine kinase
Approaches targeting inhibition of Bruton tyrosine kinase (BTK) has also shown promise. BTK plays a significant role in human innate immune responses. TLRs recognize ssRNA of viruses like SARS-CoV-2 and induce signalling via BTK- dependent activation of NF-κB, initiating a pro-inflammatory response (Feng et al., 2015; Byrne et al., 2013; Matsumoto et al., 2017; Page et al., 2018). BTK also plays a key role in the activation of the NLRP3 inflammasome, resulting in maturation and secretion of IL-1β, a key pro-inflammatory cytokine (Bittner et al., 2019; Ito et al., 2015; Liu et al., 2017). Thus, BTK seems a favourable target against the cytokine storm in COVID-19. In one study, Acalabrutinib (a selective inhibitor of BTK) was given to 19 patients with severe COVID-19 and clinical improvements were observed over a 2-week treatment period, with reduced biomarkers of inflammation (C-reactive protein and IL-6) to normal levels (Roschewski et al., 2020). Other dual inhibitors e.g. ibrutinib which target BTK/IL‐2‐inducible T‐cell kinase (ITK) signalling have also shown promise (McGee et al., 2020). In one study of patients given ibutinib for treatment of B-cell malignancies and chronic graft-versus-host disease (cGVHD), there was evidence that ibutinib may also protect against pulmonary injury in COVID-19, which these patients subsequently had, suggesting ibutinib as a possible prophylactic for vulnerable patient groups (Treon et al., 2020). Similar findings demonstrating a possible protective role of BTK inhibitors in cancer with COVID-19 have also been subsequently described (Chong et al., 2020; Paul et al., 2020; Thibaud et al., 2020). These promising findings now merit a controlled randomised trial to demonstrate efficacy and drug safety of these BTK inhibitors.
1.13.12. Intravenous immunoglobulin therapy
Intravenous immunoglobulin (IVIG) is a pooled preparation of normal IgG obtained from several thousand healthy donors. It is generally used in the immunotherapy of several autoimmune and inflammatory diseases, (Galeotti et al., 2017), and thus has been investigated for treating COVID-19 to mitigate the CSS. IVIG therapy has shown promise through several studies, although careful selection of COVID-19 patients and timing of IVIG administration appear to be the key for good clinical outcome. Preliminary findings from one multi-centre study showed that early administration of high dose IVIG improved the prognosis of critical patients with COVID-19 (Shao et al., 2020). Similarly, 3 patients with severe COVID-19 who received high-dose IVIG made a satisfactory recovery (Cao et al., 2020c). In another study, the use of IVIG as an adjuvant treatment for COVID-19 pneumonia within 48 h of admission to the ICU reduced the use of mechanical ventilation, ICU and hospital time, and the 28-day mortality rate of patients with severe COVID-19 pneumonia (Xie et al., 2020). In a case study of a COVID-19 patient with respiratory failure and shock, treatment with plasma exchange before IVIG treatment resulted in prompt recovery without the need for mechanical ventilation and may be an additional early treatment step to treat critically ill COVID-19 patients (Shi et al., 2020). IVIG treatment of severely-ill COVID-19 patients on mechanical ventilation has also shown promise. In one study of 5 patients, treatment with IVIG improved clinical and respiratory outcome, particularly saturation of O2 levels, resulting in earlier extubation of the patients (Mohtadi and Ghaysouri, 2020). Furthermore, CT graphs obtained after IVIG therapy also revealed improvements in pulmonary lesions of these patients (Mohtadi and Ghaysouri, 2020).
1.13.13. Convalescent plasma therapy
Convalescent plasma therapy (CPT) is another classical adaptive immunotherapy used for the treatment of infectious disease for over a century. It has currently been approved for COVID-19 by the FDA under compassionate use rules. The treatment involves the transfusion of high neutralizing antibody titre containing blood plasma from SARS-CoV-2 recovered patients. This provides immediate short-term immunity. This is accomplished by binding of the pathogen to the antibody, which results in the activation of the immune system causing cellular cytotoxicity, phagocytosis, or direct pathogen neutralisation. Five clinical studies, conducted involving 27 COVID-19 patients who were treated with CPT, revealed significantly lower viral titres, increased levels of neutralizing antibody, improved clinical symptoms such as apyrexia, resolved ARDS and unassisted breathing (Shen et al., 2020; Duan et al., 2020; Ye et al., 2020; Ahn et al., 2020; Zhang et al., 2020d). Among the 27 CPT-treated patients, no fatalities were recorded, and no severe adverse reactions or treatment complications associated with CPT were reported (Shen et al., 2020; Duan et al., 2020; Ye et al., 2020; Ahn et al., 2020; Zhang et al., 2020d). While providing with valuable initial data, these studies suffer from several limitations such as lack of proper control groups, non-randomized evaluations, concomitant drug treatments, poor participant selection, lack of proper CPT dosage, and duration of therapy (Rajendran et al., 2020). Three clinical trials are currently being evaluated by the FDA to test the safety and efficiency of CPT in patients who have been exposed to the virus and are at high risk of developing severe COVID-19, patients who are admitted in hospital with acute respiratory symptoms, and for COVID 19 patients under mechanical ventilation (The Lancet Haematology, 2020). Further trials are also planned or ongoing in China, Columbia, Iran, Mexico and the Netherlands (The Lancet Haematology, 2020). Early safety indicators of COVID- 19 CPT were evaluated in a study of 5000 patients and showed that the mortality rate was not unduly high and concluded that transfusion of convalescent plasma appears safe in hospitalized patients with COVID-19 (Joyner et al., 2020).
1.14. Vaccine strategies: prophylactic and therapeutic outcomes needed
While the repertoire of antivirals and repurposed drugs tested against SARS-CoV-2 are expected to help manage the disease, the development of a safe and effective vaccine would help cut down the overall number of deaths and prevent the population from getting the disease in the first place. A recent study suggested that mandatory BCG vaccination can possibly be associated in flattening the curve in the spread of COVID-19. It analysed the rate of day-wise increase in positive cases in 135 countries and deaths in 134 countries for the first 30-day period (Berg et al., 2020). While arguments for the potentially beneficial effects of pre-existing vaccines have been sporadically made, including giving MMR (Mumps, Measles and Rubella) vaccines to elderly population, generating a SARS-CoV-2 specific vaccine seems a logical and obligatory choice. As of 25 August 2020, the WHO landscape document reports 142 candidate vaccines developed on various platforms (Fig. 6 ) in preclinical stages of development: only 31 are under clinical evaluation.
Fig. 6.
Vaccine strategies for COVID-19.
(a) Viral peptide sequences can be constructed into potential epitopes with possible immunogenic capabilities to be used as a vaccine against SARS-CoV-2. (b) RNA-based vaccines such as mRNA-1273 and BNT162, which contain mRNA coding for the spike protein of SARS-CoV-2. These are often encased inside lipid vesicle. (c) DNA-based vaccine such as INO-4800 expresses variants of SARS-CoV-2 spike protein that are often inserted into cells through electroporation. (d) PiCoVacc is an inactivated virus vaccine candidate which contains purified SARS- CoV-2 virus inactivated by β-propiolactone. (e) Viral vector vaccine such as ChAdOx1-nCov19 and Ad5-nCoV are usually genetically engineered adenovirus (replication-defective) which are capable of producing SARS-CoV-2 spike protein once inside the host. (f) Once the vaccine is inside the host, antigen presenting cells (APCs) such as macrophages (MΦ) and dendritic cells engulf the virus or the proteins translated by the viral genome. SARS CoV-2 viral peptide expressed on the surface of APCs are presented to T helper (Th) cells, which further activate B cell and cytotoxic T cells (Tc). B cells secretes antibodies specific to viral S- protein which further neutralizes the virions and other viral proteins. Tc cells mount cytolytic immune response to destroy virus-infected host cells. Memory B and T cells production can further provide immunity to the host.
1.14.1. mRNA-1273
mRNA-1273 vaccine is a sequence optimized mRNA/LNP expressing a perfusion stabilized form of SARS-CoV-2 S-2 P a transmembrane anchored protein with the native furin cleavage site, developed by Moderna in collaboration with the National Institute of Allergy and Infectious Diseases Vaccine Research Center (World Health Organization, 2020c; National Institute of Allergy and Infectious Diseases (NIAID), 2020). The vaccine is undergoing an open-label phase 1 clinical trial that started in March 2020 with 45 healthy adult (18–55-year-old) volunteers for six weeks in three dose cohorts (25 μg, 100 μg and 250 μg) as two doses approximately 28 days apart via intramuscular injection in the upper arm. Three cohorts of 56−70-year-old volunteers and three cohorts of healthy volunteers aged 71 and above are being enrolled in addition to the initial volunteers. The volunteers will be followed through 12 months after the second vaccination to assess safety data, common vaccination symptoms, review trial data and advise NIAID (National Institute of Allergy and Infectious Diseases (NIAID), 2020). A Phase II trial with 600 healthy participants in two cohorts (18–55 years old adults and adults aged 55 years and above) treated with a placebo, a 50 μg or a 250 μg dose has begun from May, 29th, 2020. The in vivo studies in murine models suggested the vaccine to be immunogenic and could elicit IgG2a and IgG1 subclass S-binding antibodies. mRNA-1273 immunized mice splenocytes showed higher secretion of IFN-γ than IL-4, IL-5 or IL-3 upon re-stimulation with peptide pools (S1 and S2). A dose of 1 μg of mRNA-127 was found to induce robust CD8+T cell response to the S1 peptide pool with balanced Th1/Th2 Ab isotype response in mice. Thus, a 100 μg dose of vaccine has been decided for human trial in Phase 3 study, which is equivalent to 1 μg dose induced in mice (Corbett et al., 2020). The FDA has granted Fast Track designation for the vaccine.
1.14.2. BNT162
The Pfizer licensed BioNTech’s BNT162 vaccine development programme has developed four coronavirus vaccine candidates (World Health Organization, 2020c). Two of the vaccines contain mRNA coding for the Spike protein of SARS-CoV-2, while the other two contain only the RBD of the spike protein (Lowe, 2020). Furthermore, the four vaccine candidates are made of three different mRNA formats. Two of the vaccine are based on nucleoside modified mRNA (modRNA), which incorporates modified nucleosides in the mRNA (BioNTech, 2020). This suppresses intrinsic immune activation and the production of anti-drug antibodies against the mRNA itself (BioNTech, 2020). The suppressed immune activity against the therapeutic mRNA helps produce the antigenic protein for longer periods (BioNTech, 2020). The next vaccine candidate is based on the Optimised unmodified mRNA (uRNA) format (BioNTech, 2020). uRNA uses uridine in the mRNA, making it more immunogenic (BioNTech, 2020). Finally, the last vaccine candidate uses self-amplifying mRNA (saRNA) (BioNTech, 2020). It is based on the principle of viral replication. The saRNA, in addition to encoding a protein of interest, also encodes, replicase (BioNTech, 2020). This enables the self-amplification of the mRNA inside the cell (BioNTech, 2020). The dsRNA intermediate created during the replication of the RNA triggers an immune response making saRNA a potent activator of the immune system (BioNTech, 2020). A Phase 1/2, randomized, placebo-controlled, observer-blind, dose-finding, and vaccine candidate-selection study to evaluate the safety, tolerability, immunogenicity, and potential efficacy of the candidate in 200 healthy adult volunteers is ongoing (Zang et al., 2020).
1.14.3. Ad5-nCoV
Another frontrunner among the candidates is CanSino Bio’s Ad5-nCoV (World Health Organization, 2020c). It is a genetically engineered vaccine candidate with the replication- defective adenovirus type 5 (live virus) as the vector to express SARS-CoV-2 spike protein. This would help the body to produce neutralizing antibodies against SARS-CoV-2. It has been shown to induce a strong anti-viral activity against SARS-CoV-2 in animal and in vitro studies. A single-centre, non-random, open, and dose-escalation phase I clinical trial for recombinant novel coronavirus vaccine (adenoviral vector) in 108 healthy adults aged between 18 and 60 years were conducted. The vaccine has been administered as a liquid formulation intramuscularly in the deltoid muscle (Chinese Clinical Trial Registry, 2020). Three different doses were chosen: (a) low dose of 5 × 1010 (Widagdo et al., 2019) viral particles/0.5 mL; (b) intermediate dose of 1.5 × 1011 (World Health Organization, 2020a) viral particles/mL; and (c) high dose combines both low and intermediate dose (one in each arm). The volunteers are assessed for a period for 6 months to study any adverse reactions or other relevant outcomes (Chinese Clinical Trial Registry, 2020). Most common systematic adverse reaction observed were fever, fatigue, headache and muscular pain but with no serious adverse effect were noted within 28 days. Participants showed four-fold increase in anti-RBD antibodies in all the groups; neutralizing antibodies increased gradually being highest at 28 days post vaccination. Ad5 neutralizing antibody titres were boosted significantly post-vaccination. IL-2 and TNF-α were detected and polyfunctional memory CD4+ T cell phenotypes were higher than CD8+ T cells. This suggested Ad5 vectored COVID-19 vaccine to be immunogenic and capable of stimulating both B and T cell response. For phase 2 clinical trial, intermediate dose was chosen and is expected to be completed by 31 January 2021 (Zhu et al., 2020c). The vaccine may have some negative effects in older age people thus in the 2nd clinical trial participants above 60 years will be included. T cell response peaked earlier from 14th day after the 1st shot of vaccine whereas the antibodies production level peaked at 28th day post vaccination. The study also highlighted the possibility of negative effect on vaccine elicited immune response due to pre-existing Ad5 immunity (Zhu et al., 2020c).
1.14.4. ChAdOx1-nCov19
ChAdOx1-nCov19 is being developed by Oxford University, UK (World Health Organization, 2020c). It is a replication deficient simian adenovirus vector ChAdOx1, containing full length S-protein of SARS CoV-2 along with a tissue plasminogen activator leader sequence. The vaccine is reported to be effective in inducing an antiviral response in animal models (van et al., 2020). ChAdOx1-nCov19 was found to be immunogenic in mice mounting robust anti-viral response. Single dose of this vaccine was capable of inducing humoral and cellular immune response in rhesus macaques (van et al., 2020). A phase I/II single-blinded, randomised, multi-centre study to determine efficacy, safety and immunogenicity of the vaccine in about 1090 healthy adult volunteers aged 18–55 years was initiated on April 23rd, 2020 (University of Oxford, 2020). The volunteers have been subjected to either one dose of 5 × 1010 vp of ChAdOx1 nCoV- 19, an additional booster dose of 2.5 × 1010 vp of ChAdOx1 nCoV-19, or a control of MenACWY vaccine delivered intramuscularly (University of Oxford, 2020). The volunteers were assessed for a period for 6 months to study any adverse reactions or other relevant outcomes (Folegatti et al., 2020). The results showed increase in S-specific antibodies with a single dose by 28th day and increase in neutralizing antibodies with booster dose in all participants. ChAdOx1-nCov19 was also capable of inducing heightened effector T-cell response quite earlier than antibody response. T cell response peaked on day 14th and sustained up to 56 days. The results showed ChAdOx1 nCoV-19 vaccine to be safe, tolerant and immunogenic, which further supported phase 3 trial which is now underway (Folegatti et al., 2020).
1.14.5. PiCoVacc
PiCoVacc is a purified inactivated SARS-CoV-2 vaccine candidate which is capable of inducing neutralizing antibodies in mice, rats, and nonhuman primates specific to SARS-CoV-2. CN2 strain of SARS CoV-2 virus was chosen to develop PiCoVacc which was inactivated with β-propiolactone. This inactivated vaccine candidate was able to produce about 10- fold higher S-specific antibody titres in murine model when compared to COVID-19 recovered patients. Efficacy of PiCoVacc was also tested in rhesus macaques with an intramuscular low (1.5 μg), medium (3 μg) and high (6 μg) dose administered three times (0, 7th and 14th day) and on day 22nd SARS CoV-2 CN1 strain was inoculated through intratracheal (lungs) route. All vaccinated macaques showed protection towards SARS CoV-2 infection and their viral loads declined significantly. No notable haemato- and histopathological changes were observed; human clinical trials are awaited (Gao et al., 2020c).
1.14.6. DNA vaccine
A group of US scientists have come up with a series of prototype DNA vaccines expressing variants of the SARS-CoV-2 spike protein. The efficacy of the DNA vaccine candidates was evaluated in 35 rhesus macaques (6−12- year-old). Intramuscular dose (5 mg) of DNA vaccine was administered, followed by booster dose on 3rd week and antigenic challenge (1.5 × 108 viral particles) on 6th week (both intranasal and intratracheal route) (Hu et al., 2017). DNA vaccine was found to be protective with dramatic reduction of viral replication and enhanced production of S- specific binding as well as neutralizing antibodies compared to controls. The study has not yet addressed the possibility of mutations that may emerge in escaping neutralizing antibodies, though it seems to be protective in primates against SARS-CoV-2 (Yu et al., 2020).
1.14.7. INO-4800
INO-4800, developed by Inovio, is a DNA vaccine candidate (World Health Organization, 2020c). The optimized Spike protein of SARS-CoV-2 virus DNA plasmids are introduced into cells by the use of a proprietary platform, CELLECTRA®, via electroporation (Pharmaceuticals I, 2020). Once inserted, the plasmids are expected to strengthen the body's own natural response. A Phase I Open-label study to evaluate the safety, tolerability and immunogenicity of INO-4800 as a prophylactic vaccine against SARS-CoV-2 in 40 healthy volunteers aged 18–50 years is ongoing (Pharmaceuticals I, 2020). The volunteers will be treated with either one or two doses of 1 mg of vaccine administered intradermally followed by electroporation the following day (Pharmaceuticals I, 2020). The volunteers will be assessed for a period for 1 year to study any adverse reactions or other relevant outcomes (Pharmaceuticals I, 2020). Once the initial safety and immunogenicity of the vaccine are satisfied, Phase II efficacy studies are planned.
1.14.8. Epitope mapping
Qualitative and quantitative properties of CD4+ and CD8+ T cell responses in COVID-19 and prophylactic vaccine development necessitate identifying viral regions and potential epitopes. Thus, a total of 423 peptides (15- to 18-mer), which span the full proteome of the SARS-CoV-2 excluding ORF-1, were designed and used to assess the memory T cell responses upon challenge on 42 patients following recovery from COVID-19. 39 peptides were identified containing CD4+ and/or CD8+ epitopes. The memory of T cell responses from convalescent individuals with COVID-19 was found to be greater in severe COVID-19 cases compared to mild ones. Immunodominant epitope clusters and peptides were most markedly observed to belong to spike, M, and ORF3 proteins. In about 35 % of study groups, strong CD8+ T cell responses specific to the NP protein were observed, suggesting the possibility of inclusion of non-spike proteins in future COVID-19 vaccine design (Peng et al., 2020). In another study, a comprehensive immunogenicity map of the SARS-CoV-2 virus was carried out; 65 peptide sequences (33-mers) were generated based on computational algorithms. A single 33-mer peptide containing multiple epitopes that can possibly present on HLA class I and class II across majority of population and provide long-term immunity in most people acting as B and T cell epitopes had been identified. This in silico analysis needs further evaluation for safety and efficacy as a vaccine (Yarmarkovich et al., 2020).
2. Conclusion
In an unprecedented short span of time and speed since the beginning of the COVID-19 pandemic, significant progress has been made in our understanding of the pathogenesis of SARS-CoV-2 infection. However, there are endless unanswered questions; hopefully and most likely, they will be answered in near future. Why is there a huge population that are asymptomatic carriers? What are the genetic contributors to susceptibility and resistance to developing COVID-19? How are pregnant women and young children so resilient to developing COVID-symptoms? What happens during the period of latency, i.e. between being infected and showing symptoms? How is the lung surfactant system affected during severe symptoms? What triggers thrombotic microangiopathy in addition to complement activation? On the adaptive immune aspects, what variations exist within the population in terms of the proportion of neutralising antibodies? Persistence of neutralising antibodies and recall memory magnitude following second infection (on vaccination trials) will yield critical information about how to fine tune the dose and duration of vaccination strategies. In this acute crisis, a number of existing drugs have been repurposed empirically; clinical trials have yielded variable results. It is becoming clear that combination therapies are more likely to be successful. Deciphering, at high resolution, the mechanisms and consequences of host-pathogen interactions in COVID-19 will lead to novel therapies and preventative vaccination strategies.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
Figures 2–6 and the graphical abstract were created using BioRender.com.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.imbio.2020.152008.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Ahn J.Y., Sohn Y., Lee S.H., Cho Y., Hyun J.H., Baek Y.J., Jeong S.J., Kim J.H., Ku N.S., Yeom J.S., et al. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J. Korean Med. Sci. 2020;35 doi: 10.3346/JKMS.2020.35.E149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbar A.N., Gilroy D.W. Aging immunity may exacerbate COVID-19. Science. 2020 doi: 10.1126/science.abb0762. [DOI] [PubMed] [Google Scholar]
- Al-Bari M.A.A. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol. Res. Perspect. 2017;5 doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., Al-Dawood A., Al-Qahtani S., Al-Omari A., Al-Hameed F., et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg Infect Dis. 2016 doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assistance publique – Hôpitaux de Paris . 2020. Le Tocilizumab Améliore Significativement Le Pronostic Des Patients Avec Pneumonie COVID Moyenne Ou Sévère | APHP.https://www.aphp.fr/contenu/le-tocilizumab-ameliore-significativement-le-pronostic-des-patients-avec-pneumonie- covid Available at: [Accessed May 15, 2020] [Google Scholar]
- Aziz M., Perisetti A., Lee-Smith W.M., Gajendran M., Bansal P., Goyal H. Taste Changes (Dysgeusia) in COVID-19: a systematic review and metaanalysis. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J.A., Klinkenberg D., Wallinga J. Incubation period of 2019 novel coronavirus (2019- nCoV) infections among travellers from Wuhan, China, 20 28 January 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baergen R.N., Heller D.S. Placental pathology in Covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 2020;23:177–180. doi: 10.1177/1093526620925569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour H. Inhaled interferon beta therapy shows promise in COVID -19 trial [WWW Document] Eur. Pharm. Rev. 2020 URL https://www.europeanpharmaceuticalreview.com/news/123994/inhaled-interferon-beta-therapy-shows-promise-in-covid-19-trial/ (accessed 8.19.20) [Google Scholar]
- Bandiera L., Pavar G., Pisetta G., Otomo S., Mangano E., Seckl J.R., Digard P., Molinari E., Menolascina F., Maria I., 1# V., Viola M. Face coverings and respiratory tract droplet dispersion. medRxiv. 2020 doi: 10.1101/2020.08.11.20145086. 2020.08.11.20145086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangash M.N., Patel J., Parekh D. COVID-19 and the liver: little cause for concern. Lancet Gastroenterol. Hepatol. 2020;5:529–530. doi: 10.1016/S2468-1253(20)30084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes B.J., Adrover J.M., Baxter- Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belhadjer Z., Méot M., Bajolle F., Khraiche D., Legendre A., Abakka S., Auriau J., Grimaud M., Oualha M., Beghetti M., et al. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020 doi: 10.1161/circulationaha.120.048360. [DOI] [PubMed] [Google Scholar]
- Belot A., Antona D., Renolleau S., Javouhey E., Hentgen V., Angoulvant F., Delacourt C., Iriart X., Ovaert C., Bader-Meunier B., et al. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Eurosurveillance. 2020 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belouzard S., Chu V.C., Whittaker G.R. Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proc Natl Acad Sci U S A. 2009;106:5871–5876. doi: 10.1073/pnas.0809524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. 2020. Mandated Bacillus Calmette-guérin (BCG) Vaccination Predicts Flattened Curves for the Spread of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram S., Glowacka I., Muller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C., et al. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85:13363–13372. doi: 10.1128/jvi.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian H., Zheng Z.-H., Wei D., Zhang Z., Kang W.-Z., Hao C.-Q., Dong K., Kang W.-Z., Xia J.-L., Miao J.-L., et al. Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.21.20040691. 03.21.20040691. [DOI] [Google Scholar]
- BioNTech. mRNA therapeutics | BioNTech. Available at: https://biontech.de/how-we-translate/mrna-therapeutics [Accessed May 18, 2020].
- Bittner Z., Liu X., Dickhöfer S., Kalbacher H., Bosch K., Andreeva L., Marcu A., Stevanovic S., Mangan M., Düwell P., et al. BTK operates a phospho-tyrosine switch to regulate NLRP3 inflammasome activity. bioRxiv. 2019 doi: 10.1101/864702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitz M.J., Rochelson B., Minkoff H., Meirowitz N., Prasannan L., London V., Rafael T.J., Chakravarthy S., Bracero L.A., Wasden S.W., et al. Maternal mortality among women with COVID-19 admitted to the intensive care unit. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam S.R., Kaveri S.V., Sakuntabhai A., Gilardin L., Bayry J. Adjunct Immunotherapies for the Management of Severely Ill COVID-19 Patients. Cell Reports Med. 2020 doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomi L., Ghilardi L., Arnoldi E., Tondini Ca, Bettini Ac. A rapid fatal evolution of Coronavirus Disease-19 (COVID-19) in an advanced lung cancer patient with a long time response to nivolumab. J. Thorac. Oncol. 2020;15:e83–e85. doi: 10.1016/j.jtho.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boom B.W., Mommaas M., Daha M.R., Vermeer B.J. Complement-mediated endothelial cell damage in immune complex vasculitis of the skin: ultrastructurallocalization of the membrane attack complex. J. Invest. Dermatol. 1989;93:68S–72S. doi: 10.1111/1523-1747.ep12581073. [DOI] [PubMed] [Google Scholar]
- Borba M.G.S., Val F de A., Sampaio V.S., Alexandre M.A.A., Melo G.C., Brito M., MPG Mourão, Sousa J.D.B., Baia-da-Silva D.C., Guerra M.V.F., et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS-CoV-2) infection: preliminary safety results of a randomized, double- blinded. phase IIb cl. 2020 doi: 10.1101/2020.04.07.20056424. [DOI] [Google Scholar]
- Boriskin Y., Leneva I., Pecheur E.-I., Polyak S. Arbidol: A Broad-Spectrum Antiviral Compound that Blocks Viral Fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- Bos E.C.W., Luytjes W., Van Der Meulen H., Koerten H.K., Spaan W.J.M. The production of recombinant infectious DI-particles of a murine coronavirus in the absence of helper virus. Virology. 1996;218:52–60. doi: 10.1006/viro.1996.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino J.A., Logan H.L., Lacny J.J., Gallagher T.M. Envelope protein palmitoylations are crucial for murine coronavirus assembly. J. Virol. 2008;82:2989–2999. doi: 10.1128/jvi.01906-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böttcher-Friebertshäuser E., Klenk H.D., Garten W. Activation of influenza viruses by proteases from host cells and bacteria in the human airway epithelium. Pathog. Dis. 2013;69:87–100. doi: 10.1111/2049-632X.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard J.S., Jones N., Lake I., Hooper L., Hunter P. Facemasks and similar barriers to prevent respiratory illness such as COVID -19: A rapid systematic review. medRxiv. 2020 doi: 10.1101/2020.04.01.20049528. 2020.04.01.20049528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun J., Loyal L., Frentsch M., Wendisch D., Georg P., Kurth F., Hippenstiel S., Dingeldey M., Kruse B., Fauchere F., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020 doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- Breslin N., Baptiste C., Gyamfi-Bannerman C., Miller R., Martinez R., Bernstein K., Ring L., Landau R., Purisch S., Friedman A.M., et al. Coronavirus disease 2019 infection among asymptomatic and symptomatic pregnant women: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce C., Grimes Z., Pujadas E., Ahuja S., Beasley M.B., Albrecht R., Hernandez T., Stock A., Zhao Z., Al Rasheed M., et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. medRxiv. 2020 doi: 10.1101/2020.05.18.20099960. [DOI] [Google Scholar]
- Bugge T.H., Antalis T.M., Wu Q. Type II transmembrane serine proteases. J. Biol. Chem. 2009;284:23177–23181. doi: 10.1074/jbc.R109.021006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne J.C., Ní Gabhann J., Stacey K.B., Coffey B.M., McCarthy E., Thomas W., Jefferies C.A. Bruton’s tyrosine kinase is required for apoptotic cell uptake via regulating the phosphorylation and localization of Calreticulin. J. Immunol. 2013;190:5207–5215. doi: 10.4049/jimmunol.1300057. [DOI] [PubMed] [Google Scholar]
- Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J., Kapahi P., Lithgow G.J., Melov S., Newman J.C., Verdin E. From discoveries in ageing research to therapeutics for healthy ageing. Nature. 2019;571:183–192. doi: 10.1038/s41586-019-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. COVID-19: immunopathology and its implications for therapy. Nat. Rev. Immunol. 2020;20:269–270. doi: 10.1038/s41577-020-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Y., Li L., Feng Z., Wan S., Huang P., Sun X., Wen F., Huang X., Ning G., Wang W. Comparative genetic analysis of the novel coronavirus (2019- nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., et al. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao W., Liu X., Bai T., Fan H., Hong K., Song H., Han Y., Lin L., Ruan L., Li T. High-dose intravenous immunoglobulin as a therapeutic option for deteriorating patients with coronavirus disease 2019. Open Forum Infect. Dis. 2020 doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capes A., Bailly S., Hantson P., Gerard L., Laterre P.F. COVID-19 infection associated with autoimmune hemolytic anemia. Ann. Hematol. 2020 doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvelli J., Demaria O., Vély F., Batista L., Benmansour N.C., Fares J., Carpentier S., Thibult M.-L., Morel A., Remark R., et al. Association of COVID-19 inflammation with activation of the C5a-C5aR1 axis. Nature. 2020 doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catanzaro M., Fagiani F., Racchi M., Corsini E., Govoni S., Lanni C. Immune response in COVID-19: addressing a pharmacological challenge by targeting pathways triggered by SARS-CoV-2. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-0191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC . 2020. If You Are Pregnant, Breastfeeding, or Caring for Young Children.https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/pregnancy-breastfeeding.html? CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fprepare%2Fpregnancy-breastfeeding.html Available at: [Accessed June 22, 2020] [Google Scholar]
- Chaiworapongsa T., Espinoza J., Yoshimatsu J., Kim Y.M., Bujold E., Edwin S., Yoon B.H., Romero R. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J. Matern. Fetal. Neonatal. Med. 2002;11:368–373. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- Chan J.F.W., Yuan S., Kok K.H., To K.K.W., Chu H., Yang J., Xing F., Liu J., Yip C.C.Y., Poon R.W.S., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., Tostanoski L.H., Yu J., Maliga Z., Nekorchuk M., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science (80-) 2020 doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C. Hemostasis based on a novel “two-path unifying theory” and classification of hemostatic disorders. Blood Coagul. Fibrinolysis. 2018;29:573–584. doi: 10.1097/MBC.0000000000000765. [DOI] [PubMed] [Google Scholar]
- Chang J.C. Acute respiratory distress syndrome as an organ phenotype of vascular microthrombotic disease: based on hemostatic theory and endothelial molecular pathogenesis. Clin Appl Thromb. 2019;25 doi: 10.1177/1076029619887437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J.C. Sepsis and septic shock: endothelial molecular pathogenesis associated with vascular microthrombotic disease. Thromb. J. 2019;17:1–19. doi: 10.1186/s12959-019-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.K., Sue S.C., Yu T.H., Hsieh C.M., Tsai C.K., Chiang Y.C., Lee S.J., Hsiao H.H., Wu W.J., Chang W.L., et al. Modular organization of SARS coronavirus nucleocapsid protein. J. Biomed. Sci. 2006;13:59–72. doi: 10.1007/s11373-005-9035-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Peng H., Wang L., Zhao Y., Zeng L., Gao H., Liu Y. Infants born to mothers with a new coronavirus (COVID-19) Front. Pediatr. 2020;8 doi: 10.3389/fped.2020.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Liu M., Zhang Z., Qiao K., Huang T., Chen M., Xin N., Huang Z., Liu L., Zhang G., et al. Ocular manifestations of a hospitalised patient with confirmed 2019 novel coronavirus disease. Br. J. Ophthalmol. 2020;104:748–751. doi: 10.1136/bjophthalmol-2020-316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Guo J., Wang C., Luo F., Yu X., Zhang W., Li J., Zhao D., Xu D., Gong Q., et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395:809–815. doi: 10.1016/S0140-6736(20)30360-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Huang J., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Zhang J., et al. Favipiravir versus arbidol for COVID-19: a randomized clinical trial. medRxiv. 2020;2020 doi: 10.1101/2020.03.17.20037432. 03.17.20037432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu D., Liu L., Liu P., Xu Q., Xia L., Ling Y., Huang D., Song S., Zhang D., et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease-19 (COVID-19) J Zhejiang Univ (Med Sci) 2020;49:1–10. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Hu J., Zhang Z., Jiang S., Han S., Yan D., Zhuang R., Hu B., Zhang Z. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial. medRxiv. 2020;7 doi: 10.1101/2020.03.22.20040758. 2020.03.22.20040758. [DOI] [Google Scholar]
- Chinese Clinical Trial Registry . 2020. A randomized, Double-blinded, Placebo-controlled Phase II Clinical Trial for Recombinant Novel Coronavirus (2019-nCOV) Vaccine (Adenovirus Vector)http://www.chictr.org.cn/showprojen.aspx?proj=52006 Available at: [Accessed May 18, 2020] [Google Scholar]
- Chong E.A., Roeker L.E., Shadman M., Davids M.S., Schuster S.J., Mato A.R. BTK inhibitors in Cancer patients with COVID-19: “The winner will be the one who controls that Chaos” (Napoleon bonaparte) Clin. Cancer Res. 2020;26:3514–3516. doi: 10.1158/1078-0432.CCR-20-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy K.T., Wong A.Y.L., Kaewpreedee P., Sia S.F., Chen D., Hui K.P.Y., Chu D.K.W., Chan M.C.W., Cheung P.P.H., Huang X., et al. Remdesivir, lopinavir, emetine, and homoharringtonine inhibit SARS-CoV-2 replication in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu D.K., Akl E.A., Duda S., Solo K., Yaacoub S., Schünemann H.J. COVID-19 Systematic Urgent Review Group Effort (SURGE) study authors. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. Lancet (London, England) 2020 doi: 10.1016/S0140-6736(20)31142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu H., Chan J.F.W., Wang Y., Yuen T.T.T., Chai Y., Hou Y., Shuai H., Yang D., Hu B., Huang X., et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: an ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colavita F., Lapa D., Carletti F., Lalle E., Bordi L., Marsella P., Nicastri E., Bevilacqua N., Giancola Ml, Corpolongo A., et al. SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Intern. Med. 2020 doi: 10.7326/M20-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus C., Brust K.B., Arroliga A.C. Novel coronavirus: an emerging global threat. Baylor Univ Med Cent Proc. 2019;33(2020):209–212. doi: 10.1080/08998280.2020.1731272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett K.S., Edwards D., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., et al. SARS-CoV-2 mRNA vaccine development enabled by prototype pathogen preparedness. bioRxiv Prepr Serv Biol. 2020 doi: 10.1101/2020.06.11.145920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corse E., Machamer C.E. Infectious Bronchitis Virus E Protein Is Targeted to the Golgi Complex and Directs Release of Virus-Like Particles. J. Virol. 2000;74:4319–4326. doi: 10.1128/jvi.74.9.4319-4326.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutard B., Valle C., de Lamballerie X., Canard B., Seidah N.G., Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res. 2020;176 doi: 10.1016/j.antiviral.2020.104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., Panigada M., Aliberti S., Blasi F., Tedesco F., et al. Complement activation in patients with COVID-19: a novel therapeutic target. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico F., Baumgart D.C., Danese S., Peyrin- Biroulet L. Diarrhea during COVID-19 infection: pathogenesis, epidemiology, prevention and management. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva J.C., Mariz H.A., da Rocha Júnior L.F., de Oliveira P.S.S., Dantas A.T., ALBP Duarte, Pitta I da R., Galdino S.L., Pitta MG da R. Hydroxychloroquine decreases Th17-related cytokines in systemic lupus erythematosus and rheumatoid arthritis patients. Clinics. 2013;68:766–771. doi: 10.6061/clinics/2013(06)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlenski R., Tsankov N. COVID-19 pandemic and the skin: what should dermatologists know? Clin. Dermatol. 2020 doi: 10.1016/j.clindermatol.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daruich A., Martin D., Bremond-Gignac D. Unilateral conjunctivitis as first presentation of Coronavirus Disease 2019 (COVID-19): a telemedicine diagnosis. J. Fr. Ophtalmol. 2020;43:e167–e168. doi: 10.1016/j.jfo.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J., Abul H.T., Milton A.S., Rotondo D. Cytokines and cytokine inducers stimulate prostaglandin E2 entry into the brain. Pflugers Arch Eur J Physiol. 2001;442:526–533. doi: 10.1007/s004240100572. [DOI] [PubMed] [Google Scholar]
- de Bont C.M., Boelens W.C., Pruijn G.J.M. NETosis, complement, and coagulation: a triangular relationship. Cell. Mol. Immunol. 2019 doi: 10.1038/s41423-018-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Haan C.A.M., Rottier P.J.M. Faculty of Veterinary Medicine, Utrecht University; 3584 CL Utrecht, The Netherlands.): 2020. “Molecular Interactions in the Assembly of Coronaviruses,” in Advances in Virus Research (Virology Division, Department of Infectious Diseases and Immunology; pp. 165–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maeyer R.P.H., van de Merwe R.C., Louie R., Bracken O.V., Devine O.P., Goldstein D.R., Uddin M., Akbar A.N., Gilroy D.W. Blocking elevated p38 MAPK restores efferocytosis and inflammatory resolution in the elderly. Nat. Immunol. 2020;21:615–625. doi: 10.1038/s41590-020-0646-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E., Imbert I., Coutard B., Bouvet M., Selisko B., Alvarez K., Gorbalenya A.E., Snijder E.J., Canard B. Coronavirus nonstructural protein 16 is a Cap-0 binding enzyme possessing (Nucleoside-2′O)- Methyltransferase activity. J. Virol. 2008;82:8071–8084. doi: 10.1128/jvi.00407-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W., Bao L., Liu J., Xiao C., Liu J., Xue J., Lv Q., Qi F., Gao H., Yu P., et al. Primary exposure to SARS-CoV-2 protects against reinfection in rhesus macaques. Science. 2020 doi: 10.1126/science.abc5343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux C.A., Rolain J.M., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao B., Feng Z., Wang C., Wang H., Liu L., Wang C., Wang R., Liu Y., Liu Y., Wang G., et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. medRxiv. 2020 doi: 10.1101/2020.03.04.20031120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias F., Almeida N., Wawrzeniack I., Nery P. Role of multiple organ dysfunction syndrome in ARDS mortality. Crit Care. 2002;6:P2. doi: 10.1186/cc1662. [DOI] [Google Scholar]
- Didangelos A. COVID-19 hyperinflammation: what about neutrophils? mSphere. 2020 doi: 10.1128/mSphere.00367-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diurno F., Numis F.G., Porta G., Cirillo F., Maddaluno S., Ragozzino A., Negri P.D.E., Gennaro C.D.I., Pagano A., Allegorico E., et al. Eculizumab treatment in patients with COVID-19: preliminary results from real life ASL Napoli 2 Nord experience. Eur. Rev. Med. Pharmacol. Sci. 2020 doi: 10.26355/EURREV_202004_20875. [DOI] [PubMed] [Google Scholar]
- Dockery D.M., Rowe S.G., Murphy M.A., Krzystolik M.G. The ocular manifestations and transmission of COVID-19: recommendations for prevention. J. Emerg. Med. 2020 doi: 10.1016/j.jemermed.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Draper D., McGregor J., Hall J., Jones W., Beutz M., Phillip Heine R., Porreco R. Elevated protease activities in human amnion and chorion correlate with preterm premature rupture of membranes. Am. J. Obstet. Gynecol. 1995;173:1506–1512. doi: 10.1016/0002-9378(95)90640-1. [DOI] [PubMed] [Google Scholar]
- Drosten C., Günther S., Preiser W., Van der Werf S., Brodt H.R., Becker S., Rabenau H., Panning M., Kolesnikova L., Fouchier R.A.M., et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- Drosten C., Meyer B., Müller M.A., Corman V.M., Al-Masri M., Hossain R., Madani H., Sieberg A., Bosch B.J., Lattwein E., et al. Transmission of MERS- coronavirus in household contacts. N. Engl. J. Med. 2014 doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Du L., He Y., Zhou Y., Liu S., Zheng B.J., Jiang S. The spike protein of SARS-CoV - A target for vaccine and therapeutic development. Nat. Rev. Microbiol. 2009;7:226–236. doi: 10.1038/nrmicro2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Zhu F., Guo F., Yang B., Wang T. Detection of antibodies against SARS-CoV-2 in patients with COVID- 19. J. Med. Virol. 2020 doi: 10.1002/jmv.25820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K., Liu B., Li C., Zhang H., Yu T., Qu J., Zhou M., Chen L., Meng S., Hu Y., et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., et al. Multisystem inflammatory syndrome in children in New York State. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert D.M., Kim P.S. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 2001;70:777–810. doi: 10.1146/annurev.biochem.70.1.777. [DOI] [PubMed] [Google Scholar]
- Epand R.M. Fusion peptides and the mechanism of viral fusion. Biochim. Biophys. Acta Biomembr. 2003;1614:116–121. doi: 10.1016/s0005-2736(03)00169-x. [DOI] [PubMed] [Google Scholar]
- Escher R., Breakey N., Lämmle B. Severe COVID-19 infection associated with endothelial activation. Thromb. Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esper F., Shapiro E.D., Weibel C., Ferguson D., Landry M.L., Kahn J.S. Association between a novel human coronavirus and kawasaki disease. J. Infect. Dis. 2005 doi: 10.1086/428291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagone P., Ciurleo R., Lombardo S.D., Iacobello C., Palermo C.I., Shoenfeld Y., Bendtzen K., Bramanti P., Nicoletti F. Transcriptional landscape of SARS-CoV-2 infection dismantles pathogenic pathways activated by the virus, proposes unique sex-specific differences and predicts tailored therapeutic strategies. Autoimmun. Rev. 2020;19 doi: 10.1016/j.autrev.2020.102571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy D.H., El‐Amawy H.S., El‐Samongy M.A., Fouda A.A., Soliman S.H., El‐Kady A., Farnetani F., Conti A., Zoeir A., Eissa A., et al. COVID‐19 and dermatology: a comprehensive guide for dermatologists. J. Eur. Acad. Dermatol. Venereol. 2020 doi: 10.1111/jdv.16545. jdv.16545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr A.R., Perlman S. Department of Microbiology, University of Iowa Carver College of Medicine; Iowa City, IA, 52242, USA: 2020. “Coronaviruses: an Overview of Their Replication and Pathogenesis,” in Coronaviruses: Methods and Protocols; pp. 1–23. [DOI] [Google Scholar]
- Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., Newburger J.W., Kleinman L.C., Heidemann S.M., Martin A.A., et al. Multisystem inflammatory syndrome in U.S. Children and adolescents. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M., Chen J.Y., Weissman-Tsukamoto R., Volkmer J.P., Ho P.Y., McKenna K.M., Cheshier S., Zhang M., Guo N., Gip P., et al. Macrophages eat cancer cells using their own calreticulin as a guide: roles of TLR and Btk. Proc Natl Acad Sci U S A. 2015;112:2145–2150. doi: 10.1073/pnas.1424907112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Jia Y., Ph D., Zhu H., Ph D., Hu K., Ph D. Clinical characteristics of 82 death cases with COVID-19. medRxiv. 2020;2020 doi: 10.1101/2020.02.26.20028191. 02.26.20028191. [DOI] [Google Scholar]
- Fischer F., Stegen C.F., Masters P.S., Samsonoff W.A. Analysis of constructed e gene mutants of mouse hepatitis virus confirms a pivotal role for e protein in coronavirus assembly. J. Virol. 1998;72:7885–7894. doi: 10.1128/jvi.72.10.7885-7894.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint E.J., Cerdeira A.S., Redman C.W., Vatish M. The role of angiogenic factors in the management of preeclampsia. Acta Obstet. Gynecol. Scand. 2019;98:700–707. doi: 10.1111/aogs.13540. [DOI] [PubMed] [Google Scholar]
- Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS- CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020 doi: 10.1016/s0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Vander Heide R.S. Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. medRxiv. 2020;2020 doi: 10.1101/2020.04.06.20050575. 04.06.20050575. [DOI] [Google Scholar]
- Franceschi C., Garagnani P., Vitale G., Capri M., Salvioli S. Inflammaging and ‘Garb-aging.’. Trends Endocrinol. Metab. 2017;28:199–212. doi: 10.1016/j.tem.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Fredericksen B.L., Wei B.L., Yao J., Luo T., Garcia J.V. Inhibition of Endosomal/Lysosomal degradation increases the infectivity of human immunodeficiency virus. J. Virol. 2002;76:11440–11446. doi: 10.1128/JVI.76.22.11440-11446.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat. Rev. Rheumatol. 2020 doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeotti C., Kaveri S.V., Bayry J. IVIG-mediated effector functions in autoimmune and inflammatory diseases. Int. Immunol. 2017 doi: 10.1093/intimm/dxx039. [DOI] [PubMed] [Google Scholar]
- Galván Casas C., Català A., Carretero Hernández G., Rodríguez-Jiménez P., Fernández Nieto D., Rodríguez-Villa Lario A., Navarro Fernández I., Ruiz-Villaverde R., Falkenhain D., Llamas Velasco M., et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br. J. Dermatol. 2020 doi: 10.1111/bjd.19163. bjd.19163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gando S., Fujishima S., Saitoh D., Shiraishi A., Yamakawa K., Kushimoto S., Ogura H., Abe T., Mayumi T., Sasaki J., et al. The significance of disseminated intravascular coagulation on multiple organ dysfunction during the early stage of acute respiratory distress syndrome. Thromb. Res. 2020;191:15–21. doi: 10.1016/j.thromres.2020.03.023. [DOI] [PubMed] [Google Scholar]
- Gao J., Lu G., Qi J., Li Y., Wu Y., Deng Y., Geng H., Li H., Wang Q., Xiao H., et al. Structure of the fusion core and inhibition of fusion by a heptad repeat peptide derived from the S protein of middle east respiratory syndrome coronavirus. J. Virol. 2013;87:13134–13140. doi: 10.1128/jvi.02433-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T., Hu M., Zhang X., Li H., Zhu L., Liu H., Dong Q., Zhang Z., Wang Z., Hu Y., et al. Highly pathogenic coronavirus N protein aggravates lung injury by MASP-2-mediated complement over-activation. medRxiv. 2020;2020 doi: 10.1101/2020.03.29.20041962. 03.29.20041962. [DOI] [Google Scholar]
- Gao J., Tian Z., Yang X. Breakthrough: Chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci. Trends. 2020;14:72–73. doi: 10.5582/BST.2020.01047. [DOI] [PubMed] [Google Scholar]
- Gao Q., Bao L., Mao H., Wang L., Xu K., Yang M., Li Y., Zhu L., Wang N., Lv Z., et al. Development of an inactivated vaccine candidate for SARS-CoV- 2. Science. 2020 doi: 10.1126/science.abc1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinassi L.G., Souza C.O.S., Sales- Campos H., Fonseca S.G. Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front. Immunol. 2020 doi: 10.3389/fimmu.2020.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Sevestre J., Mailhe M., Doudier B., Aubry C., Amrane S., et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: a pilot observational study. Travel Med. Infect. Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gavriilaki E., Brodsky R.A. Severe COVID-19 infection and thrombotic microangiopathy: success doesn’t come easily. Br. J. Haematol. 2020 doi: 10.1111/bjh.16783. [DOI] [PubMed] [Google Scholar]
- Ghinai I., McPherson T.D., Hunter J.C., Kirking H.L., Christiansen D., Joshi K., Rubin R., Morales-Estrada S., Black S.R., Pacilli M., et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet. 2020;395:1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacka I., Bertram S., Muller M.A., Allen P., Soilleux E., Pfefferle S., Steffen I., Tsegaye T.S., He Y., Gnirss K., et al. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J. Virol. 2011;85:4122–4134. doi: 10.1128/jvi.02232-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman J.D., Lye D.C.B., Hui D.S., Marks K.M., Bruno R., Montejano R., Spinner C.D., Galli M., Ahn M.-Y., Nahass R.G., et al. Remdesivir for 5 or 10 Days in Patients with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., de Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.L., Lauber C., Leontovich A.M., Neuman B.W., et al. The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V.D., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement activation contributes to severe acute respiratory syndrome coronavirus pathogenesis. MBio. 2018;9 doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., et al. Compassionate use of remdesivir for patients with severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/nejmoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove J., Marsh M. The cell biology of receptor-mediated virus entry. J. Cell Biol. 2011;195:1071–1082. doi: 10.1083/jcb.201108131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guaraldi G., Meschiari M., Cozzi- Lepri A., Milic J., Tonelli R., Menozzi M., Franceschini E., Cuomo G., Orlando G., Borghi V., et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020 doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T., Yamamoto N., Sasazuki T., Ishizaka Y. Modulation of TNF-α-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-α production and facilitates viral entry. Proc Natl Acad Sci U S A. 2008;105:7809–7814. doi: 10.1073/pnas.0711241105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt D., Hudson L., Weaver L.J., Craddock P., Jacob H. Association of complement activation and elevated plasma-C5a with adult respiratory distress syndrome. Lancet. 1980;315:947–949. doi: 10.1016/s0140-6736(80)91403-8. [DOI] [PubMed] [Google Scholar]
- He Y., Wang Z., Li F., Shi Y. Public health might be endangered by possible prolonged discharge of SARS-CoV-2 in stool. J. Infect. 2020;80:e18–e19. doi: 10.1016/j.jinf.2020.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms J., Kremer S., Merdji H., Clere-Jehl R., Schenck M., Kummerlen C., Collange O., Boulay C., Fafi-Kremer S., Ohana M., et al. Neurologic features in severe SARS-COV-2 infection. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heurich A., Hofmann- Winkler H., Gierer S., Liepold T., Jahn O., Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J. Virol. 2014;88:1293–1307. doi: 10.1128/jvi.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine- Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., et al. First case of 2019 novel coronavirus in the United States. N. Engl. J. Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Whitehead E., Kumar B.N. Early recovery following new onset anosmia during the COVID-19 pandemic - an observational cohort study. J. Otolaryngol. Head Neck Surg. 2020;49:26. doi: 10.1186/s40463-020-00423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horby P., Lim W.S., Emberson J., Mafham M., Bell J., Linsell L., Staplin N., Brightling C., Ustianowski A., Elmahi E., et al. Effect of dexamethasone in hospitalized patients with COVID-19: preliminary report. medRxiv. 2020;2020 doi: 10.1101/2020.06.22.20137273. 06.22.20137273. [DOI] [Google Scholar]
- Hosier H., Farhadian S., Morotti R., Deshmukh U., Lu-Culligan A., Campbell K., Yasumoto Y., Vogels C., Casanovas-Massana A., Vijayakumar P., et al. SARS-CoV-2 infection of the placenta. medRxiv. 2020;2020 doi: 10.1101/2020.04.30.20083907. 04.30.20083907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Zeng L.P., Lou Yang X., Ge X.Y., Zhang W., Li B., Xie J.Z., Shen X.R., Zhang Y.Z., Wang N., et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., et al. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;0 doi: 10.1016/s0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst K.R., Koetzner C.A., Masters P.S. Identification of in vivo-interacting domains of the murine coronavirus nucleocapsid protein. J. Virol. 2009;83:7221–7234. doi: 10.1128/jvi.00440-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip W.K.E., Chan K.H., Law H.K.W., Tso G.H.W., Kong E.K.P., Wong W.H.S., To Y.F., Yung R.W.H., Chow E.Y., Au K.L., et al. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Shichita T., Okada M., Komine R., Noguchi Y., Yoshimura A., Morita R. Bruton’s tyrosine kinase is essential for NLRP3 inflammasome activation and contributes to ischaemic brain injury. Nat. Commun. 2015;6:1–11. doi: 10.1038/ncomms8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Java A., Apicelli A.J., Liszewski M.K., Coler-Reilly A., Atkinson J.P., Kim A.H., Kulkarni H.S. The complement system in COVID-19: friend and foe? JCI Insight. 2020 doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson T., Jones M., Al Ansari L., Bawazeer G., Beller E., Clark J., Conly J., Del Mar C., Dooley E., Ferroni E., Glasziou P., Hoffman T., Thorning S., Van Driel M. Physical interventions to interrupt or reduce the spread of respiratory viruses. Part 1 - Face masks, eye protection and person distancing: systematic review and meta-analysis. medRxiv. 2020 doi: 10.1101/2020.03.30.20047217. 2020.03.30.20047217. [DOI] [Google Scholar]
- Jiang Y., Li Y., Zhu B. T-cell exhaustion in the tumor microenvironment. Cell Death Dis. 2015 doi: 10.1038/cddis.2015.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y.H., Cai L., Cheng Z.S., Cheng H., Deng T., Fan Y.P., Fang C., Huang D., Huang L.Q., Huang L.Q., et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil. Med. Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.M., Bai P., He W., Wu F., Liu X.F., Han D.M., Liu S., Yang J.K. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Heal. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, Johnson . 2020. Lack of Evidence to Support Use of Darunavir-based Treatments for SARS-CoV-2.https://www.jnj.com/lack-of-evidence-to-support-darunavir-based-hiv-treatments-for-coronavirus Available at: [Accessed May 15, 2020] [Google Scholar]
- Joyner M.J., Wright R.S., Fairweather D., Senefeld J.W., Bruno K.A., Klassen S.A., Carter R.E., Klompas A.M., Wiggins C.C., Shepherd J.R.A., et al. Early safety indicators of COVID-19 convalescent plasma in 5,000 patients. J. Clin. Invest. 2020 doi: 10.1172/jci140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam Y.W., Okumura Y., Kido H., Ng L.F.P., Bruzzone R., Altmeyer R. Cleavage of the SARS coronavirus spike glycoprotein by airway proteases enhances virus entry into human bronchial epithelial cells in vitro. PLoS One. 2009;4:e7870. doi: 10.1371/journal.pone.0007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck J.G., Makino S., Soe L.H., Fleming J.O., Stohlman S.A., Lai M.M. University of Southern California, School of Medicine; Los Angeles 90033.): 2020. RNA Recombination of coronavirus.,” in Advances in Experimental Medicine and Biology (Department of Microbiology; pp. 99–107. [DOI] [PubMed] [Google Scholar]
- Kerr H., Richards A. Complement-mediated injury and protection of endothelium: lessons from atypical haemolytic uraemic syndrome. Immunobiology. 2012;217:195–203. doi: 10.1016/j.imbio.2011.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Jm, Chung Ys, Jo Hj, Lee Nj, Kim Jm S., Woo Sh, Park S., Kim Jm W., Kim Hm, Han Mg. Vol. 11. Osong Public Heal Res Perspect; 2020. pp. 3–7. (Identification of Coronavirus Isolated from a Patient in Korea With Covid-19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D., Lee J.Y., Yang J.S., Kim J.W., Kim V.N., Chang H. The architecture of SARS-CoV-2 transcriptome. Cell. 2020;181:914–921. doi: 10.1016/j.cell.2020.04.011. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., et al. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb. Res. 2020;191:148–150. doi: 10.1016/j.thromres.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer S., Lersy F., de Sèze J., Ferré J.-C., Maamar A., Carsin-Nicol B., Collange O., Bonneville F., Adam G., Martin-Blondel G., et al. Brain MRI findings in severe COVID-19: a retrospective observational study. Radiology. 2020 doi: 10.1148/radiol.2020202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krempl C., Schultze B., Laude H., Herrler G. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 1997;71:3285–3287. doi: 10.1128/jvi.71.4.3285-3287.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P.J.M., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 1994;124:55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksiazek T.G., Erdman D., Goldsmith C.S., Zaki S.R., Peret T., Emery S., Tong S., Urbani C., Comer J.A., Lim W., et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., Huan Y., Yang P., Zhang Y., Deng W., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J.H., Li W., Choe H., Farzan M. Angiotensin-converting enzyme 2: a functional receptor for SARS coronavirus. Cell. Mol. Life Sci. 2004;61:2738–2743. doi: 10.1007/s00018-004-4242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuri-Cervantes L., Pampena M.B., Meng W., Rosenfeld A.M., Ittner C.A.G., Weisman A.R., Agyekum R.S., Mathew D., Baxter A.E., Vella L.A., et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci. Immunol. 2020 doi: 10.1126/sciimmunol.abd7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.M., Baric R.S., Makino S., Keck J.G., Egbert J., Leibowitz J.L., Stohlman S.A. Recombination between nonsegmented RNA genomes of murine coronaviruses. J. Virol. 1985;56:449–456. doi: 10.1128/jvi.56.2.449-456.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam L.M., Murphy S.J., Kuri-Cervantes L., Weisman A.R., Ittner C.A.G., Reilly J.P., Pampena M.B., Betts M.R., Wherry E.J., Song W.-C., et al. Erythrocytes reveal complement activation in patients with COVID-19. medRxiv Prepr Serv Heal Sci. 2020 doi: 10.1101/2020.05.20.20104398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois Pf, Gawryl Ms. Accentuated formation of the terminal C5b-9 complement complex in patient plasma precedes development of the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1988;138:368–375. doi: 10.1164/ajrccm/138.2.368. [DOI] [PubMed] [Google Scholar]
- Lao W.P., Imam S.A., Nguyen S.A. Anosmia, hyposmia, and dysgeusia as indicators for positive SARS-CoV-2 infection. World J Otorhinolaryngol - Head Neck Surg. 2020 doi: 10.1016/j.wjorl.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarian G., Quinquenel A., Bellal M., Siavellis J., Jacquy C., Re D., Merabet F., Mekinian A., Braun T., Damaj G., et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br. J. Haematol. 2020 doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre A., Marteau V., Emmerich J., Zins M. Concomitant acute aortic thrombosis and pulmonary embolism complicating COVID-19 pneumonia. Diagn. Interv. Imaging. 2020;101:321–322. doi: 10.1016/j.diii.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020 doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- Lee Y.L., Liao C.H., Liu P.Y., Cheng C.Y., Chung M.Y., Liu C.E., Chang S.Y., Hsueh P.R. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J. Infect. 2020;0 doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J., Li J., Li X., Qi X. CT imaging of the 2019 novel coronavirus (2019-NCoV) pneumonia. Radiology. 2020;295:18. doi: 10.1148/radiol.2020200236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F. Evidence for a common evolutionary origin of coronavirus spike protein receptor-binding subunits. J. Virol. 2012;86:2856–2858. doi: 10.1128/jvi.06882-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Li W., Farzan M., Harrison S.C. Structural biology: structure of SARS coronavirus spike receptor-binding domain complexed with receptor. Science (80-) 2005;309:1864–1868. doi: 10.1126/science.1116480. [DOI] [PubMed] [Google Scholar]
- Li Q., Guan X., Wu P., Wang X., Zhou L., Tong Y., Ren R., Leung K.S.M., Lau E.H.Y., Wong J.Y., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N. Engl. J. Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Xu S., Yu M., Wang K., Tao Y., Zhou Y., Shi J., Zhou M., Wu B., Yang Z., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020 doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Chen Y., Liu H., Jia Y., Li F., Wang W., Wu J., Wan Z., Cao Y., Zeng R. Immune dysfunction leads to mortality and organ injury in patients with COVID-19 in China: insights from ERS-COVID-19 study. Signal Transduct. Target. Ther. 2020 doi: 10.1038/s41392-020-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Xie Z., Lin W., Cai W., Wen C., Guan Y., Mo X., Wang J., Wang Y., Peng P., et al. An exploratory randomized, controlled study on the efficacy and safety of lopinavir/ritonavir or arbidol treating adult patients hospitalized with mild/moderate COVID-19 (ELACOI) medRxiv. 2020;2020 doi: 10.1101/2020.03.19.20038984. 03.19.20038984. [DOI] [Google Scholar]
- Li N., Han L., Peng M., Lv Y., Ouyang Y., Liu K., Yue L., Li Q., Sun G., Chen L., et al. Maternal and neonatal outcomes of pregnant women with COVID-19 pneumonia: a case-control study. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian N., Xie H., Lin S., Huang J., Zhao J., Lin Q. Umifenovir treatment is not associated with improved outcomes in patients with coronavirus disease 2019: a retrospective study. Clin. Microbiol. Infect. 2020;107:84–94. doi: 10.1016/j.cmi.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Z., Xu X., Gan Q., Zhang L., Luo L., Tang X., Liu J. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur. J. Radiol. 2020;126 doi: 10.1016/j.ejrad.2020.108956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkage Linkage Camden COVID-19 Sub-study. https://www.linkage-camden.com/covid-19-sub-study/ Available at: [Accessed August 9, 2020]
- Liu X., Pichulik T., Wolz O.O., Dang T.M., Stutz A., Dillen C., Delmiro Garcia M., Kraus H., Dickhöfer S., Daiber E., et al. Human NACHT, LRR, and PYD domain–containing protein 3 (NLRP3) inflammasome activity is regulated by and potentially targetable through Bruton tyrosine kinase. J. Allergy Clin. Immunol. 2017;140:1054–1067. doi: 10.1016/j.jaci.2017.01.017. e10. [DOI] [PubMed] [Google Scholar]
- Liu L., Wei Q., Lin Q., Fang J., Wang H., Kwok H., Tang H., Nishiura K., Peng J., Tan Z., et al. Anti-spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS-CoV infection. JCI Insight. 2019 doi: 10.1172/jci.insight.123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Liu Y., Xiang P., Pu L., Xiong H., Li C., Zhang M., Tan J., Xu Y., Song R., et al. Neutrophil-to-lymphocyte ratio predicts critical illness patients with 2019 coronavirus disease in the early stage. J. Transl. Med. 2020 doi: 10.1186/s12967-020-02374-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020 doi: 10.1161/circulationaha.120.047549. CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo M.W., Kemper C., Woodruff T.M. COVID-19: complement, coagulation, and collateral damage. J. Immunol. 2020 doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loganathan S.K., Schleicher K., Malik A., Quevedo R., Langille E., Teng K., Oh R.H., Rathod B., Tsai R., Samavarchi-Tehrani P., et al. Rare driver mutations in head and neck squamous cell carcinomas converge on NOTCH signaling. Science. 2020;367:1264–1269. doi: 10.1126/science.aax0902. [DOI] [PubMed] [Google Scholar]
- Long Q.X., Liu B.Z., Deng H.J., Wu G.C., Deng K., Chen Y.K., Liao P., Qiu J.F., Lin Y., Cai X.F., et al. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020:1–4. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Lowe D. A close look at the frontrunning coronavirus vaccines As of may 1 (updated) Sci. Transl. Med. 2020 doi: 10.1101/2020.04.13.036293V1. [DOI] [Google Scholar]
- Lu G., Wang Q., Gao G.F. Bat-to- human: spike features determining “host jump” of coronaviruses SARS-CoV, MERS-CoV, and beyond. Trends Microbiol. 2015;23:468–478. doi: 10.1016/j.tim.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D., Sang L., Du S., Li T., Chang Y., Yang X.A. Asymptomatic COVID-19 infection in late pregnancy indicated no vertical transmission. J. Med. Virol. 2020 doi: 10.1002/jmv.25927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Yin J., Qian Y., Wu Y. Clinical characteristics and prognosis in cancer patients with COVID-19: a single center’s retrospective study. J. Infect. 2020;0 doi: 10.1016/j.jinf.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madu I.G., Roth S.L., Belouzard S., Whittaker G.R. Characterization of a highly conserved domain within the severe acute respiratory syndrome coronavirus spike protein S2 domain with characteristics of a viral fusion peptide. J. Virol. 2009;83:7411–7421. doi: 10.1128/jvi.00079-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei D. 2020. Gilead Announces Results from Phase 3 Trial of Investigational Antiviral Remdesivir in Patients With Severe COVID-19.https://www.gilead.com/news-and-press/press-room/press-releases/2020/4/gilead-announces-results-from-phase-3-trial-of-investigational-antiviral-remdesivir-in-patients-with-severe-covid-19 Available at: [Accessed June 4, 2020] [Google Scholar]
- Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter- Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl. Res. 2020 doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannick J.B., Morris M., Hockey H.U., Roma G., Beibel M., Kulmatycki K., Watkins M., Shavlakadze T., Zhou W., Quinn D., et al. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
- Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., Chang J., Hong C., Zhou Y., Wang D., et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason R.J. Pathogenesis of COVID-19 from a cell biology perspective. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00607-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastaglio S., Ruggeri A., Risitano A.M., Angelillo P., Yancopoulou D., Mastellos D.C., Huber-Lang M., Piemontese S., Assanelli A., Garlanda C., et al. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Takeda Y., Tatematsu M., Seya T. Toll-like receptor 3 signal in dendritic cells benefits cancer immunotherapy. Front. Immunol. 2017;8:1897. doi: 10.3389/fimmu.2017.01897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Ujike M., Morikawa S., Tashiro M., Taguchi F. Protease- mediated enhancement of severe acute respiratory syndrome coronavirus infection. Proc Natl Acad Sci U S A. 2005;102:12543–12547. doi: 10.1073/pnas.0503203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama S., Nagata N., Shirato K., Kawase M., Takeda M., Taguchi F. Efficient activation of the severe acute respiratory syndrome coronavirus spike protein by the transmembrane protease TMPRSS2. J. Virol. 2010;84:12658–12664. doi: 10.1128/jvi.01542-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumder A., Arora M., Bharadiya V., Berry P., Agarwal M., Behera P., Shewade H.D., Lohiya A., Gupta M., Rao A., et al. SARS-CoV-2 epidemic in India: epidemiological features and in silico analysis of the effect of interventions. F1000Research. 2020;9:315. doi: 10.12688/f1000research.23496.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee M.C., August A., Huang W. BTK/ITK dual inhibitors: modulating immunopathology and lymphopenia for COVID-19 therapy. J. Leukoc. Biol. 2020 doi: 10.1002/JLB.5COVR0620-306R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menter T., Haslbauer J.D., Nienhold R., Savic S., Hopfer H., Deigendesch N., Frank S., Turek D., Willi N., Pargger H., et al. Post-mortem examination of COVID19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings of lungs and other organs suggesting vascular dysfunction. Histopathology. 2020 doi: 10.1111/his.14134. his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merle N.S., Church S.E., Fremeaux- Bacchi V., Roumenina L.T. Complement system part I - molecular mechanisms of activation and regulation. Front. Immunol. 2015;6:262. doi: 10.3389/fimmu.2015.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein S. Acute anosmia from COVID-19 infection. Pract. Neurol. 2020 doi: 10.1136/practneurol-2020-002583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mille J.K., Whittaker G.R. Host cell entry of Middle East respiratory syndrome coronavirus after two-step, furin-mediated activation of the spike protein. Proc Natl Acad Sci U S A. 2014;111:15214–15219. doi: 10.1073/pnas.1407087111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohtadi N., Ghaysouri A. Shirazi S, Sara Ansari, shafiee e, Bastani e, kokhazadeh t, tavan H. Recovery of severely ill COVID-19 patients by intravenous immunoglobulin (IVIG) treatment: a case series. Virology. 2020;548:1–5. doi: 10.1016/j.virol.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon C. Fighting COVID-19 exhausts t cells. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0304-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.B., June C.H. Cytokine release syndrome in severe COVID-19. Science (80-) 2020;368:473–474. doi: 10.1126/science.abb8925. [DOI] [PubMed] [Google Scholar]
- Mueller A.L., Mcnamara M.S., Sinclair D.A. Why does COVID-19 disproportionately affect older people? Aging (Albany NY) 2020 doi: 10.18632/aging.103344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulangu S., Dodd L.E., Davey R.T., Mbaya O.T., Proschan M., Mukadi D., Manzo M.L., Nzolo D., Oloma A.T., Ibanda A., et al. A randomized, controlled trial of Ebola virus disease therapeutics. N. Engl. J. Med. 2019;381:2293–2303. doi: 10.1056/NEJMoa1910993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y. Kawasaki disease: epidemiology and the lessons from it. Int. J. Rheum. Dis. 2018 doi: 10.1111/1756-185X.13211. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Yashiro M., Uehara R., Sadakane A., Tsuboi S., Aoyama Y., Kotani K., Tsogzolbaatar E.O., Yanagawa H. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J. Epidemiol. 2012 doi: 10.2188/jea.JE20110126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nal B., Chan C., Kien F., Siu L., Tse J., Chu K., Kam J., sabelle Staropoli, Crescenzo-Chaigne B., Escriou N., et al. Differential maturation and subcellular localization of severe acute respiratory syndrome coronavirus surface proteins S, M and E. J. Gen. Virol. 2005;86:1423–1434. doi: 10.1099/vir.0.80671-0. [DOI] [PubMed] [Google Scholar]
- National Institute for Health Research . 2020. First Drug to Reduce Mortality in Hospitalised Patients With Respiratory Complications of COVID-19 Found.https://www.nihr.ac.uk/news/first-drug-to-reduce-mortality-in-hospitalised-patients-with-respiratory-complications-of-covid-19-found/25061 Available at: [Accessed June 23, 2020] [Google Scholar]
- National Institute of Allergy and Infectious Diseases (NIAID) 2020. Phase I, Open-Label, Dose-Ranging Study of the Safety and Immunogenicity of 2019-nCoV Vaccine (mRNA-1273) in Healthy Adults.https://clinicaltrials.gov/ct2/show/record/NCT04283461 Available at: [Accessed May 18, 2020] [Google Scholar]
- Navarro‐Millán I., Sattui S., Lakhanpal A., Zisa D., Siegel C., Crow M. Use of Anakinra to prevent mechanical ventilation in severe COVID‐19: a case series. Arthritis Rheumatol. 2020 doi: 10.1002/art.41422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman B.W., Kiss G., Kunding A.H., Bhella D., Baksh M.F., Connelly S., Droese B., Klaus J.P., Makino S., Sawicki S.G., et al. A structural analysis of M protein in coronavirus assembly and morphology. J. Struct. Biol. 2011;174:11–22. doi: 10.1016/j.jsb.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Zugich J., Knox K.S., Rios C.T., Natt B., Bhattacharya D., Fain M.J. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. GeroScience. 2020;42:505–514. doi: 10.1007/s11357-020-00186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan D. CEBM; Oxford: 2020. Loss of Smell and Taste As Symptoms of COVID-19: What Does the Evidence Say?https://www.cebm.net/covid-19/loss-of-smell-and-taste-as-symptoms-of-covid-19-what-does-the-evidence-say/ Available at: [Accessed June 29, 2020] [Google Scholar]
- O’Day M.P. Cardio-respiratory physiological adaptation of pregnancy. Semin. Perinatol. 1997;21:268–275. doi: 10.1016/S0146-0005(97)80069-9. [DOI] [PubMed] [Google Scholar]
- Ohkuma S., Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75:3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver C.M., Campbell M., Dulan O., Hamilton N., Birchall M. Appearance and management of COVID-19 laryngo-tracheitis: two case reports. F1000Research. 2020;9:310. doi: 10.12688/f1000research.23204.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y., Marimuthu K. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) from a Symptomatic Patient. JAMA - J Am Med Assoc. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottestad W., Seim M., Mæhlen J.O. Covid-19 med stille hypoksemi. Tidsskr. Nor. Laegeforen. 2020;140 doi: 10.4045/tidsskr.20.0299. [DOI] [PubMed] [Google Scholar]
- Page T.H., Urbaniak A.M., Espirito Santo A.I., Danks L., Smallie T., Williams L.M., Horwood N.J. Bruton’s tyrosine kinase regulates TLR7/8-induced TNF transcription via nuclear factor-κB recruitment. Biochem. Biophys. Res. Commun. 2018;499:260–266. doi: 10.1016/j.bbrc.2018.03.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Chen D., Xia Y., Wu X., Li T., Ou X., Zhou L., Liu J. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect. Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigada M., Bottino N., Tagliabue P., Grasselli G., Novembrino C., Chantarangkul V., Pesenti A., Peyvandi F., Tripodi A. Hypercoagulability of COVID-19 patients in Intensive Care Unit. A Report of Thromboelastography Findings and other Parameters of Hemostasis. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmigiani A., Alcaide M.L., Freguja R., Pallikkuth S., Frasca D., Fischl M.A., Pahwa S. Impaired antibody response to influenza vaccine in HIV-Infected and uninfected aging women is associated with immune activation and inflammation. PLoS One. 2013;8 doi: 10.1371/journal.pone.0079816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., Jayaseelan D.L., Kumar G., Raftopoulos R.E., Zambreanu L., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020 doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul S., Rausch C.R., Jain N., Kadia T., Ravandi F., DiNardo C.D., Welch M.A., Dabaja B.S., Daver N., Garcia-Manero G., et al. Treating leukemia in the time of COVID-19. Acta Haematol. 2020:1–13. doi: 10.1159/000508199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pécheur E.-I., Borisevich V., Halfmann P., Morrey J.D., Smee D.F., Prichard M., Mire C.E., Kawaoka Y., Geisbert T.W., Polyak S.J. The synthetic antiviral drug arbidol inhibits globally prevalent pathogenic viruses. J. Virol. 2016;90:3086–3092. doi: 10.1128/jvi.02077-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisajovich S.G., Shai Y. Viral fusion proteins: multiple regions contribute to membrane fusion. Biochim. Biophys. Acta Biomembr. 2003;1614:122–129. doi: 10.1016/S0005-2736(03)00170-6. [DOI] [PubMed] [Google Scholar]
- Peng Y., Mentzer A.J., Liu G., Yao X., Yin Z., Dong D., Dejnirattisai W., Rostron T., Supasa P., Liu C., et al. Broad and strong memory CD4 + and CD8 + T cells induced by SARS-CoV-2 in UK convalescent COVID-19 patients. bioRxiv Prepr Serv Biol. 2020;2020 doi: 10.1101/2020.06.05.134551. 06.05.134551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005 doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S., Netland J. Coronaviruses post-SARS: update on replication and pathogenesis. Nat. Rev. Microbiol. 2009;7:439–450. doi: 10.1038/nrmicro2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pharmaceuticals I . 2020. Innovations C for EP. Safety, Tolerability and Immunogenicity of INO-4800 for COVID-19 in Healthy Volunteers.https://clinicaltrials.gov/ct2/show/record/NCT04336410 Available at: [Accessed May 18, 2020] [Google Scholar]
- Pia L. SARS-CoV-2-reactive T cells in patients and healthy donors. Nat. Rev. Immunol. 2020 doi: 10.1038/s41577-020-0333-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouletty M., Borocco C., Ouldali N., Caseris M., Basmaci R., Lachaume N., Bensaid P., Pichard S., Kouider H., Morelle G., et al. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raamsman M.J.B., Locker J.K., de Hooge A., de Vries A.A.F., Griffiths G., Vennema H., Rottier P.J.M. Characterization of the coronavirus mouse hepatitis virus strain A59 small membrane protein e. J. Virol. 2000;74:2333–2342. doi: 10.1128/jvi.74.5.2333-2342.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rábano-Suárez P., Bermejo-Guerrero L., Méndez-Guerrero A., Parra-Serrano J., Toledo-Alfocea D., Sánchez-Tejerina D., Santos-Fernández T., Folgueira-López M.D., Gutiérrez-Gutiérrez J., Ayuso-García B., et al. Generalized myoclonus in COVID-19. Neurology. 2020 doi: 10.1212/wnl.0000000000009829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radzikowska U., Ding M., Tan G., Zhakparov D., Peng Y., Wawrzyniak P., Wang M., Li S., Morita H., Altunbulakli C., et al. Distribution of ACE2, CD147, CD26 and other SARS‐CoV‐2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID‐19 risk factors. Allergy. 2020 doi: 10.1111/all.14429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendran K., Narayanasamy K., Rangarajan J., Rathinam J., Natarajan M., Ramachandran A. Convalescent plasma transfusion for the treatment of COVID- 19: systematic review. J. Med. Virol. 2020 doi: 10.1002/jmv.25961. jmv.25961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlall V., Thangaraj P.M., Meydan C., Foox J., Butler D., Kim J., May B., De Freitas J.K., Glicksberg B.S., Mason C.E., et al. Immune complement and coagulation dysfunction in adverse outcomes of SARS-CoV-2 infection. Nat. Med. 2020:1–7. doi: 10.1038/s41591-020-1021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective. J. Eur. Acad. Dermatol. Venereol. 2020;34:e212–e213. doi: 10.1111/jdv.16387. [DOI] [PubMed] [Google Scholar]
- Reguera J., Santiago C., Mudgal G., Ordoño D., Enjuanes L., Casasnovas J.M. Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., et al. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020 doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockx B., Kuiken T., Herfst S., Bestebroer T., Lamers M.M., Oude Munnink B.B., de Meulder D., van Amerongen G., van den Brand J., Okba N.M.A., et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science (80-) 2020:368. doi: 10.1126/science.abb7314. eabb7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roschewski M., Lionakis M.S., Sharman J.P., Roswarski J., Goy A., Monticelli M.A., Roshon M., Wrzesinski S.H., Desai J.V., Zarakas M.A., et al. Inhibition of Bruton tyrosine kinase in patients with severe COVID-19. Sci. Immunol. 2020 doi: 10.1126/sciimmunol.abd0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., Dehovitz J., et al. Association of treatment with hydroxychloroquine or azithromycin with in- hospital mortality in patients with COVID-19 in New York State. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., et al. Transmission of 2019-NCOV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382:970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal College of Obstetricians and Gynaecologists . 2020. Royal College of Midwives and Royal College of Paediatrics and Child Health, with Input from the Royal College of Anaesthetists PHE and HPS. COVID-19 Virus Infection and Pregnancy.https://www.rcog.org.uk/en/guidelines-research-services/guidelines/coronavirus-pregnancy/covid-19-virus-infection-and-pregnancy/ Available at: [Google Scholar]
- Salvi R., Patankar P. Emerging pharmacotherapies for COVID-19. Biomed. Pharmacother. 2020 doi: 10.1016/j.biopha.2020.110267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar M., Niranjan N., Banyal P.K. Mechanisms of hypoxemia. Lung India. 2017;34:47–60. doi: 10.4103/0970-2113.197116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarino A., Boelaert J.R., Cassone A., Majori G., Cauda R. Effects of chloroquine on viral infections: An old drug against today’s diseases? Lancet Infect. Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawicki S.G., Sawicki D.L., Siddell S.G. A contemporary view of coronavirus transcription. J. Virol. 2007;81:20–29. doi: 10.1128/jvi.01358-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwegmann-Weßels C., Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj. J. 2006;23:51–58. doi: 10.1007/s10719-006-5437-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna P.B., Hofmann M.A., Brian D.A. Minus-strand copies of replicating coronavirus mRNAs contain antileaders. J. Virol. 1991;65:320–325. doi: 10.1128/jvi.65.1.320-325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanes E.D., Mithal L.B., Otero S., Azad H.A., Miller E.S., Goldstein J.A. Placental pathology in COVID- 19. Am. J. Clin. Pathol. 2020;154:23–32. doi: 10.1093/ajcp/aqaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020 doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., Li F. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020 doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao Z., Feng Y., Zhong L., Xie Q., Lei M., Liu Z., Wang C., Ji J., Li W., Liu H., et al. Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: a multicenter retrospective cohort study. SSRN Electron J. 2020 doi: 10.2139/ssrn.3576827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., et al. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Y., Li J., Yuan J., Wang F., Li D., Yang M., Xing L., et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA - J Am Med Assoc. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Zhou C., He P., Huang S., Duan Y., Wang X., Lin K., Zhou C., Zhang X., Zha Y. Successful treatment with plasma exchange followed by intravenous immunoglobulin in a critically ill patient with COVID-19. Int. J. Antimicrob. Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilts J., Wright G.J. No evidence for basigin/CD147 as a direct SARS-CoV-2 spike binding receptor. bioRxiv. 2020;2020 doi: 10.1101/2020.07.25.221036. 07.25.221036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020 doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirato K., Imada Y., Kawase M., Nakagaki K., Matsuyama S., Taguchi F. Possible involvement of infection with human coronavirus 229E, but not NL63, in Kawasaki disease. J. Med. Virol. 2014 doi: 10.1002/jmv.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulla A., Heald- Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85:873–882. doi: 10.1128/jvi.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman S.T., Rowley A.H. Kawasaki disease: insights into pathogenesis and approaches to treatment. Nat. Rev. Rheumatol. 2015 doi: 10.1038/nrrheum.2015.54. [DOI] [PubMed] [Google Scholar]
- Simmons G., Gosalia D.N., Rennekamp A.J., Reeves J.D., Diamond S.L., Bates P. Inhibitors of cathepsin L prevent severe acute respiratory syndrome coronavirus entry. Proc Natl Acad Sci U S A. 2005;102:11876–11881. doi: 10.1073/pnas.0505577102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N., Balayla G. Hydroxychloroquine and covid-19. Postgrad. Med. J. 2020 doi: 10.1136/postgradmedj-2020-137785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkey R.G., Guzeloglu- Kayisli O., Arlier S., Guo X., Semerci N., Moore R., Ozmen A., Larsen K., Nwabuobi C., Kumar D., et al. Thrombin-induced decidual colony-stimulating Factor-2 promotes abruption-related preterm birth by weakening fetal membranes. Am. J. Pathol. 2020;190:388–399. doi: 10.1016/j.ajpath.2019.10.020. [DOI] [PubMed] [Google Scholar]
- Snijder E.J., van der Meer Y., Zevenhoven-Dobbe J., Onderwater J.J.M., van der Meulen J., Koerten H.K., Mommaas A.M. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/jvi.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H., Yang M., Wan C., Yi L.X., Tang F., Zhu H.Y., Yi F., Yang H.C., Fogo A.B., Nie X., et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020 doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Zhang X., Chen X., Chen L., Deng C., Zou X., Liu W., Yu H. The infection evidence of SARS-COV-2 in ocular surface: a single-center cross-sectional study. medRxiv. 2020;2020 doi: 10.1101/2020.02.26.20027938. 02.26.20027938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sungnak W., Huang N., Bécavin C., Berg M., Queen R., Litvinukova M., Talavera-López C., Maatz H., Reichart D., Sampaziotis F., et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat. Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja V. Sex hormones determine immune response. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T., Bidon M., Jaimes J.A., Whittaker G.R., Daniel S. Coronavirus membrane fusion mechanism offers a potential target for antiviral development. Antiviral Res. 2020 doi: 10.1016/j.antiviral.2020.104792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay M.Z., Poh C.M., Rénia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat. Rev. Immunol. 2020:1–12. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Lancet Haematology The resurgence of convalescent plasma therapy. Lancet Haematol. 2020;7:e353. doi: 10.1016/S2352-3026(20)30117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud S., Tremblay D., Bhalla S., Zimmerman B., Sigel K., Gabrilove J. Protective role of Bruton tyrosine kinase inhibitors in patients with chronic lymphocytic leukaemia and COVID‐19. Br. J. Haematol. 2020;190:e73–e76. doi: 10.1111/bjh.16863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoms M., Buschauer R., Ameismeier M., Koepke L., Denk T., Hirschenberger M., Kratzat H., Hayn M., Mackens-Kiani T., Cheng J., et al. Structural basis for translational shutdown and immune evasion by the Nsp1 protein of SARS-CoV-2. Science (80-) 2020 doi: 10.1126/science.abc8665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To K.K.W., Tsang O.T.Y., Leung W.S., Tam A.R., Wu T.C., Lung D.C., Yip C.C.Y., Cai J.P., Chan J.M.C., Chik T.S.H., et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect. Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur. J. Cell Biol. 1984;33:281–293. http://www.ncbi.nlm.nih.gov/pubmed/6325194 Available at: [PubMed] [Google Scholar]
- Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., et al. Guillain–Barré syndrome associated with SARS-CoV-2. N. Engl. J. Med. 2020;382:2574–2576. doi: 10.1056/NEJMc2009191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toubiana J., Poirault C., Corsia A., Bajolle F., Fourgeaud J., Angoulvant F., Debray A., Basmaci R., Salvador E., Biscardi S., et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid- 19 pandemic in Paris, France: prospective observational study. BMJ. 2020 doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trad A.T.A., Ibirogba E.R., Elrefaei A., Narang K., Tonni G., Picone O., Suy A., Carreras E., Kilby M.D., Ruano R. SARS-CoV-2 in Pregnancy: Where and What Is the Evidence? SSRN Electron J. 2020:1–9. doi: 10.2139/ssrn.3576828. [DOI] [Google Scholar]
- Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L., Meid K., Yang G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020 doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University Hospital Southampton . 2020. Southampton researchers trial inhaled therapy for COVID-19 [WWW Document] URL https://www.uhs.nhs.uk/AboutTheTrust/Newsandpublications/Latestnews/2020/March/Southampton-researchers-trial-inhaled-therapy-for-COVID-19.aspx (accessed 8.19.20) [Google Scholar]
- University Hospital Southampton . 2020. Interferon beta coronavirus drugs trial expansion home test | University of Southampton [WWW Document] URL https://www.southampton.ac.uk/news/2020/05/synairgen-trial-expansion.page (accessed 8.19.20) [Google Scholar]
- University of Southampton 2020 . 2020. Encouraging Results From Trial Of Inhaled Drug To Help COVID-19 Patients | University of Southampton [WWW Document] URL https://www.southampton.ac.uk/news/2020/07/synairgen-results.page (accessed 8.19.20) [Google Scholar]
- U.S. Food & Drug Administration . 2020. FDA Drug Safety Communication: FDA Cautions Against Use of Hydroxychloroquine or Chloroquine for COVID-19 Outside of the Hospital Setting or a Clinical Trial Due to Risk of Heart Rhythm Problems.www.clinicaltrials.gov Available at: [Accessed May 15, 2020] [Google Scholar]
- Uchida H., Ma L., Ueda H. 2010. Epigenetic Gene Silencing Underlies C-Fiber Dysfunctions in Neuropathic Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- University of Oxford . 2020. A Study of a Candidate COVID-19 Vaccine (COV001) - Full Text View - ClinicalTrials.gOv.https://clinicaltrials.gov/ct2/show/NCT04324606 Available at: [Accessed May 18, 2020] [Google Scholar]
- Vaira L.A., Salzano G., Deiana G., De Riu G. Anosmia and Ageusia: Common Findings in COVID-19 Patients. Laryngoscope. 2020 doi: 10.1002/lary.28692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Doremalen N., Lambe T., Spencer A., Belij-Rammerstorfer S., Purushotham J.N., Port J.R., Avanzato V., Bushmaker T., Flaxman A., Ulaszewska M., et al. ChAdOx1 nCoV-19 vaccination prevents SARS-CoV-2 pneumonia in rhesus macaques. bioRxiv. 2020;2020 doi: 10.1101/2020.05.13.093195. 05.13.093195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varatharaj A., Thomas N., Ellul M.A., Davies N.W.S., Pollak T.A., Tenorio E.L., Sultan M., Easton A., Breen G., Zandi M., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;2:1–8. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., Mehra M.R., Schuepbach R.A., Ruschitzka F., Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M., Bonanomi E., D’Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020 doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalaín J. Membranotropic effects of arbidol, a broad anti-viral molecule, on phospholipid model membranes. J. Phys. Chem. B. 2010;114:8544–8554. doi: 10.1021/jp102619w. [DOI] [PubMed] [Google Scholar]
- von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395:e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukmanovic-Stejic M., Chambers E.S., Suárez-Fariñas M., Sandhu D., Fuentes- Duculan J., Patel N., Agius E., Lacy K.E., Turner C.T., Larbi A., et al. Enhancement of cutaneous immunity during aging by blocking p38 mitogen-activated protein (MAP) kinase–induced inflammation. J. Allergy Clin. Immunol. 2018;142:844–856. doi: 10.1016/j.jaci.2017.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Liu Z., Wang Z., Duan M., Li G., Wang S., Li W., Zhu Z., Wei Y., Christiani D.C., et al. Thrombocytopenia is associated with acute respiratory distress syndrome mortality: an international study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0094124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Gao Y.H., Lou L.L., Zhang G.J. The clinical dynamics of 18 cases of COVID-19 outside of Wuhan, China. Eur. Respir. J. 2020;55 doi: 10.1183/13993003.00398-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA - J Am Med Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe R., Matsuyama S., Shirato K., Maejima M., Fukushi S., Morikawa S., Taguchi F. Entry from the cell surface of severe acute respiratory syndrome coronavirus with cleaved S protein as revealed by pseudotype virus bearing cleaved S protein. J. Virol. 2008;82:11985–11991. doi: 10.1128/jvi.01412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss SR, Leibowitz JL. “Coronavirus pathogenesis,” in Advances in Virus Research, 85–164. doi:10.1016/B978-0-12-385885-6.00009-2. [DOI] [PMC free article] [PubMed]
- Wen W., Su W., Tang H., Le W., Zhang X., Zheng Y., Liu X., Xie L., Li J., Ye J., et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020 doi: 10.1038/s41421-020-0168-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., Ramnarayan P., Fraisse A., Miller O., Davies P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widagdo W., Na Ayudhya S.S., Hundie G.B., Haagmans B.L. Host determinants of mers-CoV transmission and pathogenesis. Viruses. 2019;11 doi: 10.3390/v11030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020 doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H.Y.F., Lam H.Y.S., Fong A.H.T., Leung S.T., Chin T.W.Y., Lo C.S.Y., Lui M.M.S., Lee J.C.Y., Chiu K.W.H., Chung T., et al. Frequency and distribution of chest radiographic findings in COVID-19 positive patients. Radiology. 2019 doi: 10.1148/radiol.2020201160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organisation . 2004. Summary of Probable SARS Cases with Onset of Illness from 1 November 2002 to 31 July 2003.http://www.who.int/csr/sars/country/table2004_04_21/en/ Available at: (Based on data as of the 31 December 2003.). [Google Scholar]
- World Health Organization . 2020. Middle East Respiratory Syndrome, MERS Situation Upadate.http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html January 2020Available at: [Google Scholar]
- World Health Organization . 2020. FREQUENTLY ASKED QUESTIONS: Breastfeeding and COVID-19 for Health Care Workers.https://www.who.int/docs/default-source/maternal-health/faqs-breastfeeding-and-covid-19.pdf?sfvrsn=d839e6c0_5 Available at: [DOI] [PubMed] [Google Scholar]
- World Health Organization . 2020. Draft Landscape of COVID-19 Candidate Vaccines - 15 May 2020.https://www.who.int/who-documents-detail/draft-landscape-of-covid-19-candidate- vaccines Available at: [Accessed May 18, 2020] [Google Scholar]
- Wu K., Li W., Peng G., Li F. Crystal structure of NL63 respiratory coronavirus receptor-binding domain complexed with its human receptor. Proc Natl Acad Sci U S A. 2009;106:19970–19974. doi: 10.1073/pnas.0908837106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A., Peng Y., Huang B., Ding X., Wang X., Niu P., Meng J., Zhu Z., Zhang Z., Wang J., et al. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27:325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P., Duan F., Luo C., Liu Q., Qu X., Liang L., Wu K. Characteristics of Ocular Findings of Patients with Coronavirus Disease 2019 (COVID-19) in Hubei Province, China. JAMA Ophthalmol. 2020;138:575–578. doi: 10.1001/jamaophthalmol.2020.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020 doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Shiu E.Y.C., Gao H., Wong J.Y., Fong M.W., Ryu S., Cowling B.J. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings --personal protective and environmental measures. Emerg. Infect. Dis. 2020;26:967–975. doi: 10.3201/eid2605.190994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K., Zhai J., Feng Y., Zhou N., Zhang X., Zou J.-J., Li N., Guo Y., Li X., Shen X., et al. Isolation and characterization of 2019-nCoV-like coronavirus from malayan PangoliN.s. bioRxiv. 2020;2020 doi: 10.1101/2020.02.17.951335. 02.17.951335. [DOI] [Google Scholar]
- Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J. Infect. 2020 doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong T.Y., Redwood S., Prendergast B., Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur. Heart J. 2020;41:1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Liu Y., Cao L., Wang D., Guo M., Jiang A., Guo D., Hu W., Yang J., Tang Z., et al. Transcriptomic characteristics of bronchoalveolar lavage fluid and peripheral blood mononuclear cells in COVID-19 patients. Emerg. Microbes Infect. 2020 doi: 10.1080/22221751.2020.1747363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R.H., He J.F., Evans M.R., Peng G.W., Field H.E., Yu DW Lee C.K., Luo H.M., Lin W.S., Lin P., et al. Epidemiologic clues to SARS origin in China. Emerg Infect Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Lou Z., Liu Y., Pang H., Tien P., Gao G.F., Rao Z. Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core. J. Biol. Chem. 2004;279:49414–49419. doi: 10.1074/jbc.M408782200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Li X., Zhu B., Liang H., Fang C., Gong Y., Guo Q., Sun X., Zhao D., Shen J., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat. Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Zhao S., Teng T., Abdalla A.E., Zhu W., Xie L., Wang Y., Guo X. Systematic comparison of two animal-to-human transmitted human coronaviruses: SARS-CoV-2 and SARS-CoV. Viruses. 2020;12:244. doi: 10.3390/v12020244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Guo J., Fan C., Juan J., Yu X., Li J., Feng L., Li C., Chen H., Qiao Y., et al. Coronavirus disease 2019 (COVID-19) in pregnant women: a report based on 116 cases. Am. J. Obstet. Gynecol. 2020 doi: 10.1016/j.ajog.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Leibowitz J.L. The structure and functions of coronavirus genomic 3’ and 5’ ends. Virus Res. 2015;206:120–133. doi: 10.1016/j.virusres.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarmarkovich M., Warrington J.M., Farrel A., Maris J.M. Identification of SARS-CoV-2 vaccine epitopes predicted to induce long-term population-scale immunity. Cell Reports Med. 2020 doi: 10.1016/j.xcrm.2020.100036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye M., Fu D., Ren Y., Wang F., Wang D., Zhang F., Xia X., Lv T. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J. Med. Virol. 2020 doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young B.E., Ong S.W.X., Kalimuddin S., Low J.G., Tan S.Y., Loh J., Ng O.T., Marimuthu K., Ang L.W., Mak T.M., et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA - J Am Med Assoc. 2020;323:1488–1494. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Tostanoski L.H., Peter L., Mercado N.B., McMahan K., Mahrokhian S.H., Nkolola J.P., Liu J., Li Z., Chandrashekar A., et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science (80-) 2020 doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorski E., Pawar T., Rahimian S., Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID‐19) Br. J. Haematol. 2020 doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaigham M., Andersson O. Maternal and perinatal outcomes with COVID-19: A systematic review of 108 pregnancies. Acta Obstet. Gynecol. Scand. 2020 doi: 10.1111/aogs.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M., Van Boheemen S., Bestebroer T.M., ADME Osterhaus, Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zang R., Gomez Castro M.F., McCune B.T., Zeng Q., Rothlauf P.W., Sonnek N.M., Liu Z., Brulois K.F., Wang X., Greenberg H.B., et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020 doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin L., Saraceno G., Panciani P.P., Renisi G., Signorini L., Migliorati K., Fontanella M.M. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (Wien) 2020 doi: 10.1007/s00701-020-04374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D., She J., Zhang Z., Yu M. Effects of acute hypoxia on heart rate variability, sample entropy and cardiorespiratory phase synchronization. Biomed. Eng. Online. 2014;13:73. doi: 10.1186/1475-925X-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Zhu F., Xie L., Wang C., Wang J., Chen R., Jia P., Guan H.Q., Peng L., Chen Y., et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.03.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yan X., Fan Q., Liu H., Liu X., Liu Z., Zhang Z. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J. Thromb. Haemost. 2020;18:1324–1329. doi: 10.1111/jth.14859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Wang Y., Qi C., Shen L., Li J. Clinical trial analysis of 2019-nCoV therapy registered in China. J. Med. Virol. 2020;92:540–545. doi: 10.1002/jmv.25733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B., Liu S., Tan T., Huang W., Dong Y., Chen L., Chen Q., Zhang L., Zhong Q., Zhang X., et al. Treatment with convalescent plasma for critically ill patients with severe acute respiratory syndrome coronavirus 2 infection. Chest. 2020 doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhang B., Li P., Ma C., Gu J., Hou P., Guo Z., Wu H., Bai Y. Cold Spring Harbor Laboratory Press; 2020. Incidence, Clinical Characteristics and Prognostic Factor of Patients With COVID-19: a Systematic Review and Meta-analysis. [DOI] [Google Scholar]
- Zheng Y.Y., Ma Y.T., Zhang J.Y., Xie X. COVID-19 and the cardiovascular system. Nat. Rev. Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Lou Yang X., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Zeng Y., Tong Y., Chen C. Cold Spring Harbor Laboratory Press; 2020. Ophthalmologic Evidence against the Interpersonal Transmission of 2019 Novel Coronavirus through Conjunctiva. [DOI] [Google Scholar]
- Zhou L., Liu K., Liu H.G. Cause analysis and treatment strategies of “recurrence” with novel coronavirus pneumonia (covid-19) patients after discharge from hospital. Chinese J Tuberc Respir Dis. 2020 doi: 10.3760/cma.j.cn112147-20200229-00219. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Ren L., Zhang L., Zhong J., Xiao Y., Jia Z., Guo L., Yang J., Wang C., Jiang S., et al. Heightened innate immune responses in the respiratory tract of COVID-19 patients. Cell Host Microbe. 2020 doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Xiao G., Xu Y., Yuan F., Zheng C., Liu Y., Yan H., Cole D.K., Bell J.I., Rao Z., et al. Following the rule: formation of the 6-helix bundle of the fusion core from severe acute respiratory syndrome coronavirus spike protein and identification of potent peptide inhibitors. Biochem. Biophys. Res. Commun. 2004;319:283–288. doi: 10.1016/j.bbrc.2004.04.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Wang L., Fang C., Peng S., Zhang L., Chang G., Xia S., Zhou W. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl. Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu F.C., Li Y.H., Guan X.H., Hou L.H., Wang W.J., Li J.X., Wu S.P., Sen Wang B., Wang Z., Wang L., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: a dose-escalation, open-label, non-randomised, first-in- human trial. Lancet. 2020 doi: 10.1016/S0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler C.G.K., Allon S.J., Nyquist S.K., Mbano I.M., Miao V.N., Tzouanas C.N., Cao Y., Yousif A.S., Bals J., Hauser B.M., et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zulfiqar Aa, Lorenzo- Villalba N., Hassler P., Andrès E. Immune thrombocytopenic purpura in a patient with covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Y., Yalavarthi S., Shi H., Gockman K., Zuo M., Madison J.A., Blair C., Weber A., Barnes B.J., Egeblad M., et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S., et al. Ribose 2’-O- methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.