Abstract
Introduction:
Chemoradiation therapy (CRT) is the standard treatment for anal squamous cell carcinoma (ASCC) but can have significant treatment related toxicities. Recent studies have demonstrated the effectiveness of local excision (LE) for stage I ASCC with comparable oncologic outcomes to CRT. We aimed to evaluate this finding in a large population-based database.
Methods:
Patients diagnosed with stage I (T1N0M0) ASCC were identified from the Surveillance, Epidemiology, and End Results database, 2004-2015. Treatment approach was categorized as CRT or LE. Factors associated with treatment approach and cause-specific survival (CSS) were analyzed for the entire cohort and after stratification by tumor size (≤1cm and 1-2cm).
Results:
Among 883 patients, 56% had ASCCs 1-2cm in size and 77% received CRT. Mean age was 60 years, 65% were female, and 89% were White. Factors independently associated with receiving CRT were, being female, higher tumor grade, and tumor size 1-2cm. Unadjusted 5-year CSS for CRT was 96% while for LE it was 98% (p=0.048). After adjusting for available confounders, treatment approach was not associated with worse CSS, however being Black (HR=8.7) and uninsured (HR=13.7) were independently associated with worse prognosis. After stratification by tumor size, there was still no significant difference in 5-year CSS by treatment approach.
Conclusions:
LE was performed in a significant proportion of patients but was not independently associated with worse CSS compared to CRT. In appropriately selected patients with well differentiated ASCCs ≤1 cm, LE could be an acceptable management option but studies measuring outcomes such as local recurrence are needed.
Keywords: anal cancer, anus neoplasms/therapy, anus neoplasms/surgery, squamous cell carcinoma
1. Introduction
Anal squamous cell cancer (ASCC) is a relatively rare malignancy, representing only 0.5 % of all new cancer diagnoses in the United States (US). In 2018, 8,580 new cases (2,960 men and 5,620 women) of ASCC were diagnosed in the US with an estimated 1,160 deaths attributed to it.1 However, its incidence has been on the rise since the 1970s, with an annual increase in incidence of 2.2% between 2001-2015 for men and 3.1% for women.2 The current National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology guidelines recommend multi-agent chemoradiation (CRT) as the primary treatment modality for localized and locally advanced ASCC including stage I disease.3,4
These guidelines are based on several randomized trials5–10 conducted over the last two decades to identify the optimal radiation strategy and chemotherapy regimen to treat patients diagnosed with ASCC. However, most of these studies included patients with advanced tumors and either did not include or had a small number of patients with stage I disease. Therefore, the external validity of these trials’ findings for treatment of early stage ASCC patients has been contested. Also, while CRT has excellent oncologic outcomes, it is associated with significant short and long-term complications that adversely impact patient quality of life. As a result of the concerns regarding the generalizability of the clinical trial results in stage I patients and CRT related toxicities, alternate approaches such as local excision (LE) have been investigated as the primary treatment for stage I ASCC.11–15
Several studies from single institutions or retrospective cohort analyses have found comparable rates of survival and disease recurrence in patients treated with LE versus CRT. The largest of these cohort studies was by Chai et al. using the National Cancer Database (NCDB) and found comparable 5-year overall survival rates of 85% vs. 87% (p=0.93) in patients treated with LE vs. CRT, respectively.12 However, the NCDB includes data from Commission on Cancer accredited hospitals only and the results may not be generalizable to the overall population. Furthermore the primary endpoint in the analysis was overall survival (OS), which can be impacted by non-disease related factors such as comorbidities particularly since ASCC is usually seen in older patients.
The present study used the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER), which is a population-based database and provides information on cause-specific survival (CSS). We evaluated the patient and tumor related factors associated with LE, and compared survival outcomes by treatment strategies in stage I ASCC. We hypothesized that CSS would not be different between patients treated with LE vs. CRT in stage I ASCC.
2. Methods
2.1. Study Population
Patients were identified from SEER, which collects population-based cancer data from 18 SEER cancer registries across the US and accounts for approximately 28% of the US population. SEER data are representative of the general U.S. population in regard to measures of poverty and education and is considered the standard for data quality given its on-going quality control and data monitoring and evaluation programs.16
Patients aged ≥18 years with histologically confirmed stage I (American Joint Committee on Cancer 6th edition) squamous cell carcinoma (International Classification of Diseases for Oncology-3 codes: 8070-8) of the anal canal (primary site code 211) diagnosed between 2004 and 2015 were included. Patients were categorized as having received CRT if they received chemotherapy and external beam radiation. Patients were categorized as having received LE if they received local excision (surgery to primary site codes 20-27) but not chemotherapy or radiation. Only patients who received CRT or LE were included in the final analyses. This study was reviewed and deemed exempt by the University of Iowa Institutional Review Board.
2.2. Study variables
Patient characteristics included age at diagnosis, sex, race/ethnicity, marital status, and insurance status (Medicaid, insured, uninsured, unknown), cause of death (ASCC), and vital status. Metropolitan residency was categorized based on the US Department of Agriculture’s Rural-Urban Continuum Codes.17 These codes categorize counties based on metropolitan areas within the county and the degree of urbanization in adjacent counties. Metropolitan residency included SEER codes 1-3 and non-metropolitan residency included codes 4-9. Tumor characteristics included tumor grade and tumor size which was categorized as ≤1cm (Collaborative Stage tumor size 2004 code 1-10, 990, 991) versus >1 to 2 cm (codes 11-20).
2.3. Statistical Analysis
Due to small numbers, on univariate analyses, three patients with unknown race and/or unknown metropolitan status were excluded and patients with undifferentiated tumors were combined with patients with poorly differentiated tumors. Two-sided student’s t-test was used to compare mean age between treatment groups. Two-sided Chi-square tests were used to compare categorical patient and tumor factors between treatment groups. Factors with p-value<0.2 on univariate analyses were included in multivariable logistic regression. The proportion of patients per treatment group was calculated for each year in the study period to assess treatment trends. Kaplan Meier survival plots and Cox hazard proportions were used to analyze CSS for all patients and then stratified by tumor size (≤1 cm versus >1 to 2 cm). A p value <0.05 was considered statistically significant. Cases with unknown tumor grade, tumor size, insurance status, or metropolitan status were excluded from multivariable models. Analyses were conducted in SAS 9.4 (SAS Institute, Cary, NC).
3. Results
3.1. Overall population and factors associated with treatment.
Among 1033 patients who met the selection criteria, 683 (67%) underwent CRT, 200 (19%) LE, and 150 (14%) underwent another treatment and were excluded yielding a final cohort of 883 who were managed with either CRT or LE alone. For the overall group the mean age at diagnosis was 60 years; 65% were female, and 89% White. Patients who underwent CRT (vs. LE) were more frequently female (68% vs. 52%), White (91% vs. 85%), and married (46% vs. 37%). They also less frequently had well differentiated tumors (12% vs. 34%) or tumors ≤1cm (29% vs. 59%, all p<0.02, Table 1).
Table 1.
Patient and tumor characteristics by treatment in stage I anal squamous cell carcinoma (2004-2015)
| N (%) | ||||
|---|---|---|---|---|
| All (n=883) | CRT (n=683) | LE (n=200) | P value* | |
| Age, mean (SD) | 60 (12) | 60 (11) | 60 (13) | 0.74 |
| Sex | <0.0001 | |||
| Female | 570 (65%) | 68% | 52% | |
| Male | 310 (35%) | 32% | 48% | |
| Race | 0.01 | |||
| Black | 68 (8%) | 6% | 12% | |
| White | 790 (90%) | 91% | 85% | |
| Other | 22 (2%) | 2% | 3% | |
| Marital Status | 0.02 | |||
| Married | 390 (44%) | 46% | 37% | |
| Unmarried/unknown | 490 (56%) | 54% | 63% | |
| Insurance | 0.51 | |||
| Insured | 597 (68%) | 69% | 65% | |
| Medicaid | 85 (10%) | 9% | 12% | |
| Uninsured | 21 (2%) | 2% | 3% | |
| Unknown | 177 (20%) | 20% | 20% | |
| Metropolitan residence | 0.65 | |||
| Yes | 778 (88%) | 89% | 88% | |
| No | 102 (12%) | 11% | 12% | |
| Tumor Grade | <0.0001 | |||
| Well differentiated | 150 (17%) | 12% | 34% | |
| Moderately differentiated | 301 (34%) | 36% | 28% | |
| Poorly differentiated/undifferentiated | 198 (23%) | 26% | 10% | |
| Unknown | 231 (26%) | 25% | 28% | |
| Tumor Size | <0.0001 | |||
| ≤ 1 cm | 313 (35%) | 29% | 59% | |
| >1 to 2 cm | 489 (56%) | 61% | 37% | |
| Unknown | 79 (9%) | 10% | 4% | |
Abbreviations: CRT=chemoradiation; LE=local excision; SD=standard deviation
Chi2 test excluded unknown values
After adjusting for age at diagnosis along with all variables associated with treatment on univariate analysis, age (OR=1.03, 95% CI: 1.01-1.05) and male sex (OR=1.89, 95% CI: 1.14-3.08) were associated with increased odds of receiving LE while moderately differentiated (OR=0.31, 95% CI:0.19-0.53) and poorly differentiated (OR=0.14, 95% CI: 0.07-0.29) tumor grades, and tumor size >1 to 2cm (OR=0.34, 95% CI:0.21-0.55) were associated with lower odds of receiving LE (all p<0.02, Table 2).
Table 2.
Odds of locale excision in stage I anal squamous cell carcinoma with adjustment of patient and tumor factors, 2004-2015 (reference group is chemoradiation therapy)
| OR (95% CI) | P value | |
|---|---|---|
| Age | 1.03 (1.01, 1.05) | 0.01 |
| Sex | ||
| Female | REF | |
| Male | 1.88 (1.14, 3.08) | 0.01 |
| Race | ||
| White | REF | |
| Black | 1.45 (0.61, 3.49) | 0.40 |
| Other | 2.36 (0.66, 8.46) | 0.19 |
| Marital status | ||
| Married | REF | |
| No/unknown | 1.48 (0.90, 2.41) | 0.12 |
| Tumor Grade* | ||
| Well differentiated | REF | |
| Moderately differentiated | 0.31 (0.19, 0.53) | <.0001 |
| Poorly differentiated | 0.14 (0.07, 0.29) | <.0001 |
| Tumor size | ||
| ≤1 cm | REF | |
| >1 to 2 cm | 0.34 (0.21, 0.55) | <.0001 |
Abbreviations: OR=odds ratio; CI=confidence interval.
Undifferentiated group excluded due to missing values.
3.2. Cause-specific survival
Kaplan-Meier survival plots for all patients showed a 5-year CSS of 96% for CRT and 98% for LE (p=0.048). When stratified by tumor size, CSS was similar between treatment groups for both strata. For tumors ≤1cm, 5-year CSS for CRT and LE were 96% and 100%, respectively; for tumors >1 to 2cm, survival was 95% and 96%, respectively (Figure 1).
Figure 1.
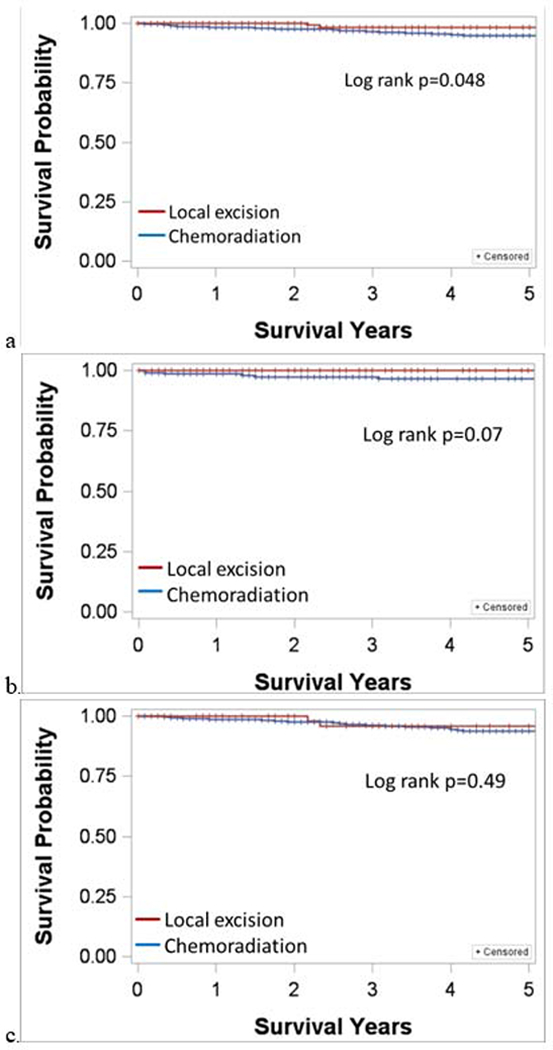
Kaplan Meier cause-specific survival plots for stage I anal squamous cell carcinoma. a) All cases; b) tumor ≤1 cm; c) tumor >1 to 2 cm.
On adjusted Cox regression for the entire cohort, Black race (HR=8.7), and uninsured status (HR=13.7) were associated with increased risk of death. LE was not associated with significant difference in risk of death (HR=0.48, CI: 0.10-2.32; Table 3).
Table 3.
Cox proportional hazards for cause-specific survival in stage I anal squamous cell carcinoma (2004-2015)
| HR (95%CI) | P value | |
|---|---|---|
| Treatment | ||
| Chemoradiation | REF | |
| Local excision | 0.48 (0.1, 2.3) | 0.36 |
| Age | 1.05 (1.0, 1.1) | 0.05 |
| Sex | ||
| Female | REF | |
| Male | 2.70 (1.0, 7.3) | 0.05 |
| Race* | ||
| White | REF | |
| Black | 8.72 (2.9, 26.7) | 0.0001 |
| Marital Status | ||
| Married | REF | |
| Unmarried/unknown | 0.39 (0.1, 1.1) | 0.08 |
| Tumor Grade** | ||
| Well differentiated | REF | |
| Moderately differentiated | 1.76 (0.4, 8.5) | 0.49 |
| Poorly differentiated | 2.48 (0.5, 13.6) | 0.30 |
| Tumor Size | ||
| ≤ 1 cm | REF | |
| >1 to 2 cm | 1.03 (0.3, 3.1) | 0.96 |
| Insurance | 0.34 | |
| Insured | REF | |
| Medicaid | 2.39 (0.5, 12.4) | 0.30 |
| Uninsured | 13.66 (3.1, 59.4) | 0.0005 |
| Metropolitan residence | ||
| Yes | REF | |
| No | 1.25 (0.3, 6.1) | 0.78 |
Abbreviations: HR=hazard ratio; CI=confidence interval
Other race group excluded due to missing values
Undifferentiated group excluded due to missing values
4. Discussion
In this population-based analysis of patients with stage I ASCC, we observed that 19% of patients underwent LE. Patients undergoing LE had smaller and well-differentiated tumors, and 5-year CSS that was not different from patients who underwent primary CRT. After controlling for available confounding variables, Black race and uninsured status were associated with increased risk of death but treatment approach (CRT vs. LE) was not.
The management of ASCC has substantially evolved over the last fifty years. Until the reports of Norman Nigro in the 1970’s regarding the efficacy of CRT in treating ASCC, abdominoperineal resection was considered the standard of care.18 Following his seminal observations, the treatment paradigm for ASCC shifted to CRT and several randomized clinical trials were conducted that established multi-agent chemotherapy with radiation as the current standard treatment.11–15 This approach has been associated with a 3-year local control rate of 62% to 83% and overall survival rate of 70 to 80% depending on the stage of disease.7–10 Despite these excellent oncological outcomes, there are significant short- and long-term treatment related toxicities associated with these regimens. Major adverse side-effects include hematologic, dermatological and gastrointestinal toxicities that can occur in up to one-third of patients receiving treatment.19,20 Furthermore, most of the randomized trials that were conducted mainly included patients with locally advanced ASCC (Stage II and III). Therefore a “one-size-fits-all” strategy particularly for early stage ASCC has been increasingly questioned as the appropriate approach to manage these patients. As a result, alternative treatment strategies have been evaluated including the use of mono-agent chemotherapy and radiation alone. Studies have shown these regimens to have comparable oncological outcomes to multi-agent CRT particularly in older patients.21 LE is another treatment option particularly for early ASCC if the entire lesion can be removed with negative margins without compromising sphincter function. Historically, LE has been reported in patients with ASCC with varied outcomes. In a report by Klas et al., twenty-one patients with ASCC underwent surgical therapy with a 5-year survival rate of 60% and a recurrence rate of 23% at 5-years.13 In a contemporary institutional series of 57 patients with stage I ASCC treated with either surgery alone (13 patients) or CRT, local recurrence was seen in two patients undergoing LE and was amenable to salvage therapy.14 However, in an analysis of 93 patients with stage I ASCC from the Nordic anal cancer database treated with either LE or CRT, LE alone was associated with a high local recurrence rate and worse survival.22 Therefore, it is likely that outcomes for LE are influenced by patient selection, with smaller and well-differentiated tumors having better outcomes. This conclusion is in line with the observation that LE in our population cohort was more commonly performed for ASCC with more favorable prognostic characteristics.
The prevalence and pattern of LE utilization in stage I ASCC is consistent with results from other large database studies from the United States. In a retrospective cohort study assessing 7223 patients in the NCDB (2003-2013) with Stage I ASCC, 24% patients were treated with LE alone.23 The analysis also found that the use of LE alone for the management of stage I ASCC had significantly increased over time with no significant impact on overall survival compared to CRT. Similarly, a SEER-Medicare study of 190 patients diagnosed with stage I ASCC between 1992 and 2009 found that surgery/ablation was used in 23% of their cohort.11 In this study after adjusting for patient and disease characteristics using propensity score, there was no statistically significant difference in survival among patients undergoing surgery/ablation compared to CRT.
The current literature including reports from SEER-Medicare, NCDB, and institutional cohorts have found LE to have similar survival outcomes to CRT in stage I ASCC. Most of these studies however may have been subject to confounding where the favorable tumor characteristics in patients undergoing LE may explain the equivalency between treatments in unadjusted analyses. In our study, in addition to the SEER-Medicare11 and NCDB studies12, similarities in survival by treatment persisted after controlling for available patient, tumor, and demographic variables. However, the available covariates were limited and key outcomes such as recurrence and sphincter preservation could not be measured. The ongoing ACT3 trial within the PLATO protocol from the United Kingdom is comparing recurrence rates between surgery alone for stage I anal cancer patients with margins >1mm and post-surgical highly selective low-dose radiotherapy with chemotherapy for patients with margins <1mm.24 Therefore, the current body of evidence does not support changes to clinical practice but does highlight the need for clinical trials that can better test the effectiveness of LE versus CRT, especially given that 19% of patients already receive LE as their primary treatment.
In our analyses, after controlling for available prognostic factors, Black race and uninsured status were the only factors significantly associated with CSS. This suggests that survival in stage I ASCC may be influenced by socioeconomic factors and access to healthcare. Survival may also be impacted by known risk factors for anal cancer, such as HPV or HIV infections and cigarette smoking, which are not collected by the SEER but may have a higher incidence in these sub group of patients.25,26 This possibility emphasizes the importance of prevention and screening efforts in at-risk patients to decrease disparity in survival for stage I ASCC.
Despite ASCC being an uncommon type of cancer, its incidence in the U.S. has increased approximately 1.6 times in both men and women from 1988 through 1992 to 2008 through 2012 and has continued to increase since that time.27 Among high risk population, the prevalence of anal high grade intra-epithelial lesions have been found to be 20-30% in men who have sex with men and 27% in women positive for human immunodeficiency virus.28,29 As a result, screening for high risk populations has become common and has been recommended by some regional societies.30 Furthermore, in recent years several trials have been initiated to determine treatment for precancerous lesions in high-risk populations including the Topical or Ablative Treatment in Preventing Anal Cancer in patients with HIV and Anal High Grade Squamous Intraepithelial Lesions (ANCHOR)31, and Laser Ablation Versus Observation to Prevent Anal Cancer (LOPAC).32 As a result of these trials, it is likely that increasingly smaller and earlier stage ASCC will be detected. Therefore, in an era of personalized medicine and patient-centered care, a more individualized approach is necessary as long as there is empiric evidence supporting it. Hence, despite the current recommendations for treating all ASCCs with CRT, there may be an opportunity of evaluating less morbid treatment strategies.
The findings of our study need to be considered in the context of the limitations of the SEER database that does not include data on disease recurrence, the need for salvage therapy, treatment related toxicities, or surgical morbidity. These outcomes need to be evaluated in order to make appropriate comparisons between LE and CRT. Additionally, chemotherapy and radiation data may be under reported due to treatment increasingly being received outside of the hospital setting.33 Furthermore, known risk factors for ASCC such as human papillomavirus infection, human immunodeficiency virus infection, and smoking could not be controlled for, which may confound the survival results. Despite these limitations this is the largest population-based study comparing LE vs. CRT in stage I ASCC in the U.S. and analyzed LE separately from other surgical approaches, which has been a limitation of previous studies.11,13 The SEER database also distinguishes tumors of the anal canal separately from those of the cloacogenic zone, overlapping lesions of the rectum, anus, and anal canal, or anal tumors whose location are not otherwise specified. This is an important strength of the SEER database as moderately differentiated T1N0 and select T2 tumors of the anal margin may be treated by local excision with 1-cm margins.3 Studies of treatment in stage I anal cancer where location of the anal canal could not be specified may bias the result towards equivalency in outcomes if anal margin cancers were also included in the analysis.13
5. Conclusion
LE was used in 19% of patients with stage I ASCC in this population-based analysis of U.S. patients. Patient who underwent LE had smaller and well-differentiated tumors, which may account for the similar 5-year CSS to patients treated with CRT. While LE may be a feasible option for select patients who cannot tolerate CRT, randomized controlled trials, particularly those that evaluate additional oncological outcomes such as disease recurrence, sphincter preservation and function and short and long-term morbidity are needed before changes in clinical practice can be recommended.
Acknowledgements:
This work was supported under National Institutes of Health (NIH) grant T32 CA 148062 (PI: R.J.W.), and NIH/National Cancer Institute contract number HHSN261201300020I (M.E.C., A.R.K).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest.
References
- 1.American Cancer Society. Key Statistics for Anal Cancer. https://www.cancer.org/cancer/anal-cancer/about/what-is-key-statistics.html Accessed August 1, 2019.
- 2.Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001-2015. J Natl Cancer Inst. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Anal Cancer. https://www.nccn.org/professionals/physician_gls/PDF/anal.pdf Accessed May 5, 2019.
- 4.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology. 2017;28(suppl_4):iv22–iv40. [DOI] [PubMed] [Google Scholar]
- 5.Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(16):1941–1948. [DOI] [PubMed] [Google Scholar]
- 6.Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomized UKCCCR Anal Cancer Trial (ACT I). British journal of cancer. 2010;102(7):1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 x 2 factorial trial. The Lancet Oncology. 2013;14(6):516–524. [DOI] [PubMed] [Google Scholar]
- 8.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1997;15(5):2040–2049. [DOI] [PubMed] [Google Scholar]
- 9.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1996;14(9):2527–2539. [DOI] [PubMed] [Google Scholar]
- 10.Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30(35):4344–4351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deshmukh AA, Zhao H, Das P, et al. Clinical and Economic Evaluation of Treatment Strategies for T1N0 Anal Canal Cancer. American journal of clinical oncology. 2018;41(7):626–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chai CY, Tran Cao HS, Awad S, Massarweh NN. Management of Stage I Squamous Cell Carcinoma of the Anal Canal. JAMA Surg. 2018;153(3):209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klas JV, Rothenberger DA, Wong WD, Madoff RD. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer. 1999;85(8):1686–1693. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti S, Jin Z, Huffman BM, et al. Local excision for patients with stage I anal canal squamous cell carcinoma can be curative. Journal of gastrointestinal oncology. 2019;10(2):171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faynsod M, Vargas HI, Tolmos J, et al. Patterns of recurrence in anal canal carcinoma. Archives of surgery (Chicago, Ill: 1960). 2000;135(9):1090–1093; discussion 1094-1095. [DOI] [PubMed] [Google Scholar]
- 16.Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. The American journal of surgical pathology. 2016;40(12):e94–e102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Department of Agriculture Economic Research Service . Rural-Urban Continuum Codes. https://www.ers.usda.gov/data-products/rural-urban-continuum-codes/ Accessed May 1, 2019.
- 18.Nigro ND, Vaitkevicius VK, Considine B Jr. Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum. 1974;17(3):354–356. [DOI] [PubMed] [Google Scholar]
- 19.Osborne MC, Maykel J, Johnson EK, Steele SR. Anal squamous cell carcinoma: an evolution in disease and management. World journal of gastroenterology. 2014;20(36):13052–13059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ludmir EB, Kachnic LA, Czito BG. Evolution and Management of Treatment-Related Toxicity in Anal Cancer. Surgical oncology clinics of North America. 2017;26(1):91–113. [DOI] [PubMed] [Google Scholar]
- 21.Charnley N, Choudhury A, Chesser P, Cooper RA, Sebag-Montefiore D. Effective treatment of anal cancer in the elderly with low-dose chemoradiotherapy. British journal of cancer. 2005;92(7):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon O, Hagberg O, Johnsson A. Primary surgery with or without postoperative radiotherapy in early stage squamous cell carcinoma in the anal canal and anal margin. Acta Oncol. 2018;57(9):1209–1215. [DOI] [PubMed] [Google Scholar]
- 23.Pricolo VE, Viani KL, Bonvini M, Abelli CF, McDuffie TJ. Challenges in Management of Squamous Cell Carcinoma of the Anus in New England and Across the United States: A Review of the National Cancer Data Base. American journal of clinical oncology. 2018;41(7):662–666. [DOI] [PubMed] [Google Scholar]
- 24.National Health Service Health Research Authority. PLATO-PersonaLising Anal cancer radioTherapy dOse (IRAS ID 204585). https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/plato-personalising-anal-cancer-radiotherapy-dose/ Accessed February 1, 2020.
- 25.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003-2006. The Journal of infectious diseases. 2011;204(4):566–573. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. Anal Cancer Prevention (PDQ)-Health Professional Version. https://www.cancer.gov/types/anal/hp/anal-prevention-pdq#_9_toc Accessed May 1, 2019.
- 27.Kang Y-J, Smith M, Canfell K. Anal cancer in high-income countries: Increasing burden of disease. PLoS One. 2018;13(10):e0205105–e0205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. The Lancet Oncology. 2012;13(5):487–500. [DOI] [PubMed] [Google Scholar]
- 29.Stier EA, Lensing SY, Darragh TM, et al. Prevalence of and Risk Factors for Anal High-grade Squamous Intraepithelial Lesions in Women Living with Human Immunodeficiency Virus. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leeds IL, Fang SH. Anal cancer and intraepithelial neoplasia screening: A review. World J Gastrointest Surg. 2016;8(1):41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.clincaltrials.gov Topical or Ablative Treatment in Preventing Anal Cancer in Patients With HIV and Anal High-Grade Squamous Intraepithelial Lesions (ANCHOR) NCT02135419. https://clinicaltrials.gov/ct2/show/NCT02135419 Accessed February 1, 2020.
- 32.National Health Service Health Research Authority. LOPAC Laser Ablation versus Observation to Prevent Anal Cancer (IRAS ID 167040). https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/lopac-laser-ablation-versus-observation-to-prevent-anal-cancer-v10/ Accessed February 1, 2020.
- 33.Surveillance, Epidemiology, and End Results Program. Data Use Agreement for SEER Radiation Therapy and Chemotherapy Information. https://seer.cancer.gov/data/ChemotherapyRadiation-SEER-DUA.pdf Published 2018 Accessed May 1, 2019.


