Abstract
Introduction:
The role of surgery for breast cancer liver metastases (BCLM) remains controversial. This study aimed to analyze survival in patients treated with hepatectomy plus systemic therapy or systemic therapy alone for BCLM and to determine selection factors to guide surgical therapy.
Materials and Methods:
Patients who underwent hepatectomy plus systemic therapy (n=136) and systemic therapy alone for isolated BCLM (n=763) were compared. Overall survival (OS) was analyzed after propensity score matching. Intrinsic subtypes were defined as: luminal A (estrogen receptor [ER]+ and/or progesterone receptor positive [PR]+, human epidermal growth factor receptor 2 [HER2]−), luminal B (ER and/or PR+, HER2+), HER2-enriched (ER and PR−, HER2+), and basal-like (ER, PR, HER2−).
Results:
After hepatectomy, independent predictors of poor OS were number and size of liver metastases, and intrinsic subtype (hazard ratios, 1.11, 1.16, and 4.28, respectively). Median OS was 75 and 81 months among patients with luminal B and HER2-enriched subtypes, compared with 17 and 53 months among patients with basal-like and luminal A subtypes (P<.001). Median progression-free survival (PFS) was 60 months with the HER2-enriched subtype, compared with 17, 16, and 5 months with luminal A, luminal B, and basal-like subtypes, respectively (P<.001). After propensity score matching, 5-year OS rates were 56% vs. 40% in the surgery vs. systemic therapy alone groups (P=.018).
Conclusion:
Surgical resection of BCLM yielded higher OS compared with systemic therapy alone and prolonged PFS among patients with the HER2-enriched subtype. These findings support the use of surgical therapy in appropriately selected patients, based on intrinsic subtypes.
Keywords: breast cancer, liver, metastases, surgery, subtypes
1. INTRODUCTION
Breast cancer is the leading cause of cancer-related mortality among women worldwide. Most deaths in breast cancer are caused by distant metastases, which occur in approximately 20% of all patients with breast cancer [1]. Up to 70% of patients with metastatic breast cancer develop liver metastases, which represent a frequent source of morbidity and mortality [2]. Systemic therapy is the mainstay of treatment for metastatic breast cancer, and advances in cytotoxic chemotherapy and hormonal therapy have led to substantial improvements in survival. Indeed, median overall survival (OS) for metastatic breast cancer has nearly tripled from 13 months in 1985 to 33 months in 2016 [3].
The liver represents the sole site of distant metastases in 10% of patients; thus, liver resection has had a limited role in treatment [4, 5]. Prior studies have shown conflicting results on the survival benefit of hepatic resection for metastatic breast cancer isolated to the liver [6, 7]. Traditional prognostic factors used to select patients for surgical therapy have included size and number of liver metastases [8, 9]. Beyond these anatomical prognostic factors, molecular classifications have been established in metastatic breast cancer that predict response to therapy and survival [10]. Intrinsic subtypes, defined by gene expression profiling, are used to stratify prognosis and guide systemic therapy [10, 11]. Due to the higher cost and limited availability of molecular profiling, immunohistochemical classification of intrinsic subtypes by estrogen and progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status is recommended to approximate intrinsic subtypes [12-14]. The purpose of this study was to compare the survival outcomes of patients with breast cancer liver metastases (BCLM) treated with hepatic resection plus systemic therapy or systemic therapy alone by using a propensity score analysis and to assess the role of intrinsic subtypes in the selection of patients for hepatic resection.
2. MATERIALS AND METHODS
2.1. Database and Patient Population
Patients diagnosed with BCLM from April 1997 to December 2016 were identified from prospectively managed Breast Medical Oncology and Surgical Oncology institutional databases at The University of Texas MD Anderson Cancer Center. Patients with unknown hormone receptor status and those who underwent liver ablation only were excluded. Among patients treated with systemic therapy alone, those with non-osseous extrahepatic metastases were excluded. Clinical tumor stage was determined at presentation by physical examination and standard-of-care imaging modalities. Synchronous BCLM were defined as liver metastases diagnosed within 6 months after the primary breast cancer [6]. The number of BCLM and size of the largest metastasis were evaluated at the time of diagnosis of BCLM and from the surgical pathology report for patients undergoing hepatic resection. Major hepatectomy was defined as resection of 3 or more contiguous Couinaud liver segments [15].
Tumor stage was determined using the seventh edition of the American Joint Committee on Cancer guidelines [16]. Systemic treatment in the metastatic setting was prescribed according to physician’s choice and/or enrollment in clinical trials. Response to systemic therapy was classified according to RECIST, version 1.1 [17].
This study was approved by the MD Anderson Cancer Center Institutional Review Board with a waiver of individual informed consent.
2.2. Assignment of Intrinsic Subtype
Intrinsic subtype classification was based on hormone receptor and HER2 status as previously reported: luminal A (estrogen receptor [ER] and/or PR+, HER2−), luminal B (ER and/or PR+, HER2+), HER2-enriched (ER and PR−, HER2+), and basal-like (ER, PR, HER2−) [18-20].
ER, PR, and HER2 status of tumors was determined by immunohistochemical analysis or fluorescence in situ hybridization using institutional laboratory thresholds and following standard current guidelines [21, 22]. When the hormone receptor or HER2 status was discordant between the primary tumor and BCLM, the BCLM status was used for analysis.
2.3. Statistical Analysis
Categorical variables were compared using the chi-square test, and continuous variables were compared using the Mann-Whitney test. OS was calculated from date of liver metastasis diagnosis or metastasectomy to the date of death or last follow-up. Progression-free survival (PFS) was calculated from date of liver metastasectomy to date of first disease relapse, death, or last follow-up. OS and PFS rates were estimated using the Kaplan-Meier method, and group comparisons performed by the log-rank test. Univariable and multivariable Cox proportional hazards regression models were assessed to determine factors associated with survival. P values < .05 were considered statistically significant; all tests were two-sided.
Propensity score analysis was performed to control for potential confounding factors and selection bias between patients who underwent surgery versus systemic therapy alone. Patients with incomplete data on systemic therapy regimens and lack of available cross-sectional imaging before and after first-line systemic therapy were excluded. From the systemic therapy alone group, patients with more than 10 or technically unresectable liver metastases and follow-up time < 1 year were excluded. A logistic regression model that predicts the probability of undergoing hepatic resection was constructed and used as the propensity score. The following variables were used for matching: primary tumor stage, grade, and histology; ER, PR, and HER2 status, resected primary tumor, BCLM number, size, and year of diagnosis; synchronous BCLM, and best RECIST response to first-line systemic therapy. A 1-to-1 matching (without replacement) by propensity score was performed using nearest neighbor method with a caliper width equal to 0.2 standard deviations. The balances of matched covariates were evaluated with standard differences.
All statistical analyses were performed using SPSS statistical software (Windows version 24.0; IBM Corp., Chicago, IL).
3. RESULTS
3.1. Patient Population
Table 1 shows patient and tumor characteristics of 136 patients who underwent resection of liver metastases plus systemic therapy and 763 patients treated with systemic therapy alone for isolated liver metastases from breast cancer. All patients were female. Among the 136 patients in the surgery group, 31 patients had metastases at extrahepatic sites, including bone (n=18), abdominal lymph nodes (n = 8), lung (n = 3), stomach (n = 1), and contralateral supraclavicular lymph node (n = 1). Median follow-up from date of liver metastasis diagnosis was 57 months (range, 9 to 229 months) in the surgical group and 18 months (range, 0 to 213 months) in the systemic therapy alone group.
TABLE 1.
Patients’ Demographic and Clinical Characteristics
| Variable | Systemic therapy alone (n = 763) |
Systemic therapy and surgery (n = 136) |
P |
|---|---|---|---|
| Age at diagnosis of liver metastases, years, median (range) | 52 (20-92) | 49 (26-71) | .025 |
| Menopausal status at diagnosis of liver metastases | 1.00 | ||
| Pre | 343 (45) | 61 (45) | |
| Post | 420 (55) | 75 (55) | |
| Median body mass index, kg/m2 (range) | 26 (14-570 | 25 (18-45) | .16 |
| Race / ethnicity | .044 | ||
| Non-Hispanic white | 579 (76) | 108 (79) | |
| Hispanic | 56 (7) | 12 (9) | |
| Black | 89 (12) | 5 (4) | |
| Asian | 28 (4) | 7 (5) | |
| Other or more than one | 11 (1) | 4 (3) | |
| ethnicity | |||
| Primary Breast Cancer | |||
| T status | < .001 | ||
| Tx | 24 (4) | 4 (3) | |
| T1 | 254 (42) | 34 (26) | |
| T2 | 234 (39) | 65 (49) | |
| T3 | 59 (10) | 19 (14) | |
| T4 | 29 (5) | 11 (8) | |
| N status | .011 | ||
| N0 | 228 (38) | 40 (30) | |
| N1 | 251 (42) | 49 (37) | |
| N2 | 74 (12) | 23 (17) | |
| N3 | 49 (8) | 21 (16) | |
| Histology | .13 | ||
| Ductal | 684 (90) | 122 (90) | |
| Lobular | 33 (4) | 10 (7) | |
| Mixed ductal-lobular | 28 (4) | 4 (3) | |
| Other | 18 (2) | 0 | |
| Grade | .001 | ||
| 1 | 11 (2) | 4 (3) | |
| 2 | 209 (30) | 57 (46) | |
| 3 | 483 (69) | 64 (51) | |
| Estrogen receptor status positive | 454 (60) | 88 (65) | .25 |
| Progesterone receptor status positive | 341 (45) | 51 (38) | .12 |
| Human epidermal growth factor receptor 2 status positive | 242 (32) | 47 (36) | .38 |
| Triple-negative breast cancer | 161 (21) | 23 (17) | .33 |
| Liver Metastases | |||
| Time interval between diagnosis of primary breast cancer and liver metastases in months | 16 (0-289) | 19 (0-305) | .91 |
| Synchronous presentation | 248 (33) | 56 (41) | .049 |
| Median number, by pathology (range) | — | 1 (0-14) | — |
| Size of largest metastasis in cm by pathology, median (range) | — | 2.2 (0-8) | — |
| Year of diagnosis | .001 | ||
| 1997-2003 | 182 (24) | 38 (28) | |
| 2004-2009 | 232 (30) | 58 (43) | |
| 2010-2016 | 349 (46) | 40 (29) | |
| Type of surgery | — | — | |
| Major hepatectomy | 57 (42) | ||
| Minor hepatectomy | 79 (58) | ||
| Liver resection margin | — | — | |
| Negative | 114 (84) | ||
| Positive | 22 (16) | ||
Data are presented as No. (%) unless indicated otherwise.
The following data are missing in surgical group: T status (n = 3), N status (n = 3), tumor grade (n = 11), HER2 status (n = 4).
The following data are missing in non-surgical group: T status (n = 163), N status (n = 161), tumor grade (n = 60).
3.2. Hormonal Status
In the liver resection group, 43 of the 47 patients with HER2-positive cancers received HER2-directed therapy. Twenty-two patients had change in receptor status between the breast primary and liver metastases. The most frequent receptor conversion was PR status from positive to negative in 13 patients.
3.3. Recurrence after Liver Resection
After liver resection, 103 of the 136 patients (76%) suffered disease relapse after median of 14 months (range, 1 to 89 months). The most common sites of recurrence were liver-only (n = 52), liver and extrahepatic site (n = 15), bone (n = 10), and brain (n = 9).
3.4. Survival
In the surgical group, 5-year and median OS rates from date of BCLM diagnosis were 53% and 69 months (range, 55 to 83 months). From date of liver metastasectomy, 5-year and median OS rates were 45% and 57 months (range, 47 to 68 months). In the non-surgical group, 5-year and median OS rates from date of BCLM diagnosis were 21% and 28 months (range, 25 to 31 months).
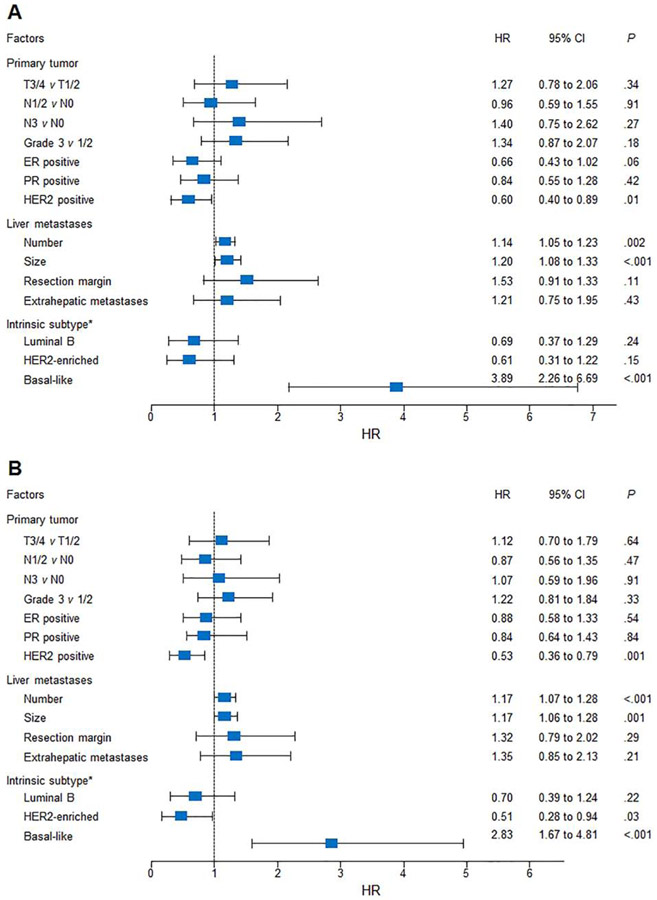
Variations in univariable hazard ratios for OS and PFS after hepatic metastasectomy were analyzed (Figure 1). HER2 positivity was significantly associated with improved OS (P = .01) and PFS (P = .001). The basal-like subtype was strongly associated with OS and PFS, with hazard ratios of 3.89 and 2.83 compared with the luminal A subtype (P < .001).
Figure 1.
Forest plot of hazard ratios (HR) for survival according to primary tumor, liver metastases, and intrinsic subtypes. (A) Overall and (B) progression-free survival after liver metastasectomy. *Luminal A subtype acts as the referent group. CI = confidence interval; ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.
On multivariable analysis, independent factors for OS were number of liver metastases (HR, 1.11; 95% CI, 1.01 to 1.22; P = .024), size of liver metastases (HR, 1.16; 95% CI, 1.01 to 1.32; P = .032), and intrinsic subtype (HR 4.28; 95% CI, 2.44 to 7.51; P < .001). Independent factors for PFS were number of liver metastases (HR, 1.16; 95% CI, 1.05 to 1.28; P = .003) and intrinsic subtype (HR, 3.42; 95% CI, 1.96 to 5.97; P <.001).
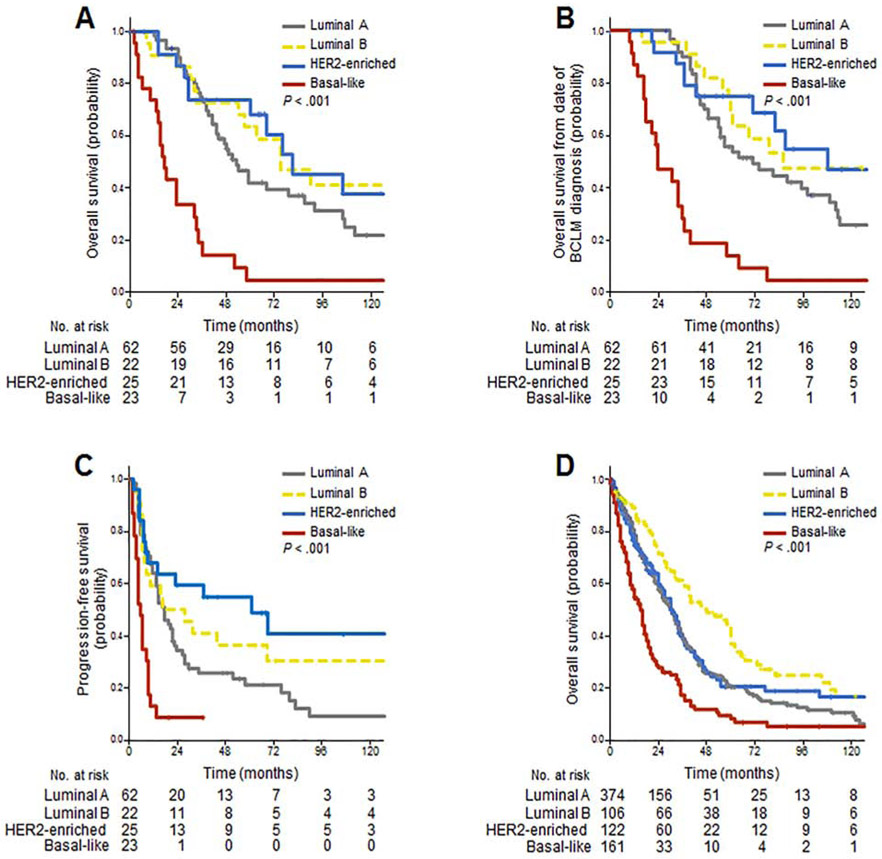
Figure 2 summarizes OS and PFS rates according to intrinsic subtypes. Among patients undergoing surgery, median OS after hepatic metastasectomy was 81 months (95% CI, 40 to 122 months) among the 25 patients with HER2-enriched tumors and 75 months (95% CI, 41 to 109 months) among the 22 patients with luminal B tumors, compared with 53 months (95% CI, 42 to 64 months) among the 62 patients with luminal A tumors and 17 months (95% CI, 12 to 22 months) among the 23 patients with basal-like tumors (P < .001; Figure 2A). Similar results were observed for OS from date of BCLM diagnosis (Figure 2B). Median PFS was 60 months (95% CI, 9 to 111 months) with HER2-enriched tumors, compared with 17 months (95% CI, 12 to 22 months) luminal A, 16 months (95% CI, 0 to 40 months) luminal B, and 5 months (95% CI, 3 to 7 months) basal-like (P < .001; Figure 2C).
Figure 2.
Survival outcomes according to intrinsic subtypes. Among surgically treated patients, overall survival from date of (A) surgery and (B) liver metastases diagnosis; (C) progression-free survival. (D) Overall survival among patients treated with systemic therapy alone for isolated breast cancer liver metastases. HER2 = human epidermal growth factor receptor 2.
Among patients treated with systemic therapy alone for isolated liver metastases, median OS was 48 months (95% CI, 29 to 67 months) among the 106 patients with luminal B tumors, compared with 30 months (95% CI, 26 to 34 months) among the 374 patients with luminal A tumors, 30 months (95% CI, 25 to 35 months) among the 122 patients with HER2-enriched tumors, and 15 months (95% CI, 12 to 18 months) among the 161 patients with basal-like tumors (P < .001; Figure 2D).
3.5. Propensity Score Matching Analysis
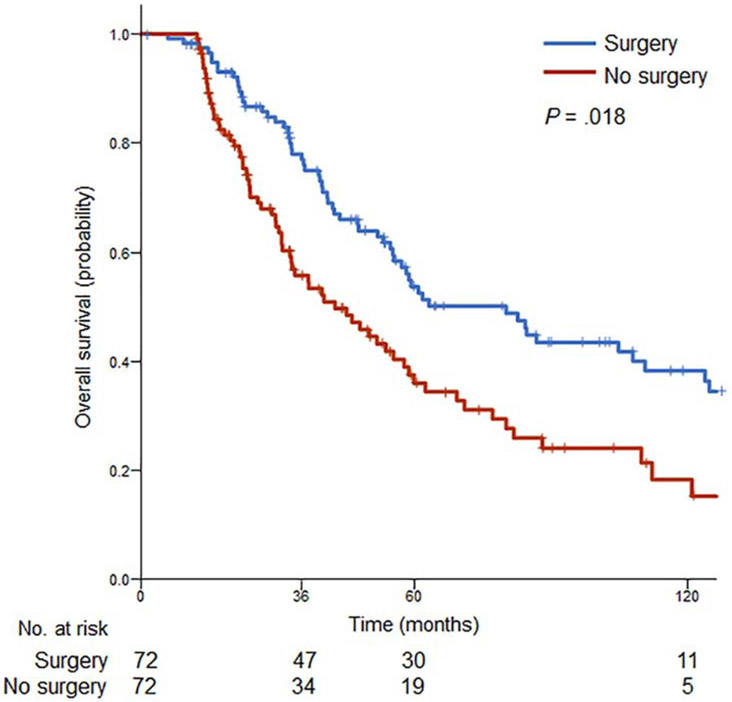
Analysis was performed of patients who had complete data on systemic therapy regimens and cross-sectional imaging before and after first-line systemic therapy available. The unmatched data set comprised 110 of the 136 patients treated with hepatic resection and 113 of the 763 patients treated with systemic therapy alone. Among the unmatched cohort, patients treated with systemic therapy alone were more likely to have higher grade primary tumors and more liver metastases (Table 2). After propensity score matching, 72 (53%) patients who underwent liver resection and systemic therapy were matched with 72 (64%) patients who received systemic therapy alone. Distribution of baseline covariates was adequately balanced in the matched data set. Patients who underwent surgery for BCLM demonstrated a statistically significant survival benefit, with 5-year OS of 56% versus 40% with medical therapy alone (P = .018; Figure 3).
Table 2.
Comparison of Baseline Variables Between Surgical and Non-Surgical Groups in Unmatched and Matched Data Sets
| Unmatched Data Set | Matched Data Set | |||||
|---|---|---|---|---|---|---|
| Surgery | No surgery | P | Surgery | No surgery | P | |
| Sample size, No. | 110 | 113 | — | 72 | 72 | — |
| Age, years, median (range) | 49 (26-71) | 52 (25-73) | .058 | 50 (26-71) | 51 (25-70) | .87 |
| Primary Breast Cancer | ||||||
| Stage at diagnosis | .25 | .39 | ||||
| 0-I | 19 (17) | 11 (10) | 10 (14) | 5 (7) | ||
| II-III | 41 (37) | 48 (42) | 27 (38) | 30 (42) | ||
| IV | 50 (46) | 54 (48) | 35 (49) | 37 (51) | ||
| Grade | .004 | .73 | ||||
| 1-2 | 51 (46) | 30 (27) | 24 (33) | 26 (36) | ||
| 3 | 59 (54) | 81 (72) | 48 (67) | 46 (64) | ||
| Unknown | 0 | 2 (2) | 0 | 0 | ||
| Estrogen receptor status positive | 73 (66) | 75 (66) | 1.00 | 45 (63) | 47 (65) | .73 |
| Progesterone receptor status positive | 46 (42) | 49 (43) | .82 | 31 (43) | 29 (40) | .74 |
| Human epidermal growth factor receptor 2 status positive | 40 (36) | 48 (42) | .35 | 27 (38) | 27 (38) | 1.00 |
| Primary tumor resected before BCLM diagnosis | 66 (60) | 64 (57) | .61 | 42 (58) | 39 (54) | .61 |
| Liver Metastases | ||||||
| Synchronous presentation | 52 (47) | 55 (49) | .89 | 37 (51) | 37 (51) | 1.00 |
| Median number by imaging (range) | 1 (1-14) | 2 (1-10) | .016 | 1 (1-14) | 2 (1-7) | .079 |
| Size of largest metastasis in cm by imaging, median (range) | 2.5 (0.9-14.0) | 2.5 (0.8-8.7) | .69 | 2.5 (0.9-14.0) | 2.5 (0.9-8.5) | .77 |
| Year of diagnosis | .34 | .97 | ||||
| 1997-2003 | 25 (23) | 27 (24) | 15 (21) | 16 (22) | ||
| 2004-2009 | 48 (44) | 39 (35) | 33 (46) | 33 (46) | ||
| 2010-2016 | 37 (34) | 47 (42) | 24 (33) | 23 (32) | ||
| Medical therapy for BCLM | ||||||
| Taxane and/or anthracycline based | 74 (67) | 66 (58) | .17 | 47 (65) | 44 (61) | .60 |
| Other cytotoxic regimen | 36 (33) | 47 (42) | 25 (35) | 28 (39) | ||
| Endocrine therapy | 16 (15) | 26 (23) | .11 | 10 (14) | 16 (22) | .19 |
| HER2-directed therapy | 34 (31) | 37 (33) | .77 | 23 (32) | 21 (29) | .72 |
| Best response to medical therapy | .24 | .71 | ||||
| Partial or complete | 81 (74) | 75 (66) | 54 (75) | 52 (72) | ||
| Stable or progressive disease | 29 (26) | 38 (34) | 18 (25) | 20 (28) | ||
Data are presented as No. (%) unless indicated otherwise
Figure 3.
Survival after propensity score matching. Overall survival outcomes of patients after liver resection versus systemic therapy alone for breast cancer liver metastases.
4. DISCUSSION
Metastatic breast cancer is considered an incurable disease with median and 5-year OS rates of 3 years and 25% [23]. Systemic therapy is the mainstay of treatment with goals of prolonging survival and palliating symptoms. A small subset of patients have oligometastatic disease confined to the liver, and retrospective series of hepatic resection have reported 5-year OS rates of 50% to 54% [4, 7]. However, matched cohort studies comparing outcomes after hepatic resection versus systemic therapy alone have shown discordant results [4, 6]. Thus, the role of surgery in the management of isolated BCLM remains controversial. The survival outcomes reported in retrospective surgical series may reflect patient selection rather than a therapeutic benefit of surgery. To reduce patient selection bias, we performed propensity score matching by the probability of undergoing hepatic resection. Other selection criteria for the systemic therapy alone group were follow up time ≥ 1 year and availability of cross-sectional imaging before and after first-line therapy to assess response. In our matched analysis, hepatic resection plus systemic therapy was associated with higher 5-year OS of 56% compared with 40% among patients treated with systemic therapy alone.
Traditionally, patient selection for hepatic resection has been guided by anatomical and temporal factors, including size and number of liver metastases, disease-free interval, and absence of extrahepatic disease [8, 9]. In addition, response to systemic therapy has been shown to be an important prerequisite for liver resection [24, 25]. Previously, we showed that response to systemic therapy, measured histologically by the residual tumor thickness at the tumor-normal tissue interface in resected liver metastases, correlated with recurrence-free survival after BCLM resection [26]. Importantly, all patients in this study, including those in the hepatectomy group, received systemic therapy. Improvements in survival are attributable to the increased efficacy of systemic treatment, particularly targeted therapies for HER2-positive disease. In this study, response to systemic therapy was included as a variable for propensity score matching, and most patients in both the surgery and non-surgery groups demonstrated partial or complete response to first-line therapy.
A secondary objective of this study was to assess biologic factors to guide selection of patients for surgical therapy. Intrinsic subtype was strongly predictive of OS and PFS after BCLM resection. Patients with luminal B and HER2-enriched subtypes had median OS rates exceeding 6 years, compared with 53 months in patients with the luminal A subtype. Despite the prolonged OS, 76% of all patients suffered disease relapse after hepatic resection, and most recurrences involved the liver, with or without extrahepatic disease. Importantly, patients with the HER2-enriched subtype achieved a durable median PFS of 60 months. Outcomes were poor after hepatic resection for patients with the basal-like subtype, with a hazard ratio for death of 3.89. These data support resection of BCLM for patients with HER2-enriched and luminal B subtypes but not the basal-like subtype. Selection of patients with luminal A tumors, the most prevalent subtype, should be made on an individual basis.
The survival advantage with hepatic resection observed in HER2-positive disease is relevant because HER2 positivity has been shown to confer tropism of breast cancer cells to the liver [2, 27]. Furthermore, HER2-enriched is the most prevalent subtype among patients whose metastatic disease is isolated to the liver [18]. Comparing unmatched cohorts in our study, the survival benefit with surgery was greatest among patients with HER2-enriched tumors, whose median OS rates were 81 and 30 months, with and without surgery, respectively. In earlier-stage breast cancer trials, the HER2-enriched subtype predicted complete pathologic response to HER2 blockade [28, 29]. The higher response with HER2-positive, hormone receptor-negative tumors is attributed to high expression of epidermal growth factor receptor and/or HER2 pathway genes. In addition, potential crosstalk between HER2 and hormone receptor pathways may lead to resistance to targeted therapies in hormone receptor-positive disease [30, 31]. Extrapolating from early stage breast cancer to BCLM, we hypothesize that the prolonged PFS in the HER2-enriched subtype reflects the additive effects of surgical removal of macroscopic disease and suppression of micrometastases through HER2 blockade.
This study has several limitations. First, immunohistochemical surrogates were used to classify intrinsic subtypes. Given the limitations of microarray-based gene expression studies in routine clinical practice, the combination of hormone receptors and HER2 status is recommended to approximate molecular subtypes [14]. Second, receptor profiles of both the primary breast cancer and liver metastases were not analyzed in every patient. Although discordant expression of receptor profiles is observed, the biology of the primary tumor is mostly preserved in distant metastases [18, 32]. Third, propensity score matching after stratifying by intrinsic subtypes was not performed. The sample size in the matched analysis was small, and the prognostic significance of intrinsic subtypes in the matched cohorts was not evaluated. However, rates of hormone receptor and HER2 positivity were similar between the matched surgery and non-surgery groups. Lastly, this study does not present randomized data, and propensity score matching does not eliminate the selection bias for patients with better biology and performance status to undergo hepatic resection.
5. CONCLUSIONS
In a propensity score matched analysis, liver resection following systemic therapy was associated with a survival advantage over systemic therapy alone for isolated BCLM. In particular, patients with the HER2-enriched subtype had durable PFS after surgery. These results suggest that, similar to systemic therapy, surgical therapy for BCLM should be guided by intrinsic subtypes, with liver resection considered for HER2-enriched and luminal B subtypes and avoided in patients with basal-like tumors.
6. ACKNOWLEDGEMENTS / ROLE OF THE FUNDING SOURCE
This work was supported by the National Institutes of Health / National Cancer Institute (P30-CA016672).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Beslija S, et al. Third consensus on medical treatment of metastatic breast cancer. Ann Oncol 2009;20:1771–85. [DOI] [PubMed] [Google Scholar]
- 2.Chikarmane SA, et al. Metastatic patterns of breast cancer subtypes: what radiologists should know in the era of personalized cancer medicine. Clin Radiol 2015;70:1–10. [DOI] [PubMed] [Google Scholar]
- 3.Sundquist M, et al. Improved survival in metastatic breast cancer 1985-2016. Breast 2017;31:46–50. [DOI] [PubMed] [Google Scholar]
- 4.Mariani P, et al. Liver metastases from breast cancer: Surgical resection or not? A case-matched control study in highly selected patients. Eur J Surg Oncol 2013;39:1377–83. [DOI] [PubMed] [Google Scholar]
- 5.Pagani O, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 2010;102:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sadot E, et al. Hepatic Resection or Ablation for Isolated Breast Cancer Liver Metastasis: A Case-control Study With Comparison to Medically Treated Patients. Ann Surg 2016;264:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruiz A, et al. Surgical resection versus systemic therapy for breast cancer liver metastases: Results of a European case matched comparison. Eur J Cancer 2018;95:1–10. [DOI] [PubMed] [Google Scholar]
- 8.Margonis GA, et al. The role of liver-directed surgery in patients with hepatic metastasis from primary breast cancer: a multi-institutional analysis. HPB (Oxford) 2016;18:700–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruiz A, et al. Predictive Profile-Nomogram for Liver Resection for Breast Cancer Metastases: An Aggressive Approach with Promising Results. Ann Surg Oncol 2017;24:535–45. [DOI] [PubMed] [Google Scholar]
- 10.Prat A, et al. Prognostic Value of Intrinsic Subtypes in Hormone Receptor-Positive Metastatic Breast Cancer Treated With Letrozole With or Without Lapatinib. JAMA Oncol 2016;2:1287–94. [DOI] [PubMed] [Google Scholar]
- 11.Perou CM, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. [DOI] [PubMed] [Google Scholar]
- 12.Russnes HG, et al. Insight into the heterogeneity of breast cancer through next-generation sequencing. J Clin Invest 2011;121:3810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fumagalli D, et al. A common language in neoadjuvant breast cancer clinical trials: proposals for standard definitions and endpoints. Lancet Oncol 2012;13:e240–8. [DOI] [PubMed] [Google Scholar]
- 14.Guiu S, et al. Molecular subclasses of breast cancer: how do we define them? The IMPAKT 2012 Working Group Statement. Ann Oncol 2012;23:2997–3006. [DOI] [PubMed] [Google Scholar]
- 15.Couinaud C [Liver lobes and segments: notes on the anatomical architecture and surgery of the liver ]. Presse Med 1954;62:709–12. [PubMed] [Google Scholar]
- 16.Edge SB and Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
- 17.Eisenhauer EA, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- 18.Soni A, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 2015;143:471–8. [DOI] [PubMed] [Google Scholar]
- 19.Haque R, et al. Impact of breast cancer subtypes and treatment on survival: an analysis spanning two decades. Cancer Epidemiol Biomarkers Prev 2012;21:1848–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiechmann L, et al. Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 2009;16:2705–10. [DOI] [PubMed] [Google Scholar]
- 21.Hammond ME, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol 2010;28:2784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolff AC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997–4013. [DOI] [PubMed] [Google Scholar]
- 23.Cardoso F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol 2018;29:1634–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abbott DE, et al. Resection of liver metastases from breast cancer: estrogen receptor status and response to chemotherapy before metastasectomy define outcome. Surgery 2012;151:710–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adam R, et al. Is liver resection justified for patients with hepatic metastases from breast cancer? Ann Surg 2006;244:897–907; discussion -8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou JH, et al. Residual tumor thickness at the tumor-normal tissue interface predicts the recurrence-free survival in patients with liver metastasis of breast cancer. Ann Diagn Pathol 2014;18:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Q, et al. Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget 2017;8:27990–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llombart-Cussac A, et al. HER2-enriched subtype as a predictor of pathological complete response following trastuzumab and lapatinib without chemotherapy in early-stage HER2-positive breast cancer (PAMELA): an open-label, single-group, multicentre, phase 2 trial. Lancet Oncol 2017;18:545–54. [DOI] [PubMed] [Google Scholar]
- 29.Prat A, et al. HER2-enriched subtype and ERBB2 expression in HER2-positive breast cancer treated with dual HER2 blockade. J Natl Cancer Inst 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harbeck N Insights into biology of luminal HER2 vs. enriched HER2 subtypes: Therapeutic implications. Breast 2015;24 Suppl 2:S44–8. [DOI] [PubMed] [Google Scholar]
- 31.Osborne CK, et al. Crosstalk between estrogen receptor and growth factor receptor pathways as a cause for endocrine therapy resistance in breast cancer. Clin Cancer Res 2005;11:865s–70s. [PubMed] [Google Scholar]
- 32.Kimbung S, et al. Contrasting breast cancer molecular subtypes across serial tumor progression stages: biological and prognostic implications. Oncotarget 2015;6:33306–18. [DOI] [PMC free article] [PubMed] [Google Scholar]