Summary
Alterations in the composition of the extracellular matrix (ECM) critically regulate the cellular responses in tissue repair, remodeling, and fibrosis. After injury, proteolytic degradation of ECM generates bioactive ECM fragments, named matricryptins, exposing cryptic sites with actions distinct from the parent molecule. Matricryptins contribute to the regulation of inflammatory, reparative, and fibrogenic cascades through effects on several different cell types both in acute and chronic settings. Fibroblasts play a major role in matricryptin generation not only as the main cellular source of ECM proteins, but also as producers of matrix-degrading proteases. Moreover, several matricryptins exert fibrogenic or reparative actions by modulating fibroblast phenotype and function. This review manuscript focuses on the mechanisms of matricyptin generation in injured and remodeling tissues with an emphasis on fibroblast-matricryptin interactions.
Keywords: Matricryptins, fibroblasts, extracellular matrix, peptides, remodeling, fibrosis
Introduction
The extracellular matrix (ECM) is the non-cellular component present in all tissues and organs. ECM is a highly dynamic 3-dimensional network that acts not only as physical scaffolding for cells but also provides mechanical support and initiates vital biochemical and biomechanical cues required for tissue homeostasis.[1] The ECM is composed mainly of water, proteins and polysaccharides; however, each tissue type has an ECM with a unique composition and topology that is generated during tissue development. Independent of tissue type, the ECM is composed of a combination of proteoglycans (such as decorin, aggrecan, and versican), hyaluronan, collagens (fibrillar, network-forming, fibril-associated, and membrane-associated), elastin and elastin-associated proteins (such as fibrillins, fibulins, and EMILIN-1), fibronectin, laminins, and variable amounts of matricellular proteins (such as SPARC/secreted protein acidic and rich in cysteine, osteopontin, periostin, tenascin-C, and thrombospondins) that typically increase following injury.[2] Although all cell types (i.e. epithelial, fibroblasts, immune cells, endothelial cells) synthesize and secrete matrix macromolecules, in most soft connective tissues the bulk of ECM is transcribed and secreted by fibroblasts. Importantly, fibroblasts not only secrete ECM but also control the overall ECM structural organization through their synthetic and mechanical machinery. Thus, fibroblasts are major effectors of tissue mechanical properties.[3] In addition, fibroblasts maintain ECM and participate in ECM turnover by secretion of proteases, most notably members of the matrix metalloproteinase (MMP) family, that degrade the various molecular components.[4] In fact, the ECM is continually being remodeled, either enzymatically or non-enzymatically, under physiologic conditions and most dramatically in pathological settings. After tissue injury, the ECM is dynamically remodeled throughout all phases of wound healing – inflammation, proliferation, and maturation.[5] Alterations in ECM composition play critical roles in the regulation of the cellular responses that mediate tissue healing. In the inflammatory phase of tissue repair, clearance of dead cells and damaged tissue is accomplished by degradation of ECM proteins. This process generates bioactive ECM fragments, named matricryptins, that interact with cell surface receptors, modulating inflammatory, fibrogenic, angiogenic, and reparative cascades.[6]
The term matricryptin was coined in 2000 by Davis and colleagues to describe proteolytically released ECM fragments that contain exposed matricryptic sites.[7] These sites contain a cryptic domain normally not exposed in the intact molecule that renders biological activity. Several years later, Ricard-Blum and colleagues extended the definition of matricryptins to include the ectodomains of membrane collagens and membrane proteoglycans, which are released in the ECM by sheddases, and fragments of ECM-associated enzymes such as lysyl oxidase and MMPs.[8, 9] Matricryptins have biological activities on their own, distinct from the parent molecule, and have been reported to regulate various physiological and pathological processes such as tissue repair, fibrosis, angiogenesis, cancer, and neurodegenerative diseases. Within the literature, the term matrikine is occasionally used interchangeably with matricryptin.[10–12] However, historically these terms describe distinct peptide origin and functions. In 1999, Maquart et al. defined matrikines as ‘ECM-derived peptides able to regulate cell activity’.[13] The term matrikine was independently used by Swindle and colleagues to describe low-affinity ligands for growth factor receptors.[14] Matrikines were defined by these authors as signaling elements that exist as subcomponents of ECM proteins and bind to cell surface receptors that belong to the cytokine, chemokine, ion channel, or growth factor receptor families.[8] Even though distinction between the terms matrikine and matricryptin is sometimes blurred; matricryptin is restricted to describing biologically active ECM fragments that contain exposed functional matricryptic sites that are not normally exposed in the full-length molecules.
Fibroblasts have a major role in matricryptin generation by serving as the main cellular source of ECM and by secreting a wide range of proteases that cleave the matrix and expose the cryptic sites. In injured tissues, fibroblasts not only serve as regulators of ECM remodeling but also represent major targets of matrix fragments. Matricryptins modulate fibroblast function either directly (by transducing signals in fibroblasts) or indirectly (through actions on immune and vascular cells that may modulate fibroblast activation). Thus, matricryptin-mediated actions are implicated in tissue remodeling and fibrosis. This review manuscript discusses the mechanisms of matricryptin generation in injured and remodeling tissues and their cellular actions that may play an important role in regulation of fibroblast phenotype, contributing to reparative and fibrotic responses.
Matricryptin generation during tissue repair
Tissue repair is a complex biological process that entails replacing damaged tissue to restore tissue integrity.[15] The reparative response involves interactions between several different cell types (including platelets, leukocytes, fibroblasts, endothelial cells (ECs), epithelial cells, and organ-specific resident parenchymal cells) and components of the ECM.[16, 17] Since all ECM components are subject to degradation and modification during tissue remodeling, matricryptins are generated throughout all phases of tissue healing and actively participate in regulation of inflammatory, fibrogenic and angiogenic responses by binding to cell surface receptors.
In all tissues, inflammation can be initiated and propagated by ECM disruption. Most types of tissue injury result in rapid activation of proteases, leading to generation and release of matricryptins. Several mechanisms have been reported to regulate the exposure of matricryptic sites within the ECM molecules; those include enzymatic proteolysis, protein multimerization, adsorption, cell-mediated mechanical forces, and denaturation.[7, 18] In addition, reactive oxygen species can also expose cryptic sites as reported for epitopes associated with the autoimmune Goodpasture syndrome.[19] Nonetheless, the primary mechanism of matricryptin generation is by enzymatic cleavage. Two main families of enzymes are responsible for ECM degradation and remodeling [20]: the MMPs and the members of the ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. To a lesser extent, serine proteinases such as plasmin and cathepsin G, which are active at neutral pH, can also degrade ECM components extracellularly.[20–22] By proteolytic cleavage, numerous matricryptins are generated with a wide range of sizes and with molecular weight ranging from 269 Da to 85 kDa. To date, the smallest matricryptins reported are made of 3 amino acids (e.g. PGP tripeptide from collagen I, GHK tripeptide from collagen Iα2)[23–25] and the longest is composed of 705 amino acids (endorepellin, a perlecan-derived matricryptin)[26]. A matricryptin may be proteolytically generated by 3 ways: 1) cleavage by a single enzyme; 2) by action of several enzymes acting independently to generate several forms (different sizes) of a matricryptin (e.g. 20, 28, and 30 kDa endostatin-related fragments);[27] or 3) sequentially via a multistep processing.[9] Table 1 lists matricryptins generated in injured tissues by ECM source and decryptive enzyme(s).
Table 1 -.
List of matricryptins and reported functions.
| ECM component | Matricryptin | Decryptive enzyme | Known functions | References |
|---|---|---|---|---|
| Collagen Iα1 | C-1158/59 * | MMP-2, MMP-9 | anti-fibrotic, pro-angiogenic | [30] |
| DGGRYY | unknown | neutrophil activator | [24, 25] | |
| Collagen I | PGP * | MMP-8, MMP-9, prolyl endopeptidase (serine protease) | chemotactic for neutrophils fibrosis | [23, 105–111,115] |
| Collagen Iα2, SPARC | GHK | unknown | chemotactic for monocytes, macrophages, and mast cells; pro-angiogenic | [25, 35] |
| Collagen IV | arresten (IVα1) | MT1-MMP, MT2-MMP | anti-angiogenic | [56–59] |
| canstatin (IVα2) * | MT1-MMP, MT2-MMP | promotes MMP-2 expression, fibroblast migration, inhibit myofibroblast contraction, fibrosis | [60–64, 67–70] | |
| tumstatin (IVα3) | MMP-9 | anti-angiogenic, fibroblast proliferation & migration | [71, 72] | |
| tetrastatin (IVα4) | MT1-MMP (expected) | anti-tumor | [65, 66] | |
| pentastatin (IVα5) | MT1-MMP (expected) | anti-tumor | [54, 55] | |
| hexastatin (IVα6) | MT1-MMP (expected) | inhibits EC migration and survival | [60, 64] | |
| Collagen XVIII | endostatin * | MMP-9 | anti-angiogenic, anti-fibrotic | [73, 74, 116–119] |
| Elastin | VGVAPG | MMP-1, MMP-2, MMP-8, MMP-9, MMP-12 | chemotactic for monocytes and fibroblasts | [43–45] |
| kappa-elastin peptides | aspartic proteases, cysteine proteases, serine proteases, and MMPs | chemotactic for monocytes, promote collagenase-1 expression, induce fibroblast proliferation | [39–42] | |
| Fibronectin | anastellin | cathepsin D and mechanical decryption | fibrillogenesis, anti-angiogenic, and binds to PDGF-BB | [76–79] |
| PHSRN | neutrophil elastase | fibroblast migration | [80, 81] | |
| Fibulin-1 | fibulin-1 peptide 1 * | unknown | fibroblast attachment, proliferation, and mitochondrial activity, fibrosis | [83, 114, 115] |
| Hyaluronic acid | LMWHA/oHA | Hyaluronidase 1 and −2 | fibroblast migration and pro-inflammatory effects | [92–97] |
| Perlecan | endorepellin | cathepsin L | anti-angiogenic, fibroblast survival | [26, 99–102] |
| Tenascin-C | YTITIRGV | MMP-2 | activates stromal fibroblasts, promotes invasion and metastasis in colon cancer cells | [89–91] |
represents matricryptins reported to have roles in fibrosis.
The enzymes responsible for decrypting the ECM to generate matricryptins are mainly produced by leukocytes; however, both fibroblasts and ECs can also secrete these enzymes. Therefore, ECM components can be degraded and remodeled throughout all the phases of wound healing, predominantly during the inflammatory and proliferative phases and to a lower extent during wound maturation. Each stage of repair may be associated with a distinct profile of matricryptin generation, dependent on the predominant proteases, the composition of the ECM, and the level of proteolytic activation of the cell types involved. During the proliferation phase, fibroblasts are the main proliferative cell and display a secretory phenotype to produce high amounts of ECM, particularly collagens, to form a new scar. The formation of the new collagenous scar is a dynamic process with constant remodeling that continues through the maturation phase. In some types of injury, uncontrolled fibroblast activation may lead to prolonged release of matricryptins, causing adverse matrix remodeling and progressive fibrosis.
ECM-derived peptides and tissue remodeling
Following tissue injury, protease-generated matricryptins act as damage-associated molecular patterns (DAMPs) by binding to pattern recognition receptors, such as toll-like receptors (TLRs) in innate immune cells.[28, 29]. Matricryptic ECM-derived fragments can also bind to integrins, growth factor receptors, CD44, purinergic receptors, and other cell-surface proteins, of both resident and infiltrating cells, through which they exert their actions.[30–34]
a. Specific cellular actions of matricryptins
A wide range of ECM proteins, including structural fibrillar proteins, non-fibrillar proteins, matricellular macromolecules, and proteoglycans, can serve as a source of matricryptins, generating fragments that modulate phenotype and function of inflammatory cells, vascular cells, and fibroblasts (Figure 1). While ECM degradation during the inflammatory phase generates matricryptins capable of interacting with leukocytes, as the healing response evolves matricryptins also interact with fibroblasts and ECs.
Figure 1.
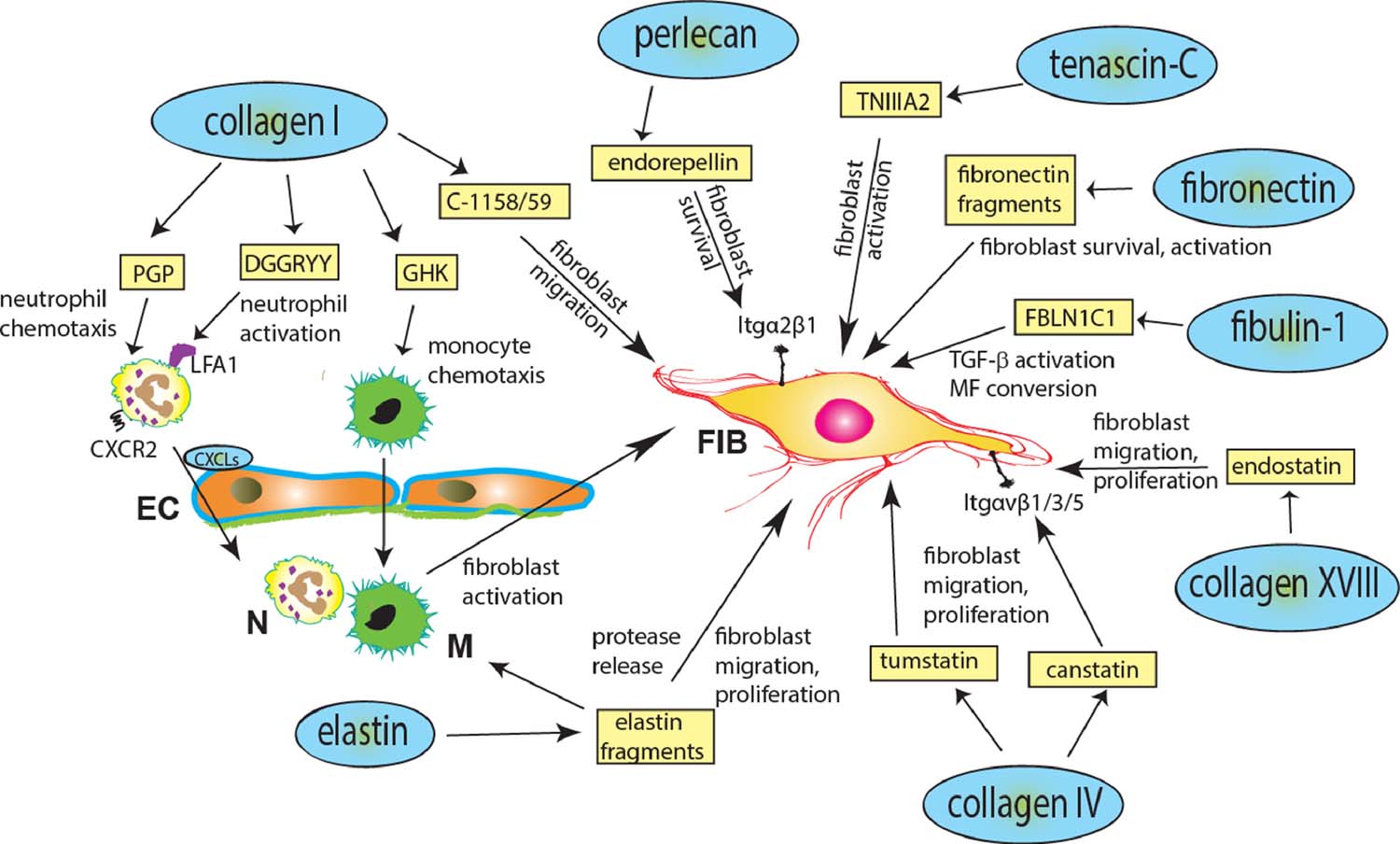
Effects of matricryptins in tissue fibrosis. Following tissue injury, proteolytic degradation of ECM proteins generates a wide range of matricryptins that may participate in the pathogenesis of fibrosis, either directly, through actions on steady state fibroblasts (FIB), that can become activated and differentiate into a myofibroblast (MF), or indirectly by recruiting or activating inflammatory cells (N, neutrophil; M, monocyte) and endothelial cells (EC).
Collagen type I, as one of the most abundant structural ECM proteins, is cleaved early by MMPs in injured tissues, generating several matricryptins that participate in the inflammatory response. The peptide DGGRYY (collagen Iα1, amino acids 1211 to 1216) binds to the lymphocyte function-associated antigen (LFA)-1 integrin to activate neutrophils and the tripeptide GHK (derived from both collagen Iα2 and the matricellular protein SPARC) is chemotactic for monocytes, macrophages, and mast cells.[24, 25]. Also collagen-derived, the tripeptide PGP was observed in cystic fibrosis patients at levels well above the control group.[23] In that study, the formation of the peptide PGP was reduced by inhibitors specific for MMP-8 and −9, suggesting that both these MMPs are responsible for its generation. In addition, the same study showed that PGP acts as a chemoattractant for neutrophils by binding to CXC chemokine receptors 1 and 2.[23]. The effects of collagen I-derived matricryptins are not limited to actions on immune cells. GHK and the collagen Iα1 C-1158/59 matricryptin have been reported to exert angiogenic properties, thus participating in scar vascularization.[30, 35] In myocardial infarction (MI), C-1158/59 generated both through MMP-2 and MMP-9 actions, has been shown to directly bind to fibroblasts to robustly induce cell migration, while reducing collagen secretion.[30]
Elastin, a polymer of tropoelastin, is characterized by domains rich in lysine and alanine and hydrophobic domains rich in glycine, valine, and proline.[36, 37] Elastolytic enzymes, including aspartic proteases, cysteine proteases, serine proteases, and metalloproteinases, are activated following injury and can generate bioactive fragments with pro-inflammatory properties.[38] Elastin-derived peptides, such as kappa-elastin,[39] have been reported to be chemotactic to monocytes.[39–42] Cleavage of the C-terminus of tropoelastin generates the matricryptin VGVAPG, a fragment with chemotactic properties for both monocytes and fibroblasts.[43–45]. Elastin-derived matricryptins have also been suggested to play an autoregulatory role. The elastin peptide VPGVG reduces elastin transcription and translation, while promoting proliferation of smooth muscle cells.[46] These matricryptin-mediated effects, i.e. simultaneous increase in cell proliferation and reduction of elastin expression, may serve as an important regulatory mechanism by which elastin synthesis is controlled in physiological and pathological conditions. Elastin-derived matricryptins have also been reported to participate in matrix remodeling by affecting protease expression. Elastin matricryptins bind to the elastin binding protein found on the surface of dermal fibroblasts, to promote fibroblast proliferation[47] and induce expression of collagenase-1 via the activation of extracellular signal-regulated kinase (ERK)1/2 in a Ras-independent manner.[48]
Collagen type IV is a major constituent of the basement membrane in all tissues and is composed of six homologous subunits that form 3 separate α-chains: α1α2α1, α3α4α5, and α5α5α6.[49] Collagen type IV has the potential to generate a vast array of bioac` tive peptides during tissue remodeling. So far 6 collagen IV-derived matricryptins have been reported to interact predominantly with fibroblasts and ECs and are known for their anti-tumor functions.[50–53] These peptides are fragments of non-collagenous domains from the α1 (arresten, 26 kDa polypeptide), α2 (canstatin, 24 kDa polypeptide), α3 (tumstatin, 28 kDa polypeptide), α4 (tetrastatin, 20 amino acids), α5 (pentastatin, 2455 Da), and α6 collagen type IV chains (hexastatin, 25 kDa polypeptide).[54, 55] Arresten is an endogenous inhibitor of angiogenesis and functions via integrin (Itg) α1β1.[56, 57] In vitro studies show that by binding to Itgα1β1, arresten inhibits angiogenesis by blocking EC proliferation, migration, and tube formation.[58, 59] Canstatin and hexastatin have also been shown to inhibit angiogenesis in tumors by binding to ECs via Itgαvβ1, Itgαvβ3, and Itgαvβ5, possibly by inactivating Akt (protein kinase B) and focal adhesion kinase (FAK) downstream signaling.[60–64] Similarly, tetrastatin also prevents tumor growth possibly via Itgαvβ3 and downstream inhibition of the FAK/PI3K/Akt pathway.[65, 66] Both canstatin and tumstatin have been shown to target fibroblasts. Specifically, canstatin, which is generated through proteolytic actions of the membrane type (MT) MMPs MT1-MMP and MT2-MMP,[67] has been suggested to modulate fibroblast migration and proliferation, exerting organ-specific actions. In cardiac fibroblasts, canstatin induces fibroblast migration via ERK-mediated actions and subsequent secretion of MMP-2.[68] In contrast, in lung fibroblasts canstatin inhibited migration.[69] Canstatin has also been reported to stimulate myofibroblast proliferation via Akt activation.[70] Moreover, in isolated lung fibroblasts, transforming growth factor (TGF) β-induced fibroblast to myofibroblast conversion was associated with canstatin release.[69] Whether canstatin contributes to myofibroblast phenoconversion, or simply accompanies the overexpression of myofibroblast markers remains unknown. Tumstatin, generated by MMP-9 cleavage, is mostly known for its anti-angiogenic properties,[71] but also promotes proliferation and migration of cardiac fibroblasts via phosphorylation (Ser473) of Akt.[72]
The peptides generated from MMP9-mediated cleavage of collagen type XVIII, termed endostatins, bind to vascular endothelial growth factor (VEGF) receptors (VEGFR-1, −2, and −3) and inhibit angiogenesis.[73] Additionally, the endostatin peptide 4 (E4, 26 monomers long) selectively phosphorylates VEGFR-3 in fibroblasts, activating downstream ERK/mitogen-activated protein kinases (MAPK) signaling.[74]
Fibronectin (Fn) is a ubiquitous glycoprotein synthesized by many cell types. Fn plays key roles in fibroblast migration through interactions involving both its cell-binding and heparin-binding domains.[75] Fibronectin-derived matricryptins have been suggested to regulate phenotype and function of fibroblasts and ECs in healing wounds. The Fn matricryptin anastellin (amino acids 630–704) promotes Fn fibrillogenesis, has anti-angiogenic activity by inhibiting the Ras/ERK pathway, and also binds to platelet-derived growth factor (PDGF)-BB, a major mitogen and survival factor for fibroblasts.[76–79] The peptide PHSRN, derived from plasma Fn, has been proposed to have a major role in acquisition of a migratory phenotype in wound fibroblasts [80], through effects that may involve Itgα5β1.[80, 81]
Fibulin-1 is normally incorporated in fibronectin-containing matrix fibers and is known to play roles in cell adhesion and migration.[82] In vitro studies have demonstrated that the fibulin-1 peptide 1 (FBLN1C1), amino acids 567 to 586, enhances pulmonary fibroblast attachment, proliferation, viability, and mitochondrial activity.[83]
The laminin family consists of at least 15 large trimeric basement membrane proteins and each is made of α, β, and γ chains.[84] Laminin-1 (α1β1γ1) is one of the best characterized laminins and plays central roles in tumor metastasis, cell spreading and attachment, and angiogenesis.[85, 86] Several synthetic peptides derived from all 3 chains have been shown to have adhesive properties, mainly through the heparin-binding domains;[87, 88]. The profile and potential role of laminin-derived matricryptins in tissue repair and remodeling is not known.
The matricellular protein tenascin-C, an important protein in fibrosis and tissue remodeling, harbors a cryptic functional site composed of the amino acid sequence YTITIRGV. Upon decryption by MMP-2,[89] the 22-mer tenascin-C peptide, termed TNIIIA2, activates stromal fibroblasts via Itgβ1.[90, 91]
Under physiologic conditions, hyaluronic acid (HA, also known as hyaluronan) exists mainly as a high molecular weight polysaccharide; however, upon injury hyaluronidases hydrolyze the linkage between N-acetyl-glucosamine and glucuronic acid residues to generate low molecular weight fragments.[92, 93] In acute lung injury models and in tumors, HA peptides have been reported to initiate inflammatory responses by activating TLR2- and TLR4-mediated MyD88-dependent signaling.[94, 95] HA oligosaccharides can also target fibroblasts and may modulate their reparative potential and inflammatory activity. In a model of cutaneous wound healing low molecular weight HA (LMWHA) prepared by γ-irradiation, improved fibroblast survival, promoting repair.[96] In contrast, in synovial fibroblasts 4-mer hyaluronan oligosaccharides stimulated inflammatory activation through TGFβ-activated kinase 1 (TAK1) and p38 MAPK pathways.[97]
The large heparan sulfate proteoglycan perlecan, expressed on cell surfaces and within basement membranes, is a well-defined pro-angiogenic molecule in its intact form. However, when processed by cathepsin L during matrix remodeling,[98] the C-terminal 85 kDa fragment of perlecan, endorepellin, has the opposite effect of its parent molecule.[99] Like other anti-angiogenic matricryptins, endorepellin acts through integrin binding. In ECs, endorepellin modulates Itgα2β1-dependent intracellular pathways, reversibly disrupting actin cytoskeleton and focal adhesions of ECs, in a Ca2+-dependent and heparan sulfate-independent manner; thus, inhibiting cell migration.[100, 101] Some of the effects of endorepellin may be mediated through interactions with other matricryptins, such as endostatin.[26] In addition to its effects on vascular cells, endorepellin has also been suggested to modulate fibroblast phenotype. One of the 3 LG domains of endorepellin, LG3, exerts anti-apoptotic effects on fibroblasts.[102] Pathologically, LG3 functions may be important in the pathogenesis of fibrosis by promoting fibroblast survival.
b. Generation and role of matricryptins in fibrotic conditions
Considering their rapid and continuous generation in fibrotic tissues, and their potent effects on phenotype and function of immune cells, vascular cells and fibroblasts, matricryptins may play an important role in the pathogenesis and progression of fibrosis. Moreover, matricryptins may be useful biomarkers, reflecting matrix-degrading activity in many fibrotic conditions.[103]
Extensive associative evidence implicates matricryptins derived from collagens I and V and fibulin-1 in diseases associated with pulmonary fibrosis. Idiopathic interstitial pneumonias, such as usual interstitial pneumonia (UIP) and organizing pneumonia (OP), exhibit early fibrotic lesions that contain small aggregates of myofibroblasts, fibroblasts, and ECM.[104] These early fibrotic lesions are thought to be the initial manifestations of lung fibrogenesis. Urushiyama and colleagues found release of the collagen type IV-derived matricryptin canstatin in the early fibrotic lesions of patients with UIP but not in patients with OP.[69] Canstatin expression in these lesions was predominantly localized in α-smooth muscle actin-expressing myofibroblast-like cells. Although in vitro canstatin has been shown to modulate migration and proliferation of lung fibroblasts,[69] the in vivo significance of these findings remains unclear.
The collagen I-derived matricryptin PGP has been also implicated in lung fibrosis. PGP levels were reported to directly correlate with disease severity in patients with a wide range of lung conditions, including chronic obstructive pulmonary disease (COPD), cystic fibrosis, bronchiolitis obliterans syndrome, and acute respiratory distress syndrome.[23, 105–107] Prolonged generation of PGP in experimental models of pulmonary injury may perpetuate neutrophilic inflammation,[108] ultimately inducing fibrotic changes. PGP generates a positive feedback inflammatory signal, triggering neutrophil influx through binding to the chemokine receptors CXCR1 and CXCR2. PGP-mediated neutrophil activation drives the release of MMP-9 from the neutrophil tertiary granules, which in turn leads to further cleavage of collagen, promotion of fibrosis, and release of additional PGP and subsequent neutrophil influx.[109, 110] While PGP is normally cleared by further enzymatic degradation, it can become stable by chemical acetylation on its N-terminus (Ac-PGP), which functions to enhance its chemotactic potential.[111] Accordingly, there has been increased emphasis on processes that both define the bioavailability of and promote the degradation of the acetylated form of PGP. Recently, O’Reilly and colleagues demonstrated Ac-PGP is degraded through action of the enzyme angiotensin-converting enzyme (ACE) and suggested the ACE pathway is aberrant in COPD, enabling accumulation of Ac-PGP.[111]
Fibulin-1 levels are elevated in the serum and lung tissue from patients with a variety of lung diseases, including idiopathic pulmonary fibrosis (IPF).[112, 113] The fibulin-1-derived matricryptin FBLN1C1 has been suggested to play an important role in the pathogenesis of pulmonary fibrosis. In a mouse model of bleomycin-induced lung fibrosis, genetic FBLN1C depletion protected animals from developing airway and lung remodeling and fibrosis by attenuation of the TGFβ signaling pathway and myofibroblast generation.[114] FBLN1C binds to latent TGFβ–binding protein 1 (LTBP1) to induce TGFβ activation and mediates downstream Smad3 phosphorylation, promoting myofibroblast conversion and collagen deposition. The clinical significance of the animal model findings are supported by evidence showing FBLN1C and LTBP1 colocalization in lung tissues from patients with IPF.[114, 115] Moreover, in fibroblasts harvested from both normal subjects and patients with lung disease (IPF or COPD), FBLN1C1 was found to stimulate fibroblast proliferation, attachment, survival, and ECM deposition.[83]
Other matricryptin-mediated actions inhibit lung fibrosis and may have therapeutic implications. The endostatin-derived peptide E4 attenuated pulmonary and cutaneous fibrosis in a bleomycin model and prevented TGFβ-induced dermal fibrosis both in vivo in a mouse model and ex vivo in human skin.[116] The antifibrotic actions of endostatin may involve modulation of PDGFR/ERK and RhoA/ROCK signaling pathways.[117, 118]. Matricryptins have also been implicated in cardiac fibrosis. PGP generation in pressure-overloaded hearts was accentuated upon disruption of the matrix-preserving properties of cardiac fibroblasts; additionally, PGP has been suggested to enhance inflammation and promote dysfunction.[115] On the other hand, the collagen I-derived matricryptin C-1158/59 reduced fibrosis in a rodent model of permanent occlusion MI.[30] Consistent with its anti-fibrotic effects in other organs, endostatin was found to inhibit fibrosis in the infarcted heart. In a rat model of MI, endostatin inhibition through administration of a neutralizing antibody worsened interstitial fibrosis, increased mortality, and accentuated cardiac hypertrophy, indicating that endostatin may have protective roles against cardiac remodeling and heart failure after MI.[119]
Conclusions and future directions
The role of the ECM is not limited to structural support of tissues and organs. In injured and remodeling tissues, proteolytic degradation of the matrix generates bioactive fragments with a critical role in regulation of inflammatory, reparative, angiogenic, and fibrogenic responses. A growing body of descriptive evidence suggests that tissue injury is associated with release of a broad range of matricryptins. In vitro, many of these bioactive fragments exhibit potent effects on immune cells, vascular cells, and fibroblasts. Considering their actions on all cells involved in fibrogenesis, matricryptins may be implicated in the pathogenesis of fibrotic conditions. Unfortunately, our understanding of the in vivo role of matricryptins in tissue fibrosis remains limited. Systematic proteomic analysis of the profile of matricryptins in fibrotic diseases is lacking. Moreover, robust in vivo studies establishing the role of specific bioactive ECM fragments and dissecting their cellular targets remain particularly challenging. The potent antifibrotic effects of certain matricryptins, such as endostatin, highlight that understanding the role of matricryptins in fibrotic conditions may also have therapeutic implications. Unfortunately, clinical evidence supporting the role of matricryptins in progression of fibrotic diseases is limited to associative data. In patients with diabetic renal disease, circulating levels of endostatin were associated with fibrosis and predicted adverse outcome.[120] Moreover, in a small population of patients with acute respiratory distress syndrome, high levels of PGP were associated with worse pulmonary function and worse prognosis.[121] This emerging evidence supports the clinical significance of matricryptins and their potential involvement in human inflammatory and fibrotic diseases.
Highlights.
Fibroblasts are essential cells for ECM homeostasis
ECM proteolytic cleavage generates biologically active peptides named matricryptins
Matricryptins modulate fibroblast functions
Matricryptins may be involved in the pathogenesis of fibrotic conditions
Acknowledgments
The authors acknowledge funding support by the American Heart Association 18AIREA33960311 [LECB], the National Institutes of Health grants R01 HL76246 [NGF], R01 HL85440 [NGF], and R01 HL149407 [NGF], and the U.S. Department of Defense grants PR151134 [NGF], PR151029 [NGF] and PR181464 [NGF].
Abbreviations:
- ACE
angiotensin-converting enzyme
- ADAMTS
a disintegrin and metalloproteinase with thrombospondin motifs
- Akt
protein kinase B
- COPD
chronic obstructive pulmonary disease
- EC
endothelial cell
- ECM
extracellular matrix
- ERK
extracellular signal-regulated kinase
- FAK
focal adhesion kinase
- Fn
fibronectin
- HA
hyaluronic acid
- IPF
idiopathic pulmonary fibrosis
- ITG
integrin
- LTBP1
latent transforming growth factor β–binding protein 1
- MAPK
mitogen-activated protein kinases
- MI
myocardial infarction
- MMP
matrix metalloproteinases
- MT-MMP
membrane-type matrix metalloproteinases
- OP
organizing pneumonia
- PDGF
platelet derived growth factor
- PDGFR
platelet derived growth factor receptor
- RhoA
ras homolog gene family, member A
- ROCK
Rho-associated protein kinase
- SPARC
secreted protein acidic and rich in cysteine
- TGFβ
transforming growth factor β
- TLR
Toll-like receptor
- UIP
usual interstitial pneumonia
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
Literature
- [1].Frantz C, Stewart KM, Weaver VM, The extracellular matrix at a glance, J Cell Sci 123(24) (2010) 4195–4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK, Extracellular matrix structure, Advanced drug delivery reviews 97 (2016) 4–27. [DOI] [PubMed] [Google Scholar]
- [3].Humphrey JD, Dufresne ER, Schwartz MA, Mechanotransduction and extracellular matrix homeostasis, Nature reviews. Molecular cell biology 15(12) (2014) 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Spinale FG, Matrix Metalloproteinases, Circ Res 90(5) (2002) 520–530. [DOI] [PubMed] [Google Scholar]
- [5].Frangogiannis NG, The extracellular matrix in myocardial injury, repair, and remodeling, J Clin Invest 127(5) (2017) 1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Frangogiannis NG, The Extracellular Matrix in Ischemic and Nonischemic Heart Failure, Circ Res 125(1) (2019) 117–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Davis GE, Bayless KJ, Davis MJ, Meininger GA, Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules, Am J Pathol 156(5) (2000) 1489–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ricard-Blum S, Salza R, Matricryptins and matrikines: biologically active fragments of the extracellular matrix, Exp Dermatol 23(7) (2014) 457–63. [DOI] [PubMed] [Google Scholar]
- [9].Ricard-Blum S, Vallet SD, Proteases decode the extracellular matrix cryptome, Biochimie 122 (2016) 300–13. [DOI] [PubMed] [Google Scholar]
- [10].Wells JM, Gaggar A, Blalock JE, MMP generated matrikines, Matrix Biol 44–46 (2015) 122–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Duca L, Floquet N, Alix AJP, Haye B, Debelle L, Elastin as a matrikine, Critical Reviews in Oncology/Hematology 49(3) (2004) 235–244. [DOI] [PubMed] [Google Scholar]
- [12].Maquart F-X, Pasco S, Ramont L, Hornebeck W, Monboisse J-C, An introduction to matrikines: extracellular matrix-derived peptides which regulate cell activity: Implication in tumor invasion, Critical Reviews in Oncology/Hematology 49(3) (2004) 199–202. [DOI] [PubMed] [Google Scholar]
- [13].Maquart FX, Siméon A, Pasco S, Monboisse JC, Regulation of cell activity by the extracellular matrix: the concept of matrikines, J Soc Biol 193(4–5) (1999) 423–8. [PubMed] [Google Scholar]
- [14].Swindle CS, Tran KT, Johnson TD, Banerjee P, Mayes AM, Griffith L, Wells A, Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor, J Cell Biol 154(2) (2001) 459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Sinno H, Prakash S, Complements and the Wound Healing Cascade: An Updated Review, Plastic Surgery International 2013 (2013) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Midwood KS, Williams LV, Schwarzbauer JE, Tissue repair and the dynamics of the extracellular matrix, The international journal of biochemistry & cell biology 36(6) (2004) 1031–1037. [DOI] [PubMed] [Google Scholar]
- [17].Raghow R, The role of extracellular matrix in postinflammatory wound healing and fibrosis, The FASEB Journal 8(11) (1994) 823–831. [DOI] [PubMed] [Google Scholar]
- [18].Ricard-Blum S, Ballut L, Matricryptins derived from collagens and proteoglycans, Front Biosci (Landmark Ed) 16 (2011) 674–97. [DOI] [PubMed] [Google Scholar]
- [19].Kalluri R, Cantley LG, Kerjaschki D, Neilson EG, Reactive oxygen species expose cryptic epitopes associated with autoimmune goodpasture syndrome, J Biol Chem 275(26) (2000) 20027–32. [DOI] [PubMed] [Google Scholar]
- [20].Cawston TE, Young DA, Proteinases involved in matrix turnover during cartilage and bone breakdown, Cell and tissue research 339(1) (2010) 221–35. [DOI] [PubMed] [Google Scholar]
- [21].Lu P, Takai K, Weaver VM, Werb Z, Extracellular matrix degradation and remodeling in development and disease, Cold Spring Harb Perspect Biol 3(12) (2011) a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Vizovišek M, Fonović M, Turk B, Cysteine cathepsins in extracellular matrix remodeling: Extracellular matrix degradation and beyond, Matrix Biology 75–76 (2019) 141–159. [DOI] [PubMed] [Google Scholar]
- [23].Gaggar A, Jackson PL, Noerager BD, O’Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE, A Novel Proteolytic Cascade Generates an Extracellular Matrix-Derived Chemoattractant in Chronic Neutrophilic Inflammation, The Journal of Immunology 180(8) (2008) 5662–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garnotel R, Monboisse JC, Randoux A, Haye B, Borel JP, The binding of type I collagen to lymphocyte function-associated antigen (LFA) 1 integrin triggers the respiratory burst of human polymorphonuclear neutrophils. Role of calcium signaling and tyrosine phosphorylation of LFA 1, J Biol Chem 270(46) (1995) 27495–503. [DOI] [PubMed] [Google Scholar]
- [25].Zetter BR, Rasmussen N, Brown L, An in vivo assay for chemoattractant activity, Lab Invest 53(3) (1985) 362–8. [PubMed] [Google Scholar]
- [26].Mongiat M, Sweeney SM, San Antonio JD, Fu J, Iozzo RV, Endorepellin, a novel inhibitor of angiogenesis derived from the C terminus of perlecan, J Biol Chem 278(6) (2003) 4238–49. [DOI] [PubMed] [Google Scholar]
- [27].Ma DH-K, Yao J-Y, Kuo M-T, See L-C, Lin K-Y, Chen S-C, Chen J-K, Chao A-S, Wang S-F, Lin K-K, Generation of Endostatin by Matrix Metalloproteinase and Cathepsin from Human Limbocorneal Epithelial Cells Cultivated on Amniotic Membrane, Invest Ophthalmol Vis Sci 48(2) (2007) 644–651. [DOI] [PubMed] [Google Scholar]
- [28].Gaudet AD, Popovich PG, Extracellular matrix regulation of inflammation in the healthy and injured spinal cord, Experimental neurology 258 (2014) 24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wight TN, Potter-Perigo S, The extracellular matrix: an active or passive player in fibrosis?, American journal of physiology. Gastrointestinal and liver physiology 301(6) (2011) G950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell KY, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Bras LE, A Novel Collagen Matricryptin Reduces Left Ventricular Dilation Post-Myocardial Infarction by Promoting Scar Formation and Angiogenesis, J Am Coll Cardiol 66(12) (2015) 1364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Frey H, Schroeder N, Manon-Jensen T, Iozzo RV, Schaefer L, Biological interplay between proteoglycans and their innate immune receptors in inflammation, Febs J 280(10) (2013) 2165–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sorokin L, The impact of the extracellular matrix on inflammation, Nat Rev Immunol 10(10) (2010) 712–23. [DOI] [PubMed] [Google Scholar]
- [33].Jiang D, Liang J, Noble PW, Hyaluronan in Tissue Injury and Repair, Annual review of cell and developmental biology 23(1) (2007) 435–461. [DOI] [PubMed] [Google Scholar]
- [34].Ricard-Blum S, Vallet SD, Matricryptins Network with Matricellular Receptors at the Surface of Endothelial and Tumor Cells, Frontiers in pharmacology 7 (2016) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA, Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis, J Biol Chem 278(39) (2003) 37849–57. [DOI] [PubMed] [Google Scholar]
- [36].Vrhovski B, Weiss AS, Biochemistry of tropoelastin, Eur J Biochem 258(1) (1998) 1–18. [DOI] [PubMed] [Google Scholar]
- [37].Vindin H, Mithieux SM, Weiss AS, Elastin architecture, Matrix Biology 84 (2019) 4–16. [DOI] [PubMed] [Google Scholar]
- [38].Werb Z, Banda MJ, McKerrow JH, Sandhaus RA, Elastases and elastin degradation, The Journal of investigative dermatology 79 Suppl 1 (1982) 154s–159s. [DOI] [PubMed] [Google Scholar]
- [39].Jacob MP, Hornebeck W, Lafuma C, Bernaudin JF, Robert L, Godeau G, Ultrastructural and biochemical modifications of rabbit arteries induced by immunization with soluble elastin peptides, Experimental and Molecular Pathology 41(2) (1984) 171–190. [DOI] [PubMed] [Google Scholar]
- [40].Robert L, Stein F, Pezess MP, Poullain N, [Immunochemical properties of elastin. Their importance in atheromatosis], Revue de l’atherosclerose et des arteriopathies peripheriques 9(1) (1967) 233–41. [PubMed] [Google Scholar]
- [41].Fulop T Jr., Jacob MP, Varga Z, Foris G, Leovey A, Robert L, Effect of elastin peptides on human monocytes: Ca2+ mobilization, stimulation of respiratory burst and enzyme secretion, Biochem Biophys Res Commun 141(1) (1986) 92–8. [DOI] [PubMed] [Google Scholar]
- [42].Le Page A, Khalil A, Vermette P, Frost EH, Larbi A, Witkowski JM, Fulop T, The role of elastin-derived peptides in human physiology and diseases, Matrix Biology 84 (2019) 81–96. [DOI] [PubMed] [Google Scholar]
- [43].Senior RM, Griffin GL, Mecham RP, Wrenn DS, Prasad KU, Urry DW, Val-Gly-Val-Ala-Pro-Gly, a repeating peptide in elastin, is chemotactic for fibroblasts and monocytes, Journal of Cell Biology 99(3) (1984) 870–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Bisaccia F, Morelli MAC, Biasi MD, Traniello S, Spisani S, Tamburro AM, Migration of monocytes in the presence of elastolytic fragments of elastin and in synthetic derivates Structure‐activity relationships, International Journal of Peptide and Protein Research 44(4) (1994) 332–341. [DOI] [PubMed] [Google Scholar]
- [45].Price LSC, Roos PJ, Shively VP, Sandberg LB, Valyl-Alanyl-Prolyl-Glycine Serves as a Quantitative Marker for Human Elastins, Matrix 13(4) (1993) 307–311. [DOI] [PubMed] [Google Scholar]
- [46].Wachi H, Seyama Y, Yamashita S, Suganami H, Uemura Y, Okamoto K, Yamada H, Tajima S, Stimulation of cell proliferation and autoregulation of elastin expression by elastin peptide VPGVG in cultured chick vascular smooth muscle cells, FEBS Lett 368(2) (1995) 215–219. [DOI] [PubMed] [Google Scholar]
- [47].Shiratsuchi E, Ura M, Nakaba M, Maeda I, Okamoto K, Elastin peptides prepared from piscine and mammalian elastic tissues inhibit collagen-induced platelet aggregation and stimulate migration and proliferation of human skin fibroblasts, Journal of peptide science : an official publication of the European Peptide Society 16(11) (2010) 652–8. [DOI] [PubMed] [Google Scholar]
- [48].Duca L, Lambert E, Debret R, Rothhut B, Blanchevoye C, Delacoux F, Hornebeck W, Martiny L, Debelle L, Elastin peptides activate extracellular signal-regulated kinase 1/2 via a Ras-independent mechanism requiring both p110gamma/Raf-1 and protein kinase A/B-Raf signaling in human skin fibroblasts, Molecular pharmacology 67(4) (2005) 1315–24. [DOI] [PubMed] [Google Scholar]
- [49].Manon-Jensen T, Kjeld NG, Karsdal MA, Collagen-mediated hemostasis, Journal of Thrombosis and Haemostasis 14(3) (2016) 438–448. [DOI] [PubMed] [Google Scholar]
- [50].Aikio M, Alahuhta I, Nurmenniemi S, Suojanen J, Palovuori R, Teppo S, Sorsa T, Lopez-Otin C, Pihlajaniemi T, Salo T, Heljasvaara R, Nyberg P, Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma, PLoS One 7(12) (2012) e51044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lambert E, Fuselier E, Ramont L, Brassart B, Dukic S, Oudart JB, Dupont-Deshorgue A, Sellier C, Machado C, Dauchez M, Monboisse JC, Maquart FX, Baud S, Brassart-Pasco S, Conformation-dependent binding of a Tetrastatin peptide to alphavbeta3 integrin decreases melanoma progression through FAK/PI3K/Akt pathway inhibition, Scientific reports 8(1) (2018) 9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Koskimaki JE, Karagiannis ED, Tang BC, Hammers H, Watkins DN, Pili R, Popel AS, Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model, BMC Cancer 10 (2010) 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gunda V, Verma RK, Sudhakar YA, Inhibition of elastin peptide-mediated angiogenic signaling mechanism(s) in choroidal endothelial cells by the alpha6(IV)NC1 collagen fragment, Invest Ophthalmol Vis Sci 54(13) (2013) 7828–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Ricard-Blum S, Vallet SD, Fragments generated upon extracellular matrix remodeling: Biological regulators and potential drugs, Matrix Biology 75–76 (2019) 170–189. [DOI] [PubMed] [Google Scholar]
- [55].Kisling A, Lust RM, Katwa LC, What is the role of peptide fragments of collagen I and IV in health and disease?, Life sciences 228 (2019) 30–34. [DOI] [PubMed] [Google Scholar]
- [56].Colorado PC, Torre A, Kamphaus G, Maeshima Y, Hopfer H, Takahashi K, Volk R, Zamborsky ED, Herman S, Sarkar PK, Ericksen MB, Dhanabal M, Simons M, Post M, Kufe DW, Weichselbaum RR, Sukhatme VP, Kalluri R, Anti-angiogenic cues from vascular basement membrane collagen, Cancer Res 60(9) (2000) 2520–6. [PubMed] [Google Scholar]
- [57].Aikio M, Alahuhta I, Nurmenniemi S, Suojanen J, Palovuori R, Teppo S, Sorsa T, López-Otín C, Pihlajaniemi T, Salo T, Heljasvaara R, Nyberg P, Arresten, a collagen-derived angiogenesis inhibitor, suppresses invasion of squamous cell carcinoma, PLoS One 7(12) (2012) e51044–e51044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Boosani CS, Sudhakar A, Cloning, purification, and characterization of a non-collagenous anti-angiogenic protein domain from human α1 type IV collagen expressed in Sf9 cells, Protein Expr Purif 49(2) (2006) 211–218. [DOI] [PubMed] [Google Scholar]
- [59].Sudhakar A, Boosani CS, Signaling Mechanisms of Endogenous Angiogenesis Inhibitors Derived from Type IV Collagen, Gene Regulation and Systems Biology 1 (2007) GRSB.S345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Petitclerc E, Boutaud A, Prestayko A, Xu J, Sado Y, Ninomiya Y, Sarras MP Jr., Hudson BG, Brooks PC, New functions for non-collagenous domains of human collagen type IV. Novel integrin ligands inhibiting angiogenesis and tumor growth in vivo, J Biol Chem 275(11) (2000) 8051–61. [DOI] [PubMed] [Google Scholar]
- [61].Panka DJ, Mier JW, Canstatin inhibits Akt activation and induces Fas-dependent apoptosis in endothelial cells, J Biol Chem 278(39) (2003) 37632–6. [DOI] [PubMed] [Google Scholar]
- [62].Roth JM, Akalu A, Zelmanovich A, Policarpio D, Ng B, MacDonald S, Formenti S, Liebes L, Brooks PC, Recombinant α2(IV)NC1 Domain Inhibits Tumor Cell-Extracellular Matrix Interactions, Induces Cellular Senescence, and Inhibits Tumor Growth in Vivo, Am J Pathol 166(3) (2005) 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Magnon C, Opolon P, Ricard M, Connault E, Ardouin P, Galaup A, Métivier D, Bidart J-M, Germain S, Perricaudet M, Schlumberger M, Radiation and inhibition of angiogenesis by canstatin synergize to induce HIF-1α–mediated tumor apoptotic switch, J Clin Invest 117(7) (2007) 1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Mundel TM, Yliniemi AM, Maeshima Y, Sugimoto H, Kieran M, Kalluri R, Type IV collagen alpha6 chain-derived noncollagenous domain 1 (alpha6(IV)NC1) inhibits angiogenesis and tumor growth, Int J Cancer 122(8) (2008) 1738–44. [DOI] [PubMed] [Google Scholar]
- [65].Brassart-Pasco S, Sénéchal K, Thevenard J, Ramont L, Devy J, Di Stefano L, Dupont-Deshorgue A, Brézillon S, Feru J, Jazeron J-F, Diebold M-D, Ricard-Blum S, Maquart F-X, Monboisse JC, Tetrastatin, the NC1 Domain of the α4(IV) Collagen Chain: A Novel Potent Anti-Tumor Matrikine, PLoS One 7(4) (2012)e29587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Lambert E, Fuselier E, Ramont L, Brassart B, Dukic S, Oudart J-B, Dupont-Deshorgue A, Sellier C, Machado C, Dauchez M, Monboisse J-C, Maquart F-X, Baud S, Brassart-Pasco S, Conformation-dependent binding of a Tetrastatin peptide to αvβ3 integrin decreases melanoma progression through FAK/PI3K/Akt pathway inhibition, Scientific reports 8(1) (2018) 9837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Okada M, Imoto K, Sugiyama A, Yasuda J, Yamawaki H, New Insights into the Role of Basement Membrane-Derived Matricryptins in the Heart, Biological and Pharmaceutical Bulletin 40(12) (2017) 2050–2060. [DOI] [PubMed] [Google Scholar]
- [68].Okada M, Murata N, Yamawaki H, Canstatin stimulates migration of rat cardiac fibroblasts via secretion of matrix metalloproteinase-2, American Journal of Physiology-Cell Physiology 312(3) (2017) C199–c208. [DOI] [PubMed] [Google Scholar]
- [69].Urushiyama H, Terasaki Y, Nagasaka S, Kokuho N, Terasaki M, Kunugi S, Mikami Y, Noguchi S, Horie M, Nagahama K, Yamauchi Y, Shimizu A, Nagase T, Role of canstatin in early fibrotic lesions of idiopathic interstitial pneumonias and migration of lung fibroblasts, International Journal of Clinical and Experimental Pathology 9(12) (2016) 12714–12722. [Google Scholar]
- [70].Sugiyama A, Okada M, Yamawaki H, Pathophysiological roles of canstatin on myofibroblasts after myocardial infarction in rats, Eur J Pharmacol 807 (2017) 32–43. [DOI] [PubMed] [Google Scholar]
- [71].Hamano Y, Zeisberg M, Sugimoto H, Lively JC, Maeshima Y, Yang C, Hynes RO, Werb Z, Sudhakar A, Kalluri R, Physiological levels of tumstatin, a fragment of collagen IV alpha3 chain, are generated by MMP-9 proteolysis and suppress angiogenesis via alphaV beta3 integrin, Cancer Cell 3(6) (2003) 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yasuda J, Fukui K, Okada M, Yamawaki H, T3 peptide, a fragment of tumstatin, stimulates proliferation and migration of cardiac fibroblasts through activation of Akt signaling pathway, Naunyn Schmiedebergs Arch Pharmacol 390(11) (2017) 1135–1144. [DOI] [PubMed] [Google Scholar]
- [73].Han KY, Azar DT, Sabri A, Lee H, Jain S, Lee BS, Chang JH, Characterization of the interaction between endostatin short peptide and VEGF receptor 3, Protein and peptide letters 19(9) (2012) 969–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Han KY, Chang M, Ying HY, Lee H, Huang YH, Chang JH, Azar DT, Selective Binding of Endostatin Peptide 4 to Recombinant VEGF Receptor 3 In Vitro, Protein and peptide letters 22(11) (2015) 1025–30. [DOI] [PubMed] [Google Scholar]
- [75].Missirlis D, Haraszti T, Kessler H, Spatz JP, Fibronectin promotes directional persistence in fibroblast migration through interactions with both its cell-binding and heparin-binding domains, Scientific reports 7(1) (2017) 3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Ohashi T, Erickson HP, Domain unfolding plays a role in superfibronectin formation, J Biol Chem 280(47) (2005) 39143–51. [DOI] [PubMed] [Google Scholar]
- [77].Yi M, Ruoslahti E, A fibronectin fragment inhibits tumor growth, angiogenesis, and metastasis, Proc Natl Acad Sci U S A 98(2) (2001) 620–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Lin F, Zhu J, Tonnesen MG, Taira BR, McClain SA, Singer AJ, Clark RAF, Fibronectin peptides that bind PDGF-BB enhance survival of cells and tissue under stress, The Journal of investigative dermatology 134(4) (2014) 1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Ambesi A, McKeown-Longo PJ, Anastellin, the angiostatic fibronectin peptide, is a selective inhibitor of lysophospholipid signaling, Molecular cancer research : MCR 7(2) (2009) 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Livant DL, Brabec RK, Kurachi K, Allen DL, Wu Y, Haaseth R, Andrews P, Ethier SP, Markwart S, The PHSRN sequence induces extracellular matrix invasion and accelerates wound healing in obese diabetic mice, J Clin Invest 105(11) (2000) 1537–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Feng Y, Mrksich M, The synergy peptide PHSRN and the adhesion peptide RGD mediate cell adhesion through a common mechanism, Biochemistry 43(50) (2004) 15811–21. [DOI] [PubMed] [Google Scholar]
- [82].National Institutes of Health. National Heart Lung and Blood Institute Factbook Fiscal Year 2008. Available at: http://www.nhlbi.nih.gov/about/factbook/FactBookFinal.pdf., (2008).
- [83].Ge Q, Chen L, Jaffar J, Argraves WS, Twal WO, Hansbro P, Black JL, Burgess JK, Oliver B, Fibulin1C peptide induces cell attachment and extracellular matrix deposition in lung fibroblasts, Scientific reports 5(1) (2015) 9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Malinda KM, Wysocki AB, Koblinski JE, Kleinman HK, Ponce ML, Angiogenic laminin-derived peptides stimulate wound healing, The international journal of biochemistry & cell biology 40(12) (2008) 2771–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Iorio V, Troughton LD, Hamill KJ, Laminins: Roles and Utility in Wound Repair, Advances in wound care 4(4) (2015) 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Kleinman HK, Weeks BS, Schnaper HW, Kibbey MC, Yamamura K, Grant DS, The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases, Vitamins and hormones 47 (1993) 161–86. [DOI] [PubMed] [Google Scholar]
- [87].Ponce ML, Nomizu M, Delgado MC, Kuratomi Y, Hoffman MP, Powell S, Yamada Y, Kleinman HK, Malinda KM, Identification of endothelial cell binding sites on the laminin gamma 1 chain, Circ Res 84(6) (1999) 688–94. [DOI] [PubMed] [Google Scholar]
- [88].Ishihara J, Ishihara A, Fukunaga K, Sasaki K, White MJV, Briquez PS, Hubbell JA, Laminin heparin-binding peptides bind to several growth factors and enhance diabetic wound healing, Nature communications 9(1) (2018) 2163–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Saito Y, Imazeki H, Miura S, Yoshimura T, Okutsu H, Harada Y, Ohwaki T, Nagao O, Kamiya S, Hayashi R, Kodama H, Handa H, Yoshida T, Fukai F, A peptide derived from tenascin-C induces beta1 integrin activation through syndecan-4, J Biol Chem 282(48) (2007) 34929–37. [DOI] [PubMed] [Google Scholar]
- [90].Fujita M, Ito-Fujita Y, Iyoda T, Sasada M, Okada Y, Ishibashi K, Osawa T, Kodama H, Fukai F, Suzuki H, Peptide TNIIIA2 Derived from Tenascin-C Contributes to Malignant Progression in Colitis-Associated Colorectal Cancer via beta1-Integrin Activation in Fibroblasts, Int J Mol Sci 20(11) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Suzuki H, Sasada M, Kamiya S, Ito Y, Watanabe H, Okada Y, Ishibashi K, Iyoda T, Yanaka A, Fukai F, The Promoting Effect of the Extracellular Matrix Peptide TNIIIA2 Derived from Tenascin-C in Colon Cancer Cell Infiltration, International journal of molecular sciences 18(1) (2017) 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Bonafè F, Govoni M, Giordano E, Caldarera CM, Guarnieri C, Muscari C, Hyaluronan and cardiac regeneration, Journal of biomedical science 21 (2014) 100–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hauser-Kawaguchi A, Luyt LG, Turley E, Design of peptide mimetics to block pro-inflammatory functions of HA fragments, Matrix Biology 78–79 (2019) 346–356. [DOI] [PubMed] [Google Scholar]
- [94].Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW, Regulation of lung injury and repair by Toll-like receptors and hyaluronan, Nat Med 11(11) (2005) 1173–1179. [DOI] [PubMed] [Google Scholar]
- [95].Bourguignon LY, Wong G, Earle CA, Xia W, Interaction of low molecular weight hyaluronan with CD44 and toll-like receptors promotes the actin filament-associated protein 110-actin binding and MyD88-NFkappaB signaling leading to proinflammatory cytokine/chemokine production and breast tumor invasion, Cytoskeleton (Hoboken) 68(12) (2011) 671–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Huang YC, Huang KY, Lew WZ, Fan KH, Chang WJ, Huang HM, Gamma-Irradiation-Prepared Low Molecular Weight Hyaluronic Acid Promotes Skin Wound Healing, Polymers 11(7) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Campo GM, Avenoso A, D’Ascola A, Prestipino V, Scuruchi M, Nastasi G, Calatroni A, Campo S, 4-mer hyaluronan oligosaccharides stimulate inflammation response in synovial fibroblasts in part via TAK-1 and in part via p38-MAPK, Curr Med Chem 20(9) (2013) 1162–72. [DOI] [PubMed] [Google Scholar]
- [98].Cailhier JF, Sirois I, Laplante P, Lepage S, Raymond MA, Brassard N, Prat A, Iozzo RV, Pshezhetsky AV, Hebert MJ, Caspase-3 activation triggers extracellular cathepsin L release and endorepellin proteolysis, J Biol Chem 283(40) (2008) 27220–9. [DOI] [PubMed] [Google Scholar]
- [99].Douglass S, Goyal A, Iozzo RV, The role of perlecan and endorepellin in the control of tumor angiogenesis and endothelial cell autophagy, Connect Tissue Res 56(5) (2015) 381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bix G, Iozzo RV, Matrix revolutions: “tails” of basement-membrane components with angiostatic functions, Trends in cell biology 15(1) (2005) 52–60. [DOI] [PubMed] [Google Scholar]
- [101].Bix G, Fu J, Gonzalez EM, Macro L, Barker A, Campbell S, utter MM, Santoro SA, Kim JK, H k M, Reed CC, Iozzo RV Endorepellin causes endothelial cell disassembly of actin cytoskeleton and focal adhesions through α2β1 integrin, Journal of Cell Biology 166(1) (2004) 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Laplante P, Raymond MA, Labelle A, Abe J, Iozzo RV, Hebert MJ, Perlecan proteolysis induces an alpha2beta1 integrin- and Src family kinase-dependent anti-apoptotic pathway in fibroblasts in the absence of focal adhesion kinase activation, J Biol Chem 281(41) (2006) 30383–92. [DOI] [PubMed] [Google Scholar]
- [103].Nielsen SH, Mouton AJ, DeLeon-Pennell KY, Genovese F, Karsdal M, Lindsey ML, Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes, Matrix Biol 75–76 (2019) 43–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Urushiyama H, Terasaki Y, Nagasaka S, Terasaki M, Kunugi S, Nagase T, Fukuda Y, Shimizu A, Role of α1 and α2 chains of type IV collagen in early fibrotic lesions of idiopathic interstitial pneumonias and migration of lung fibroblasts, Laboratory Investigation 95(8) (2015) 872–885. [DOI] [PubMed] [Google Scholar]
- [105].O’Reilly PJ, Jackson PL, Wells JM, Dransfield MT, Scanlon PD, Blalock JE, Sputum PGP is reduced by azithromycin treatment in patients with COPD and correlates with exacerbations, BMJ open 3(12) (2013) e004140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wells JM, Jackson PL, Viera L, Bhatt SP, Gautney J, Handley G, King RW, Xu X, Gaggar A, Bailey WC, Dransfield MT, Blalock JE, A Randomized, Placebo-controlled Trial of Roflumilast. Effect on Proline-Glycine-Proline and Neutrophilic Inflammation in Chronic Obstructive Pulmonary Disease, Am J Respir Crit Care Med 192(8) (2015) 934–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Hardison MT, Galin FS, Calderon CE, Djekic UV, Parker SB, Wille KM, Jackson PL, Oster RA, Young KR, Blalock JE, Gaggar A, The presence of a matrix-derived neutrophil chemoattractant in bronchiolitis obliterans syndrome after lung transplantation, J Immunol 182(7) (2009) 4423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Akthar S, Patel DF, Beale RC, Peiro T, Xu X, Gaggar A, Jackson PL, Blalock JE, Lloyd CM, Snelgrove RJ, Matrikines are key regulators in modulating the amplitude of lung inflammation in acute pulmonary infection, Nature communications 6 (2015) 8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Xu X, Jackson PL, Tanner S, Hardison MT, Abdul Roda M, Blalock JE, Gaggar A, A self-propagating matrix metalloprotease-9 (MMP-9) dependent cycle of chronic neutrophilic inflammation, PLoS One 6(1) (2011) e15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Gaggar A, Weathington N, Bioactive extracellular matrix fragments in lung health and disease, J Clin Invest 126(9) (2016) 3176–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].O’Reilly PJ, Ding Q, Akthar S, Cai G, Genschmer KR, Patel DF, Jackson PL, Viera L, Roda M, Locy ML, Bernstein EA, Lloyd CM, Bernstein KE, Snelgrove RJ, Blalock JE, Angiotensin-converting enzyme defines matrikine-regulated inflammation and fibrosis, JCI insight 2(22) (2017) e91923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Jaffar J, Unger S, Corte TJ, Keller M, Wolters PJ, Richeldi L, Cerri S, Prêle CM, Hansbro PM, Argraves WS, Oliver RA, Oliver BG, Black JL, Burgess JK, Fibulin-1 predicts disease progression in patients with idiopathic pulmonary fibrosis, Chest 146(4) (2014) 1055–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lau JY, Oliver BG, Baraket M, Beckett EL, Hansbro NG, Moir LM, Wilton SD, Williams C, Foster PS, Hansbro PM, Black JL, Burgess JK, Fibulin-1 is increased in asthma--a novel mediator of airway remodeling?, PLoS One 5(10) (2010) e13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu G, Cooley MA, Jarnicki AG, Borghuis T, Nair PM, Tjin G, Hsu AC, Haw TJ, Fricker M, Harrison CL, Jones B, Hansbro NG, Wark PA, Horvat JC, Argraves WS, Oliver BG, Knight DA, Burgess JK, Hansbro PM, Fibulin-1c regulates transforming growth factor–β activation in pulmonary tissue fibrosis, JCI Insight 4(16) (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Russo I, Cavalera M, Huang S, Su Y, Hanna A, Chen B, Shinde AV, Conway SJ, Graff J, Frangogiannis NG, Protective Effects of Activated Myofibroblasts in the Pressure-Overloaded Myocardium Are Mediated Through Smad-Dependent Activation of a Matrix-Preserving Program, Circ Res 124(8) (2019) 1214–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Yamaguchi Y, Takihara T, Chambers RA, Veraldi KL, Larregina AT, Feghali-Bostwick CA, A Peptide Derived from Endostatin Ameliorates Organ Fibrosis, Science Translational Medicine 4(136) (2012) 136ra71–136ra71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Li Y, Ren H-T, Endostatin inhibits fibrosis by modulating the PDGFR/ERK signal pathway: an in vitro study, J Zhejiang Univ Sci B 18(11) (2017) 994–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ren H, Li Y, Chen Y, Wang L, Endostatin attenuates PDGF-BB- or TGF-beta1-induced HSCs activation via suppressing RhoA/ROCK1 signal pathways, Drug Des Devel Ther 13 (2019) 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Isobe K, Kuba K, Maejima Y, Suzuki J.-i., Kubota S, Isobe M, Inhibition of Endostatin/Collagen XVIII Deteriorates Left Ventricular Remodeling and Heart Failure in Rat Myocardial Infarction Model, Circulation Journal 74(1) (2010) 109–119. [DOI] [PubMed] [Google Scholar]
- [120].Lee YH, Kim KP, Park SH, Kim DJ, Kim YG, Moon JY, Jung SW, Kim JS, Jeong KH, Lee SY, Yang DH, Lim SJ, Woo JT, Rhee SY, Chon S, Choi HY, Park HC, Jo YI, Yi JH, Han SW, Lee SH, Urinary chemokine C-X-C motif ligand 16 and endostatin as predictors of tubulointerstitial fibrosis in patients with advanced diabetic kidney disease, Nephrol Dial Transplant (2019). [DOI] [PubMed] [Google Scholar]
- [121].Sharma NS, Lal CV, Li JD, Lou XY, Viera L, Abdallah T, King RW, Sethi J, Kanagarajah P, Restrepo-Jaramillo R, Sales-Conniff A, Wei S, Jackson PL, Blalock JE, Gaggar A, Xu X, The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome, Am J Physiol Lung Cell Mol Physiol 315(5) (2018) L653–L661. [DOI] [PMC free article] [PubMed] [Google Scholar]


