Abstract
Radiation therapy is an important modality in the treatment of lung cancer, but it can lead to radiation pneumonitis, and eventually radiation fibrosis. To date, only few available drugs can effectively manage radiation-induced pulmonary fibrosis. Lipoxins are endogenous molecules exhibit anti-inflammatory and pro-resolving effects. These molecules play a vital role in reducing excessive tissue injury and chronic inflammation; however, their effects on radiation-induced lung injury (RILI) are unknown. In this study, we investigated the effects of lipoxin A4 (LXA4) on RILI using our specialized small-animal model of RILI following focal-ablative lung irradiation (IR). LXA4 significantly inhibited immune-cell recruitment and reduced IR-induced expression of pro-inflammatory cytokines and fibrotic proteins in the lung lesion sites. In addition, micro-CT revealed that LXA4 reduced IR-induced increases in lung consolidation volume. The flexiVentTM assays showed that LXA4 significantly reversed IR-induced lung function damage. Moreover, LXA4 downregulated the activities of NF-κB and the Smad-binding element promoters. The expression of FPR2, an LXA4 receptor, increased during the development of IR-induced pulmonary fibrosis, whereas silencing of endogenous LXA4 using an antagonist (WRW4) or FPR2 siRNA resulted in impaired development of pulmonary fibrosis in response to IR. Collectively, these data suggest that LXA4 could serve as a potent therapeutic agent for alleviating RILI.
Subject terms: Target identification, Respiratory tract diseases
Introduction
Radiation therapy is an important modality for treating lung cancer. Generally, it is necessary to increase the radiation dose to control the tumor by radiation, but higher radiation dose results in higher probability of developing side effects. Therefore, radiation-induced lung damage (RILI) is a major bottleneck when aiming to improve disease-free survival and well-being in patients with lung cancer1–3. RILI is classified into two types, acute phase radiation pneumonitis—that generally subsides after a few weeks—and radiation-induced pulmonary fibrosis that can develop several months or years after irradiation (IR)4,5. Modern clinical radiotherapy techniques, including stereotactic radiotherapy (SBRT)—which delivers small fractions of ablative radiation doses—are more accurate and precise than conventional radiotherapy techniques. Previously, we had developed a mouse model that simulates SBRT; we used an image-guided small-animal IR system to deliver a single high dose of radiation to the lung and verified the induction of pulmonary fibrosis in response to IR6–8.
Radiation pneumonitis develops within hours or days of lung IR and is accompanied by increased capillary permeability, leukocyte infiltration, and release of cytokines, such as transforming growth factor β (TGF-β), interleukin 6 (IL-6), tumor necrosis factor alpha, and interleukin-1 beta (IL-1β). Subsequent alveolar lung inflammation and persistent inflammation may lead to symptomatic phases, including pulmonary fibrosis9,10. TGF-β activation induces the differentiation of fibroblasts into myofibroblasts, expression of α-smooth muscle actin (α-SMA), and synthesis of extracellular matrix proteins, such as collagen11,12. Alveolar epithelial cells (AECs) are another important source of myofibroblasts and may differentiate into myofibroblasts during idiopathic pulmonary fibrosis and radiation-induced pulmonary fibrosis. This epithelial or endothelial–mesenchymal transition (EMT or EndMT) is generally known to be involved in the development of pulmonary fibrosis13. During EMT, cells undergo a morphological change from a round phenotype to a spindle-like phenotype, accompanied by a loss of epithelial cell markers—such as E-cadherin—and the gain of mesenchymal markers, such as vimentin and α-SMA14. In addition, NF-κB signaling—an important regulator of inflammatory responses—and TGF-β/Smad signaling are important regulatory mechanisms of EMT12,13,15.
The lipid mediator, LXA4, is a bioactive product of arachidonic acid that has been reported to exert a variety of activities in multiple tissues, including anti-inflammatory effects, and regulation of neutrophil infiltration, pro-resolving signaling, macrophage polarization, and the nonphlogistic uptake of apoptotic polymorphonuclear neutrophils16,17. These activities of LXA4 are regulated by the G protein-coupled ALX/FPR2 receptor18,19. LXA4 reportedly reduces DM-related renal fibrosis by targeting TGF-β/Smad signaling and suppresses liver fibrosis in experimental models by regulating the immune responses and modulating the expression of regeneration genes20,21. It also lowers TGF-β levels in bleomycin-induced pulmonary fibrosis and exhibits an anti-fibrotic effect22. Furthermore, lipoxin inhibits EMT in cancer models, such as those of pancreatic and liver cancers, and LXA4 was found to inhibit EMT in a renal fibrosis model23–25. However, the role of lipoxin in RILI has not yet been reported.
Here, we examined the anti-inflammatory and anti-fibrotic properties of LXA4 using a mouse model of RILI, wherein ablative radiation doses were delivered to the focal lung area using an image-guided IR system6,7,26–28. We also investigated whether LXA4 regulates proteins that are important for EMT, particularly whether the crosstalk between LXA4-ALX/FPR2 and TGF-β/Smad signaling plays a key role in EMT.
Results
LXA4 inhibits radiation-induced lung inflammatory responses
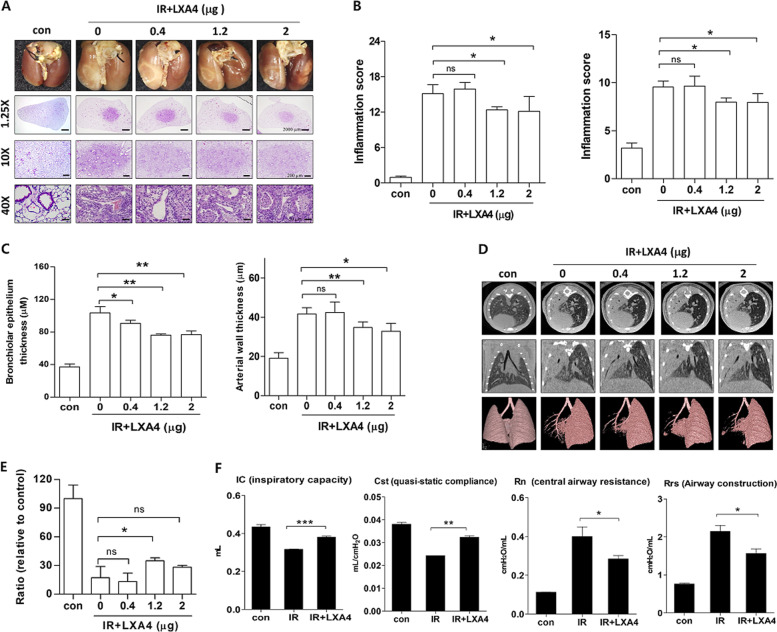
To mimic the effects of clinical radiotherapy in an animal model of IR-induced lung damage, we had previously exposed mice lungs to high-dose radiation using a small-animal micro-irradiator (X-RAD320) equipped with a collimator system (to produce focal radiation beams)7,8,28. These studies showed that radiation pneumonitis occurs in mice within 2 weeks of exposure to 60‒100 Gy of IR8,15,28. Herein, we evaluated the effects of LXA4 on pneumonitis 2 weeks after 75 Gy IR by evaluating the lung morphology and performing hematoxylin and eosin (H&E) staining and pulmonary function assays (micro-computed tomography (CT) and flexiVentTM). The normal lung was brown in color, whereas the irradiated lung exhibited a ring-like boundary with white-colored adjacent areas 2 weeks post-IR (Fig. 1a, upper). The IR group displayed significantly more inflammation at the lesion site than the control group, and inflammation was significantly blocked by LXA4 (1.2 and 2 μg per mouse) in the IR + LXA4 group, which displayed less damage than the IR group (Fig. 1b, left). Inflammatory changes in the tissues surrounding the irradiated lesion were also significantly reduced in the LXA4-treated groups (1.2 and 2 μg/mouse) compared to those in the IR group (Fig. 1b, right). Further, LXA4 significantly reduced the IR-induced increases in the thickness of the bronchiolar epithelium and arterial wall (Fig. 1c). Micro-computed tomographic (micro-CT) images taken 2 weeks after IR show that pulmonary consolidation throughout the left lung of irradiated mice makes it possible to measure high air-tissue contrast8,28. We found that LXA4 reduced IR-induced pulmonary consolidation (Fig. 1d, e); therefore, we analyzed pulmonary function—based on six parameters—using the flexiVentTM system (Supplementary Table 1). Four of these parameters showed significant improvement in the IR + LXA4 group, i.e., IC (inspiratory capacity; IR vs. IR + LXA4, 0.316 ± 0.0017 mL vs. 0.3812 ± 0.0044 mL), Cst (quasi-static compliance; IR vs. IR + LXA4, 0.02428 ± 0.00048 mL/cmH2O vs. 0.03231 ± 0.0006852 mL/cmH2O), Rn (airway construction; IR vs. IR + LXA4, 0.4000 ± 0.04930 cmH2O/mL vs. 0.2843 ± 0.01795 cmH2O/mL), and Rrs (central airway resistance; IR vs. IR + LXA4, 2.145 ± 0.1612 cmH2O/mL vs. 1.564 ± 0.1237 cmH2O/mL) (Fig. 1f).
Fig. 1. Effects of LXA4 on radiation-induced pneumonia 2 weeks after irradiation (IR).
a Representative gross (top) and H&E (bottom) images of left lung tissues 2 weeks after 75 Gy IR with or without LXA4 in the mouse model. Magnification, ×1.25, ×10, and ×40. b Quantification of inflammatory foci at the lesion site (left) and in the surrounding lung tissue (right). c Quantification of bronchiolar epithelium (left) and arterial wall (right) thickness in lung tissues. d 3-D micro-computed tomography (CT) of mouse lung tissue. Horizontal (top), trans-axial (middle), and 3-D images (bottom) acquired 2 weeks post-IR. e Normal lung volume excluding consolidation was quantified using the micro-CT images. f Several mouse lung function parameters were measured using the flexiVentTM system 2 weeks after irradiation, i.e., inspiratory capacity (IC), quasi-static compliance (Cst), central airway resistance (Rn), and airway construction (Rrs). Data are expressed as the mean ± standard error (n = 3, *p < 0.05, **p < 0.01, and ***p < 0.001).
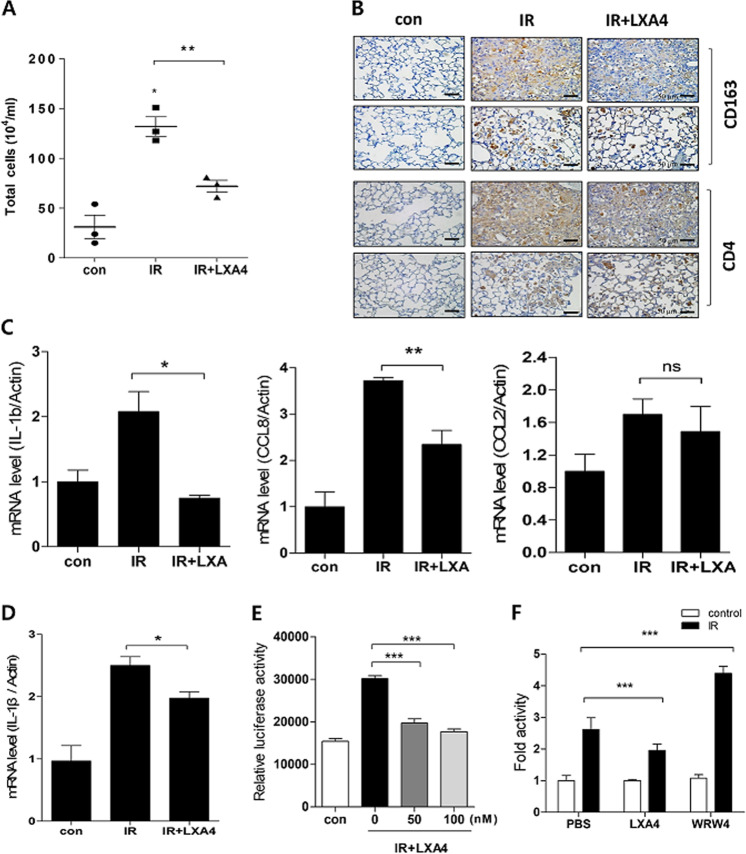
Bronchoalveolar lavage fluid (BALF) differential cell counts can provide supportive or even diagnostic information regarding various conditions, including lung inflammatory responses. IR increased the total number of BALF cells compared to that in the control group, whereas LXA4 rescued this effect and reduced the IR-induced infiltration of immune cells such as CD163+ and CD4+ cells (Fig. 2b). To confirm this anti-inflammatory effect, we evaluated inflammatory cytokine and chemokine expression, and found that while IR significantly upregulated IL-1b, CCL2, and CCL8 expression, LXA4 attenuated their expression in the mouse model (Fig. 2c). Similarly, LXA4 decreased the IR-induced increase in IL-1b expression in MLE12 cells (Fig. 2d), and significantly attenuated IR-induced NF-κB promoter activity—which mediates inflammatory gene transcription—in a dose-dependent manner (Fig. 2e). However, WRW4—a specific FPR2 antagonist—blocked the effect of endogenous LXA4 and enhanced IR-induced NF-κB promoter activity (Fig. 2f).
Fig. 2. Effects of LXA4 on radiation-induced inflammatory response.
a Total BALF-cell count in mice 2 weeks post-IR. b Representative immunohistochemical images of irradiated lung tissues using anti-CD163 and CD4 antibodies. Magnification, ×40. c IL-1b, CCL8, and CCL2 mRNA expression in irradiated lung tissue measured using quantitative real-time PCR. d IL-1b mRNA expression in MLE12 cells measured using quantitative real-time PCR. e pNF-κB reporter activity in MLE12 cells exposed to 6 Gy IR according to LXA4 dosage. f pNF-κB reporter fold activity in 6 Gy IR-exposed MLE12 cells transfected with or without WRW4. Data are expressed as the mean ± standard error of three independent experiments (*p < 0.05, **p < 0.01, and ***p < 0.001).
LXA4 attenuates radiation-induced pulmonary fibrosis
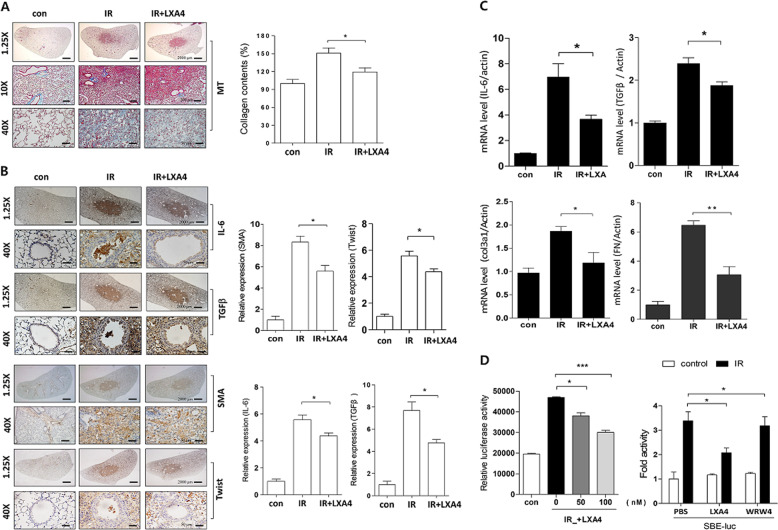
Previously, we had observed that the inflammatory response occurred for around 2 weeks, and that fibrosis was apparent 4‒6 weeks after ablative doses of IR6,8,28. Interestingly, we began to observe collagen deposition—using Masson’s trichrome (MT) staining—2 weeks after IR, with collagen deposition increasing thereafter (Fig. 3a). This early fibrosis was attenuated by LXA4 as was the expression of proteins involved in pulmonary fibrosis, including IL-6, TGF-β, α-SMA, and Twist (Fig. 3b). Moreover, LXA4 decreased IL-6, TGF-β, Col3a1, and fibronectin (FN) mRNA levels, which were increased by IR in MLE12 cells (Fig. 3c). Next, we performed a luciferase reporter assay to determine whether the effect of FPR2 on Smad-binding element (SBE) activity mediated TGF-β/Smad signaling. IR increased SBE activity, whereas LXA4 treatment significantly attenuated this increase (Fig. 3d, left). However, WRW4, a specific FPR2 antagonist, blocked the effect of endogenous LXA4 and increased IR-induced SBE-luciferase promoter activity (Fig. 3d, right). Thus, LXA4 may regulate collagen deposition and protein expression—associated with early RILI—and inhibit inflammation after IR.
Fig. 3. LXA4 inhibits IR-induced pulmonary fibrosis at 2 weeks.
a Representative MT-stained image of mouse lung tissue 2 weeks post-IR (75 Gy) with or without LXA4 in the mouse model (left). Quantification of collagen content (right). Magnification, ×1.25, ×10, and ×40. b Representative immunohistochemical image (left) and quantification (right) of irradiated lung tissues stained with antibodies against IL-6, TGF-β, α-SMA, and Twist. Magnification, ×1.25 and ×40. c IL-6, TGF-β, Col3al, and fibronectin (FN) mRNA expression in the irradiated lung tissue of mice, measured using quantitative real-time PCR. d pSmad reporter activity in 6 Gy IR-exposed MLE12 cells transfected with or without WRW4. Data are expressed as the mean ± standard error of three to five independent experiments (*p < 0.05, **p < 0.01, and ***p < 0.001).
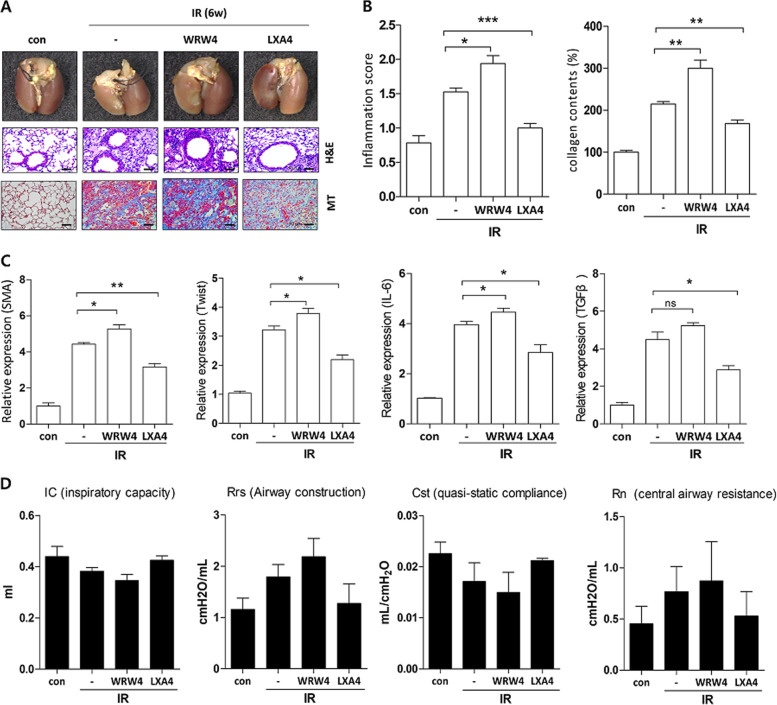
Six weeks after IR, LXA4 decreased the radiation-induced increase in immune-cell infiltration and collagen expression to match the levels in the early stage IR (Fig. 4a, b). Furthermore, we confirmed that WRW4, an FPR2 antagonist that blocks the effect of endogenous LXA4, was upregulated in the IR-treated group (Fig. 4a, b). Bronchiolar epithelium thickness and arterial wall thickness also responded to the inhibitory effects of LXA4 and WRW4 (data not shown). Moreover, the expression of α-SMA, Twist, IL-6, and TGF-β was significantly inhibited by LXA4 and increased by WRW4 (Fig. 4c). Lung function was rescued by LXA4, which significantly reversed four of the six lung function parameters (IC, Cst, Rrs, and Rn), and was worsened by WRW4, but this was not significant (Fig. 4d). These results demonstrate that LXA4 may inhibit the development of radiation-induced fibrosis via the FPR2 receptor.
Fig. 4. LXA4 attenuates IR-induced pulmonary fibrosis and rescues lung function.
a Representative gross (top), H&E (middle), and MT (bottom) images of the left lung tissue 6 weeks after 75 Gy IR with or without LXA4 in the mouse model. Magnification, ×40. b Quantification of inflammatory foci (left) and collagen content (right). c Quantification of relative α-SMA, Twist, IL-6, and TGF-β expression in the lung lesion site using immunohistochemistry. d Functional measurement of several mouse lung parameters using the flexiVentTM system 6 weeks post-IR, i.e., inspiratory capacity (IC), quasi-static compliance (Cst), central airway resistance (Rn), and airway construction (Rrs). Data are expressed as the mean ± standard error (n = 3‒5, *p < 0.05, **p < 0.01, and ***p < 0.001).
Radiation affects LXA4-FPR2 signaling
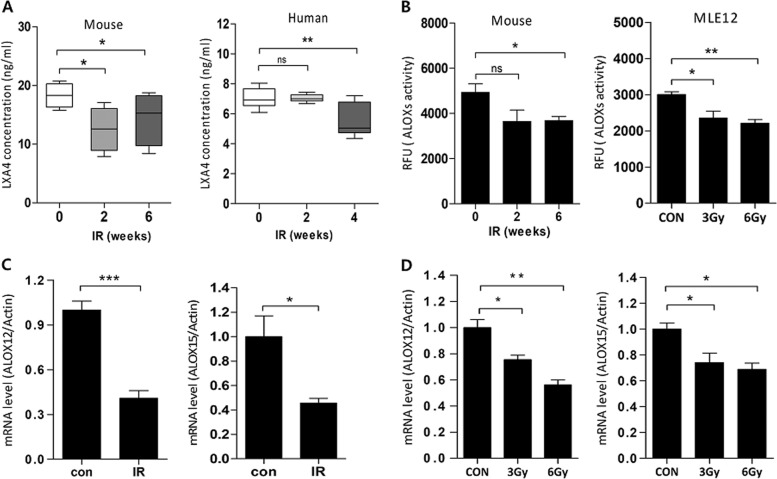
Next, we explored whether IR affects LXA4 levels using enzyme-linked immunosorbent assays (ELISA) on mouse blood samples. As shown in Fig. 5a (left), endogenous LXA4 levels were significantly lower 2 (12.56 ± 1.885 ng/mL) and 6 (14.46 ± 2.248 ng/mL) weeks after IR than those in the control group (18.33 ± 1.037 ng/mL). Similarly, LXA4 levels in the human blood (Fig. 5a, right panel) were significantly lower after 4 weeks of IR (5.527 ± 0.4614 ng/mL) than those in the control group (7.044 ± 0.2798 ng/mL). IR also reduced the lipoxygenase activity in the mouse model and MLE12 cells (Fig. 5b) and downregulated Alox12 (12-lipoxygenase) and Alox15 (15-lipoxygenase), lipoxygenase variants—involved in arachidonic acid metabolism—in mouse lung lesion sites (Fig. 5c) and MLE12 cells (Fig. 5d).
Fig. 5. IR reduces LXA4 production.
a Determination of plasma LXA4 concentration in mice. Plasma samples were collected from mice 2 and 6 weeks post-75 Gy IR and their LXA4 levels were measured by ELISA. b LOX enzyme activity was measured in mice (left) and MLE12 cells (right) using a Lipoxygenase Assay kit, according to the manufacturer’s instructions. ALOX12 and ALOX15 mRNA levels in the irradiated lung tissues of mice (c) and MLE12 cells (d) measured using quantitative real-time PCR. Data are expressed as the mean ± standard error (n = 3‒5, *p < 0.05, **p < 0.01, and ***p < 0.001).
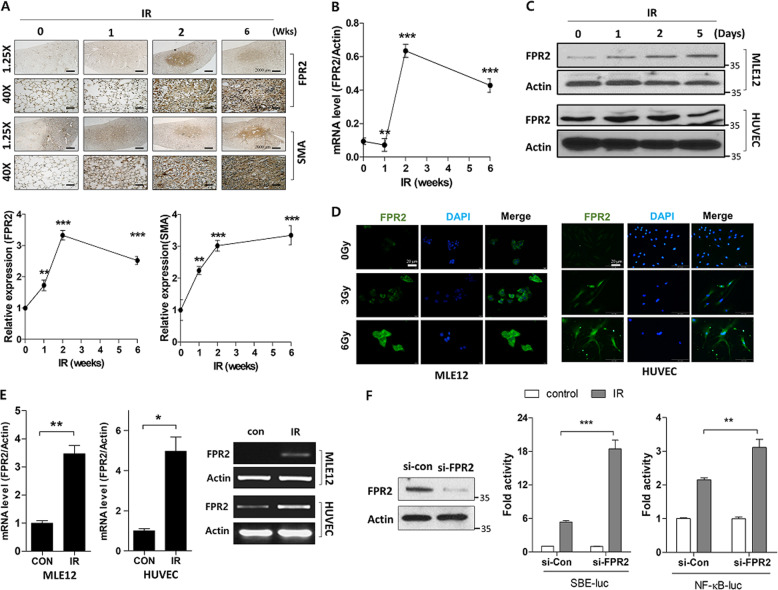
To investigate FPR2 expression, we focally irradiated the left lung of mice (3-mm diameter) with high-dose radiation (75 Gy) at the indicated times. IR increased the degree of pulmonary damage, as evidenced by inflammatory-cell infiltration—H&E staining—and collagen content—MT staining—in the IR-induced lung damage model in mice (Supplementary Fig. 1). Moreover, α-SMA expression—related to fibrosis—increased in the irradiated left lung over time. Interestingly, IR significantly increased FPR2 levels at the lesion site (Fig. 6a, 75 Gy; and Supplementary Fig. 2, 90 Gy) alongside FPR2 mRNA levels 2 weeks after IR; however, FPR2 mRNA levels were lower 6 weeks after IR (Fig. 6b). We confirmed IR-induced FPR2 expression in the mouse lung epithelial cell line—MLE12—and in human endothelial cells (HUVECs) using western blotting (Fig. 6c), immunofluorescence (Fig. 6d), and real-time quantitative RT-PCR (Fig. 6e); these showed that FPR2 levels significantly increased after 48 h of IR. Next, we performed a luciferase reporter assay to determine the effect of FPR2 on SBE and NF-κB promoter activities, which regulate TGF-β signaling and inflammatory responses. Interestingly, IR increased the activities of both promoters in MLE12 cells—as did si-FPR2—compared to those in control (Fig. 6f). Therefore, these data suggest that the altered LXA4 signaling, including increased FPR2 expression and crosstalk with TGF-β/Smad signaling, may be involved in the development of IR-induced pulmonary damage.
Fig. 6. IR regulates ALX/FPR2 expression.
a Representative image (top) and quantification (bottom) of irradiated lung tissues (75 Gy) stained for FPR2 and α-SMA at the indicated times. Magnification, ×1.25 and ×40. b FPR2 mRNA levels in IR-exposed mouse lung tissue measured using quantitative real-time PCR. c FPR2 expression measured by western blotting after exposure of MLE12 cells and HUVECs to 6 Gy IR. d FPR2 expression 24 h after 3 and 6 Gy IR in MLE12 cells and HUVECs; FPR2 (green); DAPI (blue). e FPR2 mRNA levels in MLE12 cells and HUVECs exposed to 6 Gy IR, measured using quantitative real-time PCR. f pSmad and pNF-κB promoter reporter activity in 6 Gy IR MLE12 cells transfected with or without FPR2 siRNA. Data are expressed as the mean ± standard error (n = 3‒5, *p < 0.05, **p < 0.01, and ***p < 0.001).
LXA4 blocks IR-induced cell death and oxidative response
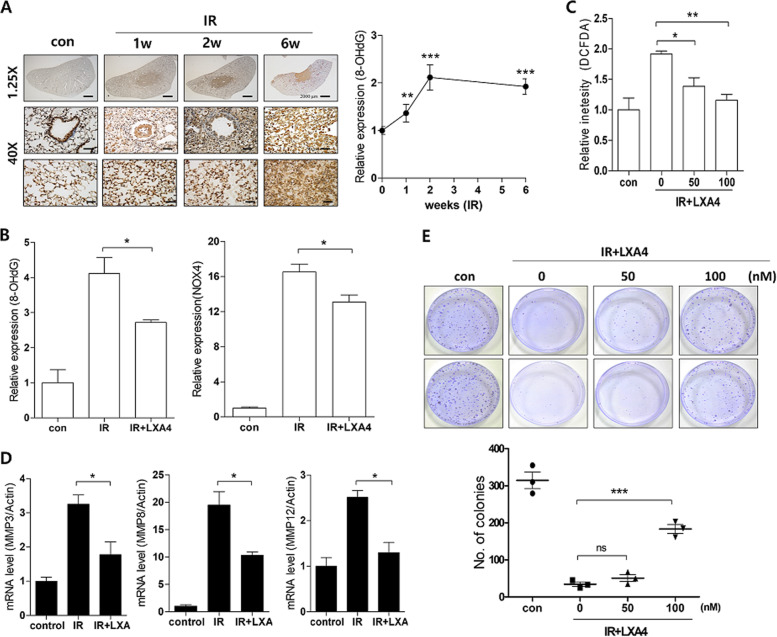
IR increased the expression of the critical free radical marker, 8-hydroxy-2′-deoxyguanosine (8-OHdG) over time (Fig. 7a); therefore, we examined 8-OHdG levels and NOX4 expression—which is involved in reactive oxygen species (ROS) modulation—using immunohistochemistry in irradiated mouse lung models to observe the effect of LXA4 on radiation-induced oxidative stress. 8-OHdG and NOX4 levels were increased 6 weeks post-IR, and were significantly decreased by LXA4 (Fig. 7b). Moreover, LXA4 reduced IR-induced ROS production in MLE12 cells—observed using a DCFDA probe (Fig. 7c)—and also reduced MMP expression (Fig. 7d) and cell death (Fig. 7e). These data suggest that LXA4 can block IR-induced increase in cell death and oxidative response.
Fig. 7. LXA4 decreases the IR-induced oxidative response.
a Representative image (right) and quantification (left) of irradiated lung tissues stained with antibodies against 8-OHdG at the indicated times. Magnification, ×1.25 and ×40. b Quantification of relative 8-OHdG and NOX4 expression in the lung lesion site 6 weeks after 75 Gy IR with or without LXA4 using immunohistochemistry. c ROS levels were measured by incubating irradiated MLE12 cells treated with or without LXA4 with a DCFDA probe. d MMP3, MMP8, and MMP12 mRNA expression in IR-exposed mouse lung tissue, measured using quantitative real-time PCR. e Colony forming activity was examined in MLE12 cells. Data are expressed as the mean ± standard error (n = 3, *p < 0.05, **p < 0.01, and ***p < 0.001).
Discussion
As the use of radiation for treating cancer—and the number of patients surviving cancer—is increasing worldwide, it has become increasingly important to improve the quality of life of these individuals and to modulate the effects of radiation therapy on normal tissues29. IR increases inflammatory reactions, thereby causing inflammatory cells to accumulate in the lesion sites of lung tissue, enhancing inflammatory cytokine expression (IL-6, IL-1b, and TGF-β) in lung tissues, and recruiting immune cells (macrophages, neutrophils, lymphocytes, and eosinophils) in BALF6,28. To our knowledge, this study is the first to demonstrate the effect of lipoxin on RILI; it reveals that LXA4 exhibits anti-inflammatory and anti-fibrotic effects in a RILI model. LXA4 not only inhibited radiation-induced pro-inflammatory cytokine production but also suppressed immune-cell recruitment into the alveolar space, downregulated fibrosis-related gene expression, and restored the damaged lung tissue and function, thereby ameliorating RILI.
Although lipoxins were discovered almost four decades ago, they have not yet reached the clinical trials stage—as potential drugs to treat inflammatory diseases—owing to their short half-life and instability on exposure to acids and light; however, stable analogs are being developed. In this study, we predominantly investigated the anti-fibrotic effects of LXA4—2 and 6 weeks after 75 Gy of IR—by immunostaining for α-SMA, TGF-β, and Twist and by MT staining for collagen expression. Two weeks after exposure to 75 Gy IR, our experimental model manifested radiation pneumonitis with radiation-induced inflammation, whereas 6 weeks after 75 Gy IR exposure, the model manifested IR-induced lung fibrosis with increased fibrosis marker and collagen expression8,26,28. To investigate the crosstalk between FPR2, IR-induced inflammatory responses, and fibrosis-related gene expression, we observed NF-κB and SBE promoter activity in the background of IR. NF-κB regulates inflammation-related signals, and its activity is known to increase during various inflammatory reactions, including radiation. We confirmed that IR increased NF-κB and SBE promoter activities in TGF-β/Smad signaling, which regulates the expression of fibrotic genes. In addition, administering si-FPR2 and WRW4 to reduce the effects of endogenous FPR2 further increased the activities of these two promoters in the IR group, whereas LXA4 suppressed their activities. To investigate the effects of LXA4 against IR-induced fibrosis, we performed a variety of assays. Immunohistochemistry for 8-OHdG and NOX4 revealed that LXA4 inhibited oxidative stress, suggesting that LXA4 may have antioxidant properties. IR-mediated ROS are known to induce apoptosis in lung epithelial cells30, which can cause pulmonary fibrosis;31 although inhibition of AEC apoptosis has been shown to attenuate fibrosis32, it did not affect IR-induced cancer cell death in A549 cells (Supplementary Fig. 4).
Chest CT and studies on pulmonary function are important factors to consider when clinically evaluating respiratory diseases. micro-CT is often used in preclinical studies to measure lung injury as it allow the therapeutic effects of drugs to be evaluated quickly and noninvasively. FlexiVentTM is a system—with a pre-programmed ventilator—that directly evaluates lung function based on the functional parameters used in humans33. Here, LXA4-treated mice displayed significantly better micro-CT finding and flexiVentTM lung function values than simply irradiated mice. Moreover, these results correlated with the histopathological findings—H&E and MT staining—suggesting that LXA4 can prevent IR-induced lung function deterioration.
LXA4 is reportedly involved in airway inflammation; its levels decrease in chronic airway inflammatory diseases, such as asthma, chronic obstructive pulmonary disease, and cystic fibrosis34,35. Moreover, a reduced proportion of pro-resolving compounds (LXA4)—compared to pro-inflammatory compounds (LTB4)—is associated with decreased lung function36. LXA4 is generated during the interaction of lipoxygenases (LOXs) via trans-cellular cooperation in several immune cells, including neutrophils, eosinophils, alveolar macrophages, and platelet airway epithelial cells37–39. Here, IR decreased LXA4 production—in the mouse model and in human blood samples—LOX activity and expression of ALOX12 and ALOX15. Conversely, IR increased the expression of the LXA4 receptor FPR2 in the mouse lung tissue model and in MLE12 cells and HUVECs. Further studies should investigate FPR2-LXA4 interactions in lung cells at different time points after IR, in addition to investigating the effect of LXA4 on aspects of radiation-induced inflammation, such as inflammasome activation and immune-cell infiltration, which promote immune resolution.
Consistent with our findings, LXA4 signaling has recently emerged as an endogenous anti-inflammation and anti-fibrosis pathway40–42; however, natural LXA4 has low biological efficacy owing to its instability. To overcome these limitations, many researchers have developed analogs—that mimic the core structure of native LXA4—as potential drugs43,22. Therefore, it may be beneficial to introduce and develop these analogs in our radiation-induced lung damage model to maximize the anti-inflammation and anti-fibrotic effects of LXA4.
In conclusion, although the precise mechanisms underlying radiation-induced lung fibrosis remain unclear, we demonstrated that LXA4 exerts protective effects by inhibiting collagen production, decreasing the expression of fibrosis-related proteins and inflammatory cytokines, and blocking NF-κB and TGF-β/Smad signaling. Collectively, these results suggest that LXA4 reduces radiation-induced lung inflammation and fibrosis and may therefore serve as a useful therapeutic agent for inhibiting radiotherapy-induced inflammatory responses and fibrosis in normal lung tissue.
Materials and methods
Animal experiments and LXA4 administration
All protocols involving the use of mice were approved by the Animal Care and Use Committees of Yonsei University Medical School, Seoul, South Korea (YUHS-IACUC; 2016-0199), and were performed in accordance with the relevant guidelines. Male C57BL/6 mice (age, 6 weeks; weight, 20–25 g) were purchased from Charles River Korea (Orient Bio, Seongnam, South Korea) and allowed to acclimatize (n = 5 per cage) for a week before IR. A single dose of 75 Gy IR was delivered to the left lung using an image-guided small-animal irradiator (X-RAD 320; Precision, North Branford, CT, USA) equipped with a collimator comprising 3.5-cm-thick copper—for producing focal radiation beams—and an imaging subsystem comprising a fluorescent screen coupled to a charge-coupled-device camera. In all experiments, 3-mm collimators were used to mimic clinical SBRT conditions with a small IR volume in lung tissues. The mice were randomly divided into the following three groups (n = 3‒5 per group): (1) control group; (2) IR group exposed to a single dose of 75 Gy delivered to the left lung in a single fraction; (3) IR + LXA4 group treated intravenously with LXA4 (0.4, 1.2, or 2 µg/ mouse) 2 weeks after IR. The mice were euthanized by CO2 inhalation, and their lung tissues were collected for analysis. LXA4 levels were measured in serum samples using an LXA4 ELISA kit (Cusabio Biotech, Wuhan, China) according to the manufacturer’s protocol.
Human blood specimens
The collection of blood samples from patients with lung cancer was approved by the Ethics Committee of Severance Hospital, Yonsei University, Seoul, South Korea (4-2014-0193). After informed consent, 10-mL blood samples were collected from each patient before radiotherapy to determine LXA4 levels in the serum, at the 2nd and 4th week after definitive radiotherapy for curative aim. The samples were centrifuged, and the serum was frozen and stored at −70 °C until further analysis. LXA4 levels were measured in serum samples using an LXA4 ELISA kit, as described above.
Cell culture
HUVECs were kindly provided by Dr. Yoon-Jin Lee (Division of Radiation Effects, Korea Institute of Radiological and Medical Sciences, Seoul, Korea) and were grown in Endothelial Growth Medium-2 (EGM-2; Promocell GmbH, Heidelberg, Germany) in 0.1% gelatin-coated dishes at 37 °C in a humidified atmosphere containing 5% (v/v) CO2. The mouse lung epithelial cell line, MLE12, obtained from the American Type Culture Collection, was grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C in a humidified atmosphere containing 5% (v/v) CO2. The cells were seeded (density, 1.0 × 106 cells) in a 60-mm plate. After 24 h, the cells were washed and maintained in serum-free medium before the experiments.
Preparation and histological evaluation of lung tissue sections
Tissues from the left lung of the irradiated mice were fixed in 4% paraformaldehyde for 24 h, dehydrated, and embedded in paraffin. Tissue sections were stained with H&E, MT, and TGF-β, α-SMA, 8-OHdG, and IL-6 antibodies (Abcam, Cambridge, MA, USA).
Immunohistochemistry and immunofluorescence
To detect TGF-β, α-SMA, 8-OHdG, and IL-6, tissue sections were probed with primary antibodies (4 °C overnight), followed by incubation with the avidin–biotin peroxidase complex (ABC Kit, Vector Laboratories, CA, USA), and were developed using 3, 3′-diaminobenzidine tetrachloride (DAB; Zymed Laboratories, CA, USA). For immunofluorescence, tissues were probed overnight with primary antibodies diluted in phosphate buffered saline (PBS) containing 0.5% bovine serum albumin (BSA) and then incubated overnight with fluorescent-conjugated secondary antibodies (Vector Laboratories, CA, USA)—diluted in the same buffer as the primary antibodies—at 4 °C. These sections were mounted on a gelatin-coated slide using a fluorescent mounting medium (Dako, Glostrup, Denmark) and dried.
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde—in PBS—permeabilized with 0.1% Triton X-100 for 10 min at room temperature, and washed several times with PBS. The cells were then incubated with pSmad2/3 primary antibodies (Abcam, Cambridge, MA, USA) at 4 °C overnight, blocked with 1% BSA in PBS, and stained with secondary Texas Red-conjugated antibodies. Next, the cells were stained with DAPI, mounted on coverslips, and observed under a confocal microscope.
Western blotting
Cells were lysed with RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM Na3VO4) containing protease inhibitors (2 mM phenylmethylsulfonyl fluoride; 100 μg/mL leupeptin; 10 μg/mL pepstatin, 1 μg/mL aprotinin; 2 mM EDTA) and a phosphatase inhibitor cocktail (GenDEPOT, Baker, TX, USA). After incubation for 20 min at 4 °C, the lysates were centrifuged at 13,000 rpm for 20 min at 4 °C, and the supernatants were collected for western blotting. Protein concentration was measured using a BCA protein kit (Bio-Rad, Hercules, USA). The lysates were subjected to SDS-PAGE gel electrophoresis, transferred onto polyvinylidene fluoride membranes (Millipore, Bedford, MA, USA), and incubated with 5% skimmed milk for 1 h at room temperature. The membranes were then probed with primary antibodies against α-SMA (Abcam, Cambridge, MA, USA), FPR2 (Novus Biological, Littleton, CO, USA), and actin (Santa Cruz Biotechnology, Dallas, TX, USA), followed by incubation with horseradish peroxidase-coupled secondary antibodies (Jackson Immunoresearch, West Grove, PA, USA). Proteins were detected using ECL Plus western blotting detection reagents (Amersham Biosciences, Piscataway, NJ, USA).
micro-CT analysis
Micro-CT images were acquired using a volumetric CT scanner (NFR-Polaris-G90MVC: NanoFocusRay, Iksan, South Korea) and reconstructed using volumetric cone-beam reconstruction (Feldkamp–Davis–Kress method) in online/offline modes. Volumetric analysis was performed using ImageJ software (http://imagej.nih.gov/ij/). To minimize inter-specimen measurement variation, the same settings were used to analyze all images.
Functional lung assessment
Lung function in irradiated mice was evaluated using a flexiVentTM system (SCIREQ, Montreal, QC, Canada), which measures flow-volume relationships in the respiratory system—including forced oscillation—to discriminate between airway and lung tissue variables27. This system was used according to the manufacturer’s instructions.
Transfection and reporter gene assay
Transient transfection was performed using Effectene (Qiagen; Santa Clara, CA, USA) according to the manufacturer’s protocol. Briefly, 1.0 × 106 cells were seeded in a 60-mm dish a day before transfection and grown to ~70% confluence. The cells were then transfected with 0.1 µg of plasmid DNA—SBE-luc from the SBE Reporter Kit (BPS Bioscience, CA, USA)—or the NF-κB-luc vector pGL4.32 [luc2P/ NF-κB-RE/Hygro] (Promega, Madison, WI, USA) along with 10 nM siRNA against FPR2 (si-FPR2) or control siRNA. The next day, the transfected cells were treated with or without radiation (6 Gy) for 48 h and harvested for protein extraction, real-time-qPCR, or further analysis. All experiments were performed in triplicate, and representative results were reported. For the reporter assay, cells were lysed and assayed for luciferase activity according to the manufacturer’s instructions (Promega, Southampton, UK).
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was isolated from cells using TRIzol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and cDNA was synthesized using a Quantifect Reverse Transcription Kit (Qiagen, Hilden, Germany). qRT-PCR was performed using SYBR Premix Ex Taq (Takara, Hercules, CA, USA), primers, RNase-free H2O, and cDNA (final reaction volume, 20 µL) under the following cycling conditions: 95 °C (10 min); and 50 cycles of 95 °C, (20 s), 55 °C (30 s), and 72 °C (20 s). The mouse primer sequences are available in the Supplementary Table 2. All experiments were performed in triplicate, and the results were normalized to glyceraldehyde 3-phosphate dehydrogenase expression. mRNA expression was calculated using the delta Ct method.
Luciferase reporter assay
MLE12 cells were cultured in 60-mm plates using complete medium until they reached ~70% confluency, and were then co-transfected with either SBE-luciferase + TK-Renilla luciferase or NF-κB-luciferase + TK-Renilla luciferase. After 24 h, the cells were irradiated using an image-guided small-animal irradiator, and the ratio of firefly/Renilla luciferase was calculated and normalized to untreated controls. Luciferase activity was measured using luciferase assay reagents (Promega Corp., Madison, Wi, USA).
Colony formation assay
A549 and MLE12 cells were cultured in 35-mm dishes using DMEM supplemented with 10% FBS in the background of IR (5 Gy) using an X-rad320 (Precision, North Branford, CT, USA). After 2 weeks—with or without LXA4 treatment—the cells were fixed, stained with 0.5% crystal violet, and quantified for colony number.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). Differences between the means of two groups were evaluated using Student’s t tests. Differences between the means of multiple groups were evaluated by one-way analysis of variance. The threshold for significance was p < 0.05, and all values were expressed as the mean ± SEM.
Supplementary information
Acknowledgements
This work was supported by the Convergence of Conventional Medicine and Traditional Korean Medicine R&D program, funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (HI15C0214), as well as by the Radiation Technology Research and Development Program (NRF-2017M2A2A7A02019612) and the Basic Science Research Program (NRF-2019R1A2C2086448) through the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MSIT).
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by A. Stephanou
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jaeho Cho, Email: jjhmd@yuhs.ac.
Jin-Mo Kim, Email: jmk0831@snu.ac.kr.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-020-02846-7).
References
- 1.Gieringer M, Gosepath J, Naim R. Radiotherapy and wound healing: principles, management and prospects (review) Oncol. Rep. 2011;26:299–307. doi: 10.3892/or.2011.1319. [DOI] [PubMed] [Google Scholar]
- 2.Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J. Am. Acad. Dermatol. 2006;54:28–46. doi: 10.1016/j.jaad.2005.08.054. [DOI] [PubMed] [Google Scholar]
- 3.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int. J. Radiat. Oncol., Biol. Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Guney Y, et al. Carnosine may reduce lung injury caused by radiation therapy. Med. Hypotheses. 2006;66:957–959. doi: 10.1016/j.mehy.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Rajan Radha R, Chandrasekharan G. Pulmonary injury associated with radiation therapy—assessment, complications and therapeutic targets. Biomed. Pharmacother. 2017;89:1092–1104. doi: 10.1016/j.biopha.2017.02.106. [DOI] [PubMed] [Google Scholar]
- 6.Hong ZY, et al. Time, dose, and volume responses in a mouse pulmonary injury model following ablative irradiation. Lung. 2016;194:81–90. doi: 10.1007/s00408-015-9825-4. [DOI] [PubMed] [Google Scholar]
- 7.Kim BY, et al. Focal exposure of limited lung volumes to high-dose irradiation down-regulated organ development-related functions and up-regulated the immune response in mouse pulmonary tissues. BMC Genet. 2016;17:29. doi: 10.1186/s12863-016-0338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim, J. Y. et al. HSP27 inhibitor attenuates radiation-induced pulmonary inflammation. Sci Rep.8, 4189 (2018). [DOI] [PMC free article] [PubMed]
- 9.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr. Drug Targets. 2013;14:1347–1356. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simone CB., 2nd Thoracic radiation normal tissue injury. Semin. Radiat. Oncol. 2017;27:370–377. doi: 10.1016/j.semradonc.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Singh V, Torricelli AA, Nayeb-Hashemi N, Agrawal V, Wilson SE. Mouse strain variation in SMA(+) myofibroblast development after corneal injury. Exp. Eye Res. 2013;115:27–30. doi: 10.1016/j.exer.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatler AL, Jenkins G. TGF-beta activation and lung fibrosis. Proc. Am. Thorac. Soc. 2012;9:130–136. doi: 10.1513/pats.201201-003AW. [DOI] [PubMed] [Google Scholar]
- 13.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng H, et al. Ac-SDKP suppresses epithelial-mesenchymal transition in A549 cells via HSP27 signaling. Exp. Mol. Pathol. 2014;97:176–183. doi: 10.1016/j.yexmp.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Sohn SH, et al. The inflammasome accelerates radiation-induced lung inflammation and fibrosis in mice. Environ. Toxicol. Pharmacol. 2015;39:917–926. doi: 10.1016/j.etap.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 16.Serhan CN, Chiang N. Endogenous pro-resolving and anti-inflammatory lipid mediators: a new pharmacologic genus. Br. J. Pharmacol. 2008;153(Suppl 1):S200–S215. doi: 10.1038/sj.bjp.0707489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nascimento-Silva V, Arruda MA, Barja-Fidalgo C, Fierro IM. Aspirin-triggered lipoxin A4 blocks reactive oxygen species generation in endothelial cells: a novel antioxidative mechanism. Thrombosis Haemost. 2007;97:88–98. [PubMed] [Google Scholar]
- 18.Krishnamoorthy S, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl Acad. Sci. USA. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brink C, et al. International Union of Pharmacology XXXVII. Nomenclature for leukotriene and lipoxin receptors. Pharmacol. Rev. 2003;55:195–227. doi: 10.1124/pr.55.1.8. [DOI] [PubMed] [Google Scholar]
- 20.Bai Y, et al. Mesenchymal stem cells reverse diabetic nephropathy disease via lipoxin A4 by TArgeting Transforming Growth Factor Beta (TGF-beta)/smad pathway and pro-inflammatory cytokines. Med. Sci. Monit. 2019;25:3069–3076. doi: 10.12659/MSM.914860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtoglu EL, et al. A bioactive product lipoxin A4 attenuates liver fibrosis in an experimental model by regulating immune response and modulating the expression of regeneration genes. Turk. J. Gastroenterol. 2019;30:745. doi: 10.5152/tjg.2019.18276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins V, et al. ATLa, an aspirin-triggered lipoxin A4 synthetic analog, prevents the inflammatory and fibrotic effects of bleomycin-induced pulmonary fibrosis. J. Immunol. 2009;182:5374–5381. doi: 10.4049/jimmunol.0802259. [DOI] [PubMed] [Google Scholar]
- 23.Zong L, et al. Lipoxin A4 reverses mesenchymal phenotypes to attenuate invasion and metastasis via the inhibition of autocrine TGF-beta1 signaling in pancreatic cancer. J. Exp. Clin. Cancer Res. 2017;36:181. doi: 10.1186/s13046-017-0655-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu SH, Zhang YM, Tao HX, Dong L, Lipoxin A. Lipoxin A4 inhibits transition of epithelial to mesenchymal cells in proximal tubules. Am. J. Nephrol. 2010;32:122–136. doi: 10.1159/000315121. [DOI] [PubMed] [Google Scholar]
- 25.Xu F, et al. Lipoxin A4 and its analog suppress hepatocarcinoma cell epithelial-mesenchymal transition, migration and metastasis via regulating integrin-linked kinase axis. Prostaglandins Other Lipid Mediat. 2018;137:9–19. doi: 10.1016/j.prostaglandins.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Kim JY, et al. The Hsp27-mediated IkBalpha-NFkappaB signaling axis promotes radiation-induced lung fibrosis. Clin. Cancer Res. 2019;25:5364–5375. doi: 10.1158/1078-0432.CCR-18-3900. [DOI] [PubMed] [Google Scholar]
- 27.Hong ZY, et al. Development of a small animal model to simulate clinical stereotactic body radiotherapy-induced central and peripheral lung injuries. J. Radiat. Res. 2014;55:648–657. doi: 10.1093/jrr/rrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JY, et al. Standardized herbal formula PM014 inhibits radiation-induced pulmonary inflammation in mice. Sci. Rep. 2017;7:45001. doi: 10.1038/srep45001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prasanna PG, et al. Normal tissue protection for improving radiotherapy: where are the gaps? Transl. Cancer Res. 2012;1:35–48. [PMC free article] [PubMed] [Google Scholar]
- 30.Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim. Biophys. Acta. 2013;1832:1028–1040. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sisson TH, et al. Targeted injury of type II alveolar epithelial cells induces pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;181:254–263. doi: 10.1164/rccm.200810-1615OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budinger GR, et al. Proapoptotic Bid is required for pulmonary fibrosis. Proc. Natl Acad. Sci. USA. 2006;103:4604–4609. doi: 10.1073/pnas.0507604103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vanoirbeek JA, et al. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am. J. Respir. Cell Mol. Biol. 2010;42:96–104. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 34.Bonnans C, et al. Lipoxins are potential endogenous antiinflammatory mediators in asthma. Am. J. Respir. Crit. Care Med. 2002;165:1531–1535. doi: 10.1164/rccm.200201-053OC. [DOI] [PubMed] [Google Scholar]
- 35.Karp CL, et al. Defective lipoxin-mediated anti-inflammatory activity in the cystic fibrosis airway. Nat. Immunol. 2004;5:388–392. doi: 10.1038/ni1056. [DOI] [PubMed] [Google Scholar]
- 36.Carlo T, Kalwa H, Levy BD. 15-Epi-lipoxin A4 inhibits human neutrophil superoxide anion generation by regulating polyisoprenyl diphosphate phosphatase 1. FASEB J. Off. Publ. Federation Am. Societies Exp. Biol. 2013;27:2733–2741. doi: 10.1096/fj.12-223982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fierro IM, Serhan CN. Mechanisms in anti-inflammation and resolution: the role of lipoxins and aspirin-triggered lipoxins. Braz. J. Med. Biol. Res. 2001;34:555–566. doi: 10.1590/s0100-879x2001000500002. [DOI] [PubMed] [Google Scholar]
- 38.Chavis C, Vachier I, Chanez P, Bousquet J, Godard P. 5(S),15(S)-dihydroxyeicosatetraenoic acid and lipoxin generation in human polymorphonuclear cells: dual specificity of 5-lipoxygenase towards endogenous and exogenous precursors. J. Exp. Med. 1996;183:1633–1643. doi: 10.1084/jem.183.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol. Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng, S. et al. Lipoxin A4 promotes lung epithelial repair whilst inhibiting fibroblast proliferation. ERJ Open Res.2, 00079–2015 (2016). [DOI] [PMC free article] [PubMed]
- 41.Gagliardo R, et al. Airway lipoxin A4/formyl peptide receptor 2-lipoxin receptor levels in pediatric patients with severe asthma. J. Allergy Clin. Immunol. 2016;137:1796–1806. doi: 10.1016/j.jaci.2015.11.045. [DOI] [PubMed] [Google Scholar]
- 42.Maderna P, Godson C. Lipoxins: resolutionary road. Br. J. Pharmacol. 2009;158:947–959. doi: 10.1111/j.1476-5381.2009.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Duffy CD, Guiry PJ. Recent advances in the chemistry and biology of stable synthetic Lipoxin analogues. MedChemComm. 2010;1:249. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.