Abstract
Proline is one of the precursors of the biosynthesis of 2-acetyl-1-pyrroline (2-AP) which is the key and characteristic volatile component of fragrant rice aroma. In order to study the effects of exogenous proline on 2-AP biosynthesis and other grain quality attributes in fragrant rice, two indica fragrant rice cultivars, “Meixiangzhan-2” and “Xiangyaxiangzhan”, and one japonica fragrant rice, “Yunjingyou”, were used in present study. At initial heading stage, proline solutions at 0 (CK), 0.10 (Pro1), 0.20 (Pro2) and 0.50 (Pro3) g L-1 were applied as foliar spray solution to fragrant rice plants. Compared with CK, Pro1, Pro2 and Pro3 treatments significantly increased the grain 2-AP content. The significant up-regulation effects due to proline treatments were observed in the contents of proline, △1-pyrrolidine-5-carboxylic acid (P5C) and △1-pyrroline which involved in 2-AP formation. Exogenous proline application also significantly decreased the grain γ-aminobutyric acid (GABA) content. Furthermore, proline treatments enhanced the activity of proline dehydrogenase (ProDH) as well as transcript level of gene PRODH. On the other hand, the transcript level of gene BADH2 and activity of betaine aldehyde dehydrogenase (BADH) decreased under proline treatments. Proline treatments (Pro2 and Pro3) also increased the grain protein content by 3.57–6.51%. Moreover, 32.03–34.25% lower chalky rice rate and 30.80–48.88% lower chalkiness were recorded in proline treatments (Pro2 and Pro3) for both Meixiangzhan and Xiangyaxiangzhan whilst for Yunjingyou, foliar application of proline had no significant effect on chalky rice rate and chalkiness. There was no remarkable difference observed in grain milled quality (brown rice rate, milled rice rate and head rice rate) and amylose content between CK and proline treatments. In conclusion, exogenous proline enhanced the 2-AP biosynthesis and promoted some grain quality characters of fragrant rice.
Subject terms: Biochemistry, Enzyme mechanisms, Enzymes, Proteins, RNA
Introduction
Fragrant rice is famous for possessing a characteristic aroma and also fetches a high price in the international market because of the good grain quality1,2. In the past two decades, many studies have conducted to investigate the compound of the aroma of fragrant rice. For example, the study of Widaja et al.3 showed that the number of volatile compounds detected in the aroma exceeds 300 in both fragrant and non-fragrant rice varieties. Hashemi et al.4 demonstrated there were more than 100 volatile compounds have been detected in the aroma of fragrant rice varieties. In recent years, with the development of many researches, it is established that 2-acetyl-1-pyrroline (2-AP) is the key compound in fragrant rice aroma1,5,6.
The process of 2-AP biosynthesis in fragrant rice is very complicated which involved many biochemical reactions while numerous studies have been conducted to understand the mechanism of 2-AP biosynthesis. An early study has evidenced that the expression of gene BADH2 which related to the betaine aldehyde dehydrogenase (BADH) activity would inhibited the 2-AP production in fragrant rice varieties7. The study of Mo et al.8 revealed a positive and significant correlation between grain 2-AP concentration and grain γ-aminobutyric acid (GABA) content. The investigation of Yoshihashi et al.9 revealed that the nitrogen in the 2-AP comes from proline in fragrant rice and demonstrated that the proline, ornithine and glutamic acid are the potential precursors of 2-AP. Moreover, Bao et al.10 and Li et al.11 demonstrated that proline is converted to 2-AP in three steps: First, proline is converted into △1-pyrrolidine-5-carboxylic acid (P5C) catalyzed by proline dehydrogenase (ProDH); Then, P5C is converted into △1-pyrroline which is the limiting substrate in 2-AP biosynthesis12; Finally, the △1-pyrroline is converted into 2-AP in fragrant rice by non-enzymatic or enzymatic reaction. Therefore, proline has an important role to play in 2-AP biosynthesis in fragrant rice.
As one of the non-essential amino acids in the human body, proline is not only one of the components of plant protein, but also one of the osmotic regulators in plant cytoplasm13. Previous studies revealed that proline plays important roles in stabilizing the structure of biomacromolecules, reducing the acidity of cells, detoxifying ammonia and regulating the redox potential of cells14,15. However, the effect of exogenous proline on fragrant rice performances especially 2-AP biosynthesis were rarely reported.
Thus, present study was conducted with the hypothesis that foliar application of proline could enhanced the 2-AP formation in fragrant rice and the objective to study the effects of exogenous proline on 2-AP biosynthesis on the physiological and molecular level.
Results
2-AP content
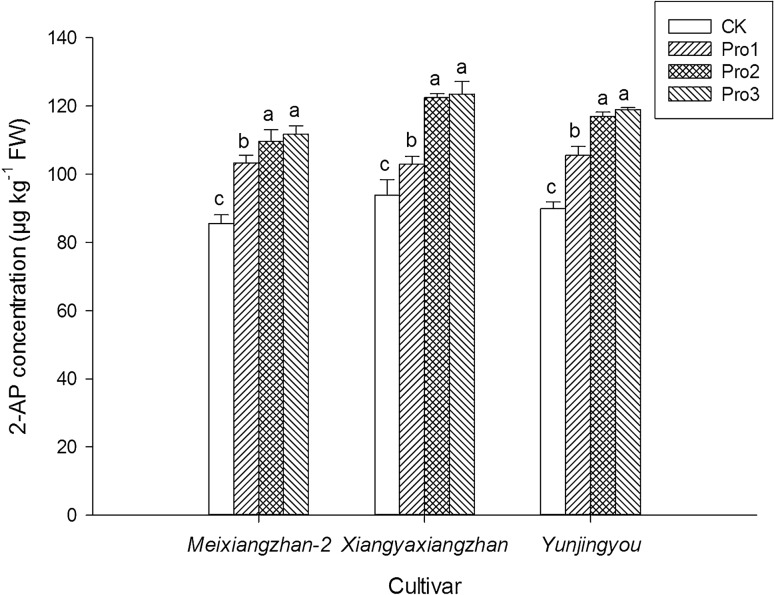
Foliar application of proline at initial heading stage significantly increased the grain 2-AP content for three fragrant rice cultivars (Fig. 1). For Meixiangzhan-2, compared with CK, Pro1, Pro2 and Pro3 significantly increased the grain 2-AP concentration by 20.63%, 23.24% and 23.94%. For Xiangyaxiangzhan, 9.65%, 27.75% and 24.18% higher 2-AP contents were recorded in Pro1, Pro2 and Pro3 than CK, respectively. For Yunjingyou, compared with CK, Pro1, Pro2 and Pro3 significantly increased the grain 2-AP concentration by 17.36%, 25.58% and 24.79%.
Figure 1.
The effect of foliage dressing with proline on grain 2-AP content of fragrant rice. Data are means and standard deviation of three replications and the columns showing different letters mean the results are statistically different.
Proline, P5C, GABA and △1-pyrroline contents
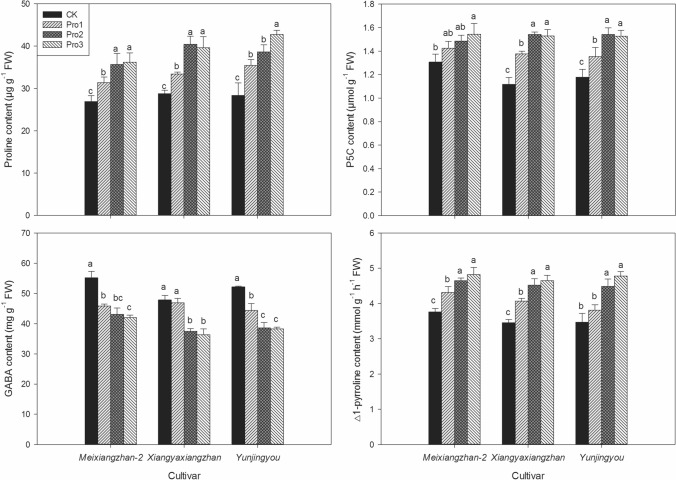
As shown in Fig. 2, foliar application of proline had impacts on grain contents of proline, P5C, GABA and △1-pyrroline in fragrant rice. For proline, compared with CK, all proline treatments significantly increased the grain proline content (except Pro1 treatment in Meixiangzhan-2) and the highest proline contents were recorded in both Pro2 and Pro3 treatments for all fragrant rice cultivars. For P5C, higher grain P5C contents were recorded in Pro2 and Pro3 treatments than CK for three cultivars whilst there was no remarkable difference between CK and Pro1 for Meixiangzhan-2 and Yunjingyou. For △1-pyrroline, higher grain △1-pyrroline concentrations were recorded in Pro1, Pro2 and Pro3 treatments than CK (except Pro1 for Yunjingyou) while the highest △1-pyrroline concentrations were recorded in both Pro2 and Pro3 treatments. For grain GABA content, exogenous application of proline significantly decreased the grain GABA concentration and the lowest or equally lowest contents were recorded in both Pro2 and Pro3 treatment.
Figure 2.
The effects of foliage dressing with proline on grain P5C, proline, GABA and △1-pyrroline contents of fragrant rice. Data are means and standard deviation of three replications and the columns showing different letters mean the results are statistically different.
Activities of ProDH, BADH, △1-pyrroline-5-carboxylic acid synthetase (P5CS) and ornithine aminotransferase (OAT)
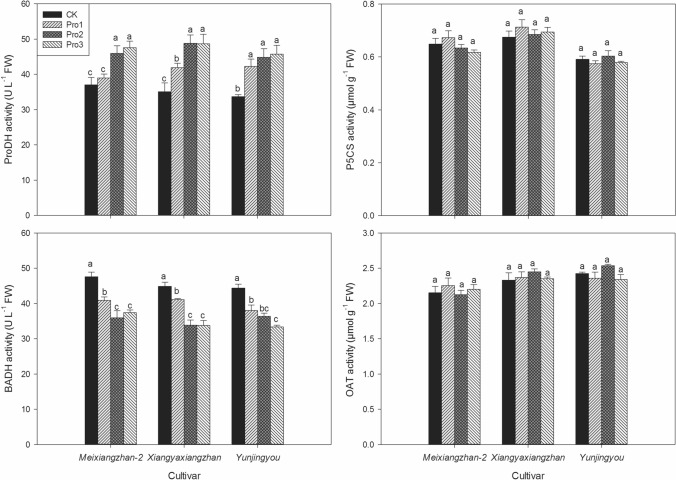
As shown in Fig. 3, foliar application of proline at initial heading stage significantly affected the activity of ProDH. For Meixiangzhan-2, there was no remarkable difference between CK and Pro1 treatment whilst 24.11% and 28.57% higher ProDH activities were recorded in Pro2 and Pro3 treatments than CK and for Xiangyaxiangzhan, compared with CK, Pro1, Pro2 and Pro3 treatments significantly improved the activity of ProDH by 19.45%, 39.10% and 38.62%, respectively; for Yunjingyou, the ProDH activities under Pro1, Pro2 and Pro3 treatments were also significantly higher than CK while there was no remarkable difference among Pro1, Pro2 and Pro3 treatments. Exogenous proline application also significantly down-regulated the activity of BADH and the lowest or equally lowest activities were recorded in both Pro2 and Pro3 treatments. On the other hand, there was no remarkable differences among all treatments in P5CS activity and similar trend was also observed in OAT activity.
Figure 3.
The effects of foliage dressing with proline on grain ProDH, BADH, OAT and P5CS activities of fragrant rice. Data are means and standard deviation of three replications and the columns showing different letters mean the results are statistically different.
Expression of genes related to 2-AP biosynthesis
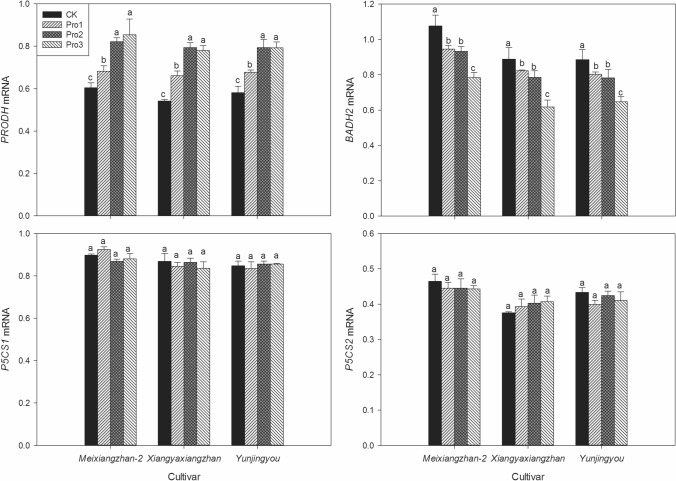
As depicted in Real-time PCR analyses (Fig. 4), the levels of PRODH transcript were higher in foliar application of proline treatments. For Meixiangzhan-2, compared with CK, Pro1, Pro2 and Pro3 treatments significantly increased the PRODH transcript by 12.83%, 35.99% and 41.35%; for Xiangyaxiangzhan, 22.11%, 46.35% and 44.05% higher PRODH transcript levels were recorded in Pro1, Pro2 and Pro3 than CK, respectively; for Yunjingyou, Pro1, Pro2 and Pro3 significantly increased the transcript level of PRODH by 16.69%, 31.48% and 26.65% compared with CK, respectively. Moreover, transcript level of gene BADH2 reduced due to exogenous proline application while the lowest or equally lowest levels were recorded in both Pro2 and Pro3 treatments. However, the transcript level of gene P5CS1 and P5CS2 remained not significantly different under all treatments.
Figure 4.
The effects of foliage dressing with proline on transcript levels of gene PRODH, BADH2, P5CS1 and P5CS2 of fragrant rice. Data are means and standard deviation of three replications and the columns showing different letters mean the results are statistically different.
Grain yield and other quality attributes
As shown in Table 1, exogenous proline had some impacts on some grain quality attributes. Compared with CK, Pro2 and Pro3 treatments significantly increased grain protein content by 3.57–6.51% and 4.39–5.58%, respectively. Lower chalky rice rate and chalkiness were also observed in Pro2 and Pro3 than CK (except for Yunjingyou). However, there was no significant difference among all treatments in grain yield and compared with CK, proline treatments (Pro1, Pro2 and Pro3) had no remarkable influence on brown rice rate, milled rice rate and head rice rate as well as amylose content.
Table 1.
The effects of foliage dressing with proline on grain yield, brown rice rate, milled rice rate, head rice rate, protein content, amylose content, chalky rice rate and chalkiness.
| Cultivar | Treatment | Grain yield (t ha-1) | Brown rice rate (%) | Milled rice rate (%) | Head rice rate (%) | Protein (%) | Amylose (%) | Chalky rice rate (%) | Chalkiness (%) |
|---|---|---|---|---|---|---|---|---|---|
| Meixiangzhan-2 | CK | 6.23a | 77.63a | 64.34a | 54.94a | 7.17b | 20.17a | 16.29a | 5.03a |
| Pro1 | 6.23a | 76.47a | 65.24a | 54.75a | 7.10b | 20.07a | 16.71a | 5.19a | |
| Pro2 | 6.20a | 76.65a | 65.74a | 55.33a | 7.63a | 20.00a | 10.93b | 3.25b | |
| Pro3 | 6.37a | 77.44a | 64.89a | 55.13a | 7.57a | 20.13a | 10.79b | 2.57b | |
| Xiangyaxiangzhan | CK | 6.30a | 76.94a | 65.20a | 50.06a | 7.47b | 20.27a | 16.23a | 5.10a |
| Pro1 | 6.30a | 76.97a | 64.60a | 49.30a | 7.43b | 20.06a | 16.29a | 4.69a | |
| Pro2 | 6.40a | 76.98a | 65.12a | 50.23a | 7.73a | 20.43a | 10.67b | 2.89b | |
| Pro3 | 6.20a | 77.00a | 64.35a | 50.09a | 7.83a | 20.23a | 11.03b | 3.53b | |
| Yunjingyou | CK | 7.00a | 81.09a | 71.89a | 62.58a | 9.10b | 16.90a | 1.51a | 0.31a |
| Pro1 | 6.80a | 81.53a | 72.12a | 63.60a | 9.07b | 17.23a | 1.64a | 0.30a | |
| Pro2 | 6.73a | 81.51a | 71.38a | 63.01a | 9.57a | 17.23a | 1.38a | 0.33a | |
| Pro3 | 6.67a | 81.01a | 71.82a | 62.44a | 9.50a | 17.07a | 1.59a | 0.29a |
The means in the same column followed by different lowercase letters for the same variety differ significantly at P < 0.05.
Discussion
Proline is a protein-derived amino acid with special conformational rigidity, which is essential for plant primary metabolism13. Early studies have revealed that proline plays multiple roles in plant physiological activities such as stress responses and protein biosynthesis16–18. In 2008, the report of Yoshihashi9 also explored that proline is one of the precursors in 2-AP production in fragrant rice cultivars. The results of present study supported the hypothesis that foliar application of proline at initial heading stage increased the grain 2-AP concentration in fragrant rice. Compared with CK, proline treatments increased the 2-AP content by 9.65–32.23%. This result agreed with the study of Yoshihashi et al.9 who demonstrated the exogenous proline greatly increased the 2-AP concentration in seedlings and callus of fragrant rice.
At the fundamental level, we observed that proline treatments increased the precursors in 2-AP biosynthesis such as proline, P5C and △1-pyrroline. Since the research of Yoshihashi et al.9, more and more studies have discovered the correlation between proline and 2-AP. For example, the study of Bao et al.10 showed that drought stress affected the 2-AP production in fragrant rice by inducing the regulation in proline biosynthesis. The investigation of Li et al.19 found a significant and positive correlation between grain 2-AP content and grain proline content under rice-duck co-culture environment. In our study, the foliar application of proline significantly increased the grain proline content and grain △1-pyrroline content in fragrant rice cultivar. Previous study revealed that △1-pyrroline is a limit factor in 2-AP production in fragrant rice12 and the increased △1-pyrroline concentration might be one of the reasons for the increment in 2-AP content. Moreover, present study showed that grain GABA content decreased while 2-AP content increased under the application of proline. The result was inconsistent with the research of Mo et al.8 which showed a positive correlation between GABA and 2-AP. The difference might be attributed to the experimental circumstances because the increased 2-AP concentration in study of Mo et al.8 was caused by stress (shading) whilst in present experiment, no any stress was made artificially and proline fitly might have effects on enhancing the stress resistance of plants17,18. As for the enzymes and genes related to 2-AP biosynthesis, significant differences were observed in the activities of some enzymes (ProDH and BADH) and transcript levels of some genes (PRODH and BADH2). Compared with control, proline treatments significantly enhanced the ProDH activity and gene PRODH expression and they were probably the further reason for increased 2-acetyl-1-pyrroline (2-AP) content under exogenous proline application. We deduced that the increased proline concentration induced the up-regulation in both ProDH activity and transcript level of PRODH and thus promoted the transformation from proline to P5C to △1-pyrroline and then to 2-AP20,21. On the other hand, as the key aroma gene in fragrant rice, the change in gene BADH2 expression cannot be neglected and the down-regulation in BADH2′s transcript level and BADH activity could be other important reason for the increased grain 2-AP concentration22,23.
Interestingly, we observed that exogenous proline also improved some other grain quality attributes of fragrant rice. The higher grain protein content, lower chalkiness and chalky rice rates were recorded in both Pro2 and Pro3 treatments than CK. The increment in grain protein content due to exogenous proline might because the proline is one of the amino to form the plant protein and the foliar application happened to provide more proline for fragrant rice to synthesis more protein in grains13. On the other hand, as a trail significantly influences the appearance of rice, chalkiness has a very complicated formation which is affected by expression of many genes24. In our study, we observed that foliar application of proline significantly decreased chalkiness of fragrant rice cultivars, “Meixiangzhan” and “Xiangyaxiangzhan”. We deduced that there were two reasons. One was that proline is a kind of nitrogen source as described as Yoshihashi et al.9 and it might also provide nitrogen to the grain filling process while the formation of chalkiness in the endosperm is suppressed by nitrogen25. The other was the proline is one of the osmotic substances in plant in defending the abiotic stress16–18 and exogenous proline application might help to improve fragrant rice stress resistance to the potential stress from the farmland microclimate. Kong et al.14 demonstrated that environmental stress during the grain filling phase would increase chalkiness in rice grain. In order to investigate the effect of exogenous proline on rice chalkiness formation, more studies should be done to at physiological and molecular level.
In addition, the highest 2-AP contents were recorded in both Pro2 and Pro3 treatments whilst no remarkable difference was observed between Pro2 and Pro3. Therefore, 0.2 g L−1 might be the most suitable concentration in the proline application to increase the fragrant rice aroma considered the cost in fragrant rice production.
Conclusion
Foliar application of proline significantly increased the grain 2-AP concentration and the related precursors including proline, P5C and △1-pyrroline in fragrant rice. At enzyme and molecular level, exogenous proline treatment significantly increased the activity of ProDH and decreased activity of BADH. The up-regulation in gene PRODH expression and down-regulation in gene BADH2 expression were also observed in the proline treatments. Exogenous proline also increased grain protein and reduced chalkiness and chalky rice rate of fragrant rice.
Methods
Plant materials and experimental details
Two indica fragrant rice cultivars, “Meixiangzhan-2” (bred and selected by Rice Research Institute, Guangdong Academy of Agricultural Sciences) and “Xiangyaxiangzhan” (bred and selected by Taishan agricultural science research institute), and one japonica fragrant rice, “Yunjingyou” (bred and selected by Institute of Grain Crops, Yunnan Academy of Agricultural Sciences), were provided by College of Agriculture, South China Agricultural University and used in present study. The field experiment was conducted in Zengcheng (23°13′ N, 113°81′ E), Guangdong, China, between July and November in 2019. The experimental soil was sandy loam with of 20.12% organic matter content, 1.408% total N, 1.068% total P, and 15.767% total K. After the soaking and germination, fragrant rice seeds were sown in polyvinyl chloride trays for nursery raising. Then 15-day-old seedlings were transplanted to the field at the planting distance of 30 × 16 cm. “Special biological organic fertilizer (Ci Tian)” manufactured by Foota (Dongguan) Biotechnology Co., Ltd China comprised of N + P2O5 + K2O ≥ 26% and organic matter ≥ 25% was applied at 900 kg ha−1 with 60% as basal dose and 40% at tillering. All other agronomic practices i.e., pest and diseases management, and weed control were the same in all treatments by following the guidelines and standards recommended by the province14.
At initial heading stage, proline solutions at 0.20 (Pro1), 0.50 (Pro2) and 1.00 (Pro3) g L-1 were applied as foliar spray solution using a special Knapsack Electric sprayer (3WBD-Qianfeng Agricultural machinery, Yangjiang, Guangdong, China). The treatment which spray the distilled water was set as control (CK). The treatments were arranged in randomized complete block design (RCBD) in triplicate with net plot size of 20 m2. At maturity stage, fresh grains from each treatment were separated from the main stem, washed with double distilled water and stored at − 80 °C for the determination of 2-AP, proline, ProDH, P5C, △1-pyrroline and molecular analysis.
Measurement of 2-AP content
Fresh grains about 1.00 g were homogenized in 5 mL of dichloromethane and treated for 4 h in oscillations instrument (HZS-H, China) using a frequency of 200 oscillations per minute. Grain 2-AP concentration was determinate using the synchronization distillation and extraction method (SDE) combined with GCMS-QP 2010 Plus (Shimadzu Corporation, Japan) and the grain 2-AP concentration was expressed as µg kg-1.
Estimation of proline, △1-pyrroline, GABA and pyrroline-5-carboxylic acid (P5C) contents
Grain proline concentration was estimated according to the methods of SAHIN26 by using ninhydrin, the absorbance was read at 520 nm and expressed as ug g-1 fresh weight (FW) of leaves. The grain P5C concentration was estimated following the method of Wu27. The 0.9 ml reaction system contained 0.2 ml enzyme extraction, 0.5 ml trichloroacetic acid (TCA) and 0.2 ml of 2-aminobenzaldehyde. Absorbance was read at 440 nm after the reaction while grain P5C concentration was expressed as μmol g−1. The △1-prroline content in grains was detected according to the method described by Hill28. The content of △1-pyrroline in reaction mixtures containing 1,4-diaminobutane was determined immediately after 30 min in 27 °C. The estimation of grain GABA content was according to the methods described by Mo8 and expressed as mg g−1 FW.
Determination of the ProDH, OAT, P5CS and BADH activity
ProDH activity was assayed following the methods of Li et al.11. The absorbance after reaction was read at 440 nm and the activity was calculated using a molar extinction coefficient. The estimations of activity of OAT, P5CS and BADH were according to the methods described by Bao et al.10 and expressed as μmol g−1 FW and U L-1 FW, respectively.
Real-time quantitative RT-PCR
The total RNA in grains was extracted with HiPure Plant RNA Mini Kit (Magen, Guangzhou, China) and the cDNA was synthesize using the Hiscript II QRT SuperMix for qPCR (+ gDNA wiper) (Vazyme, Nanjing, China). Real-time quantitative RTPCR (qRT-PCR) was carried out in CFX96 real-time PCR System (Bio-Rad, Hercules, CA, USA). Each RNA sample was performed in triplicate. Primer used for qRT-PCR were listed in Table 2.
Table 2.
Primer sequences of genes encoding enzymes involved in 2-AP synthesis in rice grains.
| Gene name | Accession No | Primer sequences |
|---|---|---|
| P5CS1 | AK102633 |
F 5′-TCTGCTCAGTGATGTGGATG-3' R 5′-CCTACACGAGATTTGTCTCC-3' |
| P5CS2 | AK101230 |
F 5′-GAGGTTGGCATAAGCACAG-3' R 5′-CTCCCTTGTCGCCGTTC-3' |
| PRODH | AK121010 |
F 5′-TCATCAGACGAGCAGAGGAGAACAGG-3' R 5′-CCCAGCATTGCAGCCTTGAACC-3' |
| BADH2 | AB096083 |
F 5′-GGTTGGTCTTCCTTCAGGTGTGC-3′ R 5′-CATCAACATCATCAAACACCACTAT-3′ |
Measurement of grain yield and other grain quality attributes
At the maturity stage, the rice was harvested from three-unit sampling area (1 m2) in each treatment to estimate the grain yield. Then, rice huller (Jiangsu, China) was used to estimate the brown rice rate while milled rice and head rice rates were determinate by using a Jingmi testing rice grader (Zhejiang, China)14. The chalkiness and chalkiness degree of fragrant rice were measured with an SDE-A light box (Guangzhou, China) and the grain amylose and protein contents was measured using an Infratec-1241 grain analyzer (FOSS-TECATOR)14.
Statistical analyses
The experiment data were subjected to analysis of variances (ANOVA) using Statistix 8 (Analytical software, Tallahassee, Florida, USA). The differences among means were separated by using least significant difference (LSD) test at 5% probability level. Graphical representation was conducted via Sigma Plot 14.0 (Systat Software Inc., California, USA).
Acknowledgements
This study was supported by the National Natural Science Foundation of China (31971843), the Technology System of Modern Agricultural Industry in Guangdong (2017 LM1098) and the World Bank Loan Agricultural Pollution Control Project in Guangdong (0724-1510A08N3684).
Author contributions
X.T. and H.L. conceived and designed the experiments. T.Z., A.Z., R.L., L.H., J.L., and P.X. carried out the experiments and analysed data. H.L. drafted the manuscript. X.T. provided the guidance during the experiment. All authors read, edited, and approved the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sakthivel K, Sundaram RM, Rani NS, Balachandran SM, Neeraja CN. Genetic and molecular basis of fragrance in rice. Biotechnol. Adv. 2009;27:468–473. doi: 10.1016/j.biotechadv.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Ashraf Y, et al. Lead (Pb) toxicity; physio-biochemical mechanisms, grain yield, quality, and Pb distribution proportions in scented rice. Front Plant Sci. 2017 doi: 10.3389/fpls.2017.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widjaja R, Craske JD, Wootton M. Changes in volatile components of paddy, brown and white fragrant rice during storage. J. Sci. Food Agric. 1996;71:218–224. doi: 10.1002/(SICI)1097-0010(199606)71:2<218::AID-JSFA570>3.0.CO;2-5. [DOI] [Google Scholar]
- 4.Hashemi FSG, et al. The genetic and molecular origin of natural variation for the fragrance trait in an elite Malaysian aromatic rice through quantitative trait loci mapping using SSR and gene-based markers. Gene. 2015;555:101–107. doi: 10.1016/j.gene.2014.10.048. [DOI] [PubMed] [Google Scholar]
- 5.Lee Y, Oh Y, Kim T, Cho Y. Quantitation of 2-acetyl-1-pyrroline in aseptic-packaged cooked fragrant rice by HS-SPME/GC-MS. Food Sci. Nutr. 2019;7:266–272. doi: 10.1002/fsn3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okpala NE, Mo Z, Duan M, Tang X. The genetics and biosynthesis of 2-acetyl-1-pyrroline in fragrant rice. Plant Physiol. Biochem. 2019;135:272–276. doi: 10.1016/j.plaphy.2018.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Chen S, et al. Badh2, encoding betaine aldehyde dehydrogenase, inhibits the biosynthesis of 2-acetyl-1-pyrroline, a major component in rice fragrance. Plant Cell. 2008;20:1850–1861. doi: 10.1105/tpc.108.058917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mo Z, et al. Shading during the grain filling period increases 2-acetyl-1-pyrroline content in fragrant rice. Rice. 2015 doi: 10.1186/s12284-015-0040-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoshihashi T, Huong NTT, Inatomi H. Precursors of 2-acetyl-1-pyrroline, a potent flavor compound of an aromatic rice variety. J. Agric. Food Chem. 2002;50:2001–2004. doi: 10.1021/jf011268s. [DOI] [PubMed] [Google Scholar]
- 10.Bao G, et al. Molecular basis for increased 2-acetyl-1-pyrroline contents under alternate wetting and drying (AWD) conditions in fragrant rice. Plant Physiol. Biochem. 2018;133:149–157. doi: 10.1016/j.plaphy.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 11.Li M, et al. Manganese-induced regulations in growth, yield formation, quality characters, rice aroma and enzyme involved in 2-acetyl-1-pyrroline biosynthesis in fragrant rice. Plant Physiol. Biochem. 2016;103:167–175. doi: 10.1016/j.plaphy.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Poonlaphdecha J, et al. Biosynthesis of 2-acetyl-1-pyrroline in rice calli cultures: demonstration of 1-pyrroline as a limiting substrate. Food Chem. 2016;197:965–971. doi: 10.1016/j.foodchem.2015.11.060. [DOI] [PubMed] [Google Scholar]
- 13.Szabados L, Savouré A. Proline: a multifunctional amino acid. Trends Plant Sci. 2010;15:89–97. doi: 10.1016/j.tplants.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Kong L, et al. Short-term water management at early filling stage improves early-season rice performance under high temperature stress in South China. Eur. J. Agron. 2017;90:117–126. doi: 10.1016/j.eja.2017.07.006. [DOI] [Google Scholar]
- 15.Rao G, et al. Ultrasonic seed treatment improved cadmium (Cd) tolerance in Brassica napus L. Ecotoxicol. Environ. Saf. 2019;185:109659. doi: 10.1016/j.ecoenv.2019.109659. [DOI] [PubMed] [Google Scholar]
- 16.Shereen A, et al. Effects of peg induced water stress on growth and physiological responses of rice genotypes at seedling stage. Pak. J. Bot. 2019;51:2013–2021. doi: 10.30848/PJB2019-6(13). [DOI] [Google Scholar]
- 17.Banerjee A, Roychoudhury A. Differential regulation of defence pathways in aromatic and non-aromatic indica rice cultivars towards fluoride toxicity. Plant Cell Rep. 2019;38:1217–1233. doi: 10.1007/s00299-019-02438-6. [DOI] [PubMed] [Google Scholar]
- 18.Chen CT, Chen L, Lin CC, Kao CH. Regulation of proline accumulation in detached rice leaves exposed to excess copper. Plant Sci. (Shannon) 2001;160:283–290. doi: 10.1016/S0168-9452(00)00393-9. [DOI] [PubMed] [Google Scholar]
- 19.Li M, et al. Rice-duck co-culture benefits grain 2-acetyl-1-pyrroline accumulation and quality and yield enhancement of fragrant rice. Crop J. 2019;7:419–430. doi: 10.1016/j.cj.2019.02.002. [DOI] [Google Scholar]
- 20.Luo H, et al. Foliar application of sodium selenate induces regulation in yield formation, grain quality characters and 2-acetyl-1-pyrroline biosynthesis in fragrant rice. BMC Plant Biol. 2019;19:502. doi: 10.1186/s12870-019-2104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen M, et al. Fragrance of the rice grain achieved via artificial microRNA-induced down-regulation of OsBADH2. Plant Breed. 2012;131:584–590. doi: 10.1111/j.1439-0523.2012.01989.x. [DOI] [Google Scholar]
- 22.Prodhan ZH, Faruq G, Taha RM, Rashid KA. Agronomic, transcriptomic and metabolomic expression analysis of aroma gene (Badh2) under different temperature regimes in rice. Int. J. Agric. Biol. 2017;19:569–576. doi: 10.17957/IJAB/15.0340. [DOI] [Google Scholar]
- 23.Sheng Z, et al. Molecular breeding of fragrant early-season hybrid rice using the Badh2 gene. Pak. J. Bot. 2019;51:2089–2095. doi: 10.30848/PJB2019-6(10). [DOI] [Google Scholar]
- 24.Lin Z, et al. Complementary proteome and transcriptome profiling in developing grains of a notched-belly rice mutant reveals key pathways involved in chalkiness formation. Plant Cell Physiol. 2017;58:560–573. doi: 10.1093/pcp/pcx001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada H, et al. Multiple strategies for heat adaptation to prevent chalkiness in the rice endosperm. J. Exp. Bot. 2019;70:1299–1311. doi: 10.1093/jxb/ery427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Şahin G. Effects of salicylic acid and heat acclimation on thermotolerance and withanolide accumulation under high temperature stress in the cape gooseberry (Physalisperuviana L.) Turk. J. Bot. 2019;43:468–474. doi: 10.3906/bot-1901-4. [DOI] [Google Scholar]
- 27.Wu M, Chou K, Wu C, Chen J, Huang T. Characterization and the possible formation mechanism of 2-acetyl-1-pyrroline in aromatic vegetable soybean (Glycine Max L.) J. Food Sci. 2009;74:S192–S197. doi: 10.1111/j.1750-3841.2009.01166.x. [DOI] [PubMed] [Google Scholar]
- 28.Hill JM. The inactivation of pea-seedling diamine oxidase by peroxidase and 1,5-diaminopentane. Biochem. J. 1967;104:1048–1055. doi: 10.1042/bj1041048. [DOI] [PMC free article] [PubMed] [Google Scholar]